Abstract
Background:
Borderline resectable pancreatic cancers are technically amenable to surgical resection, but are associated with increased risk of locoregional recurrence. Patients with these tumours may be treated with neoadjuvant therapy in an attempt to improve margin-negative resection rates.
Methods:
The University of Cincinnati Pancreatic Cancer Database was retrospectively reviewed. Borderline resectable disease was defined by the following radiographic criteria: (i) short segment occlusion of the superior mesenteric vein (SMV), portal vein (PV) or SMV/PV confluence; (ii) short segment hepatic artery encasement, or (iii) superior mesenteric artery/coeliac artery abutment of <180 degrees. Patients with resectable disease who had questionable metastatic disease or poor performance status were also included.
Results:
Twenty-nine patients met the criteria. Of these, 26 underwent a full course of neoadjuvant therapy. Twelve (46%) underwent surgical resection and 14 had tumour progression or were deemed unresectable at laparotomy. The most common neoadjuvant therapy regimen was gemcitabine-based chemotherapy alone (58%). Of those undergoing surgery, 67% had margin-negative (R0) resections and 42% required venous resection. Median survival was 15.5 months for unresected patients and 23.3 months for resected patients.
Discussion:
Borderline resectable pancreatic tumours can be treated neoadjuvantly, resulting in margin-negative resection and survival rates similar to those in initially resectable disease.
Keywords: pancreatic cancer, borderline resectable, neoadjuvant therapy
Introduction
Over the last decade, relative consensus has evolved around the definition of resectability for adenocarcinoma of the pancreatic head. These criteria are based on objective, cross-sectional imaging and include: (i) absence of extrapancreatic disease; (ii) no involvement of the superior mesenteric artery (SMA), hepatic artery and coeliac axis, and (iii) patency of the superior mesenteric vein (SMV)/portal vein (PV) confluence. There is a subgroup of tumours which have been increasingly recognized and recently described as ‘borderline resectable’. The operational definition of borderline resectable is that these tumours can be resected, albeit with an increased risk of a pathologically involved margin and therefore a higher than average risk of local recurrence.1,2 It is well established that surgical resection offers the only opportunity for cure and the median survival for unresected patients is usually <12 months. Therefore, efforts to enhance our ability to achieve margin-negative resections for borderline lesions are of considerable interest.1,2
The National Comprehensive Cancer Network (NCCN) defines borderline resectable cancer of the pancreatic head as having one of several anatomic characteristics: severe unilateral SMV/PV impingement; SMA abutment; gastroduodenal artery encasement at the level of the hepatic artery; short segment SMV occlusion; involvement of the inferior vena cava, and invasion of the colon or mesocolon.3 The MD Anderson Cancer Center has attempted to provide a more objective, computed tomography (CT)-based determination of borderline resectability, including tumours with several specific anatomic features: abutment of the SMA or coeliac axis involving <180 degrees of vessel circumference; abutment or encasement of a short segment of the hepatic artery, and short segment occlusion of the SMV, PV or SMV/PV confluence with suitable distal and proximal vein to allow for reconstruction.1
The MD Anderson group recently published a retrospective analysis of patients with borderline resectable pancreatic cancer who had been treated at their centre. Of the 160 patients included, 41% underwent resection following a neoadjuvant course of chemotherapy, chemoradiation or both. The median survival of patients undergoing resection was 40 months, compared with 13 months for patients who had progressive unresectable disease following neoadjuvant treatment. The authors concluded that the use of neoadjuvant therapy in patients with borderline resectable pancreatic cancer allowed for the selection of patients most likely to benefit from resection and supported the completion of margin-negative resections in the majority of these patients.4 The objective of this study was to retrospectively evaluate the outcomes for patients with borderline resectable pancreatic cancer treated at our centre over the past 5 years.
Materials and methods
Inclusion criteria
The diagnosis of borderline resectable pancreatic cancer was based upon the presence of at least one of four criteria identified on either contrast-enhanced thin-slice abdominal CT or endoscopic ultrasound: (i) short segment occlusion of SMV/PV confluence with suitable proximal and distal vein allowing for reconstruction; (ii) tumour encasement of a short segment of the hepatic artery; (iii) tumour abutment of the SMA, involving <180 degrees of vessel circumference, or (iv) substantial involvement of the SMV or PV of >180 degrees of vessel circumference. The first three of these criteria are based upon those described previously by Katz et al. at the MD Anderson Cancer Center.4 The fourth is modified from the NCCN definition of borderline resectable disease, which includes tumours with ‘severe unilateral SMV/PV impingement’.3 Additionally, like the MD Anderson group, we included an additional group of patients who had resectable cancer of the pancreatic head, but also had questionable metastatic or nodal disease on imaging, or who were deemed to be medically unfit for surgical resection at the time of initial presentation.4
Treatment algorithm
All patients included in our study had been histologically diagnosed with adenocarcinoma of the pancreas prior to initiation of therapy. Patients deemed to be borderline resectable anatomically, or resectable with questionable metastatic disease or performance status, underwent a course of neoadjuvant chemotherapy or chemoradiation as recommended by our multidisciplinary pancreas team. Following therapy, patients were re-staged by repeat history and physical examination, repeat carbohydrate antigen (CA) 19-9 level determination in some patients, and radiographically by contrast-enhanced CT of the chest and thin-cut CT of the abdomen and pelvis. Decisions concerning the resectability of tumours following the completion of neoadjuvant therapy were made on a case-by-case basis by the multidisciplinary pancreas treatment team. In general, patients whose tumours displayed radiographic evidence of downstaging underwent laparotomy and attempted resection. Resection was not attempted in patients with radiographically progressive locoregional or distant metastatic disease following neoadjuvant treatment. In our experience, stable tumour size following neoadjuvant therapy can indicate the replacement of tumour volume by necrosis or desmoplastic reaction, rather than failure of the tumour to respond to treatment. To that end, in patients whose tumour size did not change, additional factors, such as decreased CA 19-9 levels and decreased uptake on positron emission tomography (PET) scan were considered to indicate downstaging and resection was attempted, even when the mass appeared to abut the SMA or involve the SMV/PV. In a few instances, in which tumour size remained stable, and CA 19-9 and PET uptake did not decrease substantially, patients underwent endoscopic ultrasound and biopsy of the mass; the absence of malignant cells or the presence of necrotic or desmoplastic changes were considered to indicate downstaging and resection was attempted. All patients deemed resectable with no apparent intra-abdominal metastases at laparotomy underwent pancreaticoduodenectomy, as previously described.5 Because up to 30% of patients are noted to have metastatic disease at the time of exploration, diagnostic laparoscopy has been performed routinely for 12–24 months at our centre; some patients in this analysis were treated before this practice became routine at our institution. We consider this procedure to be appropriate both prior to laparotomy for resection and in patients who do not respond to neoadjuvant therapy; its purpose is to assess intra-abdominal metastasis and guide future treatment options. All specimens underwent standardized pathological evaluation, as described previously.4 Briefly, the bile duct and pancreatic transection margins were evaluated by frozen section intraoperatively to ensure adequate resection. The SMA margin was identified and inked by the surgeon and pathologist immediately following specimen removal. All three margins were reviewed by permanent-section microscopic examination during final pathological evaluation. If a vein resection was performed, the vein margin was inked and identified for the pathologist and permanent section analysis was undertaken.
Data collection and statistical analysis
Approval was attained for this study from the Institutional Review Board (IRB) of the University of Cincinnati. Patient databases from 2003 to 2008 were retrospectively reviewed to identify patients diagnosed with borderline resectable adenocarcinoma of the pancreatic head at the time of initial presentation at our centre.
Data including patient demographics, details of neoadjuvant treatment regimens and operative procedures, and follow-up status were collected. Pancreatic fistula was identified using previously described clinical parameters.6 Adjusted CA 19-9 levels were calculated by dividing CA 19-9 by total serum bilirubin when the bilirubin was >2 mg/dl, as previously described.7
SigmaPlot Version 11.0 (Systat Software Inc., San Jose, CA, USA) was used for statistical analysis. Student's t-test (two-tailed) analysis was used to determine significant differences between groups for parametric variables (age, tumour size, CA 19-9, duration of therapy). Fisher's exact test was utilized to determine significant differences between groups for non-parametric variables (gender, symptoms, anatomic characteristics, presence of metastatic disease or poor performance status, courses of therapy). Survival was analysed by the Kaplan–Meier log-rank method.
Results
Patient characteristics
Between 2003 and 2008, 29 patients were diagnosed with borderline resectable pancreatic cancer and underwent a course of neoadjuvant treatment at our centre. During the same period, approximately 340 patients were treated at our centre for pancreatic cancer, including about 150 patients who underwent operative resections. The operative interventions performed, and subsequent delineation into our defined resected and unresected groups, are demonstrated in Fig. 1. Of the 29 patients, 26 completed their neoadjuvant treatment courses and were included in the remainder of our study analyses. Resection was attempted in 16 patients and completed successfully in 12; the procedures were aborted in the remaining four patients after metastatic disease was seen in the liver at the time of laparotomy (three patients) or on peritoneal surfaces by diagnostic laparoscopy (one patient). These four patients, along with those in whom resection was not attempted, comprised the ‘unresected’ group in our analysis. Among the 10 patients in whom resection was not attempted, three had evidence of distant metastasis at the time of restaging (one to liver, one to lung, one to both), and seven had stable tumour size. Of these seven, three had increased CA 19-9, and the other four had evidence of continued tumour viability by either PET scan or biopsy. This is by contrast with the 12 patients who were ultimately resected; half of the tumours in these patients had decreased in size on restaging and the other half remained stable in size, but appeared to be non-viable by PET or biopsy.
Figure 1.
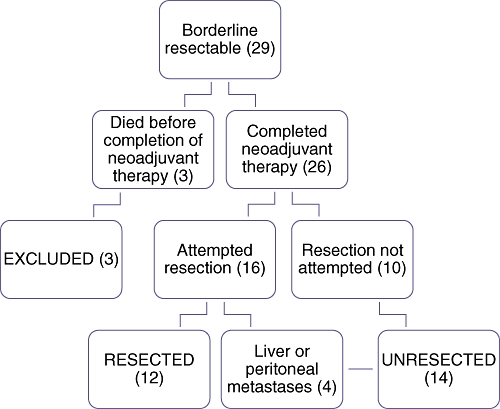
Delineation of patients into treatment groups
There were no statistically significant differences between the resected and unresected groups in terms of age, gender, symptoms, tumour size or adjusted CA 19-9 levels at presentation (Table 1). In addition, there were no differences in the anatomic characteristics of the borderline resectable tumours that qualified patients for study inclusion (58% vs. 43% for >180-degree SMV involvement, 8% vs. 29% for SMA abutment, 8% vs. 7% for SMV/PV occlusion, and 0% vs. 7% for hepatic artery encasement, of patients in the resected vs. unresected groups, respectively). Overall, 25% and 14% of patients in the resected and unresected groups, respectively, were included for questionable metastatic or nodal disease. Only one patient had been included initially for poor performance status; she died during neoadjuvant therapy and was therefore excluded from the final data analysis. Both the other two excluded patients died prior to completion of neoadjuvant therapy; one of these had SMA abutment and the other had extended SMV involvement.
Table 1.
Patient characteristics
| Resected patients (n= 12) | Unresected patients (n= 14) | P-value | |
|---|---|---|---|
| Age, years, mean ± SD | 64.6 ± 8.0 | 61.7 ± 11.3 | NS |
| Gender, % male : % female | 58 : 42 | 50 : 50 | NS |
| Symptoms at diagnosis, n (%) | |||
| Jaundice | 7 (58%) | 6 (43%) | NS |
| Abdominal pain | 8 (67%) | 10 (71%) | NS |
| Tumour size at diagnosis, cm, mean ± SD | 3.2 ± 0.8 | 3.7 ± 1.4 | NS |
| CA 19-9 at diagnosis (adjusted), U/ml, mean ± SDa | 370 ± 641 | 130 ± 189 | NS |
| ‘Borderline’ anatomic characteristic, n (%) | |||
| SMV involvement (>180) | 7 (58%) | 6 (43%) | NS |
| SMA abutment (<180) | 1 (8%) | 4 (29%) | NS |
| Short segment SMV/PV occlusion | 1 (8%) | 1 (7%) | NS |
| Hepatic artery encasement | 0 (0%) | 1 (7%) | NS |
| Possible nodal or metastatic disease, n (%) | 3 (25%) | 2 (14%) | NS |
| Poor performance status, n (%) | 0 (0%) | 0 (0%) | NS |
Adjusted by dividing CA 19-9 by total bilirubin
SD, standard deviation; NS, not significant
Neoadjuvant therapies
The neoadjuvant therapies did not differ significantly between patients who were ultimately resected compared with those who were not (Table 2). The majority of patients overall (58%) received gemcitabine-based chemotherapy; these included 75% of patients in the resected group (9/12) and 43% of patients in the unresected group (6/14). The most common chemotherapy regimens in both groups were gemcitabine alone (23%), gemcitabine with Tarceva (15%), and gemcitabine with oxaliplatin and Tarceva (12%). Our current algorithm for patients with good performance status involves doublet or triplet therapy as this has been demonstrated to be associated with increased tumour response.
Table 2.
Neoadjuvant therapies
| Resected patients (n= 12) | Unresected patients (n= 14) | P-value | |
|---|---|---|---|
| ChemoXRT alone, n (%) | 1 (8%) | 4 (29%) | NS |
| Chemotherapy alone, n (%) | 8 (67%) | 7 (50%) | NS |
| Gemcitabine alone | 4 (33%) | 2 (14%) | NS |
| Gemcitabine + cisplatin | 1 (8%) | 0 (0%) | NS |
| Gemcitabine + oxaliplatin | 0 (0%) | 1 (7%) | NS |
| Gemcitabin + Tarceva | 2 (17%) | 3 (21%) | NS |
| Gemcitabine, oxaliplatin, Tarceva | 1 (8%) | 1 (7%) | NS |
| ChemoXRT, then chemotherapy, n (%) | 2 (17%)a | 2 (14%)b | NS |
| Unknown, n (%) | 1 (8%) | 1 (7%) | NS |
| Duration of therapy, days, mean ± SD | 104 ± 37 | 121 ± 73 | NS |
One patient received gemcitabine alone after chemoXRT, one patient received gemcitabine, oxaliplatin and Tarceva after chemoXRT
Both patients received gemcitabine/Tarceva after chemoXRT
ChemoXRT, chemoradiotherapy; NS, not significant; SD, standard deviation
Although the difference was not significant, more patients in our unresected group tended to have received chemoradiation therapy alone (29%, or 4/14 patients, vs. 8%, or 1/12 patients in the resected group). Most patients receiving chemoradiation alone were treated early in the data collection period; the algorithm for neoadjuvant treatment changed to favour gemcitabine-based chemotherapy over time as it seemed to be more successful at downstaging borderline patients and controlling the progression of disease in unresectable patients at our centre. It is our current belief that radiation therapy does not cause significant tumour shrinkage and we have accordingly eliminated this from upfront therapy. This has allowed us to maximize systemic therapy dosing without increasing toxicity. The chemotherapeutic agent administered to the one resected patient during radiation was 5-fluorouracil; in the unresected group, two patients received 5-fluorouracil and two received gemcitabine. The duration of neoadjuvant treatment did not differ significantly between the two groups (104 ± 37 days in the resected group vs. 121 ± 73 days in the unresected group).
Six of the 26 patients (23%) who ultimately completed their neoadjuvant treatment were admitted to hospital during their course of therapy, four for cholangitis (15%), one with a bleeding duodenal ulcer and one with community-acquired pneumonia and pulmonary emboli. Four patients (15%) presented to the emergency department during neoadjuvant therapy and were treated as outpatients; the most common presenting symptoms were dehydration, fatigue and diarrhoea. Seventeen of the 26 patients (65%) who completed neoadjuvant therapy tolerated their treatment without hospital admission or emergency department care.
Operative characteristics for resected patients
Twelve patients were resected following neoadjuvant therapy (Table 3). Of these, five (42%) required venous resection; three patients underwent primary repair of the PV, one patient had primary repair of the SMV and PV, and one patient underwent SMV/PV reconstruction using internal jugular vein. Margin-negative (R0) resection was achieved in all five of these patients and they did not experience increased morbidity compared with the remainder of this patient population.
Table 3.
Operative and pathological characteristics for resected patients and tumours
| Operative | Resected patients (n= 12) |
|---|---|
| Venous resection, n (%) | 5 (42%) |
| Primary repaira | 4 (33%) |
| Reconstruction, n (%)b | 1 (8%) |
| Operative time, min, mean ± SD | 467 ± 105 |
| Estimated blood loss, ml, mean ± SD | 679 ± 392 |
| Length of stay, days, mean ± SD | 15.0 ± 6.7 |
| 30-day mortality, n (%) | 0 (0%) |
| 30-day morbidity, n (%) | 8 (67%) |
| Intra-abdominal abscess | 2 (17%) |
| Pancreatic fistula, type A | 2 (17%) |
| Clostridium difficile colitis | 2 (17%) |
| Urinary tract infection | 2 (17%) |
| Wound infection | 2 (17%) |
| 30-day hospital readmission, n (%) | 2 (17%) |
| Pathological | |
| Tumour size, cm, mean ± SD | 3.5 ± 1.8 |
| Tumour grade, n (%) | |
| Moderate | 6 (50%) |
| Poor | 5 (42%) |
| Lymph node positive, n (%) | 5 (42%) |
| Lymph nodes removed, mean ± SD | 12.4 ± 5.2 |
| Margin status, n (%) | |
| R0 | 8 (67%) |
| R1 | 4 (33%) |
Portal vein: three patients; superior mesenteric vein/portal vein: one patient
Superior mesenteric vein/portal vein reconstructed using internal jugular vein in one patient
SD, standard deviation
The 30-day mortality rate in our series was 0%. Eight of 12 patients (67%) experienced a complication, although all of these were minor. Two patients each suffered intra-abdominal abscess (one treated by percutaneous drainage, one by antibiotics alone), type A pancreatic fistula (drains were removed in both patients at the first postoperative visit after output had decreased substantially), Clostridium difficile colitis treated with antibiotics, urinary tract infection and wound infection. Two patients were re-admitted within 30 days of the operation, one for hypoglycaemia and one for cellulitis at the feeding tube site. No patients underwent re-operation.
The mean tumour size of resected cancers was 3.5 ± 1.8 cm. Six patients (50%) had moderate-grade tumour; five (42%) had poor differentiation. Five patients (42%) also had lymph nodes positive for disease in the resection specimen; a mean of 12.4 ± 5.2 lymph nodes were removed per operation. Eight of our operations (67%) resulted in margin-negative (R0) resections. The other four operations (33%) resulted in R1 resections; three of these reflected microscopically positive retroperitoneal margins and one resulted from a positive pancreatic margin upon final pathological analysis, which had been deemed negative by intraoperative frozen section. Of the five patients undergoing venous resection, venous structures were uninvolved by tumour in four; the fifth patient's tumour invaded through the resected portion of the PV. Our practice is to resect veins that appear abnormal during the procedure, although these findings may indicate desmoplastic reaction secondary to tumour or therapy, rather than direct tumour involvement.
Survival
Survival was analysed by the Kaplan–Meier log rank method (Table 4, Fig. 2). The median survival for resected patients was 23.3 months (range 9.8–33.4 months) vs. 15.5 months (range 6.2–18.8 months) for unresected patients, a difference that reached significance (P= 0.015). Five of the 12 resected patients are still alive, with current survivals of 14.5–27.0 months; two of 14 unresected patients are alive, with survivals of 18.2 and 18.8 months. Median survival following the completion of neoadjuvant therapy was also significantly longer in the resected group (20.0 months [range 4.7–29.5 months] vs. 9.2 months [range 2.0–14.5 months]; P= 0.023).
Table 4.
Survival rates
| Resected patients (n= 12) | Unresected patients (n= 14) | P-value | |
|---|---|---|---|
| Overall survival, months, median (range) | 23.3 (9.8–33.4)a | 15.5 (6.2–18.8)b | 0.015 |
| Survival following neoadjuvant treatment, months, median (range) | 20.0 (4.7–29.5)c | 9.2 (2.0–14.5)d | 0.023 |
| Time to recurrence, months, median (range) | 4.7 (2.1–21.4)e | – | – |
Includes five living patients, current survivals of 14.5–27.0 months
Includes two living patients, current survivals of 18.2 and 18.8 months
Includes five living patients, current post-neoadjuvant survivals of 8.9–22.7 months
Includes two living patients, current post-neoadjuvant survivals of 11.5 and 14.5 months
Two patients have not recurred at 17.1 and 19.4 months post-resection, respectively
Figure 2.
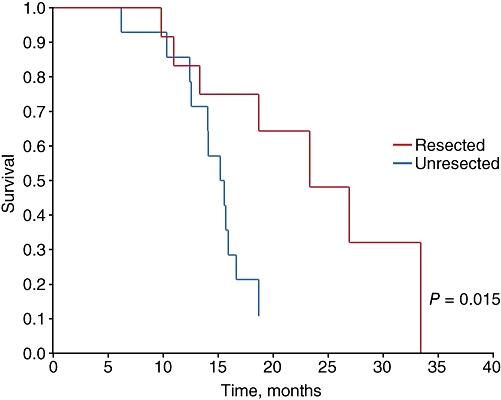
Kaplan–Meier overall survival curve. Median survival was 23.3 months (range 9.8–33.4 months) in the resected group and 15.5 months (range 6.2–18.8 months) in the unresected group
The median time to tumour recurrence in the resected group was 4.7 months. Of the nine resected patients for whom recurrence data is known, five (56%) recurred within 6 months of surgery, three in the operative bed (33%), and two as peritoneal metastases (22%); none of these patients received adjuvant therapy. Two patients (22%) received chemoradiation adjuvantly and had distant metastatic disease at 9.8 and 21.1 months postoperatively. An additional two patients (22%) have not yet recurred; they have current postoperative durations of 17.1 and 19.4 months and both received adjuvant chemoradiation. Of the three patients with positive retroperitoneal margins, one received no adjuvant therapy and recurred locally within 6 months, one received adjuvant chemoradiation and recurred distantly at 9.8 months, and one received adjuvant chemoradiation and has not yet recurred at 17.1 months postoperatively. The one patient with a positive pancreatic margin has been lost to follow-up.
Discussion
Patients with borderline resectable pancreatic cancer treated by initial surgical resection can be expected to have worse surgical and survival outcomes compared with patients with resectable disease because of the increased complexity of surgery, the advanced nature of the tumour, and the high risk for margin-positive resection. Neoadjuvant treatment for borderline resectable tumours can distinguish patients who are likely to benefit from attempted pancreaticoduodenectomy from non-responders who are unlikely to have longterm survival with or without resection. Additionally, this strategy allows for the early treatment of micrometastatic disease, increases the probability that the treatment course will be promptly completed without delays caused by postoperative recovery, and maximizes the potential for margin-negative resection in tumours that respond to treatment.1
Our radiographically defined criteria for borderline resectability include the MD Anderson criteria and an objectified definition of unilateral SMV/PV impingement, specifically those tumours involving >180 degrees of vessel circumference. In accordance with the NCCN guidelines, tumours with such substantial venous involvement should be considered to be borderline resectable because R0 resection typically requires complex venous reconstruction that can be avoided by attempted downstaging with neoadjuvant treatment. The MD Anderson group has further expanded the definition of borderline resectable disease by including patients who are anatomically resectable, but who have questionable metastatic disease or poor performance status. The inclusion of these patients obviously prevents the substage of borderline resectable disease from being defined only by objective anatomic criteria, and complicates the design of future trials to determine optimal treatment strategies for tumours bearing specific anatomic borderline characteristics. However, for the purposes of this review, as patients with questionable metastases or poor performance status are often initially treated as anatomically borderline resectable patients because of their marginally operable status, we included them in our analysis.4 In the MD Anderson retrospective analysis of patients with borderline resectable disease, outcomes for patients with questionable metastases and poor performance status were not significantly different than those for patients with anatomically borderline resectable disease in terms of the percentage of patients undergoing resection, achievement of R0 resection and nodal status at resection. Although they were not compared directly, survival rates were similar in all three groups of patients; these findings support the concept of grouping these patients clinically into a single substage of disease.4
In this series, we have established that resection following neoadjuvant treatment in borderline resectable pancreatic cancer can be accomplished with minimal mortality and reasonable morbidity. We achieved margin-negative resection in eight of 12 patients, a rate of 67%. This is lower than the 94% reported in the MD Anderson series; several factors could explain this discrepancy. One factor may concern the neoadjuvant therapies administered and their effectiveness at maximally downstaging the tumour. In our series, the majority of patients received either chemotherapy or chemoradiation alone; only four of 26 (15%) received both and all of these received chemoradiation followed by chemotherapy. By contrast, 74% and 95% of patients in the MD Anderson series received neoadjuvant chemotherapy and chemoradiation, respectively, meaning that most patients received both, with the chemotherapy occurring first in most patients. In addition, although both institutions utilized a multidisciplinary group and considered similar factors in determining which patients should undergo resection, it is possible that our group attempted resection more often in patients with less favourable tumour biology or clinical status; the MD Anderson group reported that 16% of operations were aborted on findings of abdominal metastases at laparotomy, whereas this occurred in four of our 16 operations (25%). Finally, the differences may simply reflect the small number of resected patients in our series.
The median time to recurrence following surgery in our resected patients, 4.7 months, was shorter than that reported previously. In the European Study Group for Pancreatic Cancer's ESPAC-1 trial, the median time to recurrence following resection for Stage I/II disease ranged from 9.4 to 15.3 months, with longer times achieved in patients treated with adjuvant chemotherapy.8 In the MD Anderson trial of borderline resectable patients, the median time to recurrence was 24 months.4 Overall, 20% of MD Anderson patients and five (42%) of ours were treated with adjuvant therapy, although, as mentioned above, the nature of the neoadjuvant and adjuvant therapies received are likely to have differed substantially between the two institutions. Although our median time to recurrence was low, this result reflected a bimodal distribution, in which half of our resected patients recurred within 10 months of surgery; only one of these patients received adjuvant therapy. By contrast, three patients (25%) demonstrated disease-free survival of >17 months after surgery, and two of these have not yet recurred; one received neoadjuvant and adjuvant chemotherapy and chemoradiation, whereas the other two received neoadjuvant chemotherapy and adjuvant chemoradiation. As for all patients with pancreatic adenocarcinoma, recurrence-free and overall survivals will continue to increase as chemotherapy and chemoradiation strategies are optimized. The bimodal distribution of our outcomes also suggests that data from larger sample sizes in the future may help to elucidate prognostic factors that could be used to guide individual therapy, including the decision to attempt resection.
Not surprisingly, overall survival rates were longer in patients who were successfully resected following neoadjuvant treatment (23.3 months), compared with those whose disease progressed and who did not undergo resection (15.5 months). Median survival for all unresected patients identified during the 5-year period (intent-to-treat) was 14.1 months; this is higher than that reported for patients with locally advanced disease who undergo chemoradiation or chemotherapy (6–7 months) and similar to that reported for unresected borderline resectable disease (15 months).3,4 The relatively prolonged survival that we observed in the unresected group may simply reflect favourable tumour biology in our small set of patients, an effect that may not be observed in a larger series. Our survival rates for resected patients were similar to the 20-month survival rates that have been reported in patients with Stage I/II disease treated by initial resection.8 The margin-negative resection rate in our small series of patients (67–73%) is likewise similar to that seen in previous trials of resectable patients (80–83%).1,8 As in the MD Anderson series, only about 40% of our patients presenting with borderline resectable disease were successfully resected and no clinical characteristics of the patients or tumours distinguished this group from unresectable patients at the time of initial presentation. A neoadjuvant treatment strategy for patients with borderline resectable pancreatic cancer allows for the selection of those patients who are most likely to benefit from surgery and thus spares others the morbidity of the procedure. Although we found that the results for patients with borderline resectable disease were similar to those for initially resectable patients, the results may improve as our understanding of the treatment of this subset of patients grows. Furthermore, neoadjuvant treatment does not negatively impact the survival of borderline resectable patients who are ultimately resected, compared with initially resectable patients, and should be considered standard treatment for patients presenting with borderline resectable disease.
Conflicts of interest
None declared.
References
- 1.Varadhachary GR, Tamm EP, Abbruzzese JL, Xiong HQ, Crane CH, Wang H, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13:1035–1046. doi: 10.1245/ASO.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Raut CP, Tseng JF, Sun CC, Wang H, Wolff RA, Crane CH, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246:52–60. doi: 10.1097/01.sla.0000259391.84304.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: Pancreatic Adenocarcinoma. v.1.2008. http://www.nccn.org/professionals/physician_gls/default.asp. [Accessed 1 February 2009]
- 4.Katz MHG, Pisters PWT, Evans DB, Sun CC, Lee JE, Fleming JB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. 2008;206:833–848. doi: 10.1016/j.jamcollsurg.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmad SA, Lowy AM, McIntyre BC, Matthews JB. Pancreaticoduodenectomy. J Gastrointest Surg. 2005;9:138–143. doi: 10.1016/j.gassur.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Kang CM, Kim JY, Choi GH, Kim KS, Choi JS, Lee WJ, et al. The use of adjusted preoperative CA 19-9 to predict the recurrence of resectable pancreatic cancer. J Surg Res. 2007;140:31–35. doi: 10.1016/j.jss.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Neoptolemos JP, Stocken DD, Friess H, Bass C, Dunn JA, Hickey H, et al. for the European Study Group for Pancreatic Cancer. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]


