Abstract
Background
The etiology of several common gastric motility diseases remains largely unknown. Gastric wall biopsies that include the muscularis propria to evaluate the enteric nervous system, interstitial cells of Cajal and related cells are essential to promote our understanding of the pathophysiologic mechanisms. Based on our previous work, a double EMR technique provided sufficient tissue to identify myenteric ganglia. A serious limitation to the technique was the resultant gastric wall perforation after tissue resection. The optimal procedure would seal the gastric wall defect prior to tissue resection, eliminating the risk of peritonitis.
Objectives
The aims of this study were to 1. Determine the technical feasibility and reproducibility of a full thickness gastric biopsy using a novel double EMR technique without creating a perforation (‘no hole’) and to 2. Determine safety of the procedure.
Design and Interventions
Pre-clinical study of six pigs. Each animal underwent a ‘no hole’ double EMR survival procedure. To prevent perforation, detachable endoloops and prototype T-tag tissue anchors were placed prior to resection. At 2 weeks repeat endoscopy was performed followed by necropsy.
Main Outcome Measurements
Hematoxylin and Eosin staining was used to determine which muscle layers were included in the resected specimen, and an antibody to nNOS was utilized to visualize myenteric ganglia in the sample. Technical feasibility, reproducibility and safety of the procedure were evaluated.
Results
Full thickness gastric biopsy was obtained from all animals without overt perforation. There were no procedural complications. Histology showed muscularis propria with all layers of muscle present, and immunochemical studies demonstrated myenteric ganglia in all tissue samples. Four animals had an uneventful clinical course and repeat endoscopy at week 2 showed ulceration with stellate fibrosis. Necropsy showed mild localized adhesions. Two animals were sacrificed at days 3 and 6 respectively due to suspected peritonitis. At necropsy delayed perforations at the resection sites were noted with displaced endoloops and tissue anchors.
Conclusion
This study explored the concept of obtaining deep muscle wall biopsies using a unique approach of resection without perforation. The novel ‘no hole’ double EMR technique was technically feasible and reproducible with sufficient tissue obtained to identify myenteric ganglia. However, there was a high delayed perforation rate associated with displaced endoloops and tissue anchors. Based on this early experience, improved safety data may be anticipated with future studies using improved tissue closure devices.
INTRODUCTION
The underlying mechanisms that result in upper gastrointestinal motility disorders such as gastroparesis or functional dyspepsia are not well understood1,2. Gastric wall biopsies that include the entire muscularis propria to evaluate the enteric nervous system would likely provide significant information that will likely promote our understanding of the pathophysiologic mechanisms of these diseases. Current mucosal-based biopsies are insufficient as they do not allow evaluation of the deep muscle layers, the myenteric plexus or interstitial cells of Cajal (ICC) networks. At present, full thickness biopsies require surgery as no endoscopic technique exists for safe acquisition of such tissue. We have previously evaluated different endoscopic techniques for full thickness gastric wall biopsies. Of all the techniques tried including endoscopic ultrasound (EUS) guided Tru-Cut biopsy of the gastric wall, jumbo biopsy of post-endoscopic mucosal resection (EMR) site, jumbo biopsy of gastrotomy margin and serosal-side biopsy through a gastrotomy, only a double EMR technique provided muscularis propria (oblique, circular and longitudinal muscle layers) including the myenteric plexus region with myenteric ganglia3. However, a limitation to this technique was the resultant gastric wall perforation following resection. Such a perforation can be sealed at the time of EMR using available methods such as hemoclips (Olympus America, Center Valley, PA) but the risk of peritonitis from soiling of the peritoneal cavity from leaked gastric contents at the time of perforation before resealing the hole remains. The ideal procedure would seal the gastric wall defect prior to tissue resection eliminating any risk of peritonitis.
The aims of this pre-clinical study were therefore to 1. Determine the technical feasibility and reproducibility of a full thickness gastric biopsy in a pig model using a novel double EMR technique without creating a perforation (‘no hole’), and 2. Determine the short and long term safety of the procedure
MATERIALS AND METHODS
Experimental Design
This pre-clinical survival study in a pig model was approved by the Institutional Animal Care and Use Committee. Six pigs were approved for this study. Each animal underwent a novel ‘no hole’ double EMR with use of detachable endoloops and prototype T-tag tissue anchors prior to the second resection to prevent procedural perforation. Animals were kept alive after the procedure and at two weeks repeat endoscopy was performed followed by necropsy. The resection sites were inspected for procedure related complications. Animals were sacrificed early with immediate necropsy if clinical course was complicated or animals were in significant pain. The main study outcome measurements were histologic analysis of the resected tissue to determine which wall layers were included in the resected specimen, and to determine if myenteric ganglia were present in the sample using an antibody to neuronal nitric oxide synthase (nNOS), expressed in a subpopulation of enteric neurons. Data was recorded on the clinical course of the animals, technical feasibility, reproducibility and short and long term (2 weeks) safety of the procedure. Data analysis was descriptive as this was a pilot feasibility study.
Animals
Six domestic 30 kg pigs were studied. ‘No hole’ double EMR was performed in each animal under general anesthesia and orotracheal intubation, following a 48 hour fast. Antibiotics were administered pre-procedure (cefazolin IV) and for 5 days post-procedure (cephalexin orally), and analgesia (buprenorphine hydrochloride, intramuscular) was administered immediately post-procedure. Animals were placed on a liquid diet for one day post- procedure, softened food on the second day, and by the third day all animals resumed regular feed if tolerated. After a two week survival period, repeat endoscopy was performed. Animals were chemically euthanized immediately after endoscopy followed by necropsy. Animals were euthanized and necropsy performed early if the clinical course was complicated or animals were in significant pain.
Procedure
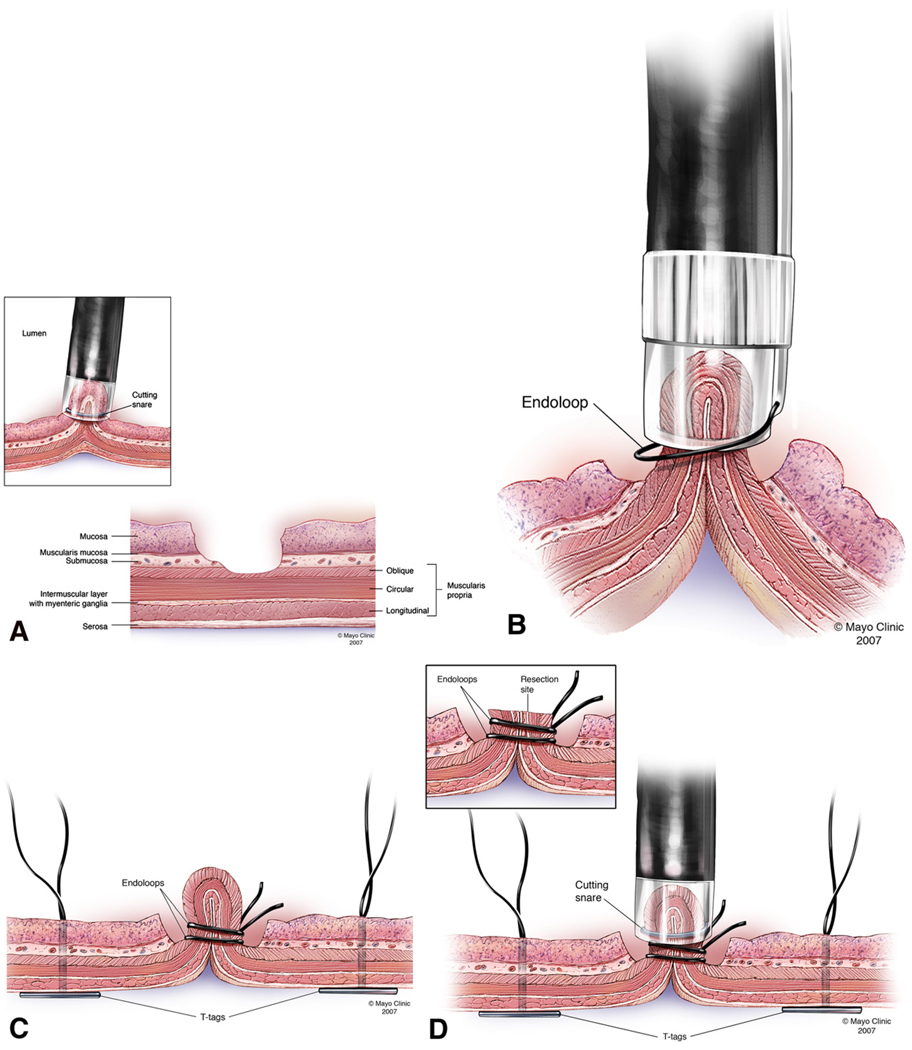
‘No hole’ double EMR technique (Figures 1A, 1B, 1C, 1D, 1E and 1F)
FIGURE 1.
FIGURE 1A: EMRC performed without a protective submucosal cushion
FIGURE 1B: Exposed muscle layer suctioned into EMRC cap while the endoloop was gently released and tightened around the pseudopolyp.
FIGURE 1C: Second endoloop and prototype T-tag tissue anchors are positioned around the base and adjacent to the pseudopolyp respectively.
FIGURE 1D: Pseudopolyp resected by snare electrocautery.
FIGURE 1E: Resection site further closed by tightening (opposing) prototype T-tag tissue anchors.
Step 1
The ‘no hole’ double EMR technique involved two cap- assisted endoscopic mucosal resections (EMRC). An oblique cap with an outer diameter of 16.1mm and length of 14mm (Olympus America, Center Valley, PA) was attached to the distal end of a therapeutic endoscope (GIF-T100; Olympus America, Center Valley, PA) and used to perform the initial EMRC4. The anterior wall of the gastric body was targeted and EMRC performed without a protective submucosal cushion in the standard manner5. Following the first resection, the underlying gastric muscle layer was exposed.
Step 2
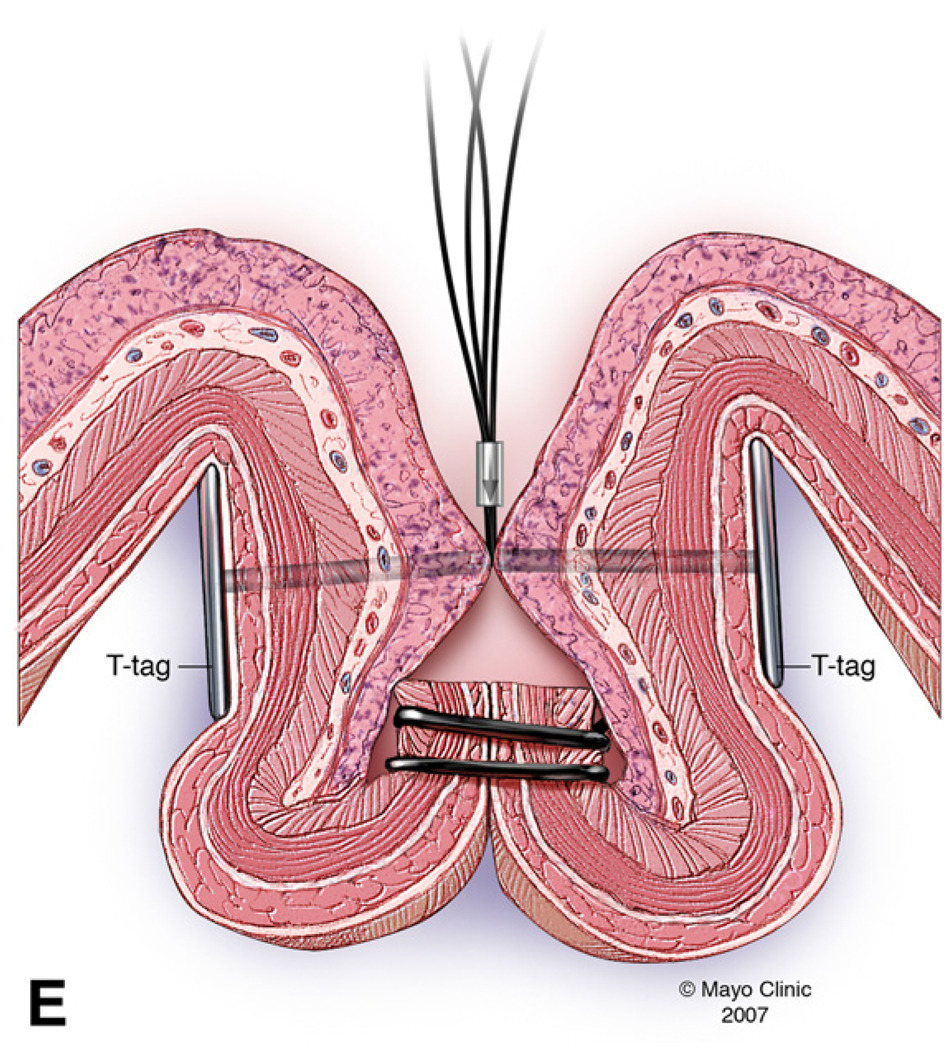
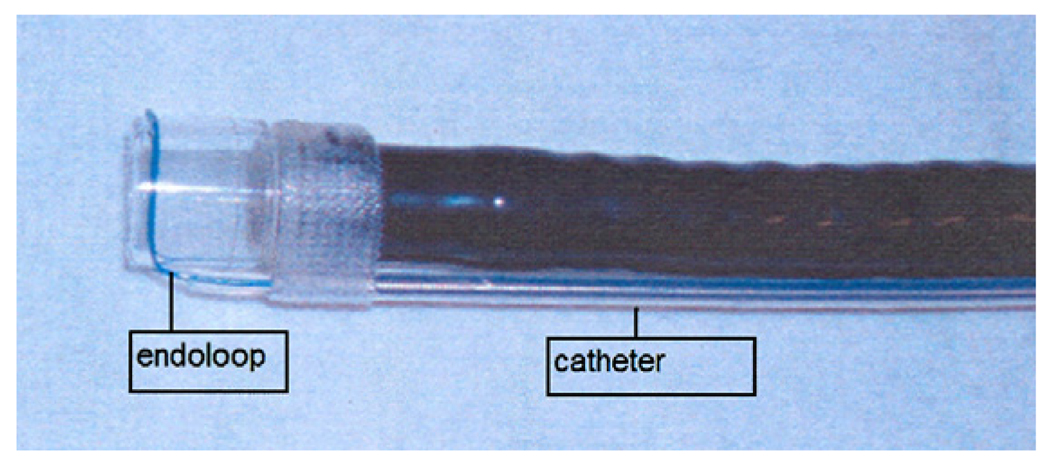
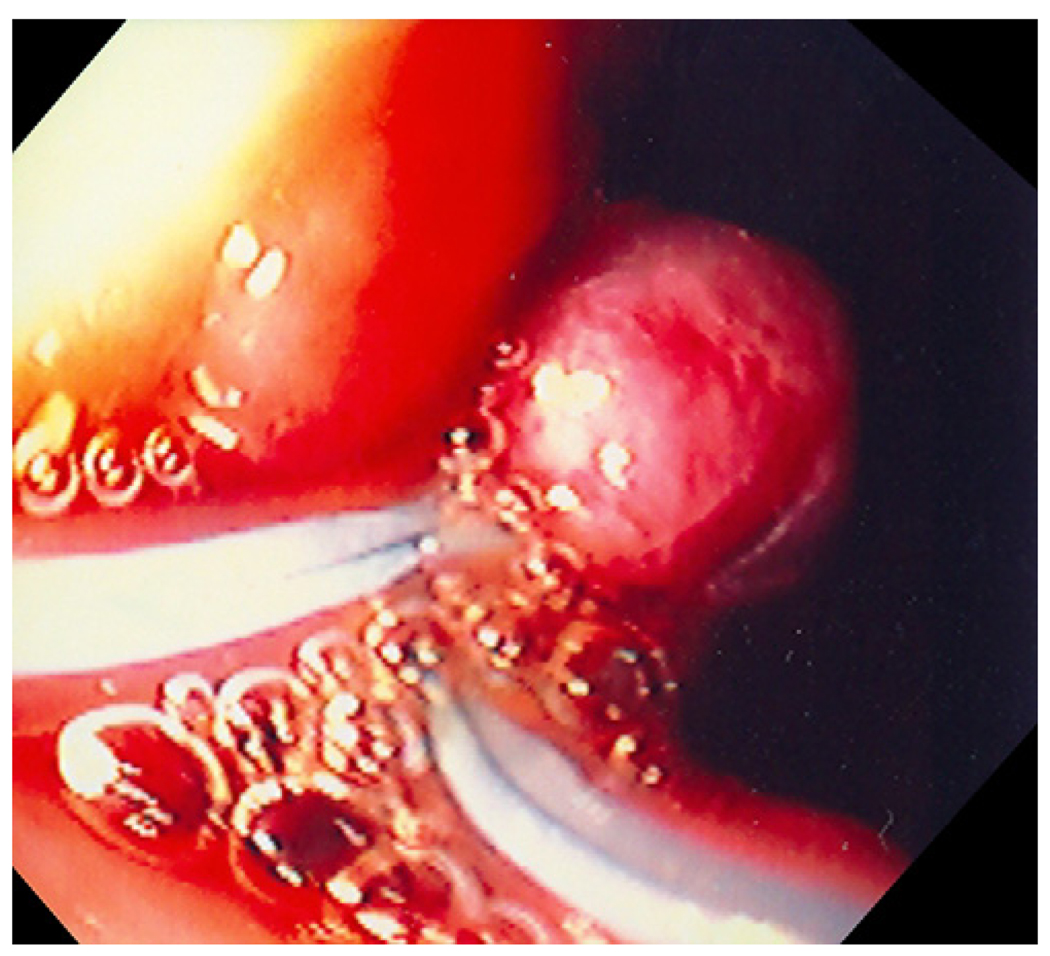
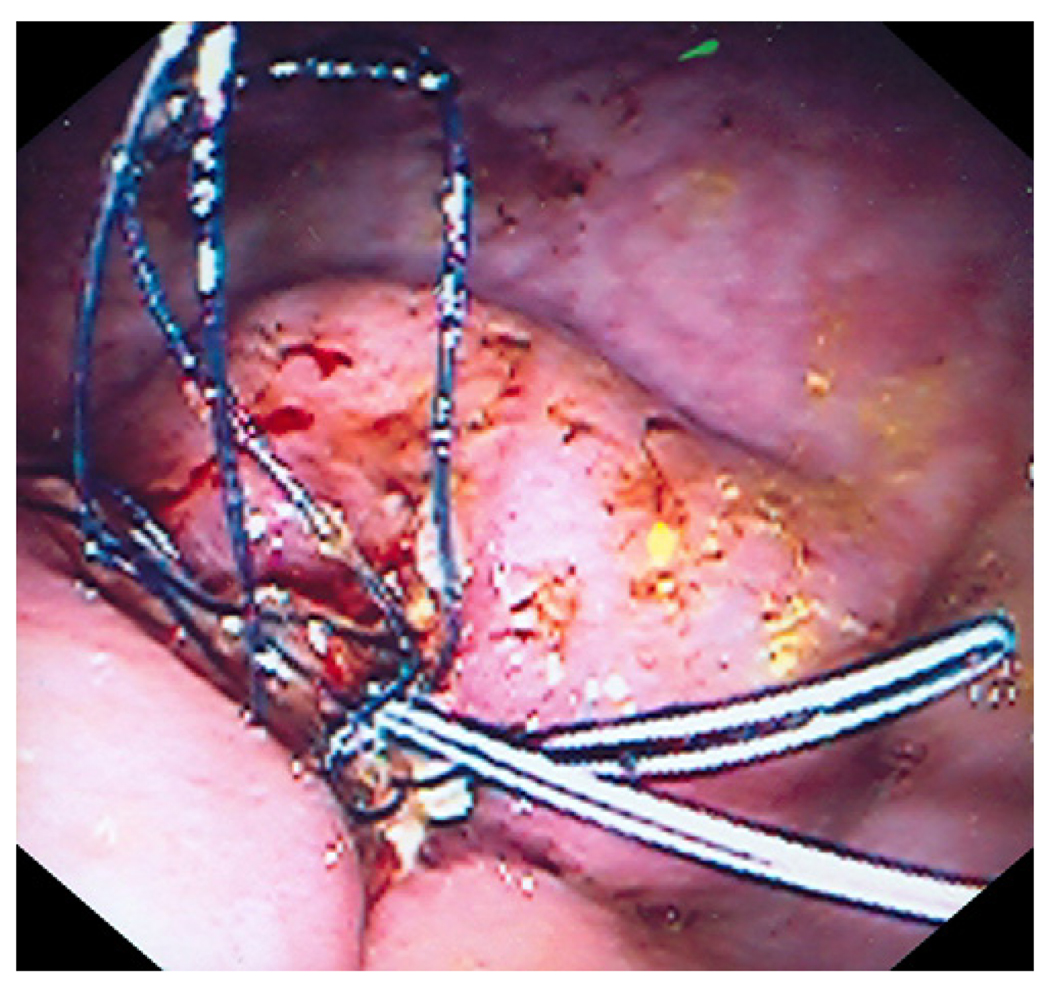
The therapeutic endoscope was removed and a smaller straight cap with an outer diameter of 13.9mm and length of 12mm (Olympus America, Center Valley, PA) was attached to a diagnostic endoscope (GIF-140; Olympus America, Center Valley, PA)2. An endoloop (MAJ-340, Olympus America, Center Valley, PA) was secured externally to the endoscope by partially opening the endoloop and tightening it around the outer aspect of the distal end of the straight cap. The catheter of the endoloop was then secured to the endoscope outer sheath using adhesive surgical tape (Figure 2). The animal was reintubated and the endoscope with endoloop advanced to the resection site. The exposed muscle layer was suctioned into the cap and once filled with tissue, the endoloop was opened and gently released from the cap. The endoloop was then tightened while still applying suction. This maneuver created a pseudopolyp of the exposed gastric muscle layer. Next a second endoloop was placed using the conventional applicator to further tighten the base of the pseudopolyp (Figure 3). Prototype needle catheter T-tag tissue anchors (Olympus America, Center Valley, PA) were then placed on either side of the pseudopolyp.
FIGURE 2.
Endoloop (A) attached around the outer aspect of the distal end of the straight EMRC cap with adhesive surgical tape securing the catheter (B) of the endoloop to the endoscope outer sheath.
FIGURE 3.
Endoscopic image showing muscle wall pseudopolyp with two endoloops at the peusopolyp base.
Step 3
The pseudopolyp was resected by snare electrocautery using an extra small snare (Olympus America, Center Valley, PA). The resected tissue was suctioned into the cap and retrieved. Finally, the resection site was further closed by tightening the prototype T-tag tissue anchors, minimizing the risk of perforation (Figure 4).
FIGURE 4.
Endoscopic image of resection site closed by prototype T-tag tissue anchors.
Tissue Samples
The resected tissues were transported over ice to the lab in Ham F12 media (Invitrogen, Carlsbad, CA). Hematoxylin and Eosin staining was used to determine which muscle layers were biopsied and an antibody to nNOS used to determine if myenteric ganglia and neurons were present in the sample and nerve fibers in the muscle layers. The biopsies were fixed overnight at 4° C in 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO) in 1X phosphate buffer solution, rinsed in 1X phosphate buffered saline (PBS), and immersed overnight at 4° C in 1X PBS solution containing 30% sucrose (Sigma-Aldrich, St. Louis, MO). The biopsies were then cut in cross-section and frozen in Tissue-Tek OCT compound (Electron Microscopy Sciences, Hatfield, PA). Cryotstat sections 12 µm in thickness were cut. A standard Hematoxylin and Eosin staining protocol was used. For the nNOS immunohistochemistry, the slides were first rinsed twice in 1X PBS followed by a blocking step for non-specific secondary antibody binding by incubating the tissue with a 1X PBS, 10% normal donkey serum (NDS; Jackson ImmunoResearch Lab, Inc., West Grove, PA) and 0.3% Triton X-100 (Pierce, Rockford, IL) solution for one hour. The primary antibody against nNOS (Chemicon International, Inc., Temecula, CA) was diluted in 1X PBS, 5% NDS, 0.3% Triton X-100 and incubated overnight at 4° C. A Cy3 conjugated donkey anti-rabbit secondary antibody (Jackson ImmunoResearch Lab, Inc., West Grove, PA) diluted in 1X PBS, 2.5% NDS, 0.3% Triton X-100 solution was used to visualize the nNOS positive neuronal fibers and myenteric ganglia.
RESULTS
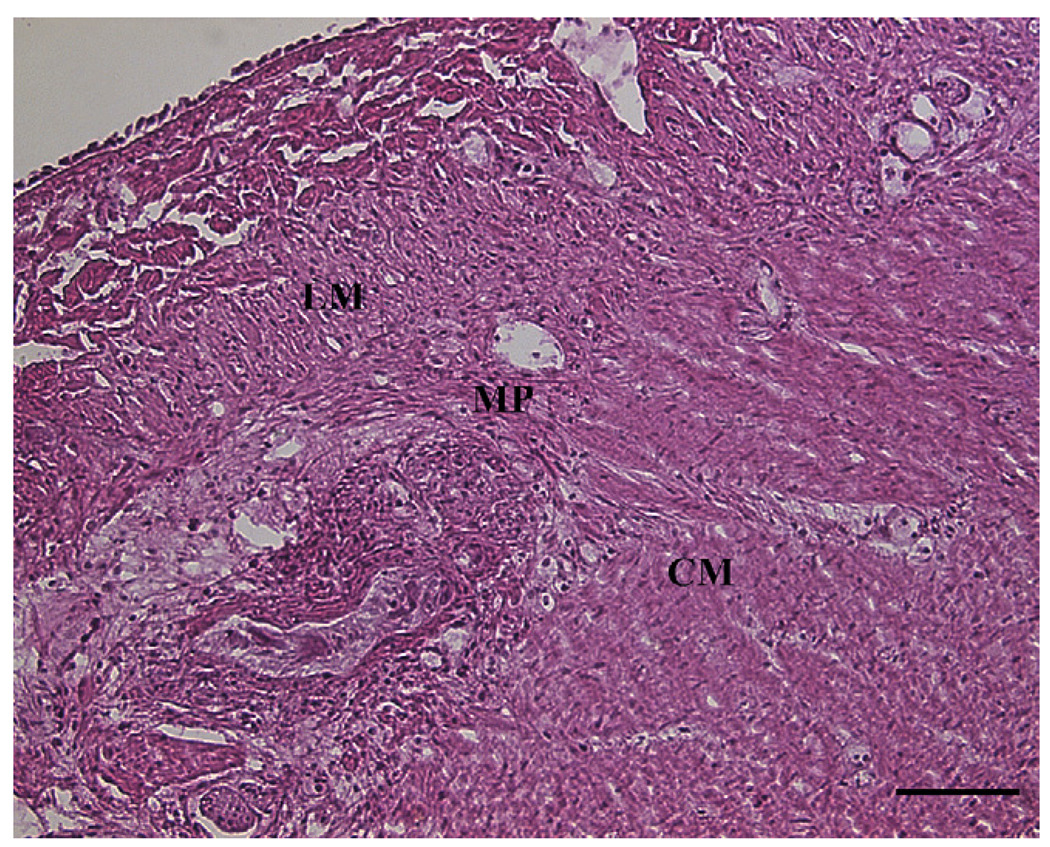
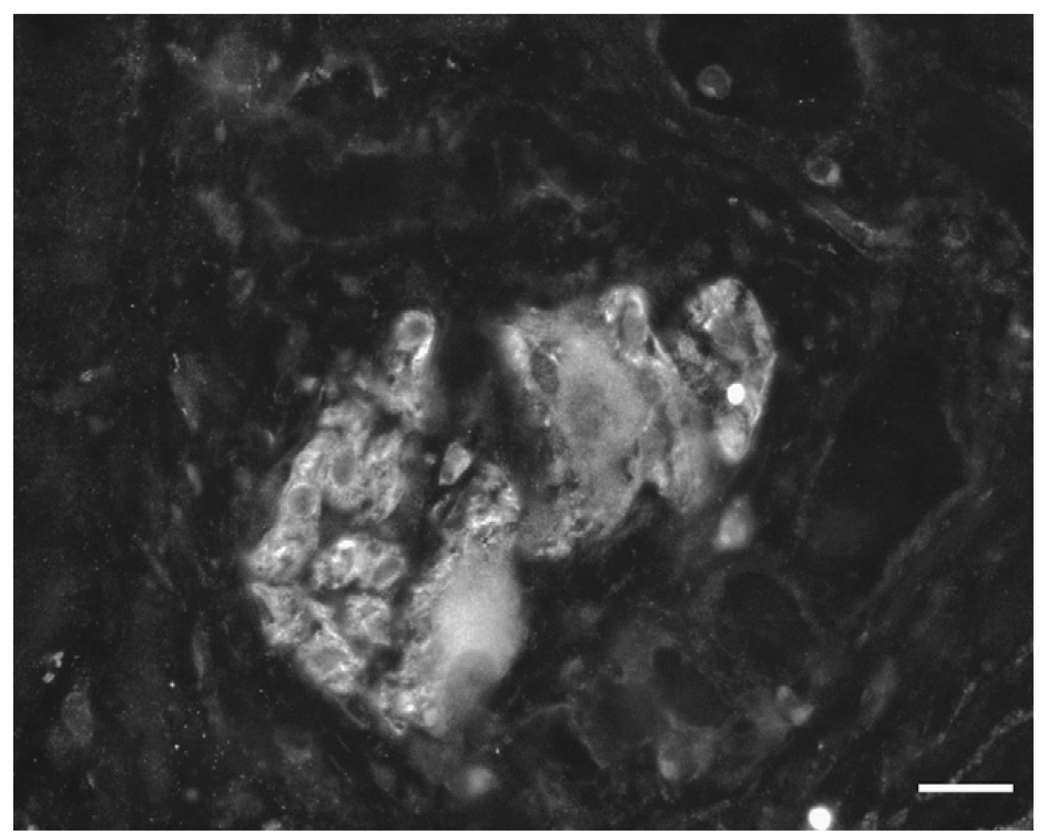
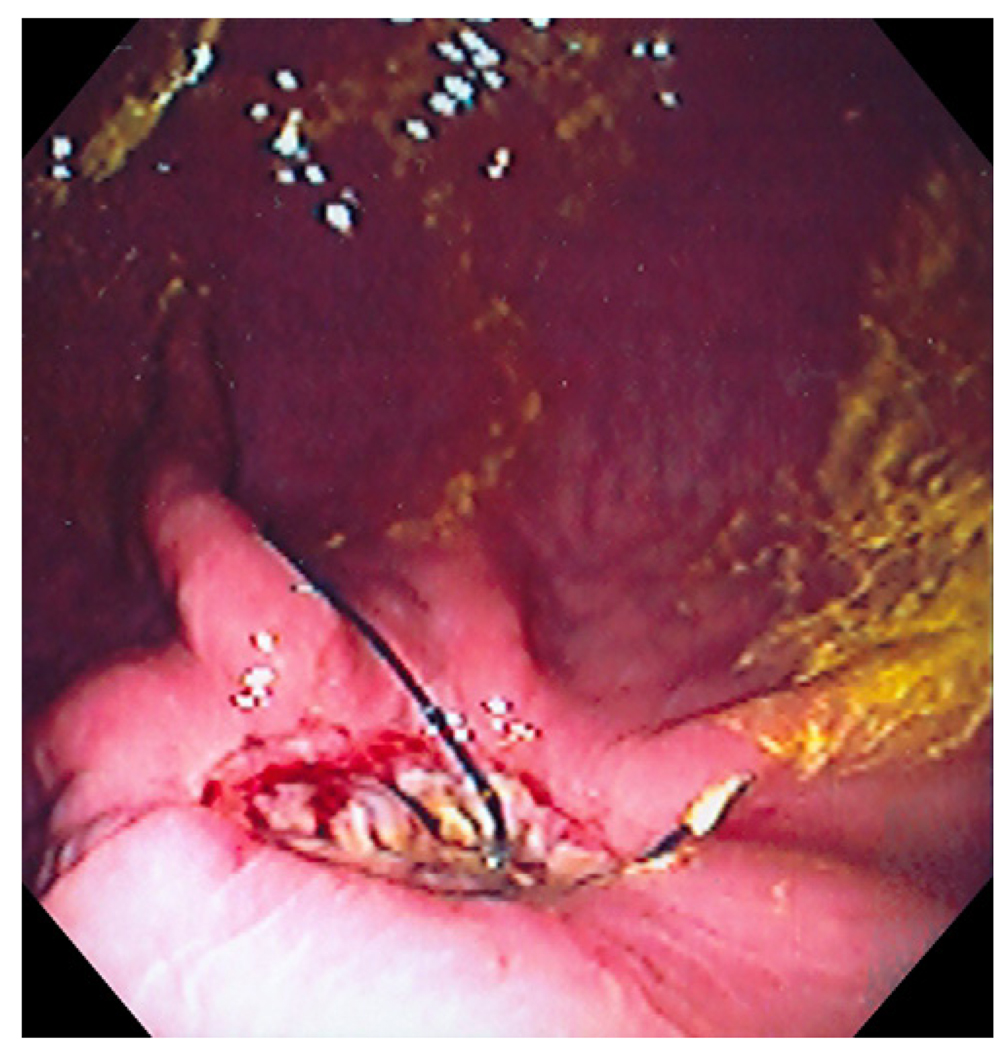
The technique of ‘no hole’ double EMR was successfully completed in all animals (100%, 6/6). Full thickness biopsy of the stomach was achieved without overt perforation. No procedural complications occurred and no adjacent organs were included in the resection. Histology showed muscularis propria (oblique, circular and longitudinal muscle layers) and serosa confirming full thickness resections in all six animals (Figure 5). Immunochemical studies demonstrated the presence of myenteric ganglia in tissue from all six animals (Figure 6). Four animals survived for 2 weeks with an uneventful clinical course. Repeat endoscopy at week 2 showed ulceration with stellate fibrosis. T-tags but no endoloops were seen, an anticipated outcome given the propensity of the endoloops to fall off as scarring occurs (Figure 7). Necropsy showed mild localized adhesions to omentum and adjacent small bowel (Figure 8). Two animals were sacrificed early at days 3 and 6 respectively due to suspected peritonitis manifest as fever, listlessness and poor appetite. At necropsy delayed perforations at the resection sites were noted with both displaced endoloops and tissue anchors.
FIGURE 5.
Hematoxylin and Eosin (H&E) staining of tissue showing the muscularis propria and myenteric plexus region. H&E stained section of the full thickness muscle resection showing the presence of the circular muscle (CM), myenteric plexus region (MP), and longitudinal muscle (LM). Scale bar = 100 µm
FIGURE 6.
Tissue showing anti-nNOS immunopositive myenteric ganglia. nNOS immunoreactivity showing the presence of nNOS positive neurons in the sample indicating that the myenteric plexus was present in the muscle resection. Scale bar = 20 µm.
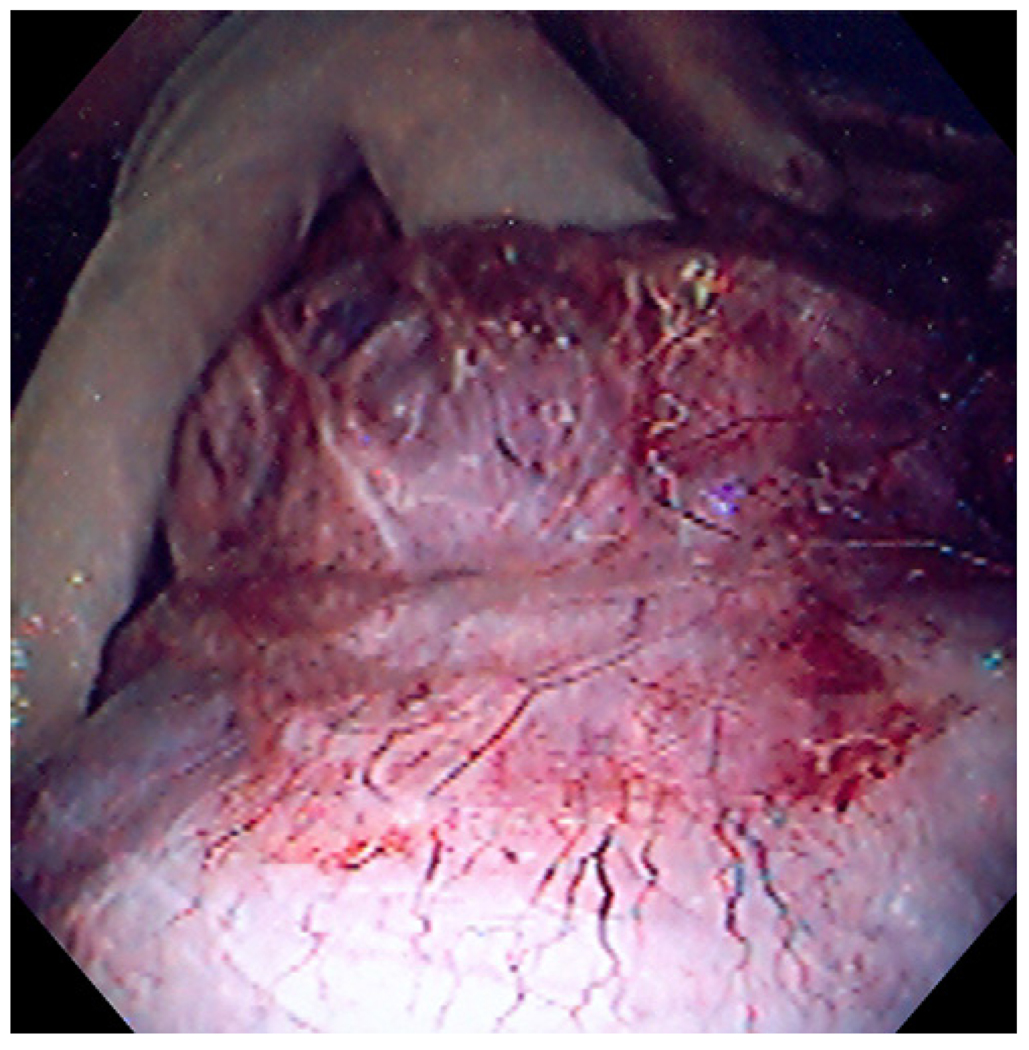
FIGURE 7.
Endoscopy at week 2 demonstrating ulceration with stellate fibrosis and retained T-tag tissue anchors.
FIGURE 8.
Necropsy showing localized adhesions between gastric wall and adjacent small bowel.
DISCUSSION
This preclinical survival study evaluated the technical feasibility and reproducibility of a full thickness biopsy of the gastric wall without causing overt perforation (‘no hole’). Using the novel ‘no hole’ double EMR technique a full thickness biopsy of the entire muscularis propria that included oblique, circular and longitudinal muscle layers could be technically achieved with sufficient tissue obtained from the intermuscular layer to identify myenteric ganglia6. The significant advantage of this technique on prior techniques is that the full thickness gastric wall defect was closed prior to tissue resection preventing leakage of any gastric contents or fluid into the peritoneal cavity. There are reports in the literature and in clinical practice of full thickness perforations closed in a timely fashion without resultant peritonitis7,8. Nonetheless, the ideal procedure should be one in which the gastric wall defect is closed prior to tissue resection eliminating the potential risk of peritonitis. There were no complications during the procedure. Four of the six animals had an uncomplicated clinical course with not unexpected mucosal ulceration with scarring and mild adhesions at follow-up. However, there was a high delayed perforation rate from displaced endoloops and T-tags with resultant peritonitis (two of the four animals). The explanation for this displacement is not entirely clear but may be due to several factors or combination of factors including the coaptive pressure exerted by the T-tags resulting in slippage of the tissue through the endoloops, local edema and inflammation loosening the endoloops or T-tags, ischemia and tissue necrosis from over-tightening and inadequately secured endoloops or T-tags. The use of the band ligator device for EMR was avoided because of the increased risk of band slippage after tissue resection.
The ‘no hole’ double EMR technique reflects an important directional shift in approaching invasive and complex endoscopic procedures. There are several effective endoscopic tools for closure of pre-existent perforations within the upper and lower gastrointestinal tract using either hemoclips, suturing devices or T-tag tissue anchors 9,10,11. We have previously reported on the performance of prototype tissue anchors including alternative designs to the T-tag12. In this experience of creating full thickness gastric plications, the more robust tissue anchor designs were most durable in maintaining a full thickness plication. It is possible that tissue anchors other than the T-tag design may be more suitable to seal a full thickness defect. There are currently no commercially available endoscopic devices to pre-seal a full thickness defect prior to resection. The ‘no hole’ double EMR technique establishes the technical feasibility of such an approach and concept, using limited commercially available endoscopic devices. There have been other approaches published on full thickness resections. Each has its own merits and disadvantages. A recent preclinical study evaluating the technical feasibility and safety of an endoscopic full thickness resection technique with a view to removing less invasive gastrointestinal cancers reported on an effective method using a prototype bidirectional cutter, snare and sphincterotome13. Resultant gastric perforations were closed using a prototype T-tag tissue anchor suturing device. This technique is limited by the presence of a gastric wall defect immediately after resection increasing the risk of peritonitis, and the use of prototype devices14. A preclinical study from our institution assessed a unique approach for entry into the peritoneal cavity as part of our natural orifice translumenal endoscopic surgery (NOTES) initiative using a ‘submucosal endoscopy with a mucosal flap’ (SEMF) technique15. In this technique, the overlying mucosal flap created by tunneling through the submucosal layer was used as a mucosal flap safety valve to seal the gastric wall perforation almost immediately after accessing the peritoneal cavity. Studies are ongoing to assess the safety of this promising approach. A recent case series reported on the use of a surgical stapling device (SurgAssist, Power Medical Interventions, Germany) for endoscopic full thickness resection of gastric tumors16. Although not yet approved for use in flexible gastrointestinal endoscopy in the US, this device has the unique capability of simultaneous full thickness tissue resection and tissue stapling in a single step. Such a device would be ideal for effective and safe acquisition of full thickness tissue specimens in clinical practice. Future iterations of a smaller caliber device with greater flexibility will provide improved maneuverability in the gastrointestinal tract. Our group reported on a device using a similar model for transmural colorectal tissue resection using a prototype full thickness resection and stapling device17. Based on the initial pre-clinical results, it was apparent that the device size and lack of flexibility would impede its clinical application.
This study explored the unique concept of obtaining deep muscle wall biopsies using a novel approach of resection without creating a perforation. Such an approach would virtually guarantee the prevention of peritonitis. This study on the innovative ‘no hole’ double EMR technique establishes the technical feasibility of this concept, and showed it was effective in providing full thickness tissue samples with myenteric ganglia. The role of inflammation and/or immune dysregulation in gastrointestinal diseases such as functional gastrointestinal disorders, gastroparesis, pseudo-obstruction and other motility disorders has not yet been adequately investigated18,19. The burden of illness is substantial, and there is no effective specific treatment to successfully alleviate patient symptoms20,21,22. Undoubtedly, this is in part because the most appropriate tissue to study, a full thickness biopsy specimen of the gastrointestinal system, is attainable only with a surgical procedure limiting availability. More studies are needed to identify the ideal endoscopic technique that would be effective in providing a full thickness biopsy, and this must be safe, easy to use and maneuverable in the upper and lower gastrointestinal tract. Better safety data may be anticipated with future studies using improved tissue acquisition and closure devices.
Acknowledgment
The authors wish to acknowledge the contributions of Robert F. Morreale Creative Services Director, Medical Illustration and Animation Mayo Clinic and Foundation, Rochester, MN.
Grant support: Funded in part by NIH grants DK68055 and DK57061.
Footnotes
Presented (Poster): Digestive Diseases Week, May 2006, Los Angeles, California. Gastrointest Endosc 2006; 63(5):AB234.
No conflict of interest
Contributor Information
E Rajan, Division of Gastroenterology and Hepatology, Developmental Endoscopy Unit, Mayo Clinic College of Medicine, 200 First St. SW, Rochester, MN 55905..
CJ Gostout, Division of Gastroenterology and Hepatology, Developmental Endoscopy Unit, Mayo Clinic College of Medicine, 200 First St. SW, Rochester, MN 55905..
MS Lurken, Enteric NeuroSciecne Program, Mayo Clinic College of Medicine, 200 First St. SW, Rochester, MN 55905..
NJ Talley, Enteric NeuroSciecne Program, Mayo Clinic College of Medicine, 200 First St. SW, Rochester, MN 55905..
GR Locke, Enteric NeuroSciecne Program, Mayo Clinic College of Medicine, 200 First St. SW, Rochester, MN 55905..
LA Szarka, Enteric NeuroSciecne Program, Mayo Clinic College of Medicine, 200 First St. SW, Rochester, MN 55905..
K Sumiyama, Division of Gastroenterology and Hepatology, Developmental Endoscopy Unit, Mayo Clinic College of Medicine, 200 First St. SW, Rochester, MN 55905..
TA Bakken, Division of Gastroenterology and Hepatology, Developmental Endoscopy Unit, Mayo Clinic College of Medicine, 200 First St. SW, Rochester, MN 55905..
GJ Stoltz, Enteric NeuroSciecne Program, Mayo Clinic College of Medicine, 200 First St. SW, Rochester, MN 55905..
MA Knipschield, Division of Gastroenterology and Hepatology, Developmental Endoscopy Unit, Mayo Clinic College of Medicine, 200 First St. SW, Rochester, MN 55905..
G Farrugia, Enteric NeuroSciecne Program, Mayo Clinic College of Medicine, 200 First St. SW, Rochester, MN 55905..
REFERENCES
- 1.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132(1):397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Camilleri M. Clinical practice. Diabetic gastroparesis. N Engl J Med. 2007;356(8):820–829. doi: 10.1056/NEJMcp062614. [DOI] [PubMed] [Google Scholar]
- 3.Rajan E, Gostout CJ, Lurken MS, Locke GR, Talley NJ, Szarka LA, et al. Different Endoscopic Approaches for Deep (Subserosal) Gastric Wall Biopsies: What Works? Gastrointest Endosc. 2006;63(5):AB233. doi: 10.1016/j.gie.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 4.Sumiyama K, Rajan E. Endosocpic caps. Techniques in Gatrointestinal Endoscopy. 2006;8:28–32. [Google Scholar]
- 5.Inoue H, Endo M, Takeshita K, Yoshino K, Muraoka Y, Yoneshima A new simplified technique of endoscopic esophageal mucosal resection using a cap-fitted panendoscope (EMRC) Surg Endosc. 1992;6:264–265. doi: 10.1007/BF02498820. [DOI] [PubMed] [Google Scholar]
- 6.Rich A, Hanani M, Ermilov LG, Malysz J, Belzer V, Szurszewski JH, et al. Physiological study of interstitial cells of Cajal identified by vital staining. Neurogastroenterol Motil. 2002;14(2):189–196. doi: 10.1046/j.1365-2982.2002.00319.x. [DOI] [PubMed] [Google Scholar]
- 7.Mana F, De Vogelaere K, Urban D. Iatrogenic perforation of the colon during diagnostic colonoscopy: endoscopic treatment with clips. Gastrointest Endosc. 2001;54(2):258–259. doi: 10.1067/mge.2001.114959. [DOI] [PubMed] [Google Scholar]
- 8.Yoshikane H, Hidano H, Sakakibara A, Ayakawa T, Mori S, Kawashima H, et al. Endoscopic repair by clipping of iatrogenic colonic perforation. Gastrointest Endosc. 1997;46(5):464–466. doi: 10.1016/s0016-5107(97)70045-2. [DOI] [PubMed] [Google Scholar]
- 9.Sumiyama K, Gostout CJ, Rajan E, Bakken TA, Deters JL, Knipschield MA. Endoscopic full-thickness closure of large gastric perforations by use of tissue anchors. Gastrointest Endosc. 2007;65(1):134–139. doi: 10.1016/j.gie.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 10.Minami S, Gotoda T, Ono H, Oda I, Hamanaka H. Complete endoscopic closure of gastric perforation induced by endoscopic resection of early gastric cancer using endoclips can prevent surgery (with video) Gastrointest Endosc. 2006;63(4):596–601. doi: 10.1016/j.gie.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 11.Hu B, Chung SC, Sun LC, Kawashima K, Yamamoto T, Cotton PB, et al. Eagle Claw II: A novel endosuture device that uses a curved needle for major arterial bleeding: a bench study. Gastrointest Endosc. 2005;62(2):266–270. doi: 10.1016/s0016-5107(05)00375-5. [DOI] [PubMed] [Google Scholar]
- 12.Seaman DL, Gostout CJ, de la Mora Levy JG, Knipschield MA. Tissue anchors for transmural gut-wall apposition. Gastrointest Endosc. 2006;64(4):577–581. doi: 10.1016/j.gie.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda K, Mosse CA, Park PO, Fritscher-Ravens A, Bergström M, Mills T, et al. Endoscopic full-thickness resection: circumferential cutting method. Gastrointest Endosc. 2006;64(1):82–89. doi: 10.1016/j.gie.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 14.Kantsevoy SV. Endoscopic full-thickness resection: new minimally invasive therapeutic alternative for GI-tract lesions. Gastrointest Endosc. 2006;64(1):90–91. doi: 10.1016/j.gie.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Sumiyama K, Gostout CJ, Rajan E, Bakken TA, Knipschield MA, Marler RJ. Submucosal endoscopy with mucosal flap safety valve. Gastrointest Endosc. 2007 doi: 10.1016/j.gie.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 16.Kaehler G, Grobholz R, Langner C, Suchan K, Post S. A new technique of endoscopic full-thickness resection using a flexible stapler. Endoscopy. 2006;38(1):86–89. doi: 10.1055/s-2005-921181. [DOI] [PubMed] [Google Scholar]
- 17.Rajan E, Gostout CJ, Burgart LJ, Leontovich ON, Knipschiel MA, Herman LJ, et al. First endoluminal system for transmural resection of colorectal tissue with a prototype full-thickness resection device in a porcine model. Gastrointest Endosc. 2002;55(7):915–920. doi: 10.1067/mge.2002.124099. [DOI] [PubMed] [Google Scholar]
- 18.Ohman L, Simren M. New insights into the pathogenesis and pathophysiology of irritable bowel syndrome. Dig Liver Dis. 2007;39(3):201–215. doi: 10.1016/j.dld.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Tornblom H, Lindberg G, Nyberg B, Veress B. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology. 2002;123(6):1972–1979. doi: 10.1053/gast.2002.37059. [DOI] [PubMed] [Google Scholar]
- 20.Talley NJ, Zinsmeister AR, Schleck CD, Melton LJ., 3rd Dyspepsia and dyspepsia subgroups: a population-based study. Gastroenterology. 1992;102:1259–1268. [PubMed] [Google Scholar]
- 21.Drossman DA, Li Z, Andruzzi E, Temple RD, Talley NJ, Thompson WG, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Digestive Diseases & Sciences. 1993;38(9):1569–1580. doi: 10.1007/BF01303162. [DOI] [PubMed] [Google Scholar]
- 22.Penston JG, Pounder RE. A survey of dyspepsia in Great Britain. Aliment Pharmacol Ther. 1996;10:83–89. doi: 10.1111/j.1365-2036.1996.tb00180.x. [DOI] [PubMed] [Google Scholar]