Abstract
The therapeutic potential of bacteriophage (phage) in a mouse model of acute B. cenocepacia pulmonary infection was assessed. Phage were administered by either intranasal (i.n.) inhalation or intraperitoneal (i.p.) injection. Bacterial density, macrophage inflammatory protein-2 (MIP-2), and tumor necrosis factor-α (TNFα) levels were significantly reduced in lungs of mice treated with i.p. phage. No significant differences in lung bacterial density or MIP-2 levels were found between untreated mice and mice treated with i.n. phage, i.p. UV-inactivated phage, or i.p. λ phage controls. Mock-infected mice treated with phage showed no significant increase in lung MIP-2 or TNFα levels compared to mock-infected / mock-treated mice. We have demonstrated the efficacy of phage therapy in an acute B. cenocepacia lung infection model. Systemic administration of phage was more effective than inhalational administration, suggesting that circulating phage have better access to bacteria in lung compared to topical phage.
Keywords: bacteriophage therapy, cystic fibrosis, respiratory pathogen
Introduction
Species within the Burkholderia cepacia complex (Bcc) are life-threatening opportunistic bacterial pathogens for persons with cystic fibrosis (CF) or chronic granulomatous disease (CGD) [1, 2]. In CF, respiratory tract infection with these species can result in acute pulmonary disease and sepsis (“cepacia syndrome”), or chronic infection characterized by an accelerated decline in lung function [3, 4]. Although most of the 17 species currently included in the Bcc have been recovered from CF patients, B. cenocepacia is particularly problematic, accounting for approximately 45% of Bcc infections in CF patients in the United States [5]. B. cenocepacia is also generally considered a contraindication to lung transplantation in CF patients with end-stage pulmonary disease [6]. Effective antimicrobial therapy of Bcc lung infection in CF and CGD is severely limited by the constitutive and inducible multi-drug resistant phenotypes exhibited by most strains [7-9]. Consequently, there is a critical need for alternative strategies to treat Bcc infection in these vulnerable patient populations.
Bacteriophages (phages) have long been envisioned as a potential therapy for bacterial infections. Indeed, after the discovery of bacteriophage in 1917, phage therapy was used to treat a variety of infectious diseases. Although this practice has continued in Eastern Europe [10, 11], a mixed record of success and the discovery of antibiotics resulted in the decline of phage therapy in Western medicine [12]. The emergence of multi-drug resistant bacteria, however, has renewed interest in phage therapy as an alternative or complement to conventional antibiotic therapy. Several recent well-controlled animal studies have demonstrated the potential of phage as antibacterial therapy in vivo [13-22].
The development of a successful phage therapy program involves the careful selection and characterization of candidate phages [23]. Lysogenic phage with potential for horizontal transfer of toxin or antibiotic resistance genes between bacteria should be avoided in favor of virulent phage, incapable of a lysogenic life-style [24]. Several virulent phages for Bcc species have been well characterized [25]. The broad-spectrum antimicrobial resistance of B. cenocepacia, an abundance of novel phages that target this clinically important species, and the availability of tools to characterize these phages make B. cenocepacia infection a reasonable choice for a trial of phage therapy.
The objective of this study was to demonstrate the in vivo efficacy of phage therapy for B. cenocepacia respiratory tract infection. Using a mouse model of acute lung infection, we examined the effect of treatment with a single phage on bacterial load and lung inflammation.
Methods
Bacterial strains and growth conditions
B. cenocepacia strains AU0728 and K56-2 were isolated from the sputum of CF patients and represent the Midwest and ET12 epidemic lineages, respectively. For infection of mice, bacteria were grown on Brain Heart Infusion agar (Fisher Scientific, Hampton, NH) then subcultured in tryptic soy broth (Fisher). Bacteria were washed in phosphate-buffered saline (PBS; Invitrogen, Carlsbad, CA) and resuspended in PBS to the desired concentration.
Bacteriophage
Phage BcepIL02 was isolated from soil collected from a corn field in Champaign County, Illinois, in 2006, by enriching 20 g of soil with B. cenocepacia PC184 (another representative of the Midwest lineage) and incubating at 37°C overnight. The culture was centrifuged and the supernatant was filter sterilized, diluted and plated to lawns of PC184. An isolated plaque was recovered and suspended in 1 mL of SM buffer (50 mM Tris-HCl pH 7.5, 100 mM NaCl, 8 mM MgSO4, 0.01 % [w/v[ gelatin). The phage isolate, designated BcepIL02, was purified by three passages through this single-plaque isolation procedure, and was routinely propagated in PC184 using standard techniques [26]. BcepIL02 is a virulent phage active against a range of B. cenocepacia strains, including the Midwest and PHDC ‘epidemic’ lineages; however, it shows no activity against B. cenocepacia in the ET12 clonal lineage.
For administration to mice, BcepIL02 was propagated in tryptone nutrient broth (TNB; 150 mM NaCl, 5 mM glucose, 5 g/L tryptone, 2.5 g/L yeast extract) using PC184 as a host. The phage liquid lysate was purified using methods modified from Sambrook et al. [27]. Endotoxin levels in the finished phage concentrates were less than 0.125 EU/mL as determined by Limulus amoebocyte gel-clotting assay (Pyrogent Plus; Lonza Biosciences, Basel, Switzerland). Phage λ was prepared by thermal induction of E. coli MC4100 containing a λ cI857 Sam7 lysogen [28]. Endotoxin content of the λ concentrate was no greater than 6 EU/mL. When necessary, phage were inactivated by exposure to a UV transilluminator for 45 min. An absence of plaques in a top agar plaque assay confirmed 100% inactivation.
Mouse model
Nine to 12 week old C57BL/6 mice were infected via tracheotomy with 1 × 107 or 1 × 108 CFU B. cenocepacia suspended in 50 μL sterile PBS. Control mice were mock-infected with 50 μL PBS without bacteria. Twenty-four h post-infection, mice were treated with either intranasal (i.n.) inhalation or intraperitoneal (i.p.) injection of phage suspended in 50 μL or 100 μL of SM buffer, respectively, at a multiplicity of infection (moi) of 100. Preliminary experiments with dye indicated that 50 μL was a sufficient volume to deliver the suspension uniformly to lower airways via inhalation. Control mice were mock-treated with 50 μL i.n. or 100 μL i.p. of SM buffer, or i.p. injection of UV-inactivated BcepIL02 or λ phage. Forty-eight h after phage treatment, mice were weighed and euthanized, and lungs were collected, weighed, and homogenized in sterile PBS. Lung homogenates were serially diluted and plated for viable bacteria counts on B. cepacia selective agar [29]. An aliquot of each homogenate was centrifuged, and supernatants were passed through a 0.45 μm filter and assayed for phage titer via top agar plaque assay. Animal experiments were approved by the University Committee on Use and Care of Animals of the University of Michigan.
MIP-2 and TNFα
MIP-2 and TNFα levels in lung were measured using Quantikine ELISA kits (R & D Systems, Minneapolis, MN) following the manufacturer's instructions. To prepare samples, Complete Protease Inhibitor (Roche, Indianapolis, IN) was added to lung homogenates. Samples were assayed in duplicate.
Antibodies
Polyclonal rabbit antibody (R418) to whole lysed B. cenocepacia has been described previously [30]. Antibody to BcepIL02 was generated in chickens using 3 × 109 PFU UV-inactivated phage as antigen (Virusys, Sykesville, MD). Total IgY was purified from egg yolk and adsorbed against heat-killed AU0728. Antigen affinity and specificity were confirmed by Western blot with AU0728 as a negative control (data not shown). Secondary antibodies were conjugated with AlexaFluor-488 or AlexaFluor-594 (Invitrogen).
Immunofluorescence
Lungs of two to three mice from each experimental group were collected 24 h or 72 h post-infection, fixed in 10% neutral buffered formalin for 24 h, and paraffin embedded. Lungs were cut into 5 μm sections, air dried, deparaffinized in xylene, and rehydrated in graded alcohols. Sections were blocked with 5% normal goat serum in tris-buffered saline for 1 h then incubated with the appropriate primary antibody overnight at 4°C. Bound antibodies were detected by anti-chicken or anti-rabbit antibody conjugated to AlexaFluor-488 or AlexaFluor-594 as indicated. Sections were counterstained with DAPI, mounted, and visualized using a Zeiss LSM 510 confocal laser microscope.
Statistical analysis
Statistical analyses were performed using SPSS (Chicago, IL). Parametric one-way ANOVA with Games-Howell post-test was used to compare three or more groups with data expressed as mean and SEM. Differences were considered statistically significant if P < 0.05. When sample distributions were not normal, a nonparametric Kruskal-Wallis test was performed. Multiple pairwise Mann-Whitney U tests with Bonferroni correction were performed as post-tests with data expressed as median values.
Results
Phage treatment of pulmonary B. cenocepacia infection
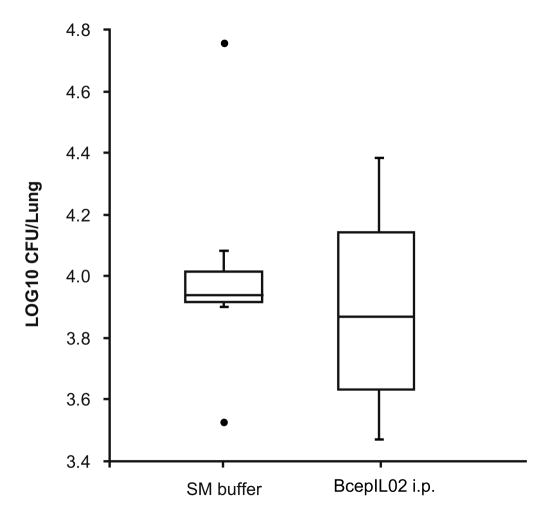
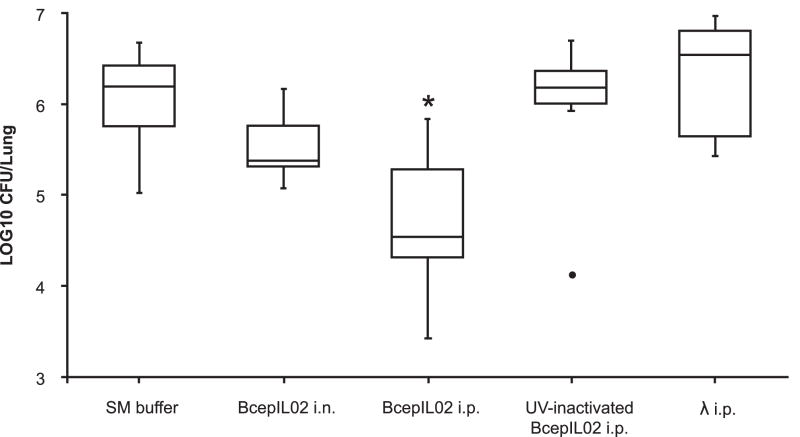
We used a model of mouse lung infection to assess the ability of phage BcepIL02 to kill B. cenocepacia in vivo. Twenty-four h after bacterial infection, mice were treated with phage at a moi of 100, administered by either i.n. inhalation or i.p. injection. Infected control mice were mock-treated with either i.n. or i.p. SM buffer without phage. Bacterial densities in lung were determined 48 h later (or 72 h post-infection). The treatment of AU0728-infected mice with BcepIL02 via i.p. injection resulted in a significant reduction in lung bacterial density 48 h after administration relative to mock-treated mice (P = 0.002; Fig. 1). AU0728-infected mice treated with i.p. BcepIL02 also had significantly lower lung weights and lung weight to body weight ratios than mock-treated mice (data not shown). A reduced density of bacteria in lungs 48 h after treatment was also observed in AU0728-infected mice treated with i.n. inhalation of BcepIL02; however, the difference between treated mice and mock-treated controls was not statistically significant.
FIG 1.
Effect of phage treatment on bacterial density. Density of bacteria in lung was determined in mice that were infected with B. cenocepacia AU0728 and treated 24 h later with i.n. inhalation (n=6) or i.p. injection (n=6) of BcepIL02. Infected control mice were mock-treated with i.n. (n=5) or i.p. (n=11) SM buffer without phage (since there was no difference between these two groups, results were combined). Other control mice were treated with i.p. injection of UV-inactivated BcepIL02 (n=6), or i.p. injection of λ phage (n=8). The horizontal line in each box is the median CFU per lung for the group, calculated from two or more independent experiments, each performed in triplicate or quadruplicate; the boxes indicate the interquartile range of CFU per lung, and the whiskers indicate the range of CFU per lung for the group. ● = extreme outlier, * P < 0.05 (Mann-Whitney) for difference between BcepIL02 i.p. treated mice versus SM buffer mock-treated control mice.
To rule out the possibility that a non-specific host immune response to the presence of phage was responsible for the decrease in bacterial density in lung, we treated AU0728-infected mice with i.p. injection of either UV-inactivated BcepIL02 or λ phage, both of which have no activity against B. cenocepacia in vitro. We observed no significant difference in lung bacterial densities 48 h after treatment, between mock-treated control mice and mice treated with either UV-inactivated BcepIL02 or λ phage (Fig. 1). In mice infected with B. cenocepacia strain K56-2 (against which BcepIL02 has no activity in vitro), we found no significant differences in lung bacterial densities between mice treated with i.p. BcepIL02 and mock-treated control mice (Fig. 2).
FIG 2.
Recovery of B. cenocepacia K56-2 from lungs 48 h after treatment. Density of bacteria was determined in mice that were infected with B. cenocepacia K56-2 and mock-treated 24 h later with SM buffer (n=7) or treated with i.p. injection of BcepIL02 (n=6). The horizontal line in each box is the median CFU per lung for the group, calculated from two independent experiments, each performed in triplicate or quadruplicate; the boxes indicate the interquartile range of CFU per lung, and the whiskers indicate the range of CFU per lung for the group ● = extreme outlier.
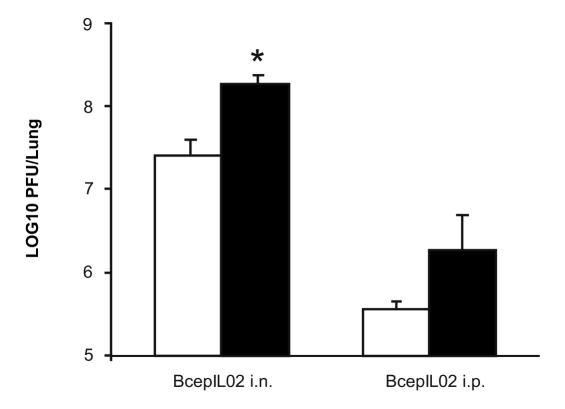
A significant advantage of therapeutic phages over conventional antibiotics is the ability of phage to replicate in the presence of a susceptible bacterial target. We measured phage titers in lungs to assess the degree of phage replication in vivo in the presence of bacteria. Phage titers in AU0728-infected mice 48 h after treatment with BcepIL02 via i.n. inhalation were significantly higher than those in mock-infected mice similarly treated with BcepIL02 (P = 0.018; Fig. 3). Phage titers in AU0728-infected mice 48 h after treatment with BcepIL02 via i.p. injection were also higher than those in mock-infected mice similarly treated with BcepIL02; however, this difference was not significant. In both B. cenocepacia-infected and mock-infected mice, approximately two logs more phage were recovered from the lungs of mice treated with BcepIL02 via i.n. inhalation than from those treated via i.p. injection (Fig. 3).
FIG 3.
Recovery of phage from treated mice. Titer of BcepIL02 was determined in mice lungs that were infected with B. cenocepacia AU0728 (black bars, n=6) or mock-infected with SM buffer (white bars, n=6) and treated 24 h later with either i.n. inhalation or i.p. injection of BcepIL02. Data are mean + SEM PFU per lung 48 h after phage treatment, calculated from two independent experiments, each performed in triplicate. * P < 0.05 (Games-Howell) for difference between i.n BcepIL02 treatment of AU0728 infected versus mock-infected mice.
Inflammatory markers in lung following phage treatment
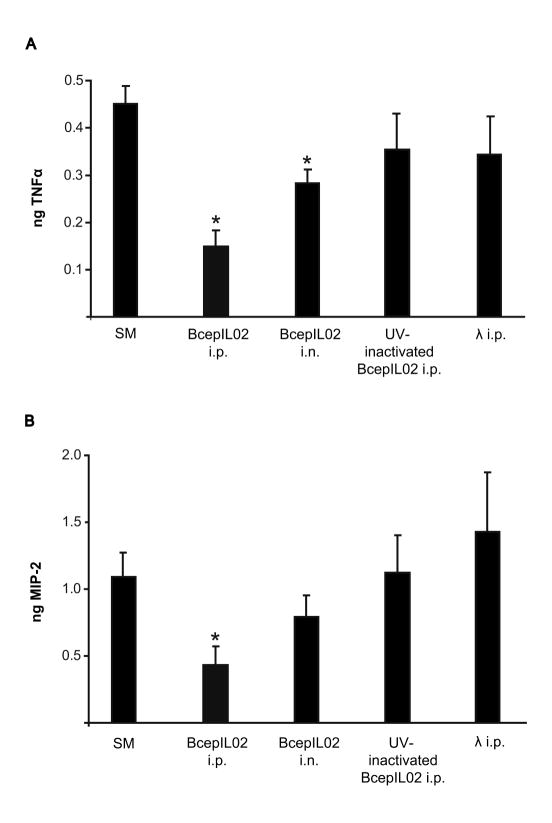
To determine if phage treatment could attenuate infection-associated lung inflammation, we measured levels of proinflammatory cytokine TNFα and neutrophil chemokine MIP-2 in lungs 48 h after treatment. In AU0728-infected mice, we observed that BcepIL02 treatment resulted in significantly reduced TNFα levels relative to mock-treated controls, whether phage were administered via i.n. inhalation (P = 0.017) or i.p. injection (P < 0.001) (Fig. 4A). We also found that AU0728-infected mice treated with BcepIL02 via i.p. injection had significantly reduced levels of MIP-2 in lung compared to mock-treated controls (P = 0.026; Fig. 4B). Treatment of AU0728-infected mice with BcepIL02 via i.n. inhalation also resulted in reduced MIP-2 levels; however, this reduction was not statistically significant (Fig. 4B).
FIG 4.
TNFα and MIP-2 levels in lungs after phage treatment. Levels of TNFα (A) and MIP-2 (B) were measured in mice lungs that were infected with AU0728. Twenty-four h after infection, mice were mock-treated with SM buffer (n=16), or treated with BcepIL02 by i.p. injection (n=6) or i.n. inhalation (n=6). Other control mice were treated via i.p. injection with UV-inactivated phage BcepIL02 (n=6) or λ phage (n=8). Data are mean + SEM ng TNFα or MIP-2 per lung, calculated from two or more independent experiments, each performed in triplicate or quadruplicate. * P < 0.05 (Games-Howell) for differences between treatments indicated and mock-treated SM buffer controls.
AU0728-infected mice treated with UV-inactivated BcepIL02 or λ phage showed no significant differences in TNFα (Fig. 5A) or MIP-2 (Fig. 4B) levels in lung compared to mock-treated controls. There were no significant differences in TNFα or MIP-2 levels in lungs of mice infected with K56-2 and treated with BcepIL02 via i.p. injection compared to mock-treated controls (data not shown). To assess the proinflammatory potential of phage alone, we measured lung cytokine levels in mock-infected mice treated with phage. Mice that were mock-infected with PBS and then treated 24 h later with BcepIL02 via either i.n or i.p. administration had no appreciable levels of either TNFα or MIP-2 in lung 48 h post-treatment (data not shown).
FIG 5.
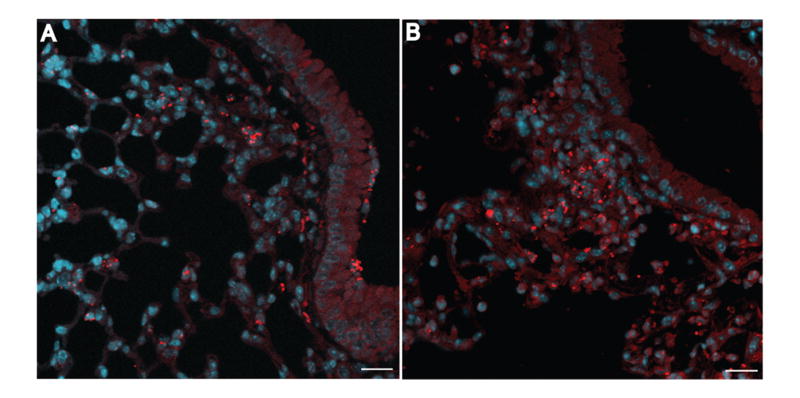
Immunofluorescent localization of B. cenocepacia AU0728. C57/BL6 mice were infected with B. cenocepacia AU0728. Lungs were removed 24 h (A) or 72 h (B) later, fixed, sectioned, stained for bacteria (red) and counterstained with DAPI (blue). A, Bacteria localized primarily in lung parenchyma with few observed in airway lumen. B, By 72 h after infection, bacteria formed microcolonies in areas of consolidated lung parenchyma. (A, B, bar = 20 μm).
Localization of bacteria and phage in lung
To evaluate how phage and bacteria interact spatially in lung, we used immunofluorescence microscopy to localize phage and bacteria in infected and phage-treated lungs. Twenty-four h post-infection with AU0728 (but prior to treatment) bacteria were found primarily in lung parenchyma in peribronchiolar and perivascular areas, occasionally co-localized with alveolar macrophages. Relatively few bacteria were located within the airway lumen (Fig. 5A). Seventy-two h post-infection, without treatment, bacteria were found predominantly in consolidated lung parenchyma, often forming microcolonies (Fig. 5B).
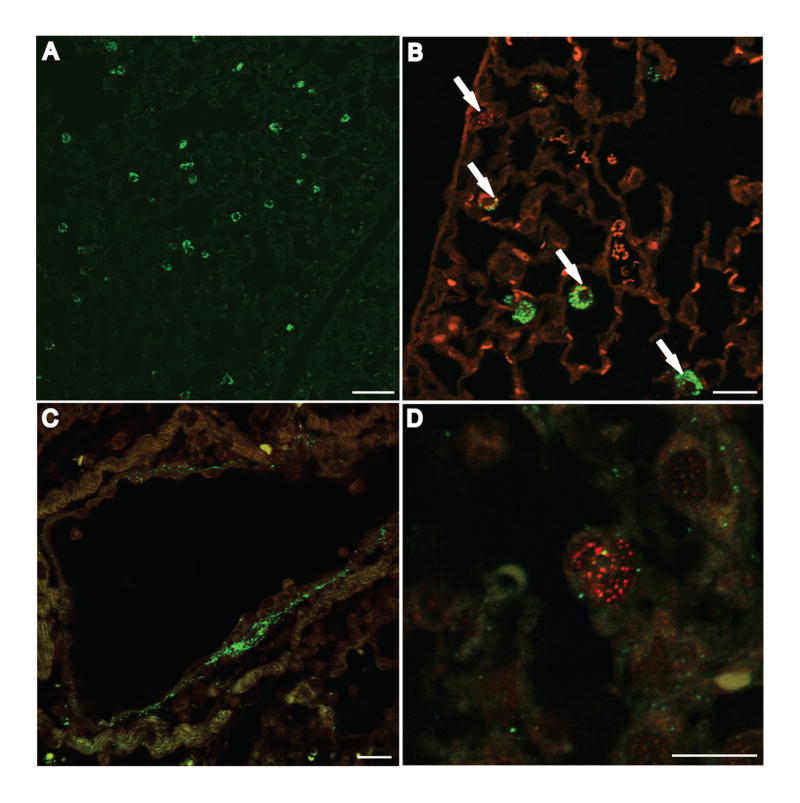
When AU0728-infected mice were treated 24 h post-infection with BcepIL02 via i.n. inhalation, most phage were co-localized with alveolar macrophages 48 h later (Fig. 6A). Phage and macrophages occasionally co-localized with degraded bacteria in alveolar septa (Fig. 6B). Few intact bacteria were observed in alveolar septa or within macrophages and no bacteria were observed in the airway lumen at this time. Mice that were mock-infected with PBS alone and then treated 24 h later with BcepIL02 via i.n. inhalation showed phage co-localized with alveolar macrophages 48 h later (data not shown). Forty-eight h after treatment of infected mice with BcepIL02 via i.p. injection, much fewer phage were observed in lung compared to lung sections from i.n. treated mice. The greatest concentration of phage in these mice was observed in perivascular areas (Fig. 6C) and alveolar septa, where phage could be found co-localized with degraded bacteria (Fig. 6D). Few intact bacteria were observed in sections from mice treated with phage via i.p. injection.
FIG 6.
Immunofluorescent localization of B. cenocepacia AU0728 and phage BcepIL02 in mouse lungs. C57BL/6 mice were infected with B. cenocepacia AU0728 and treated 24 h later by i.n. inhalation (A, B) or i.p. injection (C, D) of BcepIL02. Lungs were removed 48 h later, fixed, sectioned, and stained for phage (A-D; green) and bacteria (B, C, D; red). Phage administered via i.n. inhalation primarily localized in alveolar macrophages (A, B). B, Arrows indicate localization of degraded bacteria inside macrophages. Phage administered via i.p. injection were found primarily in perivascular areas (C) and alveolar septa, where they could be observed co-localized with degraded bacteria (D). (A, bar = 50 μm; B, C, bar = 20 μm; D, bar = 10 μm).
Discussion
Because Burkholderia typically are nonpathogenic in animals, studies investigating the virulence of these species often employ the agar bead model of respiratory infection, where bacteria are embedded in agar beads prior to instillation into murine lungs [31]. Animals develop non-lethal pulmonary disease and viable bacteria may be recovered from lungs for two to three weeks. However, because phage killing depends on unobstructed access of phage to their bacterial target, we chose to employ a model of acute infection that avoids encasing bacteria in agar. In a series of preliminary experiments, we determined that when inoculated into the lungs of mice via intratracheal instillation, B. cenocepacia causes non-lethal pulmonary disease and can be recovered for up to seven days, thus providing a tractable model to assess the relative efficacy of anti-infective treatments. Further, our previous work with AU0728 indicates that it is multi-drug resistant and capable of robust biofilm formation in vitro (unpublished data), and thus ought to provide for a rigorous test of novel anti-infective therapies.
With this acute respiratory infection model, we demonstrated in vivo efficacy of phage BcepIL02 in decreasing bacterial density and lung inflammation during infection. These positive treatment effects were not observed with either UV-inactivated phage or λ phage, and BcepIL02 was ineffective against infection with B. cenocepacia K56-2, a strain resistant to BcepIL02. These findings indicate that active and specific phage are required for effective treatment.
We found that phage were more effective when administered via i.p. injection than by i.n. inhalation, despite the significantly greater titers of phage observed in lungs after treatment via inhalation. The reasons for this observation are not clear, although previous studies have similarly shown the superiority of systemically administered phage, compared to topical phage, in treating infection [16, 18, 22]. In preliminary experiments we established that the volume used to administer phage by inhalation was sufficient to deliver the suspension to lower airways. Nevertheless, it appears that phage delivered via inhalation have less access to (and/or are less active against) bacteria than phage delivered via the systemic circulation. This suggests that the site of greatest bacterial killing may be within lung parenchyma rather than within the airway lumen. Phage delivered directly to the airway lumen may not be able to effectively penetrate the respiratory epithelium to encounter bacteria that have translocated from the lumen to the lung interstitium. Indeed, it appears that bacteriophage are not able to readily penetrate eukaryotic cells [33]. In a set of preliminary in vitro experiments using polarized 16HBE14o- human bronchial epithelial cells, which form intercellular tight junctions, we observed that BcepIL02 delivered to the apical cell surface did not penetrate the intact epithelium (data not shown). It is also possible that the lower phage titers we observed in infected lungs after i.p. injection (relative to phage titers after inhalation) reflect a more rapid decrease in bacterial density and the subsequent clearance of phage in the absence of sufficient bacterial hosts [34]. Finally, we found no significant differences between any of the treatment or control groups in the density of bacteria recovered from spleens of infected mice (data not shown). While it is possible that systemic phage may have greater access to such extrapulmonary sites than do intranasal phage, this finding suggests that bacterial re-infection of lung from infected spleen does not account for the differences in lung bacterial density observed between groups.
Several previous studies of phage therapy in animal models of infection found that delaying intervention for more than a few hours after infection mitigated or eliminated entirely any positive treatment effects [15, 16, 21, 22, 35]. In these models, which generally employ a lethality endpoint, phage intervention must be timed to interrupt the exponential expansion of the infecting bacterial population before the host succumbs. In our non-lethal infection model, in contrast, we found that phage administered well after infection was established were effective in producing positive treatment effects.
To better assess the dynamics of phage-bacteria interaction in infected lung, we employed confocal immunofluorescent microscopy to localize phage, bacteria and host cells. We found that within 24 h of intratracheal instillation, relatively few bacteria were located in the airways; rather, bacteria were found primarily in lung parenchyma occasionally co-localized with alveolar macrophages. By 72 h post-infection, bacteria were found predominantly in areas of consolidated lung, often forming large microcolonies. After phage treatment via inhalation, phage were found primarily inside alveolar macrophages, suggesting that a large proportion of inhaled phage may be sequestered in macrophages and therefore unavailable to infect and kill bacteria within the lung parenchyma. In striking contrast, phage administered via i.p. injection were not found within alveolar macrophages. Rather, phage were observed primarily in vascular and perivascular areas, and in alveolar septa, where they could be observed co-localized with degraded bacteria. Very few intact bacteria were observed in lungs from mice treated with i.p. phage.
After inhalation, phage titers in lungs were significantly greater in B. cenocepacia-infected mice than in mock-infected controls, suggesting that inhaled phage were able to reach some target bacteria and replicate. Whether phage are taken up with bacteria by macrophages after primary infection, preventing efficient secondary infection, or whether phage replicate within bacteria inside macrophages is unclear and is the subject of ongoing research. Broxmeyer et al. [36] observed that a Mycobacterium phage was able to kill Mycobacterium avium and Mycobacterium tuberculosis residing within macrophages; however, phage were carried into the intracellular compartment by phage-infected Mycobacterium smegmatis. In our study, phage were found localized in alveolar macrophages in mock-infected mice administered phage by inhalation, suggesting that a significant proportion of inhaled phage are taken up by macrophages even in the absence of bacteria. In any event, the high phage titers observed in lung 48 h after inhaled phage treatment suggests that a significant proportion of phage remain viable after uptake by macrophages.
A number of concerns have been raised regarding the therapeutic use of phages. Chief among these is that the mammalian host immune response will render phage inactive and repeat dosing ineffective. Although it has been suggested that innate immunity may be sufficient for rapid elimination of phage from circulation [19], studies have shown the persistence of high phage titers in blood as long as a suitable bacterial host was present [14, 17]. Selection of long-circulating phages is a strategy that has been suggested to avoid rapid immune clearance [19]. The development of neutralizing antibodies could also potentially inactivate phage. The presence of anti-phage antibodies following phage therapy has been reported [13, 20], although the impact these antibodies have on efficacy of repeated phage dosing is unclear. While the short course of our infection model makes it unlikely that we would have observed such an induction of humoral immunity, the effect of long-term exposure to circulating BcepIL02 is unknown. As has been suggested by others, and as we observed here, the effect of phage therapy is rapid, likely faster than specific immunity can develop [37]. Repeated dosing using phage with different antigenicities to avoid immunologic cross-reactivity is another means to evade immune inactivation. Moreover, at least with the model used here, treatment with only a single phage had a positive treatment effect, indicating that the emergence of resistant bacteria, often cited as a major weakness for phage-based treatment, might not always limit efficacy. In any case, most scenarios in which phages would be deployed in clinical settings would involve cocktails of multiple phages with different receptor specificities. Such cocktails may consist of several distinct phages, or a single phage modified to extend its host range.
In summary, we have shown that phage therapy may be a viable option for treating life-threatening B. cenocepacia respiratory infections. Future studies assessing the efficacy of phage therapy in chronic infection models of Bcc infection are worthwhile, as are studies to determine the optimal dosing and timing to achieve maximal therapeutic effect. The route of phage delivery appears to be an important determinant of successful therapy. A better understanding of the pharmacokinetics of phage treatment and the specific interactions of phage with the mammalian host immune system will be critical in optimizing a phage therapy program.
Acknowledgments
Financial support: The work was supported by the National Institutes of Health (grant R01-AI-64512 to R.F.Y., J.J.L and C.F.G.). J.J.L is also supported by the Cystic Fibrosis Foundation. The authors thank Steve Magill for providing soil samples and Tim O'Brien for technical assistance.
Footnotes
Potential conflicts of interest: None of the authors has a commercial or other association that might pose a conflict of interest
Presented in part: 48th Annual ICAAC / IDSA 46th Annual Meeting. Washington, D.C., 25-28 October 2008 (poster F1-3955)
References
- 1.Greenberg DE, Goldberg JB, Stock F, et al. Recurrent Burkholderia infection in patients with chronic granulomatous disease: 11-year experience at a large referral center. Clin Infect Dis. doi: 10.1086/598937. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LiPuma JJ. Update on the Burkholderia cepacia complex. Curr Opin Pulm Med. 2005;11:528–33. doi: 10.1097/01.mcp.0000181475.85187.ed. [DOI] [PubMed] [Google Scholar]
- 3.Courtney JM, Dunbar KE, McDowell A, et al. Clinical outcome of Burkholderia cepacia complex infection in cystic fibrosis adults. J Cyst Fibros. 2004;3:93–8. doi: 10.1016/j.jcf.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Isles A, Maclusky I, Corey M, et al. Pseudomonas cepacia infection in cystic fibrosis: An emerging problem. J Pediatr. 1984;104:206–10. doi: 10.1016/s0022-3476(84)80993-2. [DOI] [PubMed] [Google Scholar]
- 5.Reik R, Spilker T, LiPuma JJ. Distribution of Burkholderia cepacia complex species among isolates recovered from persons with or without cystic fibrosis. J Clin Microbiol. 2005;43:2926–8. doi: 10.1128/JCM.43.6.2926-2928.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray S, Charbeneau J, Marshall BC, et al. Impact of Burkholderia infection on lung transplantation in cystic fibrosis. Am J Respir Crit Care Med. 2008;178:363–71. doi: 10.1164/rccm.200712-1834OC. [DOI] [PubMed] [Google Scholar]
- 7.Aronoff SC. Outer membrane permeability in Pseudomonas cepacia: Diminished porin content in a beta-lactam-resistant mutant and in resistant cystic fibrosis isolates. Antimicrob Agents Chemother. 1988;32:1636–9. doi: 10.1128/aac.32.11.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burns JL, Wadsworth CD, Barry JJ, et al. Nucleotide sequence analysis of a gene from Burkholderia (Pseudomonas) cepacia encoding an outer membrane lipoprotein involved in multiple antibiotic resistance. Antimicrob Agents Chemother. 1996;40:307–13. doi: 10.1128/aac.40.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore RA, Hancock RE. Involvement of outer membrane of Pseudomonas cepacia in aminoglycoside and polymyxin resistance. Antimicrob Agents Chemother. 1986;30:923–6. doi: 10.1128/aac.30.6.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alisky J, Iczkowski K, Rapoport A, et al. Bacteriophages show promise as antimicrobial agents. J Infect. 1998;36:5–15. doi: 10.1016/s0163-4453(98)92874-2. [DOI] [PubMed] [Google Scholar]
- 11.Sulakvelidze A, Alavidze Z, Morris JG., Jr Bacteriophage therapy. Antimicrob Agents Chemother. 2001;45:649–59. doi: 10.1128/AAC.45.3.649-659.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merril CR, Scholl D, Adhya SL. The prospect for bacteriophage therapy in Western medicine. Nat Rev Drug Discov. 2003;2:489–97. doi: 10.1038/nrd1111. [DOI] [PubMed] [Google Scholar]
- 13.Biswas B, Adhya S, Washart P, et al. Bacteriophage therapy rescues mice bacteremic from a clinical isolate of vancomycin-resistant Enterococcus faecium. Infect Immun. 2002;70:204–10. doi: 10.1128/IAI.70.1.204-210.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capparelli R, Parlato M, Borriello G, et al. Experimental phage therapy against Staphylococcus aureus in mice. Antimicrob Agents Chemother. 2007;51:2765–73. doi: 10.1128/AAC.01513-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cerveny KE, DePaola A, Duckworth DH, et al. Phage therapy of local and systemic disease caused by Vibrio vulnificus in iron-dextran-treated mice. Infect Immun. 2002;70:6251–62. doi: 10.1128/IAI.70.11.6251-6262.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chhibber S, Kaur S, Kumari S. Therapeutic potential of bacteriophage in treating Klebsiella pneumoniae B5055-mediated lobar pneumonia in mice. J Med Microbiol. 2008;57:1508–13. doi: 10.1099/jmm.0.2008/002873-0. [DOI] [PubMed] [Google Scholar]
- 17.Matsuzaki S, Yasuda M, Nishikawa H, et al. Experimental protection of mice against lethal Staphylococcus aureus infection by novel bacteriophage phi MR11. J Infect Dis. 2003;187:613–24. doi: 10.1086/374001. [DOI] [PubMed] [Google Scholar]
- 18.McVay CS, Velasquez M, Fralick JA. Phage therapy of Pseudomonas aeruginosa infection in a mouse burn wound model. Antimicrob Agents Chemother. 2007;51:1934–8. doi: 10.1128/AAC.01028-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merril CR, Biswas B, Carlton R, et al. Long-circulating bacteriophage as antibacterial agents. Proc Natl Acad Sci U S A. 1996;93:3188–92. doi: 10.1073/pnas.93.8.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rashel M, Uchiyama J, Ujihara T, et al. Efficient elimination of multidrug-resistant Staphylococcus aureus by cloned lysin derived from bacteriophage phi MR11. J Infect Dis. 2007;196:1237–47. doi: 10.1086/521305. [DOI] [PubMed] [Google Scholar]
- 21.Smith HW, Huggins MB. Successful treatment of experimental Escherichia coli infections in mice using phage: its general superiority over antibiotics. J Gen Microbiol. 1982;128:307–18. doi: 10.1099/00221287-128-2-307. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe R, Matsumoto T, Sano G, et al. Efficacy of bacteriophage therapy against gut-derived sepsis caused by Pseudomonas aeruginosa in mice. Antimicrob Agents Chemother. 2007;51:446–52. doi: 10.1128/AAC.00635-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skurnik M, Pajunen M, Kiljunen S. Biotechnological challenges of phage therapy. Biotechnol Lett. 2007;29:995–1003. doi: 10.1007/s10529-007-9346-1. [DOI] [PubMed] [Google Scholar]
- 24.Matsuzaki S, Rashel M, Uchiyama J, et al. Bacteriophage therapy: a revitalized therapy against bacterial infectious diseases. J Infect Chemother. 2005;11:211–9. doi: 10.1007/s10156-005-0408-9. [DOI] [PubMed] [Google Scholar]
- 25.Summer EJ, Gonzalez CF, Bomer M, et al. Divergence and mosaicism among virulent soil phages of the Burkholderia cepacia complex. J Bacteriol. 2006;188:255–68. doi: 10.1128/JB.188.1.255-268.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adams MH. Bacteriophages. New York: Interscience Publishers, Inc.; 1959. [Google Scholar]
- 27.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: A laboratory manual. 2nd. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 28.Daniels DL, Schroeder JL, Szybalski W. Appendix II: Complete annotated lambda sequence. In: Hendrix RW, Roberts JW, Stahl FW, et al., editors. Lambda II. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1983. pp. 519–676. [Google Scholar]
- 29.Henry DA, Campbell ME, LiPuma JJ, et al. Identification of Burkholderia cepacia isolates from patients with cystic fibrosis and use of a simple new selective medium. J Clin Microbiol. 1997;35:614–9. doi: 10.1128/jcm.35.3.614-619.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sylvester FA, Sajjan US, Forstner JF. Burkholderia (basonym Pseudomonas) cepacia binding to lipid receptors. Infect Immun. 1996;64:1420–5. doi: 10.1128/iai.64.4.1420-1425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cash HA, Woods DE, McCullough B, et al. A rat model of chronic respiratory infection with Pseudomonas aeruginosa. Am Rev Respir Dis. 1979;119:453–9. doi: 10.1164/arrd.1979.119.3.453. [DOI] [PubMed] [Google Scholar]
- 32.Coenye T, LiPuma JJ. Population structure analysis of Burkholderia cepacia genomovar III: Varying degrees of genetic recombination characterize major clonal complexes. Microbiology. 2002;149:77–88. doi: 10.1099/mic.0.25850-0. [DOI] [PubMed] [Google Scholar]
- 33.Dabrowska K, Switala-Jelen K, Opolski A, et al. Bacteriophage penetration in vertebrates. J Appl Microbiol. 2005;98:7–13. doi: 10.1111/j.1365-2672.2004.02422.x. [DOI] [PubMed] [Google Scholar]
- 34.Payne RJ, Jansen VA. Pharmacokinetic principles of bacteriophage therapy. Clin Pharmacokinet. 2003;42:315–25. doi: 10.2165/00003088-200342040-00002. [DOI] [PubMed] [Google Scholar]
- 35.Bull JJ, Levin BR, DeRouin T, et al. Dynamics of success and failure in phage and antibiotic therapy in experimental infections. BMC Microbiol. 2002;2:35. doi: 10.1186/1471-2180-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Broxmeyer L, Sosnowska D, Miltner E, et al. Killing of Mycobacterium avium and Mycobacterium tuberculosis by a mycobacteriophage delivered by a nonvirulent mycobacterium: A model for phage therapy of intracellular bacterial pathogens. J Infect Dis. 2002;186:1155–60. doi: 10.1086/343812. [DOI] [PubMed] [Google Scholar]
- 37.Duckworth DH, Gulig PA. Bacteriophages: Potential treatment for bacterial infections. BioDrugs. 2002;16:57–62. doi: 10.2165/00063030-200216010-00006. [DOI] [PubMed] [Google Scholar]