Abstract
Objective
To evaluate the effectiveness of the 7-valent pneumococcal conjugate vaccine (PCV7) in preventing pneumonia, diagnosed radiologically according to World Health Organization (WHO) criteria, among indigenous infants in the Northern Territory of Australia.
Methods
We conducted a historical cohort study of consecutive indigenous birth cohorts between 1 April 1998 and 28 February 2005. Children were followed up to 18 months of age. The PCV7 programme commenced on 1 June 2001. All chest X-rays taken within 3 days of any hospitalization were assessed. The primary endpoint was a first episode of WHO-defined pneumonia requiring hospitalization. Cox proportional hazards models were used to compare disease incidence.
Findings
There were 526 pneumonia events among 10 600 children – an incidence of 3.3 per 1000 child-months; 183 episodes (34.8%) occurred before 5 months of age and 247 (47.0%) by 7 months. Of the children studied, 27% had received 3 doses of vaccine by 7 months of age. Hazard ratios for endpoint pneumonia were 1.01 for 1 versus 0 doses; 1.03 for 2 versus 0 doses; and 0.84 for 3 versus 0 doses.
Conclusion
There was limited evidence that PCV7 reduced the incidence of radiologically confirmed pneumonia among Northern Territory indigenous infants, although there was a non-significant trend towards an effect after receipt of the third dose. These findings might be explained by lack of timely vaccination and/or occurrence of disease at an early age. Additionally, the relative contribution of vaccine-type pneumococcus to severe pneumonia in a setting where multiple other pathogens are prevalent may differ with respect to other settings where vaccine efficacy has been clearly established.
Résumé
Objectif
Évaluer l’efficacité du vaccin antipneumococcique conjugué heptavalent (PCV7) dans la prévention de la pneumonie diagnostiquée par examen radiologique selon les critères de l’Organisation mondiale de la Santé (OMS) chez les nourrissons indigènes du Territoire du Nord en Australie.
Méthodes
Nous avons mené une étude historique sur des cohortes de naissances dans la population indigène consécutives, nées entre le 1er avril 1998 et le 28 février 2005. Les enfants ont été suivis jusqu’à l’âge de 18 mois. Le programme PCV7 a débuté le 1er juin 2001. Toutes les radiographies thoraciques réalisées dans les 3 jours suivant une hospitalisation quelconque ont été évaluées. Le principal critère de jugement était l’existence d’un premier épisode de pneumonie selon la définition de l’OMS ayant nécessité une hospitalisation. Nous avons fait appel à des modèles de Cox à risques proportionnels pour comparer les incidences de cette maladie.
Résultats
Nous avons relevé 526 cas de pneumonie parmi 10600 enfants - soit une incidence de 3,3 cas pour 1000 enfants-mois ; 183 épisodes (34,8 %) sont intervenus avant l’âge de 5 mois et 247 (47,0 %) à 7 mois. Parmi les enfants étudiés, 27 % avaient reçu 3 doses de vaccin à l’âge de 7 mois. Les rapports de risques pour le critère de jugement pneumonie valaient 1,01 pour la comparaison 1 dose de vaccin contre 0 dose ; 1,03 pour la comparaison 2 doses contre 0 dose ; et 0,84 pour la comparaison 3 doses contre 0 dose.
Conclusion
Les preuves d’une réduction par le vaccin PCV7 de l’incidence de la pneumonie diagnostiquée par examen radiologique chez les nourrissons indigènes du territoire du Nord étaient limitées, malgré la présence d’une tendance non significative à la manifestation d’un effet après l’administration de la troisième dose. Ces résultats peuvent s’expliquer par la fréquence des retards dans la vaccination et/ou par l’apparition de la maladie à un âge précoce. De plus, la contribution des germes pneumococcus de type vaccinal à la pneumonie sévère dans un contexte où l’on rencontre de nombreux autres agents pathogènes peut être différente de leur contribution dans une situation où l’efficacité du vaccin a été clairement établie.
Resumen
Objetivo
Determinar la eficacia de la vacuna antineumocócica conjugada heptavalente (PCV7) en la prevención de la neumonía diagnosticada radiológicamente de acuerdo con los criterios de la Organización Mundial de la Salud (OMS) entre lactantes indígenas del Territorio Septentrional de Australia.
Métodos
Realizamos un estudio de cohorte histórica con cohortes de nacimiento de indígenas consecutivas entre el 1 de abril de 1998 y el 28 de febrero 2005. Los niños fueron sometidos a seguimiento hasta los 18 meses de edad. El programa de administración de PCV7 comenzó el 1 de junio de 2001. Se estudiaron todas las radiografías de tórax realizadas dentro de los tres primeros días de hospitalización. La variable de evaluación principal fue un primer episodio de neumonía acorde con la definición de la OMS que requiriese hospitalización. Para comparar la incidencia de la enfermedad se usaron modelos de riesgos proporcionales de Cox.
Resultados
Se registraron 526 eventos de neumonía entre 10 600 niños, lo que supone una incidencia de 3,3 por 1000 niños‑mes; 183 episodios (34,8%) se produjeron antes de los 5 meses de edad, y 247 (47,0%) antes de los 7 meses. De los niños estudiados, un 27% habían recibido 3 dosis de vacuna antes de los 7 meses de edad. Los cocientes de riesgos instantáneos para la neumonía como variable de evaluación fueron de 1,01 para 1 frente a 0 dosis; 1,03 para 2 frente a 0 dosis; y 0,84 para 3 frente a 0 dosis.
Conclusión
Los datos obtenidos no parecen respaldar la idea de que la PCV7 reduzca la incidencia de neumonía confirmada radiológicamente entre los lactantes indígenas del Territorio Septentrional, pese a que se detecta una tendencia, no significativa, a la manifestación de un efecto después de la tercera dosis. Estos resultados podrían explicarse suponiendo que la vacunación no se hizo en su debido momento y/o la enfermedad apareció a una edad temprana. Además, la contribución relativa del neumococo del tipo vacunal a la neumonía grave en un entorno donde concurren con frecuencia muchos otros agentes patógenos puede diferir respecto a otros entornos en que la eficacia de la vacuna ha quedado claramente demostrada.
ملخص
الهدف
تقييم مدى فعالية اللقاح السباعي التكافوء المتقارن المضاد للمكورات الرئوية في الوقاية من الالتهاب الرئوي، المشخص شعاعياً وفقا لمعايير منظمة الصحة العالمية وذلك بين الأطفال الأصليين في الأراضي الشمالية لاستراليا.
الطريقة
أجريت دراسة تاريخية أترابية حول الولادات الأترابية المتتابعة للسكان الأصليين في الفترة من 1 نيسان/إبريل 1998 وحتى 28 شباط/فبراير 2005، وتمت متابعة الأطفال حتى بلوغهم 18 شهراً من العمر. وقد بدأ برنامج اللقاح السباعي التكافوء المتقارن المضاد للمكورات الرئوية في أول حزيران/يونيه 2001. وتم تقييم جميع الصور الشعاعية الصدرية المأخوذة خلال ثلاثة أيام من الاحتجاز في المستشفيات. وكانت نقطة النهاية الأولية هي النائبة الأولى من الالتهاب الرئوي وفقا لمحددات منظمة الصحة العالمية والتي تتطلب الاحتجاز في المستشفى. كما استخدمت نماذج كوكس Cox لقياس المخاطر النسبية وذلك لمقارنة وقوعات المرض.
الموجودات
كانت هناك 526 حالة من الالتهاب الرئوي بين 10600 طفل – بلغت نسبة الوقوعات 3.3 لكل 1000 طفل في الشهر؛ وحدثت 183 نائبة (34.8%) قبل الشهر الخامس من العمر، و 247 نائبة (47.0%) بحلول الشهر السابع من العمر. ومن بين الأطفال الذين خضعوا للدراسة، كان 27% منهم قد تلقوا ثلاث جرعات من اللقاح ببلوغهم الشهر السابع من العمر. أما نسبة المخاطر للنقطة النهائية من الالتهاب الرئوي فكانت 1.01 لكل جرعة مقابل صفر من الجرعات، و 1.03 لكل جرعتين مقابل صفر من الجرعات، و0.84 لكل ثلاث جرعات مقابل صفر من الجرعات.
الاستنتاج
هناك بينات محدودة على أن اللقاح السباعي التكافوء المتقارن المضاد للمكورات الرئوية يحد من حدوث الالتهاب الرئوي المؤكد بالتصوير الشعاعي بين الأطفال الأصليين في الأراضي الشمالية لأستراليا، بالرغم من وجود اتجاه لا يعتد به إحصائياً حول تأثير اللقاح بعد تلقي الجرعة الثالثة منه. ويمكن أن تعزى هذه النتائج إلى عدم إجراء التلقيح في الوقت المناسب أو حدوث المرض في عمر مبكر. هذا علاوة على أن التأثير النوعي للقاح المضاد للمكورات الرئوية بالنسبة للالتهاب الرئوي الوخيم في الأماكن التي تتنتشر فيها العديد من الممرضات الأخرى قد يختلف عنه في الأماكن الأخرى حيث تأكدت فعالية اللقاح فيها بصورة واضحة.
Introduction
Australian indigenous children suffer from extremely high rates of pneumonia and acute respiratory illness,1–3 and determining the potential for reducing disease burden with pneumococcal conjugate vaccines is therefore seen as a major health priority.4 In June 2001, the 7-valent pneumococcal conjugate vaccine (PCV7) was included in the Australian National Immunisation Program as part of a publicly funded course of primary vaccination at 2, 4 and 6 months of age, with a booster dose of the 23-valent polysaccharide pneumococcal vaccine at 18 months, for all indigenous infants born on or after 1 April 2001. Catch-up campaigns were conducted in August 2001, targeting indigenous children aged up to 2 years in the northern region and up to 5 years in the central region of the Northern Territory.
Although antibiotic use in the community was high and the consequent yield of blood cultures at the time of hospitalization was poor, data from a central Australian study that used multiple diagnostic methods (culture and pneumolysin assays) had suggested that approximately 30% of hospitalized pneumonia cases were pneumococcal.5 PCV7 covered approximately 56% of pneumococcal serotypes causing invasive disease in indigenous children6 and 60% of pneumococcal serotypes carried in the nasopharynx (K Hare, Menzies School of Health Research, Darwin, personal communication, 2009) although the contribution of these serotypes to nonbacteraemic pneumonia was unknown. Assumptions based on these data suggested that a PCV7 uptake of at least 80% offered the potential for a 17% reduction of hospitalized pneumonia cases, an effect consistent with data from the pivotal trial of the vaccine in Californian children.7
The aim of this study was to estimate the effectiveness of PCV7 in preventing radiologically diagnosed pneumonia as defined by the World Health Organization (WHO)8 among Northern Territory indigenous infants aged up to 18 months. The WHO case definition was chosen as it was the only one that could be standardized and systematically applied to the available data. For brevity, the term “pneumonia” will be used to refer to radiologically confirmed cases in the remainder of this paper.
Methods
Design
We conducted a historical cohort study of consecutive Northern Territory indigenous birth cohorts over an 8-year period. Birth cohorts were constructed from two population-based health datasets – Northern Territory Immunisation Register data for the Northern Territory and Northern Territory hospital discharge data. The study was approved by the Human Research Ethics Committee of the Northern Territory Department of Health & Family Services and the Menzies School of Health Research (approval ID #05/49).
Setting
Of the Northern Territory’s 200 000 residents, 29% identify themselves as indigenous. Approximately 1500 births occur in this population per year. There are five public hospitals in the Northern Territory and one private hospital; the latter is rarely used by indigenous persons.
Health care is readily available in the Northern Territory. Indigenous infants present on average to primary health centres in remote areas at least once every two weeks in the first year of life.9 All Northern Territory indigenous children requiring hospitalization are admitted to one of the five public hospitals. Out-of-hospital deaths are rare in the Northern Territory. Mobility is predominantly limited to within regions in the Territory; interstate migration is infrequent.
All persons born in or who receive services at any public health care facility in the Northern Territory are allocated a unique health record number. This number is used for all subsequent episodes of medical care in the Territory, and it is the basis for registration on the Northern Territory Immunisation Register – a population-based register to which all vaccine providers report routinely. Children not born in a public hospital are added to the immunization register either through compulsory registration on the Northern Territory midwives’ data collection system or at the time of their first immunization encounter or first presentation for health care.
Population studied
Children were included if they were born between 1 April 1998 and 28 February 2005 and were resident in the Northern Territory at the time they were enrolled. Children were excluded if they died during the perinatal period (0–29 days of age) or while hospitalized if the admission date had been in the perinatal period, or if they had a first episode of pneumonia in the perinatal period.
Outcomes
All chest X-rays taken within the first 3 days of any admission for any diagnosis were obtained from all Northern Territory hospital radiology departments. Films were read independently by two general paediatricians or paediatric respiratory specialists blinded to all demographic, clinical and vaccination history data. Where readings were discordant, the films were read by a panel of paediatric radiologists similarly blinded to subject data and to the reason for discordance. All readers had achieved ≥ 80% agreement with the WHO training films before the start of the study, and inter-observer agreement during the study was ≥ 90%. The primary endpoint was a first episode of pneumonia (for consistency with clinical trials of the vaccine). Data on clinical presentation and laboratory investigations were not available for this study. For children in whom more than one chest X-ray was taken, any positive film classified the episode as a pneumonia event.
Person–time under observation commenced at 29 days of age (to exclude perinatal conditions) and ceased at the earliest of the following: date of admission for the first episode of pneumonia requiring hospitalization (failure date); 31 March 2005; date of death; date on which a child reached 18 months of age; or date on which a child received the 23-valent polysaccharide pneumococcal vaccine (to reduce confounding of PCV7 effects). Follow-up time was censored at 31 March 2005. As a result, not all children were followed until 18 months of age, particularly those in the last birth cohort.
Vaccination status
Vaccination status was assigned according to Northern Territory immunization register records of PCV7 vaccination. As vaccination status varied with time, person–time was split and analysed by intervals of 0 doses of PCV7, 1 dose, 2 doses and 3 or more doses. To allow sufficient time for an adequate immune response, a dose was not considered to have been received until 14 days after actual administration.
Covariates
Data available on the children in the study were limited to demographic information, vaccination history and hospitalization data; only age, sex and region of residence could be included as covariates in the analyses. Age was considered a time-varying covariate categorized into 3-month age groups (0 to < 3 months, 3 to < 6 months, 6 to < 9 months, 9 to < 12 months, 12 to < 15 months, 15 to < 18 months).
To assess the potential for differential vaccination of children with key co-morbidities known to be associated with the risk of pneumonia (gastroenteritis, anaemia and/or malnutrition), we assessed the differences in vaccination status between hospitalized children with and without these conditions. To account for opportunity for exposure to 3 doses of vaccine, this analysis was conducted only for children born on or after 1 April 2001 who were 7 months of age or older at the time of admission.
Sample size
This study was nested within a larger burden of pneumonia study conducted in the Northern Territory over the same time period.3 On the basis of data from central Australia5 and taking into account differences in the invasive pneumococcal disease burden between Northern Territory regions,6 we assumed an incidence of 70 cases per 1000 population per year across the Territory as a whole. If 80% coverage is assumed (on the basis of routine childhood immunization data), 3 birth cohort years before and after the vaccine would provide 80% power (α = 0.05) to detect a 20% reduction in pneumonia incidence.
Statistical analyses
Crude incidence rates were calculated by dividing number of cases by person–time at risk and are presented in units per 1000 child–months with corresponding 95% confidence intervals (CIs). Cox proportional hazards models with time-varying covariates10 were used to evaluate the association between receipt of PCV7 (categorized as 0, 1, 2 or 3 doses) and the time to first pneumonia event. Vaccine effectiveness (VE) was calculated from the estimated hazard ratio (HR) for 1, 2 and 3 doses compared to zero [VE = (1−HR) × 100].
Potential predictors evaluated in the models were age, sex, birth cohort and region of residence. Schoenfeld residual tests were used to evaluate the proportional hazards assumption for each covariate.10 Likelihood ratio tests were used to assess covariate effects and potential interactions.11 Data were analysed using Stata SE v9.1 (StataCorp, College Station, Texas, United States of America).
The primary analysis evaluated the association between vaccination and pneumonia in children born on or after 1 April 1998; children born before 1 April 2001 were included as historical controls. Secondary analyses were performed including only children born on or after 1 April 2001 and with the observation period commencing at 5 months, by which time children should have received 2 doses of vaccine.
Results
A total of 10 600 children were included in the final analysis. There was no evidence of a change in all-cause hospitalization rates over time (average incidence: 66.0 per 1000 child–months, 95% CI: 64.1–68.0) or the chest X-ray rate per 1000 hospitalizations. A total of 8488 chest X-rays were taken within 3 days of admission in 6775 episodes of care. Chest X-rays were considered of inadequate quality for endpoint diagnosis in 984 (14.5%) episodes. In this analysis, these episodes were considered negative for the study endpoint.
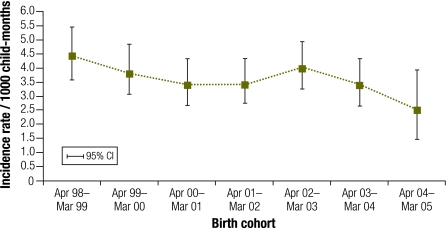
There were 526 first episodes of pneumonia – an overall incidence of 3.3 per 1000 child–months (95% CI: 3.1–3.6). Although the data were suggestive of a declining incidence over time (Fig. 1), there was insufficient statistical evidence to exclude chance as the basis for the observed change (likelihood ratio test for trend χ²: 9.98; P = 0.13). This may be due to insufficient follow-up time in the final birth cohort and an increase in incidence in the April 2002–March 2003 cohort.
Fig. 1.
Incidencea of a first episode of WHO-defined consolidated pneumonia, by birth cohort, among NT-resident Australian indigenous children aged 29 days to 18 monthsb, 1998–2005
NT, Northern Territory; WHO, World Health Organization.
a Cases per 1000 child–months.
b Mean person–time at risk in the last birth cohort was 5.5 months compared to 16.4 months in other cohorts.
There was little evidence for any patterns in incidence within birth cohorts by vaccination status (Table 1), although incidence appeared to be declining in both vaccinated and non-vaccinated children between cohorts. The mean age at first episode was 8.1 months; 183 episodes (34.8%) occurred before 5 months of age, 247 (47.0%) by 7 months, and 402 (76.4%) by 12 months. There was no difference in mean ages at the time of first episode by birth cohort. Incidence rates per 1000 child–months were highest in the two youngest age groups (Table 2).
Table 1. Incidence of WHO-defined consolidated pneumonia, by birth cohort and number of vaccine doses received, among NT‑resident Australian indigenous children aged 29 days to 18 months, 1998–2005.
| Cohort |
Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Apr 98–Mar 99 | Apr 99–Mar 00 | Apr 00–Mar 01 | Apr 01–Mar 02 | Apr 02–Mar 03 | Apr 03–Mar 04 | Apr 04–Mar 05 | |||
| 0 doses | |||||||||
| Cases | 89 | 84 | 58 | 22 | 21 | 13 | 4 | 291 | |
| Child–months | 22 514.3 | 24 312.7 | 18 779.7 | 6 571.7 | 4 915.9 | 4 129.7 | 2 288.0 | 83 512.0 | |
| Incidencea (95% CI) | 3.95 (3.21– 4.87) | 3.46 (2.79– 4.28) | 3.09 (2.39– 3.99) | 3.35 (2.20– 5.08) | 4.27 (2.79– 6.55) | 3.15 (1.83– 5.42) | 1.75 (0.66– 4.66) | 3.48 (3.10– 3.91) | |
| 1 dose | |||||||||
| Cases | NA | 0 | 8 | 28 | 15 | 17 | 7 | 75 | |
| Child–months | NA | 65.9 | 3 047.2 | 4 653.3 | 4 658.5 | 4 444.5 | 2 211.4 | 19 080.8 | |
| Incidencea (95% CI) | NA | 0 | 2.63 (1.31– 5.25) | 6.02 (4.15– 8.71) | 3.22 (1.94– 5.34) | 3.82 (2.38– 6.15) | 3.17 (1.51– 6.64) | 3.93 (3.13– 4.93) | |
| 2 doses | |||||||||
| Cases | NA | 0 | 12 | 11 | 23 | 13 | 3 | 62 | |
| Child–months | NA | 1.6 | 2 918.3 | 4 653.4 | 4 630.7 | 4 232.8 | 1 386.0 | 17 822.7 | |
| Incidencea (95% CI) | NA | 0 | 4.11 (2.34– 7.24) | 2.36 (1.31– 4.27) | 4.97 (3.30– 7.47) | 3.07 (1.78– 5.29) | 2.16 (0.69– 6.71) | 3.47 (2.71– 4.46) | |
| 3 doses | |||||||||
| Cases | NA | NA | 1 | 25 | 37 | 32 | 3 | 98 | |
| Child-months | NA | NA | 883.9 | 11 713.9 | 12 401.8 | 11 134.9 | 1 176.1 | 37 310.6 | |
| Incidencea (95% CI) | NA | NA | 1.13 (0.16– 8.03) | 2.13 (1.44– 3.16) | 2.98 (2.16– 4.12) | 2.87 (2.03– 4.06) | 2.55 (0.82– 7.90) | 2.63 (2.15– 3.20) | |
| Total | |||||||||
| Cases | 89 | 84 | 79 | 86 | 96 | 75 | 17 | 526 | |
| Child-months | 22 514.3 | 24 380.2 | 25 629.0 | 27 592.2 | 26 607.0 | 23 941.9 | 7 061.5 | 15 7726.1 | |
| Incidencea (95% CI) | 3.95 (3.21– 4.87) | 3.46 (2.78– 4.27) | 3.08 (2.47– 3.84) | 3.12 (2.52– 3.85) | 3.61 (2.95– 4.41) | 3.13 (2.50– 3.93) | 2.41 (1.49– 3.87) | 3.33 (3.06– 3.63) | |
CI, confidence interval; NT, Northern Territory; WHO, World Health Organization. a Cases per 1000 child–months.
Table 2. Incidence of WHO-defined consolidated pneumonia, by age group, among NT-resident Australian indigenous children aged 29 days to 18 months, 1998–2005.
| Age group | No. children | Cases | Child–months | Incidencea (95% CI) |
|---|---|---|---|---|
| 29 days to < 3 months | 10 600 | 128 | 30 596.2 | 4.18 (3.52–4.97) |
| 3 to < 6 months | 10 102 | 112 | 29 210.2 | 3.83 (3.19–4.61) |
| 6 to < 9 months | 9 668 | 95 | 27 921.7 | 3.40 (2.78–4.16) |
| 9 to < 12 months | 9 225 | 80 | 26 625.1 | 3.00 (2.41–3.74) |
| 12 to < 15 months | 8 766 | 74 | 25 186.2 | 2.93 (2.34–3.69) |
| 15 to < 18 months | 8 275 | 37 | 18 186.6 | 2.03 (1.47–2.81) |
| Totalb | 10 600 | 526 | 157 726.1 | 3.33 (3.06–3.63) |
CI, confidence interval; NT, Northern Territory; WHO, World Health Organization. a Cases per 1000 child–months. b Row represents the total number of children who contributed time to the study, and the total number of cases and overall incidence rate, irrespective of age.
Completeness of PCV7 vaccination among children in the study population aged 5 and 7 months by 31 March 2005 was poor: 38.1% (2365 infants) had received 2 doses by 5 months of age, and only 27.0% (1743 infants) had received 3 doses by 7 months. Coverage of 3 doses increased to 74% by 12 months of age and 82% by 18 months.
Age-adjusted hazard ratios for pneumonia comparing time vaccinated to time unvaccinated are presented in Table 3. There was limited evidence to support an effect of the vaccine in Northern Territory indigenous children for any dose level or cohort group analysed, although the evidence strengthened somewhat after the receipt of 3 doses in children beyond 5 months of age.
Table 3. Age-adjusted hazard rate ratios for WHO-defined consolidated pneumonia in vaccinated and unvaccinated NT indigenous infants aged 29 days to 18 months, by number of vaccine doses and analysis time period.
| Time period and cohort | 0 doses |
1 dose |
2 doses |
3 doses |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases (child–months) | Cases (child–months) | HRR | 95% CI | Cases (child–months) | HRR | 95% CI | Cases (child–months) | HRR | 95% CI | ||||
| Children born 1 Apr 2001 – 28 Feb 2005, from 29 days of age | 61 (17 909) | 68 (15 972) | 1.02 | 0.69–1.49 | 50 (14 907) | 1.10 | 0.72–1.67 | 97 (36 438) | 0.97 | 0.65–1.47 | |||
| Children born 1 Apr 1998 – 28 Feb 2005, from 29 days of age | 295 (83 623) | 76 (19 085) | 1.01 | 0.78–1.32 | 62 (17 827) | 1.03 | 0.72–1.47 | 98 (37 322) | 0.84 | 0.60–1.17 | |||
| All children, from 5 months of agea | 155 (49 362) | 21 (7 709) | 0.83 | 0.52–1.32 | 45 (12 697) | 1.37 | 0.81–2.32 | 97 (37 165) | 0.76 | 0.53–1.09 | |||
CI, confidence interval; HRR, hazard rate ratio; NT, Northern Territory ; WHO, World Health Organization. a Excludes 183 children who were censored before reaching 5 months of age.
There was no evidence to support a difference in vaccination status by presence or absence of key co-morbidities (gastroenteritis, anaemia and/or malnutrition) in children hospitalized for any cause (data not shown).
Discussion
To our knowledge, this is the first post-licensure field evaluation of PCV7 effectiveness in preventing a first episode of WHO-defined radiologically confirmed pneumonia in infants and the only study that has evaluated the effect of successive doses of the vaccine as children progress in age up to 18 months. It is also the first study that has measured the incidence of a first episode of WHO-defined radiologically confirmed pneumonia in an entire population from 29 days of age with detailed analyses of pneumonia risk by small time intervals. While point estimates suggested a reduction in disease incidence in both vaccinated and unvaccinated children over time and a trend towards a vaccine effectiveness of between 16% and 24% following the third dose, we were unable to exclude chance as the basis of our findings.
Vaccine efficacy estimates of 25–37% have been reported in randomized controlled trials of the 7- and 9-valent vaccines in California, South Africa, the Gambia and the Philippines.12–15 An ecological study in the United States reported a 39% decline (95% CI: 22–52) in hospitalized cases with a discharge diagnosis of pneumonia among children less than 2 years of age.16,17 Nelson et al. reported a decline of 40% (incidence rate ratio, IRR 0.60; 95% CI: 0.35–1.04) in cases with a discharge diagnosis of pneumonia among hospitalized children aged less than 1 year, with no evidence of an effect in older children.17 However, the WHO definition was not used in the latter two studies and their findings therefore cannot be readily compared to ours.
Possible explanations for the differences between our results and those reported in the clinical trials include: chance variation (our 95% CI allows the possibility of moderately substantial benefit after 3 doses), differences in case ascertainment methods, differences in vaccine schedules and serotype coverage, the possibility that the seven Streptococcus pneumoniae vaccine serotypes are not responsible for the majority of severe pneumonia in these children and/or serotype replacement. The latter may be of particular relevance given that while nasopharyngeal carriage of the serotypes targeted by the PCV7 has declined dramatically since its introduction in the Northern Territory, overall carriage of all serotypes remains unchanged.18 Similarly, rates of pneumonia due to respiratory syncytial virus and influenza virus in this population are high.5,19,20 While there are no data on its contribution to lower respiratory infection in these children, carriage of non-encapsulated Haemophilus influenzae is as high as 100% by 120 days of age.21
Data from studies published since this study was conducted suggest that the WHO definition substantially underestimates the vaccine-preventable proportion of pneumonia cases and that additional clinical data such as cross-reactive protein values may be important,22–24 although the latter may be population-dependent. In a subanalysis of data from the phase III clinical trial of the 9-valent pneumococcal conjugate vaccine in the Gambia, cross-reactive protein values did not improve estimates of vaccine efficacy or vaccine-attributable reduction in incidence.25 We did not have access to clinical data, however, and it is possible that the vaccine has prevented clinical illness not measured in our study.
The early age at which the first episode of pneumonia occurs in this population is an important finding. Half of the cases in our study occurred before 7 months of age and one quarter before 3 months of age. These data are consistent with the known early colonization by respiratory pathogens (e.g. Moraxella catarrhalis, H. influenzae and S. pneumoniae) in these children.26,27 It is clear that an effective vaccine would need to be given earlier to Australian indigenous children in order to achieve any substantial effect at the population level.
In older children aged 6 months or more, timeliness of vaccination is likely to be critical. While coverage reached over 85% by 18 months of age,28 fewer than 40% of eligible children had received 3 doses of the vaccine by 7 months of age. The necessity of 3 doses in infancy is suggested by this study’s data: while not statistically significant, the data indicated a reduction in incidence of 24% (95% CI: −9–47) after the third dose in children aged 5 months and older.
The major strength of this study is that we reviewed every hospitalization and every chest X-ray for every indigenous infant in the Northern Territory during the study period. The same person collected and processed all X-rays, and the person analysing the data was not involved in the reading of X-rays. The X-ray readers were not aware of the subject’s demographic, clinical and vaccination history.
The study design and analysis methods allowed an assessment of pneumonia as children aged over small time intervals and accounted for the potential variations in risk over time. We were able to commence measurement of person–time at risk at the same time for all individuals (29 days). In populations with rapid acquisition and nasopharyngeal colonization of respiratory pathogens early in life, as occurs in the Northern Territory indigenous population,26 variations in the age at which children enter a study may lead to differences in risk profiles between these children. This would be critical in studies without randomization of children to different groups. Accounting for the risk of infection and subsequent disease early in life is critical to the formulation of policies concerning vaccine schedules and number of doses required by specific ages.
We were able to exclude infants who had suffered a first episode of WHO-defined consolidated pneumonia in the perinatal period and who were therefore likely to have a different risk profile from those who had survived this period without contracting pneumonia. Importantly, as individual consent was not required for entry into the study, we were able to include every Northern Territory child. A major issue in clinical trials and other studies that enrol individuals is accounting for potentially important differences between those who do and do not consent to participate. Similarly, generally only healthy children are eligible for inclusion in clinical trials.
Uncertainty about the accuracy of the person–time denominator is a limitation. However, increasing vaccination coverage as children aged suggested that children were continuing to present to Northern Territory health services throughout infancy. The predominant reasons for censoring in this study were subjects reaching 18 months of age and the study reaching its end date of 31 March 2005. Both of these are administrative censoring points and the bias to the study is less important given that this type of censoring is largely independent of the characteristics of the individuals under observation.29 However, the considerably shorter person–time available in the analysis for the last birth cohort may have limited the study’s ability to detect vaccine effects. This is of particular relevance to interpreting the trend observed over time, as it is impossible to determine whether the decline in rates observed in the last year was sustained or was just a yearly variation in the incidence of disease. Moreover, declines appeared to be occurring independent of vaccination status. This underscores the need for ongoing surveillance of severe pneumonia in the Northern Territory population.
The lack of information on potentially confounding factors – particularly known risk factors such as prematurity, low birth weight, co-morbidities and exposure to household and tobacco smoke30 – necessitates a cautious approach to the interpretation of vaccine effectiveness in this study. Differences in these factors between vaccinated and unvaccinated children could have confounded the vaccine effectiveness. However, for these factors to explain our findings, vaccinated children would need to be at higher risk of exposure. Our data do suggest that vaccination status did not differ between hospitalized children with and without the other major causes of paediatric morbidity in the Northern Territory (gastroenteritis, malnutrition and anaemia).
A final limitation is the potential lack of power in this study to demonstrate an effect. Baseline disease incidence was calculated on estimates derived from a study that did not use the WHO definition for radiologically confirmed pneumonia because it was not available at the time.5 Because of our use of the WHO definition and our decision to evaluate only the first episode, disease incidence in this population was considerably lower than anticipated. Additional analyses evaluating repeated episodes of WHO- defined radiologically diagnosed pneumonia and all-cause acute lower respiratory infection and pneumonia requiring hospitalization have been performed for children from 5 to 23 months of age and have not changed our findings substantially.31
We were unable to demonstrate definitively that PCV7 had an effect in preventing a first episode of radiologically diagnosed pneumonia in a setting characterized by high rates occurring very early in infancy with delays in delivery of the primary vaccination series. This study highlights the importance of immunization timeliness to extrapolating the results of vaccine efficacy established in randomized controlled trials relative to vaccine effectiveness at the population level with vaccine delivery under routine field conditions. Optimizing the timeliness of vaccination in the Northern Territory infant population is a priority public health measure. Ongoing surveillance and further studies are required to evaluate whether the trend observed in the final year of the study was maintained. ■
Acknowledgements
The authors would like to thank the following people for their invaluable contribution to this work: Debbie Taylor-Thomson, Paul Bauert, Peter Morris, Gavin Wheaton, Grant Mackenzie, John De Campo, Margaret De Campo and Jane Benson of the study team; Vicki Krause, Christine Selvey, Linda Graham and Charles Roberts of the Centre for Disease Control, Northern Territory Department of Health and Families; Suzanna Vidmar of the Murdoch Childrens Research Institute; and Kim Mulholland, Tilman Ruff and Thomas Cherian, expert advisors.
Footnotes
Funding: Funding for this study was provided by Wyeth Vaccines. K O’Grady was supported by a National Health & Medical Research Council Post-Graduate Training Scholarship in indigenous Health and by the Australian Academy of Science’s Douglas and Lola Douglas Scholarship in Medical Research. The sponsors of this study had no role in the design, implementation, analysis, interpretation or reporting of the work.
Competing interests: None declared.
References
- 1.Carville KS, Lehmann D, Hall G, Moore H, Richmond P, de Klerk N, et al. Infection is the major component of the disease burden in Aboriginal and non-Aboriginal Australian children: a population-based study. Pediatr Infect Dis J. 2007;26:210–6. doi: 10.1097/01.inf.0000254148.09831.7f. [DOI] [PubMed] [Google Scholar]
- 2.Hanna J, Torzillo P. Acute respiratory infections in Australian Aboriginal children: current knowledge and future requirements. P N G Med J. 1991;34:204–10. [PubMed] [Google Scholar]
- 3.O’Grady K, Taylor-Thomson D, Chang A, Torzillo P, Morris P, Mackenzie G, et al. High rates of World Health Organization-defined radiologically confirmed pneumonia in Australian Indigenous children. Med J Aust. 2009 doi: 10.5694/j.1326-5377.2010.tb03644.x. In press. [DOI] [PubMed] [Google Scholar]
- 4.Torzillo PJ, Gratten M. Conjugate pneumococcal vaccines for Aboriginal children in Australia. Med J Aust. 2000;173(Suppl):S51. doi: 10.5694/j.1326-5377.2000.tb139416.x. [DOI] [PubMed] [Google Scholar]
- 5.Torzillo P, Dixon J, Manning K, Hutton S, Gratten M, Hueston L, et al. Etiology of acute lower respiratory tract infection in Central Australian Aboriginal children. Pediatr Infect Dis J. 1999;18:714–21. doi: 10.1097/00006454-199908000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Krause VL, Reid SJ, Merianos A. Invasive pneumococcal disease in the Northern Territory of Australia, 1994-1998. Med J Aust. 2000;173(Suppl):S27. doi: 10.5694/j.1326-5377.2000.tb139410.x. [DOI] [PubMed] [Google Scholar]
- 7.Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen JR, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J. 2000;19:187. doi: 10.1097/00006454-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Standardization of interpretation of chest radiographs for the diagnosis of pneumonia in children Geneva: World Health Organization; 2001. [Google Scholar]
- 9.Clucas DB, Carville K, Connors C, Currie BJ, Carapetis JR, Andrews RM. Disease burden and health-care clinic attendances for young children in remote Aboriginal communities of northern Australia. Bull World Health Organ. 2008;86:275–81. doi: 10.2471/BLT.07.043034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kleinbaum DG, Klein M. Survival analysis: a self-learning text 2nd edn. New York, NY: Springer; 2005. [Google Scholar]
- 11.Clayton D, Hills M. Statistical models in epidemiology Oxford: Oxford University Press; 2005. [Google Scholar]
- 12.Black SB, Shinefield HR, Ling S, Hansen J, Fireman B, Spring D, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr Infect Dis J. 2002;21:810. doi: 10.1097/00006454-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Cutts FT, Zaman SM, Enwere G, Jaffar S, Levine OS, Okoko JB, et al. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet. 2005;365:1139–46. doi: 10.1016/S0140-6736(05)71876-6. [DOI] [PubMed] [Google Scholar]
- 14.Klugman KP, Madhi SA, Heubner R, Kohberger R, Mbelle N, Pierce NF, et al. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med. 2003;349:1341–8. doi: 10.1056/NEJMoa035060. [DOI] [PubMed] [Google Scholar]
- 15.Lucero MG, Nohynek H, Williams G, Tallo V, Simoes EA, Lupisan S, et al. Efficacy of an 11-Valent Pneumococcal Conjugate Vaccine Against Radiologically Confirmed Pneumonia Among Children Less Than 2 Years of Age in the Philippines: A Randomized, Double-Blind, Placebo-Controlled Trial. Pediatr Infect Dis J. 2009;28:455–62. doi: 10.1097/INF.0b013e31819637af. [DOI] [PubMed] [Google Scholar]
- 16.Grijalva CG, Nuorti JP, Arbogast PG, Martin SW, Edwards KM, Griffin MR. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet. 2007;369:1179–86. doi: 10.1016/S0140-6736(07)60564-9. [DOI] [PubMed] [Google Scholar]
- 17.Nelson JC, Jackson M, Yu O, Whitney CG, Bounds L, Bittner R, et al. Impact of the introduction of pneumococcal conjugate vaccine on rates of community acquired pneumonia in children and adults. Vaccine. 2008;26:4947–54. doi: 10.1016/j.vaccine.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 18.Leach A, Mackenzie G, Mellon G, Wilson C, Hare K, Smith-Vaughan HC, et al. Emerging serotypes following universal pneumococcal conjugate vaccine (7vPCV). Paper presented at: Communicable Disease Control Conference 2007, Canberra, Australia, 4-15March2007 [Google Scholar]
- 19.Menzies R, Turnour C, Chiu C, McIntyre P. Vaccine preventable diseases and vaccination coverage in Aboriginal and Torres Strait Islander people, Australia 2003 to 2006. Commun Dis Intell. 2008;32(Suppl):S2–67. [PubMed] [Google Scholar]
- 20.Moore H, Burgner D, Carville K, Jacoby P, Richmond P, Lehmann D. Diverging trends for lower respiratory infections in non-Aboriginal and Aboriginal children. J Paediatr Child Health. 2007;43:451–7. doi: 10.1111/j.1440-1754.2007.01110.x. [DOI] [PubMed] [Google Scholar]
- 21.Smith-Vaughan HC, Leach AJ, Shelby-James TM, Kemp K, Kemp DJ, Mathews JD. Carriage of multiple ribotypes of non-encapsulated Haemophilus influenzae in Aboriginal infants with otitis media. Epidemiol Infect. 1996;116:177–83. doi: 10.1017/S0950268800052419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klugman KP, Madhi SA, Albrich WC. Novel approaches to the identification of Streptococcus pneumoniae as the cause of community-acquired pneumonia. Clin Infect Dis. 2008;47(Suppl 3):S202–6. doi: 10.1086/591405. [DOI] [PubMed] [Google Scholar]
- 23.Madhi SA, Heera JR, Kuwanda L, Klugman KP. Use of procalcitonin and C-reactive protein to evaluate vaccine efficacy against pneumonia. PLoS Med. 2005;2:e38. doi: 10.1371/journal.pmed.0020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madhi SA, Kohler M, Kuwanda L, Cutland C, Klugman KP. Usefulness of C-reactive protein to define pneumococcal conjugate vaccine efficacy in the prevention of pneumonia. Pediatr Infect Dis J. 2006;25:30–6. doi: 10.1097/01.inf.0000195787.99199.4a. [DOI] [PubMed] [Google Scholar]
- 25.Cheung YB, Zaman SM, Ruopuro ML, Enwere G, Adegbola RA, Greenwood B, et al. C-reactive protein and procalcitonin in the evaluation of the efficacy of a pneumococcal conjugate vaccine in Gambian children. Trop Med Int Health. 2008;13:603–11. doi: 10.1111/j.1365-3156.2008.02050.x. [DOI] [PubMed] [Google Scholar]
- 26.Leach AJ, Boswell JB, Asche V, Nienhuys TG, Mathews JD. Bacterial colonization of the nasopharynx predicts very early onset and persistence of otitis media in Australian aboriginal infants. Pediatr Infect Dis J. 1994;13:983–9. doi: 10.1097/00006454-199411000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Leach AJ. Otitis media in Australian Aboriginal children: an overview. Int J Pediatr Otorhinolaryngol. 1999;49(Suppl 1):S173–8. doi: 10.1016/S0165-5876(99)00156-1. [DOI] [PubMed] [Google Scholar]
- 28.O’Grady KA, Krause V, Andrews R. Immunisation coverage in Australian Indigenous children: Time to move the goal posts. Vaccine. 2009;27:307–12. doi: 10.1016/j.vaccine.2008.09.096. [DOI] [PubMed] [Google Scholar]
- 29.Szklo M, Javier Nieto F. Epidemiology: beyond the basics Gaithersburg, MD: Aspen Publishers; 2000. [Google Scholar]
- 30.Mulholland K. Global burden of acute respiratory infections in children: implications for interventions. Pediatr Pulmonol. 2003;36:469–74. doi: 10.1002/ppul.10344. [DOI] [PubMed] [Google Scholar]
- 31.O’Grady KF, Lee KJ, Carlin JB, Torzillo PJ, Chang AB, Mulholland EK, et al. Increased risk of hospitalised acute lower respiratory infections in Australian Indigenous infants aged 5–23 months following pneumococcal vaccination: a cohort study. Clin Infect Dis. 2009 doi: 10.1086/651079. In press. [DOI] [PubMed] [Google Scholar]