Abstract
Background:
To determine factors associated with outcomes and microvascular invasion (MVI) in patients undergoing liver transplantation (LT) for hepatocellular carcinoma (HCC).
Methods:
Between July 1996 and August 2008 at the Universities of Kentucky or Tennessee, LT recipients were retrospectively analysed.
Results:
One hundred and one patients had HCC in the explanted liver; one patient was excluded because of fibrolamellar histology. Seventy-nine (79%) were male and 81 (81%) were older than 50. HCC was incidental in 32 patients (32%). Median follow-up was 31 months. Ten patients (10%) developed recurrence, which was associated with poor survival (P= 0.006). Overall 1-, 3-, and 5-year survival rates were 87%, 69% and 62%, respectively. Excluding patients with lymph node metastasis (LNM) or MVI yielded 91%, 81% and 75% survival at the same time points. MVI was independently associated with recurrence (OR 28.40, 95% CI 1.77–456.48, P= 0.018) and decreased survival (OR 4.70, 95% CI 1.24–17.80, P= 0.023), and LNM with decreased survival (OR 6.05, 95% CI 1.23–29.71, P= 0.027). Tumour size (OR 4.1, 95% CI 1.2–13.5, P= 0.013) and alpha-fetoproptein (AFP) > 100 (OR 5.0, 95% CI 1.4–18.1, P= 0.006) were associated with MVI.
Conclusions:
MVI greatly increases the risk of recurrence and death after LT for HCC, and is strongly associated with tumour size and AFP > 100.
Keywords: hepatocellular carcinoma, liver transplantation, microvascular invasion
Introduction
Hepatocellular carcinoma (HCC) is the most common primary malignancy of the liver and represents the third leading cause of cancer-related deaths worldwide.1 Although many patients are candidates for surgical resection, liver transplantation (LT) is the preferred treatment for HCC as it offers not only removal of the primary lesion and any additional tumour foci but also treatment for the concomitant cirrhosis that is the setting for many HCC cases. Early experience with LT in non-selected HCC patients yielded high death rates from recurrent disease.2,3 In light of perpetual organ shortage, these results called into question the justification of LT to treat HCC. Later experience, however, showed that patients with HCC, if carefully selected on the basis of tumour size and number, could fare as well after LT as patients transplanted for other indications.4–6 Rate of tumour recurrence is much lower after LT than surgical resection7–9 but is still the major limitation for long-term survival.
Besides tumour recurrence, several other factors have been associated with decreased patient survival after LT for HCC in previous studies, namely microvascular invasion (MVI), histological grade, tumour size and elevated alpha-fetoprotein (AFP).10,11 In these studies, tumour size has been reported to be associated with MVI, but the relationship between AFP and MVI is less clear. As MVI is most consistently shown to affect survival, we wanted to evaluate outcomes of patients from two centres who underwent LT for HCC and determine factors associated with MVI in this patient population.
Materials and methods
Records of all patients who received LT at the University of Tennessee and University of Kentucky transplant centres between January 1996 and August 2008 were reviewed. Data obtained included demographic, clinical (ascites, encephalopathy, variceal bleeding and jaundice) and laboratory values [bilirubin, prothrombin time and partial thromboplastin time, albumin, blood urea nitrogen (BUN), creatinine and AFP], time on the transplant waiting list, tumour characteristics (size, number, cellular differentiation, capsular or vascular invasion and known vs. incidental presentation), aetiology of cirrhosis, hospital length of stay, adjuvant treatment, presence of recurrence, characteristics of recurrence (site and time) and survival. Histological classification was according to the tumour-node-metastasis (TNM) and International Union Against Cancer (UICC) systems, performed on explanted livers by the interpreting pathologist.
For all patients, post-transplant immunosuppression consisted of a combination of tacrolimus or cyclosporine, mycophenolate mofetil (MMF) and steroids. MMF therapy was typically discontinued at 6 months and steroids rapidly tapered; beyond 1 year, most patients were maintained on monotherapy with either tacrolimus or cyclosporine. Bridging therapy such as transarterial chemoembolization (TACE), radiofrequency ablation (RFA) or percutaneous ethanol injection (PEI) was used in patients with large, rapidly growing lesions or those with a long anticipated waiting time, at the discretion of the evaluating surgeon or hepatologist. Post-transplant surveillance consisted of AFP levels and triphasic computed tomography (CT) imaging every 3–6 months through the first year, then annually thereafter.
The date of tumour recurrence was defined in one of two ways, either by observation of newly-increased AFP values along with subsequent detection of tumour recurrence by imaging (e.g. CT, ultrasound or chest X-ray) in patients with AFP-positive tumours, or by the date of positive imaging findings and histological confirmation in patients with AFP-negative tumours.
Statistical analysis
Analyses were performed using SPSS version 16 (SPSS Inc., Chicago, IL, USA). Overall and disease-free survival rates were calculated using the Kaplan–Meier method and statistical significance defined using the log-rank test. Multivariate analyses for survival and time to recurrence were performed using Cox proportional hazards model. Fisher's exact test was used for univariate analysis of dichotomized variables, and logistic regression for multivariate analysis. Differences were considered significant at P≤ 0.05 in all cases. Results are expressed as the median or mean ± SD.
Results
Within the study timeframe, a total of 845 LT were performed at the University of Tennessee and University of Kentucky transplant centres. Of those, 101 patients had histologically-proven HCC in the explanted liver. Ninety-eight patients received deceased-donor organs, whereas three patients received grafts from live donors. One patient had fibrolamellar histology and was therefore excluded from analysis. Patient characteristics are reported in Table 1. End-stage liver disease was attributed to viral infection in 61 patients (61%), including 51 patients with hepatitis C (51%) and 10 with hepatitis B (10%). Forty-one patients (41%) had alcohol-related cirrhosis. Bridging therapy (TACE, RFA, PEI, or a combination thereof) was employed in 13 patients (13%). Twelve patients (12%) were considered Child–Pugh class A, 41 (41%) were class B and 47 (47%) were class C; 7 patients (7%) had model for end-stage liver disease (MELD) scores >15.
Table 1.
Patient characteristics
| Characteristic | No. (%) or Mean ± SD (range) |
|---|---|
| Male | 79 (79) |
| Age, years | 55.7 ± 8.0 (36–72) |
| Patients with Encephalopathy | 49 (49) |
| Patients with Variceal Bleeding | 33 (33) |
| Patients with Ascites | 59 (59) |
| AFP level (IU) | 195.6 ± 610 (1–4560) |
| Aetiology of cirrhosis: | |
| Hepatitis B | 10 (10) |
| Hepatitis C | 51 (51) |
| Alcohol | 41 (41) |
| Child–Pugh Class: | |
| A | 12 (12) |
| B | 41 (41) |
| C | 47 (47) |
| Follow-up, months | Median, 31 (1–101) |
| Patients alive | 76 (76) |
AFP, alpha-fetoprotein.
Table 2 lists tumour characteristics. In 48 patients (48%), a single tumour was present. Mean tumour size was 2.6 ± 1.5 cm (range, 0.7–10 cm). Mean AFP was 195.6 ± 610.0. Thirty-two patients (32%) had HCC that was found incidentally. There were 30 patients (30%) with MVI invasion. Nineteen patients were found to have AFP > 100, and 26 patients were found to have tumours larger than 3 cm. Median follow-up was 31 months (range, 1–101 months); of the 100 patients, 76 (76%) are currently alive. Four patients (4%) died within 30 days; causes of death were primary graft non-function, portal vein thrombosis and massive variceal haemorrhage, sepsis and massive pulmonary embolism. We did not identify a significant difference in patient survival in the pre- and post-MELD eras (P= 0.141). However, there is a long-term survival difference in favour of those in the recent MELD era.
Table 2.
Tumour characteristics
| Characteristic | No. (%) or Mean ± SD (range) |
|---|---|
| Size, cm | 2.6 ± 1.5 (0.7–10 cm) |
| MVI | 30 (30) |
| Capsular invasion | 11 (11) |
| Poorly-differentiated | 19 (19) |
| Lymph node invasion | 4 (4) |
| Number of tumours | |
| 1 | 48 (48) |
| 2 | 15 (15) |
| 3 | 6 (6) |
| ≥4 | 27 (27) |
| Data unavailable | 4 (4) |
MVI, microvascular invasion.
Causes of later death for the remaining 20 patients are listed in Table 3. All four patients who were found incidentally to have lymph node involvement on explant pathology died; two of these patients died within the first year after LT, one after 18 months and the fourth at 30 months post-LT (causes of death were stroke, liver failure and two cases of recurrent disease). Overall 1-, 3-, and 5-year survival rates were 87%, 69%, and 62%, respectively (Fig. 1). Excluding those with MVI, respective survival was higher at 92%, 78% and 72% (Fig. 2). Overall disease-free survival at 1, 3, and 5 years was 84%, 67% and 59%, respectively (Fig. 3). Ten patients subsequently experienced HCC recurrence, the sites of which are listed in Table 4. Recurrence developed within the first year after LT in four patients, between 1 and 3 years in two patients, and beyond 3 years in four patients. Median time for recurrence was 22 months (range, 4–60 months) after LT. Excluding patients with lymph node involvement, MVI yielded impressive survival rates of 91%, 81%, and 75% at 1, 3, and 5 years, respectively (Table 5).
Table 3.
Causes of patient death >30 days after transplantation
| Aetiology | No. of patients |
|---|---|
| Tumour recurrence | 9 |
| Intracranial haemorrhage | 3 |
| Sepsis/multi-organ failure | 3 |
| Chronic rejection | 2 |
| Portal hypertension | 1 |
| Pancreatic cancer | 1 |
| Unknown | 1 |
Figure 1.
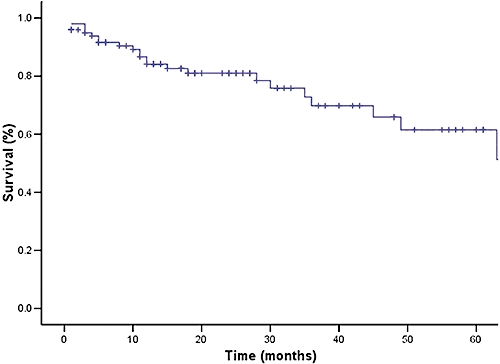
Overall survival in patients undergoing transplantation for hepatocellular carcinoma
Figure 2.
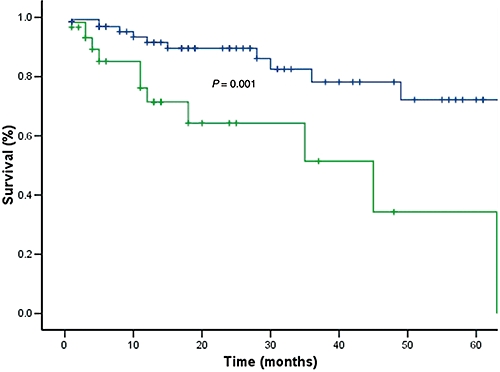
Significant difference in patient survival in the presence of microvascular invasion
Figure 3.
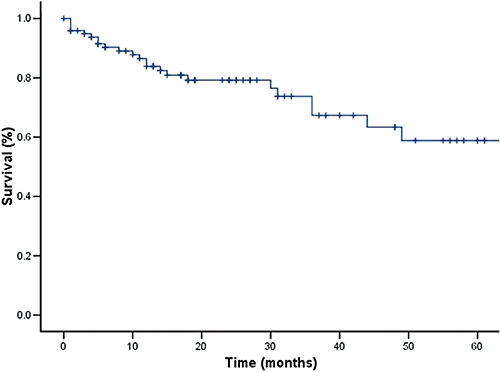
Disease-free survival in patients undergoing transplantation for hepatocellular carcinoma
Table 4.
Anatomic sites of tumour recurrence
| Site(s) | No. of patients |
|---|---|
| Liver | 3 |
| Liver with lung | 1 |
| Liver with retroperitoneal lymph nodes | 2 |
| Lungs | 2 |
| Retroperitoneum | 1 |
| Pelvis | 1 |
Table 5.
Survival
| Time | Overall | Disease free | (-) MVI | (-) MVI (-) nodal invasion |
|---|---|---|---|---|
| 1 year | 87% | 84% | 92% | 91% |
| 3 years | 69% | 67% | 78% | 81% |
| 5 years | 62% | 59% | 72% | 75% |
Analysis of recurrence
Multivariate analysis controlling for age, gender and factors found on univariate analysis to be predictors of tumour recurrence (MVI, tumour size and incidental vs. known lesion) showed MVI to be the only independent factor associated with a higher recurrence rate [odds ratio (OR) 28.40, 95% confidence interval (CI) 1.77–456.48, P= 0.018]. Out of those 10 patients with tumour recurrence, seven had MVI on pathological examination, compared with three without MVI. Tumour recurrence was strongly associated with poor patient survival in our series (P= 0.006).
Univariate and multivariate analyses of survival
Univariate analysis was performed examining age and gender; the presence of jaundice, weight loss, ascites, hepatitis B or C, elevated AFP levels; whether HCC was known or found incidentally; tumour size, tumor stage, number of lesions, MVI, lymph node invasion, histological differentiation; post-transplant chemotherapy; waiting time and MELD score. Tumour size (greater than 5 cm, P= 0.015), AFP > 100 (P= 0.021), MVI (P= 0.001) and lymph node metastasis (P < 0.001) were found to be associated with poor patient survival. We were unable to show a significant difference in survival in patients with more than three lesions compared with other patients within Milan criteria (P= NS).
Multivariate analysis including age, gender and factors identified as significant on univariate analysis (tumour size, AFP, MVI and lymph node involvement) demonstrated that MVI and lymph node metastasis are independently associated with poor survival in this patient population (MVI, OR 4.70, 95% CI 1.24–17.80, P= 0.023; lymph node involvement, OR 6.05, 95% CI 1.23–29.71, P= 0.027).
Factors associated with microvascular invasion on explanted livers
As MVI is a post-operative finding yet carries strong implications for outcome, we wanted to identify pre-operative factors that are associated with MVI. Univariate analysis showed that age (P= 0.050), tumour size greater than 3 cm (P < 0.001), AFP > 100 (P < 0.001) and the presence of multiple lesions (P= 0.004) are associated with an increased risk of developing microvascular invasion. Controlling for these factors demonstrated that tumour size and AFP > 100 are each associated with MVI (tumour size, OR 4.1, 95% CI 1.2–13.5, P= 0.013; elevated AFP, OR 5.0, 95% CI 1.4–18.1, and P= 0.006). We also repeated the analysis using only known tumours to determine predictors of MVI. In this subset, AFP > 100 continued to be an independent variable associated with MVI (P= 0.034 and OR 4.53). The positive predictive value (PPV) of large HCC and AFP > 100 for MVI is 88%, and for death is 55%.
Discussion
Liver transplantation is now a widely accepted therapy for HCC, particularly when anatomic or functional limitations preclude resection. In fact, the multifocal nature of these lesions, along with the common finding of dysplastic nodules, strongly suggest that total hepatectomy with liver replacement is the best treatment for HCC in the setting of cirrhosis. Although initial LT results in non-selected patients with HCC were discouraging, subsequent series proved that better results could be achieved by employing defined selection criteria.4–6 The criteria proposed by Mazzaferro et al.4 in 1996 are used as a basic stratification tool by numerous transplantation centres worldwide, including the two centres contributing to the present study. Using these Milan criteria, a patient with a single tumour measuring 5 cm or less, or three or fewer nodules each smaller than 3 cm, would be a candidate for LT. Excellent 5-year survival rates of 50–70% or higher have been achieved when these criteria are followed.12–14 Subsequently, the University of California at San Francisco reported 57% survival for patients with HCC who exceeded the Milan criteria but were within the limits of their expanded criteria, including patients with solitary lesions less than 6.5 cm in size, or up to three tumours with the largest no more than 4.5 cm and a combined tumour diameter of no more than 8 cm.15
Interestingly, a significant portion of our patients had previously undiagnosed HCC in the explanted liver at the time of LT. In these patients, current imaging studies (CT and MRI) failed to demonstrate the presence of malignancy. Although our percentage of incidentally found lesions is high, it is also worth noting the number of patients in our series with small tumours (16 were 1 cm or smaller), which are more difficult to detect using current imaging protocols. Llovet et al. recently published that only 30% of such lesions are confirmed by non-invasive criteria.16 This highlights the importance of improving staging techniques for patients with HCC prior to LT, to allow more accurate patient selection.
Tumour recurrence is a major problem after LT, as well as after resection.7–9 Iwatsuki et al. previously identified bilobar tumour distribution, size of the greatest lesion and vascular invasion as strong risk factors for tumour recurrence after LT17. Other strong predictors of disease recurrence identified in other studies include vascular invasion and histopathological grade.5,12 In our series, patients with MVI were more than 28 times more likely to develop recurrence. With a median follow-up of 18 months, 10 patients showed recurrent disease. MVI was also associated with poor survival using multivariate analysis. This is clearly demonstrated by the differences in survival between patients with and without MVI.
Neoadjuvant therapy or bridging intervention (e.g. TACE, RFA or PEI) is currently recommended by some institutions for patients awaiting LT. The Barcelona Clinic Liver Cancer Group published a Markov decision model on the cost-effectiveness of bridging therapy in this setting, which demonstrated that a patient expected to wait longer than 6 months for LT would gain quality-adjusted life years with PEI18 In our series, bridging therapy was employed in 13 patients, when long waiting times were expected or in patients with large or rapidly growing lesions. No improvement in survival was seen in this sub-group of patients. Only one of the seven patients who received RFA subsequently showed absolutely no remaining cancer in the explanted liver, suggesting that RFA may work by decreasing tumour burden as previously proposed.9 At our centres, chemotherapy is currently utilized in patients with large tumours, or those with capsular or vascular invasion. However, we did not observe a significant improvement in survival, in contrast to what has been reported elsewhere.19 Recently, sorafenib, an oral multikinase inhibitor, has been shown to have a significant impact on survival in patients with advanced HCC.20 This information suggests the need for new clinical trials to determine its utility and safety in patients after TACE, ablation, resection or transplantation.
In our series, all four patients with incidentally found lymph node metastases died within 30 months, and three out of the four died within 18 months after LT. Thus, finding lymph node enlargement at the time of LT presents a dilemma in these patients. Lymph nodes which are enlarged or otherwise suspicious in appearance upon exploration should be sampled and sent for frozen section, in light of the significance of invasion and its correlation with outcome. Patients without lymph node involvement or MVI experienced 5-year survival in the range of 75%, demonstrating that excellent results can be achieved in a very select group of patients. A recent meta-analysis questioned the impact of lymph node invasion on both recurrence and survival, and only five studies were found to address this issue directly.21 Considering the impact of having positive lymph nodes on outcome, and given that enlarged nodes are not uncommonly found in cirrhotic patients, the meta-analysis concluded that lymph node sampling could better identify patients with a greater likelihood of positive outcome.
Other series have determined factors associated with MVI in patients with HCC undergoing resection.10 Our results show that MVI and lymph node involvement are associated with decreased survival, with OR of 4.7 and 6.05, respectively, in agreement with other studies in patients with HCC undergoing either liver resection or LT10,22 Because of its prevalence among our patients and its implications for survival, we sought to determine factors associated with the presence of MVI. We found that both tumour size and AFP were strongly related to the presence of MVI on the explanted liver, in agreement with previous studies.23,24 In our series, size and AFP > 100 increased the risk of having concurrent MVI by 4 and 5 times, respectively. Once incidental tumours were removed from the analysis for predictors of the presence of MVI, AFP > 100 emerged as an independent factor associated with MVI. Tumour size has been found to be associated with the presence of MVI after resection and LT25,26 AFP in conjunction with other markers such as des-gamma-carboxyprothrombin and AFP-L3 has been associated with tumour burden.27 Other studies using AFP have identified significance at much higher levels, even 1000 or greater in Pawlik et al.10 We consistently found significance at much lower AFP thresholds (100 and 200), raising the possibility that AFP itself is a sensitive predictor of outcome. Interestingly, the presence of a large HCC and AFP > 100 showed a PPV of 88% for MVI. However, the PPV of these two variables to predict death is only 55%, and therefore should not be used to exclude patients for OLT.
In summary, total hepatectomy with subsequent transplantation has the inherent advantage of en bloc resection of not only the tumour, but indeed the entire liver, addressing any known (or unknown) multifocal disease. Adherence to strict selection criteria can achieve excellent results in selected patients. However, recurrence continues to be a significant problem after LT, and identification of predictive factors could prove valuable for developing therapeutic strategies. We have found that MVI is a strong predictor of tumour recurrence and thus of patient survival in our patient population. As the extent of invasion is often determined retrospectively, tumour size and AFP can be considered the best surrogates for vascular invasion and possible indicators of more aggressive lesions. This in part validates the utilization of the current criteria (e.g. Milan and UCSF) wherein tumour size and number of lesion are used to select candidates for LT. Nevertheless, we were unable to demonstrate that the number of lesions was associated with poor outcomes or a more aggressive type of cancer. The value of bridging therapy, such as chemoembolization or ablation, in conjunction with LT and adjuvant therapy to prevent tumour recurrence and improve patient survival remains uncertain. Further evaluation of these methods in large prospective series should be undertaken.
Conflicts of interest
None declared
References
- 1.Caldwell S, Park SH. The epidemiology of hepatocellular cancer: from the perspectives of public health problem to tumor biology. J Gastroenterol. 2009;44(Suppl. 19):96–101. doi: 10.1007/s00535-008-2258-6. [DOI] [PubMed] [Google Scholar]
- 2.Penn I. Hepatic transplantation for primary and metastatic cancers of the liver. Surgery. 1991;110:726–734. discussion 34-5. [PubMed] [Google Scholar]
- 3.Iwatsuki S, Gordon RD, Shaw BW, Jr, Starzl TE. Role of liver transplantation in cancer therapy. Ann Surg. 1985;202:401–407. doi: 10.1097/00000658-198510000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 5.Hemming AW, Cattral MS, Reed AI, Van Der Werf WJ, Greig PD, Howard RJ. Liver transplantation for hepatocellular carcinoma. Ann Surg. 2001;233:652–659. doi: 10.1097/00000658-200105000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Figueras J, Ibanez L, Ramos E, Jaurrieta E, Ortiz-De-Urbina J, Pardo F, et al. Selection criteria for liver transplantation in early-stage hepatocellular carcinoma with cirrhosis: results of a multicenter study. Liver Transpl. 2001;7:877–883. doi: 10.1053/jlts.2001.27856. [DOI] [PubMed] [Google Scholar]
- 7.Broelsch CE, Frilling A, Malago M. Hepatoma – resection or transplantation. Surg Clin North Am. 2004;84:495–511. doi: 10.1016/j.suc.2003.11.001. x. [DOI] [PubMed] [Google Scholar]
- 8.Helton WS, Di Bisceglie A, Chari R, Schwartz M, Bruix J. Treatment strategies for hepatocellular carcinoma in cirrhosis. J Gastrointest Surg. 2003;7:401–411. doi: 10.1016/s1091-255x(02)00161-0. [DOI] [PubMed] [Google Scholar]
- 9.Island ER, Pomposelli J, Pomfret EA, Gordon FD, Lewis WD, Jenkins RL. Twenty-year experience with liver transplantation for hepatocellular carcinoma. Arch Surg. 2005;140:353–358. doi: 10.1001/archsurg.140.4.353. [DOI] [PubMed] [Google Scholar]
- 10.Pawlik TM, Delman KA, Vauthey JN, Nagorney DM, Ng IO, Ikai I, et al. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11:1086–1092. doi: 10.1002/lt.20472. [DOI] [PubMed] [Google Scholar]
- 11.Shetty K, Timmins K, Brensinger C, Furth EE, Rattan S, Sun W, et al. Liver transplantation for hepatocellular carcinoma validation of present selection criteria in predicting outcome. Liver Transpl. 2004;10:911–918. doi: 10.1002/lt.20140. [DOI] [PubMed] [Google Scholar]
- 12.Jonas S, Bechstein WO, Steinmuller T, Herrmann M, Radke C, Berg T, et al. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33:1080–1086. doi: 10.1053/jhep.2001.23561. [DOI] [PubMed] [Google Scholar]
- 13.Colella G, De Carlis L, Rondinara GF, Sansalone CV, Belli LS, Aseni P, et al. Is hepatocellular carcinoma in cirrhosis an actual indication for liver transplantation? Transplant Proc. 1997;29:492–494. doi: 10.1016/s0041-1345(96)00221-7. [DOI] [PubMed] [Google Scholar]
- 14.Tamura S, Kato T, Berho M, Misiakos EP, O'Brien C, Reddy KR, et al. Impact of histological grade of hepatocellular carcinoma on the outcome of liver transplantation. Arch Surg. 2001;136:25–30. discussion 1. [PubMed] [Google Scholar]
- 15.Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 16.Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48(Suppl. 1):S20–S37. doi: 10.1016/j.jhep.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 17.Iwatsuki S, Dvorchik I, Marsh JW, Madariaga JR, Carr B, Fung JJ, et al. Liver transplantation for hepatocellular carcinoma: a proposal of a prognostic scoring system. J Am Coll Surg. 2000;191:389–394. doi: 10.1016/s1072-7515(00)00688-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Llovet JM, Mas X, Aponte JJ, Fuster J, Navasa M, Christensen E, et al. Cost effectiveness of adjuvant therapy for hepatocellular carcinoma during the waiting list for liver transplantation. Gut. 2002;50:123–128. doi: 10.1136/gut.50.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olthoff KM, Rosove MH, Shackleton CR, Imagawa DK, Farmer DG, Northcross P, et al. Adjuvant chemotherapy improves survival after liver transplantation for hepatocellular carcinoma. Ann Surg. 1995;221:734–741. doi: 10.1097/00000658-199506000-00012. discussion 1-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 21.Sotiropoulos GC, Malago M, Molmenti EP, Losch C, Lang H, Frilling A, et al. Hilar lymph nodes sampling at the time of liver transplantation for hepatocellular carcinoma: to do or not to do? Meta-analysis to determine the impact of hilar lymph nodes metastases on tumor recurrence and survival in patients with hepatocellular carcinoma undergoing liver transplantation. Transpl Int. 2007;20:141–146. doi: 10.1111/j.1432-2277.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- 22.Klintmalm GB. Liver transplantation for hepatocellular carcinoma: a registry report of the impact of tumor characteristics on outcome. Ann Surg. 1998;228:479–490. doi: 10.1097/00000658-199810000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marubashi S, Dono K, Sugita Y, Asaoka T, Hama N, Gotoh K, et al. Alpha-fetoprotein mRNA detection in peripheral blood for prediction of hepatocellular carcinoma recurrence after liver transplantation. Transplant Proc. 2006;38:3640–3642. doi: 10.1016/j.transproceed.2006.10.067. [DOI] [PubMed] [Google Scholar]
- 24.Sakata J, Shirai Y, Wakai T, Kaneko K, Nagahashi M, Hatakeyama K. Preoperative predictors of vascular invasion in hepatocellular carcinoma. Eur J Surg Oncol. 2008;34:900–905. doi: 10.1016/j.ejso.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 25.Lohe F, Angele MK, Rentsch M, Graeb C, Gerbes A, Lohrs U, et al. Multifocal manifestation does not affect vascular invasion of hepatocellular carcinoma: implications for patient selection in liver transplantation. Clin Transplant. 2007;21:696–701. doi: 10.1111/j.1399-0012.2007.00707.x. [DOI] [PubMed] [Google Scholar]
- 26.Esnaola NF, Lauwers GY, Mirza NQ, Nagorney DM, Doherty D, Ikai I, et al. Predictors of microvascular invasion in patients with hepatocellular carcinoma who are candidates for orthotopic liver transplantation. J Gastrointest Surg. 2002;6:224–232. doi: 10.1016/s1091-255x(01)00015-4. discussion 32. [DOI] [PubMed] [Google Scholar]
- 27.Durazo FA, Blatt LM, Corey WG, Lin JH, Han S, Saab S, et al. Des-gamma-carboxyprothrombin, alpha-fetoprotein and AFP-L3 in patients with chronic hepatitis, cirrhosis and hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23:1541–1548. doi: 10.1111/j.1440-1746.2008.05395.x. [DOI] [PubMed] [Google Scholar]


