Abstract
Loss of function and postural instability occur in Parkinson disease (PD). Dynamic exercise interventions are successful in improving motor control and physical function. However, most programs are based in a health facility or physical therapy setting and involve travel. With the limitations associated with PD (e.g. health care and medication cost as well as travel limitations) these therapies may be inaccessible and exclude some individuals from maintaining or increasing their function. The purpose of this study was to evaluate the effectiveness of a home-based exercise intervention on postural control in individuals with PD. Multivariate analysis of covariance was performed on individuals with PD (N=10) and healthy aged matched controls (N=10). Participants were assessed utilizing computerized dynamic posturography (CDP) before and after a 10-week exercise intervention. Participants were instructed on proper technique prior to the intervention, were given an illustrated home program, and were monitored weekly concerning their progress. Pre intervention assessment demonstrated that individuals with PD had statically lower scores on a Sensory Organization Test (p < .05). Following the intervention, results indicated no statistical difference between individuals with PD and aged match controls (p > .05). This initial study indicates that a home exercise intervention is an effective method of improving postural control in individuals with PD. Results from this investigation support further study to determine the extent to which both preventative and restorative home based programs can improve postural control.
Keywords: Neurodegenerative disease, rehabilitation, balance
INTRODUCTION
Parkinson disease (PD) is a chronic progressive neurological disease resulting in a reduction in the production of dopamine in the substania nigra, which subsequently leads to impairment of muscle tone and loss of voluntary movement. The four cardinal motor signs are tremor, bradykinesia, rigidity, and postural instability, and they affect the ability to initiate and carryout a movement. Specifically, postural instability is prominent and the most incapacitating feature of PD. Unfortunately, postural instability is difficult to treat and puts patients at increased risk for falls and injury. In late stage PD a fractured hip resulting from a fall can lead to death.
Several investigations have demonstrated functional deficits in PD as a result of postural instability. For example, we have previously documented altered mechanics in PD patients when performing sit to stand transfers as a result of increased muscle co-contractions as well as overall muscle weakness and motor control adaptations needed to initiate and complete the movement safely [1]. Postural instability, and the associated impairment in balance, have also been linked to increased risk of falls and identified as one of the most debilitating symptom of PD [2, 3]. Over the course of the disease, as many as 68% of patients with PD fall,_with 13% falling more than once a week [4].
Dynamic exercise interventions are successful in treating motor control and physical function [3, 5, 6]. However, most programs involve travel to a health facility or physical therapy setting. With the limitations associated with this disease (e.g health care and medication cost as well as travel limitations) these therapies may be inaccessible and exclude some individuals from maintaining or increasing their function. We set out to evaluate the effectiveness of a home-based intervention on postural control, together with the use of sensory information, to maintain balance in individuals with PD.
METHOD
Participants and Design
An age and gender matched sample of 10 individuals with PD and 10 aged match controls (AMC) were recruited for this study (Table 1). Only PD participants completed the exercise intervention, the AMC did not participate in the intervention and served to evaluate the impact of a learning effect on repeated testing. Participants with PD were referred by the same neurologist and were at Hoehn and Yahr Stage II–III (Table 2). Exclusion criteria included: fluctuating responses to medication, functionally disabling dyskinesia or dystonia, lung disease, history of cardiac disease, and major musculoskeletal or metabolic disorders.
Table 1.
Participant Demographics
| Group | Age | Height (in) | Weight (kg) |
|---|---|---|---|
| (M ± SD) | (M ± SD) | (M ± SD) | |
| PD | 73.40 ± 8.50 | 67.15 ± 2.04 | 73.24 ± 5.12 |
| AMC | 69.79± 5.24 | 67.71 ± 3.91 | 74.29 ± 11.02 |
PD = Parkinson's disease
AMC = Aged-matched controls
Table 2.
Characteristics of participants with Parkinson's disease
| Age (years) | Gender | Years since diagnosis |
H&Y score | Medications | |
|---|---|---|---|---|---|
| PD1 | 80 | M | 14 | 3 | Sinemet, Mirapex |
| PD2 | 64 | F | 11 | 3 | Sinemet, Mirapex |
| PD3 | 82 | F | 8 | 3 | Sinemet, Mirapex |
| PD4 | 66 | F | 4 | 2 | Sinemet |
| PD5 | 72 | M | 6 | 2 | Sinemet, Tolcapona |
| PD6 | 70 | M | 14 | 3 | Sinemet, Mirapex |
| PD7 | 75 | M | 11 | 2.5 | Sinemet |
| PD8 | 88 | M | 9 | 3 | Sinemet, Mirapex |
| PD9 | 61 | F | 5 | 2 | Sinemet |
| PD10 | 76 | M | 7 | 2.5 | Sinemet |
Participants with PD were tested and completed the exercise program in an “on“medication state (1–1.5 hours post ingestion) to maximize the effects of medication on functional performance. Participants received verbal explanation of the protocol and signed a consent form. All participants provided written informed consent prior to participating in the study as approved by the Institutional Review Board.
Instrumentation
Postural Control was assessed by computerized dynamic posturography performed on the NeuroCom Equi-test System (NeuroCom International, Clackamas, OR). This system utilizes transducers located in a force platform which measure vertical and horizontal forces that are produced by the body’s movement around a fixed base of support. It is equipped with standardized protocols, including the Sensory Organization Test (SOT), for use in clinical settings to determine how various sensory systems compensate for deficiencies. The SOT is used to assess balance abilities and limitations in a wide variety of populations by determining how individuals are able to respond and adapt to a variety of sensory manipulations [7]. Inaccurate information is delivered to the eyes, feet, and joints through sway referencing of the visual surround and support surface. This alteration disrupts the available sensory information presented to the participant and allows the tester to isolate sensory modalities that provide afferent information to maintain postural control. With this technique of sway referencing it is possible to determine how individuals adapt themselves to constant or changing sensory information.
Procedure
The SOT requires participants to be tested under six conditions consisting of three 20-second trials with their eyes open or closed. During the test, the participant’s visual surround, force platform, or combinations of both are sway referenced. The term sway referenced refers to the tilting of the support surface and/or visual surround to document the sway in the center of gravity [8]. In other words, as the subject sways, the walls or support surface will move forward or backward accordingly. The conditions differ, depending on the manipulation of the somatosensory, visual, and/or vestibular environments, and the participant’s resulting movements are measured and recorded. The sway referencing delivers erroneous information to the senses and the force platform measures the participant’s ability to rely on the other senses to compensate for the erroneous feedback being received. As a safety consideration, each participant used a harness during testing to eliminate the risk of falling.
Scoring
Results from the SOT were used for all comparisons of the participants’ postural stability. Effective use of the individual’s sensory inputs is determined from the overall pattern of scores on each of the six conditions. The equilibrium composite score quantifies the postural stability under each trial for all conditions. It is the weighted average of scores on all sway conditions and characterizes an individual’s overall level of performance. In addition, individual equilibrium scores on all conditions were compared to extreme responses under varying movement and sway referenced conditions. The resulting scores for each 6 conditions as well the composite score ranges from 0–100 with 100 indicating no anteroposterior sway.
Exercise Intervention Rational and Protocol
This study focused on improving postural control and the use of sensory information. These components were based on their relative importance to the diminished functional capacity and propensity for falls in individuals with PD [1, 2, 9]. The exercise interventions were also based on pre-participation interviews regarding limitations on the subjects’ daily function (e.g. going up and down stairs, getting out of bed and problems with falls). The selected exercises were also chosen based on the documented ability to improve balance in healthy older adults. The exercises included the abdominal crunch, wall squat, lunge, standing calf raise, knee flexion and extension, and step-up.
Prior to the beginning of the home exercise program, the subjects attended a practice session and were instructed on the proper method and mechanics for each exercise. During the initial session the total number of repetitions completed in 30 seconds for each exercise was recorded and used as an individualized baseline during the 10-week intervention. To improve accuracy and compliance, participants were also provided with an illustrated exercise booklet. Of note, only the PD participants carried out the 10-week exercise intervention, the AMC were used control for a learning effect of repeated testing. Each of the PD subjects completed all seven exercises each workout session. Progression of repetitions completed in 30 seconds and compliance were measured through weekly telephone calls by a physiotherapist. The results of progression as monitored throughout the 10-week intervention did not statically differ between the PD participants (p > .05).
Data Analysis
SPSS version 14.0 statistical software (SPSS, Inc., Chicago, IL) was used to conduct all descriptive statistics as well as multivariate analysis of variance (MANOVA). Statistical analyses were selected to detect group differences as well as time differences, on NeuroCom Equi-test SOT composite score and for each of the six conditions. Descriptive statistics (e.g., means, standard deviations) were calculated for all variables of interest (Table 3). All values were reported as a percentage of the participants’ body weight to allow values from both male and female participants to be compared.
Table 3.
Averages for the NeuroCom Sensory Organization Test (Mean ± SD)
| PD | AMC | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Condition 1 | 91.04 ± 3.81 | 92.04 ± 1.85 | 84.71 ± 1.13 | 94.30 ± 1.29 |
| Condition 2 | 89.91 ± 3.70 | 90.58 ± 3.81 | 91.38 ± 2.28 | 91.30 ± 2.61 |
| Condition 3 | 90.58 ± 4.05 | 91.71 ± 1.92 | 92.46 ± 3,49 | 92.52 ± 2.78 |
| Condition 4 | 60.37 ± 11.89* | 80.00 ± 4.70 | 74.29 ± 12.12* | 76.71 ± 8.41 |
| Condition 5 | 41.59 ± 10.16* | 54.21 ± 16.05 | 50.33 ± 15.83* | 53.66 ± 8.80 |
| Condition 6 | 50.00 ± 3.57* | 55.83 ± 14.42 | 56.88 ± 12.27* | 60.76 ± 6.54 |
| Composite | 50.65 ± 5.81* | 63.35 ± 9.69 | 60.50 ± 11.41* | 64.38 ± 5.69 |
PD= Parkinson disease; AMC=Aged-matched controls
Indicates a significant difference at p <.05.
RESULTS
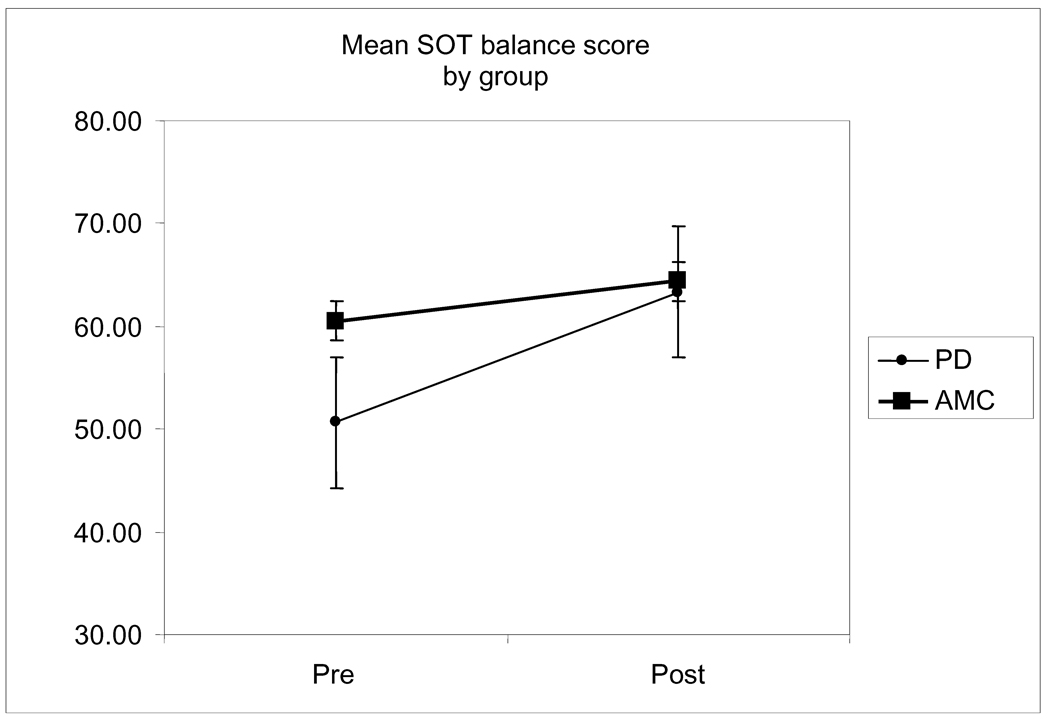
Results for pre to post NeuroCom SOT composite score measures were significantly increased for individuals with PD, F(1, 9) = 6.656, P = .026. Thus, PD composite balance scores (50.65 ± 5.81) were statistically lower at pre test when compared to the AMC composite balance score, (60.50 ± 11.41). However, at post test, there was no longer a statistical difference between the PD group (63.35 ± 9.69) and the AMC group (64.38 ± 5.69) (Figure 1).
Figure 1.
Changes in mean SOT composite score from baseline to 10-weeks of home exercise in the exercised Parkinson disease (PD) participants and aged-matched controls (AMC), non-exercised controls. The exercised PD group demonstrated a significant change from baseline, p < .05.
The results for each condition can be explained due to the similarities between conditions 1–3 and 4–6. In conditions 1–3, for example, maintaining stability is not difficult because the floor is fixed. Thus, the results indicated no significant difference between groups pre or post intervention (P > .05). However, the more difficult 4–6 conditions indicated a significant difference at pre test between the PD group and the healthy older adults [condition 4, F(1, 11) = 6.261, P = .034; condition 5, F(1, 11) = 16.914, P = .003; and condition 6, F(1, 11) = 5.289, P = .047]. Interestingly, as with the composite score, no difference existed between groups on conditions 4, 5 and 6 following the intervention (P > .05).
DISCUSSION
This study found significant increases on conditions 4, 5, and 6 as well as on the composite score of the NeuroCom SOT in the individuals with PD who completed a home-based exercise intervention. Importantly, the non-exercise AMC group demonstrated no such improvement limiting the possibility of a learning effect from repeated testing. The PD group entered the study presenting with balance impairment as measured by the NeuroCom SOT protocol. Following the intervention, the balance scores of the PD group improved to a level parallel to that of the normal controls (Figure 1).
As a multifactor problem, PD postural instability has been partially attributed to dysfunctional visual, vestibular, and proprioceptive systems [3, 6]. Consistent with these previous findings, our pre-intervention results demonstrated significant differences between groups on a destabilized surface (conditions 4, 5, and 6). However, following the exercise intervention the PD participants increased their levels of performance consistent with their age-matched peers (Figure 1 and Table 3). Similar to the work of Toole et al [6], these results suggest that several postural mechanisms were improved by the intervention. Primarily, improved stretch reflexes and increased forces in the muscular system allowed for improved perturbation response and greater postural control. Specifically, the peripheral adaptations allowed the proprioceptive, visual and vestibular feedback to be employed more accurately and efficiently to counteract perturbations in stability. Interestingly, the Toole study employed a less convenient form of therapy than our suggested home-based intervention.
The exercises in this intervention, particularly the squat, lunge, and step up were chosen based on the ability to challenge the vertical position of the body in space to increase the limits of stability. Additionally, some of the exercises were chosen based on their “closed chain” nature. These types of exercises are often used in rehabilitation because of the multi-joint, weight bearing techniques that more accurately mimic everyday movements [10]. As such, these “real” movements require the participant to use the feedback/feed forward mechanisms required to carry out functional tasks resulting in more efficient sensory afferent and efferent information processing. As such, the exercise regimen stimulated sensory-motor coordination in the basal ganglia therefore making the entire postural system more effective. Previous research indicates that functional improvements from exercise interventions stem from the activation of the motor cortex that overrides atypical basal ganglia function demonstrated in PD [11]. Thus, exercise in general can facilitate neuronal transmission and motor coordination that is essential for improved balance and overall function.
In the context of home-exercise it is important to note, in addition to the afore mentioned Toole study, the results of this study are consistent with a 2003 study by Hirsch and colleagues [3]. The Hirsch study reported balance improvements after strength and balance training in a public health facility. Specifically, Hirsch reported a 14.37 average SOT composite score improvement in the exercised PD group whereas we demonstrated a 12.70 average SOT composite score change following a home-based exercise intervention. Importantly, improvements in our sample who exercised in the home-based setting were similar to patients with PD who trained in a public health facility setting.
The primary limitation of this study was the use of a non-PD control group. Although the healthy AMC group assisted in ruling out a learning effect of repeated testing, an age and PD matched group would have allowed for comparisons specific to PD. However, due to the demographics of the community, the nature of the disease and travel limitations, recruitment was especially difficult. The demographical variation of the participants is an additional limitation of this study. The PD group’s age range was from 67 to 87 years of age and the H and Y disease stage ranged from II to III. Future studies may use a more homogenous sample, same stage and similar age and duration of disease for example, to control some of the overall variability seen in this population. Lastly, qualitative data may be utilized as a way to explore individuals’ quality of life following a home-based intervention.
In conclusion, although the exact contribution of exercise to patients with PD is uncertain it is evident that exercise can contribute to maintaining postural stability in individuals with PD and may reduce the risk of falls. Our study supports previous research demonstrating that patients with PD can benefit from training because the exercises carried out involve the practice of planning and executing movements required to keep the body in a state of equilibrium [6]. Further, our study suggests the use of an effective, cost efficient and accessible home exercise program is an alternative which can prove valuable to individuals with PD with limited access to health facilities or physical therapy settings.
ACKNOWLEDGMENTS
This study was funded in part by the Department of Kinesiology, The University of Georgia, Athens, Georgia. We would like to thank all the participations who volunteered for this study as well as Dr. Martha Trieschmann for her help with recruitment.
The project described was supported in part by Grant Number T32DC008768 from the National Institute on Deafness and other Communication Disorders. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Deafness and other Communication Disorders or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ramsey VK, Miszko TA, Horvat M. Muscle activation and force production in Parkinson's patients during sit to stand transfers. Clin Biomech (Bristol, Avon) 2004 May;19(4):377–384. doi: 10.1016/j.clinbiomech.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Hass CJ, Waddell DE, Fleming RP, Juncos JL, Gregor RJ. Gait initiation and dynamic balance control in Parkinson's disease. Arch Phys Med Rehabil. 2005 Nov;86(11):2172–2176. doi: 10.1016/j.apmr.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch MA, Toole T, Maitland CG, Rider RA. The effects of balance training and high-intensity resistance training on persons with idiopathic Parkinson's disease. Archives of Physical Medicine and Rehabilitation. 2003;84(8):1109–1117. doi: 10.1016/s0003-9993(03)00046-7. [DOI] [PubMed] [Google Scholar]
- 4.Robinson K, Dennison A, Roalf D, Noorigian J, Cianci H, Bunting-Perry L, et al. Falling risk factors in Parkinson's disease. Neuro Rehabilitation. 2005;20(3):169–182. [PubMed] [Google Scholar]
- 5.Hass CJ, Collins MA, Juncos JL. Resistance training with creatine monohydrate improves upper-body strength in patients with Parkinson disease: a randomized trial. Neurorehabil Neural Repair. 2007 Mar-Apr;21(2):107–115. doi: 10.1177/1545968306293449. [DOI] [PubMed] [Google Scholar]
- 6.Toole T, Hirsch MA, Forkink A, Lehman DA, Maitland CG. The effects of a balance and strength training program on equilibrium in Parkinsonism: A preliminary study. NeuroRehabilitation. 2000;14(3):165–174. [PubMed] [Google Scholar]
- 7.Nashner LM. Computerized dynamic posturography. In: Jacobson GP, Newman CW, Kartuch JM, editors. Handbook of Balance Function Testing. St Louis: Mosby Year Book; 1997. pp. 280–305. [Google Scholar]
- 8.Guskiewicz KM, Ross SE, Marshall SW. Postural stability and neuropsychological deficits after concussion in collegiate athletes. J Athl Train. 2001;36:10. [PMC free article] [PubMed] [Google Scholar]
- 9.Inkster LM, Eng JJ, MacIntyre DL, Stoessl AJ. Leg muscle strength is reduced in Parkinson's disease and relates to the ability to rise from a chair. Mov Disord. 2003 Feb;18(2):157–162. doi: 10.1002/mds.10299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shields RK, Madhavan S, Gregg E, Leitch J, Petersen B, Salata S, et al. Neuromuscular Control of the Knee During a Resisted Single-Limb Squat Exercise. Am J Sports Med. 2005 October 1;33(10):1520–1526. doi: 10.1177/0363546504274150. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nieuwboer A, Dom R, De Weerdt W, Desloovere K, Fieuws S, Broens-Kaucsik E. Abnormalities of the spatiotemporal characteristics of gait at the onset of freezing in Parkinson's disease. Mov Disord. 2001 Nov;16(6):1066–1075. doi: 10.1002/mds.1206. [DOI] [PubMed] [Google Scholar]