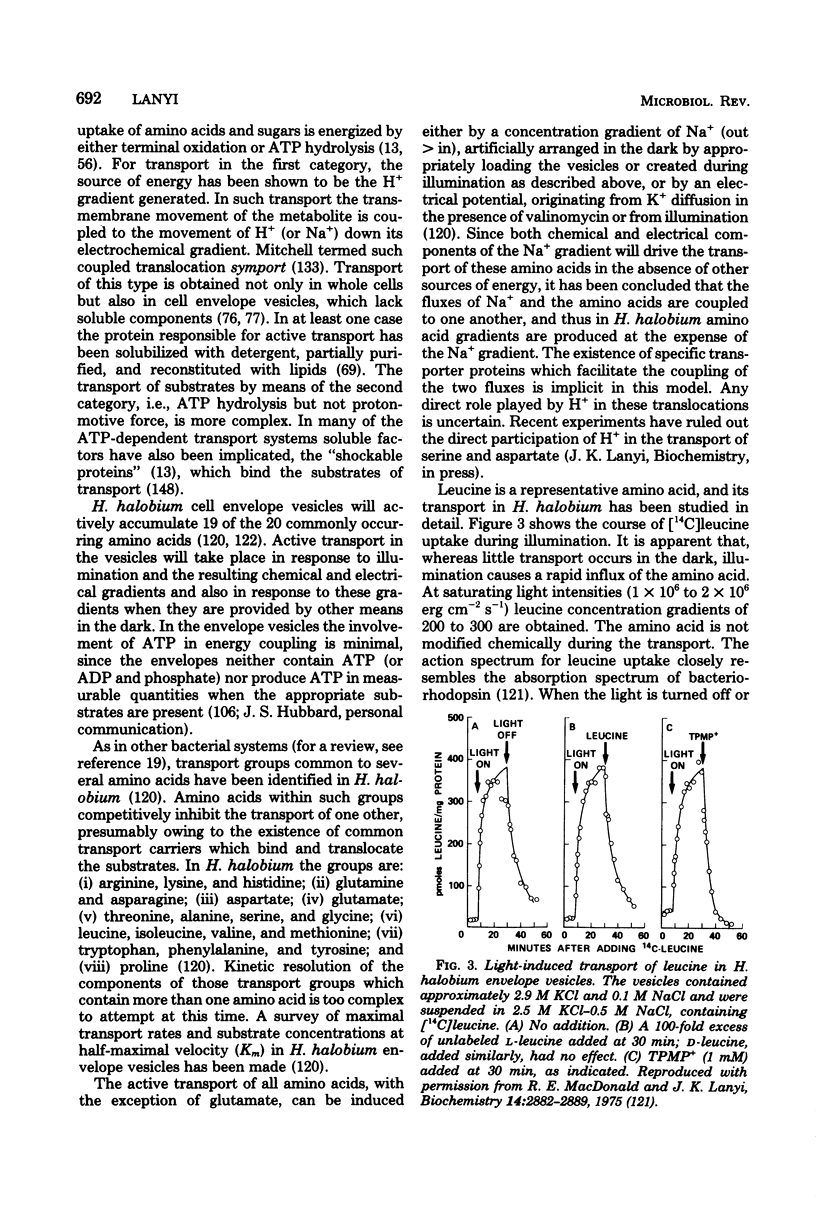
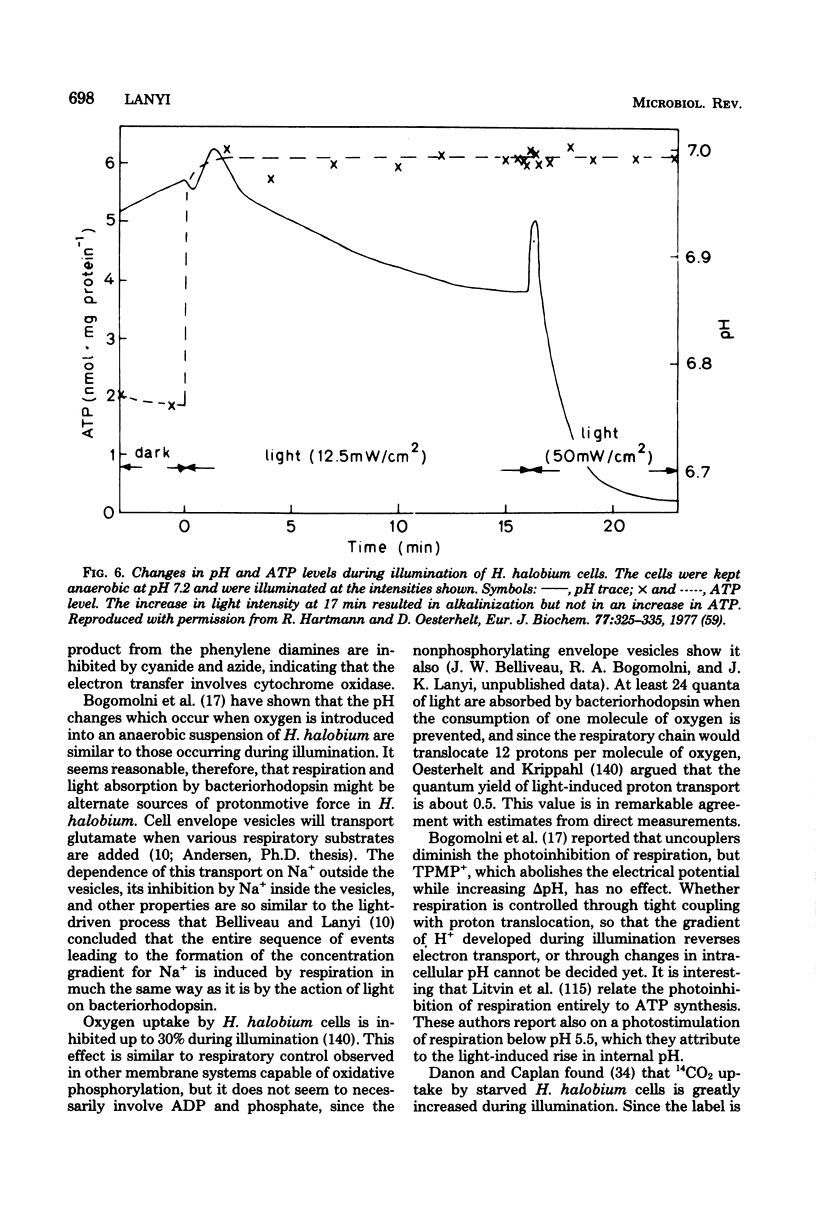
Full text
PDF






















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAM D., GIBBONS N. E. The effect of chlorides of monovalent cations, urea, detergents, and heat on morphology and the turbidity of suspensions of red halophilic bacteria. Can J Microbiol. 1961 Oct;7:741–750. doi: 10.1139/m61-088. [DOI] [PubMed] [Google Scholar]
- BAXTER R. M. An interpretation of the effects of salts on the lactic dehydrogenase of Halobacterium salinarium. Can J Microbiol. 1959 Feb;5(1):47–57. doi: 10.1139/m59-006. [DOI] [PubMed] [Google Scholar]
- BAYLEY S. T., KUSHNER D. J. THE RIBOSOMES OF THE EXTREMELY HALOPHILIC BACTERIUM, HALOBACTERIUM CUTIRUBRUM. J Mol Biol. 1964 Sep;9:654–669. doi: 10.1016/s0022-2836(64)80173-x. [DOI] [PubMed] [Google Scholar]
- BROWN A. D. ASPECTS OF BACTERIAL RESPONSE TO THE IONIC ENVIRONMENT. Bacteriol Rev. 1964 Sep;28:296–329. doi: 10.1128/br.28.3.296-329.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker E. P., Rottenberg H., Caplan S. R. An estimation of the light-induced electrochemical potential difference of protons across the membrane of Halobacterium halobium. Biochim Biophys Acta. 1976 Sep 13;440(3):557–572. doi: 10.1016/0005-2728(76)90042-6. [DOI] [PubMed] [Google Scholar]
- Bauer P. J., Dencher N. A., Heyn M. P. Evidence for chromophore-chromophore interactions in the purple membrane from reconstitution experiments of the chromophore-free membrane. Biophys Struct Mech. 1976 Apr 15;2(1):79–92. doi: 10.1007/BF00535654. [DOI] [PubMed] [Google Scholar]
- Becher B. M., Cassim J. Y. Improved isolation procedures for the purple membrane of Halobacterium halobium. Prep Biochem. 1975;5(2):161–178. doi: 10.1080/00327487508061568. [DOI] [PubMed] [Google Scholar]
- Becher B., Cassim J. Y. Effects of light adaptation on the purple membrane structure of Halobacterium halobium. Biophys J. 1976 Oct;16(10):1183–1200. doi: 10.1016/S0006-3495(76)85767-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher B., Ebrey T. G. Evidence for chromophore-chromophore (exciton) interaction in the purple membrane of Halobacterium halobium. Biochem Biophys Res Commun. 1976 Mar 8;69(1):1–6. doi: 10.1016/s0006-291x(76)80263-x. [DOI] [PubMed] [Google Scholar]
- Becher B., Ebrey T. G. The quantum efficiency for the photochemical conversion of the purple membrane protein. Biophys J. 1977 Feb;17(2):185–191. doi: 10.1016/S0006-3495(77)85636-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beliakova T. N., Kadziauskas Iu P., Skulachev V. P., Smirnova I. A., Chekulaeva L. N. Generatsiia élektrokhimicheskogo potentsiala ionov H+ i fotofosforilirovanie v kletkakh Halobacterium halobium. Dokl Akad Nauk SSSR. 1975 Jun 11;223(2):483–486. [PubMed] [Google Scholar]
- Belliveau J. W., Lanyi J. K. Analogies between respiration and a light-driven proton pump as sources of energy for active glutamate transport in Halobacterium holobium. Arch Biochem Biophys. 1977 Jan 15;178(1):308–314. doi: 10.1016/0003-9861(77)90196-5. [DOI] [PubMed] [Google Scholar]
- Belliveau J. W., Lanyi J. K. Calcium transport in Halobacterium halobium envelope vesicles. Arch Biochem Biophys. 1978 Feb;186(1):98–105. doi: 10.1016/0003-9861(78)90468-x. [DOI] [PubMed] [Google Scholar]
- Berger E. A., Heppel L. A. Different mechanisms of energy coupling for the shock-sensitive and shock-resistant amino acid permeases of Escherichia coli. J Biol Chem. 1974 Dec 25;249(24):7747–7755. [PubMed] [Google Scholar]
- Blaurock A. E. Bacteriorhodospin: a trans-membrane pump containing alpha-helix. J Mol Biol. 1975 Apr 5;93(2):139–158. doi: 10.1016/0022-2836(75)90124-2. [DOI] [PubMed] [Google Scholar]
- Blaurock A. E., Stoeckenius W. Structure of the purple membrane. Nat New Biol. 1971 Sep 29;233(39):152–155. doi: 10.1038/newbio233152a0. [DOI] [PubMed] [Google Scholar]
- Bogomolni R. A. Light energy conservation processes in Halobacterium halobium cells. Fed Proc. 1977 May;36(6):1833–1839. [PubMed] [Google Scholar]
- Bogomolni R. A., Stubbs L., Lanyi J. K. Illumination-dependent changes in the intrinsic fluorescence of bacteriorhodopsin. Biochemistry. 1978 Mar 21;17(6):1037–1041. doi: 10.1021/bi00599a015. [DOI] [PubMed] [Google Scholar]
- Boos W. Bacterial transport. Annu Rev Biochem. 1974;43(0):123–146. doi: 10.1146/annurev.bi.43.070174.001011. [DOI] [PubMed] [Google Scholar]
- Bridgen J., Walker I. D. Photoreceptor protein from the purple membrane of Halobacterium halobium. Molecular weight and retinal binding site. Biochemistry. 1976 Feb 24;15(4):792–798. doi: 10.1021/bi00649a010. [DOI] [PubMed] [Google Scholar]
- Brown A. D., Pearce R. F. Preliminary fractionation by gel electrophoresis of the membrane proteins and lipoproteins of Halobacterium halobium. Can J Biochem. 1969 Sep;47(9):833–837. doi: 10.1139/o69-131. [DOI] [PubMed] [Google Scholar]
- CHRISTIAN J. H., INGRAM M. The freezing points of bacterial cells in relation to halophilism. J Gen Microbiol. 1959 Feb;20(1):27–31. doi: 10.1099/00221287-20-1-27. [DOI] [PubMed] [Google Scholar]
- CHRISTIAN J. H., WALTHO J. A. Solute concentrations within cells of halophilic and non-halophilic bacteria. Biochim Biophys Acta. 1962 Dec 17;65:506–508. doi: 10.1016/0006-3002(62)90453-5. [DOI] [PubMed] [Google Scholar]
- Chance B., Porte M., Hess B., Oesterhelt D. Low temperature kinetics of H+ changes of bacterial rhodopsin. Biophys J. 1975 Sep;15(9):913–917. doi: 10.1016/S0006-3495(75)85865-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah K. S. Properties of electron transport particles from Halobacterium cutirubrum. The respiratory chain system. Biochim Biophys Acta. 1969 Jun 24;180(2):320–333. doi: 10.1016/0005-2728(69)90117-0. [DOI] [PubMed] [Google Scholar]
- Cheah K. S. Properties of the membrane-bound respiratory chain system of Halobacterium salinarium. Biochim Biophys Acta. 1970 Aug 4;216(1):43–53. doi: 10.1016/0005-2728(70)90157-x. [DOI] [PubMed] [Google Scholar]
- Chignell C. F., Chignell D. A. A spin label study of purple membranes from Halobacterium halobium. Biochem Biophys Res Commun. 1975 Jan 6;62(1):136–143. doi: 10.1016/s0006-291x(75)80415-3. [DOI] [PubMed] [Google Scholar]
- Chu Kung M., DeVault D., Hess B., Oesterhelt D. Photolysis of bacterial rhodopsin. Biophys J. 1975 Sep;15(9):907–911. doi: 10.1016/S0006-3495(75)85864-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane R. K. Na+ -dependent transport in the intestine and other animal tissues. Fed Proc. 1965 Sep-Oct;24(5):1000–1006. [PubMed] [Google Scholar]
- Danon A., Brith-Lindner M., Caplan S. R. Biogenesis of the purple membrane of Halobacterium halobium. Biophys Struct Mech. 1977 Apr 21;3(1):1–17. doi: 10.1007/BF00536449. [DOI] [PubMed] [Google Scholar]
- Danon A., Caplan S. R. CO2 fixation by Halobacterium halobium. FEBS Lett. 1977 Mar 1;74(2):255–258. doi: 10.1016/0014-5793(77)80858-2. [DOI] [PubMed] [Google Scholar]
- Danon A., Caplan S. R. Stimulation of ATP synthesis in Halobacterium halobium R1 by light-induced or artifically created proton electrochemical potential gradients across the cell membrane. Biochim Biophys Acta. 1976 Jan 15;423(1):133–140. doi: 10.1016/0005-2728(76)90107-9. [DOI] [PubMed] [Google Scholar]
- Danon A., Stoeckenius W. Photophosphorylation in Halobacterium halobium. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1234–1238. doi: 10.1073/pnas.71.4.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dencher N., Wilms M. Flash photometric experiments on the photochemical cycle of bacteriorhodopsin. Biophys Struct Mech. 1975 May 30;1(3):259–271. doi: 10.1007/BF00535760. [DOI] [PubMed] [Google Scholar]
- Drachev L. A., Frolov V. N., Kaulen A. D., Liberman E. A., Ostroumov S. A., Plakunova V. G., Semenov A. Y., Skulachev V. P. Reconstitution of Biological Molecular generators of electric current. Bacteriorhodopsin. J Biol Chem. 1976 Nov 25;251(22):7059–7065. [PubMed] [Google Scholar]
- Drachev L. A., Jasaitis A. A., Kaulen A. D., Kondrashin A. A., Liberman E. A., Nemecek I. B., Ostroumov S. A., Semenov AYu, Skulachev V. P. Direct measurement of electric current generation by cytochrome oxidase, H+-ATPase and bacteriorhodopsin. Nature. 1974 May 24;249(455):321–324. doi: 10.1038/249321a0. [DOI] [PubMed] [Google Scholar]
- Drachev L. A., Kaulen A. D., Ostroumov S. A., Skulachev V. P. Electrogenesis by bacteriorhodopsin incorporated in a planar phospholipid membrane. FEBS Lett. 1974 Feb 1;39(1):43–45. doi: 10.1016/0014-5793(74)80012-8. [DOI] [PubMed] [Google Scholar]
- Dundas I. E. Physiology of halobacteriaceae. Adv Microb Physiol. 1977;15:85–120. doi: 10.1016/s0065-2911(08)60315-x. [DOI] [PubMed] [Google Scholar]
- Eisenbach M., Bakker E. P., Korenstein R., Caplan S. R. Bacteriorhodopsin: biphasic kinetics of phototransients and of light-induced proton transfer by sub-bacterial Halobacterium halobium particles and by reconstituted liposomes. FEBS Lett. 1976 Dec 1;71(2):228–232. doi: 10.1016/0014-5793(76)80938-6. [DOI] [PubMed] [Google Scholar]
- Eisenbach M., Cooper S., Garty H., Johnstone R. M., Rottenberg H., Caplan S. R. Light-driven sodium transport in sub-bacterial particles of Halobacterium halobium. Biochim Biophys Acta. 1977 Mar 17;465(3):599–613. doi: 10.1016/0005-2736(77)90276-0. [DOI] [PubMed] [Google Scholar]
- Englander J. J., Englander S. W. Comparison of bacterial and animal rhodopsins by hydrogen exchange studies. Nature. 1977 Feb 17;265(5595):658–659. doi: 10.1038/265658a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher K. A., Stoeckenius W. Freeze-fractured purple membrane particles: protein content. Science. 1977 Jul 1;197(4298):72–74. doi: 10.1126/science.867052. [DOI] [PubMed] [Google Scholar]
- Garty H., Caplan S. R. Light-depending rubidium transport in intact Halobacterium halobium cells. Biochim Biophys Acta. 1977 Mar 11;459(3):532–545. doi: 10.1016/0005-2728(77)90052-4. [DOI] [PubMed] [Google Scholar]
- Garty H., Klemperer G., Eisenbach M., Caplan S. R. The direction of light-induced pH changes in purple membrane suspensions. Influence of pH and temperature. FEBS Lett. 1977 Sep 15;81(2):238–242. doi: 10.1016/0014-5793(77)80526-7. [DOI] [PubMed] [Google Scholar]
- Ginzburg M., Ginzburg B. Z. Factors influencing the retention of K in a Halobacterium. Biomembranes. 1975;7:219–251. doi: 10.1007/978-1-4684-7668-2_8. [DOI] [PubMed] [Google Scholar]
- Ginzburg M., Ginzburg B. Z. Regulation of cell volume and ion concentrations in a Halobacterium. J Membr Biol. 1976 Mar 18;26(2-3):153–171. doi: 10.1007/BF01868871. [DOI] [PubMed] [Google Scholar]
- Ginzburg M., Sachs L., Ginzburg B. Z. Ion metabolism in a Halobacterium. I. Influence of age of culture on intracellular concentrations. J Gen Physiol. 1970 Feb;55(2):187–207. doi: 10.1085/jgp.55.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginzburg M. The unusual membrane permeability of two halophilic unicellular organisms. Biochim Biophys Acta. 1969 Apr;173(3):370–376. doi: 10.1016/0005-2736(69)90002-9. [DOI] [PubMed] [Google Scholar]
- Gochnauer M. B., Kushner D. J. Growth and nutrition of extremely halophilic bacteria. Can J Microbiol. 1969 Oct;15(10):1157–1165. doi: 10.1139/m69-211. [DOI] [PubMed] [Google Scholar]
- Gochnauer M. B., Kushner D. J. Potassium binding, growth, and survival of an extremely halophilic bacterium. Can J Microbiol. 1971 Jan;17(1):17–23. doi: 10.1139/m71-004. [DOI] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann R., Oesterhelt D. Bacteriorhodopsin-mediated photophosphorylation in Halobacterium halobium. Eur J Biochem. 1977 Jul 15;77(2):325–335. doi: 10.1111/j.1432-1033.1977.tb11671.x. [DOI] [PubMed] [Google Scholar]
- Helgerson S. L., Lanyi J. K. Methionine transport in Halobacterium halobium vesicles: noncompetitive, asymmetric inhibition by L-cysteine. Biochemistry. 1978 Mar 21;17(6):1042–1046. doi: 10.1021/bi00599a016. [DOI] [PubMed] [Google Scholar]
- Henderson R. The structure of the purple membrane from Halobacterium hallobium: analysis of the X-ray diffraction pattern. J Mol Biol. 1975 Apr 5;93(2):123–138. doi: 10.1016/0022-2836(75)90123-0. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Herrmann T. R., Rayfield G. W. A measurement of the proton pump current generated by bacteriorhodopsin in black lipid membranes. Biochim Biophys Acta. 1976 Sep 7;443(3):623–628. doi: 10.1016/0005-2736(76)90482-x. [DOI] [PubMed] [Google Scholar]
- Hess B., Kuschmitz D. The photochemical reaction of the 412 nm chromophore of bacteriorhodopsin. FEBS Lett. 1977 Feb 15;74(1):29–24. doi: 10.1016/0014-5793(77)80743-6. [DOI] [PubMed] [Google Scholar]
- Heyn M. P., Bauer P. J., Dencher N. A. A natural CD label to probe the structure of the purple membrane from Halobacterium halobium by means of exciton coupling effects. Biochem Biophys Res Commun. 1975 Dec 1;67(3):897–903. doi: 10.1016/0006-291x(75)90761-5. [DOI] [PubMed] [Google Scholar]
- Heyn M. P., Cherry R. J., Müller U. Transient and linear dichroism studies on bacteriorhodopsin: determination of the orientation of the 568 nm all-trans retinal chromophore. J Mol Biol. 1977 Dec 15;117(3):607–620. doi: 10.1016/0022-2836(77)90060-2. [DOI] [PubMed] [Google Scholar]
- Hildebrand E., Dencher N. Two photosystems controlling behavioural responses of Halobacterium halobium. Nature. 1975 Sep 4;257(5521):46–48. doi: 10.1038/257046a0. [DOI] [PubMed] [Google Scholar]
- Hildebrand E. What does Halobacterium tell us about photoreception? Biophys Struct Mech. 1977 Apr 21;3(1):69–77. doi: 10.1007/BF00536457. [DOI] [PubMed] [Google Scholar]
- Hirata H., Sone N., Yoshida M., Kagawa Y. Solubilization and partial purification of alanine carrier from membranes of a thermophilic bacterium and its reconstitution into functional vesicles. Biochem Biophys Res Commun. 1976 Apr 5;69(3):665–671. doi: 10.1016/0006-291x(76)90927-x. [DOI] [PubMed] [Google Scholar]
- Hochstein L. I., Dalton B. P. Studies of a halophilic NADH dehydrogenase. I. Purification and properties of the enzyme. Biochim Biophys Acta. 1973 Apr 12;302(2):216–228. doi: 10.1016/0005-2744(73)90150-2. [DOI] [PubMed] [Google Scholar]
- Hochstein L. I. Studies of a halophilic NADH dehydrogenase. II. Kinetic properties of the enzyme in relation to salt activation. Biochim Biophys Acta. 1975 Sep 22;403(1):58–66. doi: 10.1016/0005-2744(75)90008-x. [DOI] [PubMed] [Google Scholar]
- Hubbard J. S., Rinehart C. A. Bacteriorhodopsin formation in Halobacterium halobium. Can J Microbiol. 1976 Sep;22(9):1274–1281. doi: 10.1139/m76-189. [DOI] [PubMed] [Google Scholar]
- Hubbard J. S., Rinehart C. A., Baker R. A. Energy coupling in the active transport of amino acids by bacteriohodopsin-containing cells of Halobacterium holobium. J Bacteriol. 1976 Jan;125(1):181–190. doi: 10.1128/jb.125.1.181-190.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S. B., Stoeckenius W. Purple membrane vesicles: morphology and proton translocation. J Membr Biol. 1977 May 12;33(3-4):325–350. doi: 10.1007/BF01869523. [DOI] [PubMed] [Google Scholar]
- Jan L. Y. The isomeric configuration of the bacteriorhodopsin chromophore. Vision Res. 1975 Oct;15:1081–1086. doi: 10.1016/0042-6989(75)90004-8. [DOI] [PubMed] [Google Scholar]
- KATES M., YENGOYAN L. S., SASTRY P. S. A DIETHER ANALOG OF PHOSPHATIDYL GLYCEROPHOSPHATE IN HALOBACTERIUM CUTIRUBRUM. Biochim Biophys Acta. 1965 Apr 5;98:252–268. doi: 10.1016/0005-2760(65)90119-0. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. Transport across isolated bacterial cytoplasmic membranes. Biochim Biophys Acta. 1972 Aug 4;265(3):367–416. doi: 10.1016/0304-4157(72)90014-7. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. Transport studies in bacterial membrane vesicles. Science. 1974 Dec 6;186(4167):882–892. doi: 10.1126/science.186.4167.882. [DOI] [PubMed] [Google Scholar]
- Kahane S., Marcus M., Barash H., Halpern Y. S. Sodium-dependent glutamate transport in membrane vesicles of Escherichia coli K-12. FEBS Lett. 1975 Aug 15;56(2):235–239. doi: 10.1016/0014-5793(75)81099-4. [DOI] [PubMed] [Google Scholar]
- Kanner B. I., Racker E. Light-dependent proton and rubidium translocation in membrane vesicles from Halobacterium halobium. Biochem Biophys Res Commun. 1975 Jan 2;64(3):1054–1061. doi: 10.1016/0006-291x(75)90154-0. [DOI] [PubMed] [Google Scholar]
- Kaufmann K. J., Rentzepis P. M., Stoeckenius W., Lewis A. Primary photochemical processes in bacteriorhodopsin. Biochem Biophys Res Commun. 1976 Feb 23;68(4):1109–1115. doi: 10.1016/0006-291x(76)90310-7. [DOI] [PubMed] [Google Scholar]
- Kayushin L. P., Skulachev V. P. Bacteriorhodopsin as an electrogenic proton pump: reconstitution of bacteriorhodopsin proteoliposomes generating delta psi and delta pH. FEBS Lett. 1974 Feb 1;39(1):39–42. doi: 10.1016/0014-5793(74)80011-6. [DOI] [PubMed] [Google Scholar]
- Kelly M., Norgård S., Liaaen-Jensen S. Bacterial carotenoids. 31. C50-carotenoids 5. Carotenoids of Halobacterium salinarium, especially bacterioruberin. Acta Chem Scand. 1970;24(6):2169–2182. doi: 10.3891/acta.chem.scand.24-2169. [DOI] [PubMed] [Google Scholar]
- Konishi T., Packer L. Chemical modification of bacteriorhodopsin with N-bromosuccinimide. FEBS Lett. 1977 Jul 15;79(2):369–373. doi: 10.1016/0014-5793(77)80823-5. [DOI] [PubMed] [Google Scholar]
- Konishi T., Packer L. Hydrogen exchange of dark-adapted and illuminated bacteriorhodopsin. FEBS Lett. 1977 Aug 15;80(2):455–458. doi: 10.1016/0014-5793(77)80496-1. [DOI] [PubMed] [Google Scholar]
- Kramer J. K., Kushwaha S. C., Kates M. Structure ditermination of the squalene, dihydrosqualene and tetrahydrosqualene in Halobacterium cutirubrum. Biochim Biophys Acta. 1972 May 23;270(1):103–110. doi: 10.1016/0005-2760(72)90183-x. [DOI] [PubMed] [Google Scholar]
- Kushner D. J. Lysis and dissolution of cells and envelopes of an extremely halophilic bacterium. J Bacteriol. 1964 May;87(5):1147–1156. doi: 10.1128/jb.87.5.1147-1156.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner D. J., Onishi H. Contribution of protein and lipid components to the salt response of envelopes of an extremely halophilic bacterium. J Bacteriol. 1966 Feb;91(2):653–660. doi: 10.1128/jb.91.2.653-660.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushwaha S. C., Gochnauer M. B., Kushner D. J., Kates M. Pigments and isoprenoid compounds in extremely and moderately halophilic bacteria. Can J Microbiol. 1974 Feb;20(2):241–245. doi: 10.1139/m74-038. [DOI] [PubMed] [Google Scholar]
- Kushwaha S. C., Kates M. Isolation and identification of "bacteriorhodopsin" and minor C40-carotenoids in Halobacterium cutirubrum. Biochim Biophys Acta. 1973 Aug 23;316(2):235–243. doi: 10.1016/0005-2760(73)90013-1. [DOI] [PubMed] [Google Scholar]
- Kushwaha S. C., Kates M., Martin W. G. Characterization and composition of the purple and red membrane from Halobacterium cutirubrum;. Can J Biochem. 1975 Mar;53(3):284–292. doi: 10.1139/o75-040. [DOI] [PubMed] [Google Scholar]
- Kushwaha S. C., Kramer J. K., Kates M. Isolation and characterization of C50-carotenoid pigments and other polar isoprenoids from Halobacterium cutirubrum. Biochim Biophys Acta. 1975 Aug 25;398(2):303–314. doi: 10.1016/0005-2760(75)90146-0. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K., Hilliker K. Passive potassium ion permeability of Halobacterium halobium cell envelope membranes. Biochim Biophys Acta. 1976 Sep 21;448(1):181–184. doi: 10.1016/0005-2736(76)90086-9. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K., MacDonald R. E. Existence of electrogenic hydrogen ion/sodium ion antiport in Halobacterium halobium cell envelope vesicles. Biochemistry. 1976 Oct 19;15(21):4608–4614. doi: 10.1021/bi00666a010. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K., MacDonald R. E. Light-dependent cation gradients and electrical potential in Halobacterium halobium cell envelope vesicles. Fed Proc. 1977 May;36(6):1824–1827. [PubMed] [Google Scholar]
- Lanyi J. K., Plachy W. Z., Kates M. Lipid interactions in membranes of extremely halophilic bacteria. II. Modification of the bilayer structure by squalene. Biochemistry. 1974 Nov 19;13(24):4914–4920. doi: 10.1021/bi00721a006. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K., Renthal R., MacDonald R. E. Light-induced glutamate transport in Halobacterium halobium envelope vesicles. II. Evidence that the driving force is a light-dependent sodium gradient. Biochemistry. 1976 Apr 20;15(8):1603–1610. doi: 10.1021/bi00653a002. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K. Salt-dependent properties of proteins from extremely halophilic bacteria. Bacteriol Rev. 1974 Sep;38(3):272–290. doi: 10.1128/br.38.3.272-290.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanyi J. K., Silverman M. P. The state of binding of intracellular K + in Halobacterium cutirubrum. Can J Microbiol. 1972 Jul;18(7):993–995. doi: 10.1139/m72-154. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K., Stevenson J. Studies of the electron transport chain of extremely halophilic bacteria. IV. Role of hydrophobic forces in the structure of menadione reductase. J Biol Chem. 1970 Aug 25;245(16):4074–4080. [PubMed] [Google Scholar]
- Lanyi J. K. Studies of the electron transport chain of extremely halophilic bacteria. 3. Mechanism of the effect of salt on menadione reductase. J Biol Chem. 1969 Aug 10;244(15):4168–4173. [PubMed] [Google Scholar]
- Lanyi J. K. Studies of the electron transport chain of extremely halophilic bacteria. I. Spectrophotometric identification of the cytochromes of Halobacterium cutirubrum. Arch Biochem Biophys. 1968 Dec;128(3):716–724. doi: 10.1016/0003-9861(68)90080-5. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K. Studies of the electron transport chain of extremely halophilic bacteria. II. Salt dependence of reduced diphosphopyridine nucleotide oxidase. J Biol Chem. 1969 Jun 10;244(11):2864–2869. [PubMed] [Google Scholar]
- Lanyi J. K. Studies of the electron transport chain of extremely halophilic bacteria. VII. Solubilization properties of menadione reductase. J Biol Chem. 1972 May 25;247(10):3001–3007. [PubMed] [Google Scholar]
- Lanyi J. K., Yearwood-Drayton V., MacDonald R. E. Light-induced glutamate transport in Halobacterium halobium envelope vesicles. I. Kinetics of the light-dependent and the sodium-gradient-dependent uptake. Biochemistry. 1976 Apr 20;15(8):1595–1603. doi: 10.1021/bi00653a001. [DOI] [PubMed] [Google Scholar]
- Lewis A., Spoonhower J., Bogomolni R. A., Lozier R. H., Stoeckenius W. Tunable laser resonance raman spectroscopy of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4462–4466. doi: 10.1073/pnas.71.11.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M. M., Lanyi J. K. Studies of the electron transport chain of extremely halophilic bacteria. V. Mode of action of salts on cytochrome oxidase. Biochim Biophys Acta. 1971 Aug 6;245(1):21–33. doi: 10.1016/0005-2728(71)90004-1. [DOI] [PubMed] [Google Scholar]
- Lieberman M. M., Lanyi J. K. Threonine deaminase from extremely halophilic bacteria. Cooperative substrate kinetics and salt dependence. Biochemistry. 1972 Jan 18;11(2):211–216. doi: 10.1021/bi00752a011. [DOI] [PubMed] [Google Scholar]
- Litvin F. F., Boichenko V. A., Balashov S. P., Dubrovakii V. T. Fotoindutsirovannoe ingibirovanie i stimulirovanie dykhaniia v kletkakh Halobacterium halobiums: kinetika, spektry deistviia, sviaz' s fotoinduktsiei delapH. Biofizika. 1977 Nov-Dec;22(6):1062–1071. [PubMed] [Google Scholar]
- Long M. M., Urry D. W., Stoeckenius W. Circular dichroism of biological membranes: purple membrane of Halobacterium halobium. Biochem Biophys Res Commun. 1977 Apr 11;75(3):725–731. doi: 10.1016/0006-291x(77)91532-7. [DOI] [PubMed] [Google Scholar]
- Lozier R. H., Bogomolni R. A., Stoeckenius W. Bacteriorhodopsin: a light-driven proton pump in Halobacterium Halobium. Biophys J. 1975 Sep;15(9):955–962. doi: 10.1016/S0006-3495(75)85875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozier R. H., Niederberger W., Bogomolni R. A., Hwang S., Stoeckenius W. Kinetics and stoichiometry of light-induced proton release and uptake from purple membrane fragments, Halobacterium halobium cell envelopes, and phospholipid vesicles containing oriented purple membrane. Biochim Biophys Acta. 1976 Sep 13;440(3):545–556. doi: 10.1016/0005-2728(76)90041-4. [DOI] [PubMed] [Google Scholar]
- Lozier R. H., Niederberger W. The photochemical cycle of bacteriorhodopsin. Fed Proc. 1977 May;36(6):1805–1809. [PubMed] [Google Scholar]
- MacDonald R. E., Greene R. V., Lanyi J. K. Light-activated amino acid transport systems in Halobacterium halobium envelope vesicles: role of chemical and electrical gradients. Biochemistry. 1977 Jul 12;16(14):3227–3235. doi: 10.1021/bi00633a029. [DOI] [PubMed] [Google Scholar]
- MacDonald R. E., Lanyi J. K., Greene R. V. Sodium-stimulated glutamate uptake in membrane vesicles of Escherichia coli: the role of ion gradients. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3167–3170. doi: 10.1073/pnas.74.8.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald R. E., Lanyi J. K. Light-activated amino acid transport in Halobacterium halobium envelope vesicles. Fed Proc. 1977 May;36(6):1828–1832. [PubMed] [Google Scholar]
- MacDonald R. E., Lanyi L. K. Light-induced leucine transport in Halobacterium halobium envelope vesicles: a chemiosmotic system. Biochemistry. 1975 Jul;14(13):2882–2889. doi: 10.1021/bi00684a014. [DOI] [PubMed] [Google Scholar]
- Maloney P. C., Kashket E. R., Wilson T. H. A protonmotive force drives ATP synthesis in bacteria. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3896–3900. doi: 10.1073/pnas.71.10.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney P. C., Wilson T. H. ATP synthesis driven by a protonmotive force in Streptococcus lactis. J Membr Biol. 1975;25(3-4):285–310. doi: 10.1007/BF01868580. [DOI] [PubMed] [Google Scholar]
- Marshall C. L., Wicken A. J., Brown A. D. The outer layer of the cell envelope of Halobacterium halobium. Can J Biochem. 1969 Jan;47(1):71–74. doi: 10.1139/o69-013. [DOI] [PubMed] [Google Scholar]
- Matsuno-Yagi A., Mukohata Y. Two possible roles of bacteriorhodopsin; a comparative study of strains of Halobacterium halobium differing in pigmentation. Biochem Biophys Res Commun. 1977 Sep 9;78(1):237–243. doi: 10.1016/0006-291x(77)91245-1. [DOI] [PubMed] [Google Scholar]
- Mescher M. F., Strominger J. L. Purification and characterization of a prokaryotic glucoprotein from the cell envelope of Halobacterium salinarium. J Biol Chem. 1976 Apr 10;251(7):2005–2014. [PubMed] [Google Scholar]
- Mescher M. F., Strominger J. L., Watson S. W. Protein and carbohydrate composition of the cell envelope of Halobacterium salinarium. J Bacteriol. 1974 Nov;120(2):945–954. doi: 10.1128/jb.120.2.945-954.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel H., Oesterhelt D. Light-induced changes of the pH gradient and the membrane potential in H. halobium. FEBS Lett. 1976 Jun 1;65(2):175–178. doi: 10.1016/0014-5793(76)80473-5. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in energy transduction: a logical development of biochemical knowledge. J Bioenerg. 1972 May;3(1):5–24. doi: 10.1007/BF01515993. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D. Bacteriorhodopsin as an example of a light-driven proton pump. Angew Chem Int Ed Engl. 1976 Jan;15(1):17–24. doi: 10.1002/anie.197600171. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D. Die Purpurmembran aus Holobacterium halobium. Hoppe Seylers Z Physiol Chem. 1972 Oct;353(10):1554–1555. [PubMed] [Google Scholar]
- Oesterhelt D., Hess B. Reversible photolysis of the purple complex in the purple membrane of Halobacterium halobium. Eur J Biochem. 1973 Aug 17;37(2):316–326. doi: 10.1111/j.1432-1033.1973.tb02990.x. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Krippahl G. Light inhibition of respiration in Halobacterium halobium. FEBS Lett. 1973 Oct 1;36(1):72–76. doi: 10.1016/0014-5793(73)80339-4. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Meentzen M., Schuhmann L. Reversible dissociation of the purple complex in bacteriorhodopsin and identification of 13-cis and all-trans-retinal as its chromophores. Eur J Biochem. 1973 Dec 17;40(2):453–463. doi: 10.1111/j.1432-1033.1973.tb03214.x. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Functions of a new photoreceptor membrane. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2853–2857. doi: 10.1073/pnas.70.10.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971 Sep 29;233(39):149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D. The purple membrane of Halobacterium halobium: a new system for light energy conversion. Ciba Found Symp. 1975;(31):147–167. doi: 10.1002/9780470720134.ch9. [DOI] [PubMed] [Google Scholar]
- Ohno K., Takeuchi Y., Yoshida M. Effect of light-adaptation on the photoreaction of bacteriorhodopsin from Halobacterium halobium. Biochim Biophys Acta. 1977 Dec 23;462(3):575–582. doi: 10.1016/0005-2728(77)90102-5. [DOI] [PubMed] [Google Scholar]
- Onishi H., Kushner D. J. Mechanism of dissolution of envelopes of the extreme halophile Halobacterium cutirubrum. J Bacteriol. 1966 Feb;91(2):646–652. doi: 10.1128/jb.91.2.646-652.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikov Y. A., Abdulaev N. G., Feigina M. Y., Kiselev A. V., Lobanov N. A. Recent findings in the structure-functional characteristics of bacteriorhodopsin. FEBS Lett. 1977 Dec 1;84(1):1–4. doi: 10.1016/0014-5793(77)81046-6. [DOI] [PubMed] [Google Scholar]
- Pettei M. J., Yudd A. P., Nakanishi K., Henselman R., Stoeckenius W. Identification of retinal isomers isolated from bacteriorhodopsin. Biochemistry. 1977 May 3;16(9):1955–1959. doi: 10.1021/bi00628a031. [DOI] [PubMed] [Google Scholar]
- Plachy W. Z., Lanyi J. K., Kates M. Lipid interactions in membranes of extremely halophilic bacteria. I. Electron spin resonance and dilatometric studies of bilayer structure. Biochemistry. 1974 Nov 19;13(24):4906–4913. doi: 10.1021/bi00721a005. [DOI] [PubMed] [Google Scholar]
- Racker E. A new procedure for the reconstitution of biologically active phospholipid vesicles. Biochem Biophys Res Commun. 1973 Nov 1;55(1):224–230. doi: 10.1016/s0006-291x(73)80083-x. [DOI] [PubMed] [Google Scholar]
- Racker E., Hinkle P. C. Effect of temperature on the function of a proton pump. J Membr Biol. 1974;17(2):181–188. doi: 10.1007/BF01870178. [DOI] [PubMed] [Google Scholar]
- Racker E., Stoeckenius W. Reconstitution of purple membrane vesicles catalyzing light-driven proton uptake and adenosine triphosphate formation. J Biol Chem. 1974 Jan 25;249(2):662–663. [PubMed] [Google Scholar]
- Ramos S., Schuldiner S., Kaback H. R. The electrochemical gradient of protons and its relationship to active transport in Escherichia coli membrane vesicles. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1892–1896. doi: 10.1073/pnas.73.6.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razi Naqvi K., Gonzalez-Rodriguez J., Cherry R. J., Chapman D. Spectroscopic technique for studying protein rotation in membranes. Nat New Biol. 1973 Oct 24;245(147):249–251. doi: 10.1038/newbio245249a0. [DOI] [PubMed] [Google Scholar]
- Renthal R., Lanyi J. K. Light-induced membrane potential and pH gradient in Halobacterium halobium envelope vesicles. Biochemistry. 1976 May 18;15(10):2136–2143. doi: 10.1021/bi00655a017. [DOI] [PubMed] [Google Scholar]
- Ring K., Ehle H., Foit B. Effect of alkali ions on the active transport of neutral amino acids into Streptomyces hydrogenans. Biochim Biophys Acta. 1976 May 21;433(3):615–629. doi: 10.1016/0005-2736(76)90285-6. [DOI] [PubMed] [Google Scholar]
- Ryrie I. J., Blackmore P. F. Energy-linked activities in reconstituted yeast adenosine triphosphatase proteoliposome. Adenosine triphosphate formation coupled with electron flow between ascorbate and ferricyanide. Arch Biochem Biophys. 1976 Sep;176(1):127–135. doi: 10.1016/0003-9861(76)90148-x. [DOI] [PubMed] [Google Scholar]
- Schuldiner S., Kaback H. R. Membrane potential and active transport in membrane vesicles from Escherichia coli. Biochemistry. 1975 Dec 16;14(25):5451–5461. doi: 10.1021/bi00696a011. [DOI] [PubMed] [Google Scholar]
- Schultz S. G., Curran P. F. Coupled transport of sodium and organic solutes. Physiol Rev. 1970 Oct;50(4):637–718. doi: 10.1152/physrev.1970.50.4.637. [DOI] [PubMed] [Google Scholar]
- Sherman W. V., Korenstein R., Caplan S. R. Energetics and chronology of phototransients in the light response of the purple membrane of Halobacterium halobium. Biochim Biophys Acta. 1976 Jun 8;430(3):454–458. doi: 10.1016/0005-2728(76)90021-9. [DOI] [PubMed] [Google Scholar]
- Sherman W. V., Slifkin M. A., Caplan S. R. Kinetic studies of phototransients in bacteriorhodopsin. Biochim Biophys Acta. 1976 Feb 16;423(2):238–248. doi: 10.1016/0005-2728(76)90182-1. [DOI] [PubMed] [Google Scholar]
- Simoni R. D., Postma P. W. The energetics of bacterial active transport. Annu Rev Biochem. 1975;44:523–554. doi: 10.1146/annurev.bi.44.070175.002515. [DOI] [PubMed] [Google Scholar]
- Sims P. J., Waggoner A. S., Wang C. H., Hoffman J. F. Studies on the mechanism by which cyanine dyes measure membrane potential in red blood cells and phosphatidylcholine vesicles. Biochemistry. 1974 Jul 30;13(16):3315–3330. doi: 10.1021/bi00713a022. [DOI] [PubMed] [Google Scholar]
- Slifkin M. A., Caplan S. R. Modulation excitation spectro-photometry of purple membrane of Halobacterium halobium. Nature. 1975 Jan 3;253(5486):56–58. doi: 10.1038/253056a0. [DOI] [PubMed] [Google Scholar]
- Sperling W., Carl P., Rafferty Ch, Dencher N. A. Photochemistry and dark equilibrium of retinal isomers and bacteriorhodopsin isomers. Biophys Struct Mech. 1977 Jun 29;3(2):79–94. doi: 10.1007/BF00535798. [DOI] [PubMed] [Google Scholar]
- Stock J., Roseman S. A sodium-dependent sugar co-transport system in bacteria. Biochem Biophys Res Commun. 1971 Jul 2;44(1):132–138. doi: 10.1016/s0006-291x(71)80168-7. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Rowen R. A morphological study of Halobacterium halobium and its lysis in media of low salt concentration. J Cell Biol. 1967 Jul;34(1):365–393. doi: 10.1083/jcb.34.1.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumper M., Herrmann G. Biosynthesis of purple membrane: control of retinal synthesis by bacterio-opsin. FEBS Lett. 1976 Dec 1;71(2):333–336. doi: 10.1016/0014-5793(76)80964-7. [DOI] [PubMed] [Google Scholar]
- Thompson J., MacLeod R. A. Functions of Na+ and K+ in the active transport of -aminoisobutyric acid in a marine pseudomonad. J Biol Chem. 1971 Jun 25;246(12):4066–4074. [PubMed] [Google Scholar]
- Thompson J., MacLeod R. A. Potassium transport and the relationship between intracellular potassium concentration and amino acid uptake by cells of a marine pseudomonad. J Bacteriol. 1974 Nov;120(2):598–603. doi: 10.1128/jb.120.2.598-603.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toeckenius W., Kunau W. H. Further characterization of particulate fractions from lysed cell envelopes of Halobacterium halobium and isolation of gas vacuole membranes. J Cell Biol. 1968 Aug;38(2):337–357. doi: 10.1083/jcb.38.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda H., Kaback H. R. Sodium-dependent methyl 1-thio-beta-D-galactopyranoside transport in membrane vesicles isolated from Salmonella typhimurium. Biochemistry. 1977 May 17;16(10):2130–2136. doi: 10.1021/bi00629a013. [DOI] [PubMed] [Google Scholar]
- Tomlinson G. A., Hochstein L. I. Halobacterium saccharovorum sp. nov., a carbohydrate-metabolizing, extremely halophilic bacterium. Can J Microbiol. 1976 Apr;22(4):587–591. doi: 10.1139/m76-087. [DOI] [PubMed] [Google Scholar]
- Tornabene T. G., Kates M., Gelpi E., Oro J. Occurrence of squalene, di- and tetrahydrosqualenes, and vitamin MK8 in an extremely halophilic bacterium, Halobacterium cutirubrun. J Lipid Res. 1969 May;10(3):294–303. [PubMed] [Google Scholar]
- Tsuchiya T., Hasan S. M., Raven J. Glutamate transport driven by an electrochemical gradient of sodium ions in Escherichia coli. J Bacteriol. 1977 Sep;131(3):848–853. doi: 10.1128/jb.131.3.848-853.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya T., Raven J., Wilson T. H. Co-transport of Na+ and methul-beta-D-thiogalactopyranoside mediated by the melibiose transport system of Escherichia coli. Biochem Biophys Res Commun. 1977 May 9;76(1):26–31. doi: 10.1016/0006-291x(77)91663-1. [DOI] [PubMed] [Google Scholar]
- Werber M. M., Mevarech M. Induction of a dissimilatory reduction pathway of nitrate in Halobacterium of the Dead Sea. A possible role for the 2 Fe-ferredoxin isolated from this organism. Arch Biochem Biophys. 1978 Feb;186(1):60–65. doi: 10.1016/0003-9861(78)90463-0. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Sone N., Hirata H., Kagawa Y. ATP synthesis catalyzed by purified DCCD-sensitive ATPase incorporated into reconstituted purple membrane vesicles. Biochem Biophys Res Commun. 1975 Dec 15;67(4):1295–1300. doi: 10.1016/0006-291x(75)90167-9. [DOI] [PubMed] [Google Scholar]


