Abstract
Photosynthetic thylakoid membranes in plants contain highly folded membrane layers enriched in photosystem II, which uses light energy to oxidize water and produce oxygen. The sunlight also causes quantitative phosphorylation of major photosystem II proteins. Analysis of the Arabidopsis thaliana stn7xstn8 double mutant deficient in thylakoid protein kinases STN7 and STN8 revealed light-independent phosphorylation of PsbH protein and greatly reduced N-terminal phosphorylation of D2 protein. The stn7xstn8 and stn8 mutants deficient in light-induced phosphorylation of photosystem II had increased thylakoid membrane folding compared with wild-type and stn7 plants. Significant enhancement in the size of stacked thylakoid membranes in stn7xstn8 and stn8 accelerated gravity-driven sedimentation of isolated thylakoids and was observed directly in plant leaves by transmission electron microscopy. Increased membrane folding, caused by the loss of light-induced protein phosphorylation, obstructed lateral migration of the photosystem II reaction center protein D1 and of processing protease FtsH between the stacked and unstacked membrane domains, suppressing turnover of damaged D1 in the leaves exposed to high light. These findings show that the high level of photosystem II phosphorylation in plants is required for adjustment of macroscopic folding of large photosynthetic membranes modulating lateral mobility of membrane proteins and sustained photosynthetic activity.
The use of captured sunlight energy to split water and drive oxygenic photosynthesis by photosystem II (PSII) (Barber, 2006) inevitably generates reactive oxygen species and causes oxidative damage to the PSII protein pigment complex. The light-induced damage to PSII, in particular to the D1 reaction center protein, requires PSII repair to sustain its photosynthetic function (Takahashi and Murata, 2008). Impairment and degradation of D1 increase with rising light intensities, and this protein has the fastest turnover rate among the photosynthetic proteins of plants, algae, and cyanobacteria (Yokthongwattana and Melis, 2006). However, in plants, the PSII is segregated in highly stacked membrane layers of very large thylakoid membranes (Andersson and Anderson, 1980; Kirchhoff et al., 2008), which are densely folded to fit inside chloroplasts (Mullineaux, 2005; Shimoni et al., 2005). As a consequence, the PSII repair cycle in plants is slower than in cyanobacteria (Yokthongwattana and Melis, 2006), and it includes migration of the PSII complex from the stacked membrane domains (grana) to the unstacked membranes (stroma lamellae), where proteolysis and insertion of a newly synthesized D1 protein occurs (Baena-Gonzalez and Aro, 2002; Yokthongwattana and Melis, 2006). High light also causes quantitative phosphorylation of the membrane surface–exposed regions of D1, D2, CP43, and PsbH proteins of PSII in plants (Rintamäki et al., 1997; Vener et al., 2001), but the function of this phosphorylation is largely unknown and reports on its importance for the D1 protein turnover are conflicting (Bonardi et al., 2005; Tikkanen et al., 2008).
Phosphorylation of the PSII proteins in Arabidopsis thaliana depends mostly on the light-activated protein kinase STN8 (Vainonen et al., 2005), while the STN7 kinase is essential for phosphorylation of the light-harvesting proteins of PSII (Bellafiore et al., 2005; Bonardi et al., 2005; Tikkanen et al., 2006). An earlier study on Arabidopsis mutants lacking both STN7 and STN8 (stn7xstn8), as well as only STN8, concluded that protein phosphorylation was not essential for PSII repair (Bonardi et al., 2005), while more recent work revealed a dramatic retardation in D1 degradation under high light in the stn8 and stn7xstn8 mutants (Tikkanen et al., 2008). Moreover, it was shown that the lack of PSII phosphorylation resulted in accumulation of photodamaged PSII complexes and in general oxidative damage of photosynthetic proteins in the thylakoid membranes under high light (Tikkanen et al., 2008). The other study revealed that the stn7xstn8 double mutant grown under natural field conditions produced 41% less seeds than wild-type plants (Frenkel et al., 2007), which also indicated physiological importance of thylakoid protein phosphorylation in maintenance of plant fitness.
To uncover the function of light-dependent protein phosphorylation in plant photosynthetic membranes, we performed a detailed analysis of the Arabidopsis mutants deficient in the protein kinases STN7 and STN8. The earlier published results on protein phosphorylation analyses in the stn7xstn8 mutant of Arabidopsis were restricted to antiphosphothreonine antibody-based immunodetection and did not reveal any phosphorylation of PSII core proteins (Bonardi et al., 2005; Tikkanen et al., 2008). Using a mass spectrometry (MS) approach and immunoblot analyses with two complementary antiphosphothreonine antibodies, we find remaining light-independent phosphorylation of PsbH and D2 proteins of PSII in stn7xstn8. We demonstrate that degradation and aggregation patterns of the D1 protein in stn7xstn8 differ from those in wild-type, stn7, and stn8 plants. We also observe a reproducible delay in the degradation of D1 in high light–treated leaves of stn7xstn8 and stn8 compared with the wild-type and stn7 plants. Finally, we show that phosphorylation of PSII proteins modulates macroscopic rearrangements of the entire membrane network of plant thylakoids, which facilitates lateral mobility of membrane proteins, required for repair and sustained activity of PSII.
RESULTS
Phosphorylation of PSII Proteins in stn7xstn8
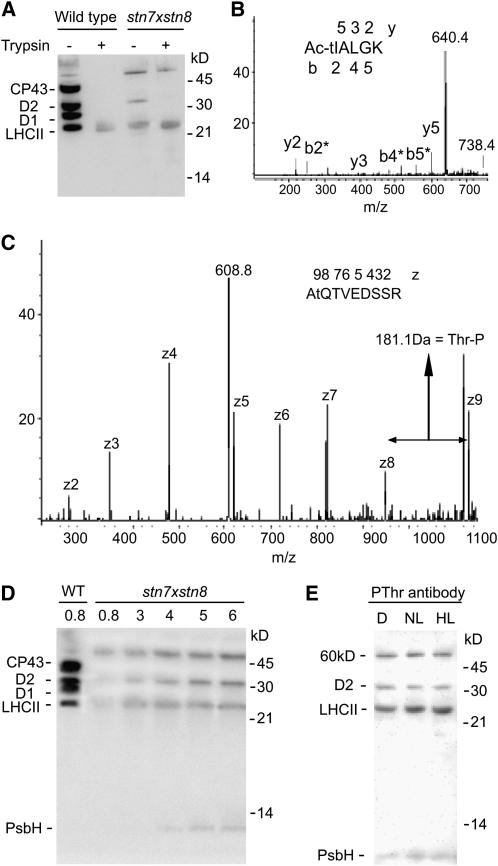
Loss of STN8 kinase causes a threefold decrease in phosphorylation of N-terminal Thr residues in D1, D2, and CP43 and abolishes phosphorylation of Thr-4 in the PsbH subunit of PSII in plants exposed to light (Vainonen et al., 2005). The STN7 kinase is required for light-induced phosphorylation of the PSII light-harvesting complex proteins (LHCII), the linker protein CP29 (Bellafiore et al., 2005; Tikkanen et al., 2006), and the regulatory protein TSP9 (Fristedt et al., 2009). The thylakoids of stn7xstn8 plants were reported to lack phosphorylation of all membrane proteins, based exclusively on the results of immunodetection with antiphosphothreonine antibodies (Bonardi et al., 2005; Tikkanen et al., 2008). To determine the precise status of protein phosphorylation in stn7xstn8, we first used an MS approach. Thylakoid membranes from the Arabidopsis stn7xstn8 mutant (see Supplemental Figure 1 online) and wild-type plants were isolated from light-exposed leaves in the presence of the phosphatase inhibitor NaF and then subjected to proteolysis of the surface-exposed phosphopeptides by trypsin (Vener et al., 2001) (Figure 1A). The peptides were analyzed by liquid chromatography–mass spectrometry using an ion trap performing alternating collision-induced dissociation and electron transfer dissociation (Syka et al., 2004); the latter is a mild fragmentation technique that does not destroy phosphorylated amino acids. In the samples from wild-type plants, we found all phosphorylated peptides characterized in our previous studies (Vener et al., 2001; Hansson and Vener, 2003; Tikkanen et al., 2006). Two phosphorylated peptides were detected from stn7xstn8: Thr-1 phosphorylated N terminus of the D2 protein (Figure 1B) and Thr-2 phosphorylated N terminus of the PsbH protein (Figure 1C). In wild-type plants, the N terminus of PsbH can be phosphorylated at residues Thr-2 and Thr-4, with phosphorylation at Thr-4 found only in light-exposed leaves (Vener et al., 2001). In the case of the PsbH N terminus from stn7xstn8, the electron transfer dissociation of the peptide ion allowed unambiguous localization of the intact phosphorylated Thr residue at the Thr-2 position (Figure 1C).
Figure 1.
Phosphorylation of Thylakoid Proteins in stn7xstn8.
(A) Immunoblotting analysis of SDS-PAGE separated thylakoid proteins from wild-type and stn7xstn8 plants with antiphosphothreonine antibody from Zymed Laboratories before (−) and after (+) the treatment of thylakoids with trypsin.
(B) The product ion MS/MS spectrum obtained by collision-induced fragmentation of a singly charged phosphopeptide with m/z = 738.4 (indicated) corresponding to the N terminus of D2 protein from stn7xstn8. The ion indicated at m/z = 604.4 corresponds to the peptide that underwent a neutral loss of phosphoric acid with a mass 98. The fragment ions b (N-terminal) and y (C-terminal) are marked below and above the peptide sequence shown with the phosphorylated and acetylated Thr indicated by the lowercase t and Ac. All b ions in the spectrum are marked with an asterisk because they lost the phosphoric acid.
(C) The product ion MS/MS spectrum obtained by electron transfer dissociation of the doubly charged phosphopeptide with m/z = 608.8 (indicated) corresponding to the N terminus of PsbH protein from stn7xstn8. The z fragment ions are labeled in the spectrum. Note that the mass increment between z8 and z9 is 181 D, which corresponds to the intact phosphorylated Thr residue at the second position from the peptide N terminus and is indicated by the lowercase t in the shown peptide sequence.
(D) Immunoblot with antiphosphothreonine antibodies from New England Biolabs. Thylakoid membrane proteins from wild-type and mutant (stn7xstn8) plants exposed to the normal growth light for 3 h were separated by SDS-PAGE. The membranes containing 0.8 μg chlorophyll were loaded for the wild type, while the samples from stn7xstn8 contained 0.8, 3, 4, 5, and 6 μg chlorophyll, as indicated.
(E) Immunoblot with antiphosphothreonine antibodies from New England Biolabs. The thylakoid membranes were isolated from the stn7xstn8 plants adapted to darkness for 15 h (D), exposed to normal light of 120 μmol photons m−2 s−1 for 3 h (NL), or to high light of 900 μmol photons m−2 s−1 for 3 h (HL). The membranes containing 2 μg chlorophyll were loaded in each lane. The positions of the major phosphorylated proteins and of the molecular markers are indicated in (A), (D), and (E).
As a next step, we performed an immunodetection analysis of thylakoid proteins with antiphosphothreonine antibodies. We used two different commercial antibodies that have previously been shown to have complementary specificities toward various thylakoid phosphoproteins (Aro et al., 2004; Vainonen et al., 2005): antibodies from New England Biolabs detected phosphorylation of the PsbH protein, while antibodies from Zymed Laboratories were more sensitive against phosphorylated LHCII but did not detect the PsbH phosphoprotein. To visualize the low phosphorylation level in stn7xstn8 in comparison to the wild-type plants, we loaded samples with increasing amounts of mutant extract (Figure 1D). Phosphorylated protein bands detected in the stn7xstn8 thylakoids were at the positions corresponding to D2, LHCII, and PsbH proteins. Phosphorylation of D2 and PsbH was confirmed by MS, while the residual LHCII phosphorylation in both stn7xstn8 and the wild type could correspond to an unidentified phosphorylation site that resists trypsin cleavage (Figure 1A) and subsequent detection by MS. Quantitative analysis revealed that phosphorylation levels of D2 and LHCII in the stn7xstn8 reached between 5 and 10% compared with wild-type plants. The immunoblot also revealed a phosphorylated 60-kD band in stn7xstn8 (Figure 1D), which was characterized as an aggregate of phosphorylated D2 with the D1 protein (see below).
To investigate if the phosphorylation in stn7xstn8 is affected by light, we analyzed the thylakoid membranes from dark-adapted plants and plants exposed for 3 h to either normal or high light. The different light conditions did not reveal any significant change in the stn7xstn8 protein phosphorylation pattern (Figure 1E). Thus, we concluded that: (1) the kinase(s) responsible for D2, PsbH, and LHCII phosphorylation in the double mutant is (are) light independent; (2) phosphorylation of the D2 protein and LHCII is more than 10 times lower in stn7xstn8 than in the wild-type plants; and (3) the N-terminal phosphorylation of the D1 and CP43 proteins of PSII is completely absent in stn7xstn8.
Aggregation and Degradation of the D1 Protein Differ in stn7xstn8 and Wild-Type Plants
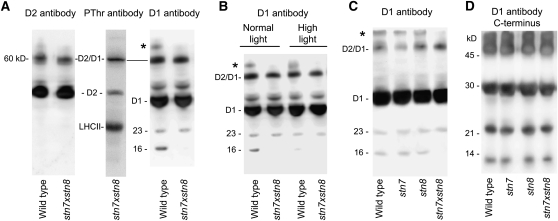
The immunoblotting revealed a phosphorylated 60-kD band, which was more pronounced in stn7xstn8 than in wild-type plants (Figures 1A, 1D, and 1E). However, the liquid chromatography–mass spectrometry analyses did not uncover any phosphopeptide from any 60-kD phosphoprotein from either stn7xstn8 or wild-type plants. The proteins of the PSII core are known to aggregate under certain conditions, and the 60-kD phosphoprotein could be an aggregate of phosphorylated D2 with D1 protein (Komayama et al., 2007). This was verified by immunoblot analysis of thylakoid proteins with specific antibodies against the D2 and D1 proteins as well as with antiphosphothreonine antibodies. Figure 2A shows representative immunoblots with each of the three antibodies that all recognized the protein band at 60 kD. This analysis also revealed differences between stn7xstn8 and the wild type in the pattern of D1 degradation and aggregation: the 16-kD proteolytic fragment of D1, as well as the D1-containing aggregate with a molecular mass higher than 60 kD, were absent in the mutant (Figure 2A). The difference between the patterns of D1 degradation and aggregation was also found in the leaves of stn7xstn8 and wild-type plants exposed to high light (Figure 2B). The signal of the 16-kD fragment was less pronounced in the membranes from the wild-type plants exposed to high light (Figure 2B), demonstrating the unstable nature of this fragment and its rapid further degradation, especially under stress conditions. The parallel immunoblotting analysis of the D1 protein from wild-type plants, stn7, stn8, and stn7xstn8 mutants revealed that disappearance of the 16-kD proteolytic fragment of D1 and of the D1 aggregate with the high molecular mass was specific to the stn7xstn8 double mutant (Figure 2C). Additional immunoblotting analysis with an antibody specific to the C terminus of D1 did not reveal the 16-kD fragment of D1 in the tested thylakoid samples (Figure 2D). Thus, we conclude that the 16-kD proteolytic fragment of D1 detected in wild-type, stn7, and stn8 plants (Figure 2C) lacks the C terminus and that it is most probably derived from the N-terminal part of D1. The existence of several pathways for D1 degradation and aggregation (Kapri-Pardes et al., 2007; Komayama et al., 2007; Komenda et al., 2007; Sun et al., 2007) reflects the importance of these processes for quality control of PSII. Our data indicate that the absence of D1 and CP43 phosphorylation and the significantly reduced phosphorylation of D2 in stn7xstn8 correlates with an alteration of the pathway for the D1 protein degradation and aggregation compared with wild-type, stn7, and stn8 plants.
Figure 2.
Specific Pattern of the D1 Protein Aggregation and Degradation in stn7xstn8.
(A) Immunoblot analysis of thylakoid membrane proteins from wild-type and stn7xstn8 plants with antibodies against D2, D1, and phosphothreonine (Zymed Laboratories), as indicated. The positions of the 60-kD aggregate of the D2 and D1 proteins (D2/D1), 23- and 16-kD fragments of the D1 protein, and of the D1 aggregate with the high molecular mass (asterisk) are indicated.
(B) Immunoblot analyses of the D1 protein in stn7xstn8 compared with wild-type plants exposed to normal light (120 μmol photons m−2 s−1) or high light (900 μmol photons m−2 s−1). The sample loading corresponding to 0.6 μg of chlorophyll was used.
(C) Immunoblot analyses of the D1 protein in stn7xstn8 compared with stn7, stn8, and wild-type plants exposed to normal light (120 μmol photons m−2 s−1).
(D) Immunoblot analysis of thylakoid membrane proteins from wild-type, stn7, stn8, and stn7xstn8 plants with antibodies against the C terminus of the D1 protein. The positions of the molecular markers are indicated.
Phosphorylation of PSII Proteins Controls Thylakoid Folding
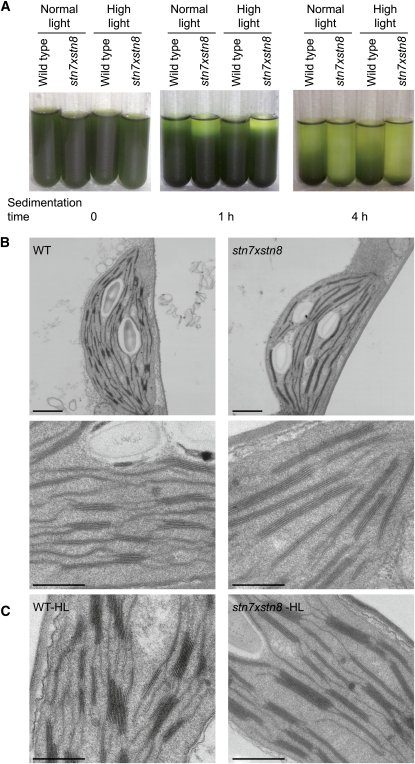
We observed that thylakoids isolated from chloroplasts of stn7xstn8, stn8-1, and stn8-2 plants had a higher rate of gravity-driven sedimentation than thylakoids from wild-type plants (Figures 3A and 4A), suggesting an increase in thylakoid folding and density. The stacking of the thylakoid membranes after breaking the chloroplasts is determined by the ionic characteristics of the resuspension medium (Izawa and Good, 1966a, 1966b). Accordingly, the gravity-driven sedimentation of isolated thylakoids is highly accelerated with increasing concentrations of MgCl2, and we performed all sedimentation experiments with thylakoids from wild-type and mutant plants in parallel at the same Mg2+ concentration. The gravity-driven sedimentation of thylakoids from the stn7 mutant was very similar to that of the thylakoids from wild-type plants, indicating a similar thylakoid folding in these two strains (Figure 4A). We also analyzed the rate of gravity-driven sedimentation for thylakoids isolated from wild-type and stn7xstn8 plants exposed to 3 h of high light treatment, which increases the extent of PSII phosphorylation in the leaves of wild-type plants (Rintamäki et al., 1997). However, exposure of leaves to the high light did not cause any apparent additional change in the density of the isolated thylakoids: their sedimentation rates were similar to those of the thylakoids isolated from the corresponding plants exposed to normal light (Figure 3A).
Figure 3.
Enhanced Thylakoid Folding in stn7xstn8.
(A) Time dependence of the gravity-driven sedimentation of thylakoid membranes isolated from the wild type and stn7xstn8. The thylakoid membranes were isolated from plants exposed to normal light (120 μmol photons m−2 s−1) or high light (900 μmol photons m−2 s−1), as indicated.
(B) Analysis of thylakoid membranes from the wild type and stn7xstn8 by electron microscopy. Leaves from 4-week-old wild-type and stn7xstn8 seedlings were directly fixed 3 h after the start of the light phase of the growth photoperiod and prepared for transmission electron microscopy. Chloroplast sections are shown for the wild type (left panels) and stn7xstn8 (right panels). Bars in the top and bottom panels = 1 and 0.5 μm, respectively.
(C) Electron microscopy analysis of thylakoid membranes from the wild type and stn7xstn8 exposed to high light of 1000 μmol photons m−2 s−1 for 3 h. Bars = 0.5 μm.
[See online article for color version of this figure.]
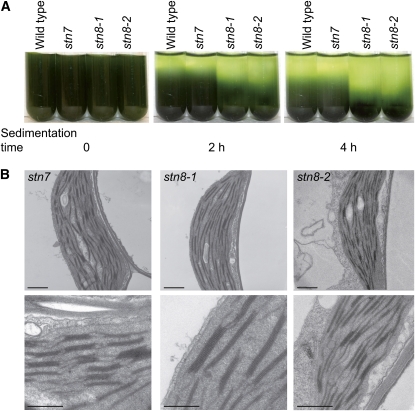
Figure 4.
Enhanced Thylakoid Folding in stn8-1 and stn8-2.
(A) Time dependence of the gravity-driven sedimentation of thylakoid membranes isolated from the wild type, stn7, stn8-1, and stn8-2.
(B) Analysis of thylakoid membranes from stn7, stn8-1, and stn8-2 by electron microscopy. Leaves from 4-week-old seedlings were directly fixed 3 h after the start of the light phase of the growth photoperiod and prepared for transmission electron microscopy. Chloroplast sections are shown for stn7, stn8-1, and stn8-2, as indicated. Bars in the top and bottom panels = 1 and 0.5 μm, respectively.
[See online article for color version of this figure.]
Direct observation of thylakoid membranes from the wild type and stn7xstn8 by transmission electron microscopy revealed an increased size of appressed membranes in the mutant (Figure 3B). Whereas the thylakoids from wild-type plants consist of many short grana stacks and stromal lamellae, a striking feature in stn7xstn8 is the presence of fewer but longer grana stacks connected by lamellar regions (Figure 3B). To obtain a quantitative estimation of the thylakoid membrane organization, the length of appressed membranes within the grana stacks was measured (see Supplemental Table 1 online; for details, see Methods). The average size of the grana stacks in wild-type thylakoids was 439 ± 155 nm, while the size of the grana in stn7xstn8 was 667 ± 223 nm. Because the stromal membranes were more difficult to trace than the stacked membranes, their measurements were not reliable for estimating the ratio between grana and stromal lamellae. Transmission electron microscopy of thylakoid membranes from stn7 and two independent stn8 lines (Figure 4B) demonstrated that the size of the grana in stn7 was rather similar to that in the wild-type thylakoids, while the grana were larger in both stn8-1 and stn8-2, although there was more size heterogeneity in stn8-2 (see Supplemental Table 1 online; Figure 4B). The average number of grana stacks was not significantly different between the wild type and the mutants (see Supplemental Table 1 online). The overall increased grana size in stn7xstn8 and stn8 explains increased sedimentation rate of their thylakoids compared with the wild type and stn7 (Figures 3A and 4A) and demonstrates significant macroscopic rearrangements of the entire membrane network as a result of the deficiency in PSII protein phosphorylation. Exposure of plants to high light did not markedly affect the proportion of grana and lamellar thylakoid regions in the wild type and stn7xstn8, although there was a slight increase in grana stacking in the wild type (Figure 3C).
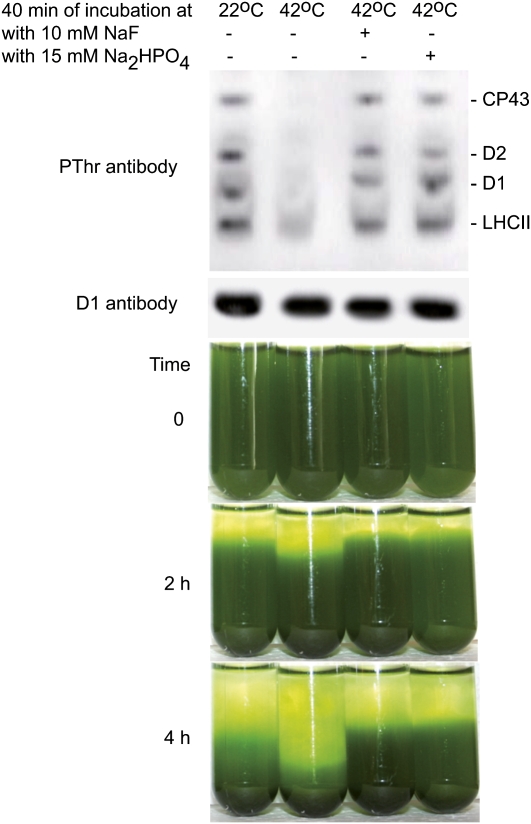
To validate our conclusion regarding a direct link between PSII phosphorylation and thylakoid membrane structure, we performed destacking of isolated wild-type thylakoids, dephosphorylation using the endogenous phosphatase, and then stacked thylakoids using increasing concentrations of MgCl2. In wild-type plants exposed to normal environmental conditions, the PSII core proteins are phosphorylated to a significant extent (Vener et al., 2001; Vener, 2007). However, when plant leaves or isolated thylakoids are subjected to a heat shock, specific and fast dephosphorylation of PSII proteins occurs (Vener et al., 1999, 2001; Rokka et al., 2000). We centrifuged isolated wild-type thylakoids and resuspended them in a buffer without MgCl2 to destabilize the stacked grana membranes by decreasing Mg2+ concentration (Arntzen and Ditto, 1976). Dephosphorylation of PSII was induced by incubation of the thylakoid suspension at 42°C for 40 min. The immunodetection analysis of thylakoid proteins with antiphosphothreonine antibodies confirmed dephosphorylation of the D1, D2, and CP43 proteins in the thylakoids treated at 42°C (Figure 5). Control and heat shock treated thylakoids were then restacked using two consecutive resuspensions in a buffer containing 5 mM MgCl2. Notably, the gravity-driven sedimentation of the dephosphorylated thylakoids was faster than that of the phosphorylated membranes (Figure 5). In two additional control experiments, we incubated thylakoids at 42°C for 40 min in the presence of either NaF or Na2HPO4, used as PSII phosphatase inhibitors (Vener et al., 1999). Each of these phosphatase inhibitors prevented dephosphorylation of thylakoid proteins, as well as the increase in the density/compactness of the thylakoid membranes after incubation at 42°C for 40 min (Figure 5). Thus, our experiments demonstrate that membrane structure of wild-type thylakoids directly depends on the state of membrane protein phosphorylation. An increased size of appressed grana membranes in stn7xstn8 and stn8 (Figures 3 and 4) could be detrimental for lateral protein diffusion, particularly for the repair cycle of PSII.
Figure 5.
Dephosphorylation of Thylakoid Proteins Increases Compactness of Thylakoid Membranes from Wild-Type Plants.
Thylakoid membranes isolated from the wild-type plants were resuspended in buffer without MgCl2, with or without NaF or Na2HPO4, and incubated in darkness at 22 or 42°C, as indicated. Then, the membranes were twice resuspended in the buffer with 5 mM MgCl2 to restack the thylakoids. Immunoblotting analysis of SDS-PAGE separated thylakoid proteins from the samples was done with antiphosphothreonine antibody from Zymed Laboratories and D1-specific antibody (loading control), as indicated. Time dependence of the gravity-driven sedimentation of the restacked phosphorylated and dephosphorylated thylakoid membranes is shown as well.
[See online article for color version of this figure.]
Deficiency in PSII Phosphorylation Impairs D1 Turnover
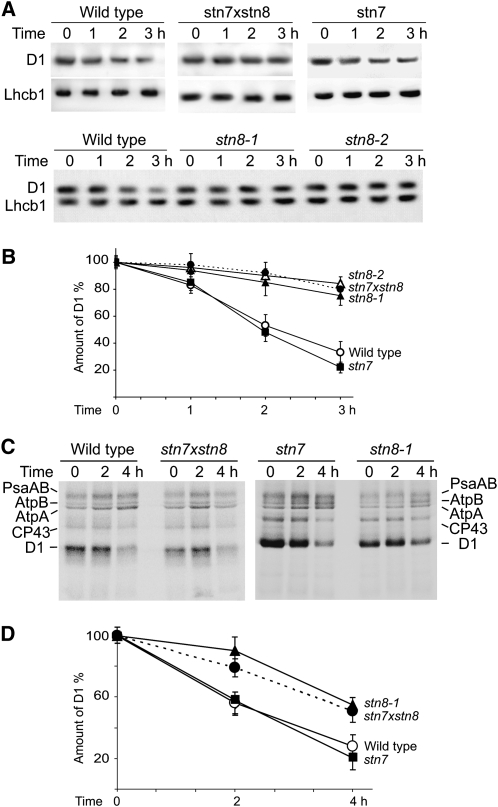
The D1 protein of the PSII reaction center is chloroplast encoded, and its translation can be blocked by lincomycin. To characterize D1 degradation induced by photodamage, we incubated cut petioles, with attached leaves, of wild-type and mutant plants overnight with lincomycin, which was followed by a 3-h high light treatment of the leaves floated in the same solution. Thylakoid proteins isolated from high light–treated leaves were analyzed by immunoblotting with specific antibodies against D1 and against a control nucleus-encoded Lhcb1 protein (Figure 6A). Half of the D1 protein was degraded in the high light–treated wild-type leaves during 1.5 h, in agreement with most of the previous studies (Yokthongwattana and Melis, 2006; Sirpio et al., 2007; Tikkanen et al., 2008), while degradation of D1 in stn7xstn8 or stn8 plants was delayed (Figures 6A and 6B). The D1 degradation in high light–treated leaves of the stn7 mutant was similar as in wild-type leaves (Figures 6A and 6B), pointing toward a specific connection between the retarded D1 turnover and deficiency in phosphorylation of PSII core proteins.
Figure 6.
Decreased D1 Turnover in stn7xstn8 and stn8.
(A) Immunoblot analysis of thylakoid proteins from wild-type, stn7, stn8-1, stn8-2, and stn7xstn8 plants with a D1-specific antibody and a control Lhcb1-specific antibody. Thylakoids were isolated from the leaves treated with lincomycin and exposed to high light of 900 μmol photons m−2 s−1 for the indicated periods of time.
(B) Time dependence of the D1 protein degradation in leaves of wild-type, stn7, stn8-1, stn8-2, and stn7xstn8 plants treated with lincomycin and exposed to high light like in (A). The values are means ± se of three independent experiments for each genotype.
(C) In vivo pulse-chase experiments with chloroplast proteins of wild-type, stn7, stn8-1, and stn7xstn8 plants labeled with [35S]Met and exposed to high light of 2000 μmol photons m−2 s−1. Four-week-old plants were labeled with [35S]Met for 2 h under dim light at room temperature in the presence of cycloheximide. After a 1-h chase period in dim light, including 30 min with lincomycin, plants were exposed to high light of 2000 μmol photons m−2 s−1 for 2 and 4 h, and proteins were analyzed by SDS-PAGE and phosphor imaging. Positions of the labeled subunits psaAB of photosystem I, AtpA and AtpB subunits of ATP synthase, and CP43 and D1 proteins of PSII are indicated.
(D) Time dependence of the labeled D1 protein degradation in leaves of wild-type, stn7, stn8-1, and stn7xstn8 plants subjected to in vivo pulse-chase experiments under high light as shown in (C). Amounts of labeled D1 protein were normalized relative to the sum of PsaAB, AtpA, AtpB, and CP43 bands. The values are means ± se of four independent experiments of each phenotype.
To verify the finding of the sluggish D1 degradation in the PSII phosphorylation-defective mutants under high light, we performed in vivo pulse-chase labeling of chloroplast proteins with radioactive Met. Wild-type and mutant plants were labeled with l-[35S]Met during 2 h, and the leaves were collected after 0, 2, and 4 h of chase under high light (2000 μmol photons m−2 s−1) in the presence of lincomycin. The latter ensured that there was no residual incorporation of label during the chase that would make the interpretation of the results ambiguous. The labeled proteins were analyzed by SDS-PAGE and phosphorimaging (Figure 6C). The results demonstrated that the turnover of D1 was diminished in the high light–treated leaves of the stn7xstn8 or stn8 mutants in comparison to wild-type or stn7 plants (Figures 6C and 6D). The D1 turnover in the stn7xstn8 mutant was also slower than in wild-type plants when the chase experiments were performed for 3 h under high light of 1000 μmol photons m−2 s−1 (see Supplemental Figure 2 online). These results are in line with the data on degradation of photodamaged D1 in wild-type plants and stn7xstn8 or stn8 mutants treated with lincomycin and analyzed by immunoblotting in this work (Figures 6A and 6B) and in the recent study by Tikkanen et al. (2008).
Segregation of D1 and FtsH Protease in stn8 and stn7xstn8
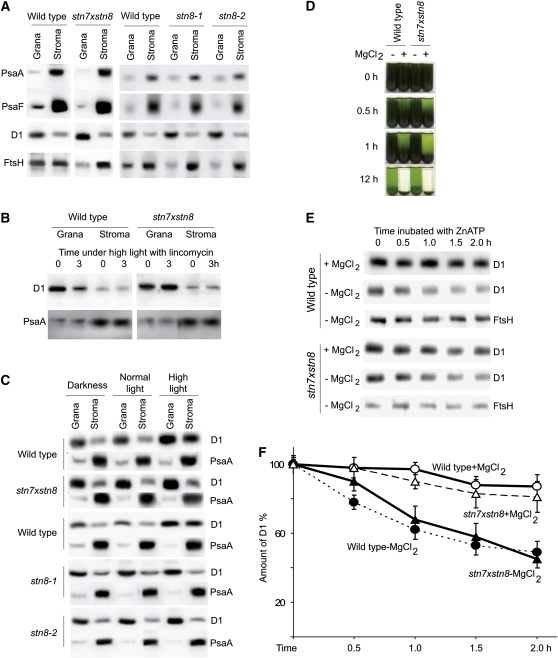
The high light–accelerated photoinhibition of PSII and rapid turnover of the D1 protein are characteristic for all oxygenic photosynthetic organisms (Yokthongwattana and Melis, 2006; Takahashi and Murata, 2008): cyanobacteria, algae, and plants. The turnover and repair of PSII depend on degradation of damaged D1 by several proteases, among which an ATP-dependent zinc metalloprotease FtsH plays a central role in all species (Nixon et al., 2005; Adam et al., 2006). However, the massive light-induced phosphorylation of PSII proteins (Rintamäki et al., 1997; Vener et al., 2001; Vener, 2007) and extensive folding of thylakoid membranes (Shimoni et al., 2005; Kirchhoff, 2008; Kirchhoff et al., 2008) are restricted to plants. Thus, maintenance of PSII in thylakoids of higher plants requires its lateral mobility between stacked grana membranes and unstacked stroma lamellae (Kirchhoff, 2008), a process that leads to a slower turnover of the D1 protein than in cyanobacteria (Yokthongwattana and Melis, 2006). We propose that decreased degradation of D1 in stn8 and stn7xstn8 plants (Figure 6) is a consequence of increased grana size in the thylakoid membranes (Figures 3 and 4), leading to decreased lateral mobility of D1 and FtsH. To test this hypothesis, we first fractionated thylakoids from wild-type, stn8, and stn7xstn8 plants into grana and stroma membranes by digitonin treatment and differential centrifugation. Figure 7A shows that in all plants, the PsaA and PsaF proteins of PSI were enriched in stroma, while the D1 protein of PSII was more abundant in the grana membranes, as expected (Andersson and Anderson, 1980; Shimoni et al., 2005). However, the allocation of FtsH protease between grana and stroma membranes in stn7xstn8 and in two independent mutant lines stn8-1 and stn8-2 differed from that in wild-type thylakoids. The ratio of FtsH between stromal and granal fractions was clearly higher in stn7xstn8, stn8-1, and stn8-2 mutants than in the wild type (Figure 7A). These results indicate that FtsH is probably further relocated from the large appressed grana to the grana margins and to the stromal lamellae in these mutants. Importantly, immunoblotting analysis of thylakoids from plants exposed to normal or high light demonstrated that the level of FtsH was not altered between wild type, stn7xstn8, and stn8-1 (see Supplemental Figure 3 online).
Figure 7.
Segregation of D1 and FtsH Protease in stn8 and stn7xstn8 Thylakoids.
(A) Immunoblot analysis of PsaA, PsaF, D1, and FtsH proteins in grana and stroma membranes fractionated by digitonin treatment and differential centrifugation of thylakoids from wild-type, stn8-1, stn8-2, and stn7xstn8 plants. Equal amounts of chlorophyll were loaded on each lane.
(B) Immunoblot analysis of D1 protein degradation in grana and stroma membranes fractionated by digitonin treatment of thylakoids from leaves of wild-type and stn7xstn8 plants treated with lincomycin and exposed to high light of 900 μmol photons m−2 s−1 during 0 or 3 h, as indicated.
(C) Immunoblot analysis of distribution of the D1 and PsaA proteins between the grana and stroma thylakoid membranes isolated from leaves of wild-type, stn8-1, stn8-2, and stn7xstn8 plants harvested in darkness or exposed for 3 h to normal light of 120 μmol photons m−2 s−1 or to high light of 900 μmol photons m−2 s−1.
(D) Time dependence of gravity-driven sedimentation of thylakoids isolated from wild-type and stn7xstn8 plants and resuspended in buffer with 5 mM MgCl2 (+MgCl2) or without MgCl2 (−MgCl2).
(E) Immunoblot analysis of D1 proteolysis in thylakoids isolated from wild-type and stn7xstn8 plants and resuspended in a buffer with 5 mM MgCl2 (+MgCl2) or without MgCl2 (−MgCl2) and supplied with 0.15 mM ZnCl2 and 2 mM ATP. Immunoblotting was done using specific antibodies against the D1 and FtsH proteins, as indicated.
(F) Time-dependent proteolysis of D1 in thylakoids isolated from wild-type and stn7xstn8 plants and resuspended in buffer with 5 mM MgCl2 (+MgCl2) or without MgCl2 (−MgCl2) and supplied with 0.15 mM ZnCl2 and 2 mM ATP. The values are mean ± se of three independent experiments for each experimental condition.
[See online article for color version of this figure.]
As a next step, we fractionated grana and stroma thylakoids from wild-type and stn7xstn8 plants after 3 h of high light in the presence of lincomycin. The high light treatment led to a substantial decrease of D1 in the grana of wild-type but not of stn7xstn8 thylakoids (Figure 7B). We also analyzed the changes in distribution of the D1 protein between the grana and stroma thylakoids isolated from plant leaves harvested in darkness, after exposure to normal growth light or high light. Transfer of plants to high light caused migration of D1 from the stacked grana to stroma membranes in wild-type plants, but not in stn8-1, stn8-2, or stn7xstn8 plants (Figure 7C). These results demonstrate that increased folding of thylakoids in the PSII phosphorylation defective mutants hindered the lateral migration of D1 between the membrane domains. Examination of the structure of the wild-type and stn7xstn8 thylakoids by electron microscopy revealed that under high light conditions, the grana fraction was not decreased (Figure 3D). The earlier electron microscopy studies of several plant species also did not find significant changes in thylakoid grana fraction after exposure of leaves to high light (Albertsson and Andreasson, 2004). Thus, the slower migration of D1 from the stacked grana to stroma membranes in stn7xstn8 plants exposed to high light may be explained by the larger layers of grana in the mutant thylakoids.
To verify the hypothesis that D1 degradation by the FtsH protease is controlled by the extent of stacking of thylakoid membranes, we induced artificial destacking of thylakoids by removing MgCl2 as explained above. Removal of MgCl2 led to a dramatic decrease in the stacking of the thylakoid membranes, as shown by the extremely slow rate of gravity-driven sedimentation of thylakoids from both wild-type and stn7xstn8 mutant plants (Figure 7D). Next, Zn2+ and ATP were supplied to the thylakoids to activate FtsH, the only Zn2+- and ATP-dependent protease in plant thylakoid membranes (Adam et al., 2006). Addition of Zn2+ and ATP to the thylakoids stacked in the presence of MgCl2 caused a very slow decrease in the amount of D1 protein but led to a significant degradation of D1 in thylakoids destacked in the absence of MgCl2 (Figure 7E). Incubation of Mg2+-depleted thylakoid membranes with Zn2+ and ATP for 2 h decreased the amount of D1 in thylakoids from wild-type and stn7xstn8 plants by 52% ± 9% and 49% ± 7%, respectively (Figure 7F). Thus, destacking of thylakoids resulted in equally fast Zn2+- and ATP-dependent in vitro degradation of D1 in wild-type and stn7xstn8 thylakoids. These findings provide experimental confirmation of our hypothesis: removal of membrane stacking underlying spatial segregation of PSII and FtsH between the thylakoid domains accelerated degradation of the D1 protein by the FtsH protease independently of the original extent of thylakoid stacking.
DISCUSSION
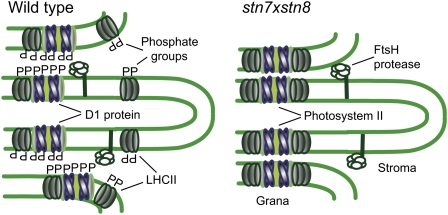
Plant chloroplasts have complex grana structures of extensively folded thylakoid membranes that accommodate large amounts of PSII and its light-harvesting antenna (Mullineaux, 2005; Kirchhoff, 2008). Photosystem I and ATP synthase are laterally segregated from PSII: they have bulky structures protruding out from the membrane into the stroma and cannot fit between the appressed grana membranes (Andersson and Anderson, 1980; Shimoni et al., 2005). FtsH protease, which degrades the photodamaged D1 protein of PSII, also forms a multisubunit complex exposed to the outer surface of thylakoid membranes (Adam et al., 2006) and is more abundant in stroma thylakoids and grana margins (Lindahl et al., 2000). The maintenance and repair of photodamaged PSII requires lateral mobility of its subunits between the stacked grana thylakoids and unstacked stroma lamellae (Kirchhoff, 2008). We found that a significant decrease in phosphorylation of PSII proteins in the stn8-1, stn8-2, and stn7xstn8 mutants of Arabidopsis led to the formation of extended appressed grana regions of thylakoids, spatial segregation of the FtsH protease from D1, and hindered lateral migration of D1 from grana to stroma membrane domains. These experimental data could explain the slow turnover of the D1 protein in the PSII phosphorylation-defective mutants exposed to high light. According to these findings, we propose a model for phosphorylation-dependent control of plant thylakoid folding and lateral migration of membrane proteins (Figure 8). We postulate that massive light-dependent phosphorylation of PSII proteins regulates functional folding and macroscopic structure of plant thylakoid membranes via repulsion of adjacent membrane layers. This model particularly predicts that thylakoid membrane folding should be altered in PSII-deficient mutants because of the absence of PSII core protein phosphorylation. Indeed, in the hcf136 mutant of Arabidopsis, which is defective in assembly of PSII, the grana of thylakoids are six- to eightfold enlarged and extend throughout the chloroplast, with grana lamellae closely appressed to each other (Meurer et al., 1998). Earlier studies of PSII-deficient mutants of barley (Hordeum vulgare; Simpson et al., 1989) also revealed a thylakoid membrane organization with enlarged grana strikingly similar to that found in stn7xstn8. The thylakoid ultrastructure of the stn7xstn8 and stn8 mutants is similar to that observed in shade plants that display large grana stacks with few interconnecting thylakoids (Guillot-Salomon et al., 1978). It is interesting to note that in shade plants, the PSII repair cycle is less important for protection against photoinhibition and that protection appears to be mediated by controlled nonphotochemical dissipation of excess excitation energy (Öquist et al., 1992). It is possible that this is due to the fact that as in stn7xstn8, the extensive grana structure impedes migration of D1 and would thus interfere with the PSII repair cycle.
Figure 8.
A Model for Macroscopic Rearrangements of Plant Thylakoid Membranes via Phosphorylation-Dependent Repulsion of Adjacent Membrane Layers.
The thylakoid margins of the wild type (left) and stn7xstn8 (right) are shown. The phosphate groups contributed by the PSII core proteins in the wild type loosen the appressed membrane regions through electrostatic repulsion and facilitate lateral migration of photodamaged PSII subunits from the stacked grana thylakoids to unstacked stroma lamellae, as well as the access of FtsH to the grana regions. Absence of PSII core protein phosphorylation in the stn7xstn8 mutant results in the higher membrane stacking, extension of grana regions, and limited lateral mobility of membrane proteins between the grana and stroma membrane domains.
[See online article for color version of this figure.]
In agreement with a recent study (Tikkanen et al., 2008) and in a contradiction to an earlier publication (Bonardi et al., 2005), our data show that the lack of PSII core protein phosphorylation disturbs the rapid degradation of D1 induced by photodamage. Tikkanen et al. (2008) analyzed D1 degradation in wild-type, stn7, stn8, and stn7xstn8 plant leaves treated with lincomycin and exposed to high light of 1000 μmol photons m−2 s−1, while Bonardi et al. (2005) worked at 2000 μmol photons m−2 s−1. We have observed reproducible differences in the rates of D1 degradation between the wild type and stn7 on one side, and stn8 and stn7xstn8 on the other under light fluencies of 900, 1000, and 2000 μmol photons m−2 s−1 (Figure 6; see Supplemental Figure 2 online). Bonardi et al. (2005) failed to find differences between wild-type plants and the mutants probably because they used inefficient vacuum infiltration of leaf discs with lincomycin or with [35S]Met, which required 4 to 6 h of high light treatment of either wild-type or mutant leaves to induce degradation of half of the D1 protein. This degradation of D1 was 3 to 4 times slower compared with that determined in many high light–treated plants in which lincomycin was introduced through cut petioles (Yokthongwattana and Melis, 2006; Sirpio et al., 2007; Tikkanen et al., 2008).
Tikkanen et al. (2008) were the first to observe retarded D1 degradation in stn8 and stn7xstn8 and proposed that the lack of PSII core protein phosphorylation disturbs the disassembly of PSII supercomplexes at high light, which is a prerequisite for efficient migration of damaged PSII complexes from grana to stroma lamellae for repair. Indeed, upon prolonged exposure to high light (24 h), the PSII complexes were nearly completely monomerized in the wild type, whereas in the stn7xstn8 mutant, most of the PSII cores were still in dimers and even in supercomplexes. However, in dark-treated plants and plants exposed to high light for 1 or 2 h, the distribution of PSII complexes between the monomers, dimers, and supercomplexes was similar between stn7xstn8 and the wild type (Tikkanen et al., 2008). We also used Blue Native gel electrophoresis to compare thylakoid protein complexes from wild-type and stn7xstn8 double mutant plants exposed to normal or high light for 3 h. In these experiments, we could not detect any significant difference in the distribution of PSII complexes between the monomers, dimers, and supercomplexes (see Supplemental Figure 4 online). Thus, we propose that extended appressed grana regions of thylakoids in stn7xstn8 and stn8 (Figures 3 and 4) could be more disadvantageous for lateral protein diffusion and for degradation of the photodamaged D1 in plants exposed to high light for a few hours (Figure 6) than the slower disassembly of PSII supercomplexes in the mutants. Moreover, the latter process could be a consequence of the macroscopic rearrangements of the thylakoid membrane network in stn7xstn8 and stn8.
Taking into consideration the importance of D1 protein turnover for PSII quality control and for the maintenance of plant photosynthetic efficiency (Yokthongwattana and Melis, 2006; Takahashi and Murata, 2008), it is not surprising that there are different pathways for degradation of damaged D1 (Kapri-Pardes et al., 2007; Komayama et al., 2007; Komenda et al., 2007; Sun et al., 2007). Our data support the theory that FtsH-mediated D1 degradation plays a central role in repair of the PSII complex in response to high light stress (Nixon et al., 2005). Slow proteolysis (Figure 5), as well as the changed patterns of D1 degradation and aggregation (Figure 2), were found in the leaves of stn7xstn8, which is consistent with the spatial segregation of the FtsH protease from D1 and hindered lateral migration of D1 from the grana to stroma membrane domains (Figure 7). An alternative proteolysis of D1, particularly by Deg1 (Kapri-Pardes et al., 2007), Deg5, and Deg8 (Sun et al., 2007) proteases, should not depend on thylakoid stacking because these three proteases are localized in the thylakoid lumen. Nevertheless, the Deg proteases could generate some of the D1 protein fragments and induce changes in the degradation pattern in stn7xstn8, in which PSII phosphorylation is significantly reduced (Figure 2). The degradation and aggregation of D1 were also suggested to represent two alternative processes influenced by protein phosphorylation (Komayama et al., 2007). In particular, a higher extent of the D1 protein aggregation was observed as a result of inhibition of protein dephosphorylation (Komayama et al., 2007) or of the missing Deg1 protease (Kapri-Pardes et al., 2007). Our results demonstrate that absence of D1 and CP43 phosphorylation and significantly reduced phosphorylation of D2 in stn7xstn8 change aggregation and degradation patterns of the D1 protein compared with wild-type plants. Phosphorylation-dependent direction of the pathway for PSII quality control determines the rate of D1 turnover, and its loss could be partly responsible for the significant decrease in plant fitness. Indeed, the stn7xstn8 double mutant grown in natural field conditions produced 41% less seeds than wild-type plants, while the stn7 mutant, deficient in phosphorylation of light-harvesting proteins and in photosynthetic state transitions, had only a 19% decrease in seed production (Frenkel et al., 2007).
Granal stacking of thylakoid membranes depends on several factors, including steric hindrance, van der Waals attraction, entropic forces, surface charge density, and electrostatic interactions (Chow et al., 2005). The light-harvesting chlorophyll a/b protein complexes play an important role in the structure of plant thylakoids. Indeed, a recent study of chlorophyll b–less mutants of Arabidopsis revealed that their thylakoids were less negatively charged than those of the wild type, and thylakoids in leaves of the mutants were not well stacked, despite the expected weaker electrostatic repulsion (Kim et al., 2009). This effect was attributed to lower van der Waals attraction, lower electrostatic attraction between opposite charges, and the absence or instability of PSII supercomplexes and peripheral LHCII trimers (Kim et al., 2009). It was proposed earlier that phosphorylation of LHCII proteins could influence surface charges, structure, and function of plant thylakoids (Barber, 1980, 1982). These publications suggested that photosynthetic state transitions depend on unstacking of thylakoids, which results from electrostatic repulsion between negative charges of membrane surfaces due to phosphorylation of LHCII polypeptides. A recent study (Chuartzman et al., 2008) of structural changes in the thylakoids of Arabidopsis, which employed atomic force microscopy, scanning and transmission electron microscopy, and confocal imaging, revealed reorganization of the membranes at the interface between the grana and stroma domains during photosynthetic state transitions. State transitions depend on the STN7 kinase (Bellafiore et al., 2005; Rochaix, 2007). We performed a parallel analysis of wild-type, stn7, stn8-1, stn8-2, and stn7xstn8 mutant plants and found significant differences in gravity-driven sedimentation rates of thylakoids, which was a consequence of larger size of the stacked thylakoids in stn8-1, stn8-2, and stn7xstn8 than in the wild type and stn7 (Figures 3 and 4). Thus, phosphorylation of the surface-exposed regions of the D1, D2, CP43, and PsbH proteins of the PSII core is more important for control of thylakoid stacking than phosphorylation of the LHCII proteins, catalyzed by the STN7 kinase. It should be noticed that the phosphorylation patterns observed in Arabidopsis are rather different from those in pumpkin (Cucurbita maxima) where transition to light and high light causes a massive increase of phosphorylation of the PSII core proteins (Rintamäki et al., 1997). The level of the PSII phosphorylation in Arabidopsis is rather stable during day or night (Vener et al., 2001), while the LHCII proteins are almost completely dephosphorylated during the night or under high light (Rintamäki et al., 1997; Vener et al., 2001; Bellafiore et al., 2005; Bonardi et al., 2005; Vainonen et al., 2005; Tikkanen et al., 2008). The stable phosphorylation state of PSII is consistent with a stable thylakoid membrane folding that is not highly affected by light intensity changes, as demonstrated by transmission electron microscopy in this work (Figures 3 and 4) and in the earlier studies of chloroplasts from 21 different plant species (Albertsson and Andreasson, 2004).
The steady state stoichiometry of in vivo PSII core protein phosphorylation in Arabidopsis corresponds to an average 40% phosphorylation of each of these proteins during growth under day/night cycles (Vener et al., 2001). The stn8-1 and stn8-2 mutants of Arabidopsis display a threefold decrease in phosphorylation of D1, D2, and CP43 and a lack of phosphorylation of Thr-4 in the PsbH subunit of PSII compared with wild-type plants (Vainonen et al., 2005). In this work, we established that phosphorylation of D1 and CP43 is completely absent, while phosphorylation of the D2 protein is more than 10 times lower in stn7xstn8 than in the wild-type plants. According to these findings, we conclude that quantitative phosphorylation of the membrane surface-exposed regions of the D1, D2, CP43, and PsbH proteins of the PSII core, corresponding to a state of at least 40% phosphorylation for each of these proteins in normal growth conditions (Vener et al., 2001), is important for control of thylakoid stacking compatible with the sustained repair cycle of PSII. The additional evidence for this conclusion has been obtained in experiments with the thylakoids isolated from wild-type Arabidopsis plants. Specific dephosphorylation of the D1, D2, and CP43 proteins induced by a heat shock treatment resulted in a faster gravity-driven sedimentation of the dephosphorylated thylakoids compared with the phosphorylated membranes. The identical heat shock treatment of thylakoids in the presence of phosphatase inhibitors prevented both dephosphoryation of thylakoid proteins and the increase in the density/compactness of the thylakoid membranes (Figure 5). Thus, our experiments demonstrate increased folding of the wild-type thylakoids caused by dephosphorylation of the D1, D2, and CP43 proteins. Although there is an increase in phosphorylation of these proteins upon transition from low light to high light, the phosphorylation mediated by STN8 in the wild type even during night time appears to be sufficient for proper thylakoid membrane folding based on the observation that we do not detect any change in folding of thylakoids from high light–treated leaves both by electron microscopy and sedimentation (Figure 3).
The evolution of oxygenic photosynthesis in plants resulted in the development of extremely large thylakoid membranes that cannot fit inside chloroplasts or cells without forming highly folded layers of grana, the characteristic trait of plants (Mullineaux, 2005). The length/diameter of grana discs in mature chloroplasts of all studied plant species is rather constant (∼400 nm), and it was proposed that a larger grana diameter could be disadvantageous for lateral protein diffusion processes (Kirchhoff, 2008). Our results confirm this hypothesis: increased length of grana discs caused by defective PSII core protein phosphorylation leads to restrictions in lateral migration of D1 and FtsH proteins between the membrane domains. In this work, we reveal a physiological function of massive phosphorylation of PSII core proteins in plant photosynthetic membranes, which was enigmatic since the original discovery of phosphorylation of chloroplast membrane proteins by Bennett (1977). We show that quantitative phosphorylation of membrane surface-exposed regions of PSII proteins is required for macroscopic rearrangements of the entire membrane network of plant thylakoids, which facilitates lateral mobility of proteins required for repair and sustained activity of PSII.
METHODS
Plant Material
Arabidopsis thaliana plants used for most experiments were grown hydroponically (Norén et al., 2004) at 23°C, 65 to 70% relative humidity, and at a photosynthetic flux of 120 μmol photons m−2 s−1 for normal light and a flux of 900 μmol photons m−2 s−1 for high light. The photoperiod was 8 h light and 16 h dark. For pulse-chase and electron microscopy experiments, the plants were grown on soil under the same photoperiod. Ecotype Columbia (Col) was used in all experiments. The stn7 (SALK 073254), stn8-1 (SALK 060869), and stn8-2 (SALK 064913) mutants in Col-0 background used in this study were characterized earlier (Bellafiore et al., 2005; Vainonen et al., 2005). The homozygous mutant T-DNA insertion lines stn7 (SALK 073254) and stn8 (SALK 060869) were crossed and the F1 progeny were selfed. DNA was extracted and stn7xstn8 homozygous plants were identified by PCR analysis. For STN7, PCR reactions were performed with oligonucleotides STN7fw (5′-TGAGGACTCATGTTTTGTGTC-3′) and STN7rev (5′-GGTGCAAACTTAATTGTTTG-3′) and with the T-DNA left border oligonucleotide LBb1 (5′-GCGTGGACCGCTTGCTGCAACT-3′) and STN7rev. For STN8 PCR reactions were performed with oligonucleotides STN8fw (5′-GGGCCACTATTGAGATGATTG-3′) and STN8rev (5′-GAATTCTACTCTTGTTGATGACG-3′) and with LB1 and STN8rev. The PCR was started by denaturing the DNA at 94°C for 5 min followed by 35 cycles with 1 min at 94°C, annealing at 49°C for 45 s, and elongation at 72°C for 45 s with Taq polymerase. At the end, the sample was incubated at 72°C for 5 min.
When D1 turnover was studied, the chloroplast-encoded protein synthesis was blocked using lincomycin. Detached leaves were incubated with their petioles submersed in 1 mM solution of lincomycin at 4°C in darkness for 16 h. The leaves were then floated in the same solution with lincomycin for 3 h under high light of 900 μmol photons m−2 s−1.
Isolation and Characterization of Thylakoids
Four-week-old plants were used for the preparation of chloroplasts and thylakoids. The thylakoid membranes were isolated from 4 g of Arabidopsis leaves harvested either after light adaptation (3 h after the light was on) or after dark adaptation (15 h after the light was off). The leaves were homogenized in 20 mL of ice-cold 25 mM Tricine, pH 7.8, 330 mM sorbitol, 1 mM EDTA, 10 mM KCl, 0.15% BSA, 4 mM sodium ascorbate, and 7 mM l-cysteine in a metal blender for four periods of 1 s at high speed. The homogenate was immediately filtered through four layers of nylon mesh (20 μm pore size), after which the filtrate was centrifuged for 3 min at 1000g. The pellet was resuspended in the same buffer to wash the chloroplasts and centrifuged for 5 min at 1000g. The chloroplast pellet was resuspended in 10 mM Tricine, 5 mM MgCl2, and 10 mM NaF and allowed to stand for 5 min in the dark on ice in order to lyse the chloroplasts. Following lysis, the thylakoids were pelleted by centrifugation for 5 min at 6000g. To wash the thylakoids, the pellet was resuspended in 100 mM sorbitol, 25 mM Tricine, pH 7.8, 5 mM MgCl2, 10 mM KCl, and 10 mM NaF and centrifuged for 5 min at 6000g. The pellet was resuspended in a small volume of the same buffer. NaF was used as a phosphatase inhibitor in the buffers when phosphorylation was quantified.
Gravity-driven sedimentation of thylakoids was analyzed at room temperature in transparent PVC tubes at a total volume of 6 mL with 0.2 mg chlorophyll/mL. The sedimentation of thylakoids was analyzed during the times indicated in the figures.
For subfractionation of thylakoids, a digitonin solution of 2% (w/v) was added to the thylakoid suspension (0.6 mg chlorophyll/mL) to a final concentration of 1% (w/v). The mixture was homogenized in a glass homogenizer five times and mixed for 5 min at room temp. The solution was centrifuged at 1000g for 5 min to pellet unsolubilized material. The supernatant was further centrifuged at 40,000g for 30 min, and the stroma lamellae were collected from the resulting supernatant by centrifugation at 140,000g for 90 min. The 40,000g pellet contained the grana stacks.
Dephosphorylation of Thylakoid Proteins in Vitro
Thylakoid membranes from the wild-type plants were prepared as described above and resuspended in either of three different incubation buffers (0.2 mg chlorophyll/mL): buffer 1 containing 100 mM sorbitol, 25 mM Tricine, pH 7.8, and 10 mM KCl; buffer 2, same as 1 plus 10 mM NaF; or buffer 3, same as 1 plus 15 mM Na2HPO4. MgCl2 was left out of the buffers to induce destacking of the thylakoid membranes; NaF and Na2HPO4 were used as phosphatase inhibitors. The membranes were incubated in darkness at 22 or 42°C for 40 min to induce dephopshorylation by the intrinsic heat-activated membrane phosphatase. Dephosphorylation was terminated by centrifugation at 1500g for 1 min at 4°C. Resuspension in the buffers 1 to 3 with 5 mM MgCl2 was done twice to restack the membranes. The thylakoid suspensions in the buffers 1 to 3 with 5 mM MgCl2 were finally studied for gravity-driven sedimentation for up to 4 h at 22°C.
Characterization of Protein Phosphorylation by MS
The thylakoids isolated from the wild-type or stn7xstn8 plants were resuspended in 25 mM NH4HCO3 and 10 mM NaF to a final concentration of 2.5 mg of chlorophyll/mL and incubated for 3 h at 22°C with a sequencing grade-modified trypsin (Promega). The peptides cleaved by trypsin were separated from the thylakoid membranes by centrifugation, lyophilized, methyl-esterified with 2 n methanolic HCl, and enriched for phosphorylated peptides by IMAC affinity chromatography (Vainonen et al., 2005).
The IMAC-enriched mixtures of phosphorylated peptides were separated using the Agilent 1100 HPLC system with the flow splitter and analyzed by electrospray ionization MS in positive ionization mode using the ion trap HCTultra PTM Discovery System (Bruker Daltonics). The peptides were separated on C18 reverse phase column (5 μm; 0.3 × 150 mm) at a flow rate of 7 μL/min. A gradient of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B) was distributed as follow: 0 to 5% B in first min; 5 to 40% B in 1 to 46 min; 40 to 100% B in 46 to 53 min; and 100% B in 53 to 60 min. The automated online tandem MS analyses were performed using alternating collision-induced dissociation and electron transfer dissociation of peptide ions.
Immunoblotting
Thylakoid membrane proteins were separated by SDS-PAGE (6% acrylamid stacking gel + 14% separation gel + 6 M urea), and the proteins were then transferred to a polyvinylidene difluoride membrane (Immobilone; Millipore). For the antiphosphothreonine antibodies, purchased both from Zymed Laboratories and New England Biolabs (Cell Signaling), the membranes were blocked with 5% BSA. For specific antibodies against the DE-loop in D1 protein (Spetea et al., 1999), D1 C terminus, PsaA, PsaF, Lhcb1 (all from Agrisera), FtsH2 (kindly provided by W Sakamoto of Okayama University, Japan) and against the residues 230 to 245 in the D2 protein (Koivuniemi et al., 1995), the blocking was done with 10% skimmed milk. The membranes were then incubated with horseradish peroxidase–conjugated secondary antibody and analyzed using the ECL detection kit (GE Healthcare) with chemiluminescence imaging using the LAS-1000 luminescent image analyzer (Fujifilm). The exposure of the membranes was within the linear range for all antibodies used in analyses. Quantification of the immunoblots was done using Fujifilm LAS-1000 software.
Pulse-Chase Experiments
In vivo pulse labeling with [35S]Met was performed according to Meurer et al. (1996). Wild-type, stn7xstn8, stn8, and stn7 4-week-old plantlets were cut between roots and stem. The stems of the plantlets were immerged for 20 min in 100 μL of 20 mM KH2PO4, pH 6.3, and 0.1% Tween 20 containing 20 μg/mL cycloheximide at room temperature under dim light. The solution was then replaced by 100 μL of the same solution containing 50 μCi l-[35S]Met (1000 Ci/mmol), and incubation was continued for 2 h. For the chase, the radioactive solution was replaced by 20 mM KH2PO4, pH 6.3; after 30 min of incubation, lincomycin (1 mM) was added and incubation was continued for 30 min. Labeled leaves were then floated in the same solution under high light (1000 μmol m−2 s−1). After indicated time, they were transferred to 20 mM KH2PO4, pH 6.3, and 10 mM DTT and ground using a plastic pestle in 500 μL of the wash solution supplemented with Sigma-Aldrich Cocktail Protease Inhibitors. The extract was centrifuged 10 min at maximal speed in an Eppendorf centrifuge at 4°C to isolate the membranes. The pellets were washed once and resuspended in 100 mM Na2CO3, 10% (w/v) sucrose, 2% SDS, 50 mM DTT, and protease inhibitors (Sigma-Aldrich) and analyzed by SDS-12% 6M urea PAGE. Gels were dried and analyzed by phosphor imaging. In each case, the label of the D1 band was normalized to the signal arising from the sum of the PsaA, AtpB, AtpA, and CP43 bands, thus correcting for unequal loading of the different lanes. The values obtained were averaged from two gel electrophoresis experimental replicates.
Electron Microscopy
Plants were grown on soil under an 8-h-light/16-h-dark regime (100 μmol m−2 s−1). After 3 h of light, pieces of leaves from 4-week-old wild-type and mutant seedlings were directly fixed at room temperature for 1 h in 2.5% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.0, containing 0.1% Tween 20. The leave pieces were then kept in the same fixative without Tween for 3 h at 4°C. The samples were washed 4 × 15 min in cacodylate and postfixed in 1% OsO4 in cacodylate for 2 h at room temperature. Samples were rinsed in cacodylate buffer 4 × 15 min and then in water and further fixed 1 h in 1% aqueous uranyl acetate. After washings in distilled water, the samples were dehydrated in a graded ethanol series and embedded in Epon 812. Ultrathin sections 80 nm thick were cut and stained classically with 2% aqueous uranyl acetate and then in Reynolds lead citrate. Sections were viewed in a transmission electron microscope, either a Phillips EM 10 at 60 kV or a FEI Tecnai G2 Sphera at 120 kV.
The lengths of appressed and stroma-exposed thylakoid membranes from electron microscope sections of wild-type and mutant plant leaves were measured using ImageJ software (http://rsbweb.nih.gov/ij/index.html). The length of the appressed regions was counted twice, whereas the outer membrane of the grana was counted once. In each case, five independent sections covering together an area of 11.1 and 9.4 μm2 in the wild type and stn7xstn8, respectively, were chosen for the measurements.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under accession numbers At1g68830 (STN7) and At5g01920 (STN8).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Locations of T-DNA Insertions within STN7 (At1g68830) and STN8 (At5g01920) Genes.
Supplemental Figure 2. In Vivo Pulse-Chase Experiments with Chloroplast Proteins of Wild-Type and stn7xstn8 Plants Labeled with [35S]Met and Exposed to High Light of 1000 μmol m−2 s−1.
Supplemental Figure 3. Immunoblotting Analysis of SDS-PAGE Separated Thylakoid Proteins from Wild-Type, stn7xstn8, and stn8-1 Plants Exposed to Normal Light of 120 μmol Photons m−2 s−1 or High Light of 900 μmol Photons m−2 s−1 with the FtsH-Specific Antibody.
Supplemental Figure 4. Blue Native PAGE and Blue Native PAGE/SDS-PAGE Gel Separations of Thylakoid Protein Complexes from Wild-Type and the stn7xstn8 Plants Exposed to Normal Light of 120 μmol Photons m−2 s−1 or High Light of 900 μmol Photons m−2 s−1 for 3 h.
Supplemental Table 1. Electron Microscope Measurements of Thylakoid Membranes in Wild-Type (Col-0), stn7, stn8-1, stn8-2, and stn7xstn8 Plant Leaves.
Supplementary Material
Acknowledgments
We thank Wataru Sakamoto for the FtsH2-specific antibody. This work was supported by grants from the Swedish Research Council, the Swedish Research Council for Environment, Agricultural Sciences, and Spatial Planning to A.V.V., and from the National Center of Competence in Research Plant Survival and Swiss National Foundation (3100AO-117712) to J.-D.R.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Alexander V. Vener (alexander.vener@liu.se).
Some figures in this article are displayed in color online but in black and white in the print edition.
Online version contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Adam, Z., Rudella, A., and van Wijk, K.J. (2006). Recent advances in the study of Clp, FtsH and other proteases located in chloroplasts. Curr. Opin. Plant Biol. 9 234–240. [DOI] [PubMed] [Google Scholar]
- Albertsson, P.A., and Andreasson, E. (2004). The constant proportion of grana and stroma lamellae in plant chloroplasts. Physiol. Plant. 121 334–342. [DOI] [PubMed] [Google Scholar]
- Andersson, B., and Anderson, J.M. (1980). Lateral heterogeneity in the distribution of chlorophyll-protein complexes of the thylakoid membranes of spinach chloroplasts. Biochim. Biophys. Acta 593 427–440. [DOI] [PubMed] [Google Scholar]
- Arntzen, C.J., and Ditto, C.L. (1976). Effects of cations upon chloroplast membrane subunit interactions and excitation energy distribution. Biochim. Biophys. Acta 449 259–274. [DOI] [PubMed] [Google Scholar]
- Aro, E.M., Rokka, A., and Vener, A.V. (2004). Determination of phosphoproteins in higher plant thylakoids. Methods Mol. Biol. 274 271–286. [DOI] [PubMed] [Google Scholar]
- Baena-Gonzalez, E., and Aro, E.M. (2002). Biogenesis, assembly and turnover of photosystem II units. Philos. Trans. R. Soc. Lond. B Biol. Sci. 357 1451–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber, J. (1980). An explanation for the relationship between salt-induced thylakoid stacking and the chlorophyll fluorescence changes associated with changes in spillover of energy from photosystem II to photosystem. FEBS Lett. 118 1–10. [Google Scholar]
- Barber, J. (1982). Influence of surface charges on thylakouid structure and function. Annu. Rev. Plant Physiol. 33 261–295. [Google Scholar]
- Barber, J. (2006). Photosystem II: An enzyme of global significance. Biochem. Soc. Trans. 34 619–631. [DOI] [PubMed] [Google Scholar]
- Bellafiore, S., Barneche, F., Peltier, G., and Rochaix, J.D. (2005). State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature 433 892–895. [DOI] [PubMed] [Google Scholar]
- Bennett, J. (1977). Phosphorylation of chloroplast membrane polypeptides. Nature 269 344–346. [Google Scholar]
- Bonardi, V., Pesaresi, P., Becker, T., Schleiff, E., Wagner, R., Pfannschmidt, T., Jahns, P., and Leister, D. (2005). Photosystem II core phosphorylation and photosynthetic acclimation require two different protein kinases. Nature 437 1179–1182. [DOI] [PubMed] [Google Scholar]
- Chow, W.S., Kim, E.H., Horton, P., and Anderson, J.M. (2005). Granal stacking of thylakoid membranes in higher plant chloroplasts: the physicochemical forces at work and the functional consequences that ensue. Photochem. Photobiol. Sci. 4 1081–1090. [DOI] [PubMed] [Google Scholar]
- Chuartzman, S.G., Nevo, R., Shimoni, E., Charuvi, D., Kiss, V., Ohad, I., Brumfeld, V., and Reich, Z. (2008). Thylakoid membrane remodeling during state transitions in Arabidopsis. Plant Cell 20 1029–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel, M., Bellafiore, S., Rochaix, J.D., and Jansson, S. (2007). Hierarchy amongst photosynthetic acclimation responses for plant fitness. Physiol. Plant. 129 455–459. [Google Scholar]
- Fristedt, R., Carlberg, I., Zygadlo, A., Piippo, M., Nurmi, M., Aro, E.M., Scheller, H.V., and Vener, A.V. (2009). Intrinsically unstructured phosphoprotein TSP9 regulates light harvesting in Arabidopsis thaliana. Biochemistry 48 499–509. [DOI] [PubMed] [Google Scholar]
- Guillot-Salomon, T., Tuquet, C., De Lubac, M., Hallais, M.F., and Signol, M. (1978). Comparative analysis of ultrastructure and lipid composition of plastids from sun and shade plants. Cytobiologie 17 442–452. [PubMed] [Google Scholar]
- Hansson, M., and Vener, A.V. (2003). Identification of three previously unknown in vivo protein phosphorylation sites in thylakoid membranes of Arabidopsis thaliana. Mol. Cell. Proteomics 2 550–559. [DOI] [PubMed] [Google Scholar]
- Izawa, S., and Good, N.E. (1966. a). Effect of salts and electron transport on the conformation of isolated chloroplasts. I. Light-scattering and volume changes. Plant Physiol. 41 533–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa, S., and Good, N.E. (1966. b). Effect of salts and electron transport on the conformation of isolated chloroplasts. II. Electron microscopy. Plant Physiol. 41 544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapri-Pardes, E., Naveh, L., and Adam, Z. (2007). The thylakoid lumen protease Deg1 is involved in the repair of photosystem II from photoinhibition in Arabidopsis. Plant Cell 19 1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, E.H., Li, X.P., Razeghifard, R., Anderson, J.M., Niyogi, K.K., Pogson, B.J., and Chow, W.S. (2009). The multiple roles of light-harvesting chlorophyll a/b-protein complexes define structure and optimize function of Arabidopsis chloroplasts: A study using two chlorophyll b-less mutants. Biochim. Biophys. Acta 1787 973–984. [DOI] [PubMed] [Google Scholar]
- Kirchhoff, H. (2008). Molecular crowding and order in photosynthetic membranes. Trends Plant Sci. 13 201–207. [DOI] [PubMed] [Google Scholar]
- Kirchhoff, H., Lenhert, S., Buchel, C., Chi, L., and Nield, J. (2008). Probing the organization of photosystem II in photosynthetic membranes by atomic force microscopy. Biochemistry 47 431–440. [DOI] [PubMed] [Google Scholar]
- Koivuniemi, A., Aro, E.-M., and Andersson, B. (1995). Degradation of the D1- and D2-proteins of photosystem II in higher plants is regulated by reversible phosphorylation. Biochemistry 34 16022–16029. [DOI] [PubMed] [Google Scholar]
- Komayama, K., et al. (2007). Quality control of photosystem II: Cleavage and aggregation of heat-damaged D1 protein in spinach thylakoids. Biochim. Biophys. Acta 1767 838–846. [DOI] [PubMed] [Google Scholar]
- Komenda, J., Tichy, M., Prasil, O., Knoppova, J., Kuvikova, S., de Vries, R., and Nixon, P.J. (2007). The exposed N-terminal tail of the D1 subunit is required for rapid D1 degradation during photosystem II repair in Synechocystis sp PCC 6803. Plant Cell 19 2839–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl, M., Spetea, C., Hundal, T., Oppenheim, A.B., Adam, Z., and Andersson, B. (2000). The thylakoid FtsH protease plays a role in the light-induced turnover of the photosystem II D1 protein. Plant Cell 12 419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurer, J., Meierhoff, K., and Westhoff, P. (1996). Isolation of high-chlorophyll-fluorescence mutants of Arabidopsis thaliana and their characterisation by spectroscopy, immunoblotting and northern hybridisation. Planta 198 385–396. [DOI] [PubMed] [Google Scholar]
- Meurer, J., Plucken, H., Kowallik, K.V., and Westhoff, P. (1998). A nuclear-encoded protein of prokaryotic origin is essential for the stability of photosystem II in Arabidopsis thaliana. EMBO J. 17 5286–5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullineaux, C.W. (2005). Function and evolution of grana. Trends Plant Sci. 10 521–525. [DOI] [PubMed] [Google Scholar]
- Nixon, P.J., Barker, M., Boehm, M., de Vries, R., and Komenda, J. (2005). FtsH-mediated repair of the photosystem II complex in response to light stress. J. Exp. Bot. 56 357–363. [DOI] [PubMed] [Google Scholar]
- Norén, H., Svensson, P., and Andersson, B. (2004). A convenient and versatile hydroponic cultivation system for Arabidopsis thaliana. Physiol. Plant. 121 343–348. [Google Scholar]
- Öquist, G., Anderson, J.M., Mc Caffery, S., and Chow, W.S. (1992). Mechanistic differences in photoinhibition of sun and shade plants. Planta 188 422–431. [DOI] [PubMed] [Google Scholar]
- Rintamäki, E., Salonen, M., Suoranta, U.M., Carlberg, I., Andersson, B., and Aro, E.-M. (1997). Phosphorylation of light-harvesting complex II and photosystem II core proteins shows different irradiance-dependent regulation in vivo. Application of phosphothreonine antibodies to analysis of thylakoid phosphoproteins. J. Biol. Chem. 272 30476–30482. [DOI] [PubMed] [Google Scholar]
- Rochaix, J.D. (2007). Role of thylakoid protein kinases in photosynthetic acclimation. FEBS Lett. 581 2768–2775. [DOI] [PubMed] [Google Scholar]
- Rokka, A., Aro, E.-M., Herrmann, R.G., Andersson, B., and Vener, A.V. (2000). Dephosphorylation of photosystem II reaction center proteins in plant photosynthetic membranes as an immediate response to abrupt elevation of temperature. Plant Physiol. 123 1525–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoni, E., Rav-Hon, O., Ohad, I., Brumfeld, V., and Reich, Z. (2005). Three-dimensional organization of higher-plant chloroplast thylakoid membranes revealed by electron tomography. Plant Cell 17 2580–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson, D.J., Vallon, O., and von Wettstein, D. (1989). Freeze-fracture studies on barley plastid membranes. VIII. In viridis-115, a mutant completely lacking photosystem II, oxygen evolution enhancer 1 (OEE1) and the alfa-subunit of cytochrome b-559 accumulate in appressed thylakoids. Biochim. Biophys. Acta 975 164–174. [Google Scholar]
- Sirpio, S., Allahverdiyeva, Y., Suorsa, M., Paakkarinen, V., Vainonen, J., Battchikova, N., and Aro, E.M. (2007). TLP18.3, a novel thylakoid lumen protein regulating photosystem II repair cycle. Biochem. J. 406 415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spetea, C., Hundal, T., Lohmann, F., and Andersson, B. (1999). GTP bound to chloroplast thylakoid membranes is required for light-induced, multienzyme degradation of the photosystem II D1 protein. Proc. Natl. Acad. Sci. USA 96 6547–6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, X., Peng, L., Guo, J., Chi, W., Ma, J., Lu, C., and Zhang, L. (2007). Formation of DEG5 and DEG8 complexes and their involvement in the degradation of photodamaged photosystem II reaction center D1 protein in Arabidopsis. Plant Cell 19 1347–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syka, J.E., Coon, J.J., Schroeder, M.J., Shabanowitz, J., and Hunt, D.F. (2004). Peptide and protein sequence analysis by electron transfer dissociation mass spectrometry. Proc. Natl. Acad. Sci. USA 101 9528–9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, S., and Murata, N. (2008). How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 13 178–182. [DOI] [PubMed] [Google Scholar]
- Tikkanen, M., Nurmi, M., Kangasjarvi, S., and Aro, E.M. (2008). Core protein phosphorylation facilitates the repair of photodamaged photosystem II at high light. Biochim. Biophys. Acta 1777 1432–1437. [DOI] [PubMed] [Google Scholar]
- Tikkanen, M., Piippo, M., Suorsa, M., Sirpiö, S., Mulo, P., Vainonen, J., Vener, A.V., Allahverdiyeva, Y., and Aro, E.-M. (2006). State transitions revisited - A buffering system for dynamic low light acclimation of Arabidopsis. Plant Mol. Biol. 62 779–793. [DOI] [PubMed] [Google Scholar]
- Vainonen, J.P., Hansson, M., and Vener, A.V. (2005). STN8 protein kinase in Arabidopsis thaliana is specific in phosphorylation of photosystem II core proteins. J. Biol. Chem. 280 33679–33686. [DOI] [PubMed] [Google Scholar]
- Vener, A.V. (2007). Environmentally modulated phosphorylation and dynamics of proteins in photosynthetic membranes. Biochim. Biophys. Acta 1767 449–457. [DOI] [PubMed] [Google Scholar]
- Vener, A.V., Harms, A., Sussman, M.R., and Vierstra, R.D. (2001). Mass spectrometric resolution of reversible protein phosphorylation in photosynthetic membranes of Arabidopsis thaliana. J. Biol. Chem. 276 6959–6966. [DOI] [PubMed] [Google Scholar]
- Vener, A.V., Rokka, A., Fulgosi, H., Andersson, B., and Herrmann, R.G. (1999). A cyclophilin-regulated PP2A-like protein phosphatase in thylakoid membranes of plant chloroplasts. Biochemistry 38 14955–14965. [DOI] [PubMed] [Google Scholar]
- Yokthongwattana, K., and Melis, A. (2006). Photoinhibition and recovery in oxygenic photosynthesis: Mechanism of a photosystem II damage and repair cycle. In Photoprotection, Photoinhibition, Gene Regulation and Environment, B. Demmig-Adams, W. Adams, and A.K. Mattoo, eds (Dordrecht, The Netherlands: Springer), pp. 175–191.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.