Abstract
Basic helix-loop-helix (bHLH) proteins are highly conserved transcription factors critical for cell proliferation and differentiation. Recent studies have implicated bHLH proteins in many plant signaling processes, including brassinosteroid (BR) signaling. Here, we report identification of two families of atypical bHLH proteins capable of modulating BR signaling. We found that activation-tagged bri1 suppressor 1-Dominant (atbs1-D), previously identified as a dominant suppressor of a weak BR receptor mutant bri1-301, was caused by overexpression of a 93–amino acid atypical bHLH protein lacking amino acids critical for DNA binding. Interestingly, atbs1-D only suppresses weak BR mutants, while overexpression of a truncated ATBS1 lacking the basic motif also rescues bri1-301, suggesting that ATBS1 likely stimulates BR signaling by sequestering negative BR signaling components. A yeast two-hybrid screen using ATBS1 as bait discovered four ATBS1-Interacting Factors (AIFs) that are members of another atypical bHLH protein subfamily. AIF1 exhibits an overlapping expression pattern with ATBS1 and its homologs and interacts with ATBS1 in vitro and in vivo. AIF1 overexpression nullifies the suppressive effect of atbs1-D on bri1-301 and results in dwarf transgenic plants resembling BR mutants. By contrast, silencing of AIF1 partially suppressed the bri1-301 phenotype. Our results suggested that plants use these atypical bHLH proteins to regulate BR signaling.
INTRODUCTION
Brassinosteroids (BRs) are a special class of plant polyhydroxysteroids that play important roles in plant growth and development (Clouse and Sasse, 1998). Unlike animal steroids that move into cells to bind intracellular receptors, BRs are perceived at the cell surface by the leucine-rich repeat receptor-like kinase BRASSINOSTEROID-INSENSITIVE1 (BRI1) that initiates a phosphorylation-mediated signaling cascade (Li and Chory, 1997; Li, 2005). In the absence of BRs, BRI1 and its coreceptor BRI1-Associated receptor Kinase (BAK1) are inactive kinases (Li et al., 2002; Nam and Li, 2002; Wang et al., 2005a), while a GSK3-like kinase BRASSINOSTEROID-INSENSITIVE2 (BIN2) is constitutively active to phosphorylate two highly similar transcription factors, bri1-EMS-Suppressor 1 (BES1) and Brassinazole-Resistant1 (BZR1) (He et al., 2002; Wang et al., 2002; Yin et al., 2002; Zhao et al., 2002), thus promoting their degradation, retaining them in cytosol, and/or inhibiting their DNA binding activity (Vert and Chory, 2006; Bai et al., 2007; Gampala et al., 2007; Ryu et al., 2007). BR binding to BRI1 triggers a rapid dissociation of BRI1 Kinase Inhibitor1 from BRI1 and promotes heterotetramerization and transphosphorylation of BRI1 and BAK1 (Wang et al., 2005b, 2008; Wang and Chory, 2006), leading to phosphorylation of BR-signaling kinases and inhibition of BIN2 (Peng et al., 2008; Tang et al., 2008). Consequently, BES1 and BZR1 accumulate in the nucleus to regulate expression of their target genes (He et al., 2005; Yin et al., 2005).
In addition to these two nuclear proteins, several other DNA binding proteins are implicated in BR signaling. These include several auxin-regulated proteins (Vert et al., 2008; Song et al., 2009b), a Myb transcription factor (Li et al., 2009), a GRAS-family protein (Tong et al., 2009), two jumonji domain-containing proteins that might affect chromatin structure (Yu et al., 2008), and at least six basic helix-loop-helix (bHLH) proteins, such as BRI1 Enhanced Expression1 (BEE1) to BEE3 (Friedrichsen et al., 2002) and BES1-Interacting Myc-like 1 (BIM1) to BIM3 (Yin et al., 2005). Biochemical studies suggested that BES1, which also contains a bHLH-like domain, interacts with BIM1 to synergistically bind to the promoter of SAUR-AC1, a well-known BR-upregulated gene (Yin et al., 2005). The identification of several bHLH proteins is consistent with genome-wide gene expression analyses that revealed enrichment of the E-box element, a well-studied regulatory element recognized by bHLH proteins, in the promoters of many BR-responsive genes (Nemhauser et al., 2004; Vert et al., 2005). These studies thus demonstrated the importance of bHLH proteins in regulating the nuclear activities of the BR signaling pathway.
The Arabidopsis thaliana genome encodes a total of 162 bHLH proteins grouped into 21 subfamilies (Toledo-Ortiz et al., 2003). Recent studies have implicated bHLH proteins in a wide range of plant developmental and physiological processes, including light signaling and stomata differentiation (Duek and Fankhauser, 2005; Nadeau, 2009). The bHLH domain that defines the family contains ∼60 amino acids with two distinctive regions, the basic region and the HLH region carrying two amphipathic α-helices linked by a variable loop (Ellenberger et al., 1994). The basic region of 15 to 17 amino acids is responsible for binding to the E-box (CANNTG) element, while the HLH region is used for homo- or heterodimerization. Based on the properties of the basic region, bHLH proteins are generally classified into two categories: DNA binding and non-DNA binding bHLH proteins. The non-DNA binding bHLH proteins (often called atypical bHLH proteins in plant literature), such as the human Id1 (Norton, 2000) and the Drosophila melanogaster EMC (Campuzano, 2001), have a less-basic region that also lacks the Glu-13 and Arg-17 residues (based on the numbering scheme of Toledo-Ortiz et al. [2003]) critical for DNA binding. Dimerization among the DNA binding bHLH proteins generates sufficient diversity to bind various variants of the E-box and to regulate cell-type/tissue-specific gene expression, whereas dimerization with a non-DNA binding bHLH protein inhibits DNA binding activity (Massari and Murre, 2000).
To identify additional components of the BR signaling pathway, we conducted an activation tagging-based genetic screen for genes that when overexpressed can suppress the dwarf phenotype of the weak bri1 mutant, bri1-301 (Xu et al., 2008), and isolated a total of six activation-tagged bri1 suppressor-Dominant (atbs-D) mutants (Kang et al., 2009). Such an approach has been successful for identifying components of the BR signaling pathway, including BRS1 (Li et al., 2001b), BAK1 (Li et al., 2002), BSU1 (Mora-Garcia et al., 2004), and BRL1 (Zhou et al., 2004). Here, we report that the Activation-Tagged Bri1-Suppressor1 (ATBS1) gene encodes an atypical 93–amino acid bHLH protein. Interestingly, overexpression of the full-length ATBS1 or its truncated form lacking the N-terminal basic motif was able to suppress the dwarf phenotype of bri1-301, suggesting that ATBS1 stimulates BR signaling by heterodimerizing and titrating off one or more inhibitory BR signaling proteins. Using a yeast two-hybrid screen, we identified four closely related bHLH proteins as ATBS1-interacting factors (AIFs). Overexpression and gene silencing experiments suggested that AIF1 likely functions as a negative regulator of the BR signaling pathway.
RESULTS
atbs1-D Is a Suppressor of bri1-301
To identify additional proteins involved in BR signaling, we previously performed an activation tagging-based genetic screen (Weigel et al., 2000) looking for genes that, when overexpressed (driven by four copies of the 35S enhancer element of cauliflower mosaic virus) suppress the dwarf phenotype of bri1-301. The bri1-301 allele is a very weak bri1 mutant that can grow as tall and produce as many seeds as the wild-type plants under certain growth conditions, yet exhibits many characteristic phenotypes of BR-deficient/signaling mutants, including a compact rosette with rounder leaves and short petiole and delayed flowering (Li and Nam, 2002). Out of ∼25,000 independent activation-tagged bri1-301 lines, we identified a total of six lines exhibiting a near-wild-type morphology when grown in soil (Kang et al., 2009), including atbs1-D, the focus of this study.
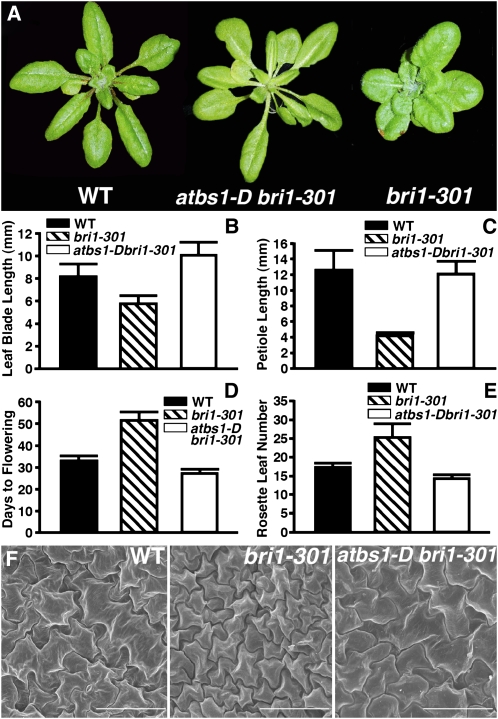
As shown in Figure 1, the atbs1-D mutation suppresses many aspects of the bri1-301 mutant phenotype. The leaf shape of atbs1-D bri1-301 mutant plants is similar to that of the wild-type plants (Figure 1A; see Supplemental Figure 1 online). The average petiole and blade length of the fifth rosette leaf, which is known to exhibit the most distinguishing features of the rosette leaves (Tsuge et al., 1996), of atbs1-D bri1-301 are similar to those of the wild-type control (Figures 1B and 1C). These morphological changes are most likely due to an increase in cell size. Scanning electron microscopy analysis of the rosette leaves showed that the leaf epidermal cells of atbs1-D bri1-301 are significantly larger than those of bri1-301 and are almost the same size as those of the wild type (Figure 1F). The atbs1-D mutation also suppresses the late flowering phenotype of bri1-301 measured by both days of flowering and rosette leaf numbers (Figures 1D and 1E).
Figure 1.
atbs1-D Is an Activation-Tagged bri1 Suppressor.
(A) Shown here are 6-week-old soil-grown plants of the wild type, bri1-301, and atbs1-D bri1-301.
(B) and (C) Comparison of leaf blade length (B) and petiole length (C) of the wild type, bri1-301, and abs1-D bri1-301. Measurements were taken for fifth leaves of 6-week-old soil-grown plants.
(D) and (E) Time to flowering was measured either as the numbers of days to bolting (D) and the numbers of rosette leaves at bolting (E). For (B) to (E), each data point represents at least 40 individual plants of duplicated experiments. Error bar denotes sd.
(F) Scanning electron microscopy analysis of rosette epidermal cells. Shown here are epidermal cells in the adaxial region of the fifth leaf of 6-week-old plants of the wild type, bri1-301, and atbs1-D bri1-301. Bars = 100 μm.
[See online article for color version of this figure.]
atbs1-D Enhances BR Signaling but Has Little Effect on BR Content
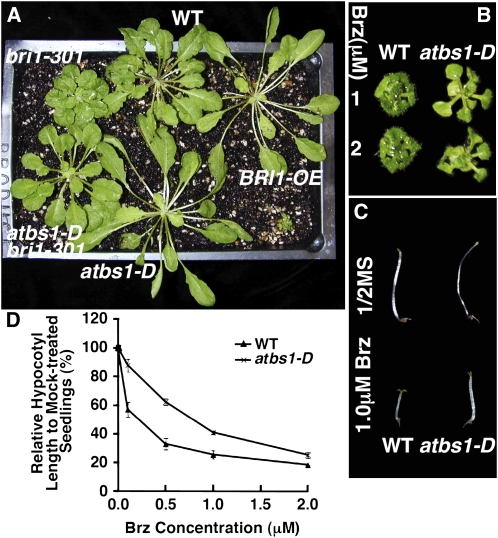
To test if atbs1-D alone has any effect on BR-mediated plant growth, we crossed atbs1-D bri1-301 with the wild type to generate the atbs1-D single mutant. As shown in Figure 2A, compared with the wild type, atbs1-D displayed longer, narrower rosette leaves in the light, similar to transgenic plants overexpressing BRI1 (Nam and Li, 2002). Such a phenotype is not caused by increased BR production but is likely due to enhanced BR signaling (see Supplemental Table 1 online). Consistent with this result, atbs1-D is more resistant to brassinazole (Brz), a well-studied BR biosynthesis inhibitor (Asami et al., 2000), under both light and dark growth conditions (Figures 2B to 2D).
Figure 2.
atbs1-D Enhances BR Signaling.
(A) Shown here are 8-week-old plants grown under short-day (8 h-light/16 h-dark) growth conditions.
(B) and (C) abs1-D exhibits enhanced resistance to Brz in both light (B) and dark (C). Wild-type and abs1-D seedlings were grown on half-strength Murashige and Skoog (MS) medium with or without Brz for 2 weeks in the light (B) or 5 d in the dark (C).
(D) Quantitative analysis of Brz resistance of wild-type and abs1-D seedlings grown on medium containing various concentrations of Brz for 5 d in the dark. Each data point represents at least 30 seedlings of duplicated experiments. Error bars denote se.
[See online article for color version of this figure.]
The Stimulatory Role of atbs1-D Requires Active BR Signaling Components
To test if the atbs1-D mutation results in constitutive activation of BR signaling or just enhances a partially active BR signaling pathway, we crossed atbs1-D into several known BR-deficient or BR signaling mutants, including det2 (Li et al., 1996), bri1-101 (Li and Chory, 1997), bri1-5 (Noguchi et al., 1999), bin2-1 (Li and Nam, 2002), and ucu1-3 (Perez-Perez et al., 2002). The det2 mutant is a BR biosynthesis mutant, while the two bri1 mutants are defective in BR receptor with bri1-5 being a weaker dwarf caused by retention of a biochemically competent BR receptor in the endoplasmic reticulum (Hong et al., 2008). Both bin2-1 and ucu1-3 are gain-of-function mutants of BIN2, with ucu1-3 being a weaker mutant. As shown in Figure 3, atbs1-D only enhanced the growth of bri1-5, ucu1-3, and heterozygous bin2-1 mutants but failed to suppress the mutant phenotypes of det2, bri1-101, and homozygous bin2 mutants, suggesting that the stimulatory effect of atbs1-D on plant growth requires the presence of active BR signaling components above certain threshold levels.
Figure 3.
The Stimulatory Effect of atbs1-D on BR Signaling Requires a Partially Active BR Signaling Pathway.
Shown here is a phenotypic comparison between single mutants of det2 (A), bri1-101 (B), bin2-1 (C), bin2-1/+ (D), bri1-5 (E), and ucu1-3 (F) and their corresponding double mutants with atbs1-D. These mutants were grown in soil for 6 weeks under the 16 h-light/8 h-dark photoperiodic condition, except det2 and atbs1-D det2 that were 8 weeks old.
[See online article for color version of this figure.]
ATBS1 Encodes an Atypical bHLH Protein
Our previous study revealed that the T-DNA was inserted 5.9 and 1.9 kb upstream of At1g74500 and At1g74510, respectively, with four copies of the 35S enhancer pointing toward the predicted start codon of At1g74500 and that At1g74500 was overexpressed in atbs1-D (Kang et al., 2009). To determine if At1g74500 is the ATBS1 gene, we overexpressed the At1g74500 cDNA using the 35S promoter in bri1-301. As shown in Figure 4A, the p35S:At1g74500 bri1-301 transgenic plants were morphologically similar to atbs1-D bri1-301, thus supporting that At1g74500 causes the atbs1-D phenotype.
Figure 4.
ATBS1 Encodes an Atypical bHLH Protein
(A) Shown here are 5-week-old soil-grown plants of bri1-301, one representative transgenic bri1301 line each for the 35S-driven transgene constructs of ATBS1 and its homologs, and the wild-type control.
(B) Alignment of ATBS1 (NP_177590) with its five Arabidopsis homologs (KIDARI, At1g26945, NP_849712; PRE1, At5g39860, NP_198802; At3g28857, NP_974372; At3g47710, NP_190355; At5g15160, NP_197020). Open box denotes the basic region, hatched bar indicates the helix motif, and the black line represents the loop region.
(C) Sequence alignment of the bHLH domain of ATBS1 and other previously studied bHLH proteins, including BEE1 (NP_173276), BEE2 (NP_849508), BEE3 (NP_177524), PHYTOCHROME INTERACTING FACTOR3 (PIF3; NP_849626), SPATULA (SPT; AAG33640), ALCATRAZ (ALC; NP_201512) from Arabidopsis, human MyoD (CCA40000), and Drosophila Single-minded (Sim; NP_524340). Stars indicate key positions where ATBS1 is different from other bHLH proteins, and the numbers in the open box show the positions of key amino acids in the basic region. For (B) and (C), alignment was performed using Tcoffee (http://tcoffee.vital-it.ch/cgi-bin/Tcoffee/tcoffee_cgi/index.cgi). Identical and similar amino acids are shaded black and gray, respectively, using the BoxShade server at http://www.ch.embnet.org/software/BOX_form.html.
(D) Shown here are 6-week-old soil-grown transgenic bri1-301 plants expressing p35S:ATBS1 or p35S:Δb-ATBS1 encoding a truncated ATBS1 lacking the basic region.
[See online article for color version of this figure.]
ATBS1 encodes a hypothetical bHLH protein of 93 amino acids that shares 61 to 74% sequence identity with KIDARI, which is thought to repress light signal transduction (Hyun and Lee, 2006), PRE1, which is involved in gibberellin signaling (Lee et al., 2006), and three other Arabidopsis proteins of 92 or 94 amino acids, At5g15160, At3g47710, and At3g28857 (Figure 4B). It is interesting to note that these six proteins are similar to the rice BRASSINOSTEROID UPREGULATED1 (BU1) that was recently implicated in BR signaling (Tanaka et al., 2009). Gene expression analysis using the Arabidopsis eFP browser, a visualization tool for exploring publicly available microarray data sets (Winter et al., 2007), revealed overlapping yet distinct expression patterns for ATBS1 and its three homologs that were printed on the Affymetrix ATH1 chip (see Supplemental Figure 2 online). Despite significant accumulation of ATBS1 and PRE1 mRNAs in roots and young siliques, respectively, low levels of their transcripts were detected in all aerial tissues that also accumulate mRNAs for BRI1 and BIN2 genes (see Supplemental Figure 2 online). This is consistent with the notion that activation tagging primarily enhances an endogenous expression pattern rather than inducing ectopic constitutive expression (Weigel et al., 2000). We suspected that these six small bHLH proteins might function redundantly in regulating BR signaling. Indeed, overexpressing each of the ATBS1 homologs driven by the 35S promoter also suppressed the bri1-301 mutation (Figure 4A), whereas T-DNA insertional mutants in KIDARI, PRE1, or At3g47710 exhibit no visible morphological change (Hyun and Lee, 2006; Lee et al., 2006). Interestingly, RNA interference (RNAi)-induced silencing of ATBS1, while having little effect on the rosette leaves, gave rise to a dwarf phenotype similar to known BR mutants (see Supplemental Figure 3 online).
Sequence alignment between ATBS1 and selected bHLH proteins revealed conservation in ATBS1 of residues that define the HLH domain. However, three amino acids (indicated by stars in Figure 4C) of the basic region of ATBS1 that contains five Arg residues, are notably different from those of most Arabidopsis DNA binding bHLH proteins (Toledo-Ortiz et al., 2003). The indicated three amino acids, Ser-13, Ser-16, and Glu-17, in ATBS1 occupy the positions of the highly conserved Glu-13, Arg-16, and Arg-17 residues of most known DNA binding bHLH proteins, respectively. Both hydrophobic and hydrophilic amino acids can be found at the Arg-17 position for some known DNA binding bHLH proteins, but no acidic amino acid has been found at this position in the known DNA binding bHLH proteins (Toledo-Ortiz et al., 2003). By contrast, Asp was found at the Arg-17 position for two previously characterized Arabidopsis proteins, HFR1 (Fairchild et al., 2000) and KIDARI (Hyun and Lee, 2006), which were known as non-DNA binding or atypical bHLH proteins. We therefore predicted that ATBS1 is also an atypical bHLH protein incapable of DNA binding and that ATBS1 might enhance BR signaling by heterodimerizing with one or more bHLH proteins that negatively regulate BR signaling. Consistent with this prediction, overexpression in bri1-301 of a truncated ATBS1 protein lacking the basic region resulted in transgenic plants resembling the atbs1-D bri1-301 mutants (Figure 4D).
ATBS1 Is a Nuclear-Localized Protein That Regulates a Subset of BR-Responsive Genes
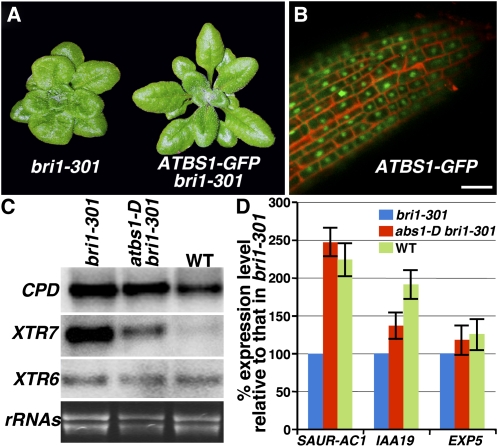
To directly examine ATBS1 subcellular localization, we generated transgenic plants that express an ATBS1-green fluorescent protein (GFP) fusion protein driven by the BRI1 promoter. The C-terminal GFP fusion did not interfere with ATBS1 function since the pBRI1:ATBS1-GFP transgene was able to suppress the bri1-301 mutation (Figure 5A). Confocal microscopy analysis of the root tips of several independent transgenic lines revealed that ATBS1 is mainly localized inside the nucleus with low GFP signals also detected in the cytosol (Figure 5B).
Figure 5.
ATBS1 Is a Nuclear-Localized Protein That Regulates Some BR-Responsive Genes.
(A) Shown here are 6-week-old soil-grown plants of bri1-301 and a representative transgenic bri1-301 line expressing GFP-tagged ATBS1 driven by the BRI1 promoter.
(B) Confocal microscopy analysis of the root tip from a pBRI1:ATBS1-GFP bri1-301 transgenic seedling. GFP fluorescence is shown in green, and cell wall staining by propidium iodide is shown in red. Bar = 50 μm.
(C) RNA gel blot analysis. Total RNAs were isolated from 4-week-old plants of bri1-301, abs1-D bri1-301, or the wild type. Hybridization was performed first with an XTR6 probe. After stripping the previous probe, the filter was rehybridized with the next probe. Ethidium bromide staining of total RNAs serves as a loading control.
(D) Real-time qRT-PCR analysis. Total RNAs isolated from 3-week-old seedlings were converted into first-strand cDNAs that were used as templates to PCR amplify transcripts of SAUR-AC1, IAA19, EXP5, or β-TUBULIN with primers listed in Supplemental Table 2 online. The comparative Ct method was used to calculate the levels of transcripts relative to that of bri1-301. Levels of β-TUBULIN transcript were used to normalize different samples. Bars represent sd (n = 3).
The revelation that ATBS1 is a nuclear-localized bHLH protein suggested that atbs1-D enhances BR signaling by modulating gene expression. We used both RNA gel blot and quantitative real-time RT-PCR (qRT-PCR) analyses to examine if atbs1-D affected expression of several known BR-responsive genes, including a BR biosynthetic gene, CPD, two auxin-inducible genes, SAUR-AC1 and IAA19, a putative expansin gene, EXP5, and two xyloglucan endotransglycosylase genes, XTR6 and XTR7. It was known that CPD and XTR7 are downregulated by BR and the other four genes are upregulated by BR (Vert et al., 2005). As shown in Figures 5C and 5D, although atbs1-D has little effect on expression of CPD, XTR6, EXP5, and IAA19, it significantly reduces the XTR7 expression and markedly increases the accumulation of the SAUR-AC1 transcripts, suggesting that atbs1-D affects a subset of the known BR-responsive genes. Immunoblot analysis using anti-BES1 antiserum revealed that the effect of atbs1-D on gene expression was unlikely caused by increased accumulation of the nonphosphorylated form of BES1 (see Supplemental Figure 4 online), which binds to the SAUR-AC1 promoter (Vert and Chory, 2006).
Identification of Four Atypical bHLH Proteins as ATBS1-Interacting Factors
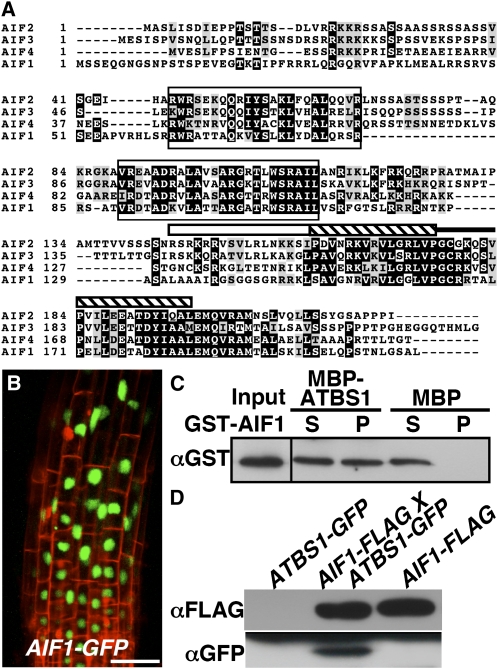
To understand more about how ATBS1 regulates BR signaling, we performed a yeast two-hybrid screen using the full-length ATBS1 as bait searching for potential ATBS1 binding proteins, some of which might be sequestered by overaccumulated ATBS1 in the atbs1-D mutant. Remarkably, 86 out of 131 positive clones were derived from cDNAs of three members of a small bHLH subfamily, At3g05800, At3g06590, and At3g17100 (also known as BHLH150, BHLH148, and BHLH147, respectively) (Figure 6A). We named them AIFs for ATBS1 interacting factors (At3g05800/BHLH150, AIF1; At3g06590/BHLH148, AIF2; At3g17100/BHLH147, AIF3). Yeast two-hybrid assay revealed that ATBS1 also interacted with the fourth member of the family, At1g09250 (BHLH149), that was named as AIF4 (see Supplemental Figure 5 online). Interestingly, BHLH150/AIF1 was also identified as a BIN2-interacting protein in our yeast two-hybrid screens and was phosphorylated by BIN2 in an in vitro kinase assay (see Supplemental Figure 6 online). Sequence alignment of the four Arabidopsis proteins revealed the highly conserved HLH domain in the C-terminal third of the proteins with a variable basic motif and two other conserved motifs in their middle regions (Figure 6A). Despite the presence of at least five basic residues in their basic regions, the lack of the conserved Glu-13 and Arg-17 residues in those ATBS1-interacting proteins suggests that these AIFs are also atypical bHLH proteins incapable of binding DNA.
Figure 6.
AIF1 Interacts with ATBS1 Both in Vitro and in Vivo.
(A) Alignment of four members of the AIF family (AIF1, At3g05800, NP_566260; AIF2, At3g06590, NP_974239; AIF3, At3g17100; NP_566567; AIF4, At1g09250, NP_563839). Alignment was performed using the Tcoffee program (http://tcoffee.vital-it.ch/cgi-bin/Tcoffee/tcoffee_cgi/index.cgi). Identical amino acids are shaded black, while similar amino acids are shaded gray using the BoxShade server at http://www.ch.embnet.org/software/BOX_form.html. The white bar indicates the basic region, the hatched bar represents the helix motif, while the black line denotes the loop region.
(B) Confocal analysis of the root tip of a pBRI1:AIF1-GFP transgenic line. GFP fluorescence is shown in green, and cell wall staining by propidium iodide is shown in red. Bar = 50 μm.
(C) A pull-down assay to test the ATBS1–AIF1 interaction. MBP, MBP-ATBS1, and GST-AIF1 were purified from E. coli. Amylose resin was used to pull down the MBP or MBP-ATBS1 and its interacting proteins. An anti-GST antibody was used to detect the copurified AIF1 by immunoblot analysis.
(D) AIF1 interacts with ATBS1 in plants. FLAG-tagged AIF1 was immunoprecipitated from total protein crude extracts isolated from the parental lines, pBRI1:ATBS1-GFP and pBRI1:AIF1-FLAG, and their F1 plants and analyzed by immunoblotting with antibody against FLAG or GFP.
AIF1 Interacts with ATBS1 in Vitro and in Vivo
Gene expression analysis using the eFP browser detected transcripts of four AIF genes in tissues that express ATBS1 and its three homologs (see Supplemental Figure 7 online), while confocal microscopy examination of transgenic plants expressing an AIF1-GFP fusion protein (Figure 6B) revealed that AIF1 is also a nuclear-localized protein, suggesting that ATBS1 and/or its homologs can interact with AIFs in plant cells. Consistent with this, an in vitro pull-down assay using Escherichia coli–expressed recombinant proteins, maltose binding protein (MBP)-fused ATBS1 (MBP-ATBS1) and glutathione S-transferase (GST)-tagged AIF1 (GST-AIF1), showed that MBP-ATBS1 but not MBP was able to pull down the GST-AIF1 fusion protein (Figure 6C). The AIF1–ATBS1 interaction was also confirmed in plant cells by coimmunoprecipitation experiments using the double transgenic plants coexpressing GFP-fused ATBS1 and FLAG-tagged AIF1 (Figure 6D).
AIF1 Negatively Regulates BR Signaling
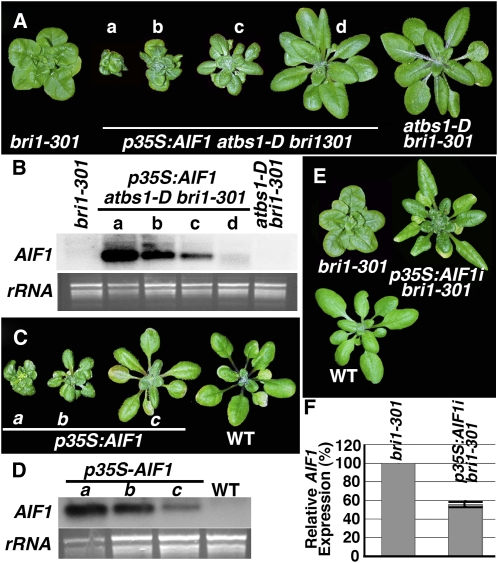
To directly test if AIFs were likely the factors sequestered by overaccumulated ATBS1 in atbs1-D, we used the 35S promoter to overexpress AIF1 in the atbs1-D bri1-301 double mutant. As shown in Figure 7A, AIF1 overexpression not only abolished the atbs1-D phenotype, but also resulted in transgenic plants resembling strong bri1 mutants. Similar results were obtained when other p35S:AIF fusion constructs were transformed into the atbs1-D bri1-301 mutants (see Supplemental Figure 8 online). More importantly, when expressed in wild-type plants, the p35S:AIF1 transgene led to a det2-like dwarf phenotype (Figure 7C; see Supplemental Figure 9 online). RNA gel blot analysis indicated that the severity of the dwarf phenotype correlated well with the expression level of the p35S:AIF1 transgene in both atbs1-D bri1-301 and wild-type backgrounds (Figures 7B and 7D). Thus, AIF1 is likely an ATBS1-sequstered negative regulator of the BR signaling pathway. Further support for the negative role of AIF1 in BR signaling came from an RNAi-mediated gene silencing experiment. Although no visible morphological change was observed with p35S:AIF1i transgenic plants (see Supplemental Figure 10 online) likely caused by functional redundancy of four AIFs, the knockdown of the AIF1 gene in bri1-301, demonstrated by qRT-PCR analysis in Figure 7F, partially suppressed the weak bri1 mutant (Figure 7E). Similar to ATBS1, overexpression or RNAi silencing of AIF1 had little effect on the phosphorylation status of BES1 (see Supplemental Figure 11 online). Our results thus suggested that AIF1 could be an important negative regulator of BR signaling to regulate gene expression in the nucleus.
Figure 7.
AIF1 Is a Negative Regulator of BR Signaling.
(A) AIF1 overexpression alters the atbs1-D bri1-301 morphology. Shown here from left to right are 6-week-old soil-grown plants of bri1-301, four independent transgenic lines of p35S:AIF1 atbs1-D bri1-301, and the wild type.
(B) RNA gel blot analysis of AIF1 overexpression in plants shown in (A).
(C) AIF1 overexpression in the wild type leads to bri1-like dwarf phenotype. Shown from left to right are 6-week-old soil-grown plants of three independent p35S:AIF1 transgenic lines and the wild type.
(D) RNA gel blot analysis of AIF1 transcript levels in plants shown in (C). For both (B) and (D), 10 μg of total RNAs of each sample were separated by agarose gel, stained with ethidium bromide for a loading control, transferred to nylon membrane, and hybridized with a 32P-labeled AIF1 cDNA fragment.
(E) Silencing of AIF1 expression suppresses the bri1-301 phenotype. Shown here are 6-week-old soil-grown plants of bri1-301, a p35S:AIF1i bri1301 transgenic line, and the wild type.
(F) Real-time qRT-PCR analysis of AIF1 expression in bri1-301 and a p35S:AIF1i bri1-301 line. Total RNAs isolated from 3-week-old seedlings were converted into cDNAs, which were used as templates to amplify transcripts of AIF1 or β-TUBULIN with primers listed in Supplemental Table 2 online. The comparative Ct method was used to calculate the levels of transcripts relative to that of bri1-301 after normalization to the β-TUBULIN control. Bars represent sd (n = 3).
[See online article for color version of this figure.]
DISCUSSION
In this study, we employed both activation tagging and yeast two-hybrid approaches to discover additional nuclear proteins involved in BR signaling. Our results strongly suggest that ATBS1 plays a role in BR signaling. First, ATBS1 overexpression suppressed multiple defects of bri1-301, including a compact rosette, short petioles, smaller cells, and delayed flowering. Second, atbs1-D in a BRI1+ background exhibits a similar morphology as a BRI1 overexpression line and is more resistant to Brz than the wild-type control. Third, atbs1-D affects expression of known BR-responsive genes. Lastly, RNAi-mediated AIF1 silencing resulted in a dwarf phenotype despite lacking a noticeable effect on rosette leaves. Our finding is consistent with a recent discovery that implicated BU1, a rice bHLH protein similar to ATBS1, in BR signaling (Tanaka et al., 2009).
Interestingly, overexpression of five ATBS1 homologs, including KIDARI and PRE1 that were previously implicated in light and gibberellin (GA) signaling, respectively (Hyun and Lee, 2006; Lee et al., 2006), also suppressed the bri1-301 mutation. Given the facts that both KIDARI and PRE1 were identified by activation tagging-based genetic screens for their long hypocotyls and early flowering phenotypes and that T-DNA insertional mutation of either gene had no effect on light or GA signaling, it is quite possible that the effect of overaccumulated KIDARI and PRE1 on light and GA signaling pathways is caused by enhanced BR signaling. This explanation is consistent with the known interactions between BRs and the signaling pathway of light or GA. For example, the first known BR biosynthesis mutant, det2, was originally identified as a deetiolation mutant that exhibits a light-grown morphology in dark (Chory et al., 1991; Li et al., 1996), while BR treatment suppresses expression of light-responsive genes and photomorphogenesis (Song et al., 2009a). Similarly, exogenous application of BR rescues the germination defects of severe GA biosynthesis/insensitive mutants in Arabidopsis (Steber and McCourt, 2001). Also, the Arabidopsis elongated mutant, which was discovered as a suppressor of a GA biosynthesis dwarf mutant (Halliday et al., 1996), was later found to carry a gain-of-function mutation in BAK1, a coreceptor for BRI1 (Whippo and Hangarter, 2005). Alternatively, the members of ATBS1/KIDARI/PRE1 might function redundantly in several different signaling pathways by regulating expression of a common gene set involved in cellular elongation, seed germination, and flowering. Further studies of ATBS1 and its homologs could lead to a better understanding of interactions between light and two growth-promoting hormones.
Our discovery that ATBS1 overexpression could only suppress weak BR-signaling mutants but had little effect on severe BR dwarf mutants suggested that ATBS1 might simply enhance a partially active BR signaling pathway. Because ATBS1 is an atypical bHLH protein lacking critical amino acids for DNA binding and overexpression of a basic region-lacking Δb-ATBS1 also suppressed bri1-301, we suspected that ATBS1 or Δb-ATBS1 might heterodimerize and hence sequester one or more negative regulators to allow positive signaling components to act on their target genes. We thus conducted a yeast two-hybrid screen and discovered four closely related bHLH proteins as ATBS1 binding proteins. Interestingly, these AIFs are also atypical bHLH proteins and are thus likely candidates for the negative regulators of the BR signaling pathway that can be sequestered by overaccumulated ATBS1 or its homologs. Not only does AIF1 share similar expression patterns with ATBS1 and its homologs, but it also interacts with ATBS1 in vitro and in plant cells. When overexpressed, AIFs nullified the suppressive activity of atbs1-D on bri1-301 and resulted in bri1/det2-like dwarf transgenic plants. More importantly, RNAi-mediated gene silencing of AIF1 can partially suppress the bri1-301 mutant phenotype. Taken together, these data strongly suggested that AIF1 is an important negative regulator in BR signaling.
It remains to be tested whether the ATBS1–AIF1 interaction is regulated by BRs. Treatment of the pBRI1:ATBS1-GFP pBRI1:AIF1-FLAG double transgenic plants with brassinolide (the most active BR) or Brz failed to reveal any change in the ATBS1–AIF1 interaction. This is likely caused by overexpression of the two interacting proteins driven by the strong BRI1 promoter. Interestingly, AIF1 was repeatedly identified as a BIN2-interacting protein by our yeast two-hybrid screens and was phosphorylated by BIN2 in an in vitro kinase assay (see Supplemental Figure 6 online). This finding not only provided additional support for AIF1 involvement in BR signaling but also suggested a potential regulatory mechanism to control its interaction with ATBS1 and other bHLH proteins. Further investigation is needed to test if BIN2 plays an additional regulatory role in BR signaling by regulating heterodimerization of AIF1 with ATBS1 or other bHLH proteins that control expression of BR-responsive genes.
How AIF1 negatively regulates BR signaling needs further investigation. As a non-DNA binding HLH protein, one likely mechanism for AIF1 to inhibit BR signaling is to heterodimerize with a DNA binding bHLH protein, thus preventing the latter from binding its target genes. Since atbs1-D accumulates the SAUR-AC1 transcripts, the most likely candidates for such DNA binding bHLH proteins are BES1 or its interaction partners BIM1-3, which were known to synergistically bind to the SAUR-AC1 promoter (Yin et al., 2005). However, our preliminary yeast two-hybrid assays revealed that AIF1 did not interact with BES1, BZR1, or three BIMs but exhibited interaction with at least two BEEs (see Supplemental Figure 12 online). A previous study showed that the Arabidopsis mutant lacking three BEEs exhibits only a very weak morphological/developmental defect compared with AIF1 transgenic plants. It is possible that other members of the extended BEE family also interact with AIFs and are involved in BR signaling. Further investigation using Arabidopsis mutants lacking multiple BEE homologs is needed to test our hypothesis. Alternatively, AIFs might directly bind to some BR-responsive promoters and inhibit their activities. AIF1 and its three Arabidopsis homologs share two other sequence motifs (Figure 6A), each containing several highly conserved Arg/Lys residues that might be involved in DNA binding. Another possibility is that a DNA binding AIF1 could recruit certain transcriptional corepressors to actively repress the expression of its target genes. Such a repression mechanism is known for certain bHLH proteins in vertebrates (Massari and Murre, 2000). Further study to test whether AIF1 is capable of DNA binding or to identify AIF1 binding proteins could lead to better knowledge of the biochemical mechanism by which AIF1 or its homologs negatively regulate BR signaling and provide a clearer picture of nuclear activities of the BR signaling process.
METHODS
Plant Materials and Growth Conditions
Arabidopsis thaliana Columbia (Col-0) ecotype was used as the wild-type control for phenotype comparison and for generating all the transgenic plants. atbs1-D bri1-301, det2-1, bin2-1, bri1-301, and bri1-101 are in Col-0 background, bri1-5 is in Wassilewskija-2 ecotype (Noguchi et al., 1999), while ucu1-3 is in Landsberg erecta background (Perez-Perez et al., 2002). The homozygous atbs1- D bri1-301 mutant was crossed with det2, bri1-101, bin2-1, bri1-5, or ucu1-3. The resulting F1 were self-fertilized, and double mutants in segregating F2 populations were identified by PCR-based genotyping using primers listed in Supplemental Table 2 online. Methods for seed sterilization and conditions for plant growth were descried previously (Li et al., 2001a).
Hypocotyl Length Measurements
Seeds were germinated on half-strength Murashige and Skoog medium containing varying concentrations of Brz in the dark. Hypocotyl lengths of 5-d-old seedlings were manually measured. For each concentration of Brz, at least 30 seedlings were measured in duplicated experiments.
Measurement of BR Contents
The rosette leaves of 5-week-old soil-grown plants of bri1-301, atbs1-D bri1-301, the wild type, and atbs1-D were collected to quantify the levels of endogenous BRs according to a previously published procedure (Fujioka et al., 2002).
RNA Gel Blot and RT-PCR Analyses
Total RNAs were extracted from plant tissues as previously described (Li et al., 2001a). Ten micrograms of RNA of each sample were separated on a 1.5% formaldehyde-agarose gel, transferred to a Hybond-XL nylon membrane (Amersham), and hybridized with 32P-labeled cDNA fragment of ATBS1, CPD, XTR6, XTR7, or AIF1. For loading controls, the filters were rehybridized with a 32P-labeled 18S rDNA probe after washing off gene-specific probes or the formaldehyde-agarose gels were stained with ethidium bromide before transferring RNAs to nylon membranes. For qRT-PCR experiments, 5 μg of total RNAs were reverse transcribed into first-strand cDNAs using the SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen) by following the manufacturer's recommended procedure. The resulting cDNAs (0.2 μL) were then used as templates for PCR amplification of transcripts of interest on the iCycler iQ5 system (Bio-Rad) using gene-specific primers (see Supplemental Table 2 online) and iQSYBR green reagent (Bio-Rad) following the manufacturer's recommended protocol. The relative transcript level (to bri1-301) was calculated from three replicates using the comparative Ct (threshold cycle) method after normalization to a β-TUBULIN control.
Plasmid Constructs
The entire insert was released from a full-length At1g74500 cDNA clone (the RIKEN Arabidopsis full-length cDNA clone RAFL14-05-N07) into pCHF1 vector (Neff et al., 1999). For making overexpressing constructs of ATBS1 homologs with the 35S promoter, a full-length cDNA or a cDNA fragment was PCR amplified from its corresponding cDNA plasmid or a cDNA library and cloned into the pCHF1 vector. To generate the ATBS1-GFP and AIF1-GFP constructs, the BRI1 coding region of the pBRI1:BRI1-GFP construct (Friedrichsen et al., 2000) was replaced with the PCR amplified open reading frame (ORF) of ATBS1 and AIF1, respectively. The AIF1 fragment was also cloned into pBRI1-FAST vector modified from p35S-FAST (Ge et al., 2005) to make the pBRI1:AIF1-FLAG construct. The entire ATBS1 ORF and a 226-bp fragment from nucleotide 291 to 516 of the At3g05800 full-length cDNA were first cloned into pHANNIBAL vector followed by cloning into pART27 vector (Wesley et al., 2001) to generate p35S:ATBS1i and p35S:AIF1i RNAi constructs, respectively.
Yeast Two-Hybrid Screening and Assay
Clontech's Matchmaker system is used for yeast two-hybrid experiments. The entire ATBS1 ORF was cloned into the pAS2-1 vector and transformed into the yeast strain Y190. The resulting yeast cells were mated with Y187 yeast cells that were previously transformed with plasmid DNAs derived from an Arabidopsis yeast two-hybrid cDNA library (Kim et al., 1997) following a previously described procedure (Ding et al., 2009). Approximately 10 million zygotic cells were spread on yeast medium lacking histidine but containing 25 to 50 mM 3-amino-1,2,4-triazol. Yeast colonies that grew on the selection medium were picked and analyzed by PCR and DNA sequencing. To test ATBS1–AIF4 interaction, the ORF of At3g06590 was PCR amplified and cloned into pACT2 vector (Clontech). The pAS2-BIN2 and pACT2-BES1/BZR1 constructs used as controls were described previously (Zhao et al., 2002).
In Vitro Protein–Protein Interaction
The entire ORF of ATBS1, AIF1, or BIN2 was cloned into pMAL-c2 (New England Biolabs) or pGEX-KG vector (Guan and Dixon, 1991). The resulting pMAL-c2 and pGEX-KG fusion constructs were transformed individually into Rosette Escherichia coli cells (Novagen). Induction and purification of MBP or GST fusion proteins were performed according to the manufacturer's recommended procedures. To test in vitro ATBS1–AIF1 interaction, ∼5 μg MBP or MBP-ATBS1 and 5 μg GST-AIF1 fusion proteins were incubated with 50 μL amylose resin (New England Biolabs) for 1 h at 4°C. After centrifugation to collect resin and three washes with the extraction buffer, the bound proteins were eluted by 1× SDS sample buffer, separated by 10% SDS-PAGE, and analyzed by immunoblot with an anti-GST antibody.
Coimmunoprecipitation
Leaves from 4-week-old plants were harvested and ground to fine powder in liquid nitrogen and mixed with the extraction buffer containing 20 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1 mM EDTA, 10% (v/v) glycerol, 0.2% (v/v) Triton X-100, 1 mM phenymethylsulfonyl fluoride, and 2 μg/mL each of aprotinin, leupeptin, and pepstatin A (all purchased from Fisher Scientific). The entire procedure was performed at 4°C. After centrifugation for 10 min at 5000g to get rid of insoluble cellular debris, the supernatant was incubated with PBS-equilibrated anti-FLAG M2 affinity gel (Sigma-Aldrich) for 2 h, followed by five washes with the PBS buffer, and dissolved in 1× SDS sample buffer. The proteins were separated by 10% SDS-PAGE, and the presence of AIF1-FLAG and ATBS1-GFP was analyzed by immunoblotting with monoclonal anti-FLAG M2 (Sigma-Aldrich) or anti-GFP antibody (MMS-118P; Covance).
Scanning Electron Microscopy
Plant materials were fixed overnight at 4°C with 4% glutaraldehyde in 25 mM NaH2PO4/Na2HPO4 buffer, pH 7, postfixed with 0.5% osmium tetroxide, and dehydrated with a graded series of ethanol. The specimens were then critical point dried with liquid CO2, mounted on scanning electron microscopy stubs, sputter-coated with gold, and examined with a Hitachi S3200N variable pressure scanning electron microscope.
Confocal Microscopy
The subcellular localizations of GFP fusion proteins were examined with root tips of 5-d-old seedlings using a Zeiss LSM 510 confocal microscope filtered with FITC10 set (488-nm excitation with emissions of 505 to 530 nm and 530 to 560 nm).
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: NP_177590 (ATBS1, At1g74500), NP_849712 (KIDARI, At1g26945), NP_198802 (PRE1, At5g39860), NP_974372 (At3g28857), NP_190355 (At3g47710), NP_197020 (At5g15160), NP_173276 (BEE1), NP_849508 (BEE2), NP_177524 (BEE3), NP_849626 (PIF3), AAG33640 (SPATULA), NP_201512 (ALCATRAZ), NP_566260 (AIF1, At3g05800), NP_974239 (AIF2, At3g06590), NP_566567 (AIF3, At3g17100), NP_563839 (AIF4, At1g09250), CCA40000 (human MyoD), and NP_524340 (Drosophila Sim).
Supplemental Data
The following materials are available in the online version of the article
Supplemental Figure 1. atbs1-D Affects Leaf Shape.
Supplemental Figure 2. Distinct and Overlapping Expression Patterns of BRI1, BIN2, ATBS1, and Three ATBS1 Homologs.
Supplemental Figure 3. The RNAi-Mediated Silencing of ATBS1 Results in a Dwarf Phenotype.
Supplemental Figure 4. atbs1-D Does Not Accumulate Nonphosphorylated BES1.
Supplemental Figure 5. ATBS1 Interacts with Four AIFs in Yeast Cells.
Supplemental Figure 6. AIF1 Is Phosphorylated by BIN2 in Vitro.
Supplemental Figure 7. Overlapping Expression Patterns of Four AIF Genes.
Supplemental Figure 8. Overexpression of Three Other AIF Genes Leads to Strong Dwarf Plants in atbs1-D bri1-301 Background.
Supplemental Figure 9. AIF1 Overexpression Leads to Strong det2-Like Dwarf Plants in the Wild-Type Background.
Supplemental Figure 10. RNAi-Mediated Silencing of AIF1 Has No Effect on Wild-Type Plants.
Supplemental Figure 11. Overexpression or RNAi-Mediated Silencing of AIF1 Has Little Effect on the BES1 Phosphorylation Status.
Supplemental Figure 12. AIF1 Interacts with BEE1 and BEE2.
Supplemental Table 1. atbs-1D Has No Effect on BR Contents
Supplemental Table 2. Oligonucleotides Used in This Study.
Supplementary Material
Acknowledgments
We thank the ABRC at Ohio State University, Columbus and RIKEN BioResource Center for seeds and DNA clones, F. Tax for seeds of bri1-5, J.M. Pérez-Pérez for ucu1-3 seeds, P.M. Waterhouse for the pHANNIBAL plasmid, S. Takatsuto for deuterium-labeled BR internal standards, and Y. Yin for anti-BES1 antiserum. We thank S. Clark, J. Scheifelbein, and T. Kerpolla for helpful suggestions/comments and members of the Li Lab for stimulating discussions throughout this study. This work was supported in part by a grant from the National Institutes of Health (GM060519) to J.L. and a Grant-in-Aid for Scientific Research (B) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Grant 19380069) to S.F.
The author responsible for distribution of materials integral to the findings reported in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Jianming Li (jian@umich.edu).
Some figures in this article are displayed in color online but in black and white in the print edition.
Online version contains Web-only data.
References
- Asami, T., Min, Y.K., Nagata, N., Yamagishi, K., Takatsuto, S., Fujioka, S., Murofushi, N., Yamaguchi, I., and Yoshida, S. (2000). Characterization of brassinazole, a triazole-type brassinosteroid biosynthesis inhibitor. Plant Physiol. 123 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, M.Y., Zhang, L.Y., Gampala, S.S., Zhu, S.W., Song, W.Y., Chong, K., and Wang, Z.Y. (2007). Functions of OsBZR1 and 14-3-3 proteins in brassinosteroid signaling in rice. Proc. Natl. Acad. Sci. USA 104 13839–13844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campuzano, S. (2001). Emc, a negative HLH regulator with multiple functions in Drosophila development. Oncogene 20 8299–8307. [DOI] [PubMed] [Google Scholar]
- Chory, J., Nagpal, P., and Peto, C.A. (1991). Phenotypic and genetic analysis of det2, a new mutant that affects light-regulated seedling development in Arabidopsis. Plant Cell 3 445–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse, S.D., and Sasse, J.M. (1998). BRASSINOSTEROIDS: Essential regulators of plant growth and development. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49 427–451. [DOI] [PubMed] [Google Scholar]
- Ding, X., et al. (2009). A rice kinase-protein interaction map. Plant Physiol. 149 1478–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duek, P.D., and Fankhauser, C. (2005). bHLH class transcription factors take centre stage in phytochrome signalling. Trends Plant Sci. 10 51–54. [DOI] [PubMed] [Google Scholar]
- Ellenberger, T., Fass, D., Arnaud, M., and Harrison, S.C. (1994). Crystal structure of transcription factor E47: E-box recognition by a basic region helix-loop-helix dimer. Genes Dev. 8 970–980. [DOI] [PubMed] [Google Scholar]
- Fairchild, C.D., Schumaker, M.A., and Quail, P.H. (2000). HFR1 encodes an atypical bHLH protein that acts in phytochrome A signal transduction. Genes Dev. 14 2377–2391. [PMC free article] [PubMed] [Google Scholar]
- Friedrichsen, D.M., Joazeiro, C.A., Li, J., Hunter, T., and Chory, J. (2000). Brassinosteroid-Insensitive-1 is a ubiquitously expressed leucine-rich repeat receptor serine/threonine kinase. Plant Physiol. 123 1247–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrichsen, D.M., Nemhauser, J., Muramitsu, T., Maloof, J.N., Alonso, J., Ecker, J.R., Furuya, M., and Chory, J. (2002). Three redundant brassinosteroid early response genes encode putative bHLH transcription factors required for normal growth. Genetics 162 1445–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka, S., Takatsuto, S., and Yoshida, S. (2002). An early C-22 oxidation branch in the brassinosteroid biosynthetic pathway. Plant Physiol. 130 930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gampala, S.S., et al. (2007). An essential role for 14–3-3 proteins in brassinosteroid signal transduction in Arabidopsis. Dev. Cell 13 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge, X., Dietrich, C., Matsuno, M., Li, G., Berg, H., and Xia, Y. (2005). An Arabidopsis aspartic protease functions as an anti-cell-death component in reproduction and embryogenesis. EMBO Rep. 6 282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, K.L., and Dixon, J.E. (1991). Eukaryotic proteins expressed in Escherichia coli: An improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal. Biochem. 192 262–267. [DOI] [PubMed] [Google Scholar]
- Halliday, K., Devlin, P.F., Whitelam, G.C., Hanhart, C., and Koornneef, M. (1996). The ELONGATED gene of Arabidopsis acts independently of light and gibberellins in the control of elongation growth. Plant J. 9 305–312. [DOI] [PubMed] [Google Scholar]
- He, J.X., Gendron, J.M., Sun, Y., Gampala, S.S., Gendron, N., Sun, C.Q., and Wang, Z.Y. (2005). BZR1 is a transcriptional repressor with dual roles in brassinosteroid homeostasis and growth responses. Science 307 1634–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, J.X., Gendron, J.M., Yang, Y., Li, J., and Wang, Z.Y. (2002). The GSK3-like kinase BIN2 phosphorylates and destabilizes BZR1, a positive regulator of the brassinosteroid signaling pathway in Arabidopsis. Proc. Natl. Acad. Sci. USA 99 10185–10190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, Z., Jin, H., Tzfira, T., and Li, J. (2008). Multiple mechanism-mediated retention of a defective brassinosteroid receptor in the endoplasmic reticulum of Arabidopsis. Plant Cell 20 3418–3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun, Y., and Lee, I. (2006). KIDARI, encoding a non-DNA binding bHLH protein, represses light signal transduction in Arabidopsis thaliana. Plant Mol. Biol. 61 283–296. [DOI] [PubMed] [Google Scholar]
- Kang, B., Wang, H., Nam, K.H., Li, J., and Li, J. (2009). Activation-tagged suppressors of a weak brassinosteroid receptor mutant. Mol. Plant, in press. [DOI] [PMC free article] [PubMed]
- Kim, J., Harter, K., and Theologis, A. (1997). Protein-protein interactions among the Aux/IAA proteins. Proc. Natl. Acad. Sci. USA 94 11786–11791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S., Yang, K.Y., Kim, Y.M., Park, S.Y., Kim, S.Y., and Soh, M.S. (2006). Overexpression of PRE1 and its homologous genes activates gibberellin-dependent responses in Arabidopsis thaliana. Plant Cell Physiol. 47 591–600. [DOI] [PubMed] [Google Scholar]
- Li, J. (2005). Brassinosteroid signaling: from receptor kinases to transcription factors. Curr. Opin. Plant Biol. 8 526–531. [DOI] [PubMed] [Google Scholar]
- Li, J., and Chory, J. (1997). A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell 90 929–938. [DOI] [PubMed] [Google Scholar]
- Li, J., Lease, K.A., Tax, F.E., and Walker, J.C. (2001. b). BRS1, a serine carboxypeptidase, regulates BRI1 signaling in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 98 5916–5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J., Nagpal, P., Vitart, V., McMorris, T.C., and Chory, J. (1996). A role for brassinosteroids in light-dependent development of Arabidopsis. Science 272 398–401. [DOI] [PubMed] [Google Scholar]
- Li, J., and Nam, K.H. (2002). Regulation of brassinosteroid signaling by a GSK3/SHAGGY-like kinase. Science 295 1299–1301. [DOI] [PubMed] [Google Scholar]
- Li, J., Nam, K.H., Vafeados, D., and Chory, J. (2001. a). BIN2, a new brassinosteroid-insensitive locus in Arabidopsis. Plant Physiol. 127 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J., Wen, J., Lease, K.A., Doke, J.T., Tax, F.E., and Walker, J.C. (2002). BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell 110 213–222. [DOI] [PubMed] [Google Scholar]
- Li, L., Yu, X., Thompson, A., Guo, M., Yoshida, S., Asami, T., Chory, J., and Yin, Y. (2009). Arabidopsis MYB30 is a direct target of BES1 and cooperates with BES1 to regulate brassinosteroid-induced gene expression. Plant J. 58 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massari, M.E., and Murre, C. (2000). Helix-loop-helix proteins: Regulators of transcription in eucaryotic organisms. Mol. Cell. Biol. 20 429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora-Garcia, S., Vert, G., Yin, Y., Cano-Delgado, A., Cheong, H., and Chory, J. (2004). Nuclear protein phosphatases with Kelch-repeat domains modulate the response to brassinosteroids in Arabidopsis. Genes Dev. 18 448–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau, J.A. (2009). Stomatal development: new signals and fate determinants. Curr. Opin. Plant Biol. 12 29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam, K.H., and Li, J. (2002). BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell 110 203–212. [DOI] [PubMed] [Google Scholar]
- Neff, M.M., Nguyen, S.M., Malancharuvil, E.J., Fujioka, S., Noguchi, T., Seto, H., Tsubuki, M., Honda, T., Takatsuto, S., Yoshida, S., and Chory, J. (1999). BAS1: A gene regulating brassinosteroid levels and light responsiveness in Arabidopsis. Proc. Natl. Acad. Sci. USA 96 15316–15323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemhauser, J.L., Mockler, T.C., and Chory, J. (2004). Interdependency of brassinosteroid and auxin signaling in Arabidopsis. PLoS Biol. 2 E258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi, T., Fujioka, S., Choe, S., Takatsuto, S., Yoshida, S., Yuan, H., Feldmann, K.A., and Tax, F.E. (1999). Brassinosteroid-insensitive dwarf mutants of Arabidopsis accumulate brassinosteroids. Plant Physiol. 121 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton, J.D. (2000). ID helix-loop-helix proteins in cell growth, differentiation and tumorigenesis. J. Cell Sci. 113 3897–3905. [DOI] [PubMed] [Google Scholar]
- Peng, P., Yan, Z., Zhu, Y., and Li, J. (2008). Regulation of the Arabidopsis GSK3-like kinase BRASSINOSTEROID-INSENSITIVE 2 through proteasome-mdiated protein degradation. Mol. Plant 1 338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Perez, J.M., Ponce, M.R., and Micol, J.L. (2002). The UCU1 Arabidopsis gene encodes a SHAGGY/GSK3-like kinase required for cell expansion along the proximodistal axis. Dev. Biol. 242 161–173. [DOI] [PubMed] [Google Scholar]
- Ryu, H., Kim, K., Cho, H., Park, J., Choe, S., and Hwang, I. (2007). Nucleocytoplasmic shuttling of BZR1 mediated by phosphorylation is essential in Arabidopsis brassinosteroid signaling. Plant Cell 19 2749–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, L., Zhou, X.-Y., Li, L., Xue, L.-J., Yang, X., and Xue, H.W. (2009. a). Genome-wide analysis revealed the complex regulatory network of brassinosteroid effects in photomorphogenesis. Mol. Plant 2 755–772. [DOI] [PubMed] [Google Scholar]
- Song, Y., You, J., and Xiong, L. (2009. b). Characterization of OsIAA1 gene, a member of rice Aux/IAA family involved in auxin and brassinosteroid hormone responses and plant morphogenesis. Plant Mol. Biol. 70 297–309. [DOI] [PubMed] [Google Scholar]
- Steber, C.M., and McCourt, P. (2001). A role for brassinosteroids in germination in Arabidopsis. Plant Physiol. 125 763–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, A., et al. (2009). BRASSINOSTEROID UPREGULATED 1, encoding a helix-loop-helix protein, is a novel gene Involved in brassinosteroid signaling and controls bending of the lamina joint in rice. Plant Physiol. 151 669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, W., Kim, T.W., Oses-Prieto, J.A., Sun, Y., Deng, Z., Zhu, S., Wang, R., Burlingame, A.L., and Wang, Z.Y. (2008). BSKs mediate signal transduction from the receptor kinase BRI1 in Arabidopsis. Science 321 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Ortiz, G., Huq, E., and Quail, P.H. (2003). The Arabidopsis basic/helix-loop-helix transcription factor family. Plant Cell 15 1749–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, H., Jin, Y., Liu, W., Li, F., Fang, J., Yin, Y., Qian, Q., Zhu, L., and Chu, C. (2009). Dwarf and low-tillering, a new member of the GRAS family, plays positive roles in brassinosteroid signaling in rice. Plant J. 58 803–816. [DOI] [PubMed] [Google Scholar]
- Tsuge, T., Tsukaya, H., and Uchimiya, H. (1996). Two independent and polarized processes of cell elongation regulate leaf blade expansion in Arabidopsis thaliana (L.) Heynh. Development 122 1589–1600. [DOI] [PubMed] [Google Scholar]
- Vert, G., and Chory, J. (2006). Downstream nuclear events in brassinosteroid signalling. Nature 441 96–100. [DOI] [PubMed] [Google Scholar]
- Vert, G., Nemhauser, J.L., Geldner, N., Hong, F., and Chory, J. (2005). Molecular mechanisms of steroid hormone signaling in plants. Annu. Rev. Cell Dev. Biol. 21 177–201. [DOI] [PubMed] [Google Scholar]
- Vert, G., Walcher, C.L., Chory, J., and Nemhauser, J.L. (2008). Integration of auxin and brassinosteroid pathways by Auxin Response Factor 2. Proc. Natl. Acad. Sci. USA 105 9829–9834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X., and Chory, J. (2006). Brassinosteroids regulate dissociation of BKI1, a negative regulator of BRI1 signaling, from the plasma membrane. Science 313 1118–1122. [DOI] [PubMed] [Google Scholar]
- Wang, X., Goshe, M.B., Soderblom, E.J., Phinney, B.S., Kuchar, J.A., Li, J., Asami, T., Yoshida, S., Huber, S.C., and Clouse, S.D. (2005. b). Identification and functional analysis of in vivo phosphorylation sites of the Arabidopsis BRASSINOSTEROID-INSENSITIVE1 receptor kinase. Plant Cell 17 1685–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X., Kota, U., He, K., Blackburn, K., Li, J., Goshe, M.B., Huber, S.C., and Clouse, S.D. (2008). Sequential transphosphorylation of the BRI1/BAK1 receptor kinase complex impacts early events in brassinosteroid signaling. Dev. Cell 15 220–235. [DOI] [PubMed] [Google Scholar]
- Wang, X., Li, X., Meisenhelder, J., Hunter, T., Yoshida, S., Asami, T., and Chory, J. (2005. a). Autoregulation and homodimerization are involved in the activation of the plant steroid receptor BRI1. Dev. Cell 8 855–865. [DOI] [PubMed] [Google Scholar]
- Wang, Z.Y., Nakano, T., Gendron, J., He, J., Chen, M., Vafeados, D., Yang, Y., Fujioka, S., Yoshida, S., Asami, T., and Chory, J. (2002). Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev. Cell 2 505–513. [DOI] [PubMed] [Google Scholar]
- Weigel, D., et al. (2000). Activation tagging in Arabidopsis. Plant Physiol. 122 1003–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley, S.V., et al. (2001). Construct design for efficient, effective and high-throughput gene silencing in plants. Plant J. 27 581–590. [DOI] [PubMed] [Google Scholar]
- Whippo, C.W., and Hangarter, R.P. (2005). A brassinosteroid-hypersensitive mutant of BAK1 indicates that a convergence of photomorphogenic and hormonal signaling modulates phototropism. Plant Physiol. 139 448–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter, D., Vinegar, B., Nahal, H., Ammar, R., Wilson, G.V., and Provart, N.J. (2007). An “electronic fluorescent pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS One 2 e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, W., Huang, J., Li, B., Li, J., and Wang, Y. (2008). Is kinase activity essential for biological functions of BRI1? Cell Res. 18 472–478. [DOI] [PubMed] [Google Scholar]
- Yin, Y., Vafeados, D., Tao, Y., Yoshida, S., Asami, T., and Chory, J. (2005). A new class of transcription factors mediates brassinosteroid-regulated gene expression in Arabidopsis. Cell 120 249–259. [DOI] [PubMed] [Google Scholar]
- Yin, Y., Wang, Z.Y., Mora-Garcia, S., Li, J., Yoshida, S., Asami, T., and Chory, J. (2002). BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell 109 181–191. [DOI] [PubMed] [Google Scholar]
- Yu, X., Li, L., Guo, M., Chory, J., and Yin, Y. (2008). Modulation of brassinosteroid-regulated gene expression by Jumonji domain-containing proteins ELF6 and REF6 in Arabidopsis. Proc. Natl. Acad. Sci. USA 105 7618–7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, J., Peng, P., Schmitz, R.J., Decker, A.D., Tax, F.E., and Li, J. (2002). Two putative BIN2 substrates are nuclear components of brassinosteroid signaling. Plant Physiol. 130 1221–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, A., Wang, H., Walker, J.C., and Li, J. (2004). BRL1, a leucine-rich repeat receptor-like protein kinase, is functionally redundant with BRI1 in regulating Arabidopsis brassinosteroid signaling. Plant J. 40 399–409. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.