Abstract
A short multi-gram process for the preparation of the analgesic compound SCP-123 (4) and its sodium salt has been developed.
Keywords: analgesic, acetaminophen, propacetamol, saccharin, hydrolysis, parenteral administration
Introduction
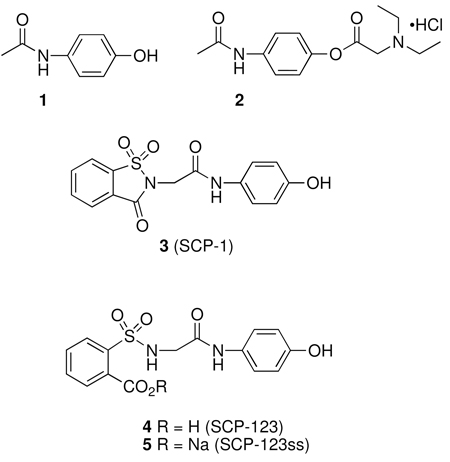
The analgesic acetaminophen (1) is widely used for the acute and chronic control of pain.1 While the therapeutic window of 1 is quite broad, the low water solubility is problematic for some delivery applications.2–4 A water-soluble analogue of 1 is the prodrug propacetamol hydrochloride (2).5,6 This form of acetaminophen is rapidly and completely hydrolyzed by plasma esterases to release 1.7 The pharmacological effects in clinical trials have shown that 2 possesses similar efficacy to 1, but due to its greater water-solubility can be parenterally administered and thus can be employed when oral administration is not possible.5,6
The recent discovery that the saccharin derivative of acetaminophen, SCP-1 (3) possesses analgesic properties of equal potency to acetaminophen with significantly diminished hepatotoxicity has prompted an extensive investigation into this class of compounds as a new generation of analgesic drugs.8–12 The lead compound 3, was found to possess an analgesic and antipyretic profile similar to 1.10–12 However, recent studies with 3 have shown that it is extensively and rapidly hydrolyzed in vivo.10 The metabolite SCP-123 (4) and corresponding sodium salt SCP-123ss (5) are equipotent on a molar basis with 3 in analgesic models. Presumably, the efficacy of 3 is derived from the hydrolysis product 4. Therefore, it was of interest to develop large-scale syntheses of 4 and 5 for further drug development studies.
Results and Discussion
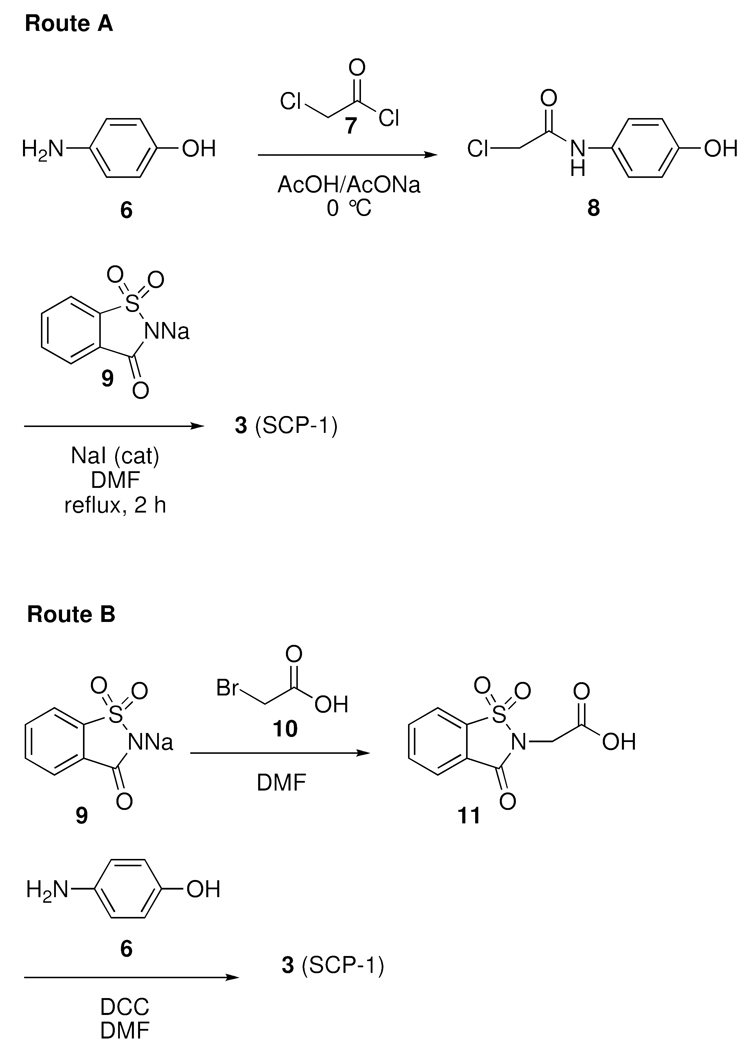
For the developmental studies, multi-gram quantities of 4 and 5 were required. Previous work with these compounds had revealed that the most efficient way to prepare the gram quantities of these metabolites was via the hydrolysis of the saccharin ring of 3.10 Therefore the design of a multi-gram synthesis focused on the initial preparation of 3, followed by the subsequent hydrolysis to afford either 4 or 5. Two synthetic routes have been established for the preparation of gram quantities of 3.8–10 As illustrated in Scheme 1, the two routes primarily differ in the sequence in which the saccharin moiety is added to the acetyl unit. In Route A,9,10 the saccharin moiety is added in the last step to the 2-chloroacetamide intermediate 8 that has been previously generated from 4-aminophenol (6) and 2-chloroacetyl chloride (7). This yields 3 via a two-step process. Alternatively in Route B,8 the intermediate acetic acid 11 is formed initially from sodium saccharin (9) and bromoacetic acid (10). The acid 11 is then coupled to 6 to furnish 3 also via a two-step process. Route A was deemed to be of greater merit due the low cost of the commercially available starting materials and that both the intermediate 8 and 3 could be obtained in a state of high purity (>95 %) by precipitation or recrystallization. Alternatively, Route B is potentially limited by the hygroscopic intermediate acid 11, which is difficult to handle, as well the multiple recrystallizations of 3 that are necessary to remove dicyclohexylurea present as a by-product from the coupling reaction.
Scheme 1.
Synthetic routes to SCP-1 (3).
Based upon our evaluation, Route A was scaled 15-fold and run on a mole scale. The 2-chloroacetyl chloride (7) was added at a controlled rate to a suspension of 6 in an acetate buffer at ≤ 5 °C. As the addition of the acid chloride progressed the reaction mixture became clear and the intermediate 2-chloroacetamide 8 began to crystallize. The 2-chloroacetamide intermediate 8 was obtained in 70% yield and no further purification was required for advancement to the next step. Preparation of 3 was routinely performed on a mole scale. The 2-chloroacetamide 8 and saccharin sodium salt (9) were heated to reflux in DMF with a catalytic amount of NaI. The saccharin derivative was then easily obtained by precipitation in ice water. A single recrystallization from ethanol/water furnished 3 in 72% yield.
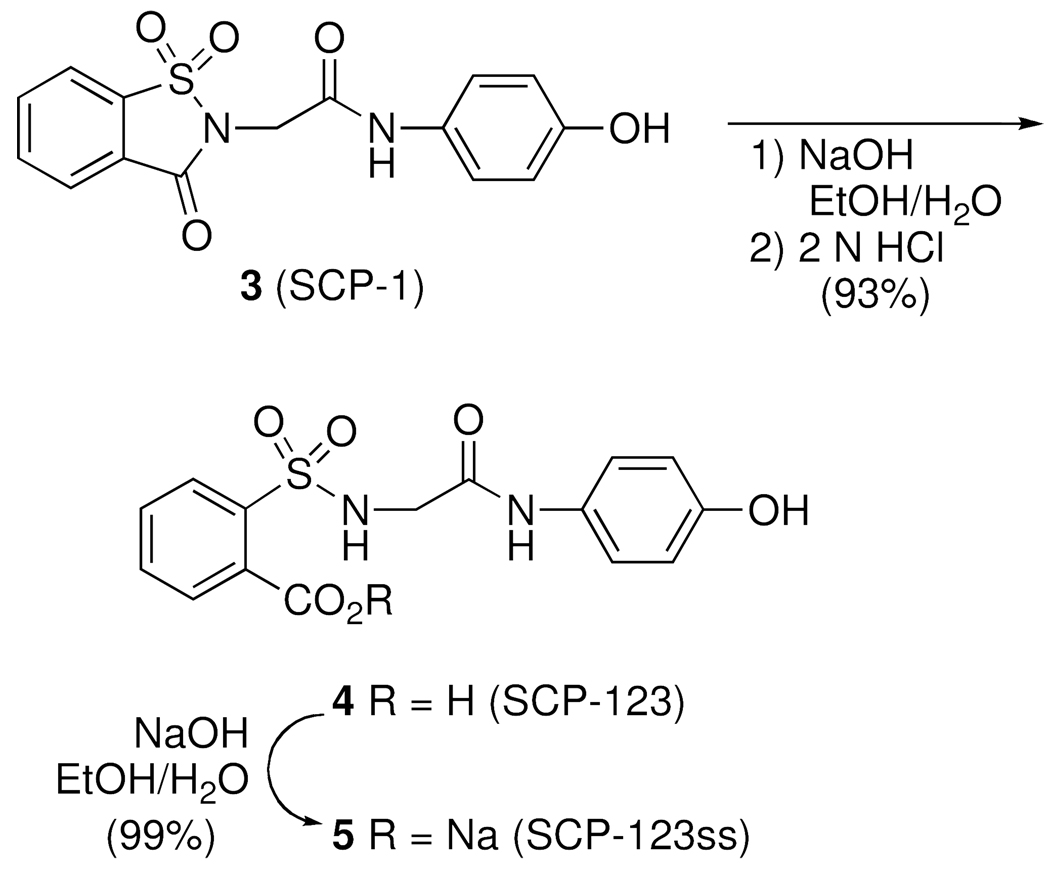
The hydrolysis of 3 was readily achieved with NaOH solution followed by treatment with 2 N HCl (Scheme 2).10,13 This afforded the corresponding acid 4 (SCP-123) in 93% yield.
Scheme 2.
Synthesis of SCP-123 (4) and SCP-123ss (5).
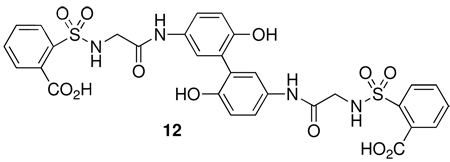
The hydrolysis step was found to be sensitive to the concentration of the saccharin derivative 3 in the basic solution. If the reaction mixture was not sufficiently dilute the formation of a side-product 12, that resulted from oxidative phenolic coupling, was obtained.14,15
This impurity 12 was present from 10–25%, depending upon the concentration of the reaction mixture relative to 3. The coupling product 12 was initially identified by LC/MS [m/z 698] but difficult to detect by NMR. However, 12 and could be clearly differentiated from 4 by HPLC. An optimized concentration of 3 in 0.5 N NaOH was determined to be 0.25 M. At this concentration, the hydrolysis of 3 proceeded cleanly and the oxidative-coupling product 12 was not observed. These conditions were preferred to using tedious degassing procedures and performing the reaction under anaerobic conditions. Due to the large reaction volumes at this concentration we were limited by our equipment and thus typically performed the hydrolysis on a 50-gram scale. The resultant hydrolysis product could be manipulated easily by precipitation with acid to give 4 in > 99% purity. Despite the smaller scale of the hydrolysis reaction, this step was typically executed in multiple simultaneous batches that could be combined to rapidly generate sub-kilogram quantities of 4. However, we have no evidence to suggest that this reaction is limited to this scale and could be performed on a larger scale if needed.
The preparation of the sodium salt 5 was achieved by titration of the acid 4 with one equivalent of NaOH (Scheme 2). The advantage of this procedure over the direct conversion of 3 into 5 was that the direct method gave an unquantifiable mixture of mono- and di-sodium salts due to the acidic phenol moiety. Alternatively, the titration of 4 with one equivalent of NaOH afforded the sodium carboxylate 5, which could be precipitated cleanly out of solution as the mono-sodium salt. Filtration and vacuum drying gave 5 as the monohydrate (5•H2O) in quantitative yield and high purity (> 99%) as determined by HPLC and combustion analysis. The monohydrate 5•H2O, albeit somewhat hygroscopic, was stable to extensive drying and gave consistent combustion analysis when stored in dry environment.
Conclusion
We have developed a multi-gram process for the preparation of 4 (SCP-123) and its sodium salt, 5•H2O. The overall yields for both 4 and 5•H2O were 47% and 46%, respectively. Both processes required no chromatography and the desired compounds 4 and 5•H2O were isolated in high purity (> 99%).
Experimental Section
General Methods
NMR were recorded on a Varian-400 MHz nuclear magnetic resonance spectrometer at ambient temperature in DMSO-d6. HPLC was used to monitor the purity of all intermediates using standard HPLC equipment with UV detection (254 nm) and data system. Separations were performed with a Waters Nova-Pak C18 (3.9 × 150 mm) steel analytical column. The mobile phases for isocratic and gradient separations were prepared using 0.01% TFA in water and 0.01% TFA in CH3CN.
N-(4-Hydroxyphenyl)-2-chloroacetamide (8)
4-Aminophenol (6, 150 g, 1.37 mol) was added to a saturated solution of sodium acetate (500 mL) followed by acetic acid (500 mL). The suspension was cooled 0 °C and the 2-chloroacetyl chloride (7, 155 g, 109 mL, 1.37 mol) was added portion-wise to the suspension at ≤ 5 °C. As the addition of 7 progressed the suspension dissipated and the mixture clarified. Prior to completion of the addition of 7, a white precipitate began to form. Upon completion of the addition, the heterogenous mixture was brought to 25 °C and stirred at room temperature for 2 h. The white precipitate was filtered, washed with distilled water solution (2 × 100 mL) and dried under vacuum to afford 177 g of 8 as a white solid (70% yield). mp 142–144 °C. 1H NMR: δ 4.17 (s, 2H), 6.70 (d, J = 8.8, 2H), 7.35 (d, J = 8.8, 2H), 9.26 (s, 1H), 10.02 (s, 1H). 13C NMR: δ 44.2, 115.9, 121.9, 130.7, 154.5, 164.6. Anal. Calcd. for C8H8ClNO2: C, 51.77; H, 4.34; N, 7.55. Found: C, 51.87; H, 4.31; N, 7.49.
SCP-1 (3)
2-Chloroacetamide (8, 326 g, 1.75 mol) and saccharin sodium salt hydrate 9 (433 g, 2.10 mol) were mixed together in the presence of NaI (1.0 g, 0.0067 mol, 0.4 mol %) in DMF (1 L). The mixture was heated to reflux for 2 h, cooled to 25 °C and poured into ice water (500 mL). A white precipitate formed and more ice (~100 g) was added until no additional precipitate formed. The sticky white precipitate was collected by vacuum filtration and allowed to dry in air for 30 min. The filter cake was dissolved in 50% ethanol-water (2 L) and recrystallized to furnish 419 g of 3 as white crystals (72% yield). mp 204–207 °C. 1H NMR: δ 4.54 (s, 2H), 6.73 (d, J = 8.8, 2H), 7.36 (d, J = 8.8, 2H), 8.00 (dt, J = 6.8, 14.3, 2H), 8.11 (d, J = 7.5, 1H), 8.30 (d, J = 7.6, 1H), 9.26 (s, 1H), 10.07 (s, 1H). 13C NMR: δ 41.2, 115.9, 121.8, 122.3, 125.8, 127.2, 130.8, 135.9, 136.5, 137.6, 154.4, 159.4, 163.3. Anal. Calcd. for C15H12N2O5S: C, 54.21; H, 3.64; N, 8.43. Found: C, 54.15; H, 3.58; N, 8.41.
SCP-123 (4)
A suspension of 3 (50 g, 0.15 mol) and aqueous 0.5 N NaOH (600 mL, 0.30 mol) was stirred at 25 °C for 1 h. Ethanol (400 mL) was added until the solution becomes clear. Stirring was continued for an additional 1 h. The solution was acidified with 2N HCl (500 mL) solution to a pH of 1 (pH meter). The white precipitate that formed was filtered and washed with distilled water (100 mL). The filter cake was dried under vacuum to afford 49 g of 4 as a white solid (93%, yield). mp 184–186 °C. 1H NMR: δ 3.72 (d, J = 5.1, 2H), 6.65 (d, J = 8.8, 2H), 7.20 (d, J = 8.8, 2H), 7.39 (s, 1H), 7.65–7.77 (m, 3H), 7.93 (m, 1H), 9.21 (s, 1H), 9.71 (s, 1H), 13.81 (b, 1H). 13C NMR: δ 46.5, 115.8, 121.7, 129.3, 130.4, 130.7, 131.7, 133.2, 133.4, 138.2, 154.2, 166.0, 169.5. Anal. Calcd. for C15H14N2O6S: C, 51.42; H, 4.03; N, 8.00. Found: C, 51.42; H, 4.15; N, 7.86.
SCP-123ss•H2O (5•H2O)
The acid 4 (71.5 g, 0.24 mol) was suspended in ethanol (400 mL) and cooled to 0 °C. A pre-cooled (0 °C) solution of NaOH (8.2 g, 0.24 mol) in distilled water (40 mL) was added drop wise to the ethanolic suspension over 10 min. After the addition of the basic solution was complete more ethanol was added to the mixture, if needed, to dissolve all the solids. The clear reaction mixture was stirred for an additional 2 h. The reaction volume was reduced by 10% (~50 mL) on a rotoevaporator without a water bath. Once a precipitate started to form, the mixture was removed from the rotoevaporator and cooled at 0 °C for 1 h. The white precipitate was filtered and washed with distilled water (100 mL). The filter cake was dried under vacuum at 60 °C to afford 93 g of 5•H2O as white solid (99% yield). mp 188–190 °C. 1H NMR: δ 3.57 (s, 2H), 6.65 (d, J = 8.8, 2H), 7.24 (d, J = 8.8, 2H), 7.39 (t, J = 7.0, 1H), 7.51 (t, J = 7.5, 1H), 7.63 (d, J = 6.6, 1H), 7.74 (d, J = 7.7, 1H), 9.00 (s, 1H), 9.58 (s, 1H), 10.03 (s, 1H). 13C NMR: δ 47.1, 115.7, 121.7, 128.0, 128.1, 130.7, 130.8, 132.9, 136.3, 142.5, 154.3, 166.4, 171.3. Anal. Calcd. for C15H13N2NaO6S·H2O: C, 46.15; H, 3.87; N, 7.18. Found: C, 46.01; H, 3.89; N, 7.14.
Supplementary Material
Acknowledgment
We are grateful to the National Institute on Neurological Disorders and Stroke for the support of this research. The project described was supported by Grant Number U44NS046891 from the National Institute of Neurological Disorders And Stroke. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders And Stroke or the National Institutes of Health.
Footnotes
Supporting Information Available
Proton NMR of compounds 4 5 and 12, HPLC conditions and retention times for compounds 3, 4, and 12, and LC-ESI MS spectrum of 12. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Prescott LF. Am. J. Ther. 2000;7:143. [PubMed] [Google Scholar]
- 2.Watkins PB, Kaplowitz N, Slattery JT, Colonese CR, Colucci SV, Stewart PW, Harris SC. J. Amer. Med. Assoc. 2006;296:87. doi: 10.1001/jama.296.1.87. [DOI] [PubMed] [Google Scholar]
- 3.Slattery JT, Nelson SD, Thummel KE. Clin. Pharmacol. Ther. 1996;60:241. doi: 10.1016/S0009-9236(96)90050-8. [DOI] [PubMed] [Google Scholar]
- 4.McGoldrick MD, Bailie GR. Ann. Pharmacother. 1997;31:221. doi: 10.1177/106002809703100214. [DOI] [PubMed] [Google Scholar]
- 5.Depré M, Van Hecken A, Verbesselt R, Tjandra-Maga TB, Gerin M, Schepper PJ. Fund. Clin. Pharmacol. 1992;6:259. doi: 10.1111/j.1472-8206.1992.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 6.Flouvat B, Leveneu A, Fitoussi S, Delhotal-Landes B, Gendron A. Int. J. Clin. Pharmacol. Ther. 2004;42:505. doi: 10.5414/cpp42050. [DOI] [PubMed] [Google Scholar]
- 7.Barcia E, Martin A, Azuara ML, Negro S. Chem. Pharm Bull. 2005;53:277. doi: 10.1248/cpb.53.277. [DOI] [PubMed] [Google Scholar]
- 8.Bazan NG, Alvarez-Builla J. Chem. Abstr. 5,554,636. U.S. Patent. 1996;125:266037. 1996.
- 9.Bazan NG, Alvarez-Builla J. Chem. Abstr. 5,621,110. U.S. Patent. 1996;125:266037. 1997.
- 10.Vaccarino AL, Paul D, Mukherjee PK, Rodríguez de Turco EB, Marcheselli VL, Xu L, Trudell ML, Minguez JM, Matía MP, Sunkel C, Alvarez-Builla J, Bazan NG. Bioorg. Med. Chem. 2007;15:2206. doi: 10.1016/j.bmc.2006.07.054. [DOI] [PubMed] [Google Scholar]
- 11.Cui J-G, Zhang X, Zhao YH, Chen C, Bazan N. Biochem. Biophys. Res. Commun. 2006;350:358–363. doi: 10.1016/j.bbrc.2006.09.055. Erratum in: Biochem. Biophys. Res. Commun. 2007, 359, 187. [DOI] [PubMed] [Google Scholar]
- 12.Bazan NG, Cui J-G. Chem. Abstr. 2008;148:276766. WIPO patent application WO 2008/021896 A2. [Google Scholar]
- 13.Xu L, Trudell ML. J. Label. Compd. Radiopharm. 2005;48:219. [Google Scholar]
- 14.Waiss AC, Kuhnle JA, Windle JJ, Wiersema AK. Tetrahedron Lett. 1966:6251. [Google Scholar]
- 15.Whiting DA. In: Comprehensive Organic Synthesis. Trost BM, Fleming I, Pattenden G, editors. Vol. 3. Oxford: Pergamon Press; 1991. pp. 659–700. and references cited therein. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.