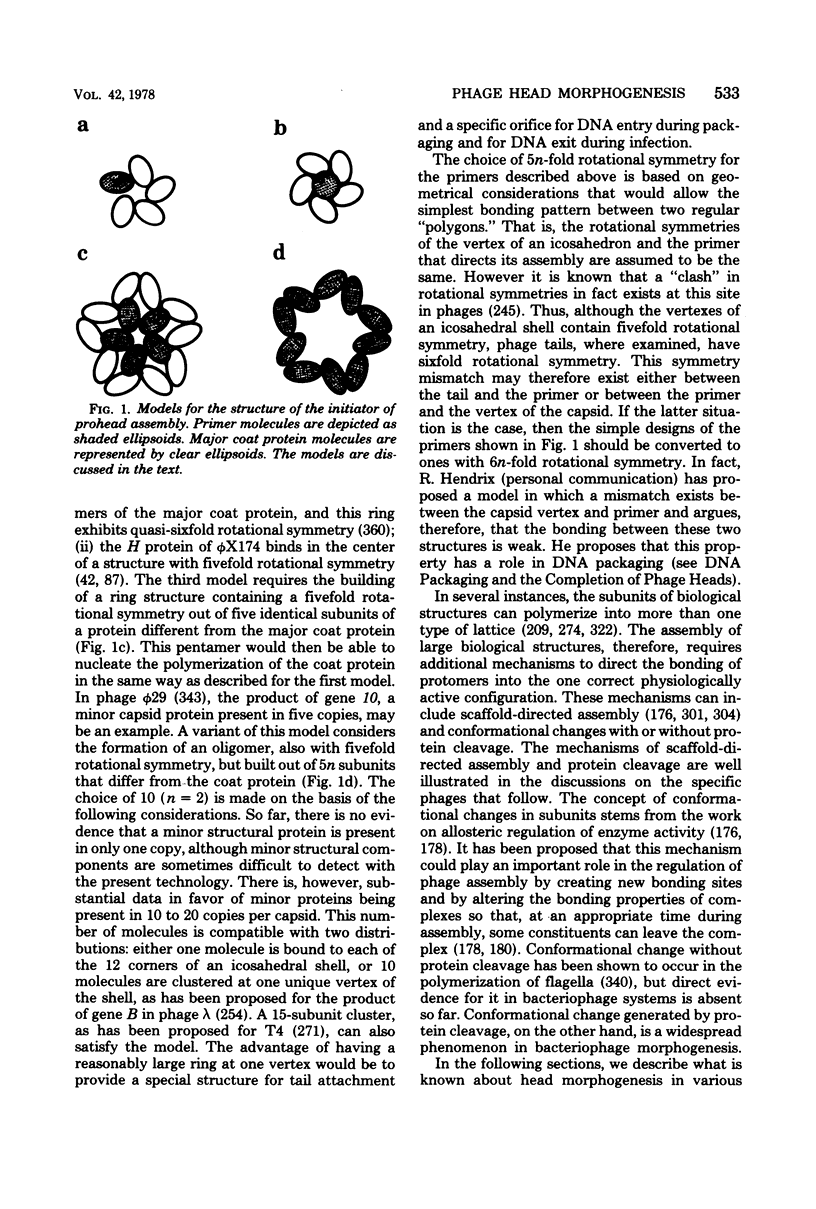
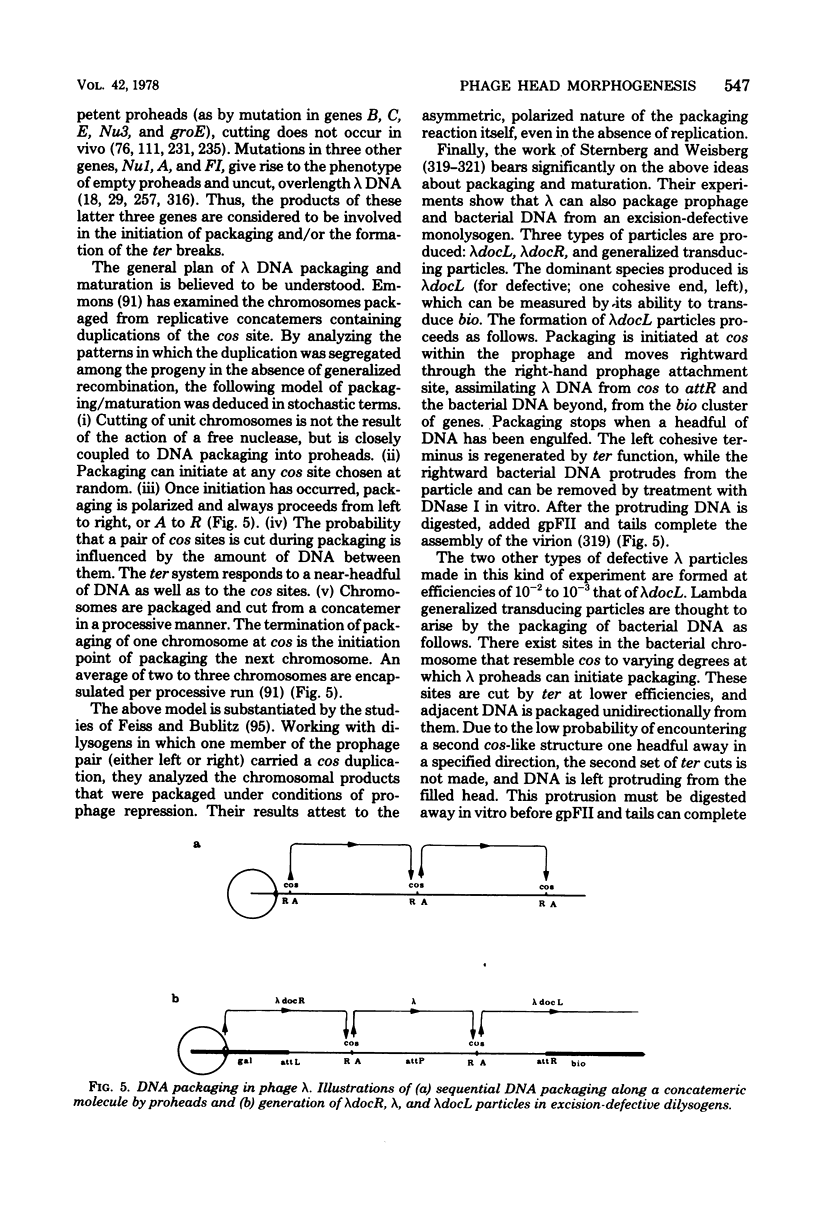
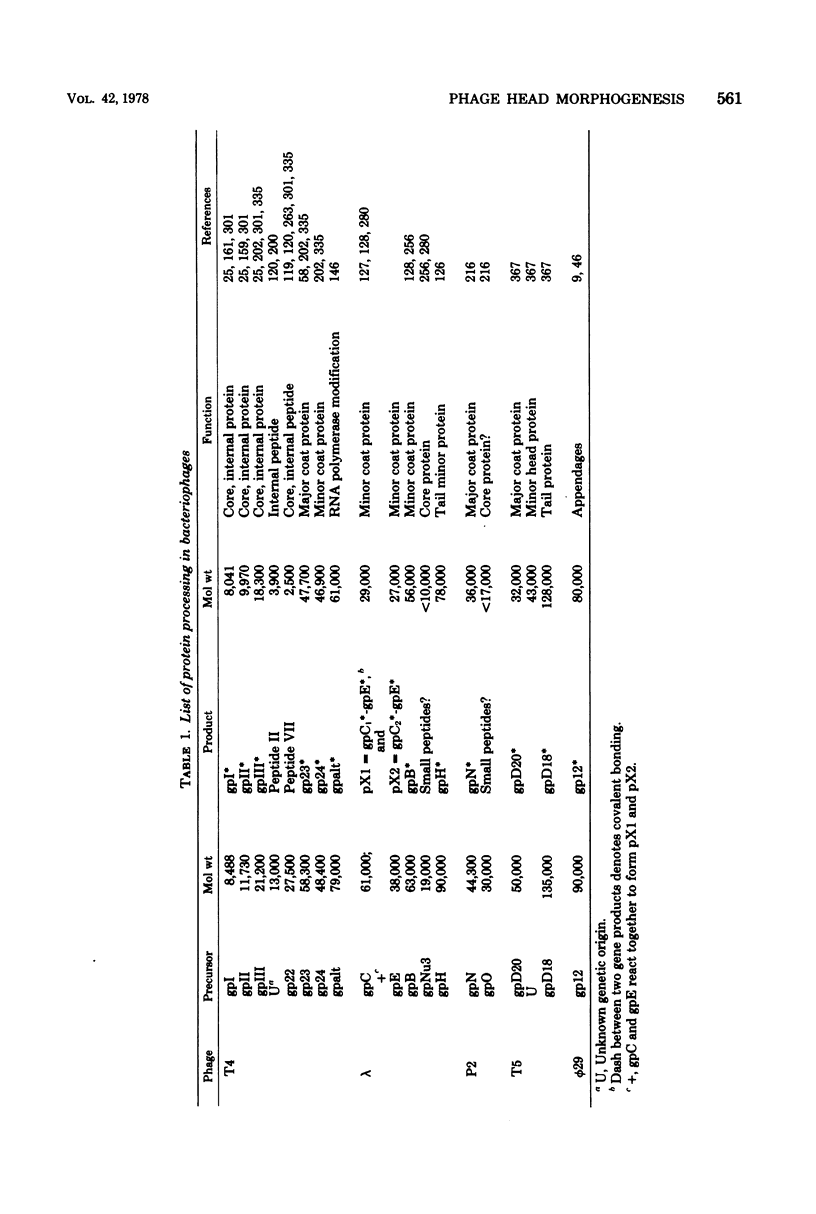
Full text
PDF












































Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Monem M., Lauppe H. F., Kartenbeck J., Dürwald H., Hoffmann-Berling H. Enzymatic unwinding of DNA. III. Mode of action of Escherichia coli DNA unwinding enzyme. J Mol Biol. 1977 Mar 15;110(4):667–685. doi: 10.1016/s0022-2836(77)80083-1. [DOI] [PubMed] [Google Scholar]
- Aebi U., Bijlenga R. K., ten Heggeler B., Kistler J., Steven A. C., Smith P. R. Comparison of the structural and chemical composition of giant T-even phage heads. J Supramol Struct. 1976;5(4):475–495. doi: 10.1002/jss.400050406. [DOI] [PubMed] [Google Scholar]
- Aebi U., Bijlenga R., v d Broek J., v d Broek H., Eiserling F., Kellenberger C., Kellenberger E., Mesyanzhinov V., Müller L., Showe M. The transformation of tau particles into T4 heads. II. Transformations of the surface lattice and related observations on form determination. J Supramol Struct. 1974;2(2-4):253–275. doi: 10.1002/jss.400020218. [DOI] [PubMed] [Google Scholar]
- Aebi U., van Driel R., Bijlenga R. K., ten Heggeler B., van den Broek R., Steven A. C., Smith P. R. Capsid fine structure of T-even bacteriophages. Binding and localization of two dispensable capsid proteins into the P23* surface lattice. J Mol Biol. 1977 Mar 15;110(4):687–698. doi: 10.1016/s0022-2836(77)80084-3. [DOI] [PubMed] [Google Scholar]
- Alberts B. M., Frey L. T4 bacteriophage gene 32: a structural protein in the replication and recombination of DNA. Nature. 1970 Sep 26;227(5265):1313–1318. doi: 10.1038/2271313a0. [DOI] [PubMed] [Google Scholar]
- Allet B., Bukhari A. I. Analysis of bacteriophage mu and lambda-mu hybrid DNAs by specific endonucleases. J Mol Biol. 1975 Mar 15;92(4):529–540. doi: 10.1016/0022-2836(75)90307-1. [DOI] [PubMed] [Google Scholar]
- Altman S., Lerman L. S. Kinetics and intermediates in the intracellular synthesis of bacteriophage T4 deoxyribonucleic acid. J Mol Biol. 1970 Jun 14;50(2):235–261. doi: 10.1016/0022-2836(70)90190-7. [DOI] [PubMed] [Google Scholar]
- Anderson D. L., Hickman D. D., Reilly B. E. Structure of Bacillus subtilis bacteriophage phi 29 and the length of phi 29 deoxyribonucleic acid. J Bacteriol. 1966 May;91(5):2081–2089. doi: 10.1128/jb.91.5.2081-2089.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. L., Reilly B. E. Analysis of bacteriophage phi 29 gene function: protein synthesis in suppressor-sensitive mutant infection of Bacillus subtilis. J Virol. 1974 Jan;13(1):211–221. doi: 10.1128/jvi.13.1.211-221.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura S., Eguchi G., Iino T. Unidirectional growth of Salmonella flagella in vitro. J Mol Biol. 1968 Jul 14;35(1):227–236. doi: 10.1016/s0022-2836(68)80050-6. [DOI] [PubMed] [Google Scholar]
- BERTANI G. Lysogenic versus lytic cycle of phage multiplication. Cold Spring Harb Symp Quant Biol. 1953;18:65–70. doi: 10.1101/sqb.1953.018.01.014. [DOI] [PubMed] [Google Scholar]
- Baas P. D., Jansz H. S. Bacteriophage phiX174 DNA synthesis in a replication-deficient host: determination of the origin of phiX DNA replication. J Mol Biol. 1976 Apr 15;102(3):633–656. doi: 10.1016/0022-2836(76)90339-9. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett K. J., Marsh M. L., Calendar R. Interactions between a satellite bacteriophage and its helper. J Mol Biol. 1976 Sep 25;106(3):683–707. doi: 10.1016/0022-2836(76)90259-x. [DOI] [PubMed] [Google Scholar]
- Bayer M. E., Bocharov A. F. The capsid structure of bacteriophage lambda. Virology. 1973 Aug;54(2):465–475. doi: 10.1016/0042-6822(73)90157-8. [DOI] [PubMed] [Google Scholar]
- Bayer M. E., Cummings D. J. Structural aberrations in T-even bacteriophage. VIII. Surface morphology of T4 lollipops. Virology. 1977 Feb;76(2):767–780. doi: 10.1016/0042-6822(77)90257-4. [DOI] [PubMed] [Google Scholar]
- Becker A., Gold M. Isolation of the bacteriophage lambda A-gene protein. Proc Natl Acad Sci U S A. 1975 Feb;72(2):581–585. doi: 10.1073/pnas.72.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker A., Marko M., Gold M. Early events in the in vitro packaging of bacteriophage lambda DNA. Virology. 1977 May 1;78(1):291–305. doi: 10.1016/0042-6822(77)90100-3. [DOI] [PubMed] [Google Scholar]
- Becker A., Murialdo H., Gold M. Studies on an in vitro system for the packaging and maturation of phage lambda DNA. Virology. 1977 May 1;78(1):277–290. doi: 10.1016/0042-6822(77)90099-x. [DOI] [PubMed] [Google Scholar]
- Bernstein C., Bernstein H. Coiled rings of DNA released from cells infected with bacteriophages T7 or T4 or from uninfected Escherichia coli. J Virol. 1974 Jun;13(6):1346–1355. doi: 10.1128/jvi.13.6.1346-1355.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein H., Bernstein C. Circular and branched circular concatenates as possible intermediates in bacteriophage T4 DNA replication. J Mol Biol. 1973 Jul 5;77(3):355–361. doi: 10.1016/0022-2836(73)90443-9. [DOI] [PubMed] [Google Scholar]
- Bijlenga R. K., Aebi U., Kellenberger E. Properties and structure of a gene 24-controlled T4 giant phage. J Mol Biol. 1976 May 25;103(3):469–498. doi: 10.1016/0022-2836(76)90213-8. [DOI] [PubMed] [Google Scholar]
- Bijlenga R. K., Broek R vd, Kellenberger E. The transformation of rho-particles into T4 heads. I. Evidence for the conservative mode of this transformation. J Supramol Struct. 1974;2(1):45–59. doi: 10.1002/jss.400020106. [DOI] [PubMed] [Google Scholar]
- Bijlenga R. K., Scraba D., Kellenberger E. Studies on the morphopoiesis of the head of T-even phage. IX. Gamma-particles: their morphology, kinetics of appearance and possible precursor function. Virology. 1973 Nov;56(1):250–267. doi: 10.1016/0042-6822(73)90304-8. [DOI] [PubMed] [Google Scholar]
- Black L. W., Abremski K. Restriction of phage T4 internal protein I mutants by a strain of Escherichia coli. Virology. 1974 Jul;60(1):180–191. doi: 10.1016/0042-6822(74)90375-4. [DOI] [PubMed] [Google Scholar]
- Black L. W. Bacteriophage T4 internal protein mutants: isolation and properties. Virology. 1974 Jul;60(1):166–179. doi: 10.1016/0042-6822(74)90374-2. [DOI] [PubMed] [Google Scholar]
- Bode V. C., Gillin F. D. The arrangement of DNA in lambda phage heads. I. Biological consequences of micrococcal nuclease attack on a portion of the chromosome exposed in tailless heads. J Mol Biol. 1971 Dec 28;62(3):493–502. doi: 10.1016/0022-2836(71)90150-1. [DOI] [PubMed] [Google Scholar]
- Bode V. C., Kaiser A. D. Changes in the structure and activity of lambda DNA in a superinfected immune bacterium. J Mol Biol. 1965 Dec;14(2):399–417. doi: 10.1016/s0022-2836(65)80190-5. [DOI] [PubMed] [Google Scholar]
- Boklage C. E., Wong E. C., Bode V. C. The lambda F mutants belong to two cistrons. Genetics. 1973 Oct;75(2):221–230. doi: 10.1093/genetics/75.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolin R. W., Cummings D. J. Structural aberrations in T-even bacteriophage. IV. Parameters of induction and formation of lollipops. J Virol. 1974 Jun;13(6):1368–1377. doi: 10.1128/jvi.13.6.1368-1377.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botstein D., Levine M. Intermediates in the synthesis of phage P22 DNA. Cold Spring Harb Symp Quant Biol. 1968;33:659–667. doi: 10.1101/sqb.1968.033.01.075. [DOI] [PubMed] [Google Scholar]
- Botstein D., Waddell C. H., King J. Mechanism of head assembly and DNA encapsulation in Salmonella phage p22. I. Genes, proteins, structures and DNA maturation. J Mol Biol. 1973 Nov 15;80(4):669–695. doi: 10.1016/0022-2836(73)90204-0. [DOI] [PubMed] [Google Scholar]
- Branton D., Klug A. Capsid geometry of bacteriophage T2: a freeze-etching study. J Mol Biol. 1975 Mar 15;92(4):559–565. doi: 10.1016/0022-2836(75)90309-5. [DOI] [PubMed] [Google Scholar]
- Brimacombe R., Nierhaus K. H., Garrett R. A., Wittmann H. G. The ribosome of Escherichia coli. Prog Nucleic Acid Res Mol Biol. 1976;18:1-44, 323-5. doi: 10.1016/s0079-6603(08)60585-1. [DOI] [PubMed] [Google Scholar]
- Brody T. A DNA-binding form of the main structural protein of lambda heads. Virology. 1973 Aug;54(2):441–451. doi: 10.1016/0042-6822(73)90155-4. [DOI] [PubMed] [Google Scholar]
- Buchwald M., Murialdo H., Siminovitch L. The morphogenesis of bacteriophage lambda. II. Identification of the principal structural proteins. Virology. 1970 Oct;42(2):390–400. doi: 10.1016/0042-6822(70)90282-5. [DOI] [PubMed] [Google Scholar]
- Buchwald M., Steed-Glaister P., Siminovitch L. The morphogenesis of bacteriophage lambda. I. Purification and characterization of lambda heads and lambda tails. Virology. 1970 Oct;42(2):375–389. doi: 10.1016/0042-6822(70)90281-3. [DOI] [PubMed] [Google Scholar]
- Bukhari A. I., Froshauer S., Botchan M. Ends of bacteriophage mu DNA. Nature. 1976 Dec 9;264(5586):580–583. doi: 10.1038/264580a0. [DOI] [PubMed] [Google Scholar]
- Bukhari A. I., Taylor A. L. Influence of insertions on packaging of host sequences covalently linked to bacteriophage Mu DNA. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4399–4403. doi: 10.1073/pnas.72.11.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess A. B. Studies on the proteins of phi X174. II. The protein composition of the phi X coat. Proc Natl Acad Sci U S A. 1969 Oct;64(2):613–617. doi: 10.1073/pnas.64.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL A. Sensitive mutants of bacteriophage lambda. Virology. 1961 May;14:22–32. doi: 10.1016/0042-6822(61)90128-3. [DOI] [PubMed] [Google Scholar]
- CASPAR D. L., KLUG A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- Carlson K. Intracellular fate of deoxyribonucleic acid from T7 bacteriophages. J Virol. 1968 Oct;2(10):1230–1233. doi: 10.1128/jvi.2.10.1230-1233.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrascosa J. L., Camacho A., Viñuela E., Salas M. A precursor of the neck appendage protein of B. subtilis phage phi 29. FEBS Lett. 1974 Aug 30;44(3):317–321. [PubMed] [Google Scholar]
- Carter B. J., Smith M. G. Intracellular pools of bacteriophage lambda deoxyribonucleic acid. J Mol Biol. 1970 Jun 28;50(3):713–718. doi: 10.1016/0022-2836(70)90096-3. [DOI] [PubMed] [Google Scholar]
- Casjens S. Bacteriophage lambda FII gene protein: role in head assembly. J Mol Biol. 1974 Nov 25;90(1):1–20. doi: 10.1016/0022-2836(74)90252-6. [DOI] [PubMed] [Google Scholar]
- Casjens S., Hohn T., Kaiser A. D. Morphological proteins of phage lambda: identification of the major head protein as the product of gene E. Virology. 1970 Oct;42(2):496–507. doi: 10.1016/0042-6822(70)90293-x. [DOI] [PubMed] [Google Scholar]
- Casjens S., Horn T., Kaiser A. D. Head assembly steps controlled by genes F and W in bacteriophage lambda. J Mol Biol. 1972 Mar 14;64(3):551–563. doi: 10.1016/0022-2836(72)90082-4. [DOI] [PubMed] [Google Scholar]
- Casjens S., King J. P22 morphogenesis. I: Catalytic scaffolding protein in capsid assembly. J Supramol Struct. 1974;2(2-4):202–224. doi: 10.1002/jss.400020215. [DOI] [PubMed] [Google Scholar]
- Casjens S., King J. Virus assembly. Annu Rev Biochem. 1975;44:555–611. doi: 10.1146/annurev.bi.44.070175.003011. [DOI] [PubMed] [Google Scholar]
- Castillo C. J., Hsiao C. L., Coon P., Black L. W. Identification and perperties of bacteriophage T4 capsid-formation gene products. J Mol Biol. 1977 Mar 5;110(3):585–601. doi: 10.1016/s0022-2836(77)80113-7. [DOI] [PubMed] [Google Scholar]
- Celis J. E., Smith J. D., Brenner S. Correlation between genetic and translational maps of gene 23 in bacteriophage T4. Nat New Biol. 1973 Jan 31;241(109):130–132. doi: 10.1038/newbio241130a0. [DOI] [PubMed] [Google Scholar]
- Chattoraj D. K., Inman R. B. Location of DNA ends in P2, 186, P4 and lambda bacteriophage heads. J Mol Biol. 1974 Jul 25;87(1):11–22. doi: 10.1016/0022-2836(74)90556-7. [DOI] [PubMed] [Google Scholar]
- Coppo A., Manzi A., Pulitzer J. F., Takahashi H. Abortive bacteriophage T4 head assembly in mutants of Escherichia coli. J Mol Biol. 1973 May 5;76(1):61–87. doi: 10.1016/0022-2836(73)90081-8. [DOI] [PubMed] [Google Scholar]
- Crowther R. A., Klug A. Structural analysis of macromolecular assemblies by image reconstruction from electron micrographs. Annu Rev Biochem. 1975;44:161–182. doi: 10.1146/annurev.bi.44.070175.001113. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., Bolin R. W. Head length control in T4 bacteriophage morphogenesis: effect of canavanine on assembly. Bacteriol Rev. 1976 Jun;40(2):314–359. doi: 10.1128/br.40.2.314-359.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings D. J., Chapman V. A., DeLong S. S., Couse N. L. Structural aberrations in T-even bacteriophage. 3. Induction of "lollipops" and their partial characterization. Virology. 1973 Jul;54(1):245–261. doi: 10.1016/0042-6822(73)90134-7. [DOI] [PubMed] [Google Scholar]
- Curtis M. J., Alberts B. Studies on the structure of intracellular bacteriophage T4 DNA. J Mol Biol. 1976 Apr 25;102(4):793–816. doi: 10.1016/0022-2836(76)90292-8. [DOI] [PubMed] [Google Scholar]
- Daniell E., Abelson J., Kim J. S., Davidson N. Heteroduplex structures of bacteriophage Mu DNA. Virology. 1973 Jan;51(1):237–239. doi: 10.1016/0042-6822(73)90385-1. [DOI] [PubMed] [Google Scholar]
- Daniell E., Boram W., Abelson J. Genetic mapping of the inversion loop in bacteriophage Mu DNA. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2153–2156. doi: 10.1073/pnas.70.7.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell E., Kohne D. E., Abelson J. Characterization of the inhomogeneous DNA in virions of bacteriophage Mu by DNA reannealing kinetics. J Virol. 1975 Apr;15(4):739–743. doi: 10.1128/jvi.15.4.739-743.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell E., Roberts R., Abelson J. Mutations in the lactose operon caused by bacteriophage Mu. J Mol Biol. 1972 Aug 14;69(1):1–8. doi: 10.1016/0022-2836(72)90019-8. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. The single-stranded DNA phages. CRC Crit Rev Microbiol. 1975 Dec;4(2):161–223. doi: 10.3109/10408417509111575. [DOI] [PubMed] [Google Scholar]
- Dewey M. J., Wiberg J. S., Frankel F. R. Genetic control of whisker antigen of bacteriophage T4D. J Mol Biol. 1974 Apr 25;84(4):625–634. doi: 10.1016/0022-2836(74)90120-x. [DOI] [PubMed] [Google Scholar]
- Dickson R. C., Barnes S. L., Eiserling F. A. Structural proteins of bacteriophage T4. J Mol Biol. 1970 Nov 14;53(3):461–474. doi: 10.1016/0022-2836(70)90077-x. [DOI] [PubMed] [Google Scholar]
- Dove W. F. Action of the lambda chromosome. I. Control of functions late in bacteriophage development. J Mol Biol. 1966 Aug;19(1):187–201. doi: 10.1016/s0022-2836(66)80060-8. [DOI] [PubMed] [Google Scholar]
- Dressler D., Wolfson J., Magazin M. Initiation and reinitiation of DNA synthesis during replication of bacteriophage T7. Proc Natl Acad Sci U S A. 1972 Apr;69(4):998–1002. doi: 10.1073/pnas.69.4.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler H. Transduction by bacteriophage T1. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1083–1088. doi: 10.1073/pnas.66.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin S. B., Benedek G. B., Bancroft F. C., Freifelder D. Molecular weights of coliphages and colip- hage DNA. II. Measurement of diffusion coefficients using optical mixing spectroscopy, and measurement of sedimentation coefficients. J Mol Biol. 1970 Dec 28;54(3):547–556. doi: 10.1016/0022-2836(70)90125-7. [DOI] [PubMed] [Google Scholar]
- Dunker A. K. Structure of isometric viruses containing nonidentical polypeptide chains. J Virol. 1974 Oct;14(4):878–885. doi: 10.1128/jvi.14.4.878-885.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykes G., Bambara R., Marians K., Wu R. On the statistical significance of primary structural features found in DNA-protein interaction sites. Nucleic Acids Res. 1975 Mar;2(3):327–345. doi: 10.1093/nar/2.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw W. C., Harrison S. C. DNA arrangement in isometric phage heads. Nature. 1977 Aug 18;268(5621):598–602. doi: 10.1038/268598a0. [DOI] [PubMed] [Google Scholar]
- Earnshaw W., Casjens S., Harrison S. C. Assembly of the head of bacteriophage P22: x-ray diffraction from heads, proheads and related structures. J Mol Biol. 1976 Jun 25;104(2):387–410. doi: 10.1016/0022-2836(76)90278-3. [DOI] [PubMed] [Google Scholar]
- Eddleman H. L., Champe S. P. Components in T4-infected cells associated with phage assembly. Virology. 1966 Nov;30(3):471–481. doi: 10.1016/0042-6822(66)90123-1. [DOI] [PubMed] [Google Scholar]
- Edgar R. S., Lielausis I. Some steps in the assembly of bacteriophage T4. J Mol Biol. 1968 Mar 14;32(2):263–276. doi: 10.1016/0022-2836(68)90008-9. [DOI] [PubMed] [Google Scholar]
- Edgar R. S., Wood W. B. Morphogenesis of bacteriophage T4 in extracts of mutant-infected cells. Proc Natl Acad Sci U S A. 1966 Mar;55(3):498–505. doi: 10.1073/pnas.55.3.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgell M. H., Hutchison C. A., 3rd, Sinsheimer R. L. The process of infection with bacteriophage phi-X174. 28. Removal of the spike proteins from the phage capsid. J Mol Biol. 1969 Jun 28;42(3):547–557. doi: 10.1016/0022-2836(69)90242-3. [DOI] [PubMed] [Google Scholar]
- Eisenberg S., Griffith J., Kornberg A. phiX174 cistron A protein is a multifunctional enzyme in DNA replication. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3198–3202. doi: 10.1073/pnas.74.8.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg S., Scott J. F., Kornberg A. Enzymatic replication of viral and complementary strands of duplex DNA of phage phiX174 proceeds by seprate mechanisms. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3151–3155. doi: 10.1073/pnas.73.9.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiserling F. A., Geiduschek E. P., Epstein R. H., Metter E. J. Capsid size and deoxyribonucleic acid length: the petite variant of bacteriophage T4. J Virol. 1970 Dec;6(6):865–876. doi: 10.1128/jvi.6.6.865-876.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons S. W. Bacteriophage lambda derivatives carrying two copies of the cohesive end site. J Mol Biol. 1974 Mar 15;83(4):511–525. doi: 10.1016/0022-2836(74)90511-7. [DOI] [PubMed] [Google Scholar]
- Enquist L. W., Skalka A. Replication of bacteriophage lambda DNA dependent on the function of host and viral genes. I. Interaction of red, gam and rec. J Mol Biol. 1973 Apr 5;75(2):185–212. doi: 10.1016/0022-2836(73)90016-8. [DOI] [PubMed] [Google Scholar]
- Favre R., Boy de la Tour E., Segrè N., Kellenberger E. Studies on the morphopoiesis of the head of phage T-even. I. Morphological, immunological, and genetic characterization of polyheads. J Ultrastruct Res. 1965 Oct;13(3):318–342. doi: 10.1016/s0022-5320(65)80080-6. [DOI] [PubMed] [Google Scholar]
- Feiss M., Bublitz A. Polarized packaging of bacteriophage lambda chromosomes. J Mol Biol. 1975 Jun 5;94(4):583–594. doi: 10.1016/0022-2836(75)90323-x. [DOI] [PubMed] [Google Scholar]
- Feiss M., Fisher R. A., Crayton M. A., Egner C. Packaging of the bacteriophage lambda chromosome: effect of chromosome length. Virology. 1977 Mar;77(1):281–293. doi: 10.1016/0042-6822(77)90425-1. [DOI] [PubMed] [Google Scholar]
- Floor E. Interaction of morphogenetic genes of bacteriophage T4. J Mol Biol. 1970 Feb 14;47(3):293–306. doi: 10.1016/0022-2836(70)90303-7. [DOI] [PubMed] [Google Scholar]
- Follansbee S. E., Vanderslice R. W., Chavez L. G., Yegian C. D. A new set of adsorption mutants of bacteriophage T4D: identification of a new gene. Virology. 1974 Mar;58(1):180–199. doi: 10.1016/0042-6822(74)90153-6. [DOI] [PubMed] [Google Scholar]
- Francke B., Ray D. S. Formation of the parental replicative form DNA of bacteriophage phi-X174 and initial events in its replication. J Mol Biol. 1971 Nov 14;61(3):565–586. doi: 10.1016/0022-2836(71)90065-9. [DOI] [PubMed] [Google Scholar]
- Frankel F. R., Batcheler M. L., Clark C. K. The role of gene 49 in DNA replication and head morphogenesis in bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):439–463. doi: 10.1016/0022-2836(71)90147-1. [DOI] [PubMed] [Google Scholar]
- Frankel F. R. DNA replication after T4 infection. Cold Spring Harb Symp Quant Biol. 1968;33:485–493. doi: 10.1101/sqb.1968.033.01.056. [DOI] [PubMed] [Google Scholar]
- Frankel F. R. Studies on the nature of replicating DNA in T4-infected Escherichia coli. J Mol Biol. 1966 Jun;18(1):127–143. doi: 10.1016/s0022-2836(66)80081-5. [DOI] [PubMed] [Google Scholar]
- Freifelder D., Chud L., Levine E. E. Requirement for maturation of Escherichia coli bacteriophage lambda. J Mol Biol. 1974 Mar 15;83(4):503–509. doi: 10.1016/0022-2836(74)90510-5. [DOI] [PubMed] [Google Scholar]
- Fröhlich B., Powling A., Knippers R. Formation of concatemeric DNA in bacteriophage T7-infected bacteria. Virology. 1975 Jun;65(2):455–468. doi: 10.1016/0042-6822(75)90051-3. [DOI] [PubMed] [Google Scholar]
- Fujisawa H., Hayashi M. Assembly of bacteriophage phi X174: identification of a virion capsid precursor and proposal of a model for the functions of bacteriophage gene products during morphogenesis. J Virol. 1977 Oct;24(1):303–313. doi: 10.1128/jvi.24.1.303-313.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa H., Hayashi M. Functions of gene C and gene D products of bacteriophage phi X 174. J Virol. 1977 Feb;21(2):506–515. doi: 10.1128/jvi.21.2.506-515.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa H., Minagawa T. Genetic control of the DNA maturation in the process of phage morphogenesis. Virology. 1971 Jul;45(1):280–291. [PubMed] [Google Scholar]
- GELLERT M., DAVIES D. R. ORGANIZATION OF DNA IN BACTERIOPHAGE T4. J Mol Biol. 1964 Mar;8:341–347. doi: 10.1016/s0022-2836(64)80197-2. [DOI] [PubMed] [Google Scholar]
- Gellert M. Formation of covalent circles of lambda DNA by E. coli extracts. Proc Natl Acad Sci U S A. 1967 Jan;57(1):148–155. doi: 10.1073/pnas.57.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos C. P., Eisen H. Bacterial mutants which block phage assembly. J Supramol Struct. 1974;2(2-4):349–359. doi: 10.1002/jss.400020224. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. P., Hendrix R. W., Casjens S. R., Kaiser A. D. Host participation in bacteriophage lambda head assembly. J Mol Biol. 1973 May 5;76(1):45–60. doi: 10.1016/0022-2836(73)90080-6. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. P., Hendrix R. W., Kaiser A. D., Wood W. B. Role of the host cell in bacteriophage morphogenesis: effects of a bacterial mutation on T4 head assembly. Nat New Biol. 1972 Sep 13;239(89):38–41. doi: 10.1038/newbio239038a0. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. P., Hohn B. Identification of a host protein necessary for bacteriophage morphogenesis (the groE gene product). Proc Natl Acad Sci U S A. 1978 Jan;75(1):131–135. doi: 10.1073/pnas.75.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs W., Goldstein R. N., Wiener R., Lindqvist B., Calendar R. Satellite bacteriophage P4: characterization of mutants in two essential genes. Virology. 1973 May;53(1):24–39. doi: 10.1016/0042-6822(73)90462-5. [DOI] [PubMed] [Google Scholar]
- Gilbert W., Dressler D. DNA replication: the rolling circle model. Cold Spring Harb Symp Quant Biol. 1968;33:473–484. doi: 10.1101/sqb.1968.033.01.055. [DOI] [PubMed] [Google Scholar]
- Gill G. S., MacHattie L. A. Limited permutations of the nucleotide sequence in bacteriophage T1 DNA. J Mol Biol. 1976 Jun 25;104(2):505–515. doi: 10.1016/0022-2836(76)90284-9. [DOI] [PubMed] [Google Scholar]
- Gill G. S., MacHattie L. A. Oriented extrusion of DNA from coliphage T1 particles. Virology. 1975 May;65(1):297–303. doi: 10.1016/0042-6822(75)90035-5. [DOI] [PubMed] [Google Scholar]
- Gillin F. D., Bode V. C. The arrangement of DNA in lambda phage heads. II. lambda DNA after exposure to micrococcal nuclease at the site of head-tail joining. J Mol Biol. 1971 Dec 28;62(3):503–511. doi: 10.1016/0022-2836(71)90151-3. [DOI] [PubMed] [Google Scholar]
- Giri J. G., McCullough J. E., Champe S. P. Identification of gene products required for in vitro formation of the internal peptides of bacteriophage T4. J Virol. 1976 Jun;18(3):894–903. doi: 10.1128/jvi.18.3.894-903.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J., Champe S. P. T4-induced activity required for specific cleavage of a bacteriophage protein in vitro. J Virol. 1974 Feb;13(2):419–427. doi: 10.1128/jvi.13.2.419-427.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein R., Lengyel J., Pruss G., Barrett K., Calendar R., Six E. Head size determination and the morphogenesis of satellite phage P4. Curr Top Microbiol Immunol. 1974;(68):59–75. doi: 10.1007/978-3-642-66044-3_3. [DOI] [PubMed] [Google Scholar]
- Gottesman M. E., Yarmolinsky M. B. The integration and excision of the bacteriophage lambda genome. Cold Spring Harb Symp Quant Biol. 1968;33:735–747. doi: 10.1101/sqb.1968.033.01.084. [DOI] [PubMed] [Google Scholar]
- Greenlee L. L., Sinsheimer R. L. The process of infection with bacteriophage phi X174. 18. Intracellular antiserum-precipitable components. J Mol Biol. 1968 Mar 14;32(2):321–326. doi: 10.1016/0022-2836(68)90012-0. [DOI] [PubMed] [Google Scholar]
- HERSHEY A. D. An upper limit to the protein content of the germinal substance of bacteriophage T2. Virology. 1955 May;1(1):108–127. doi: 10.1016/0042-6822(55)90009-x. [DOI] [PubMed] [Google Scholar]
- HERSHEY A. D., BURGI E. COMPLEMENTARY STRUCTURE OF INTERACTING SITES AT THE ENDS OF LAMBDA DNA MOLECULES. Proc Natl Acad Sci U S A. 1965 Feb;53:325–328. doi: 10.1073/pnas.53.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERSHEY A. D. Some minor components of bacteriophage T2 particles. Virology. 1957 Oct;4(2):237–264. doi: 10.1016/0042-6822(57)90061-2. [DOI] [PubMed] [Google Scholar]
- Hagen E. W., Reilly B. E., Tosi M. E., Anderson D. L. Analysis of gene function of bacteriophage phi 29 of Bacillus subtilis: identification of cistrons essential for viral assembly. J Virol. 1976 Aug;19(2):501–517. doi: 10.1128/jvi.19.2.501-517.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann R., LaRue K. Variations in sedimentation patterns among deoxyribonucleic acids synthesized after infection of Escherichia coli by different amber mutants of bacteriophage T7. J Virol. 1969 Feb;3(2):278–281. doi: 10.1128/jvi.3.2.278-281.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix R. W., Casjens S. R. Assembly of bacteriophage lambda heads: protein processing and its genetic control in petit lambda assembly. J Mol Biol. 1975 Jan 15;91(2):187–199. doi: 10.1016/0022-2836(75)90159-x. [DOI] [PubMed] [Google Scholar]
- Hendrix R. W., Casjens S. R. Protein cleavage in bacteriophage lambda tail assembly. Virology. 1974 Sep;61(1):156–159. doi: 10.1016/0042-6822(74)90250-5. [DOI] [PubMed] [Google Scholar]
- Hendrix R. W., Casjens S. R. Protein fusion: a novel reaction in bacteriophage lambda head assembly. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1451–1455. doi: 10.1073/pnas.71.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix R. W., Tsui L. Role of the host in virus assembly: cloning of the Escherichia coli groE gene and identification of its protein product. Proc Natl Acad Sci U S A. 1978 Jan;75(1):136–139. doi: 10.1073/pnas.75.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry T. J., Knippers R. Isolation and function of the gene A initiator of bacteriophage phi-chi 174, a highly specific DNA endonuclease. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1549–1553. doi: 10.1073/pnas.71.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A., Fry M. Post-translational cleavage of polypeptide chains: role in assembly. Annu Rev Biochem. 1975;44:775–797. doi: 10.1146/annurev.bi.44.070175.004015. [DOI] [PubMed] [Google Scholar]
- Hoffman B., Levine M. Bacteriophage P22 virion protein which performs an essential early function. II. Characterization of the gene 16 function. J Virol. 1975 Dec;16(6):1547–1559. doi: 10.1128/jvi.16.6.1547-1559.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B. DNA as substrate for packaging into bacteriophage lambda, in vitro. J Mol Biol. 1975 Oct 15;98(1):93–106. doi: 10.1016/s0022-2836(75)80103-3. [DOI] [PubMed] [Google Scholar]
- Hohn B., Hohn T. Activity of empty, headlike particles for packaging of DNA of bacteriophage lambda in vitro. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2372–2376. doi: 10.1073/pnas.71.6.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B., Wurtz M., Klein B., Lustig A., Hohn T. Phage lambda DNA packaging, in vitro. J Supramol Struct. 1974;2(2-4):302–317. doi: 10.1002/jss.400020220. [DOI] [PubMed] [Google Scholar]
- Hohn T., Flick H., Hohn B. Petit lambda, a family of particles from coliphage lambda infected cells. J Mol Biol. 1975 Oct 15;98(1):107–120. doi: 10.1016/s0022-2836(75)80104-5. [DOI] [PubMed] [Google Scholar]
- Hohn T., Hohn B. A minor pathway leading to plaque-forming particles in bacteriophage lambda. Studies on the function of gene D. J Mol Biol. 1973 Oct 5;79(4):649–662. doi: 10.1016/0022-2836(73)90069-7. [DOI] [PubMed] [Google Scholar]
- Hohn T., Katsura I. Structure and assembly of bacteriophage lambda. Curr Top Microbiol Immunol. 1977;78:69–110. doi: 10.1007/978-3-642-66800-5_3. [DOI] [PubMed] [Google Scholar]
- Hohn T., Morimasa T., Tsugita A. The capsid protein of bacteriophage lambda and of its prehead. J Mol Biol. 1976 Aug 5;105(2):337–342. doi: 10.1016/0022-2836(76)90117-0. [DOI] [PubMed] [Google Scholar]
- Hohn T. Packaging of genomes in bacteriophages: a comparison of ssRNA bacteriophages and dsDNA bacteriophages. Philos Trans R Soc Lond B Biol Sci. 1976 Nov 30;276(943):143–150. doi: 10.1098/rstb.1976.0105. [DOI] [PubMed] [Google Scholar]
- Hohn T., Wurtz M., Hohn B. Capsid transformation during packaging of bacteriophage lambdaDNA. Philos Trans R Soc Lond B Biol Sci. 1976 Nov 30;276(943):51–61. doi: 10.1098/rstb.1976.0097. [DOI] [PubMed] [Google Scholar]
- Hopfield J. J. Kinetic proofreading: a new mechanism for reducing errors in biosynthetic processes requiring high specificity. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4135–4139. doi: 10.1073/pnas.71.10.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz H. R. Bacteriophage T4 mutants deficient in alteration and modification of the Escherichia coli RNA polymerase. J Mol Biol. 1974 Dec 25;90(4):739–750. doi: 10.1016/0022-2836(74)90537-3. [DOI] [PubMed] [Google Scholar]
- Hosoda J., Cone R. Analysis of T4 phage proteins. I. Conversion of precursor proteins into lower molecular weight peptides during normal capsid formation. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1275–1281. doi: 10.1073/pnas.66.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howatson A. F., Kemp C. L. The structure of tubular head forms of bacteriophage lambda; relation to the capsid structure of petit lambda and normal lambda heads. Virology. 1975 Sep;67(1):80–84. doi: 10.1016/0042-6822(75)90405-5. [DOI] [PubMed] [Google Scholar]
- Hsiao C. L., Black L. W. DNA packaging and the pathway of bacteriophage T4 head assembly. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3652–3656. doi: 10.1073/pnas.74.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman J. A. Visualization of replicating mammalian and T4 bacteriophage DNA. Cold Spring Harb Symp Quant Biol. 1968;33:509–524. doi: 10.1101/sqb.1968.033.01.059. [DOI] [PubMed] [Google Scholar]
- Huntley G. H., Kemp C. L. Isolation and protein composition of normal and petit capsids of bacteriophage lambda. Virology. 1971 Nov;46(2):298–309. doi: 10.1016/0042-6822(71)90031-6. [DOI] [PubMed] [Google Scholar]
- Ihler G. M., Thomas C. A., Jr Equal incorporation of both parental bacteriophage T7 deoxyribonucleic acid strands into intracellular concatemeric deoxyribonucleic acid. J Virol. 1970 Dec;6(6):877–880. doi: 10.1128/jvi.6.6.877-880.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman R. B., Bertani G. Heat denaturation of P2 bacteriophage DNA: compositional heterogeneity. J Mol Biol. 1969 Sep 28;44(3):533–549. doi: 10.1016/0022-2836(69)90378-7. [DOI] [PubMed] [Google Scholar]
- Inman R. B., Schnös M., Howe M. Location of the "variable end" of Mu DNA within the bacteriophage particle. Virology. 1976 Jul 15;72(2):393–401. doi: 10.1016/0042-6822(76)90168-9. [DOI] [PubMed] [Google Scholar]
- Inman R. B., Schnös M., Simon L. D., Six E. W., Walker D. H., Jr Some morphological properties of P4 bacteriophage and P4 DNA. Virology. 1971 Apr;44(1):67–72. doi: 10.1016/0042-6822(71)90153-x. [DOI] [PubMed] [Google Scholar]
- Ishii T., Yanagida M. Molecular organization of the shell of the Teven bacteriophage head. J Mol Biol. 1975 Oct 5;97(4):655–660. doi: 10.1016/s0022-2836(75)80065-9. [DOI] [PubMed] [Google Scholar]
- Ishii T., Yanagida M. The two dispensable structural proteins (soc and hoc) of the T4 phage capsid; their purification and properties, isolation and characterization of the defective mutants, and their binding with the defective heads in vitro. J Mol Biol. 1977 Feb 5;109(4):487–514. doi: 10.1016/s0022-2836(77)80088-0. [DOI] [PubMed] [Google Scholar]
- Isobe T., Black L. W., Tsugita A. Complete aimino acid sequence of bacteriophage T4 internal protein I and its cleavage site on virus maturation. J Mol Biol. 1977 Feb 15;110(1):165–177. doi: 10.1016/s0022-2836(77)80104-6. [DOI] [PubMed] [Google Scholar]
- Isobe T., Black L. W., Tsugita A. Primary structure of bacteriophage T4 internal protein II and characterization of the cleavage upon phage maturation. J Mol Biol. 1976 Apr 5;102(2):349–365. doi: 10.1016/s0022-2836(76)80059-9. [DOI] [PubMed] [Google Scholar]
- Isobe T., Black L. W., Tsugita A. Protein cleavage during virus assembly: a novel specificity of assembly dependent cleavage in bacteriophage T4. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4205–4209. doi: 10.1073/pnas.73.11.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel J. V., Anderson T. F., Levine M. in vitro MORPHOGENESIS OF PHAGE P22 FROM HEADS AND BASE-PLATE PARTS. Proc Natl Acad Sci U S A. 1967 Feb;57(2):284–291. doi: 10.1073/pnas.57.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel V. E proteins of bacteriophage P22. I. Identification and ejection from wild-type and defective particles. J Virol. 1977 Jul;23(1):91–97. doi: 10.1128/jvi.23.1.91-97.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J., Kawamura F., Yanofsky S. Analysis of phi 29 and phi 15 genomes by bacterial restriction endonucleases, EcoR1 and Hpal. Virology. 1976 Mar;70(1):37–51. doi: 10.1016/0042-6822(76)90234-8. [DOI] [PubMed] [Google Scholar]
- Iwaya M., Denhardt D. T. The mechanism of replication of phi X174 single-stranded DNA. II. The role of viral proteins. J Mol Biol. 1971 Apr 28;57(2):159–175. doi: 10.1016/0022-2836(71)90339-1. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Polypeptide cleavages in the formation of poliovirus proteins. Proc Natl Acad Sci U S A. 1968 Sep;61(1):77–84. doi: 10.1073/pnas.61.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jara L., Murialdo H. Isolation of nonsense mutants in the morphogenetic region of the bacteriophage lambda chromosome. Virology. 1975 Mar;64(1):264–268. doi: 10.1016/0042-6822(75)90097-5. [DOI] [PubMed] [Google Scholar]
- Jazwinski S. M., Lindberg A. A., Kornberg A. The gene H spike protein of bacteriophages phiX174 and S13. I. Functions in phage-receptor recognition and in transfection. Virology. 1975 Jul;66(1):283–293. doi: 10.1016/0042-6822(75)90198-1. [DOI] [PubMed] [Google Scholar]
- Jovin T. M. Recognition mechanisms of DNA-specific enzymes. Annu Rev Biochem. 1976;45:889–920. doi: 10.1146/annurev.bi.45.070176.004325. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., DELATOUR E. B. ON THE FINE STRUCTURE OF NORMAL AND "POLYMERIZED" TAIL SHEATH OF PHAGE T4. J Ultrastruct Res. 1964 Dec;11:545–563. doi: 10.1016/s0022-5320(64)80081-2. [DOI] [PubMed] [Google Scholar]
- Kaiser D., Masuda T. In vitro assembly of bacteriophage Lambda heads. Proc Natl Acad Sci U S A. 1973 Jan;70(1):260–264. doi: 10.1073/pnas.70.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser D., Syvanen M., Masuda T. DNA packaging steps in bacteriophage lambda head assembly. J Mol Biol. 1975 Jan 15;91(2):175–186. doi: 10.1016/0022-2836(75)90158-8. [DOI] [PubMed] [Google Scholar]
- Kaiser D., Syvanen M., Masuda T. Processing and assembly of the head of bacteriophage lambda. J Supramol Struct. 1974;2(2-4):318–328. doi: 10.1002/jss.400020221. [DOI] [PubMed] [Google Scholar]
- Katsura I., Kühl P. W. Morphogenesis of the tail of bacteriophage lambda. III. Morphogenetic pathway. J Mol Biol. 1975 Jan 25;91(3):257–273. doi: 10.1016/0022-2836(75)90379-4. [DOI] [PubMed] [Google Scholar]
- Kayajanian G., Campbell A. The relationship between heritable physical and genetic properties of selected gal- and gal+ transducing lambda dg. Virology. 1966 Nov;30(3):482–492. doi: 10.1016/0042-6822(66)90124-3. [DOI] [PubMed] [Google Scholar]
- Kellenberger E. Assembly in biological systems. Ciba Found Symp. 1972;7:189–206. doi: 10.1002/9780470719909.ch11. [DOI] [PubMed] [Google Scholar]
- Kellenberger E. DNA viruses: cooperativity and regulation through conformational changes as features of phage assembly. Philos Trans R Soc Lond B Biol Sci. 1976 Nov 30;276(943):3–13. doi: 10.1098/rstb.1976.0092. [DOI] [PubMed] [Google Scholar]
- Kellenberger E., Der Kamp C. K.-V. On a modification of the gene product P23 according to its use as subunit of either normal capsids of phage T4 or of polyheads. FEBS Lett. 1970 Jun 1;8(3):140–144. doi: 10.1016/0014-5793(70)80247-2. [DOI] [PubMed] [Google Scholar]
- Kellenberger E., Eiserling F. A., Boy de la Tour E. Studies on the morphopoiesis of the head of phage T-even. 3. The cores of head-related structures. J Ultrastruct Res. 1967 Dec 12;21(3):335–360. doi: 10.1016/s0022-5320(67)80099-6. [DOI] [PubMed] [Google Scholar]
- Kellenberger E. Studies on the morphopoiesis of the head of phage T-even. V. The components of the T4 capsid and of other, capsid-related structures. Virology. 1968 Mar;34(3):549–561. doi: 10.1016/0042-6822(68)90074-3. [DOI] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Thomas C. A., Jr An intermediate in the replication of bacteriophage T7 DNA molecules. J Mol Biol. 1969 Sep 28;44(3):459–475. doi: 10.1016/0022-2836(69)90373-8. [DOI] [PubMed] [Google Scholar]
- Kemp C. L., Howatson A. F., Siminovitch L. Electron microscopy studies of mutants of lambada bacteriophage. I. General description and quantitation of viral products. Virology. 1968 Nov;36(3):490–502. doi: 10.1016/0042-6822(68)90174-8. [DOI] [PubMed] [Google Scholar]
- Kemp C. L. The in situ morphological transition of the bacteriophage lambda tubular head-forms. Virology. 1971 Jun;44(3):569–575. doi: 10.1016/0042-6822(71)90370-9. [DOI] [PubMed] [Google Scholar]
- Kemper B., Brown D. T. Function of gene 49 of bacteriophage T4. II. Analysis of intracellular development and the structure of very fast-sedimenting DNA. J Virol. 1976 Jun;18(3):1000–1015. doi: 10.1128/jvi.18.3.1000-1015.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr C., Sadowski P. D. Packaging and maturation of DNA of bacteriophage T7 in vitro. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3545–3549. doi: 10.1073/pnas.71.9.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi Y., King J. Genetic control of bacteriophage T4 baseplate morphogenesis. I. Sequential assembly of the major precursor, in vivo and in vitro. J Mol Biol. 1975 Dec 25;99(4):645–672. doi: 10.1016/s0022-2836(75)80178-1. [DOI] [PubMed] [Google Scholar]
- King J., Casjens S. Catalytic head assembling protein in virus morphogenesis. Nature. 1974 Sep 13;251(5471):112–119. doi: 10.1038/251112a0. [DOI] [PubMed] [Google Scholar]
- King J., Lenk E. V., Botstein D. Mechanism of head assembly and DNA encapsulation in Salmonella phage P22. II. Morphogenetic pathway. J Mol Biol. 1973 Nov 15;80(4):697–731. doi: 10.1016/0022-2836(73)90205-2. [DOI] [PubMed] [Google Scholar]
- King J., Mykolajewycz N. Bacteriophage T4 tail assembly: proteins of the sheath, core and baseplate. J Mol Biol. 1973 Apr 5;75(2):339–358. doi: 10.1016/0022-2836(73)90025-9. [DOI] [PubMed] [Google Scholar]
- Knippers R., Sinsheimer R. L. Process of infection with bacteriophage phi X174. 23. DNA synthesis in cells infected by a coat protein mutant. J Mol Biol. 1968 Aug 14;35(3):591–605. doi: 10.1016/s0022-2836(68)80016-6. [DOI] [PubMed] [Google Scholar]
- Koths K., Dressler D. Analysis of the phiX DNA replication cycle by electron microscopy. Proc Natl Acad Sci U S A. 1978 Feb;75(2):605–609. doi: 10.1073/pnas.75.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozinski A. W., Kozinski P. B., James R. Molecular recombination in T4 bacteriophage deoxyribonucleic acid. I. Tertiary structure of early replicative and recombining deoxyribonucleic acid. J Virol. 1967 Aug;1(4):758–770. doi: 10.1128/jvi.1.4.758-770.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuemmerle N. B., Masker W. E. In vitro packaging of UV radiation-damaged DNA from bacteriophage T7. J Virol. 1977 Sep;23(3):509–516. doi: 10.1128/jvi.23.3.509-516.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz M. B., Champe S. P. Precursors of the T4 internal peptides. J Virol. 1977 May;22(2):412–419. doi: 10.1128/jvi.22.2.412-419.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kül P. W., Katsura I. Morphogenesis of the tail of bacteriophage lambda. Virology. 1975 Jan;63(1):221–237. [PubMed] [Google Scholar]
- Labedan B., Crochet M., Legault-Demare J., Stevens B. J. Location of the first step transfer fragment and single-strand interruptions in T5stO bacteriophage DNA. J Mol Biol. 1973 Apr 5;75(2):213–234. doi: 10.1016/0022-2836(73)90017-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Amos L. A., Klug A. Correlation between structural transformation and cleavage of the major head protein of T4 bacteriophage. Cell. 1976 Feb;7(2):191–203. doi: 10.1016/0092-8674(76)90018-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Characterization of DNA condensates induced by poly(ethylene oxide) and polylysine. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4288–4292. doi: 10.1073/pnas.72.11.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Johnson R. A. Maturation of the head of bacteriophage T4. II. Head-related, aberrant tau-particles. J Mol Biol. 1973 Nov 15;80(4):601–611. doi: 10.1016/0022-2836(73)90199-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Mölbert E., Showe M., Kellenberger E. Form-determining function of the genes required for the assembly of the head of bacteriophage T4. J Mol Biol. 1970 Apr 14;49(1):99–113. doi: 10.1016/0022-2836(70)90379-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Paulson J. R., Hitchins V. Maturation of the head of bacteriophage T4. V. A possible DNA packaging mechanism: in vitro cleavage of the head proteins and the structure of the core of the polyhead. J Supramol Struct. 1974;2(2-4):276–301. doi: 10.1002/jss.400020219. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Quittner S. F. Maturation of the head of bacteriophage T4. IV. The proteins of the core of the tubular polyheads and in vitro cleavage of the head proteins. Virology. 1974 Dec;62(2):483–499. doi: 10.1016/0042-6822(74)90409-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Teaff N., D'Ambrosia J. Maturation of the head of bacteriophage T4. III. DNA packaging into preformed heads. J Mol Biol. 1974 Oct 5;88(4):749–765. doi: 10.1016/0022-2836(74)90397-0. [DOI] [PubMed] [Google Scholar]
- Langeveld S. A., van Mansfeld A. D., Baas P. D., Jansz H. S., van Arkel G. A., Weisbeek P. J. Nucleotide sequence of the origin of replication in bacteriophage phiX174 RF DNA. Nature. 1978 Feb 2;271(5644):417–420. doi: 10.1038/271417a0. [DOI] [PubMed] [Google Scholar]
- Leibo S. P., Mazur P. Freezing of bacteriophage T4: temperature and rate effects as a function of salt concentration. Virology. 1969 Aug;38(4):558–566. doi: 10.1016/0042-6822(69)90176-7. [DOI] [PubMed] [Google Scholar]
- Lengyel J. A., Goldstein R. N., Marsh M., Sunshine M. G., Calendar R. Bacteriophage P2 head morphogenesis: cleavage of the major capsid protein. Virology. 1973 May;53(1):1–23. doi: 10.1016/0042-6822(73)90461-3. [DOI] [PubMed] [Google Scholar]
- Lenk E., Casjens S., Weeks J., King J. Intracellular visualization of precursor capsids in phage P22 mutant infected cells. Virology. 1975 Nov;68(1):182–199. doi: 10.1016/0042-6822(75)90160-9. [DOI] [PubMed] [Google Scholar]
- Lerman L. S. A transition to a compact form of DNA in polymer solutions. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1886–1890. doi: 10.1073/pnas.68.8.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman L. S. Chromosomal analogues: long-range order in psi-condensed DNA. Cold Spring Harb Symp Quant Biol. 1974;38:59–73. doi: 10.1101/sqb.1974.038.01.009. [DOI] [PubMed] [Google Scholar]
- Lickfeld K. G., Menge B. Morphogenesis of bacteriophage lambda: electron microscopy of thin sections. J Mol Biol. 1976 May 15;103(2):299–318. doi: 10.1016/0022-2836(76)90314-4. [DOI] [PubMed] [Google Scholar]
- Lindqvist B. H. Expression of phage transcription in P2 lysogens infected with helper-dependent coliphage P4. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2752–2755. doi: 10.1073/pnas.71.7.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist B. H., Six E. W. Replication of bacteriophage P4 DNA in a nonlysogenic host. Virology. 1971 Jan;43(1):1–7. doi: 10.1016/0042-6822(71)90218-2. [DOI] [PubMed] [Google Scholar]
- Lindqvist B. H. Vegetative DNA of temperate coliphage P2. Mol Gen Genet. 1971;110(2):178–196. doi: 10.1007/BF00332647. [DOI] [PubMed] [Google Scholar]
- Ljungquist E., Bukhari A. I. State of prophage Mu DNA upon induction. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3143–3147. doi: 10.1073/pnas.74.8.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luftig R. B., Ganz C. Bacteriophage T4 head morphogenesis. II. Studies on the maturation of gene 49-defective head intermediates. J Virol. 1972 Feb;9(2):377–389. doi: 10.1128/jvi.9.2.377-389.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luftig R. B., Lundh N. P. Bacteriophage T4 head morphogenesis. Isolation, partial characterization, and fate of gene 21-defective tau-particles. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1636–1640. doi: 10.1073/pnas.70.6.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luftig R. B., Lundh N. P. Bacteriophage T4 head morphogenesis. V. The role of DNA synthesis in maturation of an intermediate in head assembly. Virology. 1973 Feb;51(2):432–442. doi: 10.1016/0042-6822(73)90442-x. [DOI] [PubMed] [Google Scholar]
- Luftig R. B., Wood W. B., Okinaka R. Bacteriophage T4 head morphogenesis. On the nature of gene 49-defective heads and their role as intermediates. J Mol Biol. 1971 May 14;57(3):555–573. doi: 10.1016/0022-2836(71)90109-4. [DOI] [PubMed] [Google Scholar]
- MACLEAN E. C., HALL C. E. Studies on bacteriophage phi-X174 and its DNA by electron microscopy. J Mol Biol. 1962 Mar;4:173–178. doi: 10.1016/s0022-2836(62)80049-7. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- MacHattie L. A., Gill G. S. DNA maturation by the "headful" mode in bacteriophage T1. J Mol Biol. 1977 Mar 5;110(3):441–465. doi: 10.1016/s0022-2836(77)80108-3. [DOI] [PubMed] [Google Scholar]
- MacHattie L. A., Ritchie D. A., Thomas C. A., Jr, Richardson C. C. Terminal repetition in permuted T2 bacteriophage DNA molecules. J Mol Biol. 1967 Feb 14;23(3):355–363. doi: 10.1016/s0022-2836(67)80110-4. [DOI] [PubMed] [Google Scholar]
- Mackinlay A. G., Kaiser A. D. DNA replication in head mutants of bacteriophage lambda. J Mol Biol. 1969 Feb 14;39(3):679–683. doi: 10.1016/0022-2836(69)90155-7. [DOI] [PubMed] [Google Scholar]
- Martuscelli J., Taylor A. L., Cummings D. J., Chapman V. A., DeLong S. S., Cañedo L. Electron microscopic evidence for linear insertion of bacteriophage MU-1 in lysogenic bacteria. J Virol. 1971 Oct;8(4):551–563. doi: 10.1128/jvi.8.4.551-563.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo-Kato H., Fujisawa H. Studies on Bacteriophage T3. III. Characterization of capsids as intermediates of the T3 head assembly. Virology. 1975 Jan;63(1):105–114. doi: 10.1016/0042-6822(75)90375-x. [DOI] [PubMed] [Google Scholar]
- Matsuo H., Fujisawa H. Studies on bacteriophage T3. I. Kinetic studies on particle formation and role of capsids as intermediates of head morphogenesis of bacteriophage T3. Virology. 1973 Aug;54(2):305–312. doi: 10.1016/0042-6822(73)90144-x. [DOI] [PubMed] [Google Scholar]
- McAllister W. T. Bacteriophage infection: which end of the SP82G genome goes in first? J Virol. 1970 Feb;5(2):194–198. doi: 10.1128/jvi.5.2.194-198.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure S. C., Gold M. Intermediates in the maturation of bacteriophage lambda DNA. Virology. 1973 Jul;54(1):19–27. doi: 10.1016/0042-6822(73)90110-4. [DOI] [PubMed] [Google Scholar]
- McClure S. C., MacHattie L., Gold M. A sedimentation analysis of DNA found in Escherichia coli infected with phage lambda mutants. Virology. 1973 Jul;54(1):1–18. doi: 10.1016/0042-6822(73)90109-8. [DOI] [PubMed] [Google Scholar]
- McGuire J. C., Pène J. J., Barrow-Carraway J. Gene expression during the development of bacteriophage phi 29. 3. Analysis of viral-specific protein synthesis with suppressible mutants. J Virol. 1974 Mar;13(3):690–698. doi: 10.1128/jvi.13.3.690-698.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNicol L. A., Simon L. E. A mutation which bypasses the requirement for p24 in bacteriophage T4 capsid morphogenesis. J Mol Biol. 1977 Oct 25;116(2):261–283. doi: 10.1016/0022-2836(77)90216-9. [DOI] [PubMed] [Google Scholar]
- Mellado R. P., Méndez E., Viñuela E., Salas M. Order of the two major head protein genes of bacteriophage phi 29 of Bacillus subtilis. J Virol. 1977 Oct;24(1):378–382. doi: 10.1128/jvi.24.1.378-382.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa T. Endonuclease of T4 ghosts. Virology. 1977 Jan;76(1):234–245. doi: 10.1016/0042-6822(77)90299-9. [DOI] [PubMed] [Google Scholar]
- Moody M. F. The shape of the T-even bacteriophage head. Virology. 1965 Aug;26(4):567–576. doi: 10.1016/0042-6822(65)90319-3. [DOI] [PubMed] [Google Scholar]
- Mosig G. A map of distances along the DNA molecule of phage T4. Genetics. 1968 Jun;59(2):137–151. doi: 10.1093/genetics/59.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount D. W., Harris A. W., Fuerst C. R., Siminovitch L. Mutations in bacteriophage lambda affecting particle morphogenesis. Virology. 1968 May;35(1):134–149. doi: 10.1016/0042-6822(68)90313-9. [DOI] [PubMed] [Google Scholar]
- Mousset S., Thomas R. Ter, a function which generates the ends of the mature lambda chromosome. Nature. 1969 Jan 18;221(5177):242–244. doi: 10.1038/221242a0. [DOI] [PubMed] [Google Scholar]
- Mukai R., Hayashi M. Synthesis of infectious phiX-174 bacteriophage in vitro. Nature. 1977 Nov 24;270(5635):364–366. doi: 10.1038/270364a0. [DOI] [PubMed] [Google Scholar]
- Murialdo H., Becker A. Assembly of biologically active proheads of bacteriophage lambda in vitro. Proc Natl Acad Sci U S A. 1977 Mar;74(3):906–910. doi: 10.1073/pnas.74.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murialdo H., Ray P. N. Model for arrangement of minor structural proteins in head of bacteriophage lambda. Nature. 1975 Oct 30;257(5529):815–817. doi: 10.1038/257815a0. [DOI] [PubMed] [Google Scholar]
- Murialdo H., Siminovitch L. The morphogenesis of bacteriophage lambda. IV. Identification of gene products and control of the expression of the morphogenetic information. Virology. 1972 Jun;48(3):785–823. doi: 10.1016/0042-6822(72)90162-6. [DOI] [PubMed] [Google Scholar]
- Murialdo H., Siminovitch L. The morphogenesis of phage lambda. V. Form-determining function of the genes required for the assembly of the head. Virology. 1972 Jun;48(3):824–835. doi: 10.1016/0042-6822(72)90163-8. [DOI] [PubMed] [Google Scholar]
- Murray K., Isaksson-Forsen A. G., Challberg M., Englund P. T. Symmetrical nucleotide sequences in the recognition sites for the ter function of bacteriophages P2, 299 and 186. J Mol Biol. 1977 May 25;112(3):471–489. doi: 10.1016/s0022-2836(77)80193-9. [DOI] [PubMed] [Google Scholar]
- Murray K., Murray N. E., Bertani G. Base changes in the recognition site for ter functions in lambdoid phage DNA. Nature. 1975 Mar 20;254(5497):262–265. doi: 10.1038/254262a0. [DOI] [PubMed] [Google Scholar]
- Murray K., Murray N. E. Terminal nucleotide sequences of DNA from temperate coliphages. Nat New Biol. 1973 May 30;243(126):134–139. doi: 10.1038/newbio243134a0. [DOI] [PubMed] [Google Scholar]
- Méndez E., Ramírez G., Salas M., Viñuela E. Structural proteins of bacteriophage phi 29. Virology. 1971 Sep;45(3):567–576. doi: 10.1016/0042-6822(71)90172-3. [DOI] [PubMed] [Google Scholar]
- Müller-Salamin L., Onorato L., Showe M. K. Localization of minor protein components of the head of bacteriophage T4. J Virol. 1977 Oct;24(1):121–134. doi: 10.1128/jvi.24.1.121-134.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. A., Reilly B. E., Anderson D. L. Morphogenesis of bacteriophage phi 29 of Bacillus subtilis: preliminary isolation and characterization of intermediate particles of the assembly pathway. J Virol. 1976 Aug;19(2):518–532. doi: 10.1128/jvi.19.2.518-532.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H., Tomizawa J. Bacteriophage lambda DNA with different structures found in infected cells. J Mol Biol. 1967 Jan 28;23(2):265–276. doi: 10.1016/s0022-2836(67)80032-9. [DOI] [PubMed] [Google Scholar]
- Onorato L., Showe M. K. Gene 21 protein-dependent proteolysis in vitro of purified gene 22 product of bacteriophage T4. J Mol Biol. 1975 Mar 5;92(3):395–412. doi: 10.1016/0022-2836(75)90288-0. [DOI] [PubMed] [Google Scholar]
- Ortin J., Viñuela E., Salas M., Vasquez C. DNA-protein complex in circular DNA from phage phi-29. Nat New Biol. 1971 Dec 29;234(52):275–277. doi: 10.1038/newbio234275a0. [DOI] [PubMed] [Google Scholar]
- Padmanabhan R., Wu R., Calendar R. Complete nucleotide sequence of the cohesive ends of bacteriophage P2 deoxyribonucleic acid. J Biol Chem. 1974 Oct 10;249(19):6197–6207. [PubMed] [Google Scholar]
- Paetkau V., Langman L., Bradley R., Scraba D., Miller R. C., Jr Folded, concatenated genomes as replication intermediates of bacteriophage T7 DNA. J Virol. 1977 Apr;22(1):130–141. doi: 10.1128/jvi.22.1.130-141.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paietta J. V., McDonald T. L., Doermann A. H. Method for the isolation of bacteriphage T4 mutants that produce particles with giant capsids. J Virol. 1976 May;18(2):785–787. doi: 10.1128/jvi.18.2.785-787.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao C. C., Speyer J. F. Order of injection of T7 bacteriophage DNA. J Virol. 1973 Jun;11(6):1024–1026. doi: 10.1128/jvi.11.6.1024-1026.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J. S. Genetics of the left arm of the chromosome of bacteriophage lambda. Genetics. 1968 Jul;59(3):311–325. doi: 10.1093/genetics/59.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson J. R., Laemmli U. K. Morphogenetic core of the bacteriophage T4 head. Structure of the core in polyheads. J Mol Biol. 1977 Apr 25;111(4):459–485. doi: 10.1016/s0022-2836(77)80064-8. [DOI] [PubMed] [Google Scholar]
- Paulson J. R., Lazaroff S., Laemmli U. K. Head length determination in bacteriophage T4: the role of the core protein P22. J Mol Biol. 1976 May 5;103(1):155–174. doi: 10.1016/0022-2836(76)90057-7. [DOI] [PubMed] [Google Scholar]
- Poteete A. R., King J. Functions of two new genes in Salmonella phage P22 assembly. Virology. 1977 Feb;76(2):725–739. doi: 10.1016/0042-6822(77)90254-9. [DOI] [PubMed] [Google Scholar]
- Pruss G. J., Calendar R. Maturation of bacteriophage P2 DNA. Virology. 1978 May 15;86(2):454–467. doi: 10.1016/0042-6822(78)90085-5. [DOI] [PubMed] [Google Scholar]
- Pruss G. J., Wang J. C., Calendar R. In vitro packaging of covalently closed circular monomers of bacteriophage DNA. J Mol Biol. 1975 Nov 5;98(3):465–478. doi: 10.1016/s0022-2836(75)80080-5. [DOI] [PubMed] [Google Scholar]
- Pruss G., Barrett K., Lengyel J., Goldstein R., Calendar R. Phage head size determination and head protein cleavage in vitro. J Supramol Struct. 1974;2(2-4):337–348. doi: 10.1002/jss.400020223. [DOI] [PubMed] [Google Scholar]
- Pruss G., Goldstein R. N., Calendar R. In vitro packaging of satellite phage P4 DNA. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2367–2371. doi: 10.1073/pnas.71.6.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulitzer J. F., Yanagida M. Inactive T4 progeny virus formation in a temperature-sensitive mutant of Escherichia coli K12. Virology. 1971 Sep;45(3):539–554. doi: 10.1016/0042-6822(71)90170-x. [DOI] [PubMed] [Google Scholar]
- Raj A. S., Raj A. Y., Schmieger H. Phage genes involved in the formation generalized transducing particles in Salmonella--Phage P22. Mol Gen Genet. 1974;135(2):175–184. doi: 10.1007/BF00264784. [DOI] [PubMed] [Google Scholar]
- Ramírez G., Méndez E., Salas M., Viñuela E. Head-neck connecting protein in phage phi29. Virology. 1972 Apr;48(1):263–265. doi: 10.1016/0042-6822(72)90134-1. [DOI] [PubMed] [Google Scholar]
- Ray P., Murialdo H. The role of gene Nu3 in bacteriophage lambda head morphogenesis. Virology. 1975 Mar;64(1):247–263. doi: 10.1016/0042-6822(75)90096-3. [DOI] [PubMed] [Google Scholar]
- Reilly B. E., Nelson R. A., Anderson D. L. Morphogenesis of bacteriophage phi 29 of Bacillus subtilis: mapping and functional analysis of the head fiber gene. J Virol. 1977 Oct;24(1):363–377. doi: 10.1128/jvi.24.1.363-377.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekosh D. M., Russell W. C., Bellet A. J., Robinson A. J. Identification of a protein linked to the ends of adenovirus DNA. Cell. 1977 Jun;11(2):283–295. doi: 10.1016/0092-8674(77)90045-9. [DOI] [PubMed] [Google Scholar]
- Rhoades M., MacHattie L. A., Thomas C. A., Jr The P22 bacteriophage DNA molecule. I. The mature form. J Mol Biol. 1968 Oct 14;37(1):21–40. doi: 10.1016/0022-2836(68)90071-5. [DOI] [PubMed] [Google Scholar]
- Richards K. E., Williams R. C., Calendar R. Mode of DNA packing within bacteriophage heads. J Mol Biol. 1973 Aug 5;78(2):255–259. doi: 10.1016/0022-2836(73)90114-9. [DOI] [PubMed] [Google Scholar]
- Ritchie D. A., Thomas C. A., Jr, MacHattie L. A., Wensink P. C. Terminal repetition in non-permuted T3 and T7 bacteriophage DNA molecules. J Mol Biol. 1967 Feb 14;23(3):365–376. doi: 10.1016/s0022-2836(67)80111-6. [DOI] [PubMed] [Google Scholar]
- Roeder G. S., Sadowski P. D. Bacteriophage T7 morphogenesis: phage-related particles in cells infected with wild-type and mutant T7 phage. Virology. 1977 Jan;76(1):263–285. doi: 10.1016/0042-6822(77)90302-6. [DOI] [PubMed] [Google Scholar]
- SARABHAI A. S., STRETTON A. O., BRENNER S., BOLLE A. CO-LINEARITY OF THE GENE WITH THE POLYPEPTIDE CHAIN. Nature. 1964 Jan 4;201:13–17. doi: 10.1038/201013a0. [DOI] [PubMed] [Google Scholar]
- STREISINGER G., EDGAR R. S., DENHARDT G. H. CHROMOSOME STRUCTURE IN PHAGE T4. I. CIRCULARITY OF THE LINKAGE MAP. Proc Natl Acad Sci U S A. 1964 May;51:775–779. doi: 10.1073/pnas.51.5.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saigo K. Tail--DNA connection and chromosome structure in bacteriophage T5. Virology. 1975 Nov;68(1):154–165. doi: 10.1016/0042-6822(75)90157-9. [DOI] [PubMed] [Google Scholar]
- Saigo K., Uchida H. Connection of the right-hand terminus of DNA to the proximal end of the tail in bacteriophage lambda. Virology. 1974 Oct;61(2):524–536. doi: 10.1016/0042-6822(74)90287-6. [DOI] [PubMed] [Google Scholar]
- Salas M., Vásquez C., Méndez E., Viñuela E. Head fibers of bacteriophage phi 29. Virology. 1972 Oct;50(1):180–188. doi: 10.1016/0042-6822(72)90358-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Air G. M., Barrell B. G., Brown N. L., Coulson A. R., Fiddes C. A., Hutchison C. A., Slocombe P. M., Smith M. Nucleotide sequence of bacteriophage phi X174 DNA. Nature. 1977 Feb 24;265(5596):687–695. doi: 10.1038/265687a0. [DOI] [PubMed] [Google Scholar]
- Schlegel R. A., Thomas C. A., Jr Some special structural features of intracellular bacteriophage T7 concatemers. J Mol Biol. 1972 Jul 21;68(2):319–345. doi: 10.1016/0022-2836(72)90216-1. [DOI] [PubMed] [Google Scholar]
- Schmieger H., Backhaus H. Altered cotransduction frequencies exhibited by HT-mutants of Salmonella-phage P22. Mol Gen Genet. 1976 Feb 2;143(3):307–309. doi: 10.1007/BF00269408. [DOI] [PubMed] [Google Scholar]
- Schmieger H. Phage P22-mutants with increased or decreased transduction abilities. Mol Gen Genet. 1972;119(1):75–88. doi: 10.1007/BF00270447. [DOI] [PubMed] [Google Scholar]
- Serwer P. Buoyant density sedimentation of macromolecules in sodium iothalamate density gradients. J Mol Biol. 1975 Mar 5;92(3):433–448. doi: 10.1016/0022-2836(75)90290-9. [DOI] [PubMed] [Google Scholar]
- Serwer P. Complexes between bacteriophage T7 capsids and T7 DNA. Virology. 1974 May;59(1):89–107. doi: 10.1016/0042-6822(74)90208-6. [DOI] [PubMed] [Google Scholar]
- Serwer P. Internal proteins of bacteriophage T7. J Mol Biol. 1976 Nov 5;107(3):271–291. doi: 10.1016/s0022-2836(76)80005-8. [DOI] [PubMed] [Google Scholar]
- Shore D., Dehò G., Tsipis J., Goldstein R. Determination of capsid size by satellite bacteriophage P4. Proc Natl Acad Sci U S A. 1978 Jan;75(1):400–404. doi: 10.1073/pnas.75.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showe M. K., Black L. W. Assembly core of bacteriophage T4: an intermediate in head formation. Nat New Biol. 1973 Mar 21;242(116):70–75. doi: 10.1038/newbio242070a0. [DOI] [PubMed] [Google Scholar]
- Showe M. K., Isobe E., Onorato L. Bacteriophage T4 prehead proteinase. I. Purification and properties of a bacteriophage enzyme which cleaves the capsid precursor proteins. J Mol Biol. 1976 Oct 15;107(1):35–54. doi: 10.1016/s0022-2836(76)80016-2. [DOI] [PubMed] [Google Scholar]
- Showe M. K., Isobe E., Onorato L. Bacteriophage T4 prehead proteinase. II. Its cleavage from the product of gene 21 and regulation in phage-infected cells. J Mol Biol. 1976 Oct 15;107(1):55–69. doi: 10.1016/s0022-2836(76)80017-4. [DOI] [PubMed] [Google Scholar]
- Siden E. J., Hayashi M. Role of the gene beta-product in bacteriophage phi-X174 development. J Mol Biol. 1974 Oct 15;89(1):1–16. doi: 10.1016/0022-2836(74)90159-4. [DOI] [PubMed] [Google Scholar]
- Siegel P. J., Schaechter M. Bacteriophage T4 head maturation: release of progeny DNA from the host cell membrane. J Virol. 1973 Mar;11(3):359–367. doi: 10.1128/jvi.11.3.359-367.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein J. L., Goldberg E. B. T4 DNA injection. I. Growth cycle of a gene 2 mutant. Virology. 1976 Jul 1;72(1):195–211. doi: 10.1016/0042-6822(76)90323-8. [DOI] [PubMed] [Google Scholar]
- Silverstein J. L., Goldberg E. B. T4 DNA injection. II. Protection of entering DNA from host exonuclease V. Virology. 1976 Jul 1;72(1):212–223. doi: 10.1016/0042-6822(76)90324-x. [DOI] [PubMed] [Google Scholar]
- Simon L. D. Infection of Escherichia coli by T2 and T4 bacteriophages as seen in the electron microscope: T4 head morphogenesis. Proc Natl Acad Sci U S A. 1972 Apr;69(4):907–911. doi: 10.1073/pnas.69.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L. D., McLaughlin T. J., Snover D., Ou J., Grisham C., Loeb M. E. coli membrane lipid alteration affecting T4 capsid morphogenesis. Nature. 1975 Jul 31;256(5516):379–383. doi: 10.1038/256379a0. [DOI] [PubMed] [Google Scholar]
- Simon L. D., Snover D., Doermann A. H. Bacterial mutation affecting T4 phage DNA synthesis and tail production. Nature. 1974 Dec 6;252(5483):451–455. doi: 10.1038/252451a0. [DOI] [PubMed] [Google Scholar]
- Simon L. D. The infection of Escherichia coli by T2 and T4 bacteriophages as seen in the electron microscope. 3. Membrane-associated intracellular bacteriophages. Virology. 1969 Jun;38(2):285–296. doi: 10.1016/0042-6822(69)90370-5. [DOI] [PubMed] [Google Scholar]
- Six E. W., Klug C. A. Bacteriophage P4: a satellite virus depending on a helper such as prophage P2. Virology. 1973 Feb;51(2):327–344. doi: 10.1016/0042-6822(73)90432-7. [DOI] [PubMed] [Google Scholar]
- Six E. W. The helper dependence of satellite bacteriophage P4: which gene functions of bacteriophage P2 are needed by P4? Virology. 1975 Sep;67(1):249–263. doi: 10.1016/0042-6822(75)90422-5. [DOI] [PubMed] [Google Scholar]
- Skalka A., Poonian M., Bartl P. Concatemers in DNA replication: electron microscopic studies of partially denatured intracellular lambda DNA. J Mol Biol. 1972 Mar 14;64(3):541–550. doi: 10.1016/0022-2836(72)90081-2. [DOI] [PubMed] [Google Scholar]
- Sternberg N. A genetic analysis of bacteriophage lambda head assembly. Virology. 1976 Jun;71(2):568–582. doi: 10.1016/0042-6822(76)90382-2. [DOI] [PubMed] [Google Scholar]
- Sternberg N. Properties of a mutant of Escherichia coli defective in bacteriophage lambda head formation (groE). II. The propagation of phage lambda. J Mol Biol. 1973 May 5;76(1):25–44. doi: 10.1016/0022-2836(73)90079-x. [DOI] [PubMed] [Google Scholar]
- Sternberg N., Weisberg R. Packaging of coliphage lambda DNA. I. The role of the cohesive end site and the gene A protein. J Mol Biol. 1977 Dec 15;117(3):717–731. doi: 10.1016/0022-2836(77)90066-3. [DOI] [PubMed] [Google Scholar]
- Sternberg N., Weisberg R. Packaging of coliphage lambda DNA. II. The role of the gene D protein. J Mol Biol. 1977 Dec 15;117(3):733–759. doi: 10.1016/0022-2836(77)90067-5. [DOI] [PubMed] [Google Scholar]
- Sternberg N., Weisberg R. Packaging of prophage and host DNA by coliphage lambda. Nature. 1975 Jul 10;256(5513):97–103. doi: 10.1038/256097a0. [DOI] [PubMed] [Google Scholar]
- Steven A. C., Aebi U., Showe M. K. Folding and capsomere morphology of the P23 surface shell of bacteriophage T4 polyheads from mutants in five different head genes. J Mol Biol. 1976 Apr 15;102(3):373–400. [PubMed] [Google Scholar]
- Steven A. C., Couture E., Aebi U., Showe M. K. Structure of T4 polyheads. II. A pathway of polyhead transformation as a model for T4 capsid maturation. J Mol Biol. 1976 Sep 5;106(1):187–221. doi: 10.1016/0022-2836(76)90307-7. [DOI] [PubMed] [Google Scholar]
- Streisinger G., Emrich J., Stahl M. M. Chromosome structure in phage t4, iii. Terminal redundancy and length determination. Proc Natl Acad Sci U S A. 1967 Feb;57(2):292–295. doi: 10.1073/pnas.57.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strätling W., Krause E., Knippers R. Fast sedimenting deoxyribonucleic acid in bacteriophage T7-infected cells. Virology. 1973 Jan;51(1):109–119. doi: 10.1016/0042-6822(73)90371-1. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Szpirer J., Brachet P. Relations physiologiques entre les phages tempérés lambda et phi80. Mol Gen Genet. 1970;108(1):78–92. doi: 10.1007/BF00343187. [DOI] [PubMed] [Google Scholar]
- TAYLOR A. L. BACTERIOPHAGE-INDUCED MUTATION IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1963 Dec;50:1043–1051. doi: 10.1073/pnas.50.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TROMANS W. J., HORNE R. W. The structure of bacteriophage phi-X174. Virology. 1961 Sep;15:1–7. doi: 10.1016/0042-6822(61)90069-1. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Coppo A., Manzi A., Martire G., Pulitzer J. F. Design of a system of conditional lethal mutations (tab/k/com) affecting protein-protein interactions in bacteriophage T4-infected Escherichia coli. J Mol Biol. 1975 Aug 25;96(4):563–578. doi: 10.1016/0022-2836(75)90139-4. [DOI] [PubMed] [Google Scholar]
- Takano T., Kakefuda T. Involvement of a bacterial factor in morphogenesis of bacteriophage capsid. Nat New Biol. 1972 Sep 13;239(89):34–37. doi: 10.1038/newbio239034a0. [DOI] [PubMed] [Google Scholar]
- Thomas J. O. Chemical linkage of the tail to the right-hand end of bacteriophage lambda DNA. J Mol Biol. 1974 Jul 25;87(1):1–9. doi: 10.1016/0022-2836(74)90555-5. [DOI] [PubMed] [Google Scholar]
- Tonegawa S., Hayashi M. Intermediates in the assembly of phi X174. J Mol Biol. 1970 Mar 14;48(2):219–242. doi: 10.1016/0022-2836(70)90158-0. [DOI] [PubMed] [Google Scholar]
- Tye B. K. A mutant of phage P22 with randomly permuted DNA. J Mol Biol. 1976 Jan 25;100(3):421–426. doi: 10.1016/s0022-2836(76)80073-3. [DOI] [PubMed] [Google Scholar]
- Tye B. K., Chan R. K., Botstein D. Packaging of an oversize transducing genome by Salmonella phage P22. J Mol Biol. 1974 Jan 5;85(4):485–500. doi: 10.1016/0022-2836(74)90311-8. [DOI] [PubMed] [Google Scholar]
- Tye B. K., Huberman J. A., Botstein D. Non-random circular permutation of phage P22 DNA. J Mol Biol. 1974 Jan 5;85(4):501–528. doi: 10.1016/0022-2836(74)90312-x. [DOI] [PubMed] [Google Scholar]
- Uhlenhopp E. L., Zimm B. H., Cummings D. J. Structural aberrations in T-even bacteriophage. VI. Molecular weight of DNA from giant heads. J Mol Biol. 1974 Nov 15;89(4):689–702. doi: 10.1016/0022-2836(74)90045-x. [DOI] [PubMed] [Google Scholar]
- Uratani Y., Asakura S., Imahori K. A circular dichroism study of Salmonella flagellin: evidence for conformational change on polymerization. J Mol Biol. 1972 Jun 14;67(1):85–98. doi: 10.1016/0022-2836(72)90388-9. [DOI] [PubMed] [Google Scholar]
- Vanderslice R. W., Yegian C. D. The identification of late bacteriophage T4 proteins on sodium dodecyl sulfate polyacrylamide gels. Virology. 1974 Jul;60(1):265–275. doi: 10.1016/0042-6822(74)90384-5. [DOI] [PubMed] [Google Scholar]
- Viñuela E., Camacho A., Jiménez F., Carrascosa J. L., Ramírez G., Salas M. Structure and assembly of phage phi29. Philos Trans R Soc Lond B Biol Sci. 1976 Nov 30;276(943):29–35. doi: 10.1098/rstb.1976.0095. [DOI] [PubMed] [Google Scholar]
- Wake R. G., Kaiser A. D., Inman R. B. Isolation and structure of phage lambda head-mutant DNA. J Mol Biol. 1972 Mar 14;64(3):519–540. doi: 10.1016/0022-2836(72)90080-0. [DOI] [PubMed] [Google Scholar]
- Wang J. C., Brezinski D. P. Alignment of two DNA helices: a model for recognition of DNA base sequences by the termini-generating enzymes of phage lambda, 186, and P2. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2667–2670. doi: 10.1073/pnas.70.9.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C., Kaiser A. D. Evidence that the cohesive ends of mature lambda DNA are generated by the gene A product. Nat New Biol. 1973 Jan 3;241(105):16–17. doi: 10.1038/newbio241016a0. [DOI] [PubMed] [Google Scholar]
- Wang J. C., Martin K. V., Calendar R. On the sequence similarity of the cohesive ends of coliphage P4, P2, and 186 deoxyribonucleic acid. Biochemistry. 1973 May 22;12(11):2119–2123. doi: 10.1021/bi00735a016. [DOI] [PubMed] [Google Scholar]
- Watson J. D. Origin of concatemeric T7 DNA. Nat New Biol. 1972 Oct 18;239(94):197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- Weigel P. H., Englund P. T., Murray K., Old R. W. The 3'-terminal nucleotide sequences of bacteriophage lambda DNA. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1151–1155. doi: 10.1073/pnas.70.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigle J. Assembly of phage lambda in vitro. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1462–1466. doi: 10.1073/pnas.55.6.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil J., deWein N., Casale A. Morphogenesis of lambda with genomes containing excess DNA: functional particles containing 12 and 15 per cent excess DNA. Virology. 1975 Feb;63(2):352–366. doi: 10.1016/0042-6822(75)90309-8. [DOI] [PubMed] [Google Scholar]
- Weisbeek P. J., Sinsheimer R. L. A DNA-protein complex involved in bacteriophage phi chi 174 particle formation. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3054–3058. doi: 10.1073/pnas.71.8.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Richards K. E. Letter: Capsid structure of bacteriophage lambda. J Mol Biol. 1974 Sep 15;88(2):547–550. doi: 10.1016/0022-2836(74)90501-4. [DOI] [PubMed] [Google Scholar]
- Wolfson J., Dressler D., Magazin M. Bacteriophage T7 DNA replication: a linear replicating intermediate (gradient centrifugation-electron microscopy-E. coli-DNA partial denaturation). Proc Natl Acad Sci U S A. 1972 Feb;69(2):499–504. doi: 10.1073/pnas.69.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. B., Henninger M. Attachment of tail fibers in bacteriophage T4 assembly: some properties of the reaction in vitro and its genetic control. J Mol Biol. 1969 Feb 14;39(3):603–618. doi: 10.1016/0022-2836(69)90148-x. [DOI] [PubMed] [Google Scholar]
- Wood W. B., Revel H. R. The genome of bacteriophage T4. Bacteriol Rev. 1976 Dec;40(4):847–868. doi: 10.1128/br.40.4.847-868.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R., Taylor E. Nucleotide sequence analysis of DNA. II. Complete nucleotide sequence of the cohesive ends of bacteriophage lambda DNA. J Mol Biol. 1971 May 14;57(3):491–511. doi: 10.1016/0022-2836(71)90105-7. [DOI] [PubMed] [Google Scholar]
- Wurtz M., Kistler J., Hohn T. Surface structure of in vitro assembled bacteriophage lambda polyheads. J Mol Biol. 1976 Feb 15;101(1):39–56. doi: 10.1016/0022-2836(76)90065-6. [DOI] [PubMed] [Google Scholar]
- Yanagida M., Ahmad-Zadeh C. Determination of gene product positions in bacteriophage T4 by specific antibody association. J Mol Biol. 1970 Jul 28;51(2):411–421. doi: 10.1016/0022-2836(70)90151-8. [DOI] [PubMed] [Google Scholar]
- Yanagida M., Boy de la Tour E., Alff-Steinberger C., Kellenberger E. Studies on the morphopoiesis of the head of bacteriophage T-even. 8. Multilayered polyheads. J Mol Biol. 1970 May 28;50(1):35–58. doi: 10.1016/0022-2836(70)90102-6. [DOI] [PubMed] [Google Scholar]
- Yanagida M. Molecular organization of the shell of T-even bacteriophage head. II. Arrangement of subunits in the head shell of giant phages. J Mol Biol. 1977 Feb 5;109(4):515–537. doi: 10.1016/s0022-2836(77)80089-2. [DOI] [PubMed] [Google Scholar]
- Yanofsky S., Kawamura F., Ito J. Thermolabile transfecting DNA from temperature-sensitive mutant of phage phi29. Nature. 1976 Jan 1;259(5538):60–63. doi: 10.1038/259060a0. [DOI] [PubMed] [Google Scholar]
- Zachary A., Simon L. D., Litwin S. Lambda head morphogenesis as seen in the electron microscope. Virology. 1976 Jul 15;72(2):429–442. doi: 10.1016/0042-6822(76)90172-0. [DOI] [PubMed] [Google Scholar]
- Zachary A., Simon L. D. Size changes among lambda capsid precursors. Virology. 1977 Aug;81(1):107–116. doi: 10.1016/0042-6822(77)90062-9. [DOI] [PubMed] [Google Scholar]
- Zweig M., Cummings D. J. Cleavage of head and tail proteins during bacteriophage T5 assembly: selective host involvement in the cleavage of a tail protein. J Mol Biol. 1973 Nov 5;80(3):505–518. doi: 10.1016/0022-2836(73)90418-x. [DOI] [PubMed] [Google Scholar]
- Zweig M., Rosenkranz H. S., Morgan C. Development of coliphage T5: ultrastructural and biochemical studies. J Virol. 1972 Mar;9(3):526–543. doi: 10.1128/jvi.9.3.526-543.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Driel R. Assembly of bacteriophage T4 head-related structures. Assembly of polyheads in vitro. J Mol Biol. 1977 Jul;114(1):61–72. doi: 10.1016/0022-2836(77)90283-2. [DOI] [PubMed] [Google Scholar]


