Abstract
Aging is regulated by modifications in single genes and by simple changes in the environment. The signaling pathway connecting insulin to FoxO transcription factors integrates environmental stimuli to regulate lifespan. FoxO transcription factors are directly phosphorylated in response to insulin/growth factor signaling by the protein kinase Akt, thereby causing their sequestration in the cytoplasm. In the absence of insulin/growth factors, FoxO factors translocate to the nucleus where they trigger a range of cellular responses, including resistance to oxidative stress—a phenotype highly coupled with lifespan extension. Our recent results indicate that FoxO transcription factors are also regulated in response to nutrient deprivation by the AMP-activated protein kinase (AMPK) pathway. The energy-sensing AMPK directly phosphorylates FoxO transcription factors at six regulatory sites. AMPK phosphorylation enhances FoxO transcriptional activity, leading to the expression of specific target genes involved in stress resistance and changes in energy metabolism. The AMPK–FoxO pathway plays a crucial role in the ability of a dietary restriction regimen to extend lifespan in Caenorhabditis elegans. Understanding the intricate signaling networks that translate environmental conditions like dietary restriction into changes in gene expression that extend lifespan will be of critical importance to identify ways to delay the onset of aging and age-dependent diseases.
Keywords: AMPK, FoxO, dietary restriction, caloric restriction, longevity, aging
Introduction
One of the most potent interventions to extend lifespan is dietary restriction (DR), the reduction of food intake without malnutrition. DR extends both the mean and maximal lifespan in a wide range of species.1 DR not only extends lifespan, but also decreases the incidence of age-dependent diseases and age-dependent traits. In humans and mice, DR decreases the incidence of cancer.2,3 In rodents, DR prevents the age-dependent decline in learning and memory.4,5 Understanding the mechanisms that mediate the beneficial effects of DR on longevity and age-dependent traits will help harness the benefits of DR.
AMPK: A Protein Kinase that Senses Low Energy Levels
A series of genes have recently been found to be critical in mediating longevity induced by DR.6–17 In our studies, we have focused on the importance of the energy-sensing AMP-activated protein kinase (AMPK) in response to DR.14 AMPK is activated in response to a variety of stimuli associated with DR, including decreased energy or glucose levels and decreased leptin levels.18 AMPK is also activated in response to exercise and the antidiabetic drug metformin, both of which also affect overall lifespan.19 AMPK is activated by all these diverse stimuli because the γ subunit of this heterotrimeric protein kinase senses cellular energy levels by monitoring the ratio of AMP to ATP levels in cells. AMP binding to the γ subunit induces a conformational change that allosterically activates the catalytic α subunit.18 AMPK is well known to regulate lipid metabolism and protein synthesis in response to low energy to restore energy levels.18
AMPK Is Necessary for Longevity Induced by a Method of DR in C. elegans
We asked whether AMPK also mediated the ability of DR to extend lifespan, using the nematode Caenorhabditis elegans. We developed a DR regimen in worms, termed sDR (for solid DR), by restricting the amount of feeding bacteria seeded on plates. Using reverse-phase high-performance liquid chromatography, we found that sDR increased the AMP:ATP ratio in C. elegans. These findings suggest that sDR activates AMPK in worms. To determine if AMPK was necessary for longevity induced by sDR, we used a mutant strain of worms carrying a deletion in the gene encoding one of the catalytic α AMPK subunits (aak-2). We found that AMPK/aak-2 was necessary to mediate the ability of sDR to extend lifespan. In addition to extending lifespan, sDR also prevents the age-dependent decline in the worm’s activity, in an AMPK/aak-2-dependent manner. Thus, AMPK is necessary for sDR to extend lifespan and healthspan in C. elegans.14
AMPK Acts in Part via the FoxO Transcription Factor DAF-16 in C. elegans
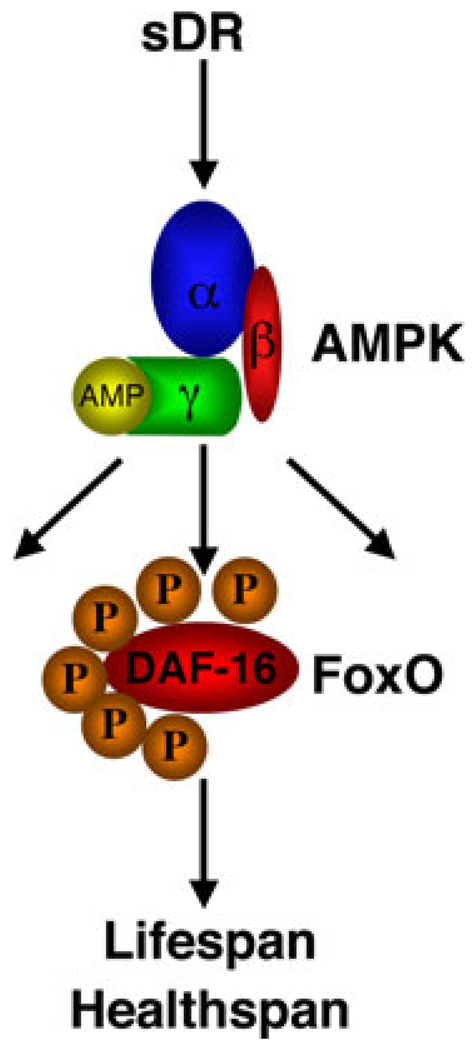
We then examined the possible mechanisms of action of AMPK in C. elegans. We tested if the FoxO family of Forkhead transcription factors, which is well known to integrate a wide range of external stimuli, including insulin and oxidative stress, also responded to DR. While several studies have reported that longevity induced by other DR regimens was not dependent on FoxO/daf-16 in worms,12,16,20 we found that FoxO/daf-16, like AMPK/aak-2, was necessary for longevity induced by sDR.14 To test if the effects of AMPK on lifespan were indeed mediated by FoxO, we generated transgenic worms expressing a constitutively active form of AMPK. Expression of constitutively active AMPK extended lifespan and resistance to oxidative stress, in a FoxO-dependent manner.14 These findings suggest that AMPK acts via FoxO to extend lifespan in response to sDR. We next asked whether the interaction between AMPK and FoxO was direct. We found that AMPK could directly phosphorylate FoxO/daf-16 in vitro.14 Using tandem mass spectrometry, we identified six previously unidentified sites of phosphorylation of DAF-16 by AMPK. These results suggest that one way in which sDR extends lifespan is by inducing a signaling pathway that activates AMPK. AMPK would in turn phosphorylate and activate FoxO/daf-16 to induce genes involved in stress resistance and longevity (Fig. 1).
Figure 1.
Model for the mechanisms underlying longevity in response to sDR in C. elegans. sDR would activate AMPK by increasing the AMP:ATP ratio. Activated AMPK would in turn phosphorylate and activate FoxO/ daf-16 and possibly other substrates. FoxO would induce the expression of a program of genes involved in stress resistance and alternative energy metabolism, which would lead to beneficial effects on lifespan and healthspan. (In color in Annals online.)
The AMPK-FoxO Pathway Is Necessary for Longevity Induced by Some, but Not All, Methods of DR in C. elegans
Our most recent findings indicate that AMPK and FoxO are necessary for longevity induced by some, but not all, methods of DR in C. elegans,21 confirming that there are additional genetic pathways mediating DR-induced longevity in this organism, including mTOR, FoxA/pha-4, NFE2/skn-1, and Sir2 pathways.11–13,15–17,21–24 The observation that distinct genetic pathways mediate longevity in response to a variety of DR regimens in C. elegans may be due to the differences in the way these regimens trigger DR. Indeed DR methods differ in the type of food that is limiting (bacteria versus defined chemicals), the time at which DR is applied (development versus adult), and by the type of medium in which DR is induced (solid versus liquid). For example, specific nutrients may be more limiting in one DR regimen than in another, which could evoke different energy-sensing pathways to extend lifespan. Alternatively, the reduction of nutrients might be sensed by different tissues (neurons or intestine) and therefore activate distinct genetic pathways. Understanding the genetic pathways that mediate longevity induced by different DR regimens will help harness the full benefits of this intervention on lifespan and healthspan.
The AMPK-FoxO Pathway Also Functions in Mammalian Cells to Regulate Gene Expression
An important question is whether the link between AMPK and FoxO is conserved across species. In vitro kinase assays revealed that AMPK could also phosphorylate all four human FoxO family members (FoxO1, FoxO3, FoxO4, and FoxO6), with a preference for FoxO3. Using tandem mass spectrometry, we identified the residues phosphorylated by AMPK on human FoxO3 (T179, S399, S413, S555, S588, and S626).25 Using phosphospecific antibodies for these sites, we showed that FoxO3 was phosphorylated by AMPK in mammalian cells in response to stimuli that activate AMPK (such as 2-deoxyglucose). The finding that AMPK can phosphorylate human FoxO3 prompted us to examine the importance of this phosphorylation for FoxO3 action in mammalian cells. Using luciferase reporter assays, we found that mutating the AMPK phosphorylation sites on FoxO3 led to a decrease of FoxO transcriptional activity, at least on an artificial promoter. We next reconstituted FoxO3−/− mouse fibroblast cells with either wild-type FoxO3 or a form of FoxO3 that was mutated at all the AMPK phosphorylation sites. Genomewide microarray analysis of these cells revealed that FoxO3, when mutated at the AMPK sites, induced different sets of genes than wild-type FoxO3, indicating that AMPK phosphorylation modified FoxO3 transcriptional activity, and perhaps its recruitment to specific promoters in vivo.25 A GO-term analysis revealed that the cluster containing the genes affected by themutant of FoxO3 that can no longer be phosphorylated by AMPK was significantly enriched for genes involved in oxidative stress resistance and energy metabolism using sources other than glucose. Taken together, these observations raise the possibility that AMPK phosphorylation of FoxO3 is required to counteract the effects of nutrient deprivation and to induce stress resistance, a cellular function highly coupled with organismal longevity. Our findings suggest the following model: sDR activates AMPK by increasing the AMP:ATP ratio. Active AMPK phosphorylates FoxO transcription factors, which activates the transcription of FoxO target genes that enable the organism to resist cumulative damage caused by oxidative stress and to live longer (Fig. 1).
Conclusion
The AMPK-FoxO pathway might also mediate lifespan extension in response to DR in other species. In mammals, glucose levels and leptin levels decrease when animals are subjected to DR, and these two conditions have been reported to activate AMPK in vivo.26 DR was also reported to activate AMPK (AMPK α2) in the mammalian brain,27,28 though another study reported no change in AMPK activation by short-term DR in the liver.29 Compounds that activate AMPK, such as 2-deoxyglucose, phenformin, and metformin, have been proposed to act as “caloric restriction mimetics,” based on their ability to mimic low glucose levels, a consequence of DR, and have been proposed as a strategy to slow aging.30–32 Molecules that activate AMPK have recently been found to decrease the incidence of cancer in rodents and to prevent the appearance of some biomarkers of aging,19,33,34 suggesting that AMPK activation may be important to extend longevity and delay age-dependent diseases in a wide range of species. Identifying the relevance of AMPK and FoxO transcription factors in mediating DR across species will be pivotal for understanding why DR is such a conserved and efficient intervention for increasing lifespan and decreasing the risk of age-dependent diseases.
Acknowledgments
This work was supported by NIH/NIA R01 AG026648-01, the American Institute for Cancer Research, the American Association for Aging Research, the NIH/NCI training Grant 5T32CA09302-28 (E.L.G.), the NIH/NIA National Research Service Award 1F31AG032837-01 (M.R.B.), an NSF graduate fellowship (E.L.G.), and a Stanford Graduate Fellowship (M.R.B).
Footnotes
Additional Note Since this manuscript was accepted another method of DR in C. elegans, termed IF for intermittent fasting, has been reported. This DR method is dependent on RHEB-1 and FoxO/ daf-16, but independent of AMPK/aak-2 (Honjoh S., T. Yamamoto, M. Uno & E. Nishida E. 2009. Signalling through RHEB-1 mediates intermittent fasting-induced longevity in C. elegans. Nature 457: 726–730.)
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Mair W, Dillin A. Aging and survival: the genetics of life span extension by dietary restriction. Annu. Rev. Biochem. 2008;77:727–754. doi: 10.1146/annurev.biochem.77.061206.171059. [DOI] [PubMed] [Google Scholar]
- 2.Fontana L, et al. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc. Natl. Acad. Sci. USA. 2004;101:6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michels KB, Ekbom A. Caloric restriction and incidence of breast cancer. Jama. 2004;291:1226–1230. doi: 10.1001/jama.291.10.1226. [DOI] [PubMed] [Google Scholar]
- 4.Ingram DK, et al. Dietary restriction benefits learning and motor performance of aged mice. J. Gerontol. 1987;42:78–81. doi: 10.1093/geronj/42.1.78. [DOI] [PubMed] [Google Scholar]
- 5.Stewart J, Mitchell J, Kalant N. The effects of life-long food restriction on spatial memory in young and aged Fischer 344 rats measured in the eight-arm radial and the Morris water mazes. Neurobiol. Aging. 1989;10:669–675. doi: 10.1016/0197-4580(89)90003-1. [DOI] [PubMed] [Google Scholar]
- 6.Bauer JH, et al. Neuronal expression of p53 dominant-negative proteins in adult Drosophila melanogaster extends life span. Curr. Biol. 2005;15:2063–2068. doi: 10.1016/j.cub.2005.10.051. [DOI] [PubMed] [Google Scholar]
- 7.Kaeberlein M, et al. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- 8.Kapahi P, et al. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr. Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- 10.Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc. Natl. Acad. Sci. USA. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen M, et al. New genes tied to endocrine, metabolic, and dietary regulation of lifespan from a Caenorhabditis elegans genomic RNAi screen. PLoS Genet. 2005;1:119–128. doi: 10.1371/journal.pgen.0010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lakowski B, Hekimi S. The genetics of caloric restriction in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 1998;95:13091–13096. doi: 10.1073/pnas.95.22.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Tissenbaum HA. Overlapping and distinct functions for a Caenorhabditis elegans SIR2 and DAF-16/FOXO. Mech. Ageing Dev. 2006;127:48–56. doi: 10.1016/j.mad.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Greer EL, et al. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr. Biol. 2007;17:1646–1656. doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinkraus KA, et al. Dietary restriction suppresses proteotoxicity and enhances longevity by an hsf-1-dependent mechanism in Caenorhabditis elegans. Aging Cell. 2008;7:394–404. doi: 10.1111/j.1474-9726.2008.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panowski SH, et al. PHA-4/Foxa mediates diet-restriction-induced longevity of C. elegans. Nature. 2007;447:550–555. doi: 10.1038/nature05837. [DOI] [PubMed] [Google Scholar]
- 17.Bishop NA, Guarente L. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature. 2007;447:545–549. doi: 10.1038/nature05904. [DOI] [PubMed] [Google Scholar]
- 18.Kahn BB, et al. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Anisimov VN, et al. Effect of metformin on life span and on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Exp. Gerontol. 2005;40:685–693. doi: 10.1016/j.exger.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Houthoofd K, et al. Life extension via dietary restriction is independent of the Ins/IGF-1 signalling pathway in Caenorhabditis elegans. Exp. Gerontol. 2003;38:947–954. doi: 10.1016/s0531-5565(03)00161-x. [DOI] [PubMed] [Google Scholar]
- 21.Greer EL, Brunet A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell. 2009;2:113–127. doi: 10.1111/j.1474-9726.2009.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curtis R, O’Connor G, DiStefano PS. Aging networks in Caenorhabditis elegans: AMP-activated protein kinase (aak-2) links multiple aging and metabolism pathways. Aging Cell. 2006;5:119–126. doi: 10.1111/j.1474-9726.2006.00205.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee GD, et al. Dietary deprivation extends lifespan in Caenorhabditis elegans. Aging Cell. 2006;5:515–524. doi: 10.1111/j.1474-9726.2006.00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaeberlein TL, et al. Lifespan extension in Caenorhabditis elegans by complete removal of food. Aging Cell. 2006;5:487–494. doi: 10.1111/j.1474-9726.2006.00238.x. [DOI] [PubMed] [Google Scholar]
- 25.Greer EL, et al. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J. Biol. Chem. 2007;282:30107–30119. doi: 10.1074/jbc.M705325200. [DOI] [PubMed] [Google Scholar]
- 26.Minokoshi Y, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 27.Barazzoni R, et al. Ghrelin regulates mitochondrial-lipid metabolism gene expression and tissue fat distribution in liver and skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2005;288:E228–E235. doi: 10.1152/ajpendo.00115.2004. [DOI] [PubMed] [Google Scholar]
- 28.Pallottini V, et al. Mechanisms underlying the impaired regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase in aged rat liver. Mech. Ageing Dev. 2004;125:633–639. doi: 10.1016/j.mad.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez AA, et al. Metabolic adaptations to fasting and chronic caloric restriction in heart, muscle, and liver do not include changes in AMPK activity. Am. J. Physiol. Endocrinol.Metab. 2004;287:E1032–E1037. doi: 10.1152/ajpendo.00172.2004. [DOI] [PubMed] [Google Scholar]
- 30.McCarty MF. Chronic activation of AMP-activated kinase as a strategy for slowing aging. Med. Hypotheses. 2004;63:334–339. doi: 10.1016/j.mehy.2004.01.043. [DOI] [PubMed] [Google Scholar]
- 31.Hawley SA, et al. The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes. 2002;51:2420–2425. doi: 10.2337/diabetes.51.8.2420. [DOI] [PubMed] [Google Scholar]
- 32.Ingram DK, et al. Development of calorie restriction mimetics as a prolongevity strategy. Ann. N. Y. Acad. Sci. 2004;1019:412–423. doi: 10.1196/annals.1297.074. [DOI] [PubMed] [Google Scholar]
- 33.Zhu Z, et al. 2-Deoxyglucose as an energy restriction mimetic agent: effects on mammary carcinogenesis and on mammary tumor cell growth in vitro. Cancer Res. 2005;65:7023–7030. doi: 10.1158/0008-5472.CAN-05-0453. [DOI] [PubMed] [Google Scholar]
- 34.Anisimov VN, et al. Effects of phentermine and phenformin on biomarkers of aging in rats. Gerontology. 2005;51:19–28. doi: 10.1159/000081430. [DOI] [PubMed] [Google Scholar]