Abstract
An R0 margin width of 1 cm has traditionally been considered a prerequisite to minimize local recurrence and optimize survival following hepatic resection for metastatic colorectal cancer. However, recent data have called into question the prognostic importance of the ‘1-cm rule’. Specifically, several studies have noted that, although an R0 resection is important, the actual margin width may not be as critical. We provide a brief overview of the impact of an R1 vs. an R0 resection on local recurrence and overall survival. In addition, we specifically review the impact of margin width in patients who have undergone an R0 resection. Finally, we highlight those factors most associated with an increased likelihood of an R1 resection and provide recommendations for avoiding and dealing with microscopic carcinoma discovered intraoperatively at the cut parenchymal transection margin.
Keywords: colorectal, liver, metastases, margin, outcome, recurrence
Introduction
Hepatic resection is the most effective therapy for patients with colorectal liver metastases (CLM) confined to the liver, with reported actuarial survival rates approaching 58% at 5 years,1–3 and 22–26% at 10 years.1,4–6 Several preoperative clinicopathological factors have traditionally been utilized to identify the patients who might benefit most from liver resection. These include size and number of liver tumours, primary tumour nodal status, length of disease-free interval and pre-resection carcinoembryonic antigen (CEA) level.5,7 Although they are predictive of prognosis and indicative of tumour biology, these factors are fixed and are outside the direct control of the surgeon. By contrast, the status of the resection margin is one of the few modifiable factors that have been investigated as being of prognostic importance. Although the adverse impact of leaving gross residual disease at the time of resection (R2) has been well documented, the prognostic implications of a microscopically positive surgical margin (R1) and of the width of a microscopically negative surgical margin (R0) remain controversial.
Despite the lack of extensive evidence, since the early 1980s there had been a general consensus that the optimal surgical margin during resection of CLM should measure ≥1 cm. In fact, some authors even suggested that inability to accommodate a 1-cm margin should perhaps preclude a patient from being considered for hepatic resection.8–10 Despite small patient numbers, these initial reports led to a de facto acceptance of a ‘1-cm rule’ with regard to the margin, which was utilized to guide the selection of patients for hepatic resection. More recently, however, multiple reports have questioned whether margin width has any effect on outcome as long as a negative margin is achieved.3,11–15 In fact, pathological studies from Japan have suggested that that the 1-cm rule should be completely abandoned, as micrometastases, satellitosis and Glisson sheath extension associated with CLM are exceptionally rare.16,17 Perhaps even more controversial, de Haas and colleagues recently reported similar overall survival rates following margin-negative (R0) and margin-positive (R1) hepatectomy for CLM using an aggressive approach combining chemotherapy and repeat surgery.18 To complicate the matter further, accurate assessment of margin status can sometimes be difficult as the techniques used to transect the parenchyma may vaporize, aspirate, ablate or fracture the tissue on the parenchyma edge, thereby leading to an overestimation of the resection margin.
We herein present a brief overview of the current existing literature on the topic of surgical resection margin status. Specifically, we assess the impact of a positive (R1) margin, as well as the impact of a sub-centimetre R0 resection margin on both survival and recurrence following resection of CLM.
Microscopically positive R1 margin status
Multiple studies on the resection of CLM have specifically examined the role of a microscopically positive margin on overall survival.1,3,5,8,18–20 With the exception of one recent report,18 all previous studies (Table 1) have demonstrated consistently that a microscopically positive R1 margin is strongly correlated with worse overall survival. Specifically, 5-year survival following a microscopically negative R0 resection has been reported to range from 37% to 64%, whereas 5-year survival rates after an R1 resection are less than 20%. Interestingly, the data on whether R1 margin status is an independent predictor of overall survival have been conflicting. Although several trials1,5,8 have found R1 margin status to be associated with survival on multivariate analysis, other studies3,18,19 have noted that R1 margin status was not associated with survival after controlling for competing risk factors. Because of the lack of association of R1 status with survival on multivariate analysis, some investigators have suggested that, rather than being an independent predictor of survival, R1 margin status may instead be a surrogate indicator of advanced and/or more extensive disease. As such, the negative impact of R1 status on overall survival may not derive from the leaving of microscopic tumour cells at the surgical margin, but, rather, from the more aggressive biological phenotype that makes extirpation of the tumour with negative surgical margins more difficult.
Table 1.
Reported differences in overall survival based on margin status (R0 vs. R1 resection) after hepatic resection for colorectal liver metastases
| Author(s) | Year | n | Study period | Follow-up, months | R1 rate |
Survival |
||||
|---|---|---|---|---|---|---|---|---|---|---|
|
5-year |
Median, months |
P-value | ||||||||
| R0 | R1 | R0 | R1 | |||||||
| Steele et al.20 | 1991 | 87 | 1984–1988 | 37 | 21% | – | – | 37 | 21 | <0.01 |
| Cady et al.8 | 1998 | 244 | – | 37 | 16% | – | – | 18a | 9a | <0.05b |
| Fong et al.5 | 1999 | 1001 | 1985–1998 | – | 11% | 37% | 20% | 45 | 23 | <0.001b |
| Choti et al.1 | 2002 | 226 | 1984–1999 | – | 5% | – | – | 46 | 24 | 0.04b |
| Pawlik et al.3 | 2005 | 557 | 1990–2004 | 29 | 8% | 64% | 17% | NR | 49 | 0.01 |
| Nuzzo et al.19 | 2008 | 185 | 1992–2005 | 39 | 5% | 39% | 0% | 48 | 22 | 0.01 |
| de Haas et al.18 | 2008 | 436 | 1990–2006 | 40 | 46% | 61% | 57% | 77 | 84 | 0.27 |
Indicates disease-free survival
P remained significant (<0.05) on multivariate analysis
P-values shown in bold indicate <0.05
NR, not reached
Recently, de Haas and colleagues18 published a provocative study reporting that patients undergoing R1 vs. R0 resection had comparable longterm survival outcomes. In this study, 436 patients with CLM were treated with combined modality therapy utilizing modern cytotoxic chemotherapy and surgical resection; mean follow-up was 40 months. The authors noted that CEA >10 ng/ml and receipt of major hepatectomy, but not R1 margin status, were independent predictors of poor overall survival.18 Unlike in many previous studies, 5-year overall survival rates were similar between patients who had undergone an R0 vs. an R1 resection (61% vs. 57%, respectively). The study has been criticized for its unusually high incidence of R1 resection (46%), which may have resulted in part from the aggressive surgical approach adopted by the investigators. Although the results of this study need to be corroborated, the data provide further evidence that R1 status may not be the main determinant of overall survival. Rather, it may be that emerging, more efficacious chemotherapy will provide patients who undergo an R1 resection with worse associated tumour biology better longterm outcomes.
R1 surgical resection margin status has also been strongly associated with an increased risk of both true margin (e.g. cut parenchymal transection edge) as well as ‘any-site’ intrahepatic recurrence (Table 2).3,8,16,18,19,21,22 Whereas local recurrence at the surgical margin has been reported to occur in only 3–8% of cases following an R0 resection, the rate of local recurrence has been noted to be as high as 9–55% following an R1 resection.3,16,18,19,22 The largest series to address the specific issue of margin status and local recurrence investigated 557 patients from three major hepatobiliary centres. The authors reported that CEA >200 ng/ml, tumour size >5 cm and R1 margin status were each associated with a higher overall recurrence rate, but only R1 margin status predicted true margin recurrence.3 Similarly, any-site recurrence in the liver has also been reported to be higher following an R1 resection (22–78%) compared with an R0 resection (14–38%).3,8,18,19,21 In a study of 436 patients at Hôpital Paul-Brousse in France, any-site intrahepatic recurrence was significantly higher following R1 resection.18 The finding that R1 margin status increased the risk of discontiguous any-site recurrence again implies that patients who undergo an R1 resection may have more extensive liver disease and perhaps an inherently worse overall biology that predisposes them to intrahepatic recurrence.
Table 2.
Reported differences in marginal and overall intrahepatic recurrence based on margin status (R0 vs. R1 resection) after hepatic resection for colorectal metastases
| Author(s) | Year | n | Study period | Median follow-up, months | R1 Rate |
Recurrence |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Marginal |
Overall intrahepatic |
||||||||||
| R0 | R1 | P-value | R0 | R1 | P-value | ||||||
| Hughes et al.21 | 1986 | 607 | – | – | 6% | – | – | – | 38% | 68% | <0.05b |
| Cady et al.8 | 1998 | 244 | – | 37 | 16% | – | – | – | 23% | 43% | 0.03 |
| Kokudo et al.16 | 2002 | 183 | 1980–2000 | 29 | 25%a | 6% | 20% | – | – | – | – |
| Pawlik et al.3 | 2005 | 557 | 1990–2004 | 29 | 8% | 3% | 11% | 0.003 | 14% | 22% | – |
| Wakai et al.22 | 2008 | 90 | 1989–2004 | – | 11% | 3% | 30% | 0.001b | – | – | – |
| Nuzzo et al.19 | 2008 | 185 | 1992–2005 | 39 | 5% | 4% | 55% | <0.01 | 27% | 78% | <0.01 |
| de Haas et al.18 | 2008 | 436 | 1990–2006 | 40 | 46% | 8% | 9% | 0.72 | 17% | 28% | 0.004 |
R1 resection was defined in this study as margin clearance <2 mm
P remained significant (<0.05) on multivariate analysis
P-values shown in bold indicate <0.05
Cumulative data from the literature would strongly suggest that an R1 margin status is associated with worse overall longterm survival, as well as an increased risk of margin site and intrahepatic recurrence. Although true margin recurrence may be associated with residual microscopic disease at the cut parenchymal edge, the worse overall survival and increased rate of any-site intrahepatic recurrence may reflect a worse tumour biology in patients with more extensive disease in whom it is more difficult to achieve an R0 margin. The fact remains, however, that surgeons should strive assiduously to achieve complete macro- and microscopic resection of CLM to help ensure the best overall and local outcomes for the patient.
Microscopically negative R0 margin status: is there an optimal margin width?
Although surgeons strive to achieve a complete resection with negative margins during hepatectomy for CLM, the ideal margin width to optimize longterm survival and minimize local recurrence has been the subject of considerable debate. In the mid-1980s, Ekberg et al. reported a series of 72 resected patients. In this series, the authors noted poor outcomes associated with sub-centimetre resections and therefore concluded that liver resection for CLM should not be performed if a margin ≥1 cm could not be anticipated.9 Although other studies have noted similar findings with regard to the 1-cm rule,8,10,22–24 these studies had several limitations, including small sample sizes, lack of multivariate analysis, or the inappropriate inclusion of patients with positive margins in the sub-centimetre margin category. More recently, emerging contemporary data from several institutions have begun to call the 1-cm rule into serious question. For example, Are et al.25 and Elias et al.26 demonstrated that, although a 1-cm margin should be attempted whenever possible, sub-centimetre resections were also associated with favourable outcomes and should not preclude patients from undergoing resection. In two separate studies, Kokudo et al.16 and Nuzzo et al.19 proposed that margin widths of 2 mm and 5 mm, respectively, were acceptable and led to similar outcomes compared with 1-cm margin resections. More recently, a considerable body of literature3,11–15 has emerged which strongly suggests that survival following hepatic resection for CLM is similar among patients who have undergone an R0 resection, regardless of the width of the negative margin. These investigators note that complete R0 resection – not millimetres of margin width – determines the outcome.27
In a multi-institutional study of 557 patients, Pawlik et al.3 reported similar outcomes in patients with negative margins, regardless of margin width (Fig. 1). Specifically, after hepatic resection, 225 (40.4%) patients had recurrence. This recurrence was seen at the surgical margin in 21 patients, at another intrahepatic site in 56, at an extrahepatic site in 82, and at both intrahepatic and extrahepatic sites in 66. Patients with negative margins of 1–4 mm, 5–9 mm and ≥1 cm had similar rates of true margin recurrence, as well as overall recurrence. In addition, overall survival among patients undergoing an R0 resection was similar, regardless of the width of the surgical margin. The authors concluded that the width of a negative surgical margin does not affect survival, recurrence risk or site of recurrence. A predicted margin of <1 cm after resection of hepatic CLM should not be used as an exclusion criterion for resection.
Figure 1.
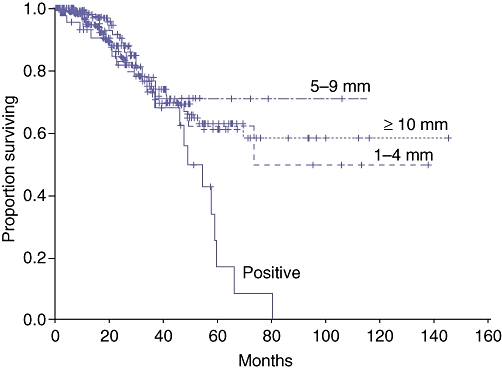
Survival stratified by margin status in 557 patients who underwent liver resection for colorectal metastases. The width of margin clearance did not affect survival as long as the margin was negative. Reproduced with permission from Pawlik et al.3
In a separate study from the Memorial Sloan–Kettering Cancer Center, Are et al. reported on 1019 patients who underwent resection with a uniform method of parenchymal transection (Kelly clamp-crush technique).25 The authors reported an R1 resection rate of 11%; 33% of patients had an R0 margin >10 mm, whereas 56% of patients had an R0 margin of ≤10 mm. When margin width was examined as a continuous variable, an incremental increase in median survival was shown as margin width increased, with two inflection points on the curve: one at approximately 0 mm and one at 10 mm (Fig. 2). On the basis of this analysis, the authors subsequently stratified patients into three groups (R1 margin, R0 margin of 1–10 mm, R0 margin >10 mm). On multivariate analysis, patients with a margin of >10 mm had better longterm survival compared with patients with an R0 resection with a margin of <10 mm. However, of note was the finding of no difference in survival among patients with a <10-mm margin, regardless of the width of the sub-centimetre margin. In fact, among patients with a sub-centimetre R0 margin, longterm survival was quite favourable, with a median survival of 42 months. As such, the authors concluded that failure to anticipate a 1-cm margin as a result of anatomic constraints or by misjudgement of the actual margin based on preoperative imaging should not preclude a patient from undergoing resection.
Figure 2.
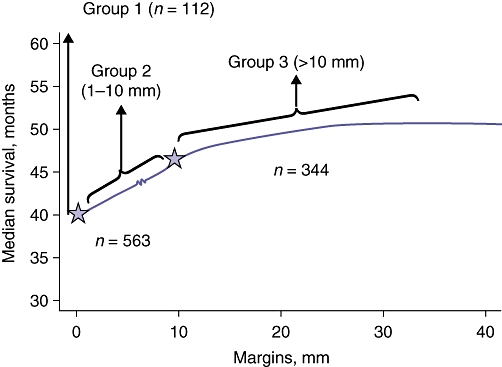
Influence of margin width (analysed as a continuous variable) on survival in 1019 patients who underwent hepatic resection for metastatic colorectal cancer. Based on the two inflection points noted on the curve at approximately 0 mm and 10 mm, patients were stratified into three groups (involved margin, 1–10 mm and >10 mm). Reproduced with permission from Are et al.25
Other studies have further corroborated that a margin width of <1 cm is acceptable as it does not necessarily lead to a worse local or overall outcome. In a study of 523 patients who had undergone R0 resection, Figueras et al.11 reported that the existence of more than three tumours and the presence of extrahepatic disease, but not margin width of <1 cm, were independently associated with hepatic recurrence. Similarly, Scheele et al.15 reported on 350 patients who underwent R0 resection. On multivariate analysis, factors including synchronous presentation, primary tumour lymph node status, poor tumour differentiation, presence of satellite lesions, and the number and size of the liver metastasis were each associated with a worse outcome. A margin width of <1 cm was, however, not associated with an adverse outcome. These data strongly suggest that biology, rather than millimetres, dictates outcome following resection of CLM.
Several studies from Japan have begun to provide molecular clues into the issue of margin width and CLM resection.16,17 Kokudo and colleagues examined specimens from 58 patients who underwent hepatic resection of CLM for K-ras and p53 mutations. In this study, the authors noted that micro-metastases adjacent to the index CLM were very uncommon, occurring in only 2% of 199 tested tumours. In addition, in the 2% of tumours that showed evidence of micro-metastatic disease adjacent to the index lesion, all were confined to within 4 mm of the tumour border.16 Although micrometastases along the Glisson pedicle were somewhat more common (14%), these were also confined within a short distance from the tumour edge (<5 mm).16 In a separate study, Yamamoto et al. analysed 89 CLM lesions resected from 40 patients.17 These authors noted that all the lesions were well circumscribed and that satellitosis was present in only one tumour.17 Taken together, these data strongly suggest that aggressive biological factors, and not necessarily margin width, dictate outcome following hepatic resection of CLM. Although achieving an R0 surgical margin remains important, the actual surgical margin width may not be as critical. Surgeons should strive to perform resections with a margin of non-tumorous liver tissue, but a predicted margin of <1 cm should not be used as a contraindication for resection. Rather, the currently available data support the concept of limited complete resection, utilizing a planned approach that encompasses all tumour-bearing hepatic parenchyma.27
Planning the hepatic resection: avoidance of the R1 margin
The presence of multiple tumours and bilateral distribution are the two most common factors associated with an increased risk of R1 resection.3,11,18,19,25,26 In addition, large tumours18 and tumours located centrally or in proximity to a major vessel24 have also been shown to be more difficult to extirpate with negative surgical margins.
In an attempt to increase the chance of an R0 resection, some investigators have advocated the use of neoadjuvant chemotherapy.28 The University of Texas MD Anderson Cancer Center reported on a series of 108 patients who underwent hepatectomy, 61 of whom had received preoperative chemotherapy. The authors reported that those patients who received preoperative chemotherapy had a significantly higher incidence of multiple tumours. Despite this, patients who had received preoperative chemotherapy had a higher rate of R0 resection compared with those who did not, although the difference failed to reach statistical significance.28 In a separate study from Memorial Sloan–Kettering Cancer Center, the use of preoperative chemotherapy was not associated with an increased rate of R0 resection with >1 cm margin width.25 Therefore, although the administration of systemic chemotherapy prior to liver resection for CLM may sometimes allow for a more parenchyma-sparing procedure, there is no definitive evidence that it necessarily leads to an increase in the likelihood of R0 surgical margins. In fact, recent data on the pathological pattern of CLM response to chemotherapy have suggested that, in addition to the dominant pattern of centripetal tumour contraction, regional differences in chemosensitivity within a single metastasis can lead to random tumour cell death throughout the tumour and the persistence of islands of viable tumour cells outside the edge of the contracted tumour.29 Such data may provide a potential explanation for the comparable R1 margin rates following resection of CLM irrespective of the use of preoperative chemotherapy.
As well as preoperative tumour-related features, intraoperative technical factors may be associated with a higher likelihood of an R1 margin status. Although some studies have suggested that non-anatomic resections24,30 may be associated with an increased risk of R1 resection, other investigators have found this not to be the case.31 Based on a retrospective series of 267 patients who underwent liver resection for CLM, DeMatteo et al. reported that anatomic segmental resection had a lower rate of positive margins compared with wedge hepatectomy (2% vs. 16%, respectively).30 However, in a separate study, Zorzi et al. reported that anatomic resection was not superior to non-anatomic resection in terms of surgical margin clearance, site of recurrence or survival.31 In this study, the authors concluded that resection with a clear surgical margin – irrespective of whether an anatomic or non-anatomic resection is performed – leads to the same acceptable outcome.
In addition, the technique of parenchymal transection does not appear to influence the likelihood of an R1 resection. In a prospective randomized trial of 132 patients undergoing partial hepatectomy for primary and metastatic liver cancer, Takayama et al. compared parenchymal transection utilizing the clamp-crush technique with ultrasonic dissection.32 The authors noted no significant difference in the incidence of margin recurrence between the two techniques.32 Other authors have also noted that the addition of saline-linked cautery to ultrasonic dissection does not decrease the likelihood of an R1 resection.33
Occasionally, following resection a positive margin is recognized intraoperatively. When gross residual disease remains (R2 resection), re-resection of the area should be undertaken because leaving gross residual disease behind should be avoided in all circumstances. If microscopic carcinoma is noted at the parenchymal transection margin based on intraoperative frozen section analysis, strategies to extend the resection margin are reasonable, but their benefit remains unproven. Fastidious intraoperative orientation and marking of the specimen for pathological assessment is critical at the time the specimen is removed. Close attention to the area along the transection line, which is worrisome for a close margin, can help direct where to re-treat the liver edge if the margin proves to be microscopically positive for carcinoma. In the study by Pawlik et al.3 different techniques were used to treat positive margins at the time of resection. When additional surgical resection was not feasible, ablation with radiofrequency or cautery was used to treat the positive margin. We currently prefer to re-resect or, when this is not feasible, to use saline-linked cautery to treat positive margins. Animal data have suggested that saline-linked surface radiofrequency ablation can achieve destruction of ≥1 cm of hepatic tissue in a porcine model.34
Conclusions
A 1-cm R0 surgical margin width has been traditionally considered necessary to avoid local intrahepatic recurrence and optimize longterm survival after hepatic resection for CLM. Recently, more rigorous multi-institutional data have reported that the likelihood of local recurrence is independent of margin width. Rather than millimetres, tumour biology is a more important predictor of both intrahepatic any-site recurrence and worse overall survival. Although an R1 resection should clearly be avoided, the actual margin width of an R0 resection does not impact on outcome after resection of CLM. Data have shown that CLM are overwhelmingly well circumscribed and are associated with very low incidences of satellitosis or micro-metastasis. Surgeons should employ a systematic approach, which should include high-quality preoperative cross-sectional imaging as well as the use of intraoperative ultrasonography. Although surgeons should not strive to achieve a ‘minimal margin’, a limited negative margin resection in patients with hepatic CRM does not seem to affect survival, local recurrence risk or site of recurrence. As such, failure to comply with the 1-cm rule can no longer be considered a contraindication for the surgical resection of CLM.
Acknowledgments
TMP is supported by a grant (no. 1KL2RR025006-01) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NCRR or the NIH.
Conflicts of interest
None declared.
References
- 1.Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, et al. Trends in longterm survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernandez FG, Drebin JA, Linehan DC, Dehdashti F, Siegel BA, Strasberg SM. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET) Ann Surg. 2004;240:438–447. doi: 10.1097/01.sla.0000138076.72547.b1. discussion 447–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715–722. doi: 10.1097/01.sla.0000160703.75808.7d. discussion 722–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict longterm survival. Ann Surg. 2004;240:644–657. doi: 10.1097/01.sla.0000141198.92114.f6. discussion 657–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. discussion 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minagawa M, Makuuchi M, Torzilli G, Takayama T, Kawasaki S, Kosuge T, et al. Extension of the frontiers of surgical indications in the treatment of liver metastases from colorectal cancer: longterm results. Ann Surg. 2000;231:487–499. doi: 10.1097/00000658-200004000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 8.Cady B, Jenkins RL, Steele GD, Jr, Lewis WD, Stone MD, McDermott WV, et al. Surgical margin in hepatic resection for colorectal metastasis: a critical and improvable determinant of outcome. Ann Surg. 1998;227:566–571. doi: 10.1097/00000658-199804000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ekberg H, Tranberg KG, Andersson R, Lundstedt C, Hagerstrand I, Ranstam J, et al. Determinants of survival in liver resection for colorectal secondaries. Br J Surg. 1986;73:727–731. doi: 10.1002/bjs.1800730917. [DOI] [PubMed] [Google Scholar]
- 10.Shirabe K, Takenaka K, Gion T, Fujiwara Y, Shimada M, Yanaga K, et al. Analysis of prognostic risk factors in hepatic resection for metastatic colorectal carcinoma with special reference to the surgical margin. Br J Surg. 1997;84:1077–1080. [PubMed] [Google Scholar]
- 11.Figueras J, Burdio F, Ramos E, Torras J, Llado L, Lopez-Ben S, et al. Effect of sub-centimetre non-positive resection margin on hepatic recurrence in patients undergoing hepatectomy for colorectal liver metastases. Evidences from 663 liver resections. Ann Oncol. 2007;18:1190–1195. doi: 10.1093/annonc/mdm106. [DOI] [PubMed] [Google Scholar]
- 12.Hamady ZZ, Cameron IC, Wyatt J, Prasad RK, Toogood GJ, Lodge JP. Resection margin in patients undergoing hepatectomy for colorectal liver metastasis: a critical appraisal of the 1-cm rule. Eur J Surg Oncol. 2006;32:557–563. doi: 10.1016/j.ejso.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Bodingbauer M, Tamandl D, Schmid K, Plank C, Schima W, Gruenberger T. Size of surgical margin does not influence recurrence rates after curative liver resection for colorectal cancer liver metastases. Br J Surg. 2007;94:1133–1138. doi: 10.1002/bjs.5762. [DOI] [PubMed] [Google Scholar]
- 14.Altendorf-Hofmann A, Scheele J. A critical review of the major indicators of prognosis after resection of hepatic metastases from colorectal carcinoma. Surg Oncol Clin N Am. 2003;12:165–192. xi. doi: 10.1016/s1055-3207(02)00091-1. [DOI] [PubMed] [Google Scholar]
- 15.Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg. 1995;19:59–71. doi: 10.1007/BF00316981. [DOI] [PubMed] [Google Scholar]
- 16.Kokudo N, Miki Y, Sugai S, Yanagisawa A, Kato Y, Sakamoto Y, et al. Genetic and histological assessment of surgical margins in resected liver metastases from colorectal carcinoma: minimum surgical margins for successful resection. Arch Surg. 2002;137:833–840. doi: 10.1001/archsurg.137.7.833. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto J, Sugihara K, Kosuge T, Takayama T, Shimada K, Yamasaki S, et al. Pathologic support for limited hepatectomy in the treatment of liver metastases from colorectal cancer. Ann Surg. 1995;221:74–78. doi: 10.1097/00000658-199501000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Haas RJ, Wicherts DA, Flores E, Azoulay D, Castaing D, Adam R. R1 resection by necessity for colorectal liver metastases: is it still a contraindication to surgery? Ann Surg. 2008;248:626–637. doi: 10.1097/SLA.0b013e31818a07f1. [DOI] [PubMed] [Google Scholar]
- 19.Nuzzo G, Giuliante F, Ardito F, Vellone M, Giovannini I, Federico B, et al. Influence of surgical margin on type of recurrence after liver resection for colorectal metastases: a single-centre experience. Surgery. 2008;143:384–393. doi: 10.1016/j.surg.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 20.Steele G, Jr, Bleday R, Mayer RJ, Lindblad A, Petrelli N, Weaver D. A prospective evaluation of hepatic resection for colorectal carcinoma metastases to the liver: Gastrointestinal Tumor Study Group Protocol 6584. J Clin Oncol. 1991;9:1105–1112. doi: 10.1200/JCO.1991.9.7.1105. [DOI] [PubMed] [Google Scholar]
- 21.Hughes KS, Simon R, Songhorabodi S, Adson MA, Ilstrup DM, Fortner JG, et al. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of patterns of recurrence. Surgery. 1986;100:278–284. [PubMed] [Google Scholar]
- 22.Wakai T, Shirai Y, Sakata J, Valera VA, Korita PV, Akazawa K, et al. Appraisal of 1-cm hepatectomy margins for intrahepatic micrometastases in patients with colorectal carcinoma liver metastasis. Ann Surg Oncol. 2008;15:2472–2481. doi: 10.1245/s10434-008-0023-y. [DOI] [PubMed] [Google Scholar]
- 23.Kato T, Yasui K, Hirai T, Kanemitsu Y, Mori T, Sugihara K, et al. Therapeutic results for hepatic metastasis of colorectal cancer with special reference to effectiveness of hepatectomy: analysis of prognostic factors for 763 cases recorded at 18 institutions. Dis Colon Rectum. 2003;46(10) Suppl.:22–31. doi: 10.1097/01.DCR.0000089106.71914.00. [DOI] [PubMed] [Google Scholar]
- 24.Wray CJ, Lowy AM, Mathews JB, Park S, Choe KA, Hanto DW, et al. The significance and clinical factors associated with a sub-centimetre resection of colorectal liver metastases. Ann Surg Oncol. 2005;12:374–380. doi: 10.1245/ASO.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 25.Are C, Gonen M, Zazzali K, Dematteo RP, Jarnagin WR, Fong Y, et al. The impact of margins on outcome after hepatic resection for colorectal metastasis. Ann Surg. 2007;246:295–300. doi: 10.1097/SLA.0b013e31811ea962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elias D, Cavalcanti A, Sabourin JC, Lassau N, Pignon JP, Ducreux M, et al. Resection of liver metastases from colorectal cancer: the real impact of the surgical margin. Eur J Surg Oncol. 1998;24:174–179. doi: 10.1016/s0748-7983(98)92878-5. [DOI] [PubMed] [Google Scholar]
- 27.Pawlik TM, Vauthey JN. Surgical margins during hepatic surgery for colorectal liver metastases: complete resection not millimetres defines outcome. Ann Surg Oncol. 2008;15:677–679. doi: 10.1245/s10434-007-9703-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parikh AA, Gentner B, Wu TT, Curley SA, Ellis LM, Vauthey JN. Perioperative complications in patients undergoing major liver resection with or without neoadjuvant chemotherapy. J Gastrointest Surg. 2003;7:1082–1088. doi: 10.1016/j.gassur.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Ng JK, Urbanski SJ, Mangat N, McKay A, Sutherland FR, Dixon E, et al. Colorectal liver metastases contract centripetally with a response to chemotherapy: a histomorphologic study. Cancer. 2008;112:362–371. doi: 10.1002/cncr.23184. [DOI] [PubMed] [Google Scholar]
- 30.DeMatteo RP, Palese C, Jarnagin WR, Sun RL, Blumgart LH, Fong Y. Anatomic segmental hepatic resection is superior to wedge resection as an oncologic operation for colorectal liver metastases. J Gastrointest Surg. 2000;4:178–184. doi: 10.1016/s1091-255x(00)80054-2. [DOI] [PubMed] [Google Scholar]
- 31.Zorzi D, Mullen JT, Abdalla EK, Pawlik TM, Andres A, Muratore A, et al. Comparison between hepatic wedge resection and anatomic resection for colorectal liver metastases. J Gastrointest Surg. 2006;10:86–94. doi: 10.1016/j.gassur.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 32.Takayama T, Makuuchi M, Kubota K, Harihara Y, Hui AM, Sano K, et al. Randomized comparison of ultrasonic vs. clamp transection of the liver. Arch Surg. 2001;136:922–928. doi: 10.1001/archsurg.136.8.922. [DOI] [PubMed] [Google Scholar]
- 33.Aloia TA, Zorzi D, Abdalla EK, Vauthey JN. Two-surgeon technique for hepatic parenchymal transection of the non-cirrhotic liver using saline-linked cautery and ultrasonic dissection. Ann Surg. 2005;242:172–177. doi: 10.1097/01.sla.0000171300.62318.f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Topp SA, McClurken M, Lipson D, Upadhya GA, Ritter JH, Linehan D, et al. Saline-linked surface radiofrequency ablation: factors affecting steam popping and depth of injury in the pig liver. Ann Surg. 2004;239:518–527. doi: 10.1097/01.sla.0000118927.83650.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]


