Abstract
We investigated the distribution of natural killer (NK) cell subsets, their activating and inhibitory receptors, and their cytolytic potential, in primary human immunodeficiency virus (HIV)-infected (PHI) individuals at baseline and during 1 year of follow-up with or without antiretroviral therapy, and compared the results with those obtained in treatment-naïve, chronically HIV-infected (CHI) individuals, and HIV-seronegative (HN) healthy individuals. The proportion of the CD56dim and CD56bright subsets decreased with disease progression, whereas that of the CD56− CD16+ subset increased. In the CD56dim subset, the proportion of cells with natural cytotoxicity receptors (NCRs) decreased with disease progression, and their cytolytic potential was reduced. Conversely, the CD56bright subset was characterized by a high proportion of NCR-positive, killer cell immunoglobulin-like receptor (KIR)-positive NKG2A+ cells in both CHI and PHI individuals, which was associated with an increase in their cytolytic potential. During the 1 year of follow-up, the PHI individuals with high viraemia levels and low CD4+ T-cell counts who received highly active antiretroviral therapy (HAART) had a similar proportion of NK subsets to CHI individuals, while patients with low viraemia levels and high CD4+ T-cell counts who remained untreated had values similar to those of the HN individuals. Our results indicate a marked perturbation of the NK cell compartment during HIV-1 infection that is multifaceted, starts early and is progressive, primarily involves the CD56bright subset, and is partially corrected by effective HAART.
Keywords: activation, cell surface molecules, flow cytometry/fluorescence-activated cell sorting, human immunodeficiency virus, natural killer cells
Introduction
Successful host defence against invading pathogens relies on the concerted action of innate and adaptive immunity. Natural killer (NK) cells participate in innate immunity mainly by directly killing infected cells, but also play an important role in determining the outcome of the adaptive immune response by releasing cytokines and chemokines, and co-operating with dendritic cells (DCs).1
The effector function of NK cells is finely tuned by the opposite signals delivered by inhibitory and activating receptors.2 The principal inhibitory receptors in humans are killer cell immunoglobulin-like receptors (KIRs), which recognize polymorphic determinants on major histocompatibility complex (MHC) class I molecules, and the NKG2A C-type lectin receptor, which recognizes non-conventional MHC class-1b ligands.3,4 The principal activating receptors are NKG2D,5 and the natural cytotoxicity receptors (NCRs) NKp30, NKp46 and NKp44 [the last being expressed only by interleukin (IL)-2-activated NK cells].6 Human NKG2D ligands are self molecules induced on target cells by ‘stress signals’7, while cellular ligands for NCRs remain currently elusive.8–10
More than 90% of mature circulating NK cells in healthy humans are phenotypically characterized as CD16+CD56dim; the other 10% consist of CD16− CD56bright and a very small subset of CD16+ CD56− cells.11 Resting CD56dim NK cells have abundant intracellular perforin, show enhanced cytotoxicity, express clonally distributed KIRs, NCRs and C-type lectin receptors, and carry homing receptors for inflamed peripheral sites.12 Resting CD56bright NK cells lack perforin, but secrete large amounts of a wide range of cytokines and express high levels of C-type lectin receptors and no surface NCRs, and a very small fraction express KIRs. They express homing receptors for secondary lymphoid organs, where they may have immune-regulatory functions.13 After activation, the cytotoxic activity of CD56bright cells is similar to or greater than that of CD56dim cells, and they start expressing KIRs, NCRs and homing receptors for inflamed tissues.13,14
NK cells may play a critical role in the natural history of human immunodeficiency virus (HIV) infection, and in protecting individuals at high risk of infection.15,16 Two recent studies have shown that individuals expressing the human leucocyte antigen (HLA)-Bw4 epitope and certain alleles of the KIR3DL1 locus, as well as those expressing the activating KIR3DS1 receptor in the presence of its ligand Bw4-80I, show better control of HIV viraemia and progress to acquired immune deficiency syndrome (AIDS) more slowly.17–19
The number of circulating NK cells may increase early during primary HIV infection and decline with disease progression,20–22 particularly for the CD16+ CD56dim subset, whereas the proportion of the CD16+ CD56− subset increases in individuals with high viremia levels.23–26 Some studies have shown that the number of NK cells expressing inhibitory KIRs increases in individuals with HIV viraemia, but others have reported no change.25–27 The decrease in the expression of NCRs has been more consistently described, and is associated with an impairment of the cytolytic function of total NK cells that normalizes after effective highly active antiretroviral therapy (HAART).25,28
A few studies have analysed the impact of early HIV infection on the NK cell compartment. Alter et al.29,30 found that there was a significant expansion of the CD56dim NK subset during acute infection, before seroconversion, with a rapid decline after the start of HAART. Ongoing viral replication led to the depletion of the CD56bright subset and a parallel increase in the CD16+ CD56− subset, accompanied by reduced functional NK cell activity.30
The aim of the present study was to analyse the distribution of NK subsets and the expression of their main activating and inhibitory receptors, as well as their cytolytic potential, at baseline and during a 1-year follow-up period in primary HIV-infected (PHI) individuals, starting HAART or not, and to compare the results with those obtained in treatment-naïve, chronically HIV-infected (CHI) individuals and HIV-seronegative (HN) healthy individuals.
Subjects and methods
Study population
Thirty-four PHI individuals were consecutively enrolled from October 2004 to March 2006 at the Clinic of Infectious Diseases, San Raffaele Scientific Institute, Milan, Italy. The study was approved by the Institutional Review Board.
Primary HIV infection was defined as recent (< 3 months) exposure to HIV-1, and the presence of clinical findings consisting of an acute retroviral syndrome regardless of its severity, or laboratory findings consisting of detectable plasma HIV-RNA or HIV-1 p24 Gag antigenaemia, associated with a negative or weakly positive enzyme-linked immunosorbent assay (ELISA) with increasing reactivity over time, and a western blot assay with ≤ 3 bands for anti-HIV-1 antibodies (Abs). These individuals were followed up for 12 months, during which time, on the basis of the clinical, immunological and virological findings, 18 individuals remained untreated and 16 initiated a HAART regimen consisting of a combination of two nucleotide reverse transcriptase inhibitors (NRTIs) and either one HIV protease inhibitor (PI) or a non-NRTI (NNRTI). HAART was offered to individuals with ≤ 300 CD4 cells/μl and/or HIV-RNA levels ≥ 105 copies/ml, and to those with persistently high-grade symptoms of acute retroviral syndrome. Peripheral venous blood was drawn for analysis at the time of the first visit (baseline), and after 4, 24 and 48 weeks.
Forty-seven treatment-naïve CHI individuals (some of whom had a recent diagnosis of HIV infection, but were likely to have a long-standing infection according to the medical history, the complete pattern of anti-HIV-1 Abs and the time of exposure; mean time from complete seroconversion = 30 months) and 34 HN individuals were enrolled and tested as control groups. All of the enrolled individuals gave written informed consent.
Monoclonal antibodies (mAbs)
The mAbs used were: fluorescein isothiocyanate (FITC)-conjugated anti-CD3 (UCHT1; Beckman-Coulter, Marseille, France); phycoerythrin-cyanin-5 (PC5)-conjugated anti-CD56 (NKH-1; Beckman-Coulter); phycoerythrin (PE)-conjugated anti-CD4 (RPA-T4), anti-CD8 (RPA-T8), anti-CD16 (3G8) and anti-CD107a (H4A3) (BD Pharmingen, San Diego, CA), and anti-NKG2A (Z199), anti-NKG2D (ON72), anti-NKp30 (Z25), anti-NKp46 (BAB281), anti-NKp44 (Z231), anti-CD158b (GL183 or KIR2DL2), anti-CD158e (Z27·3·7 or KIR3DL1) and anti-CD158i (FES172 or KIR2DS4) (Beckman-Coulter). Appropriate anti-isotypic mAbs (Beckman-Coulter) were used as negative controls.
The BAB281, AZ20 (anti-NKp30), Z231, ON72 and Z199 mAbs used at saturation in the redirected CD107a expression assay were provided by A. Moretta and D. Pende (University of Genoa, Genoa, Italy), and the MOV10 anti-human ovary carcinoma mAb, used as an irrelevant Ab, was a generous gift of S. Menard (National Cancer Institute, Milan, Italy).
Flow cytometry
Peripheral venous blood was collected in ethylenediaminetetraacetic acid (EDTA)-containing tubes and centrifuged over a Ficoll gradient to separate the peripheral blood mononuclear cells (PBMCs), which were divided into aliquots and cryopreserved. After thawing, viable PBMCs (2 × 106 cells/ml) in RPMI-1640 culture medium (BioWhittaker Europe, Verviers, Belgium) plus 10% fetal bovine serum (FBS; Cambrex, Verviers, Belgium), 2 mm l-glutamine and 50 U/ml penicillin/streptomycin were incubated with saturating concentrations of human immunoglobulin G (IgG) (Endobulin; Immuno S.r.l., Pisa, Italy) for 20 min on ice, and then stained with the appropriate fluorochrome-conjugated mAbs. Based on forward and side scatter analysis, we gated first on lymphocytes and then on CD3− cells. NK cells were defined as CD3− CD56+ lymphocytes, and their expression of different NK receptors (NKRs) or CD16 molecules was analysed using three-colour staining. A representative dot plot of the expression of some NKRs and CD16 is shown in Fig. 1.
Figure 1.
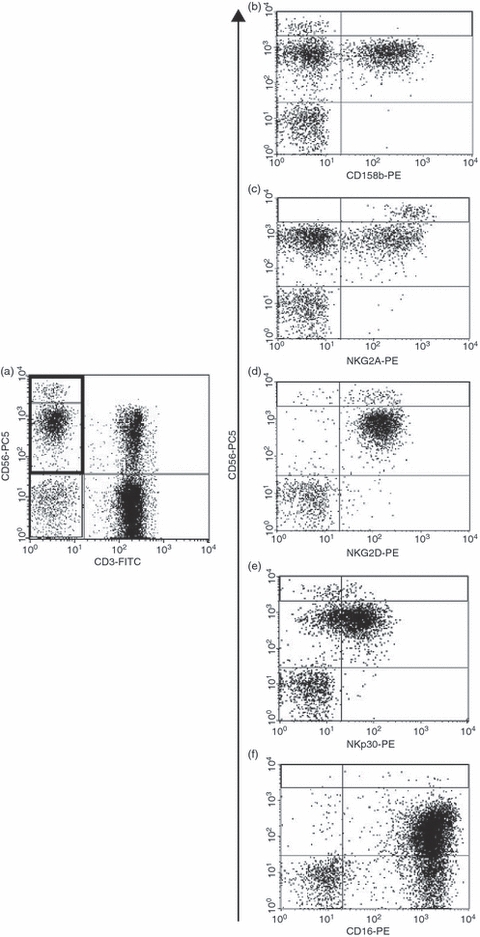
Representative dot plots of the expression of surface receptors on natural killer (NK) cells. Peripheral blood mononuclear cells were stained with anti-CD3-fluorescein isothiocyanate (FITC), anti-CD56-phycoerythrin-cyanin-5 (PC5), and a series of phycoerythrin (PE)-conjugated anti-NK cell receptor monoclonal antibodies (mAbs). Based on forward and side scatter analysis, we first gated on lymphocytes (not shown) and then on CD3− cells (a), and the expression of the different NK cell receptors in the CD56dim or CD56bright subsets was analysed (b–f).
CD107a expression
The frequency of degranulating NK cells (CD107a expression) was quantified by means of multiparametric flow cytometry, in part according to Alter et al.31 Briefly, frozen PBMCs were thawed in RPMI-1640 medium plus 10% FBS with 20 μg/ml DNase (Sigma-Aldrich, Milan, Italy), suspended at 1 × 106 cells/ml in a 48-well plate and stimulated overnight with 200 U/ml recombinant (r)IL-2 (Boehringer-Mannheim, Monza, Italy). Then PBMCs were co-cultured with the MHC class I-negative K562 cell line or the FcγR+ P815 cell line at an effector to target ratio of 5 : 1, in the presence of the anti-CD107a mAb (20 μl/ml); the negative controls were PBMCs in medium alone. For the redirected assay, P815 cells were previously incubated with the appropriate anti-NKR or MOV10 mAbs for 20 min on ice. After the addition of PBMCs, the co-cultures were incubated for 1 hr at 37°, followed by an additional 5 hr in the presence of the secretion-inhibitor monensin (Golgi-Stop; BD-Pharmingen). Cultured cells were then stained for CD3 and CD56 surface markers, and fixed with 1% paraformaldehyde.
The multiparametric flow cytometric analysis was performed using a BD-FACSCalibur instrument (BD Biosciences, San Diego, CA, USA) by gating on CD3− CD56dim or CD3− CD56bright subsets, and the percentage of CD107a+ cells was calculated by subtracting the value for the negative control. A representative dot plot of the expression of CD107a on both CD56dim and CD56bright subsets is shown in Fig. 2.
Figure 2.
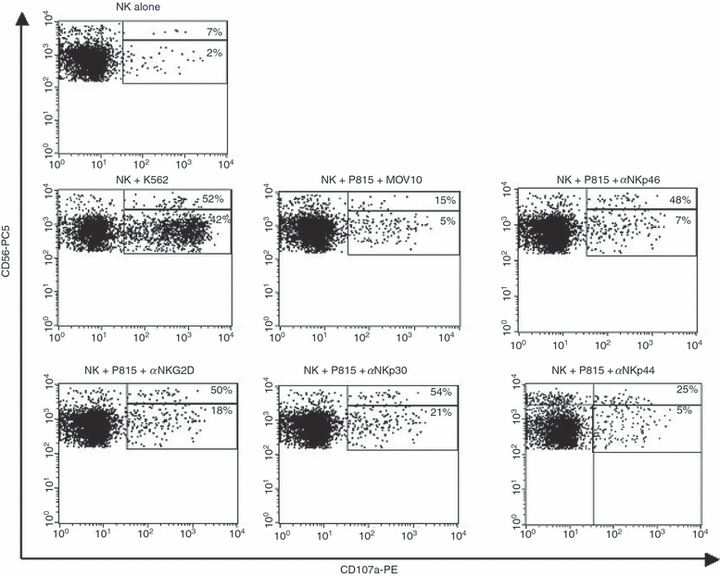
Representative dot plots of CD107a expression on CD56dim and CD56bright natural killer (NK) cell subsets. Interleukin (IL)-2-stimulated peripheral blood mononuclear cells were cultured in medium alone or with the K562 or P185 cell line in the presence of an irrelevant monoclonal antibody (mAb) (MOV10) or anti-(α-)activating NK-receptor mAbs (NKp46, NKp30, NKp44 and NKG2D), and stained with anti-CD3-fluorescein isothiocyanate (FITC), anti-CD56-phycoerythrin-cyanin-5 (PC5) and anti-CD107a-phycoerythrin (PE) mAbs. After gating on lymphocytes (not shown), the CD3+ cells were excluded from the analysis, and the percentage of CD107a+ cells in the CD56dim and CD56bright subsets was determined.
Statistical analysis
The normality of distribution of continuous variables was evaluated using the Kolmogorov–Smirnov test. The data are given as median values with interquartile ranges (25–75th percentiles). The values of the continuous variables were compared among subject groups (after the log-transformation of non-normally distributed data) using one-way analysis of variance (anova), with subject group as the grouping factor. In the case of variables for which significant differences were found in anova, Student’s t-test for independent data was used for the between-group comparisons.
The χ2 test (with Yates’ correction for continuity) and Fisher’s exact test were used to compare discrete variables.
Changes from baseline in the NK cell variables were evaluated using one-way anova for repeated measures, with the antiretroviral treatment as grouping factor. Changes from baseline at a specific time-point were calculated and tested using Student’s t-test for paired data.
Bonferroni’s correction was used to adjust the P-values for multiple comparisons (in relation to the three groups and to the study time-points).
We determined whether a significant linear relationship between continuous variables existed by means of Spearman’s correlation coefficient.
The analyses were performed using sas software (version 8.2; SAS Institute, Cary, NC). All tests of significance were two-sided, and a P-value of < 0·05 was considered to be statistically significant.
Results
Circulating CD3− CD56dim and CD3− CD56bright NK subsets decrease with disease progression, while the CD3− CD16+ CD56− subset increases
Baseline plasma viraemia levels were significantly higher in PHI than in CHI individuals (P = 0·0016), but there were no significant differences in the numbers and percentages of CD4+ or CD8+ T lymphocytes. The percentages (as percentages of total PBMCs) of circulating CD3− CD56+ NK cells in PHI and CHI individuals were significantly lower (P = 0·0003 and P < 0·0001, respectively) than in HN individuals; within the HIV-infected individuals, both the number and the percentage of NK cells were generally higher in PHI than in CHI individuals, although this difference did not reach statistical significance (P = 0·07) (Table 1).
Table 1.
Virological and immunological characteristics of the individuals at baseline
| Viraemia |
CD3+ CD4+ T cells |
CD3+ CD8+ T cells |
CD4+/CD8+ |
CD3− CD56+ NK cells |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Age (years) | (log10 copies/ml)1 | Abs1 | %1 | Abs1 | %1 | Ratio1 | Abs1 | %1 | P (% NK cells) | |
| PHI | 34 | 39·0 (32–42) | 4·98 (4·49–5·61) | 443 (304–800) | 18·7 (13·6–30·0) | 1329 (919–1911) | 56 (43·4–69·4) | 0·33 (0·20–0·73) | 199 (126–363) | 8·5 (6·4–11·5) | PHI versus HN = 0·0003 |
| CHI | 47 | 32·5 (30–37 | 4·34 (3·85–4·99) | 531 (412–705) | 22·4 (17·5–29·1) | 1251 (902–1620) | 55·5 (44·7–62·2) | 0·41 (0·28–0·65) | 138 (83–252) | 7·2 (4·0–11·2) | CHI versus HN < 0·0001 |
| HN | 34 | 36·5 (29–45) | nd | nd | nd | nd | nd | nd | nd | 14·4 (10·1–21·9) | PHI versus CHI = 0·07 |
Viraemia, PHI versus CHI: P = 0·0016.
Abs, absolute number; CHI, chronic HIV-infected individuals; HN, HIV-negative healthy individuals; PHI, primary HIV-infected individuals; N, number of individuals; NK, natural killer; nd, not done.
Median (25th and 75th percentiles).
On the basis of their clinical, immunological and virological parameters, 18 of the 34 PHI individuals remained untreated during the 1 year of follow-up. As expected, the 16 who received HAART had significantly lower circulating CD4+ T-cell counts and percentages, and higher viraemia levels at baseline (Table 2). Their CD4+ T-cell counts and percentages increased and viraemia significantly decreased to undetectable levels (HIV-RNA < 50 copies/ml) from week 24 (P = 0·0009). The baseline levels of CD3− CD56+ NK cells were comparable in the two groups but, during follow-up, their numbers and percentages (as percentages of total PBMCs) increased in the treated group and tended to decrease in the untreated group (ANOVA; P = 0·037 and P = 0·007, respectively). The HAART-induced control of viraemia was therefore characterized by increases in both CD3+ CD4+ T lymphocytes and CD3− CD56+ NK cells.
Table 2.
Comparison of immunological and virological parameters in highly active antiretroviral therapy (HAART)-treated and untreated primary HIV-1-infected individuals at baseline and during follow-up
| Viraemia |
CD3+ CD4+ T cells |
CD3+ CD8+ T cells |
CD4+/CD8+ |
CD3− CD56+ NK cells |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time | HAART | N | (log10 copies/ml)1 | Abs1 | %1 | Abs1 | %1 | Ratio1 | Abs1 | %1 |
| Time 0 | Yes | 16 | 5·54 (4·88–5·91) | 400 (275–456) | 15·9 (10·4–18·6) | 1762 (1232–2410) | 63·6 (56·0–75·9) | 0·30 (0·13–0·35) | 240 (158–363) | 9·0 (6·8–13·7) |
| No | 18 | 4·68 (4·41–4·98) | 790 (431–886) | 29·4 (16·7–36·9) | 1042 (890–1350) | 44·4 (39·4–63·4) | 0·66 (0·26–0·91) | 191 (91–380) | 8·5 (5·1–11·5) | |
| P = 0·0022 | P = 0·0022 | P = 0·0032 | P = 0·0282 | P = 0·0042 | P = 0·0232 | P = ns2 | P = ns2 | |||
| Week 4 | Yes | 15 | 3·84 (3·25–4·61) | 589 (365–742) | 23·7 (17·9–28·1) | 1503 (942–1806) | 56·6 (41·4–63·3) | 0·42 (0·28–0·59) | 222 (99–364) | 9·4 (7·1–13·5) |
| No | 15 | 4·55 (3·88–4·92) | 756 (524–1150) | 31·8 (23·6–42·0) | 926 8563–1246) | 44·4 (40·2–55·0) | 0·66 (0·4–1·0) | 233 (169–329) | 9·6 (6·7–12·9) | |
| P = 0·00022 | P = ns2 | P = ns2 | P = ns2 | P = 0·0142 | P = ns2 | P = ns2 | P = ns2 | |||
| Week 24 | Yes | 12 | Undetectable | 638 (480–866) | 28·2 (25·1–34·2) | 730 (639–1304) | 41·3 (35·0–46·2) | 0·66 (0·48–0·96) | 294 (248–407) | 15·8 (12·8–19·4) |
| No | 13 | 4·40 (3·85–4·74) | 674 (446–817) | 34·3 (25·4–36·5) | 960 (628–1262) | 43·5 (35·1–47·2) | 0·74 (0·54–1·04) | 158 (120–262) | 8·1 (5·0–9·8) | |
| P = 0·00082 | P = 0·0162 | P = 0·0042 | P = 0·0292 | P = 0·0012 | P = 0·0052 | P = 0·00212 | P = 0·00042 | |||
| Week 48 | Yes | 13 | Undetectable | 682 (498–867) | 29·3 (26·1–33·9) | 898 (650–1392) | 37·2 834·2–49·4) | 0·75 (0·56–0·98) | 288 (260–450) | 15·9 (11·8–20·5) |
| No | 12 | 4·21 (3·86–4·56) | 791 (476–983) | 37·2 (29·3–39·6) | 1105 (686–1178) | 44·2 (39·3–48·8) | 0·82 (0·60–0·94) | 153 (136–223) | 6·1 (5·3–11·5) | |
| P = 0·00072 | P = 0·0132 | P = 0·0242 | P = 0·082 | P = 0·0052 | P = 0·0242 | P = 0·00712 | P = 0·00252 | |||
| anova: P | Therapy | 0·011 | 0·21 | 0·05 | 0·4 | 0·4 | 0·2 | 0·1 | 0·034 | |
| Time | 0·0001 | 0·001 | < 0·0001 | 0·048 | < 0·0001 | < 0·0001 | 0·7 | 0·2 | ||
| Time × therapy | 0·0009 | 0·07 | 0·02 | 0·1 | 0·0002 | 0·005 | 0·037 | 0·007 | ||
Abs, absolute number; anova, analysis of variance for repeated measures; HAART, highly active antiretroviral therapy; N, number of individuals; NK, natural killer; ns, not significant.
Median (25th and 75th percentiles).
Treated versus untreated individuals.
Consistent with previous reports,24–26 the percentages (as percentages of total PBMCs) of the CD3− CD56dim and CD3− CD56bright subsets were significantly lower in CHI than in PHI and HN individuals (CHI versus PHI, P = 0·005; CHI versus HN, P < 0·0001), and that of the CD3− CD16+ CD56− subset was significantly higher in CHI than in PHI individuals (P = 0·0005) and in PHI than in HN individuals (P = 0·003; data not shown). The absolute number (calculated from the number of blood lymphocytes) of CD56bright (but not CD56dim) cells was significantly lower in the CHI than in the PHI group (P = 0·01). In contrast, the absolute number of cells in the CD3− CD56− CD16+ subset was significantly higher in CHI than in PHI individuals (P = 0·009).
The baseline percentage of the CD3− CD56bright subset was lower in HAART-treated PHI individuals than in those who remained untreated (P = 0·03; not significant when adjusted for multiple comparisons) and similar to that found in CHI individuals (P = 0·3), but significantly different from that found in HN individuals (P = 0·017); in the group not treated with HAART, this percentage was similar to that found in HN individuals (P = 0·77) but significantly different from that observed in CHI individuals (P = 0·0007). The values of the other subsets were comparable (data not shown).
Among the CD3− CD56+ NK cells, the percentage of the CD56dim subset increases, and that of the CD56bright subset decreases in HIV-infected individuals in comparison with HN individuals
Having shown that both the CD56dim and CD56bright subsets decrease with HIV-disease progression, we analysed their proportions among CD3− CD56+ NK cells.
The percentage of the CD56dim subset was higher in CHI than in HN individuals, and that of the CD56bright subset was lower; PHI individuals showed intermediate values (Fig. 3a).
Figure 3.
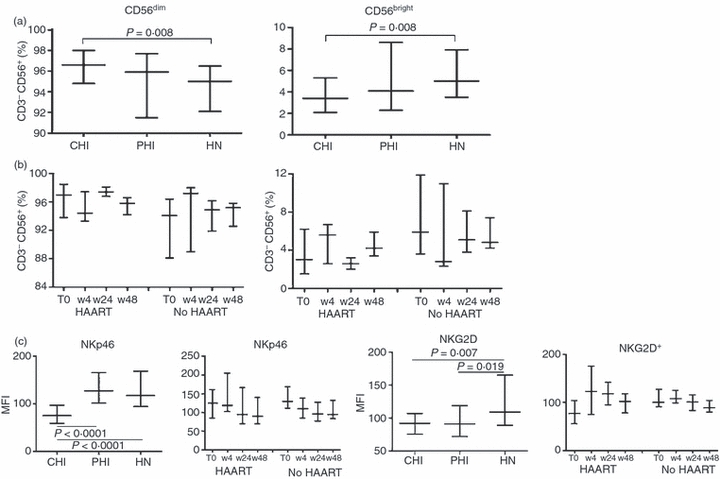
Frequency of CD56dim and CD56bright subsets in CD3− CD56+ blood natural killer (NK) cells. (a, b) Proportion of CD56dim and CD56bright subsets among the CD3− CD56+ NK cells analysed in (a) chronic HIV-infected (CHI) individuals, primary HIV-infected (PHI) individuals and HIV-negative (HN) healthy individuals, and in (b) PHI individuals treated with highly active antiretroviral therapy (HAART) and not treated with HAART (No HAART) at baseline (T0) and during follow-up at weeks 4, 24 and 48 (w4, w24 and w48, respectively). (c) Surface density of NKp46 and NKG2D molecules, expressed as mean fluorescence intensity (MFI), in the three groups, and in the treated (HAART) and untreated (No HAART) PHI individuals at baseline (T0) and during follow-up (w4, w24 and w48). Data are expressed as median values, with 25th and 75th percentiles.
PHI individuals receiving HAART had higher baseline percentages of CD56dim cells, and lower percentages of CD56bright cells (P = 0·03) than those who remained untreated, although the difference was not significant when adjusted for multiple comparisons (Fig. 3b). These values were not different from those found in CHI individuals (P = 0·55), but significantly different from those found in HN individuals (P = 0·022); however, in the group not treated with HAART, the percentages of the CD56dim and CD56bright subsets were similar to those found in HN individuals (P = 0·56), but significantly different from those found in CHI individuals (P = 0·017).
In the HAART group, there was an inverse correlation between the percentage of CD56bright cells at baseline and the variations from baseline in viral load (VL) at weeks 24 and 48 (ΔVL0–24: r= 0·713, P = 0·009; ΔVL0–48: r = 0·608, P = 0·036).
The percentages of CD56dim NK cells with NKp46 or NKp30 receptors decrease with disease progression
The function of NK cells is modulated by different surface receptors that transduce either inhibitory or activating signals. In particular, NKp30, NKp46, NKp44 and NKG2D are the main activating receptors. We therefore analysed their expression on both CD56dim and CD56bright subsets.
The percentage of NKp46+ or NKp30+ CD3− CD56dim cells significantly decreased with disease progression (CHI ≤ PHI ≤ HN; Fig. 4a, top). The percentages of NKp44+ and NKG2D+ cells were not significantly different in the three groups.
Figure 4.
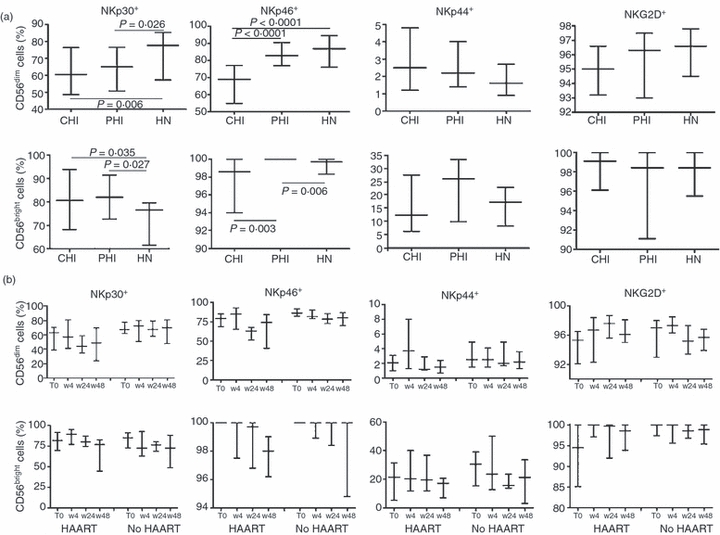
Frequency of NKp30+, NKp46+, NKp44+ and NKG2D+ cells in CD56dim and CD56bright blood natural killer (NK) cell subsets. Gating on CD3− CD56dim or CD3− CD56bright NK subsets, we analysed the proportion of CD56dim cells (a and b, top) or CD56bright cells (a and b, bottom) showing the surface expression of NKp30, NKp46, NKp44 or NKG2D receptors. (a) Chronic HIV-infected (CHI) individuals, primary HIV-infected (PHI) individuals and HIV-negative (HN) healthy individuals. (b) PHI individuals treated with highly active antiretroviral therapy (HAART) and not treated with HAART (No HAART) at baseline (T0) and during follow-up at weeks 4, 24 and 48 (w4, w24 and w48, respectively). Data are expressed as median values, with 25th and 75th percentiles.
PHI individuals have a higher percentage of CD56bright NK cells with NKp46 receptors than CHI and HN individuals
Within the CD3− CD56bright subset, the percentage of NKp30+ cells was significantly higher in both CHI and PHI individuals than in HN individuals (Fig. 4a, bottom). It is worth noting that NKp46 receptors were expressed on 100% of the CD56bright cells in all of the PHI individuals, a significantly higher percentage than that found in the CHI and HN groups (PHI > HN ≥CHI). There were no between-group differences in the percentages of NKG2D+ and NKp44+ cells, although the percentage of the latter was moderately, but not significantly, higher in PHI individuals than in CHI or HN individuals.
There were no significant differences in the percentages of CD56dim or CD56bright cells bearing NCRs between HAART-treated and untreated PHI individuals at baseline or during follow-up (Fig. 4b).
The expression of NKp46 and NKG2D receptors on the surface of NK cells is lower in CHI than in HN individuals
The function of NK cells depends not only on the presence of particular NKRs, but also on their surface density. We therefore evaluated the surface density of the different NKRs on NK cells, expressed as mean fluorescence intensity (MFI).
The MFIs of NKp46 and NKG2D (but not NKp30) receptors were significantly lower on NK cells of CHI individuals than on those of HN individuals (Fig. 3c); no differences in the other NKRs were found (data not shown).
In PHI individuals, the MFI of NKp46 was similar to that found in HN individuals, and that of NKG2D was similar to that found in CHI individuals. At baseline, there were no significant differences between treated and untreated individuals, but HAART induced a significant increase in the MFI of NKG2D over time (P = 0·016).
The percentage of KIR+ CD56bright NK cells increases with disease progression, and PHI individuals show a higher percentage of NKG2A+ cells than the other groups
KIRs and NKG2A are the main class-I-restricted NKRs. We therefore analysed their expression on both CD56dim and CD56bright NK subsets.
The percentage of CD56dim cells with CD158b, CD158e or NKG2A inhibitory receptors was similar in the three groups, whereas that of cells with the CD158i activating receptor was higher in CHI individuals than in PHI or HN individuals (P = 0·034 and P = 0·05, respectively; Fig. 5, top).
Figure 5.
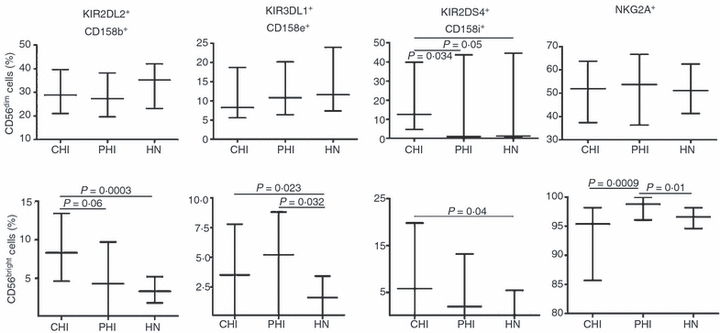
Frequency of killer cell immunoglobulin-like receptor-positive (KIR+) or NKG2A+ cells in CD56dim and CD56bright blood natural killer (NK) cell subsets. Gating on CD3− CD56dim or CD3− CD56bright NK cell subsets, we analysed the proportion of CD56dim cells (top) or CD56bright cells (bottom) showing CD158b, CD158e and CD158i KIRs, or NKG2A receptors in chronic HIV-infected (CHI) individuals, primary HIV-infected (PHI) individuals and HIV-negative (HN) healthy individuals. Data are expressed as median values, with 25th and 75th percentiles.
Interestingly, a small percentage of CD56bright cells expressed KIRs, with significant differences among groups (CHI > PHI = HN for CD158b; CHI = PHI > HN for CD158e; CHI > HN = PHI for CD158i); the percentage of NKG2A+ cells was significantly higher in PHI individuals than in CHI and HN individuals (Fig. 5, bottom). Thus, the greatest differences among groups were observed within the CD56bright subset also in terms of expression of KIRs and NKG2A receptors.
The percentages of CD56dim and CD56bright cells with KIRs or NKG2A were similar in the HAART-treated and untreated PHI individuals at baseline and during follow-up (data not shown).
Functional capacity of NK subsets
In order to determine whether the among-group differences in the expression of activating receptors on NK subsets corresponded to functional differences, we analysed the expression of CD107a (a marker of degranulation that correlates with the lytic potential of cytotoxic cells31) on CD56dim and CD56bright cells co-cultured with the K562, or P815 cell lines after cross-linking of NKp46, NKp30, NKp44 or NKG2D receptors. Baseline PBMCs taken from four CHI individuals, seven PHI individuals (four receiving HAART and three untreated; two HAART-treated and two untreated patients were also tested at week 48) and eight HN individuals were cultured with rIL-2 overnight to stimulate the cytotoxic activity of the CD56bright cells also, and to induce the expression of NKp44 receptors.
The expression of CD107a was significantly lower on the CD56dim cells of CHI individuals co-cultured with K562 cells than on those of PHI or HN individuals (P = 0·0035; Fig. 6a, top). There was no difference for CD56bright cells, although the percentage of CD107a+ cells was high and comparable with that of CD56dim cells from HN individuals (Fig. 6a, bottom).
Figure 6.
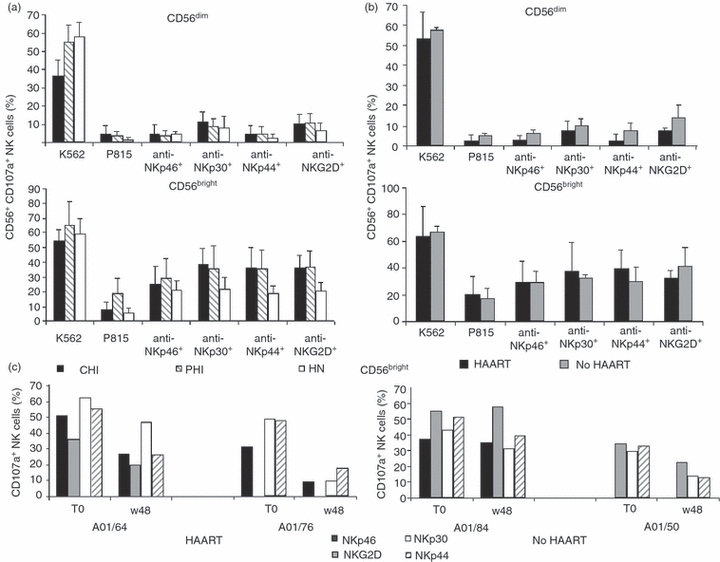
Functional capacity of natural killer (NK) cell subsets. Expression of CD107a on CD56dim (a and b, top) and CD56bright (a and b, bottom) NK cell subsets after co-culturing interleukin (IL)-2-stimulated peripheral blood mononuclear cells with the K562 cell-line or the P185 cell-line in the presence or absence of anti-NKp46, anti-NKp30, anti-NKp44 or anti-NKG2D monoclonal antibodies (mAbs) was determined. (a) Chronic HIV-infected (CHI) individuals (n = 4), primary HIV-infected (PHI) individuals (n = 7), and HIV-negative (HN) healthy individuals (n = 8). (b) PHI individuals treated with highly active antiretroviral therapy (HAART; n = 4) and not treated with HAART (No HAART; n = 3) at baseline. Data are expressed as mean values plus standard deviation. (c) Percentage of CD56bright NK cells expressing CD107a molecules in four representative PHI individuals treated with HAART (HAART) or untreated (No HAART) at baseline and after 48 weeks of follow-up. IL-2-stimulated peripheral blood mononuclear cells were co-cultured with the P815 cell line in the presence of anti-NKp46 (black), anti-NKG2D (grey), anti-NKp30 (white) or anti-NKp44 (hatched) mAbs.
There was no among-group difference in the functional activity of the activating receptors on CD56dim cells (Fig. 6a, top), but the functional activity of NKp30, NKp44 and NKG2D receptors on CD56bright cells was significantly greater in the HIV-infected individuals than in HN individuals (P = 0·0486, P = 0·0129 and P = 0·0045, respectively); that of NKp46 was also increased, but not significantly (Fig. 6a, bottom). Interestingly, baseline CD107a expression on the CD56bright cells co-cultured with P815 cells was significantly higher in PHI individuals than in CHI or HN individuals (P = 0·0059).
There were no differences in baseline CD107a expression on the CD56dim or CD56bright subsets between the HAART-treated and untreated PHI individuals under any of the experimental conditions (Fig. 6b). However, after the engagement of activating receptors on CD56bright cells there was a reduction in CD107a expression at week 48 in comparison with baseline that correlated with the reduction in the percentage of CD56bright cells with activating receptors in the two tested HAART patients, but not in the untreated patients (Fig.6c). There was no difference in CD107a expression on the CD56dim cells taken from either subject (data not shown).
Discussion
We investigated the distribution of the principal circulating NK cell subsets, and the expression of their activating and inhibitory receptors, in HAART-treated and untreated PHI individuals at baseline and during 1-year of follow-up, and compared the results with those observed in treatment-naïve CHI individuals and HN individuals. To the best of our knowledge, this is the first study to analyse the distribution of NK cell receptors and their cytolytic capacity on CD56dim and CD56bright subsets at different stages of HIV-1 infection.
Our findings indicate a marked perturbation of the NK cell compartment that is multifaceted, starts early, progressively increases, primarily involves the CD56bright subset, and is only partially corrected by effective HAART.
We found that the percentage and number of circulating CD3− CD56+ NK cells decreased with the progression of HIV infection, depending on both CD56dim and particularly CD56bright subsets, while the percentage and number of the anergic CD16+ CD56− subset increased. Furthermore, PHI subjects with high viraemia and a low number of CD4+ T lymphocytes (the HAART-treated group, undergoing therapy during the 1 year of follow-up) had a lower percentage and number of CD56bright cells (with values close to those of CHI individuals, but significantly different from those of HN individuals) than those with low viraemia and a high number of CD4+ T lymphocytes (the untreated group, who remained untreated during follow-up), who showed values close to those of HN individuals, but significantly different from those of CHI individuals.
The involvement of the CD56bright subset in HIV-1 infection was highlighted when the analysis was performed within CD3− CD56+ NK cells, with the proportion of the CD56bright subset decreasing and, conversely, that of the CD56dim subset increasing, with disease progression. Again, the levels in PHI individuals were intermediate between those in CHI and HN individuals, with the HAART group showing levels similar to those in CHI and the NO HAART group to HN individuals. At baseline, PHI subjects receiving therapy show a decreased proportion of CD56bright cells compared with those who remained untreated. Thus, the CD56bright NK subset is the one primarily involved in the early stages of HIV-1 infection.
These observations confirm previous reports on the variations of CD56dim and CD16+ CD56− subsets during HIV infection. In contrast, CHI patients showed higher proportion of CD56bright subset24–26,32 than HN individuals. The reasons for this discrepancy may be related to the high number of treatment-naïve CHI individuals in our study, differences in the virological and immunological features of the patients, or the fact that some of these studies phenotyped CD3− CD56+ cells23,31 and others selected CD56+ NK cells,24,25 whereas we analysed both.
The HIV progression-related decrease in the CD56bright subset was not merely attributable to the decrease in the total NK cell population, because this was the only subset in which such a decrease was found. Recent findings suggest that secondary lymphoid tissue (SLT) may provide a unique site for the development of CD56bright NK cells,33 and as SLT is the principal site of early HIV replication and spread,34 and its architecture is progressively disrupted during disease progression,35 the maturation and production of CD56bright cells might also be involved. Early and effective antiretroviral therapy might benefit CD56bright cells by reducing SLT damage.
NK cell function is finely modulated by the engagement of inhibitory and activating receptors. Previous studies found a decrease in NK cells displaying NCRs in viraemic CHI individuals, associated with decreased cytolytic function, without any change in NKG2D expression.25,28,36 We have shed light on and extended these observations by showing that the reduced percentage of NKp46+ and NKp30+ NK cells in CHI individuals compared with HN individuals is restricted to the CD56dim subset, with PHI individuals showing intermediate values. Furthermore, in comparison with HN individuals, surface NKp46 and NKG2D receptors were down-modulated in CHI individuals. The expression of NKG2D in PHI individuals was comparable to that in CHI individuals, with therapy inducing a significant up-regulation over time. Various factors might be responsible for these effects, including transforming growth factor (TGF)-β, which has been reported to modulate the expression of NKG2D receptors37 and to be increased in the plasma of HIV-infected individuals,38 or direct engagement of the Toll-like receptors expressed by NK cells.39
Conversely, the proportion of NKp46+ and NKp30+ cells within the CD56bright subset was significantly higher in the HIV-infected individuals than in the HN individuals, and it is worth noting that 100% of the CD56bright cells of all of the PHI individuals expressed NKp46 receptors, indicating that this phenotype can be considered a hallmark of early infection. The proportion of CD56bright NKp44+ cells was higher in the PHI individuals than in the other groups (although not significantly), whereas there was no difference in the proportion of CD56bright NKG2D+ cells. The induced expression of NCRs is considered to be a marker of NK cell activation,13 and so our results corroborate previous observations of the early activation of NK cells during HIV infection, mainly restricted to the CD56bright subset.36
Another peculiarity of HIV-1 infection, to our knowledge never reported before, was the significant increase in CD56bright cells displaying KIRs in CHI individuals, and the higher percentage of NKG2A+ cells in PHI individuals, in comparison with HN individuals. Resting CD56bright NK cells residing in the SLT can interact with, and be activated by, incoming pathogen-capturing DCs. At the same time, activated CD56bright cells stimulate DCs by means of cell–cell contacts, thus representing an important bridge between innate and adaptive immune responses.40,41 After activation, CD56bright cells may leave SLT and start to express perforin, KIRs, NCRs and homing receptors for inflamed tissues, where they accumulate and perform effector functions.13,14,42 The increase in circulating NCR+ and KIR+ CD56bright NK cells in PHI individuals, as well as the high percentage of NKG2A+ cells, confirms their early activation and release from SLT. However, it is possible that CD56bright cells derive directly from activated CD56dim cells.
Conversely, there were no significant among-group differences in the percentages of KIR+ and NKG2A+ cells within the CD56dim subset. Mavilio et al.25 have reported a decrease in the percentage of NKG2A+ cells among the enriched NK cells of HIV viraemic patients in comparison with aviraemic patients and healthy controls, whereas, consistent with our results, Ravet et al.43 found no differences in the levels of CD56+ NKG2A+ NK cells among HIV-infected individuals, exposed uninfected individuals and healthy controls. Once again, it is necessary to analyse NK subsets in a larger number of selected individuals before drawing any final conclusions.
Consistent with previous observations,25,28–30,44,45 we found that CHI individuals showed reduced NK cell functional activity (reduced CD107a granule exocytosis) against K562 targets in comparison with PHI and HN individuals, and this was restricted to the CD56dim subset. This may be partially explained by the reduction in the percentage of CD56dim NK cells with NCRs that occurs with the progression of HIV infection, as well as by the reduced surface expression of NKG2D and NKp46 receptors.
IL-2-stimulated CD56bright cells taken from HIV-infected individuals and HN individuals, and co-cultured with K562 cells, showed conserved granule exocytosis, whereas the CD56bright cells of HIV-infected individuals co-cultured with P815 cells after the engagement of activating receptors showed increased granule exocytosis in comparison with those of HN individuals. These findings are consistent with the activation of CD56bright cells and the high percentage of cells displaying NCRs. Moreover, the CD56bright cells of PHI individuals showed more spontaneous granule exocytosis than those of CHI or HN individuals, which correlates with their higher percentage of NKp46+ cells. As reported previously, NKp46 engagement by ligands expressed on P815 cells is responsible for their spontaneous NK-mediated killing.46 Interestingly, CD56bright cells from two PHI patients on HAART (but not those from two untreated patients) showed a reduction in CD107a expression at the end of the 1-year follow-up period, which is consistent with a reduced percentage of cells bearing activating receptors (Fig. 6c).
Our functional results should be considered preliminary because of the small number of individuals included, but they do seem to support the hypothesis that CD56bright NK cells play a particular role in HIV-1 infection.
In conclusion, our findings confirm that there is an early and progressive perturbation of the NK cell compartment during HIV-1 infection, mainly involving the CD56bright subset. Early ad hoc antiviral and/or immune-based therapies capable of strengthening the NK cell compartment may therefore play a significant role in controlling HIV infection, as recently suggested by others.47,48
Acknowledgments
We are grateful to A. Moretta and D. Pende (University of Genoa, Genoa, Italy) for their kind gift of mAbs for the redirected CD107a expression assay, and to S. Menard (National Cancer Institute, Milan, Italy) for the MOV10 mAb. We would like to thank G. Poli for reading the manuscript.
Disclosures
None.
References
- 1.Andoniou CE, Andrews DM, Degli-Esposti MA. Natural killer cells in viral infection: more than just killers. Immunol Rev. 2006;214:239–50. doi: 10.1111/j.1600-065X.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- 2.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–53. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 3.Moretta L, Moretta A. Killer immunoglobulin-like receptors. Curr Opin Immunol. 2004;16:626–33. doi: 10.1016/j.coi.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Borrego F, Ulbrecht M, Weiss EH, Coligan JE, Brooks AG. Recognition of human histocompatibility leukocyte antigen (HLA)-E complexed with HLA class I signal sequence-derived peptides by CD94/NKG2 confers protection from natural killer cell-mediated lysis. J Exp Med. 1998;187:813–18. doi: 10.1084/jem.187.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–29. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 6.Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, Biassoni R, Moretta L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 7.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev. 2003;3:781–90. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 8.Ward J, Bonaparte M, Sacks J, Guterman J, Fogli M, Mavilio D, Barker E. HIV modulates the expression of ligands important in triggering natural killer cell cytotoxic responses on infected primary T-cell blasts. Blood. 2007;110:1207–14. doi: 10.1182/blood-2006-06-028175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vieillard V, Strominger JL, Debre P. NK cytotoxicity against CD4+ T cells during HIV-1 infection: a gp41 peptide induces the expression of an NKp44 ligand. Proc Natl Acad Sci USA. 2005;102:10981–86. doi: 10.1073/pnas.0504315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byrd A, Hoffmann SC, Jarahian M, Momburg F, Watzl C. Expression analysis of the ligands for the Natural Killer cell receptors NKp30 and NKp44. PLoS ONE. 2007;2:e1339. doi: 10.1371/journal.pone.0001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–40. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 12.Ferlazzo G, Munz C. NK cell compartments and their activation by dendritic cells. J Immunol. 2004;172:1333–39. doi: 10.4049/jimmunol.172.3.1333. [DOI] [PubMed] [Google Scholar]
- 13.Ferlazzo G, Thomas D, Lin SL, Goodman K, Morandi B, Muller WA, Moretta A, Munz C. The abundant NK cells in human secondary lymphoid tissues require activation to express killer cell Ig-like receptors and become cytolytic. J Immunol. 2004;172:1455–62. doi: 10.4049/jimmunol.172.3.1455. [DOI] [PubMed] [Google Scholar]
- 14.Dalbeth N, Gundle R, Davies RJ, Lee YC, McMichael AJ, Callan MF. CD56bright NK cells are enriched at inflammatory sites and can engage with monocytes in a reciprocal program of activation. J Immunol. 2004;173:6418–26. doi: 10.4049/jimmunol.173.10.6418. [DOI] [PubMed] [Google Scholar]
- 15.Scott-Algara D, Truong LX, Versmisse P, et al. Cutting edge: increased NK cell activity in HIV-1-exposed but uninfected Vietnamese intravascular drug users. J Immunol. 2003;171:5663–67. doi: 10.4049/jimmunol.171.11.5663. [DOI] [PubMed] [Google Scholar]
- 16.Tiemessen CT, Shalekoff S, Meddows-Taylor S, et al. Cutting edge: unusual NK cell responses to HIV-1 peptides are associated with protection against maternal-infant transmission of HIV-1. J Immunol. 2009;182:5914–18. doi: 10.4049/jimmunol.0900419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flores-Villanueva PO, Yunis EJ, Delgado JC, et al. Control of HIV-1 viremia and protection from AIDS are associated with HLA-Bw4 homozygosity. Proc Natl Acad Sci USA. 2001;98:5140–45. doi: 10.1073/pnas.071548198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin MP, Gao X, Lee JH, et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet. 2002;31:429–34. doi: 10.1038/ng934. [DOI] [PubMed] [Google Scholar]
- 19.Martin MP, Qi Y, Gao X, et al. Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nat Genet. 2007;39:733–40. doi: 10.1038/ng2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fortis C, Poli G. Dendritic cells and natural killer cells in the pathogenesis of HIV infection. Immunol Res. 2005;33:1–22. doi: 10.1385/IR:33:1:001. [DOI] [PubMed] [Google Scholar]
- 21.Douglas SD, Durako SJ, Tustin NB, Houser J, Muenz L, Starr SE, Wilson C. Natural killer cell enumeration and function in HIV-infected and high-risk uninfected adolescents. AIDS Res Hum Retroviruses. 2001;17:543–52. doi: 10.1089/08892220151126643. [DOI] [PubMed] [Google Scholar]
- 22.Hu PF, Hultin LE, Hultin P, et al. Natural killer cell immunodeficiency in HIV disease is manifest by profoundly decreased numbers of CD16+ CD56+ cells and expansion of a population of CD16dim CD56- cells with low lytic activity. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:331–40. [PubMed] [Google Scholar]
- 23.Titanji K, Sammicheli S, De Milito A, et al. Altered distribution of natural killer cell subsets identified by CD56, CD27 and CD70 in primary and chronic human immunodeficiency virus-1 infection. Immunology. 2008;123:164–70. doi: 10.1111/j.1365-2567.2007.02657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tarazona R, Casado JG, Delarosa O, et al. Selective depletion of CD56(dim) NK cell subsets and maintenance of CD56(bright) NK cells in treatment-naive HIV-1-seropositive individuals. J Clin Immunol. 2002;22:176–83. doi: 10.1023/a:1015476114409. [DOI] [PubMed] [Google Scholar]
- 25.Mavilio D, Benjamin J, Daucher M, et al. Natural killer cells in HIV-1 infection: dichotomous effects of viremia on inhibitory and activating receptors and their functional correlates. Proc Natl Acad Sci USA. 2003;100:15011–16. doi: 10.1073/pnas.2336091100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mavilio D, Lombardo G, Benjamin J, et al. Characterization of CD56−/CD16+ natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc Natl Acad Sci USA. 2005;102:2886–91. doi: 10.1073/pnas.0409872102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kottilil S, Shin K, Planta M, et al. Expression of chemokine and inhibitory receptors on natural killer cells: effect of immune activation and HIV viremia. J Infect Dis. 2004;189:1193–98. doi: 10.1086/382090. [DOI] [PubMed] [Google Scholar]
- 28.De Maria A, Fogli M, Costa P, Murdaca G, Puppo F, Mavilio D, Moretta A, Moretta L. The impaired NK cell cytolytic function in viremic HIV-1 infection is associated with a reduced surface expression of natural cytotoxicity receptors (NKp46, NKp30 and NKp44) Eur J Immunol. 2003;33:2410–18. doi: 10.1002/eji.200324141. [DOI] [PubMed] [Google Scholar]
- 29.Alter G, Teigen N, Ahern R, Streeck H, Meier A, Rosenberg ES, Altfeld M. Evolution of innate and adaptive effector cell functions during acute HIV-1 infection. J Infect Dis. 2007;195:1452–60. doi: 10.1086/513878. [DOI] [PubMed] [Google Scholar]
- 30.Alter G, Teigen N, Davis BT, et al. Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood. 2005;106:3366–69. doi: 10.1182/blood-2005-03-1100. [DOI] [PubMed] [Google Scholar]
- 31.Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294:15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Meier UC, Owen RE, Taylor E, et al. Shared alterations in NK cell frequency, phenotype, and function in chronic human immunodeficiency virus and hepatitis C virus infections. J Virol. 2005;79:12365–74. doi: 10.1128/JVI.79.19.12365-12374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freud AG, Becknell B, Roychowdhury S, et al. A human CD34(+) subset resides in lymph nodes and differentiates into CD56bright natural killer cells. Immunity. 2005;22:295–304. doi: 10.1016/j.immuni.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 34.Smith BA, Gartner S, Liu Y, et al. Persistence of infectious HIV on follicular dendritic cells. J Immunol. 2001;166:690–96. doi: 10.4049/jimmunol.166.1.690. [DOI] [PubMed] [Google Scholar]
- 35.Pantaleo G, Graziosi C, Demarest JF, Cohen OJ, Vaccarezza M, Gantt K, Muro-Cacho C, Fauci AS. Role of lymphoid organs in the pathogenesis of human immunodeficiency virus (HIV) infection. Immunol Rev. 1994;140:105–30. doi: 10.1111/j.1600-065x.1994.tb00867.x. [DOI] [PubMed] [Google Scholar]
- 36.Fogli M, Costa P, Murdaca G, Setti M, Mingari MC, Moretta L, Moretta A, De MariaA. Significant NK cell activation associated with decreased cytolytic function in peripheral blood of HIV-1-infected patients. Eur J Immunol. 2004;34:2313–21. doi: 10.1002/eji.200425251. [DOI] [PubMed] [Google Scholar]
- 37.Castriconi R, Cantoni C, Della Chiesa M, et al. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: consequences for the NK-mediated killing of dendritic cells. Proc Natl Acad Sci USA. 2003;100:4120–25. doi: 10.1073/pnas.0730640100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lotz M, Seth P. TGF beta and HIV infection. Ann N Y Acad Sci. 1993;685:501–11. doi: 10.1111/j.1749-6632.1993.tb35912.x. [DOI] [PubMed] [Google Scholar]
- 39.Alter G, Suscovich TJ, Teigen N, Meier A, Streeck H, Brander C, Altfeld M. Single-stranded RNA derived from HIV-1 serves as a potent activator of NK cells. J Immunol. 2007;178:7658–66. doi: 10.4049/jimmunol.178.12.7658. [DOI] [PubMed] [Google Scholar]
- 40.Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Munz C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med. 2002;195:343–51. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002;195:327–33. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strowig T, Brilot F, Munz C. Noncytotoxic functions of NK cells: direct pathogen restriction and assistance to adaptive immunity. J Immunol. 2008;180:7785–91. doi: 10.4049/jimmunol.180.12.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ravet S, Scott-Algara D, Bonnet E, et al. Distinctive NK cell receptor repertoires sustain high-level constitutive NK cell activation in HIV-exposed uninfected individuals. Blood. 2007;109:4296–305. doi: 10.1182/blood-2006-08-040238. [DOI] [PubMed] [Google Scholar]
- 44.Morsica G, Tasca S, Biswas P, et al. Natural killer-cell cytotoxicity in HIV-positive and HIV-negative patients with and without severe course of Hepatitis B virus infection. Scand J Immunol. 2005;62:318–24. doi: 10.1111/j.1365-3083.2005.01659.x. [DOI] [PubMed] [Google Scholar]
- 45.Tasca S, Tambussi G, Nozza S, et al. Escape of monocyte-derived dendritic cells of HIV-1 infected individuals from natural killer cell-mediated lysis. Aids. 2003;17:2291–98. doi: 10.1097/00002030-200311070-00003. [DOI] [PubMed] [Google Scholar]
- 46.Sivori S, Pende D, Bottino C, Marcenaro E, Pessino A, Biassoni R, Moretta L, Moretta A. NKp46 is the major triggering receptor involved in the natural cytotoxicity of fresh or cultured human NK cells. Correlation between surface density of NKp46 and natural cytotoxicity against autologous, allogeneic or xenogeneic target cells. Eur J Immunol. 1999;29:1656–66. doi: 10.1002/(SICI)1521-4141(199905)29:05<1656::AID-IMMU1656>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 47.Fogli M, Mavilio D, Brunetta E, et al. Lysis of endogenously infected CD4+ T cell blasts by rIL-2 activated autologous natural killer cells from HIV-infected viremic individuals. PLoS Pathog. 2008;4:e1000101. doi: 10.1371/journal.ppat.1000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Michaelsson J, Long BR, Loo CP, Lanier LL, Spotts G, Hecht FM, Nixon DF. Immune reconstitution of CD56(dim) NK cells in individuals with primary HIV-1 infection treated with interleukin-2. J Infect Dis. 2008;197:117–25. doi: 10.1086/524141. [DOI] [PMC free article] [PubMed] [Google Scholar]


