Abstract
Natural killer (NK) cells bridge the interface between innate and adaptive immunity and are implicated in the control of herpes simplex virus 2 (HSV-2) infection. In subjects infected with human immunodeficiency virus 1 (HIV-1), the critical impact of the innate immune response on disease progression has recently come into focus. Higher numbers of NK cells are associated with lower HIV-1 plasma viraemia. Individuals with the compound genotype of killer cell immunoglobulin-like receptor (KIR) 3DS1 and human leucocyte antigen (HLA)-Bw4-80I, or who have alleles of KIR3DL1 that encode proteins highly expressed on the NK cell surface, have a significant delay in disease progression. We studied the effect of HSV-2 co-infection in HIV-1-infected subjects, and show that HSV-2 co-infection results in a pan-lymphocytosis, with elevated absolute numbers of CD4+ and CD8+ T cells, and NK cells. The NK cells in HSV-2 co-infected subjects functioned more efficiently, with an increase in degranulation after in vitro stimulation. The number of NK cells expressing the activating receptors NKp30 and NKp46, and expressing KIR3DL1 or KIR3DS1, was inversely correlated with HIV-1 plasma viral load in subjects mono-infected with HIV-1, but not in subjects co-infected with HSV-2. This suggests that HSV-2 infection mediates changes within the NK cell population that may affect immunity in HIV-1 infection.
Keywords: CD107, herpes simplex virus 2, human immunodeficiency virus, killer cell immunoglobulin-like receptor, natural killer cells
Introduction
Natural killer (NK) cells are critical effectors of the innate immune response to viral infections, including infection with human immunodeficiency virus 1 (HIV-1; reviewed in ref. 1). NK cell function is regulated by a balance of activating and inhibitory signals received through distinct families of cell surface receptors. These receptors are segregated into several molecular groups, including the killer cell immunoglobulin-like receptors (KIRs), the C-type lectin receptors NKG2A, NKG2C, NKG2D and CD161, and a family of natural cytotoxicity receptors containing NKp30, NKp44 and NKp46.2 KIRs themselves may be activating or inhibitory, and are critical for recognition of cells that have down-regulated major histocompatibility complex (MHC) class I expression, the basis for the missing self hypothesis.3 Genetic studies linking the compound genotype of KIR3DS1 and human leucocyte antigen (HLA)-Bw4-80I with delayed disease progression in HIV-infected individuals,4 and the more recent finding that alleles of KIR3DL1 encoding proteins expressed at high levels on NK cells5 or the presence of KIR3DS1 alone6 influences both HIV-1 viral load and disease progression, further highlight the importance of NK cells in HIV-1 infection. There is evidence for NK cell-mediated control of HIV-1 in both primary and chronic HIV-1 infection, as well as in perinatally infected children, where the expression of particular NK cell receptors correlates with disease severity.7 Therapeutic intervention with cytokine treatment, including treatment with interleukin (IL)-2, boosts both the number and function of circulating NK cells.8
Infection with herpes simplex virus 2 (HSV-2) has become an important consideration for the clinical management of HIV-1 infection, where 50–90% of HIV-1-infected subjects are seropositive for HSV-2.9 HSV-2 infection is associated with increased genital shedding of HIV-1 and increases HIV-1 transmissibility.10,11 Valacyclovir (a nucleoside analogue) therapy to treat HSV-2 infection significantly reduces HIV-1 RNA levels in both plasma and genital secretions.12
Previous studies have shown the involvement of NK cell function in containment of HSV-2 infection, and case studies correlate severe HSV-2 pathology with absent or defective NK cells.13,14 Interestingly, the NK cell response to herpesvirus infections may impact susceptibility to bacterial infections. In a mouse model of gamma-herpesvirus infection, latent infection was associated with elevated levels of interferon (IFN)-γ production and enhanced basal activation of innate immune cells, rendering the mice resistant to infection with certain bacterial pathogens.15 Evidence from mouse models also suggests that NK cells are of importance for protection from HSV infection.16–18 IL-15-deficient mice lack NK cells and are not protected from infection by immunization with recombinant HSV-2 glycoprotein-G.19 In this case, protection is deficient despite both similar levels of specific antibody production and CD8+ T-cell function, but is restored upon reconstitution of the NK cell population with recombinant IL-15 (rIL-15).
In a previous study of HIV-1-seropositive subjects in São Paulo, Brazil, we observed that subjects co-infected with HSV-2 maintained higher numbers of circulating CD4+ T cells.20 As immune protection from HSV-2 infection might be dependent upon NK cells, we reasoned that the effect on circulating CD4+ T-cell numbers might, in part, be mediated by the NK cell response to HSV-2 infection. Although most HSV-2-infected individuals are asymptomatic, nearly all continuously shed HSV-2 virions in mucosal genitalia,9,21 suggesting latent HSV-2 infection may have properties of a subclinical infection. Significantly, a higher rate of mucosal HSV-2 shedding is associated with increased HIV-1 viral load and decreased CD4+ T-cell counts.11 Here, we sought to examine the effects of HSV-2 co-infection in the NK cell population of HIV-1-infected individuals.
Materials and methods
Study subjects
We examined CD4+ and CD8+ T-cell counts, HIV-1 viral load, and NK cell number and function in a cohort of 31 treatment-naïve HIV-1-positive subjects identified during early HIV-1 infection (study entry within 170 days of seroconversion) by serologic testing algorithm for recent HIV seroconversion (STARHS).22 These patients were enrolled and followed at the Federal University of São Paulo, São Paulo, Brazil. We collected information on participant age and gender, and determined HSV-2 co-infection serology using an indirect enzyme-linked immunosorbent assay (ELISA) (Dia Sorin, Saluggia, Italy) as previously described.20 Of these patients, 16 were serologically positive for HSV-2. Symptomatic genital herpes was not reported at the time of sample collection. Subjects were followed over time and removed from the study at the time at which they started antiretroviral therapy or were lost to follow-up. Ten age-matched HIV-1-seronegative subjects residing in Brazil were used as controls. Complete blood counts (CBCs) were performed at the time of sample collection, and the results were subsequently used to calculate the absolute number of NK cells following flow cytometric analysis. Ethical approval was obtained from the Federal University of São Paulo IRB, and patients gave informed consent.
Cell culture and antigenic stimulation
Cryopreserved peripheral blood mononuclear cells (PBMCs) were thawed and used for measurements of NK cell frequency, number and receptor expression. The thawed cells were washed with RPMI-1640 medium supplemented with 15% fetal bovine serum (FBS) before staining or stimulation. NK cell function was assessed by cytokine flow cytometry (CFC). To measure NK cell function, PBMCs were cultured in medium alone, or stimulated with K-562 cells (10 : 1 effector to target ratio). The PBMCs cultured in medium alone were taken as a measure of ‘spontaneous’ NK cell function. Briefly, 100 μl of thawed PBMCs was stimulated at 5 × 106 cells/ml in 96-well plates (5·0 × 105/well) in the presence of 10 μg/ml fluorescein isothiocyanate (FITC)-conjugated anti-CD107a antibody for 24 hr; during the last 6 hr of culture, monensin and brefeldin-A were added to block trans-Golgi transport and allow intracellular accumulation of cytokines. The cells were then harvested, washed in buffer and prepared for antibody staining and flow cytometry.
Cell staining and flow cytometric analysis
Cryopreserved specimens were used for measurements of NK cell frequency, number and receptor expression. The thawed cells were washed with phosphate-buffered saline (PBS) supplemented with 1% bovine serum albumin (BSA) and 2 mm ethylenediaminetetraacetic acid (EDTA) [fluorescence-activated cell sorting (FACS) buffer] before staining. For staining, 5 × 105 cells were incubated with purified human immunoglobulin G (IgG; 100 μg/ml) to block non-specific binding. For the gating strategy, doublets were excluded based on forward scatter (FSC) height and FSC area (Fig. 1a). A broad PBMC gate was then used based on FSC height and side light scatter (SSC). Monocytes, B cells and T cells were excluded based on CD14, CD19 and CD3 gating, respectively (Fig. 1a). NK cells were gated from the CD14-, CD19-, CD3-negative lymphocyte population and were then subdivided into CD56bright, CD56dim and CD56neg populations and analysed for the expression of the NK cell activating receptors NKp30 and NKp46, and for CD107 expression. We used commercially available anti-KIR antibodies DX9 and Z27 to further phenotype the NK cells (BD Biosciences, San Jose, CA). Fluorescence minus one (FMO) samples were prepared for each fluorochrome to facilitate gating. All cells were analysed by flow cytometry using a two-laser FACSCanto instrument running facs-diva software (BD Biosciences). Anti-mouse IgG-coated beads (BD Biosciences) were stained with each fluorochrome separately and used for software-based compensation. Data analysis was carried out using flowjo flow cytometric analysis software (FlowJo Software; Tree Star, Ashland, OR). Statistical analysis was performed using graphpad prism statistical software (GraphPad Software, San Diego, CA). Non-parametric Mann–Whitney U-tests were used to determine differences between groups of subjects, and a two-tailed Spearman correlation at the 95% confidence interval was used to assess the relationship between two groups of variables.
Figure 1.
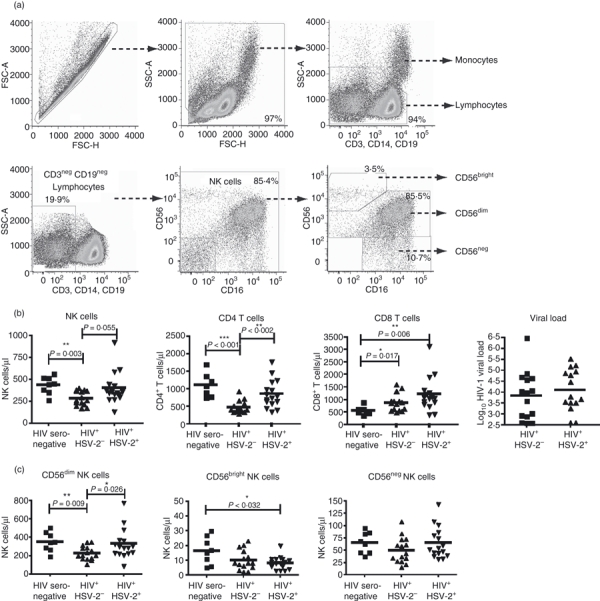
Flow cytometric analysis, lymphocyte numbers, and natural killer (NK) cell subset distribution. (a) Cryopreserved peripheral blood mononuclear cells (PBMCs) were stained with a panel of monoclonal antibodies to distinguish NK cells from other lymphocytes and monocytes. Cells were gated using forward scatter (FSC-H) versus area (FSC-A), and live PBMCs were gated using FSC-H and side scatter (SSC-A). Monocyte and lymphocyte populations were defined using the combination of SSC-A and CD3, CD14 and CD19. NK cells were gated as CD3-negative, CD19-negative lymphocytes, and further defined using CD56 and CD16. NK cells were then further subgrouped based on the level of CD56 staining (CD56bright, CD56dim or CD56neg) as shown in the lower right panel. (b) Human immunodeficiency virus 1 (HIV-1) viral load and the numbers of NK cells and CD4+ and CD8+ T cells were compared among HIV-1-seronegative (n = 11), HIV-1 mono-infected (n = 15), and HIV-1 and herpes simplex virus 2 (HSV-2) co-infected subjects (n = 16). (c) NK cell subgrouping among subjects. A Mann–Whitney U-test was used to determine differences between the means. Significance is indicated by asterisks: *P < 0·05; **P < 0·01; ***P < 0·001.
Results
Pan-lymphocytosis in HIV-1-infected subjects seropositive for HSV-2
We examined the number of NK cells and CD4+ and CD8+ T cells in a Brazilian cohort of HIV-1-seropositive subjects. The cohort included 31 HIV-1-infected patients, of whom 16 patients were seropositive for HSV-2. A description of the cohort may be found in Table 1. These subjects were subdivided into individuals who were seropositive (n = 15) or seronegative (n = 16) for HSV-2 (HSV-2-negative subjects are hereafter referred to as ‘HIV-1 mono-infected’). Although there was no difference in the mean HIV-1 plasma viral load between these two groups, subjects co-infected with HSV-2 showed a pan-lymphocytosis, with elevated numbers of NK cells, CD4+ T cells, and CD8+ T cells relative to HIV-1 mono-infected subjects, but this difference was not significant except for CD4+ T cells (Fig. 1b). The numbers of both NK cells and CD4+ T cells were not significantly different for HIV-1-seronegative controls. Similar to our previous study,20 we observed a significantly elevated number of CD4+ T cells in subjects co-infected with HSV-2 relative to HIV-1 mono-infected subjects (mean = 859 cells/μl versus 474 cells/μl for HIV-1 mono-infected patients; P = 0·002). Furthermore, the number of CD8+ T cells was higher than for seronegative controls for both HIV-1 mono-infected subjects (558 cells/μl versus 866 cells/μl; P = 0·017) and HSV-2 co-infected subjects (1215 cells/μl; P = 0·006). As a result, NK cell and T-cell levels in HSV-2 co-infected subjects were increased beyond the elevated levels seen in HIV-1 mono-infected subjects.
Table 1.
Clinical characteristics and demographics of patients in the study cohort at study entry
| Controls | HIV-1+ | HIV-1+/HSV-2+ | |
|---|---|---|---|
| Number | 10 | 15 | 16 |
| Demographics | |||
| Sex (male:female) | 5 : 5 | 14 : 1 | 15 : 1 |
| Age (years) [mean (± SD)] | 35·2 (± 9·4) | 32·4 (± 5·9) | 35·8 (± 11·6) |
| Blood T-cell counts (cells/μl) | |||
| CD4+ T lymphocytes [median (IQR)] | 1064 (776·0, 1258·0) | 408 (353·0, 570·0) | 772 (605·5,1141) |
| CD8+ T lymphocytes [median (IQR)] | 477 (466·5, 603·5) | 856 (559·0, 927·0) | 1079 (841·5, 1347) |
| HIV-1 RNA concentrations | |||
| Plasma HIV-1 RNA (log10 copies/ml) [median (IQR)] | NA | 3·98 (2·890, 4·630) | 3·74 (3·318, 4·910) |
IQR, interquartile range; NA, not applicable; SD, standard deviation.
We next evaluated the subset distribution of NK cells with respect to the level of CD56 expression. NK cells were separated into CD56bright CD16neg; CD56dim CD16pos; or CD56neg CD16pos subsets. The last group has been described as both increased in number and dysfunctional in HIV-1-infected subjects.23 The frequency of these populations was examined as a percentage of total NK cells, and no significant difference was detected in the mean subset frequencies between subject groups (data not shown). We then used this percentage to calculate the absolute number of NK cells present in these subsets. The results of this calculation demonstrate that the elevated number of total NK cells is primarily attributable to an increase in the number of CD56dim NK cells (Fig. 1c). HIV-1 mono-infected subjects had a decreased mean number of CD56dim NK cells (227 cells/μl) relative to uninfected controls (354 cells/μl; P = 0·009), and HSV-2 co-infected subjects (331 cells/μl; P = 0·026). Interestingly, the number of CD56bright NK cells was reduced in the both the HIV-1 mono-infected group (10 cells/μl) and the HSV-2 co-infected group (8 cells/μl) relative to uninfected controls (17 cells/μl; P = 0·032 for HSV-2 co-infected versus uninfected) (Fig. 1c). As CD56dim NK cells are the major population of NK cells, it was not surprising to find reduced numbers in the HIV-1 mono-infected group, mirroring the results obtained for total NK cells (Fig. 1b). Although previous studies have indicated an increase in the frequency of CD56neg NK cells in HIV-1-infected subjects,23 we did not observe either an elevated number (Fig. 1c) or an elevated frequency (data not shown) in this population of Brazilian subjects, either with or without concomitant HSV-2 infection.
Increased number of functional NK cells in HSV-2 co-infection
It is well established that NK cells are important in the immune response controlling herpesviruses. In particular, HSV pathogenesis in both humans and mouse models is enhanced in the absence of NK cells, when NK cell function is inhibited, or when innate accessory cells required for activation of dendritic cells (DCs), plasmacytoid DCs and macrophages are absent or dysfunctional. In HIV-1 infection, alterations in the number and function of NK cells have been described previously.1,24–29 We evaluated the function of NK cells from HIV-1 mono-infected subjects, HSV-2 co-infected subjects, and healthy HIV-1-seronegative controls. We stimulated PBMCs from these individuals by co-culture with the MHC class I-deficient K-562 erythroleukemia cell line.30 K-562 cells do not express MHC class I proteins (HLA-A, HLA-B, HLA-C, HLA-E or HLA-G) on their surface; therefore, they fail to express any known ligands for the inhibitory and activating KIRs. We detected an increased absolute number of degranulating NK cells, as indicated by levels of CD107 antibody staining (Fig. 2). The number of CD107+ NK cells in HSV-2 co-infected subjects was increased relative to HIV-1 mono-infected subjects in stimulated cultures (263 cells/μl versus 195 cells/μl, respectively; P = 0·018) and was not different from that in HIV-1-seronegative healthy control subjects. Furthermore, in cultures with no stimulation, the number of functional NK cells was significantly depressed in HIV-1 mono-infected subjects compared with healthy control subjects (121 cells/μl versus 198 cells/μl, respectively; P = 0·007).
Figure 2.
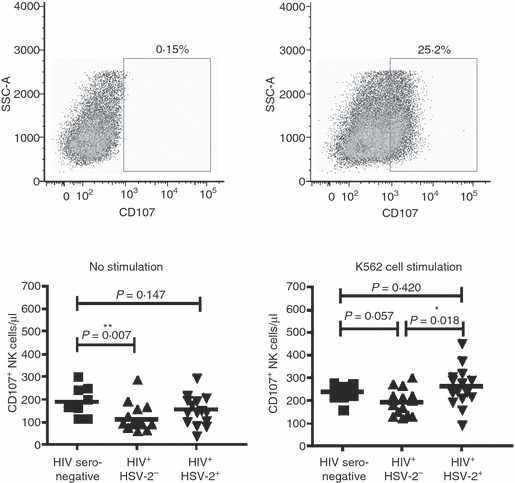
Functional analysis of natural killer (NK) cells. Cryopreserved peripheral blood mononuclear cells (PMBCs) were cultured overnight without stimulation or were stimulated with K-562 cells. Brefeldin-A and Monensin were added for the last 5 hr, and cells were stained with the panel of antibodies described in Fig. 1a. A fluorescence minus one (FMO) control was used to set the CD107 gate (upper left), and the percentage of CD107+ NK cells was determined (upper right). The percentage of CD107+ cells was used to calculate the absolute number of CD107+ NK cells per μl of whole blood. Differences in the number of CD107+ NK cells are shown for each group without stimulation (lower left) and following K-562 cell stimulation (lower right). Mann–Whitney U-test results are shown. Significance is indicated by asterisks: *P < 0·05; **P < 0·01; ***P < 0·001.
NKp30- and NKp46-expressing NK cells are negatively correlated with viral load in HIV-1 mono-infected subjects
We assessed the relationship between HIV-1 plasma viral load and the number of NK cells expressing the natural cytotoxicity receptors NKp30 and NKp46 in HIV-1 mono-infected and HSV-2 co-infected subjects (Fig. 3). Although there was no difference in the mean number or frequency of NKp30- or NKp46-positive cells between groups (Fig. 3a), or in HIV-1 plasma viral load (Fig. 1a), an inverse correlation was observed in HIV-1 mono-infected subjects for both NK cells receptors (Fig. 3b,c). This correlation was not observed in HSV-2 co-infected subjects. In HIV-1 mono-infected subjects, this inverse correlation was significant for NKp46 (P= 0·046). Consequently, the number of NKp30+ and NKp46+ NK cells correlates with reduced viral load in HIV-1 mono-infected subjects, yet this does not appear to be the case in subjects co-infected with HSV-2.
Figure 3.
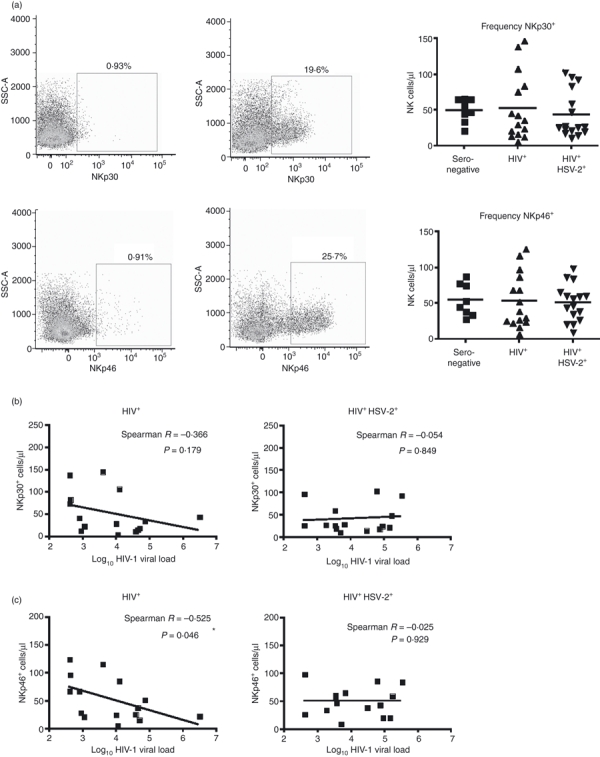
Correlation of NKp30- and NKp46-expressing natural killer (NK) cells and human immunodeficiency virus 1 (HIV-1) viral load. (a) A fluorescence minus one (FMO) control was used to set the gates for NK cells expressing NKp30 and NKp46 (left panels), and the frequency of receptor-positive cells was determined (right panel). The frequencies were used to calculate the number of NKp30- and NKp46-positive NK cells, and no significant difference was observed among the subject groups (b) Spearman correlation of the number of NKp30-expressing NK cells and log10 HIV-1 viral load shown for HIV mono-infected and HIV-1 and herpes simplex virus 2 (HSV-2) co-infected groups. (c) Spearman correlation for NKp46-expressing NK cells and log10 HIV-1 viral load.
KIR expression pattern on NK cells affects viral load
The presence of particular combinations of HLA and KIR genes impacts the rate of HIV-1 disease progression.4–6 In particular, the combination of KIR3DS1 with HLA-Bw4 molecules possessing isoleucine at position 80 (Bw4-80I) is linked with a delayed progression to AIDS.4 More recently, our group has published data indicating that the presence of KIR3DS1 alone may be sufficient to affect NK cell function in HIV-1 infection.6 The issue is further complicated by data suggesting that the presence of alleles of KIR3DL1 encoding proteins expressed at high levels on the cell surface of NK cells in combination with HLA-Bw4-80I is strongly associated with delayed HIV-1 disease progression.5
Previous studies have suggested that the presence of alleles for KIR3DS1 or KIR3DL1 may also lead to delayed HIV-1 disease progression. KIR3DS1 is expressed at the cell surface, and can be discriminated from KIR3DL1 by flow cytometry with the use of two KIR-specific antibodies (i.e. DX9 and Z27).31 As we do not know the KIR genotype of this cohort of Brazilian subjects, and certain alleles of KIR3DL1 that are expressed in low amounts (similar to KIR3DS1) can be misassigned as KIR3DS1, we have used nomenclature to reflect the relative levels of binding of the DX9 and Z27 antibodies. In previous studies in which the KIR genotype was known, NK cells that were DX9-negative and Z27-low were defined as KIR3DS1+ cells, whereas NK cells positive for DX9 only, or both DX9 and Z27, were defined as KIR3DL1+. Although this is probably correct, we have chosen to define our populations as KIR3D-positive to reflect either DX9 and/or Z27 binding, and segregated this group into populations that are KIR3Dhigh or KIR3Dlow based on Z27 staining characteristics (Fig. 4a). No significant differences were seen in the number or frequency of KIR3D+ NK cells among seronegative, HIV-1 mono-infected, and HIV-1 and HSV-2 co-infected subjects (Fig. 4b). However, we then correlated the number of KIR3D+ NK cells with HIV-1 viral load and noted an inverse correlation (Fig. 4c). The number of KIR3D+ NK cells correlated inversely with HIV-1 viral load in all HIV-positive subjects combined, and this correlation became significant when the HIV-1 mono-infected subjects were segregated as a group (P = 0·029). However, this correlation was lost in the HSV-2 co-infected group (P = 0·634). When KIR3D+ NK cells were segregated into KIR3Dhigh and KIR3Dlow expression groups, a stronger inverse correlation with viral load was observed in the KIR3Dlow population (P = 0·043 and P < 0·1 for all groups and HIV-1 mono-infected individuals, respectively), and this correlation was again lost in the HSV-2 co-infected group (P = 0·969).
Figure 4.
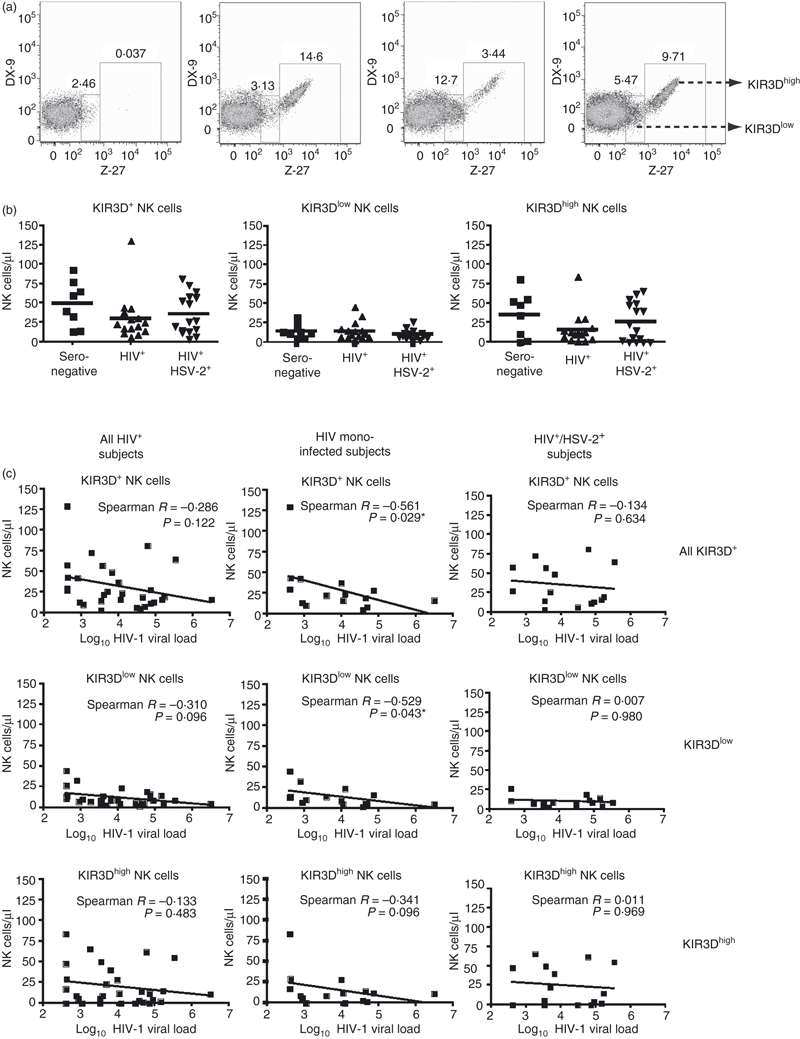
(a) Phenotypic flow cytometric analysis of natural killer (NK) cells using killer cell immunoglobulin-like receptor 3D (KIR3D)-specific antibodies DX9 and Z-27. DX9 antibody binds the proteins encoded by most alleles of KIR3DL1, whereas Z-27 binds both KIR3DL1 and KIR3DS1. Gates were drawn to differentiate the level of KIR3D expression defined as KIR3Dhigh or KIR3Dlow. From the left to right, the panels depict NK cells taken from a patient with no KIR3D expression, a patient with predominantly KIR3Dhigh expression, a patient with predominantly KIR3Dlow expression, and a patient expressing both KIR3Dhigh and KIR3Dlow. Numbers indicate the frequency of NK cells. (b) Total number of KIR3D-expressing NK cells, and number of KIR3Dlow and KIR3Dhigh NK cells among subject groups. (c) Spearman correlations of total KIR3D, KIR3Dlow and KIR3Dhigh NK cells with log10 HIV-1 viral load. Significance is indicated by asterisks: *P < 0·05; **P< 0·01; ***P < 0·001.
Discussion
We show here that HSV-2 co-infection of HIV-1-infected subjects has a considerable impact on the number of circulating peripheral NK cells, and that these NK cells display increased function in response to stimulation. Previous studies have shown that the frequency and absolute numbers of NK cells are decreased in chronic HIV infection and the function of remaining NK cells is impaired.32,33 In the current study, increased numbers of NK cells correlated with increased NK cell function, and we found greater numbers of CD107+ NK cells in HSV-2 co-infected subjects. Of greatest interest is that the number of NK cells expressing the receptors NKp30, NKp46 and low-level KIR3D was strongly and inversely correlated with viral load in HIV-1-infected subjects. This suggests that increased numbers of functional NK cells negatively impact HIV-1 viral load, and that NK cells might mediate some level of control of HIV-1, although this will require further study to determine causality and potential mechanisms. Conversely, in the context of HSV-2 co-infection, there are greater numbers of functional NK cells, yet this increase in NK cell functional capacity has no impact on HIV-1 viral load, as the correlation with the numbers of NK cells expressing activating receptors is lost. These data suggest a model whereby HSV-2 co-infection results in an increased number of functional NK cells, but this increased function is possibly directed towards HSV-2 at the expense of HIV-1 recognition and control. In this model, prophylactic control of HSV-2 infection may allow NK cells to resume effective control of HIV-1 viraemia, resulting in reduced HIV-1 viral load. Importantly, however, we have not formally demonstrated either HIV-1 or HSV-2 specificity of NK cell function, leaving our results open to other interpretations.
In previous studies of HSV-2 co-infection in HIV-1-positive subjects, reactivation of HSV-2 was associated with increased HIV-1 viral load, and was more common in subjects with lower CD4+ T-cell counts.21,34 Conversely, no significant correlation was observed between HIV-1 viral load and HSV-2 infection in the absence of HSV-2 lesions. Subjects infected with HSV-2 are at greater risk for HIV-1 acquisition,35 providing the impetus for the study of HSV-2 prophylaxis in preventing HIV-1 infection. However, treatment with acyclovir has not been demonstrated to be effective in preventing HIV-1 acquisition in HSV-2-positive subjects,36 but was effective in reducing HIV-1 viral load in co-infected women.37 More recent evidence has shown that acyclovir itself strongly inhibits HIV-1 reverse transcriptase, and may account for the reduced HIV-1 viral load observed in response to HSV-2 prophylaxis.38 In the previous study evaluating CD4+ T-cell numbers in co-infected subjects by Barbour et al.,20 it was noted that subjects who had acquired HSV-2 prior to HIV-1 infection had elevated numbers of CD4+ T cells; however, this was not the case in subjects who acquired HSV-2 subsequent to HIV-1 infection. This led to speculation that HSV-2 infection has a lasting effect on the immune system, and that the higher numbers of CD4+ T cells in HSV-2-infected subjects may increase the risk for HIV-1 acquisition by providing a greater number of potential target cells. As it is likely that HSV-2 infection preceded HIV-1 acquisition in the subjects included in the current study, the elevated number of NK cells we observed may be attributable to an imprinting effect of HSV-2 on the immune system that remains throughout the early stages of HIV-1 infection.
Herpesvirus infection can have significant and sustained effects on the expression of NK cell receptors on both NK cells and CD8+ T cells. Studies examining the effects of infection with cytomegalovirus (CMV), a β herpes virus, have noted an imprinting effect resulting in a lasting increase in the frequency of NK cells expressing the activating receptor NKG2C.39 More recently, a longitudinal study of subjects recently exposed to CMV revealed increased expression of both activating and inhibitory NK receptors on CMV-specific CD8+ T cells that remained for at least 1 year following the acute phase of the infection.40 These results raise the possibility that HSV-2 infection may be having immunomodulatory effects on NK cells that affect the host response to HIV-1.
Several mouse models of HSV infection have shown that NK cells are involved in the immune control of HSV, and severe HSV-2 infection has been described in human case studies of persons lacking functional NK cells.13,14 NK cells are effector lymphocytes of the innate immune response important for recognition of virally infected and transformed cells. Further, in HIV-1 infection, alterations in the number and function of NK cells have been described previously.1,24–29 As essential early effector cells, one of their critical functions is the production of cytokines to support the development of antigen-specific cellular immunity. Production of IFN-γ by NK cells promotes the development of T helper type 1 (Th1) cytotoxic T lymphocyte (CTL) responses and eventual development of immune memory. A recent study of mouse gamma-herpesvirus infection demonstrates that latent infection imparts enhanced IFN-γ secretion by NK cells, and renders the mice resistant to bacterial infections.15 In this model, latent herpesvirus infection increases the basal activation state of NK cells, protecting the host from subsequent infections. As nearly all humans become infected with HSVs during their lifetime, it has been suggested that HSV infection, and the resulting increase in basal activation, may encompass part of the natural function of the host immune system. Although no such role has been established for HSV-2, it may nonetheless be the case that minimal levels of HSV-2 replication elevate the basal activation status of innate immune cells, such as NK cells. This enhanced activation may produce benefits for subjects infected with HIV-1, such as the pan-lymphocytosis described here, or alternatively may distract immune effector cells away from HIV-1-infected targets. The factors leading to increased numbers of lymphocytes are not clear, and require further study. Additionally, more investigation is needed to define how HSV-2 infection might modulate HIV-1 pathology.
Acknowledgments
Support for this work was provided by the National Institute of Allergies and Infectious Diseases (grants NIAID AI060379, AI052731 and AI064520 to DFN and AI64520 to LLL). JDB is supported by AI-066917 and AI-076014 (NIAID). Additional support was provided by the Brazilian Program for STD and AIDS, Ministry of Health (914/BRA/3014 – UNESCO/Kallas), the São Paulo City Health Department (2004-0·168·922-7/Kallas), Fundação de Amparo a Pesquisa do Estado de São Paulo (04/15 856-9/Kallas), the John E. Fogarty International Center (D43 TW00003) and the AIDS Research Institute of the AIDS Biology Program at UCSF (grant to DFN). MMS and KIC’s scholarships were supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazilian Ministry of Education. LLL is an American Cancer Society Research Professor. We thank Skip Virgin for helpful discussions.
Disclosures
The authors declare no conflict of interest.
References
- 1.Alter G, Altfeld M. NK cell function in HIV-1 infection. Curr Mol Med. 2006;6(6):621–9. doi: 10.2174/156652406778195035. [DOI] [PubMed] [Google Scholar]
- 2.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–74. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 3.Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–8. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 4.Martin MP, Gao X, Lee JH, et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet. 2002;31:429–34. doi: 10.1038/ng934. [DOI] [PubMed] [Google Scholar]
- 5.Martin MP, Qi Y, Gao X, et al. Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nat Genet. 2007;39:733–40. doi: 10.1038/ng2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Long BR, Ndhlovu LC, Oksenberg JR, Lanier LL, Hecht FM, Nixon DF, Barbour JD. KIR3DS1 Conferral of Enhanced Natural Killer Cell Function in Early HIV-1 Infection. J Virol. 2008;82:4785–92. doi: 10.1128/JVI.02449-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballan WM, Vu BA, Long BR, et al. Natural killer cells in perinatally HIV-1-infected children exhibit less degranulation compared to HIV-1-exposed uninfected children and their expression of KIR2DL3, NKG2C, and NKp46 correlates with disease severity. J Immunol. 2007;179:3362–70. doi: 10.4049/jimmunol.179.5.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michaelsson J, Long BR, Loo CP, Lanier LL, Spotts G, Hecht FM, Nixon DF. Immune reconstitution of CD56(dim) NK cells in individuals with primary HIV-1 infection treated with interleukin-2. J Infect Dis. 2008;197:117–25. doi: 10.1086/524141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strick LB, Wald A, Celum C. Management of herpes simplex virus type 2 infection in HIV type 1-infected persons. Clin Infect Dis. 2006;43:347–56. doi: 10.1086/505496. [DOI] [PubMed] [Google Scholar]
- 10.Corey L, Wald A, Celum CL, Quinn TC. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J Acquir Immune Defic Syndr. 2004;35:435–45. doi: 10.1097/00126334-200404150-00001. [DOI] [PubMed] [Google Scholar]
- 11.Wright PW, Hoesley CJ, Squires KE, Croom-Rivers A, Weiss HL, Gnann JW., Jr A prospective study of genital herpes simplex virus type 2 infection in human immunodeficiency virus type 1 (HIV-1)-seropositive women: correlations with CD4 cell count and plasma HIV-1 RNA level. Clin Infect Dis. 2003;36:207–11. doi: 10.1086/345440. [DOI] [PubMed] [Google Scholar]
- 12.Nagot N, Ouedraogo A, Foulongne V, et al. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N Engl J Med. 2007;356:790–9. doi: 10.1056/NEJMoa062607. [DOI] [PubMed] [Google Scholar]
- 13.Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320:1731–5. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 14.Dalloul A, Oksenhendler E, Chosidow O, et al. Severe herpes virus (HSV-2) infection in two patients with myelodysplasia and undetectable NK cells and plasmacytoid dendritic cells in the blood. J Clin Virol. 2004;30:329–36. doi: 10.1016/j.jcv.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 15.Barton ES, White DW, Cathelyn JS, Brett-McClellan KA, Engle M, Diamond MS, Miller VL, Virgin HWt. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature. 2007;447:326–9. doi: 10.1038/nature05762. [DOI] [PubMed] [Google Scholar]
- 16.Ashkar AA, Rosenthal KL. Interleukin-15 and natural killer and NKT cells play a critical role in innate protection against genital herpes simplex virus type 2 infection. J Virol. 2003;77:10168–71. doi: 10.1128/JVI.77.18.10168-10171.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 18.Ching C, Lopez C. Natural killing of herpes simplex virus type 1-infected target cells: normal human responses and influence of antiviral antibody. Infect Immun. 1979;26:49–56. doi: 10.1128/iai.26.1.49-56.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gill N, Ashkar AA. Adaptive immune responses fail to provide protection against genital HSV-2 infection in the absence of IL-15. Eur J Immunol. 2007;37:2529–38. doi: 10.1002/eji.200636997. [DOI] [PubMed] [Google Scholar]
- 20.Barbour JD, Sauer MM, Sharp ER, et al. HIV-1/HSV-2 Co-Infected Adults in Early HIV-1 Infection Have Elevated CD4+ T Cell Counts. PLoS ONE. 2007;2:e1080. doi: 10.1371/journal.pone.0001080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schacker T, Zeh J, Hu HL, Hill E, Corey L. Frequency of symptomatic and asymptomatic herpes simplex virus type 2 reactivations among human immunodeficiency virus-infected men. J Infect Dis. 1998;178:1616–22. doi: 10.1086/314486. [DOI] [PubMed] [Google Scholar]
- 22.Kallas EG, Bassichetto KC, Oliveira SM, et al. Establishment of the serologic testing algorithm for recent human immunodeficiency virus (HIV) seroconversion (STARHS) strategy in the city of Sao Paulo, Brazil. Braz J Infect Dis. 2004;8:399–406. doi: 10.1590/s1413-86702004000600003. [DOI] [PubMed] [Google Scholar]
- 23.Mavilio D, Lombardo G, Benjamin J, et al. Characterization of CD56-/CD16+ natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc Natl Acad Sci USA. 2005;102:2886–91. doi: 10.1073/pnas.0409872102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kottilil S, Chun TW, Moir S, et al. Innate immunity in human immunodeficiency virus infection: effect of viremia on natural killer cell function. J Infect Dis. 2003;187:1038–45. doi: 10.1086/368222. [DOI] [PubMed] [Google Scholar]
- 25.Mavilio D, Benjamin J, Daucher M, et al. Natural killer cells in HIV-1 infection: dichotomous effects of viremia on inhibitory and activating receptors and their functional correlates. Proc Natl Acad Sci USA. 2003;100:15011–6. doi: 10.1073/pnas.2336091100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alter G, Malenfant JM, Delabre RM, Burgett NC, Yu XG, Lichterfeld M, Zaunders J, Altfeld M. Increased natural killer cell activity in viremic HIV-1 infection. J Immunol. 2004;173:5305–11. doi: 10.4049/jimmunol.173.8.5305. [DOI] [PubMed] [Google Scholar]
- 27.Alter G, Suscovich TJ, Teigen N, Meier A, Streeck H, Brander C, Altfeld M. Single-stranded RNA derived from HIV-1 serves as a potent activator of NK cells. J Immunol. 2007;178:7658–66. doi: 10.4049/jimmunol.178.12.7658. [DOI] [PubMed] [Google Scholar]
- 28.Alter G, Teigen N, Ahern R, Streeck H, Meier A, Rosenberg ES, Altfeld M. Evolution of innate and adaptive effector cell functions during acute HIV-1 infection. J Infect Dis. 2007;195:1452–60. doi: 10.1086/513878. [DOI] [PubMed] [Google Scholar]
- 29.Alter G, Teigen N, Davis BT, et al. Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood. 2005;106:3366–9. doi: 10.1182/blood-2005-03-1100. [DOI] [PubMed] [Google Scholar]
- 30.Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294:15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Carr WH, Rosen DB, Arase H, Nixon DF, Michaelsson J, Lanier LL. Cutting edge: KIR3DS1, a gene implicated in resistance to progression to AIDS, encodes a DAP12-associated receptor expressed on NK cells that triggers NK cell activation. J Immunol. 2007;178:647–51. doi: 10.4049/jimmunol.178.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruunsgaard H, Pedersen C, Skinhoj P, Pedersen BK. Clinical progression of HIV infection: role of NK cells. Scand J Immunol. 1997;46:91–5. doi: 10.1046/j.1365-3083.1997.d01-98.x. [DOI] [PubMed] [Google Scholar]
- 33.Meier UC, Owen RE, Taylor E, et al. Shared alterations in NK cell frequency, phenotype, and function in chronic human immunodeficiency virus and hepatitis C virus infections. J Virol. 2005;79:12365–74. doi: 10.1128/JVI.79.19.12365-12374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schacker T, Zeh J, Hu H, Shaughnessy M, Corey L. Changes in plasma human immunodeficiency virus type 1 RNA associated with herpes simplex virus reactivation and suppression. J Infect Dis. 2002;186:1718–25. doi: 10.1086/345771. [DOI] [PubMed] [Google Scholar]
- 35.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 36.Celum C, Wald A, Hughes J, et al. Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:2109–19. doi: 10.1016/S0140-6736(08)60920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baeten JM, Strick LB, Lucchetti A, et al. Herpes simplex virus (HSV)-suppressive therapy decreases plasma and genital HIV-1 levels in HSV-2/HIV-1 coinfected women: a randomized, placebo-controlled, cross-over trial. J Infect Dis. 2008;198:1804–8. doi: 10.1086/593214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McMahon MA, Siliciano JD, Lai J, Liu JO, Stivers JT, Siliciano RF, Kohli RM. The antiherpetic drug acyclovir inhibits HIV replication and selects the V75I reverse transcriptase multidrug resistance mutation. J Biol Chem. 2008;283:31289–93. doi: 10.1074/jbc.C800188200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guma M, Budt M, Saez A, Brckalo T, Hengel H, Angulo A, Lopez-Botet M. Expansion of CD94/NKG2C+ NK cells in response to human cytomegalovirus-infected fibroblasts. Blood. 2006;107:3624–31. doi: 10.1182/blood-2005-09-3682. [DOI] [PubMed] [Google Scholar]
- 40.van Stijn A, Rowshani AT, Yong SL, Baas F, Roosnek E, Ten BergeIJ, van Lier RA. Human Cytomegalovirus Infection Induces a Rapid and Sustained Change in the Expression of NK Cell Receptors on CD8+ T Cells. J Immunol. 2008;180:4550–60. doi: 10.4049/jimmunol.180.7.4550. [DOI] [PubMed] [Google Scholar]


