Abstract
The NACHT, LRR and PYD domains containing protein (NALP3) inflammasome is a key regulator of interleukin-1β (IL-1β) secretion. As there is strong evidence for a pro-inflammatory role of IL-1β in rheumatoid arthritis (RA) and in murine models of arthritis, we explored the expression of the different components of the NALP3 inflammasome as well as other nucleotide oligomerization domain (NOD)-like receptors (NLRs) in synovium obtained from patients with RA. The expression of NLRs was also studied in fibroblast lines derived from joint tissue. By immunohistology, NALP3 and apoptosis-associated speck-like protein containing a CARD domain (ASC) were expressed in myeloid and endothelial cells and B cells. T cells expressed ASC but lacked NALP3. In synovial fibroblast lines, NALP3 expression was not detected at the RNA and protein levels and stimulation with known NALP3 agonists failed to induce IL-1β secretion. Interestingly, we were unable to distinguish RA from osteoarthritis synovial samples on the basis of their basal level of RNA expression of known NLR proteins, though RA samples contained higher levels of caspase-1 assayed by enzyme-linked immunsorbent assay. These results indicate that myeloid and endothelial cells are the principal sources of inflammasome-mediated IL-1β production in the synovium, and that synovial fibroblasts are unable to activate caspase-1 because they lack NALP3. The NALP3 inflammasome activity does not account for the difference in level of inflammation between RA and osteoarthritis.
Keywords: caspase-1, inflammasome, interleukin-1β, rheumatoid arthritis, synovitis
Introduction
Clinical and experimental studies point to a key role for interleukin-1β (IL-1β) in the pathophysiology of rheumatoid arthritis (RA) and inhibition of IL-1 reduces signs and symptoms of RA as well as radiological damage. Animal models of RA, such as collagen-induced arthritis and antigen-induced arthritis, also respond to IL-1 inhibition.1,2 Interleukin-1β is produced as an inactive pro-molecule by immune cells such as macrophages, monocytes and dendritic cells; as well as by other cell types such as keratinocytes. The pro-molecule (p35) must be cleaved into active IL-1β (p17), which is then released from the cell. Cleavage of pro-IL-1β is catalysed by the enzyme caspase-1 (also known as IL-1-converting enzyme) and therefore the biological activity of IL-1β is directly dependent on the activity of caspase-1. Recent work established that caspase-1 activation requires the recruitment and dimerization of the enzyme within a molecular platform known as the inflammasome. Briefly, the inflammasome is a cytoplasmic complex formed by the intracellular receptor NACHT, LRR and PYD domains containing protein (NALP), the apoptosis-associated speck-like protein containing a CARD domain (ASC) adapter protein and pro-caspase-1. The NALPs belong to the nucleotide oligomerization domain (NOD)-like receptor (NLR) family of intracellular pattern-recognition receptors that to date includes 22 members, among which NALP1, NALP3 and NALP12 and ICE-protease activating factor are capable of forming functional caspase-1 complexes. The inflammasome links the sensing of pathogen and danger signals to pro-IL-1β processing. The NALP3 inflammasome is the best-known inflammasome, detecting bacterial wall components or the bacteria themselves. In addition, NALP3 can be activated by signals that induce potassium efflux, such as ATP, via its P2X7 receptor.3
The importance of the inflammasomes in human disease is illustrated by the discovery that cryopyrin-associated periodic syndromes are the result of mutations in the NALP3 gene4 and that monosodium urate (MSU) crystals induce inflammation through the NALP3 inflammasome.5 There are scant data on inflammasome expression in RA. Rosengren et al. showed that NALP3 RNA levels were increased in RA synovium and that macrophages differentiated in vitro increased NALP3 expression when stimulated by tumour necrosis factor (TNF).6
We therefore analysed the expression NALP3 and ASC in the synovium as well as examining the capacity of RA synovial fibroblasts to produce active IL-1β. Synovial tissues from patients with RA and patients with osteoarthritis (OA) were also compared for the expression of NLR proteins and their production of IL-1β and caspase-1.
Materials and methods
Tissue samples
Synovial tissues were obtained from nine patients with RA (nine women, mean age 58·6 ± 11·6 years) and 11 patients with OA (five women, six men, mean age 74·6 ± 11·7 years) undergoing joint replacement surgery of the knee or the hip (Department of Orthopaedics, CHUV). Osteoarthritis was diagnosed by clinical and radiological criteria and RA patients fulfilled the American Rheumatism Association revised criteria for RA. All tissues were cut into small pieces and immediately frozen in pre-cooled hexane and stored at −70° until use, or fixed in formol and embedded in paraffin. Ethical committee approval was obtained for these experiments.
Fibroblast-like synoviocyte cultures
Fibroblast-like synoviocyte (FLS) lines were established as described previously.7 Cells were used between the third and seventh passages. Synoviocyte cell cultures or, as positive control, THP-1 cells (2 × 105 cells/well) were incubated in Dulbecco’s modified Eagle’s minimal essential medium or RPMI-1640 medium containing 0·5% fetal calf serum, with or without the following stimuli: lipopolysaccharide (LPS; 10 μg/ml), ATP (5 mm), H2O2 (30 μm), TNF-α (10 ng/ml) and MSU (200 μg/ml). After 24 hr incubation, culture supernatants were harvested, and cells were suspended for 20 min in 200 μl ice-cold lysis buffer [50 mm Tris–HCl pH 7·4, 110 mm NaCl, 10 mm ethylenediaminetetraacetic acid (EDTA), 0·1% nonidet P-40 (NP-40)] containing a protease inhibitor cocktail (Sigma-Aldrich Chemie GmbH, Buchs, Switzerland). The detergent-soluble proteins were separated by centrifugation (14 000 g for 15 min at 4°).
Antibodies and immunohistology
Murine monoclonal antibodies against NALP1 and NALP3 were used at 10 μg/ml final concentration (Alexis Biochemicals, Lausanne, Switzerland; ALX-804-803 and ALX-804-819, respectively). For NALP12 and ASC, rabbit polyclonal antibodies at 1 μg/ml (Abnova GmbH, Heidelberg, Germany and Alexis Biochemicals, ALX-210-905, respectively) were used. Immunohistochemistry was performed on air-dried 5-μm cryostat tissue sections, fixed for 10 min in acetone at 4° before use, using an established protocol.8 For specificity control, we used isotype-matched immunoglobulin gG or pre-immune rabbit serum. Double staining was performed to characterize NALP3 and ASC-expressing cells. Antibodies against CD3, CD31, CD68, CD20 and myeloperoxidase (MPO) (all from Sigma-Aldrich, Buchs, Switzerland) were detected, as described above, using Vector VIP (Reactolab, Servion, Switzerland) as substrate (red staining). The NLR or ASC staining was revealed, as described above, using Vector SG (Reactolab) substrate (grey staining). Immunohistochemistry-positive staining was evaluated using a microscope (Olympus, Mont-sur-Lausanne, Switzerland) coupled to a colour video camera (Intas, Gottingen, Germany). Image analysis was performed using the Nuance analysis software (Intas).
Preparation of tissue extracts
Synovial tissues were homogenized in protein extraction buffer (50 mm Tris–HCl pH 7·4, 110 mm NaCl, 10 mm EDTA, 0·1% NP-40, cocktail protease inhibitor (Sigma)], using the TissueLyser system (Qiagen, Basel, Switzerland). The homogenates were centrifuged at 14 000 g for 15 min at 4° and the supernatants were stored at −80°.
Caspase-1 and IL-1β enzyme-linked immunosorbent assays
Tissue extracts were tested by enzyme-linked immunosorbent assay (ELISA) for IL-1β (Bioscience, San Diego, CA) and caspase-1 (BMS250, Bender MedSystems GmbH Vienna, Austria) levels, according to the manufacturer’s instructions. These IL-1β and caspase-1 ELISA do not discriminate between the pro-forms or active forms of IL-1β and caspase-1, respectively.
Western blotting
Tissue lysates were subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis and transferred electrophoretically to nitrocellulose membranes. Membranes were blocked using 5% bovine serum albumin in phosphate-buffered saline for 1 hr at 25°. The blots were then incubated overnight at 4° with anti-NALP1, anti-NALP3, anti-NALP12 or anti-ASC antibodies in phosphate-buffered saline containing 0·1% Tween-20, followed by horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit immunoglobulin G (2 hr at 25°) and detected by Uptilight HRP Blot (Interchim, Montlucon, France).
Reverse transcription–polymerase chain reaction
About 200–300 mg of tissues from OA and RA synovial membranes or 106 cells (FLS or THP-1) were homogenized in 1 ml Trizol reagent (Invitrogen, Basel, Switzerland) and total RNA extractions were performed. RNA (1 μg) was reverse transcribed and amplified. The primers used for inflammasome components and conditions have been published elsewhere.9 The glyceraldehyde 3-phosphate dehydrogenase primers were 5′-tttgacgctggggctgg-3′ and 5′-ttactccttggaggccatg-3′.
Statistical analysis
The statistical analyses were performed using prism (GraphPad Prism software, version 4, La Jolla, CA, USA).
Results
Cellular expression of NALP3 and ASC in synovium
We localized the cellular expression of NALP3 and ASC in RA and OA synovium by immunohistology. NALP3 was widely expressed in the lining and sub-lining areas (Fig. 1a). Double labelling studies were performed and showed that NALP3 was expressed by a proportion of CD31+ endothelial cells, CD68+ cells, CD20+ B cells and almost all MPO-positive neutrophils, but was not found in CD3+ T cells (Fig. 1b). As for ASC, it was also abundantly detected (Fig. 2a) in T and B cells, macrophages, neutrophils and endothelial cells (Fig. 2b). Taken together, these results indicate that in RA and OA synovial tissue, many different cell types express NALP3 and ASC, but T cells did not express NALP3.
Figure 1.
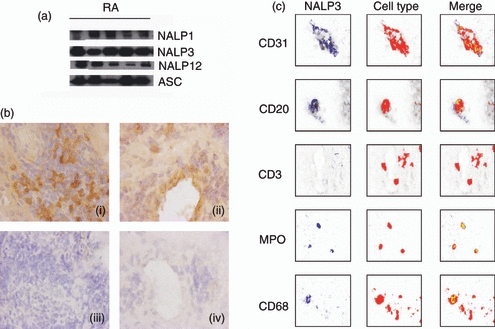
Inflammasome protein components detection in human rheumatoid arthritis (RA) synovial tissue. (a) Synovial tissues collected from RA patients were homogenized and subjected to Western blot analysis, with antibodies against NACHT, LRR and PYD domains containing protein 3 (NALP3) or apoptosis-associated speck-like protein containing a CARD domain (ASC) as indicated. (b) NALP3 immunohistochemical analysis of RA synovial membrane. Left panels (i, iii) show the synovial lining layer area, whereas endothelium of blood vessels in the sublining is shown in right panels (ii, iv). Upper panels (i, ii) show tissue sections incubated with anti-NALP3 antibodies. Lower panels (iii, iv) show negative control section incubated with isotype-matched antibodies. Original magnification × 100. (c) Characterization of NALP3-expressing cells within the RA synovial membrane. Staining for CD1 (endothelial cells), CD20 (B cells), CD3 (T cells), myeloperoxidase (MPO; neutrophils) and CD68 (macrophages and fibroblasts) is detected in red. NALP3 staining is shown in blue. Double-staining analysis (merge) demonstrated NALP3 expression in a subset of endothelial cells, B cells and macrophages, in almost all neutrophils, but in none of the T cells.
Figure 2.

Apoptosis-associated speck-like protein containing a CARD domain (ASC) immunohistochemistry in human rheumatoid arthritis (RA) synovial tissue. (a) Left panels (i, iii) show synovial lining layer area, whereas endothelium of blood vessels in the sublining is shown in right panels (ii, iv). Upper panels (i, ii) show tissue sections incubated with anti-ASC antibodies. Lower panels (iii, iv) show negative control section incubated with preimmune serum. Original magnification × 100. (b) Characterization of ASC-expressing cells within the RA synovial membrane. Staining for CD31 (endothelial cells), CD20 (B cells), CD3 (T cells), myeloperoxidase (MPO; neutrophils) and CD68 (macrophages and fibroblasts) is detected in red. ASC staining appears in blue. Double-staining analysis (merge) demonstrated ASC expression in all the cell types analysed.
Expression of NLRs and inflammasome-associated genes in the synovium
The expression of messenger RNAs (mRNAs) encoding the different NLRs, ASC as well as caspase-1, caspase-5 was examined by reverse transcription–polymerase chain reaction (RT-PCR). NALP1, NALP3, NALP6, NALP10, NALP12 and NALP14 were readily detected in both RA and OA synovium (Table 1), whereas no expression of NALP5 and NALP13 was found in any of the samples analysed. Expression of the other NALPs (2, 4, 7, 8, 9, 11) was not ubiquitous, and was positive in a proportion of the samples analysed. Both caspase-1 and caspase-5 were expressed. Western blots confirmed the protein expression of ASC and NALP1, NALP3 and NALP12 in the synovium. (Fig. 1).
Table 1.
Expression of inflammasome components examined by reverse transcription–polymerase chain reaction in arthritic synovium from osteoarthritis (OA) and rheumatoid arthritis (RA) patients
| OA | RA | OA | RA | ||
|---|---|---|---|---|---|
| NALP1 | 11/11 | 8/9 | NALP10 | 10/10 | 7/9 |
| NALP2 | 3/11 | 5/9 | NALP11 | 3/10 | 5/9 |
| NALP3 | 11/11 | 9/9 | NALP12 | 8/11 | 7/9 |
| NALP4 | 4/11 | 5/9 | NALP13 | 0/11 | 0/9 |
| NALP5 | 0/11 | 0/9 | NALP14 | 9/10 | 8/9 |
| NALP6 | 9/11 | 9/9 | ASC | 11/11 | 9/9 |
| NALP7 | 3/10 | 3/9 | Caspase-1 | 8/11 | 7/9 |
| NALP8 | 4/11 | 5/9 | Caspase-5 | 7/9 | 8/9 |
| NALP9 | 1/10 | 1/9 | Pro-IL-18 | 10/10 | 9/9 |
| Pro-IL-1β | 7/11 | 6/9 |
Expression of NACHT, LRR and PYD domains containing proteins (NALPs) 1–14, apoptosis-associated speck-like protein containing a CARD domain (ASC), caspase 1 and caspase 5 messenger RNA in synovial tissue from patients with RA and OA undergoing joint replacement surgery, as determined by reverse transcription–polymerase chain reaction. Before this, normalization of the samples was performed with glyceraldehyde 3-phosphate dehydrogenase to control for cellularity. Data are presented as the number of positive expressing tissues over the total number of biopsies analysed (at least nine patients per group).
Expression and function of inflammasome related proteins in synovial fibroblasts
In macrophages and keratinocytes, IL-1β processing is dependent on the inflammasome. As fibroblasts comprise a major resident cell population in the synovium, they may play a part in the production of inflammatory cytokines from the results described above. We first assessed the presence of the molecular components of the inflammasome by RT-PCR. The FLS from RA patients (n = 3) were cultured in the presence or absence of crude LPS, a known activator of the NALP3 inflammasome. We found expression of NALPs 1, 2, 3, 8, 10, 12 and 14 as well as of ASC, caspase-1 and caspase-5 in both unstimulated and LPS-stimulated cells (Fig. 3a). Under the same conditions, NALPs 4, 5, 6, 9, 11 and 13 were not detected and a variable expression of NALP7 and NALP8 was observed. Expression of ASC was confirmed by Western blot of unstimulated and LPS-stimulated FLS (Fig. 3b) as well as by immunohistochemistry (Fig. 3c). Although NALP3 mRNA was readily detectable in FLS, no NALP3 protein could be demonstrated by Western blot or immunohistochemistry (Fig. 3b,c).
Figure 3.

Expression of inflammasome components in rheumatoid arthritis (RA) synovial fibroblasts. (a) Expression of NACHT, LRR and PYD domains containing proteins (NALPs) 1–14, apoptosis-associated speck-like protein containing a CARD domain (ASC), caspase 1 and caspase 5 messenger RNA assessed by reverse transcription–polymerase chain reaction and two of their substrates interleukin-1β (IL-1β) and IL-18 in RA fibroblast-like synoviocytes (FLS) stimulated with lipopolysaccharide (LPS; 10 μg/ml) for 24 hr (lane 1) or unstimulated (lane 2). Results are representative data obtained with cells isolated from one RA patients out of the three tested. (b) Expression of ASC and NALP3 by Western blot. The RA FLS cellular extracts, stimulated with LPS (10 μg/ml) for 24 hr (lane 1) or unstimulated (lane 2) were analysed by Western blot with anti-ASC or anti-NALP3 antibodies. (c) Expression of ASC and NALP3 by immunohistochemistry. Unstimulated RA FLS were stained with anti-ASC or anti-NALP3 antibodies. A negative control section (control) incubated with pre-immune serum is shown at the bottom. Original magnification × 400.
Activated human synoviocytes are unable to process and secrete IL-1β
We investigated if FLS could process and secrete IL-1β when activated by stimuli that are known to induce IL-1β secretion in macrophages. Interleukin-1β levels were measured in cell lysates and in supernatants. Intracellular levels of IL-1β increased in response to the different stimuli, except for ATP and H2O2 (Table 2). However, this was not paralleled by secretion of IL-1β into the culture supernatant, as no IL-1β was detected by ELISA (detection limit 2 pg/ml) or by Western blotting (results not shown). Similarly, intracellular levels of caspase-1 were elevated when FLS were stimulated, but secreted caspase-1 was not detected in the supernatants. By contrast, stimulation of monocytic THP-1 cells by LPS or MSU led to secretion of large amounts of IL-1β in the culture medium (unstimulated cells 100 pg/ml, LPS stimulation 5730 pg/ml, MSU stimulation 1710 pg/ml, in one representative experiment). Finally, under the same conditions, secretion of IL-6 was induced by stimulating FLS, indicating a functional cellular response and ruling out a general toxic effect on secretory pathways.
Table 2.
Modulation of interleukin-1β (IL-1β) and caspase-1 by inflammasome activators in rheumatoid arthritis (RA) and osteoarthritis (OA) synovial fibroblasts
| IL-6 (ng/105 cells) |
IL-1β (pg/105 cells) |
Caspase-1 (pg/105 cells) |
||||
|---|---|---|---|---|---|---|
| Stimulation | N | Extracellular | Intracellular | Extracellular | Intracellular | Extracellular |
| Control | 3 | ND ND | ND 1·8 ± 1·8 | ND ND | 83 ± 51 | ND |
| LPS 10 μg/ml | 3 | 20 ± 5 1·3 ± 0·5 | 6 ± 6 1·8 ± 1·8 | ND ND | 81 ± 21 | ND |
| MSU 200 μg/ml | 3 | 6 ± 5 0·1 ± 0·1 | 5 ± 5 5 ± 1·4 | ND ND | Not measured | Not measured |
| ATP 5 mm | 3 | ND 0·1 ± 0·1 | ND 6·2 ± 2·6 | ND ND | 196 ± 14 | ND |
| LPS 10 μg/ml + ATP 5 mm | 3 | 6 ± 1 6·2 ± 1·2 | ND 11·5 ± 7·8 | ND ND | 326 ± 255 | ND |
| LPS 10 μg/ml + MSU 200 μg/ml | 3 | 6 ± 2 3·4 ± 1·1 | 32 ± 12 4·4 ± 0·7 | ND ND | 193 ± 14 | ND |
| LPS 10 μg/ml + TNF 10 ng/ml | 2 | 59 ± 11 | 13 ± 7 | ND | Not measured | Not measured |
| H2O2 | 2 | ND 0·7 ± 0·7 | ND 1·8 ± 1·8 | ND ND | ND | ND |
Primary RA and OA synoviocytes were exposed to different known inflammasome activators for 24 hr. Cell extracts and culture supernatants were recovered and analysed for the presence of IL-6, IL-1β and caspase-1 levels by enzyme-linked immunosorbent assay. OA values for IL-6 and IL-1β are presented in italics. Data are presented as mean ± SD of three independent fibroblast-like synoviocyte (FLS) cultures [except for lipopolysaccharide (LPS) + tumour necrosis factor (TNF) and H2O2 where n = 2]. ND indicates not detected. In both RA and OA FLS, all stimuli (except ATP alone) increased the secretion of IL-6, but not that of extracellular IL-1β.
Comparison of expression of inflammasome components between RA and OA synovium
The expression of inflammasome components in RA and OA synovium (n = 9 and n = 11 for RA and OA, respectively, see Table 1) was compared by RT-PCR and by Western blotting. By Western blot, we found that NALP1, NALP3, NALP12 and ASC were expressed in all the OA and RA biopsies examined (n = 8 for each group), and no differences in protein expression were seen by densitometric analysis of the Western blots (Fig. 4a).
Figure 4.

Expression of inflammasome components in tissue extracts from osteoarthritis (OA) and rheumatoid arthritis (RA) synovial tissues. (a) Densitometric analysis of NACHT, LRR and PYD domains containing protein 1 (NALP1), NALP3, NALP12 or apoptosis-associated speck-like protein containing a CARD domain (ASC) expression on Western blots performed on synovial tissues from OA and RA patients (at least eight patients per group). (b) Quantification of caspase-1 and interleukin-1β (IL-1β) in OA and RA synovial tissue extracts, as determined by specific enzyme-linked immunosorbent assays (at least eight patients per group). Caspase-1 levels were found to be significantly increased in synovial tissue from RA patients. *P < 0·05.
As a result of their low expression levels, we were unable to detect caspase-1 and IL-1β by Western blotting. However, by ELISA, we found that caspase-1 levels were significantly enhanced in RA synovial tissues compared with OA samples, whereas no difference in IL-1β concentrations was detected (Fig. 4b).
Discussion
Results from animal models of arthritis as well as clinical studies in man suggest that IL-1β plays an important role in synovial inflammation and cartilage destruction. A severe form of deforming arthritis is also observed in some patients with cryopyrin-associated periodic syndromes, a condition caused by excessive IL-1β production.10 Interleukin-18 has also been reported to mediate inflammation and cartilage erosion in experimental arthritis.11 Both IL-1β and IL-18 require processing by pro-inflammatory caspases to become bioactive. The emergence of NLR proteins and the inflammasome complex as critical components for this processing step suggests that they may have a role in human joint diseases.
In contrast to previous reports of differences in mRNA expression, we found that NALP3 protein expression was similar in RA and OA. This could be accounted for by the expression of NALP3 mRNA by FLS, which was not reflected by protein expression. At a histological level, endothelial and myeloid cells expressed both ASC and NALP3. Among lymphoid cells, B cells stained positively for both, but T cells in the synovium did not express NALP3. These results are similar to those obtained by Kummer et al.12 who observed expression of NALP3 by neutrophils, macrophages, T cells and B cells. The lack of synovial T-cell staining for NALP3 in our hands remains to be explained, but one possible explanation is the selective migration of a distinct T-cell subset to the synovium that lacks NALP3, in contrast to T cells in the peripheral blood, which express the protein.
Based on the results from functional studies on synovial fibroblast lines, we conclude that FLS cannot assemble a fully functional inflammasome complex made up of NALP3, and do not respond to disease-associated molecular patterns, which are triggers of the inflammasome in macrophages. In this context, it is interesting to note that IL-18, the secretion of which depends also on inflammasome-induced caspase-1 activation, is not released from activated synoviocytes.13 Taken together with the immunohistological and Western blot data, our results suggest that the main cell types that process and secrete IL-1β (and by inference IL-18) in the arthritic synovium are myeloid cells, endothelial cells and possibly B cells. Synovial fibroblasts do not appear to be a source of mature secreted IL-1β. Our findings are consistent with previous observations showing that FLS expressed detectable levels of IL-1β mRNA upon stimulation with TNF-α or direct T-cell membrane contact, but did not release bioactive IL-1β.14
When we compared and contrasted the expression of different NALPs and inflammasome components between RA and OA synovia, we were surprised that there were few differences in mRNA expression between the two pathologies, nor in the protein expression measured by Western blotting. Rosengren et al. found increased levels of NALP3 mRNA in RA synovia, but did not perform any Western blot analysis. The only difference we found was a higher concentration of caspase-1 in the synovium as measured by ELISA in RA samples, whereas IL-1β protein levels were similar. As currently available ELISAs do not discriminate between the pro-forms or active forms of caspase-1 and IL-1β, it is impossible to extrapolate from increased caspase-1 levels to increased IL-1β activity. In our study, the higher levels of caspase-1 observed in RA were not associated with increased inflammasome expression, suggesting that its regulation is distinct from that of ASC and NALP3. In this context, it is interesting to note that as IL-1β plays an important role in murine arthritis, we investigated the contribution of NALP3, studying the phenotype of NALP3-deficient mice (NALP3−/−) and wild-type (+/+) mice during antigen-induced arthritis (AIA). As expected, IL-1β−/− mice showed reduced severity of AIA. By contrast, NALP3−/− mice did not show any alteration of joint inflammation, indicating that IL-1β activation during AIA is independent of the classical NALP3 inflammasome.15 Taken together, our results on human and experimental arthritis suggest that activation of IL-1β does not seem to occur through the NALP3 inflammasone.
Finally, the finding that OA synovial membranes express similar levels of inflammasome components as well as similar IL-1β concentrations compared with RA is interesting, and suggests that synovial IL-1β production does not account for the clear differences in pathology between these two diseases. However, these results should be taken with caution as OA synovial samples were obtained at end-stage disease during joint replacement surgery, where there is often a considerable degree of synovial inflammation reflecting chronic joint injury, and therefore there may not be representative of OA as a whole.
Conclusion
Components of the NALP3 inflammasome are widely expressed in RA synovial tissues, and are found predominantly in myeloid cells, endothelial cells and B cells. The FLS lack NALP3 protein expression despite the presence of NALP3 mRNA, and activators of the NALP3 inflammasome were unable to induce functional IL-1β secretion. Finally, the pattern of expression of known NLRs are comparable in RA and OA synovium, suggesting that NLRs are not a critical determinant of the pathology of these two diseases.
Acknowledgments
This work was supported by grants from the Fonds National Suisse de la Recherche Scientifique (K-32K1-116460 to N.B. and 320000-120319/1 to G.P.) and by the Jean and Linette Warnery foundation. We are indebted to Monica Azevedo for excellent technical support.
Glossary
Abbreviations:
- ASC
apoptosis-associated speck-like protein containing a CARD domain
- ELISA
enzyme-linked immunosorbent assay
- FLS
fibroblast-like synoviocytes
- IL-1β
interleukin-1β
- LPS
lipopolysaccharide
- MPO
myeloperoxidase
- MSU
monosodium urate
- NALP
NACHT, LRR and PYD domains containing protein
- NLR
NOD-like receptor
- NOD
nucleotide oligomerization domain
- NP-40
nonidet-P40
- OA
osteoarthritis
- PCR
polymerase chain reaction
- RA
rheumatoid arthritis
- RT-PCR
reverse transcription–polymerase chain reaction
- TNF
tumour necrosis factor
Disclosures
The authors declare that they have no competing interests.
Authors’contributions
L.K. was responsible for the majority of the practical work and for the writing of the manuscript. The study was originally designed by A.S. and N.B. G.P., D.T. and V.C. were involved in different methodological parts and interpretation of the data. A.S. and N.B. were involved in interpretation of the results and manuscript writing. All authors read and approved the final manuscript.
References
- 1.Bresnihan B, Alvaro-Gracia JM, Cobby M, et al. Treatment of rheumatoid arthritis with recombinant human interleukin-1 receptor antagonist. Arthritis Rheum. 1998;41:2196–204. doi: 10.1002/1529-0131(199812)41:12<2196::AID-ART15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 2.Alten R, Gram H, Joosten LA, et al. The human anti-IL-1 beta monoclonal antibody ACZ885 is effective in joint inflammation models in mice and in a proof-of-concept study in patients with rheumatoid arthritis. Arthritis Res Ther. 2008;10:R67. doi: 10.1186/ar2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mariathasan S, Weiss DS, Newton K, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–32. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 4.Hawkins PN, Lachmann HJ, Aganna E, McDermott MF. Spectrum of clinical features in Muckle–Wells syndrome and response to anakinra. Arthritis Rheum. 2004;50:607–12. doi: 10.1002/art.20033. [DOI] [PubMed] [Google Scholar]
- 5.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–41. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 6.Rosengren S, Hoffman HM, Bugbee W, Boyle DL. Expression and regulation of cryopyrin and related proteins in rheumatoid arthritis synovium. Ann Rheum Dis. 2005;64:708–14. doi: 10.1136/ard.2004.025577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmer G, Busso N, Aurrand-Lions M, et al. Expression and function of junctional adhesion molecule-C in human and experimental arthritis. Arthritis Res Ther. 2007;9:R65. doi: 10.1186/ar2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Busso N, Frasnelli M, Feifel R, Cenni B, Steinhoff M, Hamilton J, So A. Evaluation of protease-activated receptor 2 in murine models of arthritis. Arthritis Rheum. 2007;56:101–7. doi: 10.1002/art.22312. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe H, Gaide O, Petrilli V, et al. Activation of the IL-1beta-processing inflammasome is involved in contact hypersensitivity. J Invest Dermatol. 2007;127:1956–63. doi: 10.1038/sj.jid.5700819. [DOI] [PubMed] [Google Scholar]
- 10.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle–Wells autoinflammatory disorder. Immunity. 2004;20:319–25. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 11.Plater-Zyberk C, Joosten LA, Helsen MM, et al. Therapeutic effect of neutralizing endogenous IL-18 activity in the collagen-induced model of arthritis. J Clin Invest. 2001;108:1825–32. doi: 10.1172/JCI12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kummer JA, Broekhuizen R, Everett H, Agostini L, Kuijk L, Martinon F, van Bruggen R, Tschopp J. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J Histochem Cytochem. 2007;55:443–52. doi: 10.1369/jhc.6A7101.2006. [DOI] [PubMed] [Google Scholar]
- 13.Zeisel MB, Neff LA, Randle J, Klein JP, Sibilia J, Wachsmann D. Impaired release of IL-18 from fibroblast-like synoviocytes activated with protein I/II, a pathogen-associated molecular pattern from oral streptococci, results from defective translation of IL-18 mRNA in pro-IL-18. Cell Microbiol. 2004;6:593–8. doi: 10.1111/j.1462-5822.2004.00385.x. [DOI] [PubMed] [Google Scholar]
- 14.Maret M, Chicheportiche R, Dayer JM, Gabay C. Production of intracellular IL-1alpha, IL-1beta, and IL-1Ra isoforms by activated human dermal and synovial fibroblasts: phenotypic differences between human dermal and synovial fibroblasts. Cytokine. 2004;25:193–203. doi: 10.1016/j.cyto.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Kolly L, Karababa M, Joosten LA, et al. Inflammatory role of ASC in antigen-induced arthritis is independent of caspase-1, NALP-3 and IPAF. J immunol. 2009 doi: 10.4049/jimmunol.0802173. (in press) [DOI] [PubMed] [Google Scholar]


