Abstract
Trappin-2/Elafin is a serine protease inhibitor that plays a major role as an anti-inflammatory mediator at mucosal surfaces. In addition, Trappin-2/Elafin has antibacterial activity against Gram-positive and Gram-negative bacterial and fungal pathogens. In this study we examined the production of Trappin-2/Elafin by epithelial cells from the human upper and lower female reproductive tract as well as its activity as an anti-human immunodeficiency virus (HIV)-1 molecule. We found that primary uterine, Fallopian tube, cervical and ectocervical epithelial cells produce Trappin-2/Elafin constitutively and that production of Trappin-2/Elafin is enhanced following stimulation with Poly(I:C), especially by the uterine cells. Given the presence of Trappin-2/Elafin in the reproductive tract, we tested the ability of recombinant Trappin-2/Elafin to inhibit HIV-1, an important sexually transmitted pathogen. We found that recombinant Trappin-2/Elafin was able to inhibit both T-cell-tropic X4/IIIB and macrophage-tropic R5/BaL HIV-1 in a dose-dependent manner. The inhibitory activity was observed when virus was incubated with Trappin-2/Elafin but not when Trappin-2/Elafin was added to cells either before infection or after infection. This suggests that the mechanism of inhibition is likely to be a direct interaction between HIV-1 and Trappin-2/Elafin. Additionally, we measured the levels of secreted Trappin-2/Elafin in cervico-vaginal lavages (CVL) from both HIV-positive and HIV-negative women and found that average levels of secreted Trappin-2/Elafin were higher in the CVL from HIV-negative women, although the values did not reach statistical significance. We also found that women at the secretory phase of the menstrual cycle produced more Trappin-2/Elafin in CVL relative to women at the proliferative phase of the menstrual cycle. Our data suggest that Trappin-2/Elafin might be an important endogenous microbicide of the female reproductive tract that is protective against HIV-1.
Keywords: antiviral, female reproductive tract, human immunodeficiency virus, Poly(I:C), Trappin-2/Elafin
Introduction
As the human immunodeficiency virus (HIV)/acquired immune-deficiency syndrome (AIDS) pandemic continues, and with the recent failures in vaccine and microbicide trials,1–5 the need for innovative solutions has become essential. Currently, heterosexual transmission accounts for more than 80% of new infections.6,7 Although several studies have found that women are more likely than men to be infected with HIV during vaginal intercourse,8 the transmission rate of HIV from a man to a woman per act of sexual intercourse is still relatively low, ranging from 1:122 to 1:1000.9,10 One reason for this might be that cells of the female reproductive tract (FRT) produce and secrete a number of endogenous antimicrobials that are protective against HIV.11–15 The mucosal innate immune system of the FRT has to perform the complex immune function of accepting allogeneic sperm and a semi-allogeneic fetus while preventing pathogen infection. Epithelial cells that line the FRT are the first line of host defense. In addition to presenting a physical barrier, these cells perform a multitude of immune functions. FRT epithelial cells from both the upper and the lower tract express innate immune sensors, such as toll-like receptors (TLR),11,12,16,17 and secretions from these cells have been demonstrated to be antimicrobial.13,18,19
Evidence of innate immune protection has also been described in vivo. In secretions recovered by cervico-vaginal lavage (CVL) the presence of cationic polypeptides has been linked to anti-HSV and anti-HIV activity.14,20 In many HIV-infected women, the plasma viral load (PVL) has not been found to correlate with genital tract viral load (GTVL) and has also been found to be genetically distinct.21–23 Genital tract viral shedding can be highly localized and can change with the menstrual cycle (S. Cu-Uvin, unpublished data,24,25). In addition, the proportion of virus in the genital tract that is actually infectious and capable of transmission seems to be very low, irrespective of the PVL and the GTVL (M. Ghosh and J. V. Fahey, unpublished data;26,27). In a study by Keller et al.,14 the CVL was collected from normal women throughout the course of the menstrual cycle and assayed for a number of immune activators, antimicrobials and antibodies. CVL samples were found to contain a spectrum of factors, most of which changed with the menstrual cycle, specifically dipping at mid-cycle to the early secretory phase, which has been proposed as a ‘window of vulnerability’ through which women become infected with HIV.28
Many of the innate immune molecules that are known to protect the FRT18,19,29 are regulated by the sex hormones oestradiol and progesterone during the menstrual cycle.30–32 Two such molecules are the anti-proteases secretory leucocyte protease inhibitor (SLPI) and Trappin-2/Elafin. SLPI and Trappin-2/Elafin are members of the whey acidic protein (WAP) family. They are produced by multiple cell types, secreted in mucosal secretions constitutively and can be elevated in the presence of inflammatory stimuli.33–37 These molecules are anti-inflammatory; they function by inhibiting specific neutrophil proteases. Trappin-2/Elafin has been demonstrated to inhibit neutrophil elastase and proteinase 3.19 In addition, both SLPI and Trappin-2/Elafin have been demonstrated to have antimicrobial activity.38–41 The main mechanism for this activity is predicted to be the cationic nature of these molecules, which destabilizes the negative charges of the bacterial cell wall or the viral envelope.40,41
Trappin-2/Elafin has specifically been shown to have antimicrobial activity against both Gram-positive and Gram-negative bacteria, and fungi.39 Trappin-2/Elafin is unique in that it can be biologically active as both cell-associated and secreted protein. The precursor of Trappin-2/Elafin is known as Trappin-2, which contains a transglutaminase substrate-binding domain (TSBD) that is cleaved from the processed Trappin-2/Elafin molecule. The TSBD is involved in covalent binding to extracellular matrix proteins, including laminin, fibronectin, collagen IV, elastin and fibrinogen.33,40 This might provide local protection from proteolytic activity by endogenous proteases, whereas the cleaved soluble form can act at distant sites. Trappin-2/Elafin has been found to be involved in immune disorders of the skin, such as psoriasis42 and lung chronic obstructive pulmonary disorder (COPD43). Whereas SLPI has been demonstrated to be an important inhibitor of HIV,44,45 there are no published reports on Trappin-2/Elafin being an anti-HIV molecule.
In this study we demonstrated that the epithelial cells of the upper tract (Fallopian tube, uterus and cervix) and of the lower tract (ectocervix) constitutively produce Trappin-2/Elafin messenger RNA (mRNA) and protein. However, only the uterine cells consistently up-regulate Trappin-2/Elafin production upon stimulation with Poly(I:C), a synthetic viral double-stranded RNA (dsRNA) mimic. We also demonstrated that recombinant Trappin-2/Elafin can inhibit both X4/T-tropic IIIB and R5/M-tropic BaL HIV-1 in a dose-dependent manner, possibly through a mechanism that involves direct interaction of virus and Trappin-2/Elafin. Finally, we demonstrated that CVL from both HIV-positive and HIV-negative women contain Trappin-2/Elafin, with higher amounts present in HIV-negative women. In addition, women in the secretory phase of the menstrual cycle produced significantly more Trappin-2/Elafin than women in the proliferative phase, suggesting hormonal regulation of this molecule in the FRT.
Materials and methods
Source of tissues
Human uterine and Fallopian tube tissue was obtained from women undergoing hysterectomy at Dartmouth-Hitchcock Medical Center (Lebanon, NH). Tissues used in this study were collected from patients with benign conditions, such as fibroids, distal from the site of pathology. The sections were examined by a pathologist and identified to be free of pathological lesions. A total of 11 different patients were used to obtain epithelial cells from the uterus, Fallopian tube, endocervix and ectocervix. All work on human subjects was carried out with the approval of the Dartmouth College and Miriam Hospital, Brown University Institutional Review Boards. Approval to use tissues was previously obtained from the Committee for the Protection of Human Subjects (CPHS).
Isolation of primary uterine, Fallopian tube, cervical and ectocervical epithelial cells
Epithelial cells were isolated as previously described elsewhere.46,47 Briefly, tissues were rinsed with 1 × phosphate-buffered saline (PBS) and minced into 1–2 mm fragments before subjecting them to enzymatic digestion for 2 hr at 37°. The enzyme mixture contained 3·4 mg/ml of pancreatin (Invitrogen Life Technologies, Carlsbad, CA), 0·1 mg/ml of hyaluronidase (Worthington Biochemical, Lakewood, NJ), 1·6 mg/ml of collagenase (Worthington Biochemical) and 2 mg/ml of d-glucose, in 1 × Hanks’ balanced salt solution (HBSS) (Invitrogen Life Technologies). After digestion, cells were dispersed through a 250-mm mesh screen, washed and resuspended in Dulbecco’s modified Eagle’s minimal essential medium (DMEM)/F12 complete medium without phenol red, supplemented with 20 mm HEPES, 2 mm l-glutamine (all from Invitrogen Life Technologies), 50 μg/ml of primocin (Invivogen, San Diego, CA) and 10% defined fetal bovine serum (FBS) (Hyclone, Logan, UT). Epithelial sheets were separated from stromal cells by filtration through a 20-mm nylon mesh filter (Small Parts, Miami Lakes, FL). Epithelial cell sheets retained on the filter were recovered by rinsing and backwashing, centrifuged at 500 g for 10 min and analyzed for cell number and viability. By this procedure, we isolated epithelial cells that stained positive for the epithelial antigens Ber-EP4 and cytokeratin and stained negative for CD4, CD45 and vimentin.48
Cell culture
To establish a cell culture system of polarized human uterine, Fallopian tube and endocervical epithelial cells with both apical and basolateral compartments, primary cells were cultured in human extracellular matrix (Becton Dickinson, Franklin Lakes, NJ)-coated Falcon cell culture inserts in 24-well culture plates (Fisher Scientific, Pittsburgh, PA). For these experiments, apical and basolateral compartments contained 300 and 850 μl of complete medium, respectively. In order to keep the culture conditions similar, the same procedure was followed for culturing the squamous ectocervical epithelial cells, which do not polarize. The medium was changed every 2 days. The cells were treated with 25 μg/ml of TLR3 agonist Poly(I:C) (Invivogen) for 24 hr, after which apical and basolateral conditioned media (CM) were collected, centrifuged for 5 min at 10 000 g and stored at −80° until use.
Measurement of transepithelial resistance
Tight junction formation of cultured epithelial cell monolayers was assessed by periodically measuring transepithelial resistance (TER) using an EVOM electrode and Voltohmmeter (World Precision Instruments, Sarasota, FL), as described previously.49 TER is a functional measurement of the integrity of tight junctions in an epithelial cell monolayer. The presence of non-epithelial cells in the culture interferes with the formation of tight junctions and therefore prevents an increase in TER. TER is also an indicator for the purity of the epithelial monolayer.
Measurement of Trappin-2/Elafin protein secretion
The concentrations of Trappin-2/Elafin in the apical and basolateral supernatants from primary FRT epithelial cells and CVL from HIV-positive and HIV-negative women were determined using an enzyme-linked immunosorbent assay (ELISA) Duoset kit (R&D Systems, Minneapolis, MN), according to the manufacturer’s protocol. This assay measures both Trappin-2 and Elafin. The amounts of Trappin-2/Elafin were measured based on a standard curve after measuring the absorbance at 450 nm on an ELISA reader (Dynex, Chantilly, VA).
TaqMan real-time polymerase chain reaction
Real-time reverse transcription–polymerase chain reaction (RT-PCR) was performed using a two-step protocol, as described previously.50 Total RNA was isolated from cells using TRIzol Reagent according to the manufacturer’s recommendations (Invitrogen Life Technologies) and purified by elution through RNeasy columns (Qiagen, Valencia, CA). Coincident with RNA purification was on-column DNAse digestion using the RNAse-Free DNAse set (Qiagen). For each specimen, 400 ng of total RNA was reverse-transcribed using the iScript complementary DNA (cDNA) synthesis kit (Bio-Rad, Hercules, CA), according to the manufacturer’s recommendations, in a 20 μl volume. Relative mRNA expression levels of Trappin-2/Elafin were measured using the 5′ fluorogenic nuclease assay in real-time quantitative PCR using TaqMan chemistry on the ABI 7300 Prism real-time PCR instrument (Applied Biosystems, Foster City, CA). The Trappin-2/Elafin and β-actin primer/MGB probe sets were obtained from Applied Biosystems assays-on-demand (ID nos Hs00160066_m1 and 4333762T, respectively). This primer-probe set recognizes both Trappin-2 and Elafin. PCR was conducted using the following cycle parameters: 12 min at 95° for one cycle, followed by 40 cycles of 20 seconds at 95° and 1 min at 60°. Analysis was conducted using the sequence detection software supplied with the ABI 7300. The software calculates the threshold cycle (Ct) for each reaction, which was used to quantify the amount of starting template in the reaction. The Ct values for each set of duplicate reactions were averaged for all subsequent calculations. A difference in Ct values (ΔCt) was calculated for each gene by taking the mean Ct of each gene of interest and subtracting the mean Ct for the housekeeping gene β-actin for each cDNA sample. Assuming that each reaction functions at 100% PCR efficiency, a difference of one Ct represents a twofold difference. Relative expression levels were expressed as a fold-increase in mRNA expression and were calculated using the formula 2–ΔΔCt.
Trappin-2/Elafin inhibition of HIV-1: TZM-bl assay
The TZM indicator cell line (kindly provided by Dr Phalguni Gupta, University of Pittsburgh) is a HeLa cell derivative that expresses high levels of CD4, CCR5 and CXCR4.51 The cells contain HIV long terminal repeat (LTR)-driven β-galactosidase and luciferase reporter cassettes that are activated by HIV tat expression. TZM cells were routinely subcultured every 3–4 days by trypsinization and were maintained in TZM media consisting of DMEM (Invitrogen Life Technologies) supplemented with 10% defined FBS (HyClone), 2 mm l-glutamine (Invitrogen Life Technologies) and 50 μg/ml of primocin (Invivogen), and did not contain phenol red.
TZM cells were seeded at 2 × 104 cells per well in a 96-well microtiter plate and allowed to adhere overnight at 37°. Varying doses of recombinant human Trappin-2/Elafin (Peprotech, Rocky Hill, NJ) were incubated with HIV-1 IIIB and BaL at a multiplicity of infection (MOI) of 1 for 1 hr at 37° in a final volume of 100 μl. Following incubation, the media was aspirated from TZM cells, and the virus plus Trappin-2/Elafin was added to the cells along with 100 μl of TZM medium. Luciferase activity was measured after 48 hr at 37° with 5% CO2 in a humidified incubator. Briefly, the supernatants were aspirated and the cells were lysed using a Beta Glo luciferase assay substrate (Promega, Madis, WI). The light intensity of each well was measured using a luminometer. Uninfected cells were used to determine background luminescence. All infectivity assays were performed in quadruplicate.
Other experiments were conducted in order to determine whether the inhibitory effects of Trappin-2/Elafin were at the cell-surface level, such as the blocking of a co-receptor. For these experiments, varying amounts of Trappin-2/Elafin were directly added to TZM cells and incubated for 1 hr at 37° before washing out with 1 × PBS and addition of IIIB or BaL viruses. In additional experiments to determine possible postinfection mechanisms of inhibition by Trappin-2/Elafin, TZM cells were infected with HIV-1 IIIB and BaL at an MOI of 1 and were washed out at 6 and 24 hr postinfection followed by the addition of 10 ng/ml of recombinant Trappin-2/Elafin (rTrappin-2/Elafin). Assays were developed at 48 hr by addition of the Beta-Glo substrate and measurement of relative light units using a luminometer.
Viability
Viability of TZM cells upon treatment with Trappin-2/Elafin and CVL was quantified using the CellTiter 96® AQueous One Solution Cell Proliferation assay (Promega) according to the manufacturer’s instructions. Briefly, reagent was added directly to cell cultures and incubated for 1 hr at 37°, after which the absorbance of each well was read at 490 nm in a plate reader.
Source and characteristics of CVL samples
CVL samples from 32 HIV-positive women (12 Black, nine Hispanic and 12 White) were provided by Dr S. Cu-Uvin (Brown University, Providence, RI). Fifteen CVL samples from HIV-negative women (five Black, five Hispanic and five White) were obtained from the Rhode Island HIV Epidemiology Research Study (HERS). All sample collections were carried out in accordance with human experimentation guidelines of Miriam Hospital (Brown University, Providence, RI). CVL from women were catalogued by race based on self-identification. The HIV-positive and HIV-negative women were in the same age range (18–50 years). The HIV-positive women were relatively healthy with average CD4 counts of 712 cells/mm3 blood, an average plasma viral load of 12 666 copies/ml and were not on any antiretroviral therapy. Only six out of 32 women showed a detectable genital tract viral load. CVL was collected by washing the cervical-vaginal area with 10 ml of sterile saline (pH 7·0) and collecting the fluid, which was then centrifuged at 10 000 g for 5 min and separated from the cellular fraction. The supernatants were aliquoted and stored frozen at −80° until use. For the HIV-negative samples used in this study, CVL were collected and frozen immediately at −80°. Before assaying the supernatants, samples were thawed, centrifuged at 10 000 g for 5 min and separated from the cellular fraction.
Statistics
A two-tailed paired t-test or a one-way analysis of variance (anova) with Bonferonni’s post-test was performed using GraphPad InStat version 3.0a (GraphPad Software, San Diego, CA). A P-value of < 0·05 was taken as indicative of statistical significance.
Results
Constitutive Trappin-2/Elafin production by epithelial cells from the upper and lower female reproductive tract
Epithelial cells were isolated from uterus (UT), Fallopian tube (FT), endocervix (Cx) and ectocervix (Ecx) FRT tissues, grown to confluence and, in the case of epithelial cell (EC) from the upper FRT, high TER (> 500 ohms/well). CM was collected at 24 hr and cells were harvested to isolate RNA. TaqMan real-time RT-PCR was performed on untreated RNA samples obtained from matched tissues from different patients to observe comparative levels of Trappin-2/Elafin mRNA production by the FRT compartments. The data were normalized to Trappin-2/Elafin levels in the Ecx, which typically expressed low amounts of Trappin-2/Elafin mRNA. As shown in Fig. 1a, in all four patients, FT had the highest levels of Trappin-2/Elafin expression – 10–368-fold higher than that seen in Ecx – set at 1. Trappin-2/Elafin mRNA levels in the Cx were also greater than the Ecx, being 2–36-fold higher. UT epithelial cells, however, typically showed very low Trappin-2/Elafin mRNA expression, which was significantly lower than epithelial cells from all the other compartments (FT, Cx, Ecx).
Figure 1.
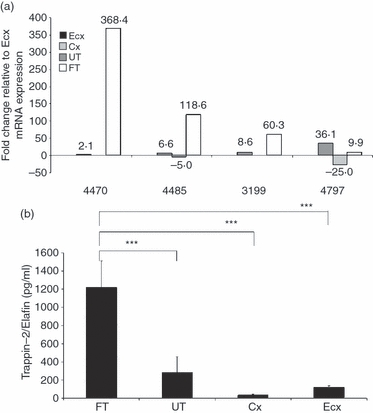
Constitutive production of Trappin-2/Elafin by epithelial cells of the female reproductive tract. (a) Primary uterine (UT), Fallopian tube (FT), cervical (Cx) and ectocervical (Ecx) epithelial cells were isolated from four patients and cultured until confluence and high transepithelial resistance (TER) was reached (with the exception of Ecx cells, which do not polarize). RNA was extracted from the cells and real-time reverse transcription–polymerase chain reaction (RT-PCR) was used to determine the relative expression levels of Trappin-2/Elafin. After normalization to endogenous control β-actin, each patient sample was further calibrated to Ecx cells, which typically produced low levels of Trappin-2/Elafin. The data are shown relative to Ecx, which was set at 1. Generally, FT cells produced significantly higher levels of Trappin-2/Elafin mRNA compared with Cx, Ecx and UT cells. All values twofold higher or lower were considered to be significant. (b) After 24 hr, accumulation of constitutive apical and basolateral conditioned media was collected from female reproductive tract (FRT) epithelial cells from patients and assayed for Trappin-2/Elafin protein secretion by enzyme-linked immunosorbent assay (ELISA). An average of three to five patients per each tissue is shown. Significantly higher levels of constitutive Trappin-2/Elafin secretion were observed in the fallopian tube epithelial cells (FTEC) compared with UT, Cx and Ecx cells. The P-values were calculated using analysis of variance (anova); ***significantly greater than control, P < 0·001.
In order to determine whether this pattern of mRNA expression would match that of protein expression, we analyzed the CM collected from FRT epithelial cells from FT, UT, Cx and Ecx, by Trappin-2/Elafin ELISA. As shown in Fig. 1b, when CM from multiple patients was analyzed, we found that FT epithelial cells secreted the highest levels of Trappin-2/Elafin, significantly higher than that of UT, Cx and Ecx. The average of three to five patients per tissue is shown in Fig. 1b.
Up-regulation of Trappin-2/Elafin by epithelial cells of the FRT upon stimulation with Poly(I:C)
Our laboratory has previously reported that the FRT epithelial cells can mount an antiviral response upon stimulation with Poly(I:C), a synthetic mimic for viral dsRNA.11,12 Therefore, we were interested in determining whether Trappin-2/Elafin, a known antimicrobial, would also be produced in response to Poly(I:C) stimulation. As shown in Fig. 2a, when UT epithelial cells were treated with Poly(I:C) for 24 hr, Trappin-2/Elafin mRNA expression was significantly up-regulated by four- to 95-fold when compared with control cells whose expression was set at 1 (six out of six patients). In a time–course experiment where cells were treated with Poly(I:C) and harvested 3, 6 and 24 hr after treatment, we observed that Poly(I:C) treatment up-regulated Trappin-2/Elafin mRNA expression at 6 hr, with continued increases seen at 24 hr (Fig. 2b). To demonstrate whether Poly(I:C) also stimulated secretion of Trappin-2/Elafin protein we analyzed 24 hr CM by ELISA. As shown for a representative patient (Fig. 2c), we found that Trappin-2/Elafin secretion by UT epithelial cells is significantly increased upon Poly(I:C) stimulation. Furthermore, when apical and basolateral secretions were analyzed, we found that the secretion of Trappin-2/Elafin was preferentially apical. The concentration of Trappin-2/Elafin was measurable in basolateral secretions, but very low relative to apical secretions (data not shown).
Figure 2.
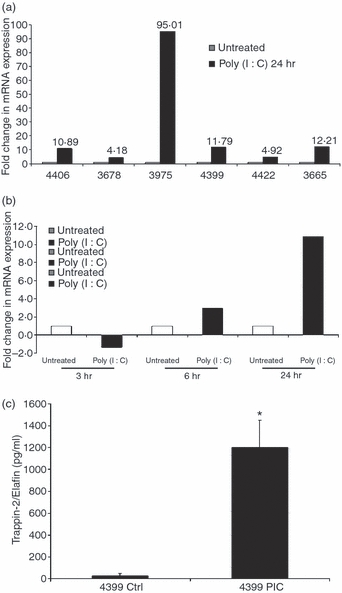
Induction of Trappin-2/Elafin messenger RNA (mRNA) and protein by uterine (UT) epithelial cells upon treatment with Toll-like receptor 3 (TLR3) agonist Poly(I:C). (a) Primary UT epithelial cells from six different patients were treated with Poly(I:C) for 24 hr after which the cells were harvested and RNA extracted. Real-time reverse transcription–polymerase chain reaction (RT-PCR) was used to determine the relative expression levels of Trappin-2/Elafin. After normalization to endogenous control β-actin, each patient sample was further calibrated to its own untreated control. In all six UT samples, Trappin-2/Elafin mRNA levels were enhanced by at least twofold. (b) Primary UT epithelial cells were treated with Poly(I:C) for 3, 6 and 24 hr before the cells were harvested and RNA was extracted. Real-time RT-PCR was used to determine the relative expression levels of Trappin-2/Elafin. Data were normalized to endogenous control β-actin and further calibrated to its own untreated control. Upon Poly(I:C) treatment, Trappin-2/Elafin mRNA was found not to be upregulated at 3 hr, but was upregulated at 6 hr and the upregulation was maintained at 24 hr. Data are shown from one out of three representative patients. (c) UT epithelial cells were treated with Poly(I:C) for 24 hr. Apical and basolateral conditioned media (CM) were collected and assayed for Trappin-2/Elafin protein secretion by enzyme-linked immunosorbent assay (ELISA). Secretion of Trappin-2/Elafin upon Poly(I:C) treatment was significantly enhanced when compared with untreated controls. Data from one representative patient out of four is shown. The P-values were calculated using a two-tailed paired t-test. *Significantly greater than control, P < 0·05.
To evaluate more fully the extent of Poly(I:C)-mediated Trappin-2/Elafin secretion throughout the FRT, similar analyses were carried out with FT, Cx and Ecx epithelial cells. Unexpectedly, we found that whereas cells from all compartments constitutively produced Trappin-2/Elafin both at the mRNA and the protein levels (Fig. 1a,b), Poly(I:C) stimulation enhanced RNA expression and protein secretion in only a fraction of the patients tested. Out of four to six patients tested for each compartment, approximately one-third typically responded to Poly(I:C) by up-regulating Trappin-2/Elafin.
Anti-HIV activity of Trappin-2/Elafin: direct and indirect effects
Trappin-2/Elafin is a known antibacterial molecule that has been shown to be effective against both Gram-positive and Gram-negative bacteria.39 As we demonstrated that a synthetic dsRNA analog Poly(I:C) enhances Trappin-2/Elafin production/secretion from FRT epithelial cells, we investigated whether Trappin-2/Elafin could have direct antiviral activity. Because HIV-1 is an important sexually transmitted pathogen, we tested the activity of rTrappin-2/Elafin against HIV-1 X4/T-tropic IIIB and R5/M-tropic BaL. HIV-1 IIIB and BaL were incubated with rTrappin-2/Elafin at 0·01, 0·1, 1 or 10 ng/ml for 1 hr at 37°. TZM-bl indicator cells were plated the previous day at 25 000 cells per well and grown to 70–80% confluence. The virus–Trappin-2/Elafin mixture was added to the TZM cells and incubated for 48 hr at 37°. At the end of the incubation period, Beta-Glo substrate was added to the cells and viral infection was quantified in relative light units using a luminometer. The data were expressed as per cent of control with the virus-only control set at 100%. As shown in Fig. 3, rTrappin-2/Elafin significantly inhibited both IIIB and BaL at all the concentrations tested, achieving up to 80% inhibition of IIIB and up to 60% inhibition of BaL. We demonstrated, by ELISA, that the biological concentrations of Trappin-2/Elafin secreted by epithelial cells, both constitutively and upon Poly(I:C) stimulation, ranged between 0·25 and 9 ng/ml. Therefore, the concentrations of Trappin-2/Elafin showing anti-HIV-1 activity were in the range of physiological levels of this molecule that are secreted by the FRT epithelial cells. Because the inhibitory activity was observed as a result of pre-incubation of HIV-1 with rTrappin-2/Elafin, we believe that the effect of Trappin-2/Elafin on viral infection was direct. Viability studies were conducted in parallel to demonstrate that the inhibitory activity observed was not caused by the toxic effect of rTrappin-2/Elafin on the TZM cells (data not shown).
Figure 3.
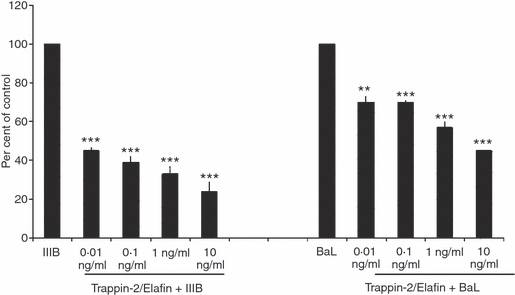
Direct anti-human immunodeficiency virus-1 (HIV-1) activity of recombinant Trappin-2/Elafin. Human recombinant Trappin-2/Elafin at 0·01, 0·1, 1 and 10 ng/ml was incubated with HIV-1 IIIB and BaL at a multiplicity of infection (MOI) of 1 for 1 hr at 37° before addition onto TZM-bl cells. Substrate β-Glo was added 48 hr postinfection and the level of viral infection was quantified using a luminometer. Data were expressed as ‘per cent of control’ with the ‘virus-only’ samples set at 100%. Trappin-2/Elafin was found to significantly inhibit both HIV-1 IIIB and BaL at all doses tested. The P-values were calculated using a two-tailed paired t-test. Significantly greater than control: ***, P < 0·001.
Anti-HIV factors have been shown to inhibit HIV by multiple mechanisms, including through direct interaction with HIV, by blocking cell-surface receptors (CXCR4, CCR5) and by affecting postinfection steps.40,52,53 To demonstrate whether rTrappin-2/Elafin might also have indirect effects on HIV-1 infection by blocking any cell-surface receptors or molecules, we pre-incubated the TZM cells with 0·1 and 1 ng/ml of rTrappin-2/Elafin for 1 hr at 37°. Following incubation, cells were washed repeatedly with 1 × PBS before the addition of HIV-1 IIIB and BaL after which the cells were incubated for 48 hr and infectivity assessed. In contrast to the pre-incubation experiments described above, we observed that pre-exposure of the cells to Trappin-2/Elafin at any of the doses tested had no effect on infectivity of TZM cells by IIIB and BaL (Fig. 4a). This indicates that the inhibitory activity of Trappin-2/Elafin occurs through a direct interaction with the virus rather than at the level of the target cell surface, for example, through the blocking of receptors.
Figure 4.
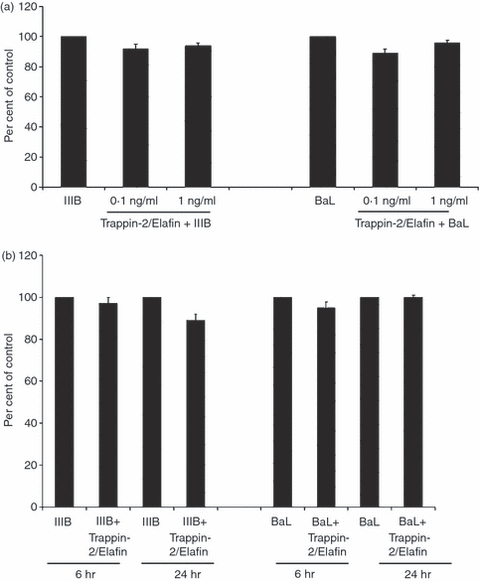
Anti-human immunodeficiency virus-1 (HIV-1) activity of Trappin-2/Elafin did not occur through interaction with TZM-bl cells or through postinfection inhibition mechanisms. (a) Human recombinant Trappin-2/Elafin at 0·1 and 1 ng/ml was added to TZM-bl cells for 1 hr at 37° followed by washing the cells and adding HIV-1 IIIB and BaL at a multiplicity of infection (MOI) of 1. Substrate β-Glo was added 48 hr postinfection and the level of viral infection was quantified using a luminometer. Data were expressed as ‘per cent of control’ with the ‘virus-only’ samples set at 100%. Trappin-2/Elafin did not significantly inhibit either IIIB or BaL HIV-1 when added to the cells before infection. (b) TZM-bl cells were infected with HIV-1 IIIB and BaL at MOI of 1. At 6 and 24 hr postinfection, cells were washed and 1 ng/ml of recombinant Trappin-2/Elafin was added. Assays were developed by addition of substrate β-Glo and viral infection was quantified using a luminometer. Data were expressed as ‘per cent of control’ with the ‘virus-only’ samples set at 100%. With the exception of a slight inhibition observed 24 hr after infection with HIV-1 IIIB, no anti-HIV-1 activity was observed under any of the conditions.
To determine whether Trappin-2/Elafin acts through postinfection mechanisms in addition to directly interacting with the virus, TZM cells were infected with IIIB and/or BaL, washed out at 6 and 24 hr postinfection to remove free virus, after which rTrappin-2/Elafin (1 ng/ml) was added to TZM cells. Other than a slight inhibition observed 24 hr after infection with the IIIB virus, we observed no significant postinfection inhibition (Fig. 4b). Overall, these data indicate that the inhibitory activity of Trappin-2/Elafin occurs through direct interactions with the virus rather than at the level of the cell surface, or through the disabling of postinfection steps.
Because these experiments suggested that antiviral activity might be caused by epithelial cell production of Trappin-2/Elafin, studies were undertaken to remove Trappin-2/Elafin by antibody neutralization. To ensure that the antibody used was sufficient to remove Trappin-2/Elafin, we first attempted to neutralize known amounts of Trappin-2/Elafin. We found that neutralization with rTrappin-2/Elafin (1 ng/ml) resulted in a complete reversal of anti-HIV activity. However, when we attempted to neutralize secretions from primary EM epithelial cell cultures, known to contain Trappin-2/Elafin (0·1 ng/ml), we obtained a statistically significant 20% reversal (data not shown). This finding fits with several studies showing that secretions from the FRT contain between 12 and 20 known antimicrobial factors, many of which has anti-HIV-1 activity.11–14,20,54 These results indicate that Trappin-2/Elafin produced by human uterine epithelial cells in culture is responsible for some of the antiviral activity measured in apical secretions.
Measurement of Trappin-2/Elafin in the CVL of HIV-positive and HIV-negative women
To determine whether Trappin-2/Elafin might be important for protection in vivo, we measured Trappin-2/Elafin levels in CVL from both HIV-positive and HIV-negative women. As seen in Fig. 5, we found Trappin-2/Elafin protein in CVL from both groups of women, at concentrations ranging from 4 to 8 ng/ml. Moreover, while not statistically different, Trappin-2/Elafin levels in HIV-negative women tended to be higher than that measured in HIV-positive women. The differences did not reach statistical significance, possibly because of variation within patient groups (P=0·09). The higher levels of Trappin-2/Elafin measured in HIV-negative women might indicate a protective role that is compromised when the levels are lowered upon infection. When we stratified the data according to race (Fig. 5b), no significant differences were found when HIV-negative Black, Hispanic and White women were compared with HIV-positive women in terms of Trappin-2/Elafin levels. Within each racial group, Trappin-2/Elafin levels were higher in the HIV-negative CVL when compared with the HIV-positive CVL. Again, the differences did not reach statistical significance, possibly because of the variability among patients, despite a general trend towards elevated values in the HIV-negative women compared with the HIV-positive women. When we stratified the HIV-positive CVL according to menstrual status, we observed a significant increase of Trappin-2/Elafin secretion in the secretory phase of the cycle, suggesting that this molecule might be hormonally regulated (Fig. 5c). The presence of Trappin-2/Elafin in CVL suggests that Trappin-2/Elafin might be a relevant molecule for in vivo protection against HIV-1.
Figure 5.
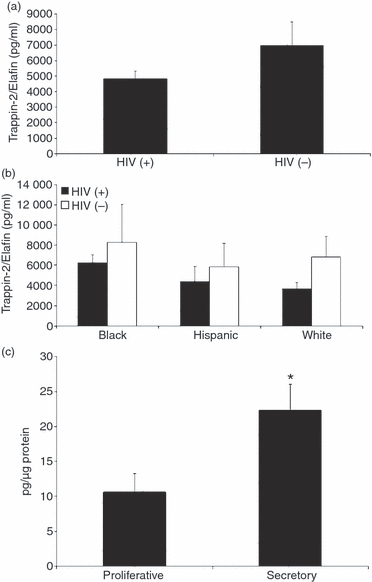
Trappin-2/Elafin protein levels were higher in cervico-vaginal lavages (CVL) obtained from human immunodeficiency virus (HIV)-negative women when compared with HIV-positive women and regulated by the menstrual cycle. (a) Supernatants from CVL obtained from 32 HIV-positive women and 15 HIV-negative women were tested for Trappin-2/Elafin protein by enzyme-linked immunosorbent assay (ELISA). The average for the HIV-negative women was found to be higher than the average for the HIV-positive women. (b) When the patients were stratified by race, the same trend was observed; in each case average levels of Trappin-2/Elafin for the HIV-negative women were higher than those for the HIV-positive women. (c) When HIV-positive patients were stratified according to menstrual status, those in the secretory phase of the cycle produced significantly more Trappin-2/Elafin than those in the proliferative phase of the cycle. The data in this case were normalized to total protein content in each sample and are expressed as pg of Trappin-2/Elafin per μg of protein.
Discussion
The research presented demonstrates that epithelial cells from the upper and lower FRT synthesize and secrete Trappin-2/Elafin. We also found that rTrappin-2/Elafin has potent anti-HIV activity against both X4/T-tropic IIIB and R5/M-tropic BaL HIV-1. To our knowledge this is the first published report of anti-HIV activity of rTrappin-2/Elafin against HIV-1. Furthermore, unlike epithelial cells from the Fallopian tubes, cervix and vagina, uterine epithelial cells respond to Poly(I:C) by secreting increased amounts of Trappin-2/Elafin. Lastly, we observed that Trappin-2/Elafin is present in CVL from both HIV-positive and HIV-negative women, and generally higher levels, although not statistically significant, were observed in HIV-negative women, suggesting that this molecule is normally found in FRT secretions and that it might have anti-HIV protective functions in vivo. Another possible explanation might be that HIV-1 infection can inhibit production of Trappin-2/Elafin.
Previous work from our laboratory has demonstrated that epithelial cells from the upper human and rodent female reproductive tract in culture synthesize and secrete antimicrobials that bathe the mucosal surfaces of the FRT.11–13,54–56 As part of the first line of immune protection, secretions from polarized epithelial cells from the Fallopian tubes, uterus and cervix contain a spectrum of antimicrobials, including SLPI, macrophage inflammatory protein (MIP)-3α, defensins and lactoferrin14,18 (M. Ghosh, unpublished data). Our findings indicate that, as a part of this protection, Trappin-2/Elafin is produced by epithelial cells throughout the upper FRT. Others have shown, by immunohistochemistry, that Trappin-2/Elafin is present in neutrophils and glandular epithelial cells in the uterus during the menstrual cycle30 and in the CVL.57 Our findings extend these observations in several ways. First, this study demonstrates the production of Trappin-2/Elafin by epithelial cells throughout the FRT. Second, our studies suggest that some, if not all, Trappin-2/Elafin in the CVL is the result of the downstream movement of secretions from the upper FRT to the lower FRT. Third, whereas others have reported Trappin-2/Elafin in the CVL of HIV-positive women, our findings demonstrate that Trappin-2/Elafin is present in the CVL of healthy women.57
With regard to hormonal control, King and colleagues reported that expression of Trappin-2/Elafin mRNA peaked during menstruation and was associated with the presence of neutrophils.30 Our previous findings demonstrate that SLPI, as well as other innate immune molecules in the CVL, vary during the menstrual cycle, with the levels of several factors reduced at midcycle.14 The present study extends these findings by demonstrating that Trappin-2/Elafin is present in the CVL from healthy women as well as from HIV-positive women and that Trappin-2/Elafin levels in the CVL vary with the menstrual cycle. We found significantly higher levels of Trappin-2/Elafin during the secretory phase of the menstrual cycle compared with the proliferative phase of the menstrual cycle. Whether these changes are caused by the direct effects of oestradiol on epithelial cells through estrogen receptor α/β (ERα/β) receptors or are the result of hormonally regulated growth factors, such as hepatocyte growth factor (HGF) made by underlying stromal cells49, Fertility and Sterility), remains to be determined.
Our studies indicate that Trappin-2/Elafin is a potent inhibitor of HIV-1 infection. Whereas others have shown that Trappin-2/Elafin has antibacterial activity,39,40 to the best of our knowledge, our study is the first published demonstration that Trappin-2/Elafin blocks both X4 and R5 infectivity of target cells, although Moreau et al.40 refers to a patent application that discusses some aspects of anti-HIV activity of Elafin. We and others have shown that interference with viral infectivity in the FRT is probably caused by a spectrum of endogenous antimicrobials in FRT secretions.11,12,14 For example, HIV inhibition has been reported for the well-characterized anti-HIV molecule SLPI,40 which is homologous to Trappin-2/Elafin.40 While the mechanism of Trappin-2/Elafin inhibition of HIV-1 remains to be determined, its homology with SLPI suggests a similar mechanism of action. SLPI interacts with cell-membrane proteins and can disrupt both viral entry and fusion.40,52 Our findings of anti-HIV activity in the present study indicate that Trappin-2/Elafin contributes to the spectrum of endogenously produced microbicides present in secretions throughout the FRT. That Trappin-2/Elafin anti-HIV-1 activity is direct is suggested from our studies in which pre-incubation of HIV with Trappin-2/Elafin, but not pre-incubation of target cells, blocked target cell infection. Further studies are needed to define more fully the mechanism(s) through which Trappin-2/Elafin protects against viral infection.
Whether protection in the FRT is the result of a single molecule or several acting in synergy remains to be determined. Extensive previous studies from our laboratory have demonstrated that the epithelial cells of the FRT express and produce 10–20 cytokines/chemokines/antimicrobials constitutively and upon stimulation.12,54 Beyond the contribution of Trappin-2/Elafin towards anti-HIV-1 activity, we and others have found that FRT molecules, including SLPI, beta defensins, lactoferrin and MIP3α, have potent anti-HIV activity11–13,50). Venkataraman et al.20 demonstrated that anti-HIV activity in CVL can be attributed to multiple cationic peptides, as the removal of cationic components abrogates this activity. Singh et al. and Chen et al.58,59 reported that in lungs and skin, some cationic peptides act synergistically, whereas others cancel each other out and still others have no effect on each other. Further studies in the FRT are required to determine the contributions of individual molecules towards overall anti-HIV activity. As mucosal antimicrobials interact in a very complex manner, it is unlikely that deletion of single molecules would affect the overall antimicrobial activity of the secretions.41,60
What remains to be determined is why, in spite of the presence of Trappin-2/Elafin and other endogenous antiviral molecules, women become infected with HIV. As discussed elsewhere, we have reviewed the literature and concluded that multiple immunological parameters in the upper and lower FRT are suppressed at midcycle, between the time of ovulation and implantation, to optimize the conditions for fertilization and implantation.28 As a result, we postulate that for a 7-day time-period beginning with ovulation, there exists a window of vulnerability when a woman might be more likely to be infected by HIV.28 With specific reference to the innate immune system, we and others have reported that antiviral molecules, including SLPI, defensins, etc., are lowest at this time relative to early proliferative and late secretory stages of the menstrual cycle.28 It remains to be determined whether nadir levels are below the threshold of immune protection as a result of the direct effects of sex hormones on immune cell synthesis and secretion. Beyond the absolute level of these molecules in CVL, the biological activity of each must also be considered. For example, others have recently reported that kallikreins, a family of serine proteases known for their influence on the development of innate antimicrobial peptide function, are present in FRT secretions.61 As kallikreins vary with stage of the menstrual cycle,62,63 these findings suggest that conversion of inactive molecules to biologically active ones may be as important as the levels of antimicrobials present in FRT secretions. Another processing molecule is the serine protease CD26, which is important for activating chemokines such as stromal derived growth factor-1 (SDF-1) and MIP1β that block the cell-surface receptors required for HIV entry.64,65
Our finding of Trappin-2/Elafin and other antimicrobials being produced and secreted into the lumen by upper FRT cells provides an explanation for what has been a paradoxical observation. It is well established that bacteria reach the upper FRT within minutes of vaginal deposition.66 This is in contrast to findings that culturable bacteria from the upper FRT are only infrequently recovered.67 Our findings, in the present study, that Trappin-2/Elafin is secreted throughout the FRT along with other microbicides, suggests that entry of pathogens to the upper tract may lead to rapid inactivation by the first-line defenders of the innate immune system.
An unexpected finding in our studies was that only UT epithelial cells consistently responded to Poly(I:C), a viral dsRNA analog, whereas epithelial cells from the FT and Cx were unresponsive. Previously, we and others demonstrated that epithelial cells throughout the FRT (FT, UT and Cx) respond to Poly(I:C) by producing a spectrum of cytokines and chemokines.11,12,56 Our findings suggest a specialized function of UT epithelial cells not previously appreciated. UT epithelial cell responsiveness to Poly(I:C) may be related to the uterus being an implantation site, to protect against potential pathogens that enter along with sperm. As Trappin-2/Elafin has important anti-inflammatory functions,40 and is expressed at high levels in normal pregnant uterus,68 it may be that this molecule dampens immune responses in preparation for the implantation of an allogeneic fetus. Whether unresponsiveness of FT and Cx epithelial cells is a result of these cells being fully activated in terms of antimicrobial production before exposure to Poly(I:C) remains to be determined. What is clear is that FT cells are selectively responsive in that, while unresponsive in terms of Trappin-2/Elafin, Poly(I:C) increases intracellular interferon-β (IFN-β)-induced gene expression of 2′-5′-oligoadenylate synthetase (2′5′-OAS) and MxA, the pro-inflammatory cytokines interleukin-8 (IL-8) and tumour necrosis factor-α (TNF-α) as well as the innate immune factor human β-defensin 2.11
The present study demonstrates that Trappin-2/Elafin is present in CVL secretions collected from HIV-positive and HIV-negative women. We have recently found that CVL from both populations have anti-HIV activity against X4 and R5 HIV-1 (M. Ghosh and J. V. Fahey, unpublished data). These findings suggest that Trappin-2/Elafin may play an important protective role in vivo against the transmission of HIV from men to women. Furthermore, it suggests an explanation for the low amounts of infectious HIV typically found in CVL samples, irrespective of viral load.26,27 The role of Trappin-2/Elafin in HIV-1 infection could be further defined by studying discordant couples and highly exposed seronegative women. Although such studies will provide important insights, they are beyond the scope of this investigation.
In conclusion, our studies have identified Trappin-2/Elafin as a novel endogenous anti-HIV-1 factor of the female reproductive tract. We have established that Trappin-2/Elafin is produced constitutively by upper and lower FRT epithelial cells and that the uterine epithelial cells can be consistently stimulated by Poly(I:C) to produce elevated levels of Trappin-2/Elafin that are inhibitory to HIV-1. We have also shown that this molecule might be important in vivo because CVL of HIV-negative women generally show higher levels of Trappin-2/Elafin compared with CVL of HIV-positive women. Similar studies are likely to identify other such endogenous molecules that can act in a complex synergy to protect the FRT from harmful pathogens.
Acknowledgments
The authors thank Richard Rossoll, MS (Dartmouth Medical School), Deena Ratner, BS (University of Pittsburgh), Irma Rodriguez (Brown University) and Jessica Ingersoll, MS (Emory University), for excellent technical assistance in the preparation of samples, cells and virus stocks. The authors also thank Dr Phalguni Gupta (University of Pittsburgh) for generous sharing of reagents and information. Additionally, the authors thank Vincent Memoli, MD, Section Chief of Anatomical Pathology, for procuring tissues; other members of the Department of Pathology for inspecting and dissecting tissue specimens: Jorge Gonzalez, MD, Alan Schned, MD, Peter Seery, Shannon Schutz, Elizabeth Rizzo, Richard Merrill, Charles-Robert Moultry, Patricia Larkin, Aimee Larson, Jennifer Simonton and Dawn Maddaline; for clinical support and scheduling: Laura Wolfe, Linda Hallock, Kathleen Pilchman, Karen Carter, Kris Ramsey, Tamara Krivit and Joanne Lavin; surgeons: Barry Smith, Joan Barthold, Jackson Beecham, John Currie, Leslie Demars, Paul Hanissian, John Ketterer, Benjamin Mahlab, Paul Manganiello, Misty Porter, Karen George, William Young, Kris Strohbehn, Roger Young, Stephen Andrews and Eric Sailer; and OR nurses: Jeanette Sawyer, Tracy Stokes, Fran Reinfrank and Jaclyn Logan. This work was supported by AI51877 awarded to Dr Charles Wira from National Institute of Health; by AI40350 and AI066884 awarded to Dr Susan Cu-Uvin from National Institute of Health; and by Lifespan/Tufts/Brown CFAR P30AI42853 and CDC CCU106795 awarded to Dr Susan Cu-Uvin and Dr Kenneth Mayer.
Disclosures
The authors have no conflicts of interest to declare.
References
- 1.Padian NS, van der Straten A, Ramjee G, et al. Diaphragm and lubricant gel for prevention of HIV acquisition in southern African women: a randomised controlled trial. Lancet. 2007;370:251–61. doi: 10.1016/S0140-6736(07)60950-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen J. AIDS research. Promising AIDS vaccine’s failure leaves field reeling. Science. 2007;318:28–9. doi: 10.1126/science.318.5847.28. [DOI] [PubMed] [Google Scholar]
- 3.Van Damme L, Govinden R, Mirembe FM, et al. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. N Engl J Med. 2008;359:463–72. doi: 10.1056/NEJMoa0707957. [DOI] [PubMed] [Google Scholar]
- 4.Bolognesi N. AIDS gel’s failure calls prevention approach into question. Nat Med. 2007;13:230. doi: 10.1038/nm0307-230b. [DOI] [PubMed] [Google Scholar]
- 5.Honey K. Microbicide trial screeches to a halt. J Clin Invest. 2007;117:1116. doi: 10.1172/JCI32291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CDC . HIV/AIDS Among Women. http://wwwcdcgov/hiv/topics/women/resources/factsheets/pdf/womenpdf 2007. [Google Scholar]
- 7.Comparison of female to male and male to female transmission of HIV in 563 stable couples. European Study Group on Heterosexual Transmission of HIV. BMJ. 1992;304:809–13. doi: 10.1136/bmj.304.6830.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ndesendo VM, Pillay V, Choonara YE, Buchmann E, Bayever DN, Meyer LC. A review of current intravaginal drug delivery approaches employed for the prophylaxis of HIV/AIDS and prevention of sexually transmitted infections. AAPS PharmSciTech. 2008;9:505–20. doi: 10.1208/s12249-008-9073-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gray RH, Wawer MJ, Brookmeyer R, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1discordant couples in Rakai, Uganda. Lancet. 2001;357:1149–53. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 10.Wawer MJ, Gray RH, Sewankambo NK, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–9. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 11.Ghosh M, Schaefer TM, Fahey JV, Wright JA, Wira CR. Antiviral responses of human Fallopian tube epithelial cells to toll-like receptor 3 agonist poly(I:C) Fertil Steril. 2008;89(Suppl. 5):1497–506. doi: 10.1016/j.fertnstert.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaefer TM, Fahey JV, Wright JA, Wira CR. Innate immunity in the human female reproductive tract: antiviral response of uterine epithelial cells to the TLR3 agonist poly(I:C) J Immunol. 2005;174:992–1002. doi: 10.4049/jimmunol.174.2.992. [DOI] [PubMed] [Google Scholar]
- 13.Fahey JV, Wira CR. Effect of menstrual status on antibacterial activity and secretory leukocyte protease inhibitor production by human uterine epithelial cells in culture. J Infect Dis. 2002;185:1606–13. doi: 10.1086/340512. [DOI] [PubMed] [Google Scholar]
- 14.Keller MJ, Guzman E, Hazrati E, et al. PRO 2000 elicits a decline in genital tract immune mediators without compromising intrinsic antimicrobial activity. AIDS. 2007;21:467–76. doi: 10.1097/QAD.0b013e328013d9b5. [DOI] [PubMed] [Google Scholar]
- 15.Cole AM. Innate host defense of human vaginal and cervical mucosae. Curr Top Microbiol Immunol. 2006;306:199–230. [PubMed] [Google Scholar]
- 16.Fichorova RN, Cronin AO, Lien E, Anderson DJ, Ingalls RR. Response to Neisseria gonorrhoeae by cervicovaginal epithelial cells occurs in the absence of toll-like receptor 4-mediated signaling. J Immunol. 2002;168:2424–32. doi: 10.4049/jimmunol.168.5.2424. [DOI] [PubMed] [Google Scholar]
- 17.O’Connell CM, Ionova IA, Quayle AJ, Visintin A, Ingalls RR. Localization of TLR2 and MyD88 to Chlamydia trachomatis inclusions. Evidence for signaling by intracellular TLR2 during infection with an obligate intracellular pathogen. J Biol Chem. 2006;281:1652–9. doi: 10.1074/jbc.M510182200. [DOI] [PubMed] [Google Scholar]
- 18.Ochiel D, Fahey JV, Ghosh M, Haddad SN, Wira CR. Innate immunity in the female reproductive tract: role of sex hormones in regulating uterine epithelial cell protection against pathogens. Curr Womens Health Rev. 2008;4:102–17. doi: 10.2174/157340408784246395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horne AW, Stock SJ, King AE. Innate immunity and disorders of the female reproductive tract. Reproduction. 2008;135:739–49. doi: 10.1530/REP-07-0564. [DOI] [PubMed] [Google Scholar]
- 20.Venkataraman N, Cole AL, Svoboda P, Pohl J, Cole AM. Cationic polypeptides are required for anti-HIV-1 activity of human vaginal fluid. J Immunol. 2005;175:7560–7. doi: 10.4049/jimmunol.175.11.7560. [DOI] [PubMed] [Google Scholar]
- 21.Fiore JR, Suligoi B, Saracino A, et al. Correlates of HIV-1 shedding in cervicovaginal secretions and effects of antiretroviral therapies. AIDS. 2003;17:2169–76. doi: 10.1097/00002030-200310170-00004. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan ST, Mandava U, Evans-Strickfaden T, Lennox JL, Ellerbrock TV, Hart CE. Diversity, divergence, and evolution of cell-free human immunodeficiency virus type 1 in vaginal secretions and blood of chronically infected women: associations with immune status. J Virol. 2005;79:9799–809. doi: 10.1128/JVI.79.15.9799-9809.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Philpott S, Burger H, Tsoukas C, Foley B, Anastos K, Kitchen C, Weiser B. Human immunodeficiency virus type 1 genomic RNA sequences in the female genital tract and blood: compartmentalization and intrapatient recombination. J Virol. 2005;79:353–63. doi: 10.1128/JVI.79.1.353-363.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benki S, Mostad SB, Richardson BA, Mandaliya K, Kreiss JK, Overbaugh J. Increased levels of HIV-1-infected cells in endocervical secretions after the luteinizing hormone surge. J Acquir Immune Defic Syndr. 2008;47:529–34. doi: 10.1097/QAI.0b013e318165b952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reichelderfer PS, Coombs RW, Wright DJ, et al. Effect of menstrual cycle on HIV-1 levels in the peripheral blood and genital tract. WHS 001 Study Team. AIDS. 2000;14:2101–7. doi: 10.1097/00002030-200009290-00005. [DOI] [PubMed] [Google Scholar]
- 26.Cummins JE, Jr, Villanueva JM, Evans-Strickfaden T, et al. Detection of infectious human immunodeficiency virus type 1 in female genital secretions by a short-term culture method. J Clin Microbiol. 2003;41:4081–8. doi: 10.1128/JCM.41.9.4081-4088.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovacs A, Chan LS, Chen ZC, Meyer WA, III, Muderspach L, Young M, Anastos K, Levine AM. HIV-1 RNA in plasma and genital tract secretions in women infected with HIV-1. J Acquir Immune Defic Syndr. 1999;22:124–31. doi: 10.1097/00126334-199910010-00003. [DOI] [PubMed] [Google Scholar]
- 28.Wira CR, Fahey JV. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. AIDS. 2008;22:1909–17. doi: 10.1097/QAD.0b013e3283060ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghosh M. Endogenous Microbicides of the Human Female Reproductive Tract: Innate Anti-HIV-1 Activity in Epithelial Cell Secretions. New Delhi, India: Microbicides 2008; 2008. [Google Scholar]
- 30.King AE, Critchley HO, Kelly RW. Innate immune defences in the human endometrium. Reprod Biol Endocrinol. 2003;1:116. doi: 10.1186/1477-7827-1-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pioli PA, Weaver LK, Schaefer TM, Wright JA, Wira CR, Guyre PM. Lipopolysaccharide-induced IL-1 beta production by human uterine macrophages up-regulates uterine epithelial cell expression of human beta-defensin 2. J Immunol. 2006;176:6647–55. doi: 10.4049/jimmunol.176.11.6647. [DOI] [PubMed] [Google Scholar]
- 32.Hirata T, Osuga Y, Hamasaki K, et al. Expression of toll-like receptors 2, 3, 4, and 9 genes in the human endometrium during the menstrual cycle. J Reprod Immunol. 2007;2:53–60. doi: 10.1016/j.jri.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 33.Bingle CD, Vyakarnam A. Novel innate immune functions of the whey acidic protein family. Trends Immunol. 2008;29:444–53. doi: 10.1016/j.it.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Bingle L, Tetley TD, Bingle CD. Cytokine-mediated induction of the human elafin gene in pulmonary epithelial cells is regulated by nuclear factor-kappaB. Am J Respir Cell Mol Biol. 2001;25:84–91. doi: 10.1165/ajrcmb.25.1.4341. [DOI] [PubMed] [Google Scholar]
- 35.Schalkwijk J, Wiedow O, Hirose S. The trappin gene family: proteins defined by an N-terminal transglutaminase substrate domain and a C-terminal four-disulphide core. Biochem J. 1999;3:569–77. [PMC free article] [PubMed] [Google Scholar]
- 36.Pfundt R, van Ruissen F, van Vlijmen-Willems IM, et al. Constitutive and inducible expression of SKALP/elafin provides anti-elastase defense in human epithelia. J Clin Invest. 1996;98:1389–99. doi: 10.1172/JCI118926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sallenave JM, Shulmann J, Crossley J, Jordana M, Gauldie J. Regulation of secretory leukocyte proteinase inhibitor (SLPI) and elastase-specific inhibitor (ESI/elafin) in human airway epithelial cells by cytokines and neutrophilic enzymes. Am J Respir Cell Mol Biol. 1994;11:733–41. doi: 10.1165/ajrcmb.11.6.7946401. [DOI] [PubMed] [Google Scholar]
- 38.Simpson AJ, Maxwell AI, Govan JR, Haslett C, Sallenave JM. Elafin (elastase-specific inhibitor) has anti-microbial activity against gram-positive and gram-negative respiratory pathogens. FEBS Lett. 1999;452:309–13. doi: 10.1016/s0014-5793(99)00670-5. [DOI] [PubMed] [Google Scholar]
- 39.Baranger K, Zani ML, Chandenier J, Dallet-Choisy S, Moreau T. The antibacterial and antifungal properties of trappin-2 (pre-elafin) do not depend on its protease inhibitory function. FEBS J. 2008;275:2008–20. doi: 10.1111/j.1742-4658.2008.06355.x. [DOI] [PubMed] [Google Scholar]
- 40.Moreau T, Baranger K, Dade S, Dallet-Choisy S, Guyot N, Zani ML. Multifaceted roles of human elafin and secretory leukocyte proteinase inhibitor (SLPI), two serine protease inhibitors of the chelonianin family. Biochimie. 2008;90:284–95. doi: 10.1016/j.biochi.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 41.Williams SE, Brown TI, Roghanian A, Sallenave JM. SLPI and elafin: one glove, many fingers. Clin Sci (Lond) 2006;110:21–35. doi: 10.1042/CS20050115. [DOI] [PubMed] [Google Scholar]
- 42.Pol A, Pfundt R, Zeeuwen P, Molhuizen H, Schalkwijk J. Transcriptional regulation of the elafin gene in human keratinocytes. J Invest Dermatol. 2003;120:301–7. doi: 10.1046/j.1523-1747.2003.12043.x. [DOI] [PubMed] [Google Scholar]
- 43.Roghanian A, Williams SE, Sheldrake TA, Brown TI, Oberheim K, Xing Z, Howie SE, Sallenave JM. The antimicrobial/elastase inhibitor elafin regulates lung dendritic cells and adaptive immunity. Am J Respir Cell Mol Biol. 2006;34:634–42. doi: 10.1165/rcmb.2005-0405OC. [DOI] [PubMed] [Google Scholar]
- 44.Moutsopoulos NM, Greenwell-Wild T, Wahl SM. Differential mucosal susceptibility in HIV-1 transmission and infection. Adv Dent Res. 2006;19:52–6. doi: 10.1177/154407370601900111. [DOI] [PubMed] [Google Scholar]
- 45.Skott P, Lucht E, Ehnlund M, Bjorling E. Inhibitory function of secretory leukocyte proteinase inhibitor (SLPI) in human saliva is HIV-1 specific and varies with virus tropism. Oral Dis. 2002;8:160–7. doi: 10.1034/j.1601-0825.2002.01807.x. [DOI] [PubMed] [Google Scholar]
- 46.Fahey JV, Prabhala RH, Guyre PM, Wira CR. Antigen-presenting cells in the human female reproductive tract: analysis of antigen presentation in pre- and post-menopausal women. Am J Reprod Immunol. 1999;42:49–57. doi: 10.1111/j.1600-0897.1999.tb00465.x. [DOI] [PubMed] [Google Scholar]
- 47.Fahey JV, Humphrey SL, Stern JE, Wira CR. Secretory component production by polarized epithelial cells from the human female reproductive tract. Immunol Invest. 1998;27:167–80. doi: 10.3109/08820139809089454. [DOI] [PubMed] [Google Scholar]
- 48.Wallace PK, Yeaman GR, Johnson K, Collins JE, Guyre PM, Wira CR. MHC class II expression and antigen presentation by human endometrial cells. J Steroid Biochem Mol Biol. 2001;5:203–11. doi: 10.1016/s0960-0760(00)00149-7. [DOI] [PubMed] [Google Scholar]
- 49.Richardson JM, Kaushic C, Wira CR. Polymeric immunoglobin (Ig) receptor production and IgA transcytosis in polarized primary cultures of mature rat uterine epithelial cells. Biol Reprod. 1995;53:488–98. doi: 10.1095/biolreprod53.3.488. [DOI] [PubMed] [Google Scholar]
- 50.Godfrey TE, Kim SH, Chavira M, Ruff DW, Warren RS, Gray JW, Jensen RH. Quantitative mRNA expression analysis from formalin-fixed, paraffin-embedded tissues using 5′ nuclease quantitative reverse transcription-polymerase chain reaction. J Mol Diagn. 2000;2:84–91. doi: 10.1016/S1525-1578(10)60621-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei X, Decker JM, Liu H, et al. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother. 2002;46:1896–905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma G, Greenwell-Wild T, Lei K, Jin W, Swisher J, Hardegen N, Wild CT, Wahl SM. Secretory leukocyte protease inhibitor binds to annexin II, a cofactor for macrophage HIV-1 infection. J Exp Med. 2004;200:1337–46. doi: 10.1084/jem.20041115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amella CA, Sherry B, Shepp DH, Schmidtmayerova H. Macrophage inflammatory protein 1alpha inhibits postentry steps of human immunodeficiency virus type 1 infection via suppression of intracellular cyclic AMP. J Virol. 2005;79:5625–31. doi: 10.1128/JVI.79.9.5625-5631.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fahey JV, Schaefer TM, Channon JY, Wira CR. Secretion of cytokines and chemokines by polarized human epithelial cells from the female reproductive tract. Hum Reprod. 2005;20:1439–46. doi: 10.1093/humrep/deh806. [DOI] [PubMed] [Google Scholar]
- 55.Soboll G, Crane-Godreau MA, Lyimo MA, Wira CR. Effect of oestradiol on PAMP-mediated CCL20/MIP-3 alpha production by mouse uterine epithelial cells in culture. Immunology. 2006;118:185–94. doi: 10.1111/j.1365-2567.2006.02353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soboll G, Shen L, Wira CR. Expression of Toll-like receptors (TLR) and responsiveness to TLR agonists by polarized mouse uterine epithelial cells in culture. Biol Reprod. 2006;75:131–9. doi: 10.1095/biolreprod.106.050690. [DOI] [PubMed] [Google Scholar]
- 57.Mlinar D, Ball TB, Iqbal SM, Kimani J, Wachihi C, Plummer FA. The Role of Genital Tract Trappin-2/Elafin And RANTES in Mediating Resistance to HIV-1 Infection. Montreal, Quebec, Canada: CAHR; 2008. [Google Scholar]
- 58.Singh PK, Tack BF, McCray PB, Jr, Welsh MJ. Synergistic and additive killing by antimicrobial factors found in human airway surface liquid. Am J Physiol Lung Cell Mol Physiol. 2000;279:L799–805. doi: 10.1152/ajplung.2000.279.5.L799. [DOI] [PubMed] [Google Scholar]
- 59.Chen X, Niyonsaba F, Ushio H, Okuda D, Nagaoka I, Ikeda S, Okumura K, Ogawa H. Synergistic effect of antibacterial agents human beta-defensins, cathelicidin LL-37 and lysozyme against Staphylococcus aureus and Escherichia coli. J Dermatol Sci. 2005;40:123–32. doi: 10.1016/j.jdermsci.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 60.Levy O. Antimicrobial proteins and peptides: anti-infective molecules of mammalian leukocytes. J Leukoc Biol. 2004;76:909–25. doi: 10.1189/jlb.0604320. [DOI] [PubMed] [Google Scholar]
- 61.Yamasaki K, Schauber J, Coda A, et al. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB J. 2006;20:2068–80. doi: 10.1096/fj.06-6075com. [DOI] [PubMed] [Google Scholar]
- 62.Clements J, Mukhtar A, Yan S, Holland A. Kallikreins and kinins in inflammatory-like events in the reproductive tract. Pharmacol Res. 1997;35:537–40. doi: 10.1006/phrs.1997.0183. [DOI] [PubMed] [Google Scholar]
- 63.Shaw JL, Diamandis EP. A potential role for tissue kallikrein-related peptidases in human cervico-vaginal physiology. Biol Chem. 2008;389:681–8. doi: 10.1515/BC.2008.069. [DOI] [PubMed] [Google Scholar]
- 64.Ajami K, Pitman MR, Wilson CH, et al. Stromal cell-derived factors 1alpha and 1beta, inflammatory protein-10 and interferon-inducible T cell chemo-attractant are novel substrates of dipeptidyl peptidase 8. FEBS Lett. 2008;582:819–25. doi: 10.1016/j.febslet.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 65.Wolf M, Albrecht S, Marki C. Proteolytic processing of chemokines: implications in physiological and pathological conditions. Int J Biochem Cell Biol. 2008;7:1185–98. doi: 10.1016/j.biocel.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 66.Yeaman GR, Howell AL, Weldon S, et al. Human immunodeficiency virus receptor and coreceptor expression on human uterine epithelial cells: regulation of expression during the menstrual cycle and implications for human immunodeficiency virus infection. Immunology. 2003;109:137–46. doi: 10.1046/j.1365-2567.2003.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wira CR, Grant-Tschudy KS, Crane-Godreau MA. Epithelial cells in the female reproductive tract: a central role as sentinels of immune protection. Am J Reprod Immunol. 2005;53:65–76. doi: 10.1111/j.1600-0897.2004.00248.x. [DOI] [PubMed] [Google Scholar]
- 68.King AE, Kelly RW, Sallenave JM, Bocking AD, Challis JR. Innate immune defences in the human uterus during pregnancy. Placenta. 2007;12:1099–106. doi: 10.1016/j.placenta.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 69.Coleman KD, Wright JA, Ghosh M, Wira CR, Fahey JV. Estradiol modulation of hepatocyte growth factor by stromal fibroblasts in the female reproductive tract. Fertil Steril. doi: 10.1016/j.fertnstert.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ghosh M, Shen Z, Schaefer TM, Fahey JV, Gupta P, Wira CR. CCL20/MIP3alpha is a novel anti-HIV-1 molecule of the human female reproductive tract. Am J Reprod Immunol. 2009;62:60–71. doi: 10.1111/j.1600-0897.2009.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


