Abstract
We are interested in determining the signaling pathways for 1,25-dihydroxyvitamin D3 (1,25D)-induced differentiation of HL60 leukemic cells. One possible candidate is Raf-1, which is known to signal cell proliferation and neoplastic transformation through MEK, ERK, and downstream targets. It can also participate in the regulation of cell survival and various forms of cell differentiation, though the precise pathways are less well delineated. Here we report that Raf-1 has a role in monocytic differentiation of human myeloid leukemia HL60, which is not mediated by MEK and ERK, but likely by direct interaction with p90RSK. Specifically, we show that Raf-1 and p90RSK are increasingly activated in the later stages of differentiation of HL60 cells, at the same time as activation of MEK and ERK is decreasing. Transfection of a wild-type Raf-1 construct enhances 1,25D-induced differentiation, while antisense Raf-1 or short interfering (si) Raf-1 reduces 1,25D-induced differentiation. In contrast, antisense oligodeoxynucleotides (ODN) and siRNAs to MEK or ERK have no detectable effect on differentiation. In late stage differentiating cells Raf-1 and p90RSK are found as a complex, and inhibition of Raf-1, but not MEK or ERK expression reduces the levels of phosphorylatedp90RSK. These findings support the thesis that Raf-1 signals cell proliferation and cell differentiation through different intermediary proteins
Involvement of mitogen-activated protein kinases (MAPKs) in signaling of monocyte/macrophage differentiation induced by 1,25-dihydroxyvitamin D3 (1,25D) has been well documented (e.g., Wang et al., 2000; Marcinkowska, 2001; Wang and Studzinski, 2001a; Kim et al., 2002; Studzinski et al., 2005), but the exact sequence of signaling steps has not been precisely elucidated. Early studies described MAPK activity as the basis of rapid, membrane-related effects of 1,25D on human acute promyelocytic leukemia NB4 cells (Song et al., 1998) and other cell types (Buitrago et al., 2006). These effects occurred in the time frame of seconds and minutes, but have not been universally observed in other cell types. More recently, studies in leukemia cells showed involvement of several more slowly activated MAPK cascades in signaling of 1,25D effects on cell proliferation (Wang and Studzinski, 2001a; Ji et al., 2002), survival (Wang and Studzinski, 1997; Pepper et al., 2003; Zhang et al., 2006), and differentiation (Wang et al., 2000; Marcinkowska, 2001; Wang and Studzinski, 2001a; Studzinski et al., 2005). For instance, we and others have shown that in HL60 cells ERK1/2 is activated in the early phase of 1,25D-induced differentiation, and this can be accentuated by serum starving the cells prior to exposure to 1,25D (Marcinkowska et al., 1997; Marcinkowska, 2001; Wang and Studzinski, 2001a). Other studies have implicated JNK (Ji et al., 2002; Wang et al., 2003; Buitrago et al., 2006), p38 (Wang and Studzinski, 2001a,b; Ji et al., 2002) and AKT (Zhang et al., 2006) pathways in the transmission of the 1,25D-generated signals to the transcriptional machinery in the nucleus. However, the Raf-MEK-ERK pathways remains firmly established as a principal all-purpose signaling cascade (Chang et al., 2003), and as such deserve additional attention regarding its role in the induction of differentiation.
In an attempt to examine the upstream regulators of MEK-ERK activation previously shown by us to characterize the initial phase of 1,25D-induced monocytic differentiation (Wang and Studzinski, 2001a), we studied the role of kinase suppressor of ras-1 (KSR-1) in this process (Wang and Studzinski, 2001c, 2004). KSR-1 gene can be directly regulated by liganded vitamin D receptor (Wang et al., 2006) and encodes a mainly membrane-associated protein. This protein has been reported to phosphorylate Raf-1 in several model systems (Xing and Kolesnick, 2001; Yan and Polk, 2001; Wang and Studzinski, 2004) and can also act as a scaffold which facilitates the assembly of Raf-1 protein with its downstream targets at the cell membrane (Brennan et al., 2002; Ory et al., 2003). By either mechanism it can modulate the efficiency of Raf-1 signaling, and we previously found that KSR-1 amplified the differentiation signal provided by low concentrations of 1,25D, and was required for optimal monocytic differentiation (Wang and Studzinski, 2004).
Surprisingly, however, the time course of the gradually increasing expression of KSR-1 and Raf-1 activation did not coincide with the maximal activation of ERK, which took place within the first 24 h of exposure to 1,25D (Wang and Studzinski, 2001a). We therefore investigated in detail the role of Raf-1 in 1,25D-induced differentiation of HL60 cells, and found that its activation correlated with the appearance of the monocytic phenotype. Further, its ectopic expression enhanced differentiation, while the inhibition of Raf-1 expression diminished 1,25D-induced differentiation. However, the increased Raf-1 activation in the later stages of differentiation was accompanied by decreased activation of MEK and ERK, suggesting that Raf-1 participates in 1,25D-induced monocytic differentiation by regulating other targets in this system, perhaps p90RSK. Thus, our data and reports in the literature (Porras et al., 1994; Kuo et al., 1996; Lenormand et al., 1996; Yen and Varvayanis, 2000; Hong et al., 2001; Akimov and Belkin, 2003; Dhillon et al., 2003) lead to the hypothesis that proliferative and pro-differentiation signals from active Raf-1 are channeled through different downstream targets.
Materials and Methods
Chemicals and plasmids
1,25-Dihydroxyvitamin D3 was provided by Dr. Milan Uskokovic, Bioxell, Nutley, NJ. ERK MAP kinase sampler kit was purchased from Cell Signaling (Beverly, MA). Plasmid constructs pcDNAHA-Raf1 and pcDNAHA-Raf1-C4 were kind gifts from Dr. Ulf Rapp. HA-tagged Raf-C4 is a dominant negative carboxyl-terminus deletion mutant of Raf-1 and contains the Ras-binding domain (Bruder et al., 1992). Transient transfections were performed as described before (Wang and Studzinski, 2004) using the Raf-1 plasmid (10 μg) along with 2 μg pEGFP1 plasmid (BD BioSciences Clontech, Palo Alto, CA) with efficiency of 18.2 ± 3.2%. The antibodies against Raf-1 (c-12, rabbit polyclonal), MEK-1 (c-18, rabbit polyclonal), ERK1/2 (k-23, rabbit polyclonal), and p90RSK (c-21, rabbit polyclonal) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Tissue culture
HL60-G cells (Wajchmann et al., 1996) were grown in the suspension culture at 37°C in RPMI 1640 medium with 10% iron-supplemented bovine calf serum (Hyclone, Logan, UT). For all experiments, HL60 cells were suspended at 3 × 105 cells/ml of fresh medium, and 1,25D or all trans retinoic acid (RA) was added for the indicated times.
Determination of the markers of differentiation
The procedures were performed as described before (Wang et al., 1997). Aliquots of 1 × 106 cells were harvested, washed, and stained with MY4-RD1 (anti-CD14) and M01-FITC (anti-CD11b) antibodies from Beckman Coulter (Miami, FL). Two parameter analysis was performed using a Beckman Coulter EPICS XL Flow Cytometer. Isotypic mouse Ig G1 was used to set threshold parameters.
Antisense oligodeoxynucleotides (ODN) and small interfering (si) RNA
Phosphorothioate antisense ODNs to Raf-1 (5′-GCTCCCTG TATGTGCTCCAT-3′, corresponding to the start codon of Raf-1), sense ODN: ATGGAGCACATACAGGGAGC); antisense ODN to MEK1 (5′-GCTTCTTCTTGGGCATCT-3′), sense ODN (5′-AGATGCCCAAGAAGAAGC-3′), antisense ODN to ERK (5′-CCGCCGCCGCCGCCGCC AT-3′), and sense ODN (5′-ATGGCGGCGGCGGCGGCGG-3′) were synthesized by Molecular Resource Facility of the New Jersey Medical School. Each ODN was incubated with the cells at 10 μM for 48 h before adding 1,25D. We designed double-stranded 21-mer siRNA targeting Raf-1(913-933) at the sequence 5′-AAUGUCCACAUGGUCAGCACC-3′. β-Galactosidase siRNA duplex (234-254) was used as a nonspecific (NS) control for RAf-1 and directed against the sequence: 5′-AACUUAAUCGCCUU GCAGCAC-3′. siMEK-1 and siERK-2 were purchased from Santa Cruz Biotechnology, Inc. along with NS siRNA for MEK-1 and ERK-2 controls. siRNAs were transfected into HL60 cells using Amaxa nucleofector and incubated for 48 h before adding 1,25D.
Immunoblotting (IB)
Immunoblotting was performed using whole cell extracts. The cells were harvested and washed twice with ice-cold 1× PBS. The washed cell pellets were solubilized with a lysis buffer containing 20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 mM PMSF, 1 μg/ml leupeptin, 1 μg/ml aprotinin, followed by centrifugation at 12,000g for 5 min. The protein concentrations of the extracts were determined by usingBio-Rad protein assay kit. Equal amounts of 3× SDS sample buffer containing 150 mM Tris-HCl, pH 6.8, 30% glycerol, 3% SDS, 1.5 mg/ml bromophenol blue dye, and 100 mM dithiothreitol were then added to each sample. Forty micrograms of whole cell extracts were separated on 10% SDS–PAGE gel and transferred to nitrocellulose membranes. The membranes were blocked with 5% milk in TBS/0.1% Tween 20 for 1 h, subsequently blotted overnight with primary antibodies, then blotted with a horseradish-linked secondary antibody for 1 h. The protein bands were visualized with a chemiluminescence assay system (Amersham, Piscataway, NJ). The optical density (OD) of each band was quantitated using an image quantitator (Molecular Dynamics, Sunnyvale, CA).
Kinase reaction assay
Total lysates were prepared and immunoprecipitated with the Raf-1 antibody. The beads were washed three times in immunoprecipitation buffer and then three times in kinase buffer (50 mM HEPES, 10 mM MgCl2, 5 mM MnCl2, 1 mM DTT, 0.2 mM NaVO4, 1 mM phenylmethlsulfonyl fluoride, 25 μg/ml leupeptin, 25 μg/ml aprotinin, and 25 μg/ml pepstatin A). The kinase reaction was carried out at 37°C for 30 min in 40 μl of kinase reaction buffer containing 10 μM ATP, 0.4 mCi [γ-32 P] and 2 μg of specific substrate myelin basic protein (MBP). The reaction was stopped by adding 3× SDS sample buffer. After boiling for 5 min and centrifugation at 3,000g for 2 min, the supernatant proteins were resolved on a 20% SDS– PAGE gel. The radioactivity was detected by autoradiography.
Co-immunoprecipitation (Co-IP)
Co-immunoprecipitation was performed by using Seize Primary Mammalian Immunoprecipitation Kit (Pierce Biotechnology, Rockford, IL) according to the manufacturer's recommended procedure. First, 50 μg of anti-Raf1 or anti-p90RSK antibody was coupled to 100 μl of AminoLink Plus coupling gel in a spin column and incubated overnight at 4°C, then a 200 μl aliquot of the whole cell extract was incubated with the antibody-coupling gel at 4°C overnight. Next, the immunoprecipitated proteins were eluted from the column and run on 10% SDS–PAGE gel, transferred to a membrane, and probed with specified antibodies. The protein bands were visualized with a chemiluminescence assay system (Amersham Pharmacia Biotech, Inc).
Statistical methods
All experiments were repeated for a minimum of three times. Significance of differences between mean values was assessed by two-tailed Student's t-test. All computations were performed with an IBM-compatible personal computer using Microsoft EXCEL.
Results
Kinase activity and phosphorylation of RAf-1 dramatically increase in a sustained manner after exposure to 1,25D
We first inquired if exposure of HL60 cells to low, near-physiological concentration (1 nM) of 1,25D activates the signaling kinase Raf-1. As shown in Figure 1, addition of 1,25D to HL60 cells resulted in a marked and sustained increase in enzyme activity of Raf-1, which was paralleled by both the expression and the phosphorylation of this protein. This confirmed that the 1,25D-induced phosphorylation was activating Raf-1 protein as an enzyme (Diaz et al., 1997; Xing and Kolesnick, 2001), rather than decreasing its kinase activity (Morrison et al., 1993; Dhillon et al., 2002), as shown in some other systems. This increase in the levels of phosphorylated Raf-1 was also observed when HL60 cells were exposed to all-trans RA at 1μM concentration, documented in previous studies to induce granulocytic differentiation of HL60 cells (Hong et al., 2001). However, in RA-treated cells the increase in P-Raf-1 was less marked (Fig. 1B, quantified in Fig. 2D). Together, these experiments demonstrate that differentiation of HL60 cells is accompanied by increased levels of P-Raf-1, and that enhanced kinase activity correlates with Raf-1 Ser338 phosphorylation.
Fig. 1.
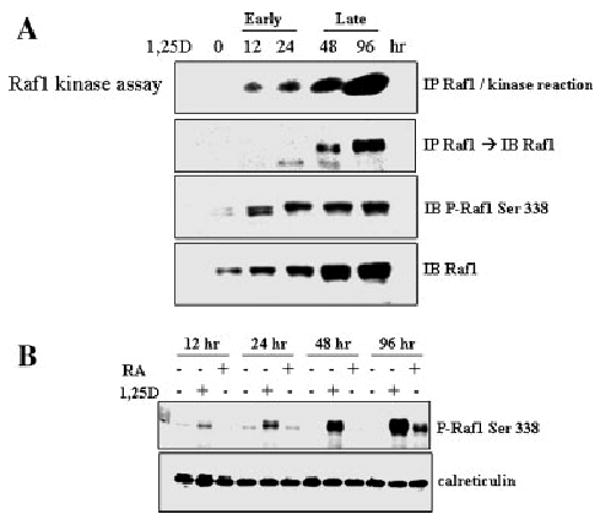
Kinase activity of Raf-1 increases in a sustained manner after exposure to 1,25D. A: The top two panel show the time course of Raf-1 kinase assay using extracts of HL60 cells exposed to 1 nM 1,25D for the indicated times. Note that the sensitivity of the radioactive assay (top panel) is higher than the sensitivity of protein detection by immunoblotting in the second panel. The kinase assay is paralleled by the detection of the phosphorylated Raf-1 (Ser 338) by immunoblotting (third panel). The increasing expression of Raf-1 protein is also shown. B: Comparison of the effects of 1 nM 1,25D with 1 μM RA on the time course of the increase in P-Raf-1 levels in HL60 cells.
Fig. 2.

Comparison and quantitation of the effects of 1,25D and retinoic acid on activation by phosphorylation of Raf-1, MEK-1, ERK1/2, and p90RSK. A: HL60 cells were treated with 1 nM 1,25D for the indicated times, and monocytic markers CD11b and CD14 were determined by flow cytometry. B: Immunoblots of whole cell extracts. HL60 cells were treated with 1,25D and also with retinoic acid for comparison. p90RSK showed different activation patterns compared to MEK-1 and ERKs. C: Quantitation by optical density (OD) of signals of the phosphorylated forms of the components of ERK MAP kinase pathway after 1,25D treatment. The signals for the phosphorylated Raf-1 (primary data shown in Fig. 1B) and p90RSK were continuously increasing and correlated with each other, while the intensity of signals for P-MEK and P-ERK showed an initial slight increase, followed by a sustained decrease. D: Quantitation of signals of the phosphorylated forms of components of ERK MAP kinase pathway after retinoic acid (RA, 1 μM) treatment showing different patterns of activation compared with those of 1,25D-treated HL60 cells.
Increased levels of phosphorylated Raf-1 and p90RSK, but not of phosphorylated MEK-1 and ERK1/2 proteins, parallel 1,25D-induced monocytic phenotype of HL60 cells
HL60 cells exposed to 1,25D express the morphology and various markers of monocytic phenotype, including the surface markers CD11b and CD14 (Wang et al., 1997). To determine if the induction of differentiation by 1,25D is associated with the classical MAPK phosphorylation cascade of Raf-MEK-ERK-RSK, we compared the kinetics of 1,25D-induced differentiation with the phosphorylation of the down-stream components of this cascade (Fig. 2). In order to see if a similar phosphorylation cascade takes place in granulocytic differentiation we again included in these experiments an exposure to RA. Surprisingly, although 1,25D induced increasing levels of P-p90RSK with kinetics that were tightly coupled to the emerging monocytic phenotype and P-Raf-1activation (shown in Fig.1A), the kinetics of MEK and ERK phosphorylation were starkly different (Fig. 2). As previously reported (Wang and Studzinski, 2001a), MEK and ERK showed an initially slightly increased level of activation as determined by phosphorylation, followed by a gradual decrease at later stages of differentiation, while phosphorylated p90RSK continued to increase in the presence of 1,25D (Fig. 2C). In contrast, activation of all four members of the Raf/MEK-1/ERK/RSK cascade was apparent in RA-treated HL60 cells during the 96 h period of observation (Fig. 2B,D, and data in Fig. 1B). Thus, although induction of monocytic differentiation by 1,25D is accompanied by increased Raf-1 activity, its downstream targets do not appear to be MEK and ERK in this system.
Ectopic expression of wild-type (wt) Raf-1, but not RAf-1 lacking the kinase domain, accelerates 1,25D-induced differentiation
When HL60 cells were transfected with a construct that expresses wild-type Raf-1 differentiation was not induced, but when 1 nM 1,25D was added to the transfected cells the appearance of monocytic phenotype was accelerated (Fig. 3). In these experiments the effects were modest due to low transfection efficiencies. On the other hand, transfection of the Raf-1 C4 construct, which lacks the kinase domain of Raf-1, had no significant effect on 1,25D-induced differentiation (Fig. 3). This shows that the increased levels of wild-type Raf-1 have a positive effect on 1,25D-induced differentiation, and although Raf-1 is not sufficient for this process to take place it appears to be required.
Fig. 3.

Ectopic expression of wild-type (wt) Raf-1, but not Raf-1 C4, increases 1,25D-induced monocytic differentiation. HL60 cells were transiently transfected with either Raf-C4 which lacks the kinase domain, or with the full-length wt Raf-1 plasmid, then 1 nM 1,25D was added for 48 h, and monocytic markers CD11b and CD14 were determined by flow cytometry. Full length Raf-1 significantly increased 1,25D-induced expression of both markers. *P < 0.05; **P < 0.01; n = 3.
Antisense Raf-1, but not antisense MEK1 or ERK1/2, inhibits 1,25D-induced differentiation
Transfection of HL60 cells with plasmids is difficult and less efficient than the uptake of oligonucleotides (Ferrari et al., 1992; Melkonyan et al., 1996; Manfredini et al., 1997). To further analyze this system we therefore utilized antisense technology which gives excellent results in this laboratory (Wang et al., 1998). As shown in Figure 4A, antisense (AS) Raf-1, but not mismatched (MM) Raf-1, or AS-MEK-1 and AS-ERK2 (the predominant form of ERK in HL60 cells), significantly reduced differentiation induced by 1 nM 1,25D. Western blot analysis showed that as expected, AS-Raf-1 reduced the cellular level of P-Raf-1 (and Raf-1 protein, data not shown) but the levels of P-MEK1 and P-ERK-2 were unchanged (Fig. 4), further suggesting that Raf-1 does not signal differentiation through the MEK/ERK module in this system. Interestingly, the phosphorylation of p90RSK, the downstream component of the typical Raf-1 signaling cascade, was reduced by AS-Raf-1 (Fig. 4B), suggesting that signaling of 1,25D-induced differentiation by Raf-1 bypasses the MEK/ERK module. Consistent with this notion, AS-MEK or AS-ERK had no observable effect on 1,25D-induced differentiation (Fig. 4A), or levels of P-p90RSK (Fig. 4B), although the total cell number was modestly reduced (data not shown), as expected from a role of ERK in promoting cell proliferation.
Fig. 4.

Antisense oligonucleotides to Raf-1, but not to MEK or ERK, decrease 1,25D-induced monocytic differentiation. A: HL60 cells were pretreated with the indicated oligonucleotides (10 μM) for 48 h before adding 1 nM 1,25D for another 48 h. Monocytic differentiation markers CD11b and CD14 were decreased in cells pretreated with antisense Raf1, but not when pretreated with MEK or ERK antisense oligonucleotides. B: The phosphorylation levels of the components of the Raf-1 signaling cascade demonstrating that antisense oligos decreased the corresponding phosphorylated protein levels. The ratios of each protein band intensity relative to the internal control calreticulin are shown below each band. Note that knock-down of Raf-1 activation does not reduce the phosphorylation of MEK or ERK in this system, but does reduce the phosphorylation of p90RSK. “Ratios” = OD of signals of the phosphorylated forms of the components of ERK MAP kinase pathway to OD of the internal control calreticulin.
Inhibition of 1,25D-induced differentiation by siRNA to Raf-1, but not by MEK-1 or ERK-2 siRNA
In view of the unexpected results described above, we used yet another approach to confirm these results. Again, knock-down of P-Raf-1 levels by siRaf-1 resulted in reduction of 1,25D-induced differentiation, but knock-down of P-MEK-1 or P-ERK2 by the corresponding siRNAs did not (Fig. 5). Together, these experiments provide compelling evidence that activated Raf-1, but not MEK-1 or ERK-2, are required for late stages of 1,25D-induced monocytic differentiation.
Fig. 5.

Inhibition of Raf-1 expression in HL60 by silencing Raf-1 RNA (siRaf-1), but not by silencing MEK or ERK RNA, partially blocks 1,25D-induced differentiation. A: siRNAs were transfected into HL60 cells using Amaxa nucleofector and incubated for 48 h, then 1 nM 1,25D was added for 48 h. The cells were harvested to determine CD11b, CD14, and monocyte-specific esterase (MSE). “NS-siRNA” is nonspecific siRNA used here as a control. B: Immunoblots for the phosphorylated target proteins after transfection with the different silencing RNAs showed that siRNAs effectively knocked down their target proteins. “Ratios” = OD of signals of the phosphorylated forms of the components of ERK MAP kinase pathway to internal control Crk-L protein.
Raf-1 and p90RSK interact with each other in differentiating HL60 cells
Given that the kinetics of Raf-1 and p90RSK activation closely parallel each other and differentiation of 1,25D-treated HL60 cells (Fig. 2C), and that the levels of P-p90RSK are reduced following inhibition of Raf-1 expression by its antisense oligonucleotide (Fig. 4B), we enquired if there is a direct interaction between these two protein kinases. Indeed, reciprocal immunoprecipitation/immunoblotting experiments demonstrate that Raf-1 and p90RSK strongly interact with each other in a 1,25D-dependent manner (Fig. 6). The well-recognized interaction of Raf-1 with MEK-1 (Jelinek et al., 1994) was also detected in this experiment, but the MEK-1 signal was less intense. Interestingly, we also detected a previously unknown association between p90RSK and MEK-1, the significance of which is currently unknown.
Fig. 6.

Interaction of Raf1, p90RSK, and MEK1 in differentiating HL60 cells. A: HL60 cells were treated with 1 nM 1,25D for 48 h. Whole cell lysates of HL60 cells were immunoprecipitated with either Raf-1 or p90RSK antibody, then immunoblotting was performed to detect Raf-1, p90RSK, and MEK1. B: Quantitation of the experiments. Raf-1 immunoprecipitation showed that binding of MEK-1 to Raf-1 in HL60 cells increases only modestly after 1,25D treatment, but binding of p90RSK to Raf-1 after treatment with 1,25D is markedly increased. Reciprocal co-immunoprecipitation confirmed increased Raf-1 and MEK-1 binding to p90RSK in differentiating cells. Mean ± SD, n = 3, are shown. C, untreated control; D, 1,25D. Numbers under each bar represent groups illustrated in part A.
Discussion
This report provides the first evidence that Raf-1 does not appear to signal through MEK and ERK in the context of 1,25D-induced monocytic differentiation of myeloid leukemia HL60 cells. Other deviations from the classical Raf/MEK/ERK signaling sequence were previously noted in other experimental models. Porras et al. (1994) reported that there is a dissociation between the activation of Raf-1 and ERK2/p90RSK signaling of adipocytic differentiation of 3T3 L1 cells, and Lenormand et al. (1996) showed that oncogenic Raf-1 activates p70S6K via a MAPK-independent pathway. Also, Kuo et al. (1996) showed that Raf-1, but not MEK or ERK, is sufficient for differentiation of neuronal cells, while Yen et al. found a disparity between the activation of Raf-1 and ERK by RA in HL60 cells, both as regards the timing (Hong et al., 2001), and the extent of activation (Yen and Varvayanis, 2000). Remarkably, it was also reported that the Raf oncogene and the MEK1/ERK signaling module have opposing roles in the regulation of surface transglutaminase in fibroblasts (Akimov and Belkin, 2003), while others described a Raf-1 mutant that disassociates MEK/ERK activation from differentiation but not from proliferation (Dhillon et al., 2003). Of special interest to our work is the report that a Raf-1 mutant that could not interact with MEK did not interfere with p90RSK phosphorylation or normal differentiation of PC12 cells (Pearson et al., 2000). It was also noted that a pharmacological inhibitor of the MEK pathway PD 184321 (Cl 1040) inhibited Raf-1 catalyzed phosphorylation of MEK in vitro without any effect on the Raf-1 catalyzed phosphorylation of MBP (Davies et al., 2000). Similarly, Raf inhibitor BAY 43-9006 was equipotent with Cl-1040 in an in vitro assay that detects phosphorylation of ERK which is dependent on Raf and MEK activity, but in contrast to Cl-1040, was ineffective in an in vivo Raf-dependent lung tumor mouse model (Kramer et al., 2004; Konopleva et al., 2005). As recently discussed (Hindley and Kolch, 2002), studies in Raf-1 knock-out mice further support the view that the phenotype of these animals cannot be totally explained by regulation of ERK by Raf-1 (Huser et al., 2001). On the basis of these and our results, we are proposing that while the Raf/MEK/ERK/RSK cascade may be of paramount importance for cell proliferation, transformation, and in the early stages of differentiation when the cells are still undergoing amplifying cell divisions, in at least some forms, and at some stages, of cell differentiation the MEK/ERK module is bypassed, and p90RSK is directly or indirectly phosphorylated by Raf-1. This hypothesis is schematically illustrated in Figure 7, and it remains to be further tested. Precisely what governs the switch from MEK-1 to p90RSK as the target for Raf-1 is also a matter for future extensive investigations. At this time our hypothesis is that as the level of the scaffold/kinase protein KSR-1 rises due to the direct upregulation of its expression by the liganded VDR (Wang et al., 2006), the affinity of KSR-1 for association with p90RSK increases. This is also depicted in Figure 7.
Fig. 7.

Schematic representation of the postulated signaling of MAP kinase pathway in proliferating or differentiating HL60 cells. A: In the early stage of HL60 cell differentiation, when proliferation still continues (hematopoietic lineage amplification), activation of ERK MAP kinase pathway is important in regulating proliferation-related genes, such as c-myc, Ets, and other targets. B: In the later stages of HL60 cell differentiation activation of both MEK-1 and ERK kinases is low, while the activation of RAf-1 signaling is continuously increasing, and appears to activate downstream targets such as p90RSK, while bypassing the MEK/ERK module. KSR-1 may amplify the Raf-RSK pathway, and result in phosphorylation of targets such as C/EBPβ. The C/EBPβ is one of the effectors of monocytic differentiation, perhaps through a C/EBP element present in the promoter of the monocyte marker CD14.
As in other systems (Khanim et al., 2004; Narayanan et al., 2004; Banwell et al., 2006), the mechanisms underlying signaling by 1,25D are likely to be cell specific, and it is already clear that several diverse signaling pathways are involved in the effects of 1,25D on HL60 cells. These include the JNK (Wang and Studzinski, 2001b; Ji et al., 2002; Wang et al., 2003) and the AKT (Zhang et al., 2006) pathways, and as described here, the Raf-1/p90RSK pathway. It is important to elucidate the precise detailed steps of this pathway in leukemia cells, as it has potential to be targeted for therapeutic intervention (Milella et al., 2001; Chang et al., 2003; Konopleva et al., 2005), and it is essential to identify the correct targets.
Acknowledgments
We thank Dr. Milan Uskokovic, Bioxell, Nutley, NJ for the gift of 1,25D and Dr. Ulf Rapp, University of Wurzburg, Germany, for the Raf-1 constructs. We also are grateful to Dr. Michael Danilenko, Ben-Gurion University, Israel, and Dr. Ewa Marcinkowska, University of Wroclaw, Poland for help with the manuscript.
Contract grant sponsor: National Cancer Institute, NIH; Contract grant number: R01-CA44722-16; Contract grant sponsor: American Institute for Cancer Research (AICR); Contract grant number: 05A022.
Literature Cited
- Akimov SS, Belkin AM. Opposing roles of Ras/Raf oncogenes and the MEK1/ERK signaling module in regulation of expression and adhesive function of surface transglutaminase. J Biol Chem. 2003;278(37):35609–35619. doi: 10.1074/jbc.M303488200. [DOI] [PubMed] [Google Scholar]
- Banwell CM, MacCartney DP, Guy M, Miles AE, Uskokovic MR, Mansi J, Stewart PM, O'Neill LP, Turner BM, Colston KW, Campbell MJ. Altered nuclear receptor corepressor expression attenuates vitamin D receptor signaling in breast cancer cells. Clin Cancer Res. 2006;12(7 Pt 1):2004–2013. doi: 10.1158/1078-0432.CCR-05-1218. [DOI] [PubMed] [Google Scholar]
- Brennan JA, Volle DJ, Chaika OV, Lewis RE. Phosphorylation regulates the nucleocytoplasmic distribution of kinase suppressor of Ras. J Biol Chem. 2002;277(7):5369–5377. doi: 10.1074/jbc.M109875200. [DOI] [PubMed] [Google Scholar]
- Bruder JT, Heidecker G, Rapp UR. Serum-, TPA-, and Ras-induced expression from Ap-1/Ets-driven promoters requires Raf-1 kinase. Genes Dev. 1992;6(4):545–556. doi: 10.1101/gad.6.4.545. [DOI] [PubMed] [Google Scholar]
- Buitrago CG, Ronda AC, Boland AR, Boland R. MAP kinases p38 and JNK are activated by the steroid hormone 1alpha,25(OH)(2)-vitamin D(3) in the C2C12 muscle cell line. J Cell Biochem. 2006;97(4):698–708. doi: 10.1002/jcb.20639. [DOI] [PubMed] [Google Scholar]
- Chang F, Steelman LS, Lee JT, Shelton JG, Navolanic PM, Blalock WL, Franklin RA, McCubrey JA. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: Potential targeting for therapeutic intervention. Leukemia. 2003;17(7):1263–1293. doi: 10.1038/sj.leu.2402945. [DOI] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351(Pt 1):95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon AS, Pollock C, Steen H, Shaw PE, Mischak H, Kolch W. Cyclic AMP-dependent kinase regulates Raf-1 kinase mainly by phosphorylation of serine 259. Mol Cell Biol. 2002;22(10):3237–3246. doi: 10.1128/MCB.22.10.3237-3246.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon AS, Meikle S, Peyssonnaux C, Grindlay J, Kaiser C, Steen H, Shaw PE, Mischak H, Eychene A, Kolch W. A Raf-1 mutant that dissociates MEK/extracellular signal-regulated kinase activation from malignant transformation and differentiation but not proliferation. Mol Cell Biol. 2003;23(6):1983–1993. doi: 10.1128/MCB.23.6.1983-1993.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz B, Barnard D, Filson A, MacDonald S, King A, Marshall M. Phosphorylation of Raf-1 serine 338-serine 339 is an essential regulatory event for Ras-dependent activation and biological signaling. Mol Cell Biol. 1997;17(8):4509–4516. doi: 10.1128/mcb.17.8.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S, Manfredini R, Grande A, Torelli U. Antisense strategies to characterize the role of genes and oncogenes involved in myeloid differentiation. Ann N Y Acad Sci. 1992;660:11–26. doi: 10.1111/j.1749-6632.1992.tb21053.x. [DOI] [PubMed] [Google Scholar]
- Hindley A, Kolch W. Extracellular signal regulated kinase (ERK)/mitogen activated protein kinase (MAPK)-independent functions of Raf kinases. J Cell Sci. 2002;115(Pt 8):1575–1581. doi: 10.1242/jcs.115.8.1575. [DOI] [PubMed] [Google Scholar]
- Hong HY, Varvayanis S, Yen A. Retinoic acid causes MEK-dependent RAF phosphorylation through RARalpha plus RXR activation in HL-60 cells. Differentiation. 2001;68(1):55–66. doi: 10.1046/j.1432-0436.2001.068001055.x. [DOI] [PubMed] [Google Scholar]
- Huser M, Luckett J, Chiloeches A, Mercer K, Iwobi M, Giblett S, Sun XM, Brown J, Marais R, Pritchard C. MEK kinase activity is not necessary for Raf-1 function. EMBO J. 2001;20(8):1940–1951. doi: 10.1093/emboj/20.8.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek T, Catling AD, Reuter CW, Moodie SA, Wolfman A, Weber MJ. RAS and RAF-1 form a signalling complex with MEK-1 but not MEK-2. Mol Cell Biol. 1994;14(12):8212–8218. doi: 10.1128/mcb.14.12.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Kutner A, Verstuyf A, Verlinden L, Studzinski GP. Derivatives of vitamins D2 and D3 activate three MAPK pathways and upregulate pRb expression in differentiating HL60 cells. Cell Cycle. 2002;1(6):410–415. doi: 10.4161/cc.1.6.269. [DOI] [PubMed] [Google Scholar]
- Khanim FL, Gommersall LM, Wood VH, Smith KL, Montalvo L, O'Neill LP, Xu Y, Peehl DM, Stewart PM, Turner BM, Campbell MJ. Altered SMRT levels disrupt vitamin D3 receptor signalling in prostate cancer cells. Oncogene. 2004;23(40):6712–6725. doi: 10.1038/sj.onc.1207772. [DOI] [PubMed] [Google Scholar]
- Kim SH, Kang SN, Kim HJ, Kim TS. Potentiation of 1,25-dihydroxyvitamin D3-induced differentiation of human promyelocytic leukemia cells into monocytes by costunolide, a germacranolide sesquiterpene lactone. Biochem Pharmacol. 2002;64(8):1233–1242. doi: 10.1016/s0006-2952(02)01292-3. [DOI] [PubMed] [Google Scholar]
- Konopleva M, Shi Y, Steelman LS, Shelton JG, Munsell M, Marini F, McQueen T, Contractor R, McCubrey JA, Andreeff M. Development of a conditional in vivo model to evaluate the efficacy of small molecule inhibitors for the treatment of Raf-transformed hematopoietic cells. Cancer Res. 2005;65(21):9962–9970. doi: 10.1158/0008-5472.CAN-05-1068. [DOI] [PubMed] [Google Scholar]
- Kramer BW, Gotz R, Rapp UR. Use of mitogenic cascade blockers for treatment of C-Raf induced lung adenoma in vivo: CI-1040 strongly reduces growth and improves lung structure. BMC Cancer. 2004;4:24. doi: 10.1186/1471-2407-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo WL, Abe M, Rhee J, Eves EM, McCarthy SA, Yan M, Templeton DJ, McMahon M, Rosner MR. Raf, but not MEK or ERK, is sufficient for differentiation of hippocampal neuronal cells. Mol Cell Biol. 1996;16(4):1458–1470. doi: 10.1128/mcb.16.4.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenormand P, McMahon M, Pouyssegur J. Oncogenic Raf-1 activates p70 S6 kinase via a mitogen-activated protein kinase-independent pathway. J Biol Chem. 1996;271(26):15762–15768. doi: 10.1074/jbc.271.26.15762. [DOI] [PubMed] [Google Scholar]
- Manfredini R, Balestri R, Tagliafico E, Trevisan F, Pizzanelli M, Grande A, Barbieri D, Zucchini P, Citro G, Franceschi C, Ferrari S. Antisense inhibition of c-fes proto-oncogene blocks PMA-induced macrophage differentiation in HL60 and in FDC-P1/MAC-11 cells. Blood. 1997;89(1):135–145. [PubMed] [Google Scholar]
- Marcinkowska E. Evidence that activation of MEK1,2/erk1,2 signal transduction pathway is necessary for calcitriol-induced differentiation of HL-60 cells. Anticancer Res. 2001;21(1A):499–504. [PubMed] [Google Scholar]
- Marcinkowska E, Wiedlocha A, Radzikowski C. 1,25-Dihydroxyvitamin D3 induced activation and subsequent nuclear translocation of MAPK is upstream regulated by PKC in HL-60 cells. Biochem Biophys Res Commun. 1997;241(2):419–426. doi: 10.1006/bbrc.1997.7832. [DOI] [PubMed] [Google Scholar]
- Melkonyan H, Sorg C, Klempt M. Electroporation efficiency in mammalian cells is increased by dimethyl sulfoxide (DMSO) Nucleic Acids Res. 1996;24(21):4356–4357. doi: 10.1093/nar/24.21.4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milella M, Kornblau SM, Estrov Z, Carter BZ, Lapillonne H, Harris D, Konopleva M, Zhao S, Estey E, Andreeff M. Therapeutic targeting of the MEK/MAPK signal transduction module in acute myeloid leukemia. J Clin Invest. 2001;108(6):851–859. doi: 10.1172/JCI12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison DK, Heidecker G, Rapp UR, Copeland TD. Identification of the major phosphorylation sites of the Raf-1 kinase. J Biol Chem. 1993;268(23):17309–17316. [PubMed] [Google Scholar]
- Narayanan R, Sepulveda VA, Falzon M, Weigel NL. The functional consequences of cross-talk between the vitamin D receptor and ERK signaling pathways are cell-specific. J Biol Chem. 2004;279(45):47298–47310. doi: 10.1074/jbc.M404101200. [DOI] [PubMed] [Google Scholar]
- Ory S, Zhou M, Conrads TP, Veenstra TD, Morrison DK. Protein phosphatase 2A positively regulates Ras signaling by dephosphorylating KSR1 and Raf-1 on critical 14-3-3 binding sites. Curr Biol. 2003;13(16):1356–1364. doi: 10.1016/s0960-9822(03)00535-9. [DOI] [PubMed] [Google Scholar]
- Pearson G, Bumeister R, Henry DO, Cobb MH, White MA. Uncoupling Raf1 from MEK1/2 impairs only a subset of cellular responses to Raf activation. J Biol Chem. 2000;275(48):37303–37306. doi: 10.1074/jbc.C000570200. [DOI] [PubMed] [Google Scholar]
- Pepper C, Thomas A, Hoy T, Milligan D, Bentley P, Fegan C. The vitamin D3 analog EB1089 induces apoptosis via a p53-independent mechanism involving p38 MAP kinase activation and suppression of ERK activity in B-cell chronic lymphocytic leukemia cells in vitro. Blood. 2003;101(7):2454–2460. doi: 10.1182/blood-2002-07-1984. [DOI] [PubMed] [Google Scholar]
- Porras A, Muszynski K, Rapp UR, Santos E. Dissociation between activation of Raf-1 kinase and the 42-kDa mitogen-activated protein kinase/90-kDa S6 kinase (MAPK/RSK) cascade in the insulin/Ras pathway of adipocytic differentiation of 3T3 L1 cells. J Biol Chem. 1994;269(17):12741–12748. [PubMed] [Google Scholar]
- Song X, Bishop JE, Okamura WH, Norman AW. Stimulation of phosphorylation of mitogen-activated protein kinase by 1alpha,25-dihydroxyvitamin D3 in promyelocytic NB4 leukemia cells: A structure-function study. Endocrinology. 1998;139(2):457–465. doi: 10.1210/endo.139.2.5747. [DOI] [PubMed] [Google Scholar]
- Studzinski GP, Wang X, Ji Y, Wang Q, Zhang Y, Kutner A, Harrison JS. The rationale for deltanoids in therapy for myeloid leukemia: Role of KSR-MAPK-C/EBP pathway. J Steroid Biochem Mol Biol. 2005;97(1–2):47–55. doi: 10.1016/j.jsbmb.2005.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajchmann HJ, Rathod B, Song S, Xu H, Wang X, Uskokovic MR, Studzinski GP. Loss of deoxycytidine kinase expression and tetraploidization of HL60 cells following long-term culture in 1,25-dihydroxyvitamin D3. Exp Cell Res. 1996;224(2):312–322. doi: 10.1006/excr.1996.0141. [DOI] [PubMed] [Google Scholar]
- Wang X, Studzinski GP. Antiapoptotic action of 1,25-dihydroxyvitamin D3 is associated with increased mitochondrial MCL-1 and RAF-1 proteins and reduced release of cytochrome c. Exp Cell Res. 1997;235(1):210–217. doi: 10.1006/excr.1997.3667. [DOI] [PubMed] [Google Scholar]
- Wang X, Studzinski GP. Activation of extracellular signal-regulated kinases (ERKs) defines the first phase of 1,25-dihydroxyvitamin D3-induced differentiation of HL60 cells. J Cell Biochem. 2001a;80(4):471–482. doi: 10.1002/1097-4644(20010315)80:4<471::aid-jcb1001>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Wang X, Studzinski GP. Inhibition of p38MAP kinase potentiates the JNK/SAPK pathway and AP-1 activity in monocytic but not in macrophage or granulocytic differentiation of HL60 cells. J Cell Biochem. 2001b;82(1):68–77. doi: 10.1002/jcb.1141. [DOI] [PubMed] [Google Scholar]
- Wang X, Studzinski GP. Phosphorylation of raf-1 by kinase suppressor of ras is inhibited by “MEK-specific” inhibitors PD 098059 and U0126 in differentiating HL60 cells. Exp Cell Res. 2001c;268(2):294–300. doi: 10.1006/excr.2001.5292. [DOI] [PubMed] [Google Scholar]
- Wang X, Studzinski GP. Kinase suppressor of RAS (KSR) amplifies the differentiation signal provided by low concentrations 1,25-dihydroxyvitamin D3. J Cell Physiol. 2004;198(3):333–342. doi: 10.1002/jcp.10443. [DOI] [PubMed] [Google Scholar]
- Wang X, Gardner JP, Kheir A, Uskokovic MR, Studzinski GP. Synergistic induction of HL60 cell differentiation by ketoconazole and 1-desoxy analogues of vitamin D3. J Natl Cancer Inst. 1997;89(16):1199–1206. doi: 10.1093/jnci/89.16.1199. [DOI] [PubMed] [Google Scholar]
- Wang QM, Chen F, Luo X, Moore DC, Flanagan M, Studzinski GP. Lowering of p27Kip1 levels by its antisense or by development of resistance to 1,25-dihydroxyvitamin D3 reverses the G1 block but not differentiation of HL60 cells. Leukemia. 1998;12(8):1256–1265. doi: 10.1038/sj.leu.2401088. [DOI] [PubMed] [Google Scholar]
- Wang X, Rao J, Studzinski GP. Inhibition of p38 MAP kinase activity up-regulates multiple MAP kinase pathways and potentiates 1,25-dihydroxyvitamin D(3)-induced differentiation of human leukemia HL60 cells. Exp Cell Res. 2000;258(2):425–437. doi: 10.1006/excr.2000.4939. [DOI] [PubMed] [Google Scholar]
- Wang Q, Wang X, Studzinski GP. Jun N-terminal kinase pathway enhances signaling of monocytic differentiation of human leukemia cells induced by 1,25-dihydroxyvitamin D3. J Cell Biochem. 2003;89(6):1087–1101. doi: 10.1002/jcb.10595. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang TT, White JH, Studzinski GP. Induction of kinase suppressor of RAS-1 (KSR-1) gene by 1, 25-dihydroxyvitamin D3 in human leukemia HL60 cells through a vitamin D response element in the 5′-flanking region. Oncogene. 2006 doi: 10.1038/sj.onc.1209697. May 29, 2006. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing HR, Kolesnick R. Kinase suppressor of Ras signals through Thr269 of c-Raf-1. J Biol Chem. 2001;276(13):9733–9741. doi: 10.1074/jbc.M008096200. [DOI] [PubMed] [Google Scholar]
- Yan F, Polk DB. Kinase suppressor of ras is necessary for tumor necrosis factor alpha activation of extracellular signal-regulated kinase/mitogen-activated protein kinase in intestinal epithelial cells. Cancer Res. 2001;61(3):963–969. [PubMed] [Google Scholar]
- Yen A, Varvayanis S. Retinoic acid increases amount of phosphorylated RAF; ectopic expression of cFMS reveals that retinoic acid-induced differentiation is more strongly dependent on ERK2 signaling than induced GO arrest is. In Vitro Cell Dev Biol Anim. 2000;36(4):249–255. doi: 10.1290/1071-2690(2000)036<0249:raiaop>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang J, Studzinski GP. AKT pathway is activated by 1, 25-dihydroxyvitamin D3 and participates in its anti-apoptotic effect and cell cycle control in differentiating HL60 cells. Cell Cycle. 2006;5(4):447–451. doi: 10.4161/cc.5.4.2467. [DOI] [PubMed] [Google Scholar]


