A biological understanding of memory remains one of the great quests of neuroscience. For over 30 years the fruit fly Drosophila melanogaster has primarily been viewed as an excellent vehicle to find ‘memory genes’. However, the recent advent of sophisticated genetic tools to manipulate neural activity has meant that these genes can now be viewed within the context of functioning neural circuits. A holistic understanding of memory in flies is therefore now a realistic goal. Larvae and adult flies exhibit remarkable behavioral complexity and they can both be trained in a number of ways. In this review, our intention is to summarize the many assays that have been developed to study plastic behaviors in flies. More specific and detailed reviews have been published by us and others, reviewed in refs. 1–6. While our bias for olfactory conditioning paradigms is obvious, our purpose here is not to pass judgment on each method. We would rather leave that to those readers who might be inspired to try each assay for themselves.
Learning in Adult Flies
Olfactory avoidance learning
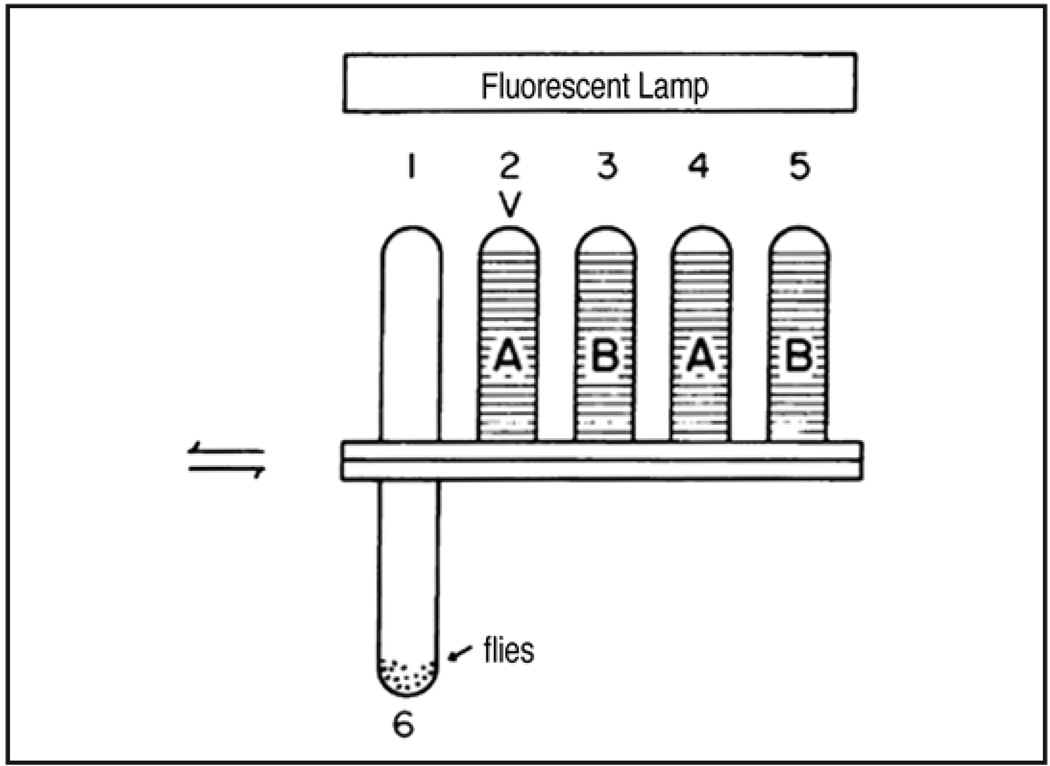
In the early 1970s Chip Quinn and Bill Harris converted Seymour Benzer’s iconic countercurrent apparatus for fractionating flies according to their phototactic capability7 into a machine to study olfactory learning—from here referred to as the QHB assay8 (Fig. 1). In their apparatus approximately 40 flies are given 15 seconds to run (drawn by the light) into an illuminated tube lined with an electrified grid and painted with an odor (conditioned odor, CS+). They are then knocked back into the starting tube, given 60 seconds of rest and then allowed 15 seconds to run into another tube containing a non-electrified grid painted with a new odor (CS−). Training is complete following three trials of CS+/shock and CS−/no shock. Odor memory is tested at given times by allowing the flies 15 seconds to run into either a new non-electrified tube containing the CS+ or CS− odor and in each case the number of flies avoiding the tube is counted. A learning index is calculated by subtracting the number of flies that avoided the CS− from the number of flies that avoided the CS+, divided by the total number of flies. To reduce non-associative effects, different populations of flies of the same genotype are trained and tested with the CS+ and CS− odors reversed and a single learning index score is the average of the two reciprocal half experiments. Control assays for odor and shock acuity/avoidance were also devised. Although typical learning index scores are relatively low (~0.3), this assay allowed the isolation of the first learning and memory mutants in the field; dunce, rutabaga and amnesiac being the most lauded.9–11
Figure 1.
Olfactory avoidance learning in the QHB assay. 40 flies are loaded into Tube 6. Tube 1 is the rest tube, tube 2 is used for shock training with the CS+, tube 3 for training with CS−. Tubes 4 and 5 are used for memory testing. V = voltage. Figure taken from reference 8 with the permission of W.G. Quinn.
Olfactory aversive conditioning
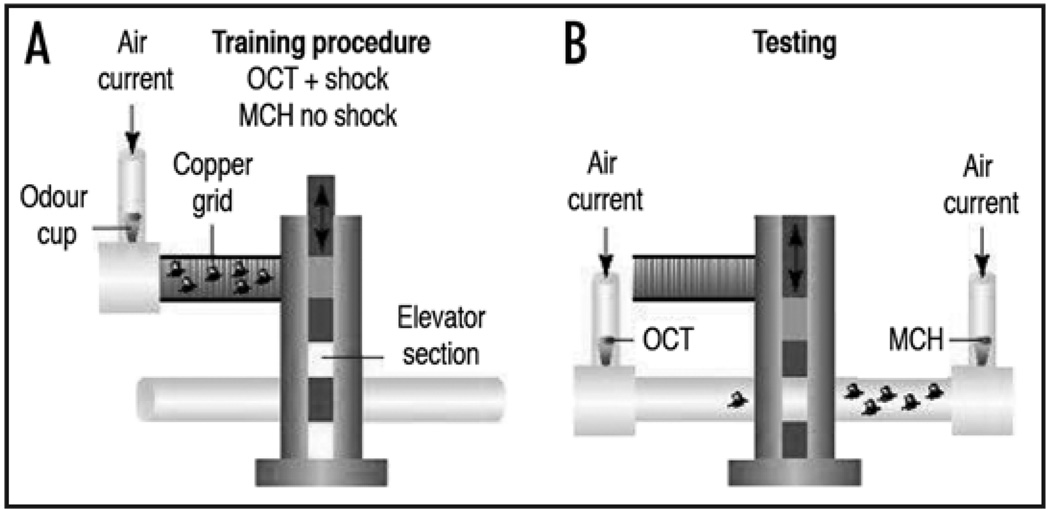
The Drosophila olfactory memory field took a significant step forward with the development of a classical conditioning assay that involves a binary T-maze choice,12 after Jellies13—from here referred to as the TQ assay (Fig. 2) that is performed under dim-red light or darkness. In this paradigm 100 flies are trapped in the training tube that has an electrifiable grid and the experimenter therefore has complete control over shock presentation, intensity and duration. Odors on an air current are piped into the training chamber. Training consists of a 1 minute presentation of an odor (CS+) with twelve one second electric shocks (at 5 second intervals), followed by 30 seconds of fresh air and another 1 minute presentation of a different odor without electric shock (CS−). Odor memory is tested at given times thereafter by transporting flies in the elevator to the T-maze where they are allowed 2 minutes to choose between tubes containing either of the two odorants they experienced during conditioning. A performance index (PI) is calculated by subtracting the number of flies avoiding the CS− from the number of flies avoiding the CS+, divided by the total number of flies. As with the previous assay, a different population of the same genotype of flies is trained with the CS+ and CS− odors reversed and a final performance index is the average of the two reciprocal half experiments. PI scores using the TQ paradigm can be higher than 0.9 (with a score of 1 representing learning in every fly) but generally range from 0.6–0.9. Memory can either be tested immediately after training (3 minute memory, referred to as ‘learning’ or short-term memory) or the flies can be transferred to food vials and housed until being tested at later time points to assess different memory phases (e.g., middle-term and long-term memory). A single training session does not form persistent memory in the TQ paradigm and performance is essentially absent 24 hr after training. However, 6–10 training sessions with or without rest intervals forms memory that lasts for days.14
Figure 2.
Olfactory conditioning in the TQ assay. (A) 100 flies are loaded into the training tube (top), which can either be electrified for aversive conditioning, or contain a filter paper with dried sucrose for appetitive conditioning. (B) Following training, flies are moved to the “elevator” and lowered to the T-maze where they choose between the arms of the maze that contain either of the odors used during conditioning. Odors (OCT-octanol, MCH-methylscyclohexanol) are drawn through the machine by vacuum (not shown). Figure taken from reference 59.
Olfactory appetitive conditioning
Although training flies to avoid electric shock is effective, shock is not an ecologically relevant reinforcer. Tempel et al.15 described conditioning with odorants and sucrose reward using a variation of the QHB apparatus. They used light (and negative geotaxis) to attract food-deprived flies into a training tube painted with a band of sucrose and odor (CS+). Training consisted of two rounds of a 30 second exposure to odor A (CS−) with no reward, 30 seconds of rest, followed by odor B (CS+) with sucrose reward. Memory was assayed by allowing the flies 15 seconds to choose between the CS+ and CS− odor in a T-maze. Performance scores were calculated by subtracting the number of flies approaching the CS− from the number approaching the CS+, divided by the total number of flies tested. Once again, the final PI score is the average of two reciprocal experiments where the CS+ and CS− odorants are swapped.
Flies have to be hungry to learn and retrieve memory efficiently in the sugar rewarded paradigm. Tempel et al.15 concluded that flies exhibit optimal learning after 19–20 hours of starvation; a treatment that did not affect their intrinsic odor preference or their learning performance in the TQ assay. They also found that sugar reinforced memory persists much longer than shock reinforced memory. Appetitive conditioning is becoming more popular and is now routinely performed in a TQ-like manner.16–18 The primary difference between shock and sucrose training in the TQ machine is that flies are trained in two separate tubes for appetitive conditioning; one lined with crystallized sucrose on filter paper and one lined with blank filter paper. Using this approach we have recently shown that a single 2-minute pairing of odorant and sucrose forms protein synthesis-dependent long-term memory that lasts for days.18 Extended periods of starvation can confound the appetitive paradigm. However, it is possible to extend the use of the assay to two-three days following a single session of training by feeding the flies after training and re-starving them before testing memory.18
Olfactory conditioning of the proboscis extension reflex
The Proboscis extension reflex (PER) has been used to study gustatory behaviors in several insects. Hungry flies instinctively extend their proboscis when sugar is presented to gustatory receptors on the foreleg tarsi. The probability of this extension increases as a function of sugar concentration and decreases as increasing concentrations of a bitter substance are added to a fixed concentration of sugar.19 Newly eclosed flies are fed on normal food for 24 hrs and starved for 24 hrs to induce a hunger state. They are then immobilized on slides where the responders are separated from non-responders (potentially sick flies) based on whether or not they extend their proboscis when water is applied to the leg.20 They are then fed water to satiation and tested with sugar solutions applied to the leg. Sugar sated flies do not extend their proboscis in response to sugar solutions, implying a motivational component exists for this assay much like that observed in appetitive olfactory conditioning.15,18,19
The PER can be associatively conditioned in flies21 using an appetitive paradigm established in bees (reviewed in ref. 22). Flies that have been prepared and immobilized, as above for PER, receive five spaced presentations of odor paired with a sucrose reward administered to the labellum of the proboscis. Flies are tested for memory by exposing them to the CS+ odor or CS− odor. They extend their proboscis in response to the CS+ odor, demonstrating authentic associative conditioning. Separate flies are trained in a reciprocal manner. Surprisingly, the memory only lasts a few minutes, and is absent 1 hour after the last training trial. Drosophila can be conditioned to inhibit PER if the bitter tastant quinine is presented in the sugar23 and can also be trained to withdraw their proboscis in response to electric shock.24
Visual learning in the flight simulator
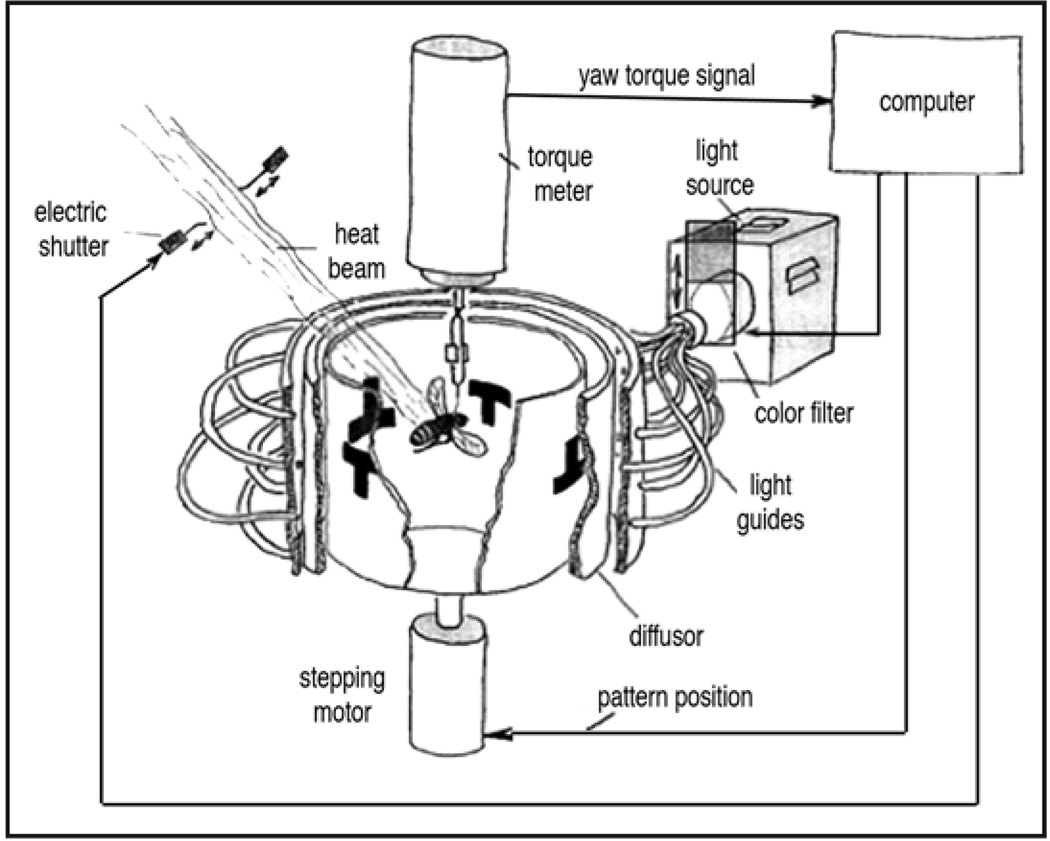
Flies can discriminate between different shapes and colors and these visual parameters can be utilized in a learning paradigm based on a flight simulator (Fig. 3). A fly, tethered on a copper wire glued between the head and thorax, is hung in the middle of a cylindrical arena where it can fly and generate horizontal yaw torque, but it cannot pitch or roll. The wire is connected to a meter that measures the direction and force of the exerted yaw torque and a computer translates that information into a precise rotation of the arena in the opposite direction. This tuning allows the fly to control its position relative to the visual panorama; if it torques left, the drum rotates right (or vice versa). Colored card, banks of light-emitting diodes or computer screens can be used to represent simple images/patterns and different colors and/or patterns.
Figure 3.
The flight simulator. A single fly, tethered on a copper wire, is hung in the middle of a cylindrical arena. The fly controls its position relative to the panorama, in this case consisting of a pattern of upright and inverted “T” shapes. The fly is trained, using either an operant or classical paradigm, to avoid one pattern (e.g., upright T), by punishing that direction of flight with a heat beam. Figure taken from reference 27 with the permission of Martin Heisenberg.
Two different modes of operation are routinely used in the flight arena. In ‘flight simulator’ or ‘closed-loop’ mode the fly controls its position relative to the arena. This provides the fly operant control of learning and allows it to selectively attend to particular landmarks in the arena. In ‘open-loop’ mode the experimenter controls the position of the panorama with respect to the direction of the fly, allowing classical conditioning.
Flies can be trained to avoid a particular landmark (a pattern e.g., an upright ‘T’) by punishing the fly when it approaches that pattern, either with heat25 or with a plume of an aversive odor, such as benzaldehyde.26 A different ‘safe’ unpunished landmark (e.g., an upside down ‘T’) is also presented. During the memory test phase, the fly is given several minutes to display preference for one of the visual cues and trained flies selectively avoid the conditioned landmark. Memory in these two paradigms lasts for ~20 minutes27 but can be lengthened by repetitive training.28 Context complexity can be added to the visual paradigm for example, by changing the color of the background illumination between training and testing.29
Motor learning in the flight simulator
The flight simulator can also be used without visual cues to assess motor learning.30 If the arena is evenly illuminated, the fly can be conditioned to avoid turning (yawing) right or left, by punishing it with heat when it torques in that particular direction. The flies learn to avoid the heat by directing their yaw torque in the safe range.
Spatial orientation memory
Named after the 14th century French philosopher Jean Buridan, “Buridan’s ass” describes a conflict of free-will where a donkey that is placed equidistant between two equally delectable piles of hay starves to death, because it is unable to choose one over the other.
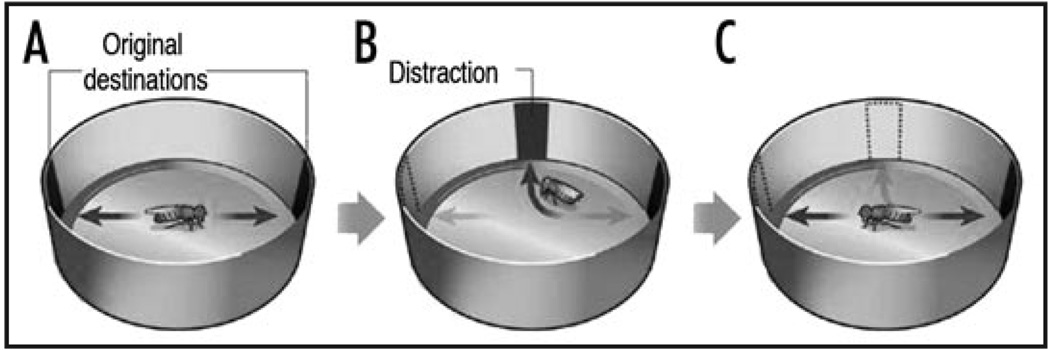
In the ‘Buridan’s paradigm’, a single fly with clipped wings is placed on a platform in the middle of a circular arena, separated from white featureless walls by a water moat. If two dark vertical stripes are introduced on opposing faces of the arena the fly walks continuously back and forth between the stripes (Fig. 4).31 Strauss and Pichler32 observed that a fly walking between two vertical stripes continues to walk for 2–8 seconds even when the previously attractive landmark is removed. In a recent paper, Neuser et al.33 developed new variations on the Buridan’s paradigm to investigate orientation memory. In their assay, when the fly crosses the midline of the arena, the stripes disappear and another stripe appears at a position that is perpendicular to the original orientation of the fly. In most cases, the fly turns towards the new stripe. However, as soon as the fly faces the new stripe, the new stripe is also made to disappear. Interestingly, Neuser et al.33 found that when the perpendicular stripe disappears, most flies reorient to approach the initial, but now invisible, target, indicating that the fly can remember the location of the original target stripe. Memory in this ‘detour paradigm’ lasts for a few seconds, consistent with it representing something akin to working memory.
Figure 4.
Buridan’s paradigm and spatial orientation memory. An individual fly with clipped wings is placed on a circular platform surrounded by a moat, in a large white arena. In the regular Buridan’s set-up, two black vertical stripes are presented on opposite sides of the arena (A) and the fly paces back and forth between the stripes. (B) To assay working memory one stripe disappears and is replaced by a distractor stripe. (C) Following the disappearance of the distractor, flies continue along their original path. Figure taken from reference 60 with the permission of Ronald L. Davis.
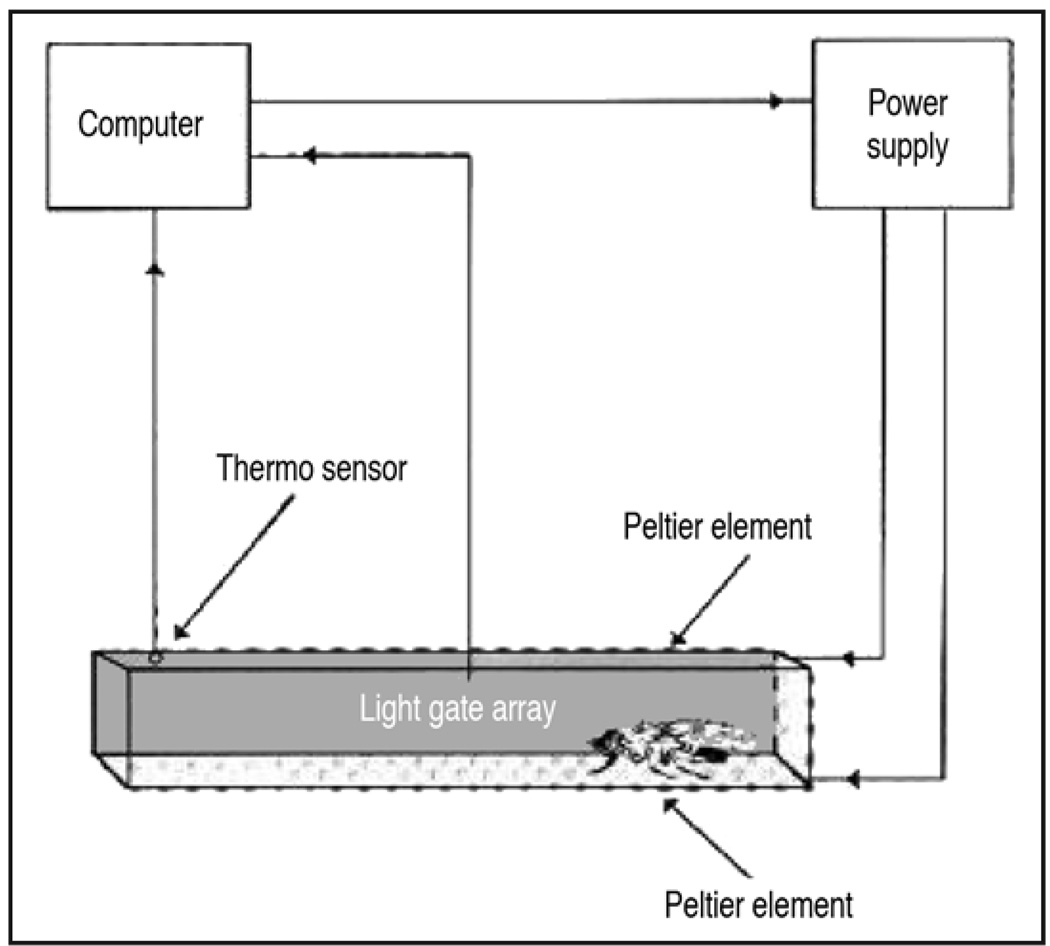
Heat box
The heat-box (Fig. 5) was also designed to assess spatial memory.34,35 An individual freely walking fly is trained for 4 minutes to avoid one half of a long dark chamber. Using Peltier elements to quickly heat the chamber, the fly is punished with temperature above 33°C when it enters one half of the chamber and not when it occupies the other half. After training the heat is turned off, and the position of the fly is tracked for 3 minutes to determine the amount of time the fly spends in each side of the chamber. A performance index is calculated as the time in ‘safe’ side subtracted from the time in punished side, divided by the total time. Since the flies are trained and tested in darkness, the memory formed is believed to result from the integration of tactile information and path length/body orientation.
Figure 5.
The heat-box assay. An individual fly is placed into a small dark chamber, and its position within is monitored. The chamber is split into two virtual halves by an infra-red light gate that is also sensitive to direction. During training, when the fly enters the side of the chamber that the investigator has chosen as the ‘punished’ side, breaking the light gate, peltier elements heat the chamber. When the fly crosses back into the ‘safe’ side the heat switches off. Figure taken from reference 35 with the permission of Martin Heisenberg.
‘Winner or loser mentality’
Aggressive behavior in fruit flies was observed as early as 1915,36 and has been formally studied since the 1960’s.37 Both male and female flies fight, although the things they fight over and their fighting styles differ. The aggression assay is simple to set up and reveals remarkable complexity in the behavioral repertoire. A typical “fighting arena” consists of a small food cup placed in the center of a round covered Petri-dish. Aggression is induced between pairs of male or female flies by adding a resource (e.g., food, yeast paste) to fight over. Males will also fight for a live, or decapitated, female fly. Behavior is video-recorded while the flies are in the arena (2-minutes to >1 hour), and aggressive encounters are scored by observation. Three behaviors can be scored unambiguously as aggressive; wing threat (both wings raised to a 45° angle), charging, and boxing (rearing up on the hind legs and striking the opponent).37 Other aggressive behaviors include fencing, lunging, holding and tussling.6 Males exhibit all of these behaviors, and fight frequently. Female flies fight less often, nand ever engage in high-intensity aggressive behaviors such as boxing or tussling, but they employ additional female-specific moves such as head-butting and shoving.38
Similar to the ‘learned helplessness’ observed in courtship conditioning (covered in the following section), male flies adapt their fighting strategy depending on whether they emerge as the ‘winner’ or ‘loser’ of their first fight.39 In general, during the first bout, the male who initiated and who fought with the greatest intensity from the start is ultimately the victor. More specifically, Yurkovic et al.39 found that over a series of encounters between the same pair of flies, the winner lunged more and retreated less, whereas the loser did the opposite. When the combatants were given time to recover before being put back into the fighting arena, an interesting pattern emerged that is suggestive of the development of a hierarchical structure. Opponents who previously fought each other spent less time fighting when re-paired than flies that were paired with unfamiliar opponents, suggesting that they remembered their opponents, and had no desire to reinitiate their previous brawl. Perhaps even more interesting, flies who lost their first fight never won their second fight, when paired with either their previous opponent, an unfamiliar winner, or a naïve opponent. In their second fight, winners always defeated losers, but won or lost with equal frequency against naïve opponents. Loser flies were sometimes able to win fights against other losers, but never against a former winner. Female flies do not develop a winner-loser hierarchy.
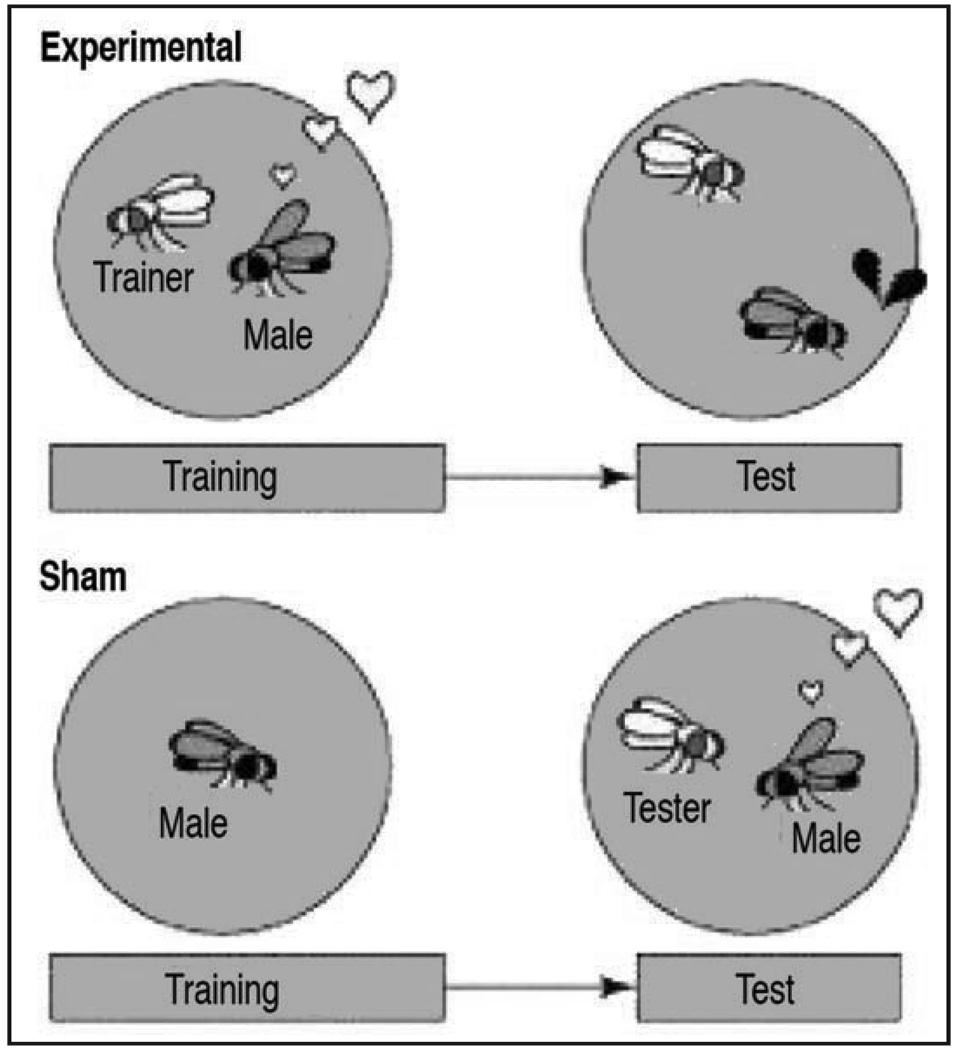
Courtship conditioning
Even the most inexperienced fly pusher can tell you that one thing that fruit flies do very well is mate—in any given bottle of flies there are always a few pairs of flies in flagrante delicto. So it may come as a surprise that the seemingly irrepressible enthusiasm with which male fruit flies pursue females can be modified by extended exposure to a previously mated female. Learned suppression of the male courtship response is known as courtship conditioning (Fig. 6), and it has many of the same properties as the other forms of learning discussed here.40–42 When a sexually naïve male is placed with a recently mated female in a small chamber, his courtship vigor rapidly declines with continued exposure to (and rejection by) the female. If the trained male is then paired with a virgin female, normally an object of vigorous courtship, he courts her far less than a control male that was not trained. Male courtship activity is quantified by the courtship index (CI), which is the fraction of time that a male spends courting up to (but not including) copulation. Procedurally, learned suppression is measured as a ratio between the courtship indices (CIs) of two trained flies of the same genotype, where one has been trained with a mated female, and the other is “sham” trained by housing it in an empty courtship chamber for the same amount of time as its counterpart. If a particular genotype can learn, the CI(trained)/CI(sham) ratio should be much less than 1; a CI(trained)/CI(sham) ratio close to 1 indicates a lack of learning, or memory, depending on the amount of time elapsed. Similar to other forms of associative learning, courtship suppression can last anywhere from hours to days depending on the nature of the training protocol. An hour of training with a mated female results in a short-term suppression of courtship that lasts for approximately 3 hours.40 Long-term courtship suppression memories, which last for days, are formed by pairing the male with a mated female for 5 hours continuously or 3 spaced 1-hour sessions with isolation of the male for 30–60 minutes between each session.43
Figure 6.
Courtship conditioning. Male flies are placed into a chamber with a ‘trainer’ fly (mated female, Experimental), or alone (Sham) for a defined period of time (1 to several hours depending on the desired length of the memory). Courtship index is the comparison of courtship activity between the first and last period of training in experimental males. Experimental and sham males are then given a tester fly (virgin female), and courtship index is compared between experimental and sham males. Wild-type males suppress their courtship activity over the period of training and maintain the suppression during the test. Figure modified from reference 4 with permission of Leslie C. Griffith.
While the precise nature of the learning cues is not known, it is believed that the male associates the pheromones the mated female emits (CS) with the inability to mate (US) and thus learns not to court when presented with a second virgin female. This training does not cause general courtship suppression, because males trained with mated females do not reduce their courtship towards immature males.41 More recently it was shown that if males are trained with live, decapitated females (to prevent copulation) of a specific sexual maturity—immature virgin, mature virgin or mature mated female—in subsequent testing they will show the strongest courtship suppression towards the type of female they were trained against.42 Female flies of differing sexual maturity have markedly differing cuticular hydrocarbon profiles,42,44 so it is likely that male flies use their sense of smell to determine the sexual maturity of a female.45–47
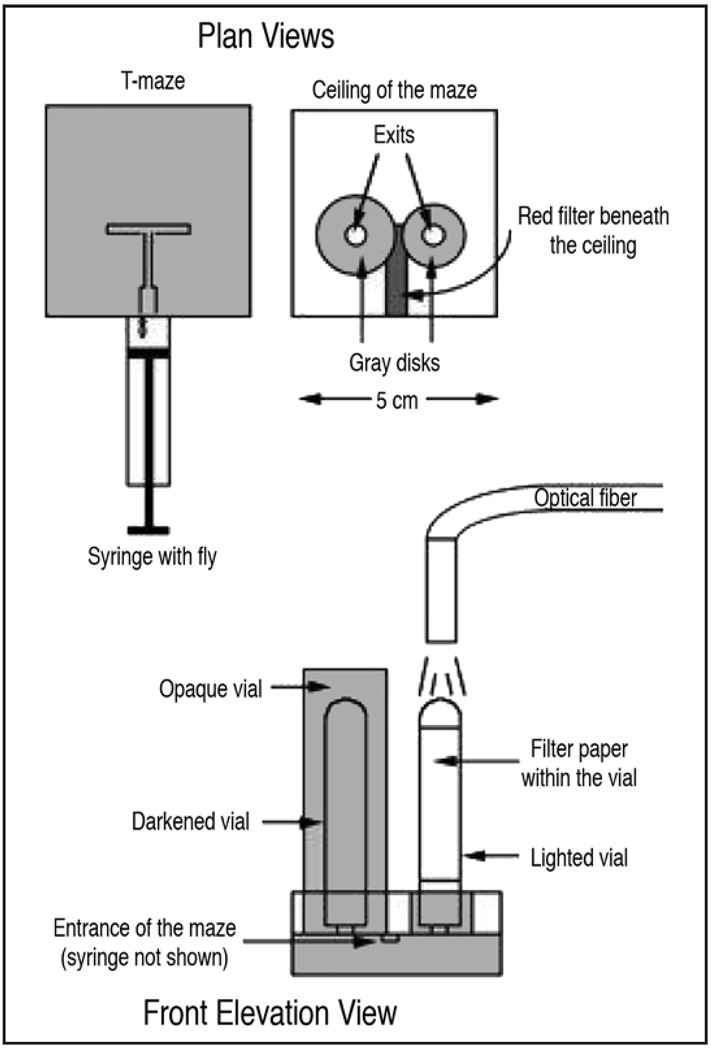
Aversive phototaxic suppression
Adult fruit flies are strongly phototaxic, preferring to approach light rather than dark. However, flies suppress their attraction to light if the light is presented with aversive quinine and/or humidity.48,49 The assay involves allowing a fly to choose between a tube that is lit and a tube that is dark (Fig. 7). Those who choose the light encounter a quinine soaked filter paper. Light/Dark sides are alternated to avoid the potential confound of flies following a previously laid odor trace, or exhibiting an innate left or right turning bias. Quinine reinforcement is present at all times, and flies are given 16 training/testing trials, which are broken into 4 blocks of 4 trials for analysis. A ‘learning score’ is calculated for each block of 4 trials as the number, or percentage, of photonegative choices. Learning can be observed within an experimental group by comparing scores between the first and last block of 4 choices, or between groups by comparing scores in the last block.48 Learning does not improve with more training trials. It is noteworthy that an experienced fly makes a roughly 50:50 choice between dark and light and very rarely goes to the dark or ‘correct’ tube in all 4 of the sessions of the final testing block.
Figure 7.
Aversive phototaxic suppression. Schematic showing plan and elevation views. An individual fly is placed into the maze by syringe, and given 16 trials to choose between the darkened vial, or the lit vial containing quinine. Figure taken from reference 49 with permission from Eric Le Bourg.
Learning in Larvae
Olfactory conditioning in larvae
Drosophila larvae possess 21 pairs of olfactory sensory neurons, 80 pairs of gustatory sensory neurons and only 12 neurons for vision.3 The adult fly in comparison has approximately 1300, 650 and 6000, respectively.50,51 The reduction in the complexity of the larval nervous system has recently inspired some investigators to revisit pursuit of an understanding of behavioral plasticity in the larvae rather than the adult fly.
The first description of larval olfactory learning52 was also the brainchild of Chip Quinn and was clearly inspired by adult fly learning.8 80 to 100 larvae were placed in a Petri dish containing an electrically conductive agarose gel. Larvae were exposed to an odor (CS+) for 30 seconds in the presence of electric shock (voltage was applied across the plate with electrodes on either side). Following 90-seconds of fresh air, the larvae were exposed to another odor (CS−) without shock. As with adult learning,8 the training was repeated three times. To test memory, 30–40 larvae were transferred to the center of a new agarose plate with the CS+ and CS− odors spotted on filter papers at opposite sides. Memory was observed as a preferential movement away from the CS+. A different population of the same genotype of larvae were trained with the CS+ and CS− odors reversed and, as in the adult assay, the final learning index score was the average of the two reciprocal experiments. The assay was validated by the demonstration that dunce and turnip mutants that were defective in olfactory learning as adults also exhibited a learning defect as larvae.52
Olfactory conditioning with gustatory reinforcement in larvae
Larvae can also be trained to associate odors with gustatory reinforcement 53 In the first published report of appetitive conditioning, individual larvae were trained for 1 minute to associate Odor A with a positive reinforcer (fructose) added to an agarose plate and were then transferred to a second plate and trained to associate a second odor, Odor B, with a negative reinforcer (quinine or salt) for 1 minute. This training cycle was repeated 10 times. Separate larvae were reciprocally trained to control for odor bias. During testing, larvae were placed in the center of an agarose plate without reinforcers, with odors A and B on opposite sides of the plate. The position of the larva on the plate was noted every 20 or 60 seconds for 5 minutes. Trials from many individual larvae were grouped for analysis. Memory was measured by calculating the proportion of larvae in the positively reinforced side at each time point (# larvae in Odor A - # in Odor B/Total # larvae), or by defining overall odor preference for each larvae (# of counts in Odor A - # counts in Odor B/Total # of counts).53
It was subsequently found that larvae could learn as efficiently if odor was paired with positive reinforcement alone.54–56 However, memory formed with punishment alone can only be observed if the negative reinforcer is present on the test plate.54 Additionally, larvae can be trained and tested en masse in groups of 30.57 Training in groups is similar to that in individuals. During testing, larvae are given 3 minutes to disperse between the two odors used during training, and the number of larvae are counted on each side of the plate after 3 minutes and divided by the total number of larvae.
Visual learning in larvae
Larvae, unlike adult flies, have an innate preference for darkness. It is possible to train individual larvae to favor either light or dark by associating light with a sugar reward and dark with quinine/salt negative reinforcement, and vice versa. Light/dark preference is tested by placing individual larvae on a plate with two lighted and two shaded quadrants and recording the position of the larva every 10 seconds for 5 minutes.58
Concluding Remarks
Fortunately, it is no longer necessary to propose that all animals, other than humans, are automata. However, in the minds of many established neuroscientists, the stigma remains for invertebrates and the notion that they provide a useful model system to understand cognition is not universally appreciated. In this review, we hope to have at least conveyed the message that fruit fly behavior is complex and plastic.
Acknowledgements
Research in the Waddell lab is supported by NIH grants R01 MH069883, R01 MH081982 and R01 GM085788.
References
- 1.Keene AC, Waddell S. Drosophila olfactory memory: single genes to complex neural circuits. Nat Rev Neurosci. 2007;8:341–354. doi: 10.1038/nrn2098. [DOI] [PubMed] [Google Scholar]
- 2.Heisenberg M, Wolf R, Brembs B. Flexibility in a single behavioral variable of Drosophila. Learn Mem. 2001;8:1–10. doi: 10.1101/lm.8.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Gerber B, Stocker RF. The Drosophila larva as a model for studying chemosensation and chemosensory learning: a review. Chem Senses. 2007;32:65–89. doi: 10.1093/chemse/bjl030. [DOI] [PubMed] [Google Scholar]
- 4.Mehren JE, Ejima A, Griffith LC. Unconventional sex: fresh approaches to courtship learning. Curr Opin Neurobiol. 2004;14:745–750. doi: 10.1016/j.conb.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Brembs B. Operant conditioning in invertebrates. Curr Opin Neurobiol. 2003;13:710–717. doi: 10.1016/j.conb.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Chen S, Lee AY, Bowens NM, Huber R, Kravitz EA. Fighting fruit flies: a model system for the study of aggression. Proc Natl Acad Sci USA. 2002;99:5664–5668. doi: 10.1073/pnas.082102599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benzer S. Behavioral mutants of Drosophila isolated by countercurrent distribution. Proc Natl Acad Sci USA. 1967;58:1112–1119. doi: 10.1073/pnas.58.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quinn WG, Harris WA, Benzer S. Conditioned behavior in Drosophila melanogaster. Proc Natl Acad Sci USA. 1974;71:708–712. doi: 10.1073/pnas.71.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dudai Y, Jan YN, Byers D, Quinn WG, Benzer S. dunce, a mutant of Drosophila deficient in learning. Proc Natl Acad Sci USA. 1976;73:1684–1688. doi: 10.1073/pnas.73.5.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Livingstone MS, Sziber PP, Quinn WG. Loss of calcium/calmodulin responsiveness in adenylate cyclase of rutabaga, a Drosophila learning mutant. Cell. 1984;37:205–215. doi: 10.1016/0092-8674(84)90316-7. [DOI] [PubMed] [Google Scholar]
- 11.Quinn WG, Sziber PP, Booker R. The Drosophila memory mutant amnesiac. Nature. 1979;277:212–214. doi: 10.1038/277212a0. [DOI] [PubMed] [Google Scholar]
- 12.Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol [A] 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- 13.Jellies JA. (Master thesis) Normal: Illinois State University; 1981. Associative olfactory conditioning in Drosophila melanogaster and memory retention through metamorphosis. [Google Scholar]
- 14.Tully T, Preat T, Boynton SC, Del VM. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79:35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 15.Tempel BL, Bonini N, Dawson DR, Quinn WG. Reward learning in normal and mutant Drosophila. Proc Natl Acad Sci USA. 1983;80:1482–1486. doi: 10.1073/pnas.80.5.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci. 2003;23:10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keene AC, Krashes MJ, Leung B, Bernard JA, Waddell S. Drosophila dorsal paired medial neurons provide a general mechanism for memory consolidation. Curr Biol. 2006;16:1524–1530. doi: 10.1016/j.cub.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 18.Krashes MJ, Waddell S. Rapid consolidation to a radish and protein synthesis-dependent long-term memory after single-session appetitive olfactory conditioning in Drosophila. J Neurosci. 2008;28:3103–3113. doi: 10.1523/JNEUROSCI.5333-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dethier VG. The Hungry Fly—A physiological study of the behavior associated with feeding. Cambridge: Harvard University Press; 1976. [Google Scholar]
- 20.Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Chabaud MA, Devaud JM, Pham-Delegue MH, Preat T, Kaiser L. Olfactory conditioning of proboscis activity in Drosophila melanogaster. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006 doi: 10.1007/s00359-006-0160-3. [DOI] [PubMed] [Google Scholar]
- 22.Menzel R. Searching for the memory trace in a mini-brain, the honeybee. Learn Mem. 2001;8:53–62. doi: 10.1101/lm.38801. [DOI] [PubMed] [Google Scholar]
- 23.DeJianne D, McGuire TR, Pruzan-Hotchkiss A. Conditioned suppression of proboscis extension in Drosophila melanogaster. J Comp Psychol. 1985;99:74–80. [PubMed] [Google Scholar]
- 24.Medioni J, Vaysse G. Conditional suppression of a reflex in Drosophila melanogaster: acquisition and extinction. C R Seances Soc Biol Fil. 1975;169:1386–1391. [PubMed] [Google Scholar]
- 25.Dill M, Wolf R, Heisenberg M. Visual pattern recognition in Drosophila involves retinotopic matching. Nature. 1993;365:751–753. doi: 10.1038/365751a0. [DOI] [PubMed] [Google Scholar]
- 26.Guo A, Gotz KG. Association of visual objects and olfactory cues in Drosophila. Learn Mem. 1997;4:192–204. doi: 10.1101/lm.4.2.192. [DOI] [PubMed] [Google Scholar]
- 27.Brembs B, Heisenberg M. The operant and the classical in conditioned orientation of Drosophila melanogaster at the flight simulator. Learn Mem. 2000;7:104–115. doi: 10.1101/lm.7.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xia S, Liu L, Feng C, Guo A. Memory consolidation in Drosophila operant visual learning. Learn Mem. 1997;4:205–218. doi: 10.1101/lm.4.2.205. [DOI] [PubMed] [Google Scholar]
- 29.Liu L, Wolf R, Ernst R, Heisenberg M. Context generalization in Drosophila visual learning requires the mushroom bodies. Nature. 1999;400:753–756. doi: 10.1038/23456. [DOI] [PubMed] [Google Scholar]
- 30.Wolf R, Wittig T, Liu L, Wustmann G, Eyding D, Heisenberg M. Drosophila mushroom bodies are dispensable for visual, tactile and motor learning. Learn Mem. 1998;5:166–178. [PMC free article] [PubMed] [Google Scholar]
- 31.Bülthoff H, Götz KG, Herre M. Recurrent inversion of visual orientation in the walking fly, Drosophila melanogaster. J Comp Physiol [A] 1982;148:471–481. [Google Scholar]
- 32.Strauss R, Pichler J. Persistence of orientation toward a temporarily invisible landmark in Drosophila melanogaster. J Comp Physiol [A] 1998;182:411–423. doi: 10.1007/s003590050190. [DOI] [PubMed] [Google Scholar]
- 33.Neuser K, Triphan T, Mronz M, Poeck B, Strauss R. Analysis of a spatial orientation memory in Drosophila. Nature. 2008;453:1244–1247. doi: 10.1038/nature07003. [DOI] [PubMed] [Google Scholar]
- 34.Wustmann G, Heisenberg M. Behavioral manipulation of retrieval in a spatial memory task for Drosophila melanogaster. Learn Mem. 1997;4:328–336. doi: 10.1101/lm.4.4.328. [DOI] [PubMed] [Google Scholar]
- 35.Putz G, Heisenberg M. Memories in Drosophila heat-box learning. Learn Mem. 2002;9:349–359. doi: 10.1101/lm.50402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sturtevant AH. Experiments on sex recognition and the problem of sexual selection in Drosophila. J Animal Behav. 1915;5:351–366. [Google Scholar]
- 37.Jacobs ME. Influence of light on mating of Drosophila melanogaster. Ecology. 1960;41:182–188. [Google Scholar]
- 38.Nilsen SP, Chan YB, Huber R, Kravitz EA. Gender-selective patterns of aggressive behavior in Drosophila melanogaster. Proc Natl Acad Sci USA. 2004;101:12342–12347. doi: 10.1073/pnas.0404693101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yurkovic A, Wang O, Basu AC, Kravitz EA. Learning and memory associated with aggression in Drosophila melanogaster. Proc Natl Acad Sci USA. 2006;103:17519–17524. doi: 10.1073/pnas.0608211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siegel RW, Hall JC. Conditioned Responses in Courtship Behavior of Normal and Mutant Drosophila. Proc Natl Acad Sci USA. 1979;76:3430–3434. doi: 10.1073/pnas.76.7.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gailey DA, Jackson FR, Siegel RW. Conditioning mutations in Drosophila melanogaster affect an experience-dependent behavioral modification in courting males. Genetics. 1984;106:613–623. doi: 10.1093/genetics/106.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ejima A, Smith BP, Lucas C, Levine JD, Griffith LC. Sequential learning of pheromonal cues modulates memory consolidation in trainer-specific associative courtship conditioning. Curr Biol. 2005;15:194–206. doi: 10.1016/j.cub.2005.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McBride SM, Giuliani G, Choi C, Krause P, Correale D, Watson K, Baker G, Siwicki KK. Mushroom body ablation impairs short-term memory and long-term memory of courtship conditioning in Drosophila melanogaster. Neuron. 1999;24:967–977. doi: 10.1016/s0896-6273(00)81043-0. [DOI] [PubMed] [Google Scholar]
- 44.Tompkins L, Hall JC. The different effects on courtship of volatile compounds from mated and virgin Drosophila females. Journal of Insect Physiology. 1981;27:17–21. [Google Scholar]
- 45.Kurtovic A, Widmer A, Dickson BJ. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 2007;446:542–546. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- 46.Ejima A, Smith BP, Lucas C, van der Goes van Naters W, Miller CJ, Carlson JR, Levine JD, Griffith LC. Generalization of courtship learning in Drosophila is mediated by cis-vaccenyl acetate. Curr Biol. 2007;17:599–605. doi: 10.1016/j.cub.2007.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Goes van Naters W, Carlson JR. Receptors and neurons for fly odors in Drosophila. Curr Biol. 2007;17:606–612. doi: 10.1016/j.cub.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Le Bourg E, Buecher C. Learned suppression of photopositive tendencies in Drosophila melanogaster. Anim Learn Behav. 2002;30:330–341. doi: 10.3758/bf03195958. [DOI] [PubMed] [Google Scholar]
- 49.Le Bourg E. Humidity as an aversive stimulus in learning in Drosophila melanogaster. Learn Behav. 2005;33:265–276. doi: 10.3758/bf03192856. [DOI] [PubMed] [Google Scholar]
- 50.Stocker RF. The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res. 1994;275:3–26. doi: 10.1007/BF00305372. [DOI] [PubMed] [Google Scholar]
- 51.Stocker RF. Drosophila as a focus in olfactory research: mapping of olfactory sensilla by fine structure, odor specificity, odorant receptor expression and central connectivity. Microsc Res Tech. 2001;55:284–296. doi: 10.1002/jemt.1178. [DOI] [PubMed] [Google Scholar]
- 52.Aceves-Pina EO, Quinn WG. Learning in normal and mutant Drosophila larvae. Science. 1979;206:93–96. doi: 10.1126/science.206.4414.93. [DOI] [PubMed] [Google Scholar]
- 53.Scherer S, Stocker RF, Gerber B. Olfactory learning in individually assayed Drosophila larvae. Learn Mem. 2003;10:217–225. doi: 10.1101/lm.57903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hendel T, Michels B, Neuser K, Schipanski A, Kaun K, Sokolowski MB, Marohn F, Michel R, Heisenberg M, Gerber B. The carrot, not the stick: appetitive rather than aversive gustatory stimuli support associative olfactory learning in individually assayed Drosophila larvae. J Comp Physiol A. 2005;191:265–279. doi: 10.1007/s00359-004-0574-8. [DOI] [PubMed] [Google Scholar]
- 55.Michels B, Diegelmann S, Tanimoto H, Schwenkert I, Buchner E, Gerber B. A role for Synapsin in associative learning: the Drosophila larva as a study case. Learn Mem. 2005;12:224–231. doi: 10.1101/lm.92805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yarali A, Hendel T, Gerber B. Olfactory learning and behaviour are ‘insulated’ against visual processing in larval Drosophila. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006;192:1133–1145. doi: 10.1007/s00359-006-0140-7. [DOI] [PubMed] [Google Scholar]
- 57.Neuser K, Husse J, Stock P, Gerber B. Appetitive olfactory learning in Drosophila larvae: effects of repetition, reward strength, age, gender, assay type and memory span. Anim Behav. 2005;69:891–898. [Google Scholar]
- 58.Gerber B, Scherer S, Neuser K, Michels B, Hendel T, Stocker RF, Heisenberg M. Visual learning in individually assayed Drosophila larvae. J Exp Biol. 2004;207:179–188. doi: 10.1242/jeb.00718. [DOI] [PubMed] [Google Scholar]
- 59.Waddell S, Quinn WG. What can we teach Drosophila? What can they teach us? Trends Genet. 2001;17:719–726. doi: 10.1016/s0168-9525(01)02526-4. [DOI] [PubMed] [Google Scholar]
- 60.Tomchik SM, Davis RL. Behavioural neuroscience: Out of sight, but not out of mind. Nature. 2008;453:1192–1194. doi: 10.1038/4531192a. [DOI] [PubMed] [Google Scholar]