Abstract
1,3-Dihydro-1-[2-hydroxy-5-(trifluoromethyl)phenyl]-5-(trifluoromethyl)-2H-benzimidazol-2-one (NS1619), a potent activator of the large conductance Ca2+ activated potassium (BKCa) channel, has been demonstrated to induce preconditioning (PC) in the heart. The aim of our study was to test the delayed PC effect of NS1619 in rat cortical neuronal cultures against oxygen-glucose deprivation, H2O2, or glutamate excitotoxicity. We also investigated its actions on reactive oxygen species (ROS) generation, and on mitochondrial and plasma membrane potentials. Furthermore, we tested the activation of the phosphoinositide 3-kinase (PI3K) signaling pathway, and the effect of NS1619 on caspase-3/7. NS1619 dose-dependently protected the cells against the toxic insults, and the protection was completely blocked by a superoxide dismutase mimetic and a PI3K antagonist, but not by BKCa channel inhibitors. Application of NS1619 increased ROS generation, depolarized isolated mitochondria, hyperpolarized the neuronal cell membrane, and activated the PI3K signaling cascade. However, only the effect on the cell membrane potential was antagonized by BKCa channel blockers. NS1619 inhibited the activation of capase-3/7. In summary, NS1619 is a potent inducer of delayed neuronal PC. However, the neuroprotective effect seems to be independent of cell membrane and mitochondrial BKCa channels. Rather it is the consequence of ROS generation, activation of the PI3K pathway, and inhibition of caspase activation.
Keywords: BKCa channel, mitochondria, neuronal culture, neuroprotection, phosphoinositide 3-kinase, reactive oxygen species
Mitochondria play a central role in the life and death of neurons as well as other cells. Diminished mitochondrial energetics with increased reactive oxygen species (ROS) generation and Ca2+ overload are initiators of cascades leading toward cellular disintegration. Mitochondria are also major targets of cytoprotective studies. One of the most promising approaches is preconditioning (PC), in which a non-injurious stress renders cells, tissues, and organs tolerant to a subsequent, otherwise damaging insult.
Mitochondrial potassium channels have long been proposed to play a fundamental role in the initiation and mediation of PC. Following the first report on the presence of ATP sensitive K+ channels in the inner mitochondrial membrane (Inoue et al. 1991), several studies have demonstrated neuronal PC and protection against numerous neurotoxic insults using selective pharmacological activators of these channels (for a review see Busija et al. 2004). Neurons, on the other hand, also express another K+ channel that may mediate potential neuroprotective effects. The large conductance Ca2+ activated K+ (BKCa) channel which is activated by depolarization and increased cytosolic Ca2+ concentration plays a regulatory role in many physiological processes including neurotransmitter release, and neuronal excitability. The BKCa channel is composed of a pore-forming α subunit (BKCaα) and an auxiliary β subunit (BKCaβ) which modulates channel activity and sensitivity to specific antagonists (Vergara et al. 1998). Following the demonstration of the presence of BKCa channels in the inner membrane of mitochondria (mitoBKCa) (Siemen et al. 1999; Xu et al. 2002; Douglas et al. 2006), an increasing number of studies have reported immediate and delayed PC induced cardio-protection via the activation of BKCa channels (Xu et al. 2002; Shintani et al. 2004; Wang et al. 2004; Cao et al. 2005; Sato et al. 2005). In most of these studies, PC was induced by the benzimidazole derivative 1,3-dihydro-1-[2-hydroxy-5-(trifluoromethyl)phenyl]-5-(trifluoromethyl)-2H-benzimidazol-2-one (NS1619) (Olesen et al. 1994). The protective effect of the activation of BKCa channels after the injury was also shown in the brain (Cheney et al. 2001; Runden-Pran et al. 2002; Hepp et al. 2005). However, whether neuronal PC can be induced with a BKCa channel opener has not yet been investigated.
The aim of our present study was to examine whether the BKCa channel opener NS1619 induces delayed PC in rat cortical neuronal cultures. We also studied the effects of NS1619 on mitochondrial and plasma membrane potential, and on ROS generation. Furthermore, we examined the role of the phosphoinositide 3-kinase (PI3K) signaling pathway, cellular antioxidants, and caspases in the mediation of delayed PC induced by NS1619.
Materials and methods
Materials
Cell culture plastics were purchased from Becton-Dickinson (San Jose, CA, USA). Dulbecco’s modified Eagle medium, Neurobasal medium, B27 Supplement, 2-mercaptoethanol, and horse serum were obtained from Gibco BRL (Grand Island, NY, USA). Percoll was purchased from Amersham Biosciences (Uppsala, Sweden), dispase I from Roche (Mannheim, Germany), and M40401 from Metaphore Pharmaceuticals (St. Louis, MO, USA). NS1619, paxilline (Pax), 4-aminopyridine (4AP), and wortmannin (Wrt) were purchased from Sigma (St. Louis, MO, USA), and iberiotoxin (IbTx) and charybdotoxin (ChTx) from California Peptide Research (Napa, CA, USA). Hybernate A was obtained from BrainBits LLC (Springfield, IL, USA). CellTiter-Glo Luminescent Assay and CellTiter 96 AQueous One Solution Assay were both procured from Promega (Madison, WI, USA). Hydroethidine (HEt), tetramethylrhodamine ethyl ester (TMRE), 4-{2-[6-(dioctylamino)-2-naphthalenyl]ethenyl}-1-(3-sulfopropyl)-pyridinium (di-8-ANEPPS), and Amplex Red Catalase Assay Kit were obtained from Molecular Probes (Eugene, OR, USA). The SOD Assay Kit was purchased from Fluka (Buchs, Switzerland), Glutathione Peroxidase Assay Kit from Cayman Chemical Company (Ann Arbor, MI, USA), and SensoLyte TM Homogenous AMC Caspase-3/7 Assay Kit from AnaSpec (San Jose, CA, USA). The Bio-Rad DC Protein Assay was procured from Bio-Rad (Hercules, CA, USA). Antibodies were obtained from the following sources: anti-glial fibrillary acidic protein antibody from Chemicon (Temecula, CA, USA); anti-microtubule associated protein-2, monoclonal anti-protein kinase Bα/Akt, monoclonal anti-BKCa channel α subunit, monoclonal anti-cytochrome c antibodies from Becton-Dickinson; polyclonal anti-pS473 Akt antibody from Promega; polyclonal anti-calreticulin antibody from Stressgen (Victoria, BC, Canada); polyclonal anti-phospho-glycogen synthase kinase (Gsk) 3α/β (Ser 21/9) antibody, and monoclonal anti-Gsk3β antibody from Cell Signaling Technology (Danvers, MA, USA); polyclonal anti-BKCa channel β4 subunit antibody from Sigma; and anti-rabbit IgG and anti-mouse IgG from Jackson Immuno-Research (West Grove, PA, USA). All other chemicals were from Sigma.
Primary rat cortical neuronal culture
Timed pregnant Sprague–Dawley rats were obtained from Harlan (Indianapolis, IN, USA) and were maintained and used in compliance with the principles set forth by the Animal Care and Use Committee of Wake Forest University Health Sciences. Primary rat cortical neurons were isolated from E18 Sprague–Dawley fetuses as described in Gaspar et al. (2006). The cells were plated at a density of 2 × 105 cells/cm2 onto poly-l-lysine coated glass coverslips for confocal microscopic analysis while 106 cells/cm2 were placed onto poly-d-lysine coated plates or dishes for the other experiments in plating medium which consisted of 60% Dulbecco’s modified Eagle medium, 20% Ham’s F-12 Nutrient Mixture, 20% horse serum, and l-glutamine (0.5 mM). After cell attachment, the plating medium was replaced with regular cell culture medium (‘feeding medium’; FM) which consisted of Neurobasal medium supplemented with B27 (2%), l-glutamine (0.5 mM), 2-mercaptoethanol (55 µM), and KCl (25 mM). Positive immunostaining for microtubule-associated protein-2 and negative immunostaining for glial fibrillary acidic protein verified that the cultures consisted of more than 98% of neurons on day 7 in vitro. Experiments were carried out on 7 to 9-day-old cultures, during which period neurons expressed N-methyl-d-aspartate, α-amino-3-hydroxy-5-methylisoxazole-4-propionate, and kainate receptors and were vulnerable to glucose deprivation (Mattson et al. 1991, 1993).
Treatment with NS1619
To induce delayed PC, 7-day-old neuronal cultures were treated with increasing doses of NS1619 (10, 25, 50, 100, 150, and 200 µM) for 6 h in FM once a day for three consecutive days. In other sets of the experiments, the cells were treated with NS1619 (100 µM) and the K+ channel antagonists, IbTx (1 µM), ChTx (1 µM), Pax (4 µM), 4AP (1 mM), the PI3K inhibitor Wrt (100 nM), or with the Mn-based non-peptidyl S,S-dimethyl substituted bis-cyclohexylpyridine derivative superoxide dismutase mimetic M40401 (50 µM) which has no reactivity towards hydrogen peroxide, nitric oxide, peroxynitrite, and hypochlorite (Salvemini et al. 1999; Cuzzocrea et al. 2001). After each treatment, the drugs were washed out thoroughly, and the cells were kept in FM. The selected doses of these compounds showed no toxicity in preliminary experiments.
Combined oxygen and glucose deprivation
Neurons in 96-well plates were exposed to oxygen and glucose deprivation (OGD) for 180 min at 37°C using a protocol described previously (Goldberg and Choi 1993; Kis et al. 2003). Briefly, one day after the last treatment, the cells were rinsed, the medium was replaced with glucose-free Earle’s balanced salt solution (EBSS) and then the cultures were placed in a ShelLab Bactron Anaerobic Chamber (Sheldon Manufacturing Inc., Cornelius, OR, USA) filled with a humidified anaerobic gas mixture (5% CO2, 5% H2 and 90% N2) at 37°C. Control cell cultures were incubated in glucose-containing (5.5 mM) EBSS in a regular 5% CO2 cell culture incubator for 180 min. OGD was terminated by replacing the glucose-free EBSS with FM and thereafter the cultures were maintained in the regular 5% CO2 incubator within normoxic conditions.
Exogenous hydrogen peroxide toxicity
The FM on neuronal cultures in 96-well plates was replaced with Neurobasal medium containing H2O2 (80 µM) 1 day after the last treatment and the cells were incubated at 37°C in the 5% CO2 incubator. After 6 h of incubation the cultures were washed, FM was restored, and the cultures were returned to the 5% CO2 incubator.
Glutamate excitotoxicity
Cell cultures in 96-well plates were exposed to glutamate (200 µM) 24 h after the last NS1619 treatment in FM for 60 min at 37°C in the 5% CO2 incubator. Afterward the cells were rinsed and returned to the 5% CO2 incubator in FM.
Quantification of neuronal survival
The cell viability in neuronal cultures was determined 24 h after the neurotoxic insults using the tetrazolium-based CellTiter 96 AQueous One Solution assay. Absorbance at 492 nm was measured with a microplate reader (FLUOstar OPTIMA, BMG Labtech GmbH, Offenburg, Germany). Comparisons were always made in the same manner, between sister cultures exposed to the neurotoxic stimulus on the same day, and cell viability was expressed as a percentage of the corresponding control culture (untreated, and not exposed to the lethal insult) as follows:
Isolation of rat brain mitochondria and mitochondria enriched liver preparations
Brain mitochondria and mitochondria rich liver preparations were isolated using a discontinuous Percoll gradient as described previously (Rajapakse et al. 2001; Gaspar et al. 2007). All the instruments and buffers were kept ice-cold during the procedure. Male Sprague–Dawley rats (280–320 g; Harlan) were over-anesthetized with isoflurane and decapitated. The brain, without the cerebellum, and the liver were removed and weighed, and were then homogenized in mitochondrial isolation buffer (MIB) containing sucrose (250 mM), K+-EDTA (0.5 mM), Tris-HCl (10 mM), and 1% bovine serum albumin (pH 7.4) using Dounce homogenizers and glass pestles (Kontes Glass Co., Vineland, NJ, USA). The homogenates were centrifuged for 3 min at 500 g, and then the supernatants were collected and resuspended in equal amounts of 24% Percoll in MIB. This suspension was layered onto a discontinuous Percoll gradient (24% and 40% in MIB). The gradient was centrifuged for 5 min at 28 000 g and the layer between the 24% and 40% Percoll suspensions containing the purified mitochondria was collected. The preparation was washed in MIB and centrifuged for 12 min at 15 000 g. For fluorescent plate reader measurements, protein content was determined using the Bio-Rad DC Protein Assay (λabs = 650 nm), and then equal amounts of mitochondria were transferred in MIB into black-walled, clear-bottomed 96-well plates and were used within 3 h.
Isolation of rat cardiac mitochondria
Heart mitochondria were isolated from male Sprague–Dawley rats using a previously described method (Katakam et al. 2007). Briefly, hearts were homogenized in ice-cold isolation buffer containing mannitol (225 mM), sucrose (75 mM), 3-(N-morpholino)propane-sulfonic acid (5 mM), EGTA (0.5 mM), taurine (2 mM), and 0.2% bovine serum albumin (pH 7.25). The homogenate was centrifuged twice at 1000 g for 5 min (4°C) and the supernatant was then centrifuged at 10 000 g for 10 min (4°C). After washing, the pellet was re-suspended in a buffer containing mannitol (225 mM), sucrose (25 mM), 3-(N-morpholino)propanesulfonic acid (5 mM), EGTA (1 mM), KH2PO4 (5 mM), and taurine (2 mM) supplemented with 0.2% bovine serum albumin (pH = 7.4), placed on ice and used within 3 h.
Determination of mitochondrial membrane potential
The changes of mitochondrial membrane potential were analyzed using TMRE. Measurements were performed in phosphate buffered saline (PBS) containing 1 mg/ml glucose (neurons) or in MIB (mitochondria) at 37°C. Isolated rat brain mitochondria in MIB and neurons in FM were loaded with NS1619 (10–200 µM) and TMRE (0.5 µM). A second set of isolated mitochondria were co-treated with IbTx (1 µM), ChTx (1 µM), Pax (4 µM), or 4AP (1 mM). After 20 min of incubation in the dark at 4°C (mitochondria) or 37°C (neurons), the drugs were washed out thoroughly, and TMRE-fluorescence was measured in each well using the same microplate reader that was used for viability measurements (λex = 510 nm, λem = 590 nm). Data were expressed as a percentage of the intensity of the untreated control culture:
Analysis of ROS formation
Reactive oxygen species generation was assessed in black-walled 96-well plates with HEt using the same microplate reader used in the TMRE measurements. Isolated rat brain mitochondria in MIB, and neurons in transport medium (Hibernate A supplemented with 2% B27, 0.5 mM l-glutamine, 55 µM 2-mercaptoethanol, and 25 mM KCl) were loaded with HEt (5 µM) and were treated with NS1619 (10–200 µM) 1 min before the assay. In another experiment, rat brain mitochondria were loaded with HEt (5 µM) and were treated with NS1619 (100 µM), and one of the following K+ channel blockers: IbTx (1 µM), ChTx (1 µM), Pax (4 µM), or 4AP (1 mM) 1 min before the assay. HEt-fluorescence (λex = 510, λem = 590 nm) in each well was measured at 37°C every minute for 30 min. Data were expressed as a change in relative fluorescent intensity/minute or as a percentage of the starting intensity of the untreated control as follows:
Measurement of plasma membrane potential
Plasma membrane potential was monitored using the voltage sensitive dye di-8-ANEPPS. Neuronal cultures in FM were loaded with 1 µM di-8-ANEPPS in PBS in the dark for 20 min at 37°C, then were washed three times with PBS and placed into transport medium. Confocal images of cellular di-8-ANEPPS fluorescence were acquired using a laser scanning microscope (LSM 510; Zeiss, Jena, Germany) with a 63× water immersion objective (Zeiss). Fluorescent images (λex = 488 nm, λem1 > 650 nm, and λem2 = 500–550 nm) of randomly selected fields were recorded every 20 s. NS1619 (10–200 µM) or vehicle was administered after 1 min of measurement. In other experiments, IbTx (1 µM), ChTx (1 µM), or 4AP (1 mM) was co-applied with 100 µM NS1619. The average pixel intensity of individual cell bodies was determined using the software supplied by the manufacturer (LSM 510; Zeiss). Ratio of emissions was calculated and data were expressed as a percentage of the starting ratio of the corresponding control culture using the following equations:
and
ATP assay
The ATP level of neurons was measured with the glow-type CellTiter-Glo Luminescent Assay. Cortical neurons cultured in opaque-walled 96-well plates were equilibrated to room temperature (21°C) for 30 min. CellTiter-Glo was added to each well, and the plates were incubated at room temperature for 10 min to stabilize the luminescent signal, which was then measured with a FLUOstar OPTIMA microplate reader. An ATP standard curve was generated for each measurement to calculate the ATP contents of wells.
Western blotting for cytochrome c, calreticulin, α and β4 subunits of the BKCa channel, total and phosphorylated Akt, and Gsk3β
Cultured cells were washed twice in ice-cold PBS and then were harvested by scraping in ice-cold Nonidet P40 lysis buffer supplemented with proteinase inhibitors (1 µg/mL aprotinin, 50 µg/mL phenylmethylsulfonyl fluoride, and 1 µg/mL leupeptin), and a phosphatase inhibitor cocktail (1 mM EDTA, 1 mM sodium orthovanadate, 10 µg/mL benzamidine, 1 mM sodium pyrophosphate, and 1 mM sodium fluoride). Equal amounts of protein for each sample were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a polyvinylidine difluoride sheet (Polyscreen PVDF; Perkin Elmer Life Sciences, Boston, MA, USA). Membranes were incubated in a blocking buffer (Tris-buffered saline, 0.1% Tween 20 and 5% skimmed milk powder) for 1 h at room temperature (21°C) after which the blots were incubated with polyclonal anti-calreticulin (1 : 5000), monoclonal anti-cytochrome c (1 : 1000), monoclonal anti-BKCa α subunit (1 : 500), polyclonal anti-BKCa channel β4 subunit (1 : 400), monoclonal anti-Akt (1 : 1000), polyclonal anti-pS473 Akt (1 : 2500), monoclonal anti-Gsk3β (1 : 1000), and polyclonal anti-phospho-Gsk3α/β (1 : 1000) antibodies overnight at 4°C. The membranes were then washed three times in Tris-buffered saline with 0.1% Tween 20 and incubated for 1 h in the blocking buffer with anti-rabbit IgG (1 : 50 000) or anti-mouse IgG (1 : 5000) conjugated to horseradish peroxidase. The final reaction products were visualized using enhanced chemiluminescence (SuperSignal West Pico; Pierce, Rockford, IL, USA) and recorded on X-ray film. Films were digitalized using a FOTO/Analyst® Investigator-PC Electronic Documentation System and the provided Image v5.00 software (FOTODYNE Inc., Hartland, WI, USA). Densitometry was performed with the public domain ImageJ 1.30v software (http://rsb.info.nih.gov/ij; National Institutes of Health, USA).
Catalase assay
Catalase activity was assessed with the Amplex Red Catalase Activity Assay Kit. Neurons on 96-well plates were pre-treated with NS1619 (50, 100 µM) for 3 days, then 24 h after the last treatment the plates were rinsed with PBS, and enzyme activity was measured with the same microplate reader that was used for other fluorescent measurements (λex = 555 nm and λem = 590 nm). A standard curve was generated for each measurement to calculate catalase activity of wells. Data were expressed as a percentage of the activity of the untreated control culture using the following equation:
Enzyme activity assay for manganese superoxide dismutase
Manganese superoxide dismutase (MnSOD) activity was measured using the tetrazolium-based SOD Assay Kit as directed by the manufacturer. Neurons on 96-well plates were pre-treated with NS1619 (50, 100 µM) for 3 days, then 24 h after the last treatment the cells were incubated with NaCN (5 mM) for 10 min to inhibit CuZnSOD activity. Subsequently the plates were rinsed with PBS, and enzyme activity was measured with the same microplate reader that was used for the viability measurements (λabs = 460 nm). Data were expressed as a percentage of the activity of the untreated control culture using the following equations:
and
Glutathione peroxidase assay
Glutathione peroxidase (GPx) activity was measured with the Glutathione Peroxidase Assay Kit as directed by the manufacturer. The assay measures GPx activity indirectly by the oxidation of NADPH to NADP+. Neurons on 96-well plates were pre-treated with NS1619 (50, 100 µM) for 3 days, then 24 h after last treatment the plates were rinsed with PBS, and changes in absorbance at 350 nm were measured every minute for 10 min with the same microplate reader as that used for the viability measurements. Data were expressed as a percentage of the activity of the untreated control culture using the following equations:
(where 0.00373 µM−1 is the adjusted NADPH extinction coefficient, 0.02 ml is the sample volume, and 0.19 ml is the final volume) and
Caspase-3/7 activity assay
Caspase-3/7 activity was assessed using the SensoLyte TM Homogenous AMC Caspase-3/7 Assay Kit as directed by the manufacturer. Neuronal cells on 96-well plates were treated with NS1619 (10, 25, 50, 100 µM) for 3 days, then 24 h after last treatment the plates were exposed to OGD, H2O2, or glutamate, as in the viability experiments. Caspase activity was measured every 5 min for 1 h starting immediately after the insults with the same microplate reader as that used for other fluorescent measurements (λex = 355 nm and λem = 460 nm). Caspase activity in corresponding control cultures was monitored without exposure to neurotoxic insults. The change of activity was calculated from the linear part of the curves using the following equation:
Statistical analysis
Statistical analyses were performed with SigmaStat (SPSS, Chicago, IL, USA). Data are presented as means ± SEM. Differences between groups were assessed by one way anova or two way repeated measures anova, where appropriate, followed by Tukey comparison tests. A value of p < 0.05 was considered to be statistically significant.
Results
NS1619 induced delayed PC
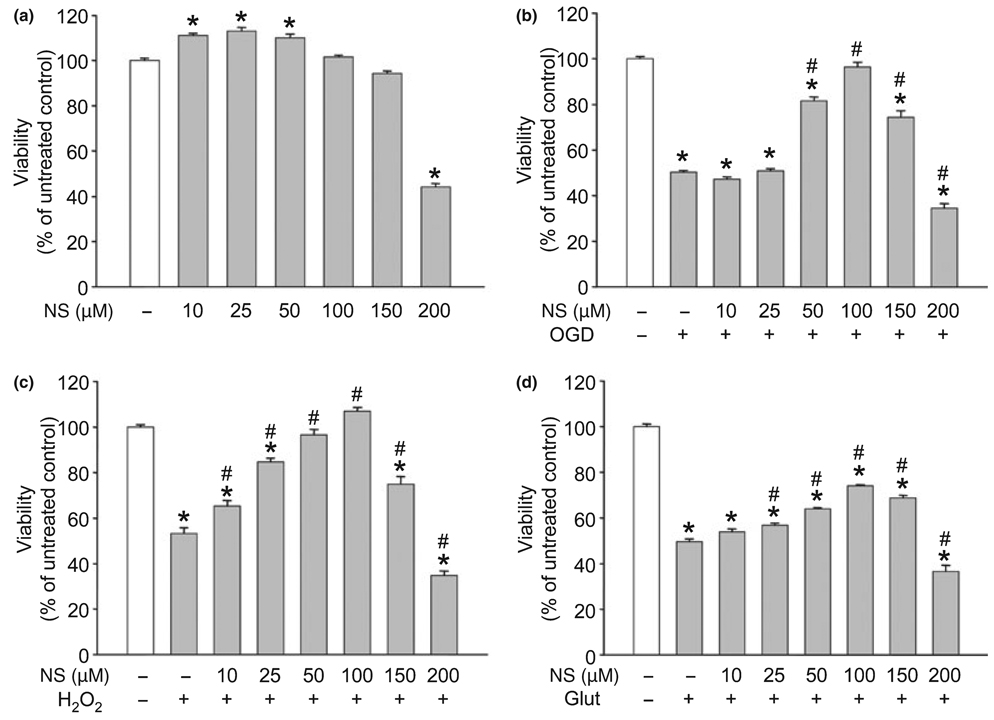
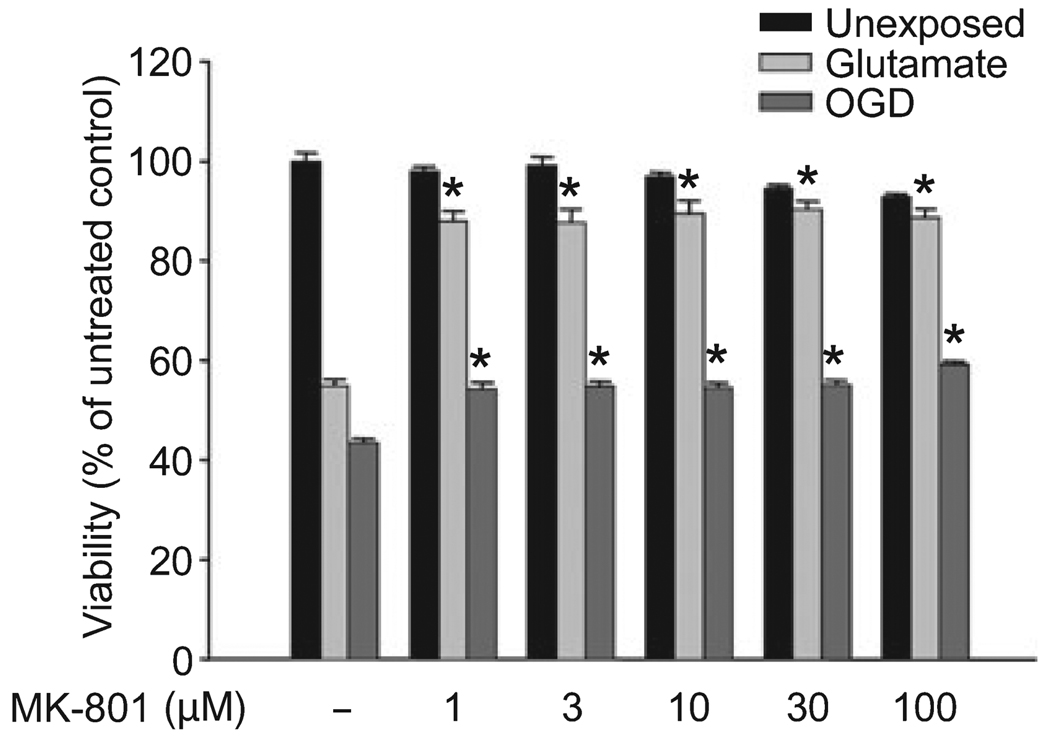
One-day treatment with NS1619 induced minimal neuroprotection against OGD, hydrogen peroxide, and glutamate excitotoxicity (data not shown); therefore, a once-daily treatment protocol was used for 3 days to induce delayed PC. Treatment for 6 h each day with concentrations of NS1619 below 150 µM had no toxic effect on the viability of quiescent cells, whereas doses of 200 µM and above resulted in significant cell death (Fig. 1a). This treatment protocol resulted in a robust and dose-dependent protection against both OGD (Fig. 1b) and hydrogen peroxide toxicity (Fig. 1c). In both cases, the best protection was achieved using 100 µM NS1619 but lower doses of the drug provided better survival after the H2O2 challenge. The improvement of neuronal viability after glutamate excitotoxicity showed similar dose-dependency, but NS1619 proved to be less protective in this paradigm (Fig. 1d). Because of the apparent difference between the protection against OGD and glutamate toxicity, to test the involvement of NMDA receptors in the mediation of these two cell death paradigms, we performed experiments in which increasing doses of the NMDA receptor antagonist MK-801 (1–100 µM) were applied to the cells during exposure to either OGD (180 min) or glutamate (200 µM, 60 min), and found that MK-801 almost completely antagonized glutamate excitotoxicity-induced cell death while it was much less effective against OGD (Fig. 2). In addition, no dose-dependence of MK-801 was seen with the tested concentrations. As NS1619 showed the best protection against hydrogen peroxide, this experimental setup was used in further experiments to study the protective mechanism of the compound.
Fig. 1.
NS1619 (NS) induced delayed neuronal preconditioning. Cultured cortical neurons were treated with NS1619 for 6 h daily on three consecutive days. The effect of NS1619 per se on cell survival was assessed 48 h after the last treatment (a). To test the protective effect of NS1619, 24 h after the last treatment other cultures were exposed to oxygen and glucose deprivation (OGD) for 180 min (b), 80 µM hydrogen peroxide (H2O2) for 6 h (c), or 200 µM glutamate (Glut) for 60 min (d). Cell viability was then measured on the next day. In all experiments, viability was expressed as a percent of the viability of control cultures which were not treated with NS1619 and were not exposed to any of the toxic insults (white bars). *Significant difference (p < 0.05) compared to untreated control cultures which were not exposed to any of the insults. #Significant difference (p < 0.05) compared to untreated cultures which were exposed to the corresponding toxic insult. Data are expressed as mean ± SEM; cells from at least two individual cultures (n = 16–32).
Fig. 2.
The differential involvement of NMDA receptors in glutamate and oxygen-glucose deprivation (OGD)-induced cell death. Cortical neuronal cultures were exposed to glutamate (200 µM) for 60 min or to OGD for 180 min in the presence of increasing doses of the NMDA receptor antagonist MK-801. *Significant difference (p ≤ 0.05) compared to untreated control cultures which were exposed to the same cytotoxic paradigm. Data are expressed as mean ± SEM; cells from at least two individual cultures (n = 8–16).
The K+ channel antagonists could not abolish the PC effect of NS1619
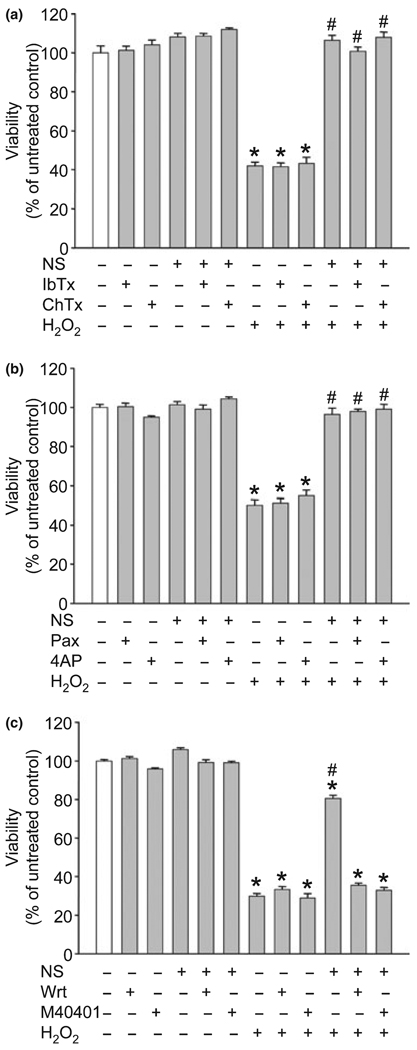
To identify the underlying mechanisms of delayed PC, we co-treated neuronal cultures with NS1619 (100 µM), and with the BKCa channel inhibitors IbTx (Fig. 3a), ChTx (Fig. 3a), or the lipid soluble Pax (Fig. 3b). Furthermore, we tested whether the voltage sensitive potassium (KV) channel inhibitor 4AP (Fig. 3b) had any antagonizing effect on the neuroprotection. The selected doses of these compounds showed no effect on neuronal viability. Moreover, none of them blocked the delayed PC effect of NS1619.
Fig. 3.
The phosphoinositide 3-kinase inhibitor wortmannin (Wrt) and the superoxide dismutase mimetic M40401 but not the K+ channel blockers antagonized the preconditioning effect of NS1619 (NS). Cultured cortical neurons were treated with NS1619 (100 µM) with or without the large conductance Ca2+ sensitive K+ channel inhibitors iberiotoxin (IbTx; 1 µM), charybdotoxin (ChTx; 1 µM) (a), paxilline (Pax; 4 µM), the voltage sensitive K+ channel blocker 4-aminopyridine (4AP; 1 mM) (b), Wrt (100 nM), or M40401 (50 µM) (c) for 6 h daily on three consecutive days. On the next day, the cultures were exposed to 80 µM H2O2 for 6 h. Cell viability was measured 24 h later and was expressed as a percent of control cultures which were not treated with any of the compounds and were not exposed to H2O2. *Significant difference (p < 0.05) compared to untreated control cultures which were not exposed to H2O2. #Significant difference (p < 0.05) compared to untreated cultures which were exposed to H2O2. Data are expressed as mean ± SEM; cells from at least two individual cultures (n = 8–32).
Reactive oxygen species generation and the activation of the PI3K signaling pathway were essential in the initiation of delayed PC
The PI3K inhibitor Wrt proved to be safe within a dose range between 10 nM and 100 nM and had little effect on cell survival (Fig. 3c). On the other hand, co-application of Wrt with NS1619 was effective in antagonizing the neuroprotective effect. In fact, 100 nM Wrt completely eliminated the protection. Treatment of neurons with doses of the superoxide dismutase mimetic M40401 between 10 µM and 50 µM did not influence viability of control neurons and did not affect cell survival after exposure to H2O2 (Fig. 3c). However, co-treatment with M40401 completely abolished the delayed PC effect of NS1619.
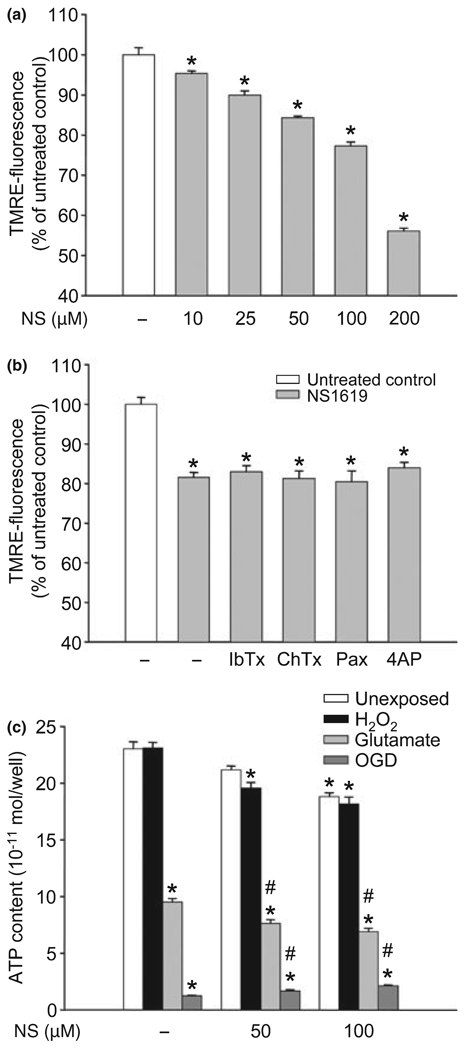
NS1619 induced mitochondrial depolarization and ROS generation
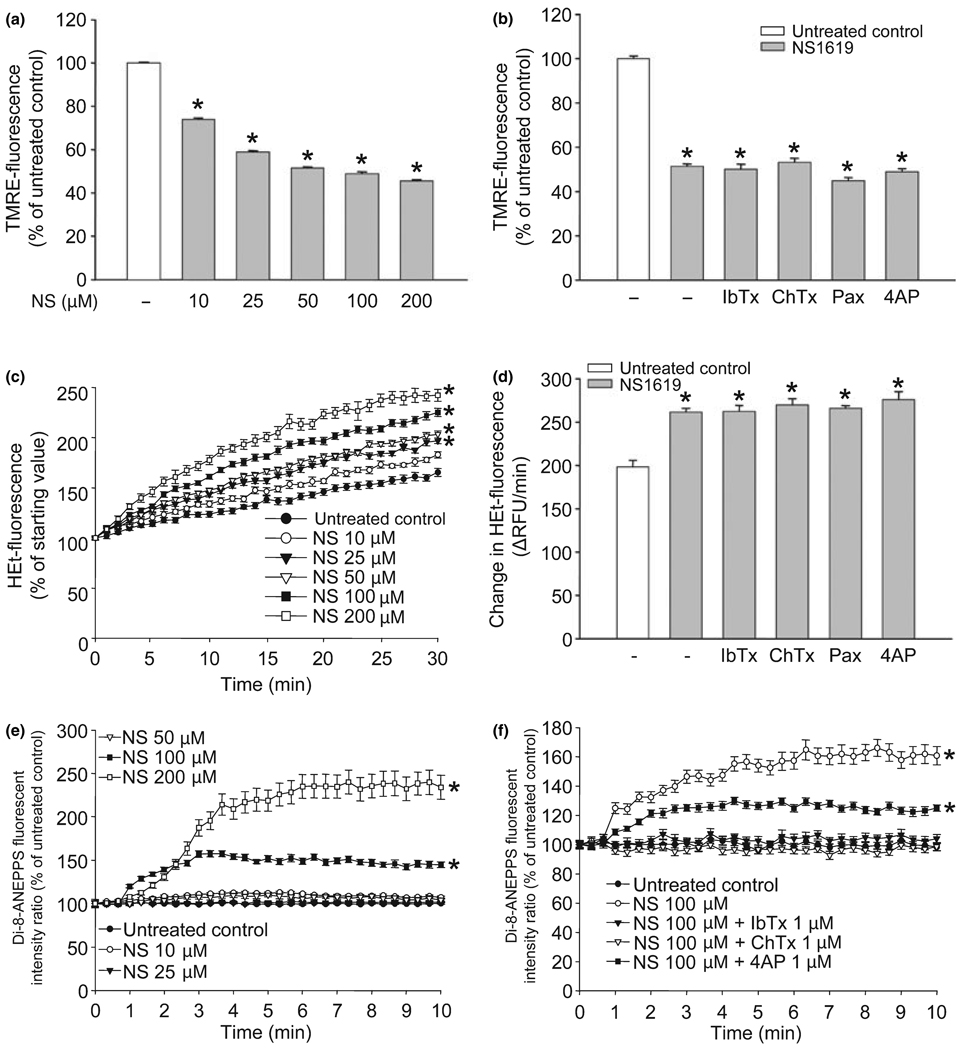
We tested the immediate effects of NS1619 on both isolated brain mitochondria and cultured neurons. The compound dose-dependently depolarized isolated mitochondria (Fig. 4a) indicated by the decrease of TMRE-fluorescence intensity. Mitochondrial depolarization was also seen, although to a lesser extent, in cultured neurons (TMRE-fluorescence: untreated control, 100.00 ± 2.10%; NS1619 25 µM, 97.49 ± 1.62%; NS1619 50 µM, 88.01 ± 2.28%*; NS1619 100 µM, 79.02 ± 1.68%*; NS1619 150 µM, 70.79 ± 1.56%*; NS1619 200 µM, 67.44 ± 1.16%*; *p < 0.05 vs. untreated control; data presented as mean ± SEM, n = 16). However, mitochondrial depolarization could not be antagonized using BKCa channel antagonists, or a KV channel blocker (Fig. 4b). Similar dose-response curves of ROS production were generated in isolated mitochondria (Fig. 4c) which showed a marked increase in HEt-fluorescence over time depending on the dose of NS1619 applied. NS1619 also increased ROS generation in cultured neurons (HEt-fluorescence of neurons after 30 min of measurement, expressed as the % of starting value: untreated control, 205.60 ± 6.62%; NS1619 10 µM, 211.40 ± 4.70%; NS1619 25 µM, 234.80 ± 4.03%*; NS1619 50 µM, 267.60 ± 7.08%*; NS1619 100 µM, 322.60 ± 5.85%*; NS1619 200 µM, 402.30 ± 7.66%*; *p < 0.05 vs. untreated control; values presented as mean ± - SEM, n = 32). Again, the K+ channel blockers did not reduce the ROS surge elicited by NS1619 in mitochondria (Fig. 4d).
Fig. 4.
NS1619 (NS) induced mitochondrial depolarization, reactive oxygen species (ROS) generation, and plasma membrane hyperpolarization. To assess mitochondrial membrane potential, isolated rat brain mitochondria were treated with NS1619 (10–200 µM) and loaded with tetramethylrhodamine ethyl ester (TMRE) (a). Another set of isolated mitochondria was treated with NS1619 (100 µM) with or without iberiotoxin (IbTx; 1 µM), charybdotoxin (ChTx; 1 µM), paxilline (Pax; 4 µM), or 4-aminopyridine (4AP; 1 mM) during loading with TMRE (b). After 20 min, mitochondria were washed, and TMRE fluorescence was measured using a fluorescent microplate reader. *Significant difference (p < 0.05) compared to untreated control cultures (n = 14–24). Mitochondrial ROS production was monitored using the fluorescent dye, hydroethidine (HEt). Isolated rat brain mitochondria were treated with NS1619 (10–200 µM) 1 min before loading with HEt (c). The changes of HEt-fluorescence were recorded for 30 min using a fluorescent microplate reader. *Significant difference (p < 0.05) compared to the fluorescent intensity of untreated control mitochondria after 30 min of measurement (n = 14–16). To evaluate the effect of K+ channel inhibitors on NS1619 induced ROS generation, isolated mitochondria were co-treated with NS1619 (100 µM) and IbTx (1 µM), ChTx (1 µM), Pax (4 µM), or 4AP (1 mM) 1 min before loading with HEt (d). HEt fluorescent intensity was measured for 30 min with a fluorescent microplate reader and was expressed as a change in relative fluorescent units/minute (ΔRFU/min). *Significant difference (p < 0.05) compared to the fluorescent intensity of untreated control mitochondria (n = 12–16). Plasma membrane potential of cultured cortical neurons was measured with the voltage sensitive dye, 4-{2-[6-(dioctylamino)-2-naphthalenyl]ethenyl}-1-(3-sulfopropyl)-pyridinium (di-8-ANEPPS). The intensity of di-8-ANEPPS fluorescence was recorded using confocal microscopy. Neuronal cultures were challenged with either NS1619 (10–200 µM) alone (e) or with NS1619 (100 µM) and IbTx (1 µM), ChTx (1 µM), or 4AP (1 mM) (f) 1 min after the initiation of measurement. The ratio of emissions was calculated and the fluorescent intensity ratio of the starting value was considered 100%. *Significant difference (p < 0.05) compared to untreated control; n = 30–37. All data are expressed as mean ± SEM.
NS1619 resulted in plasma membrane hyperpolarization
Lower doses of NS1619 did not change plasma membrane potential significantly (Fig. 4e). To induce marked hyperpolarization, shown as an increase in the di-8-ANEPPS fluorescent ratio, the administration of at least 100 µM NS1619 was needed. IbTx (1 µM) and ChTx (1 µM) completely, and 4AP (1 mM) partially counteracted the effect of 100 µM NS1619 on the cell membrane (Fig. 4f). The K+ channel antagonists per se had no effect on the plasma membrane potential of cortical neurons (data not shown).
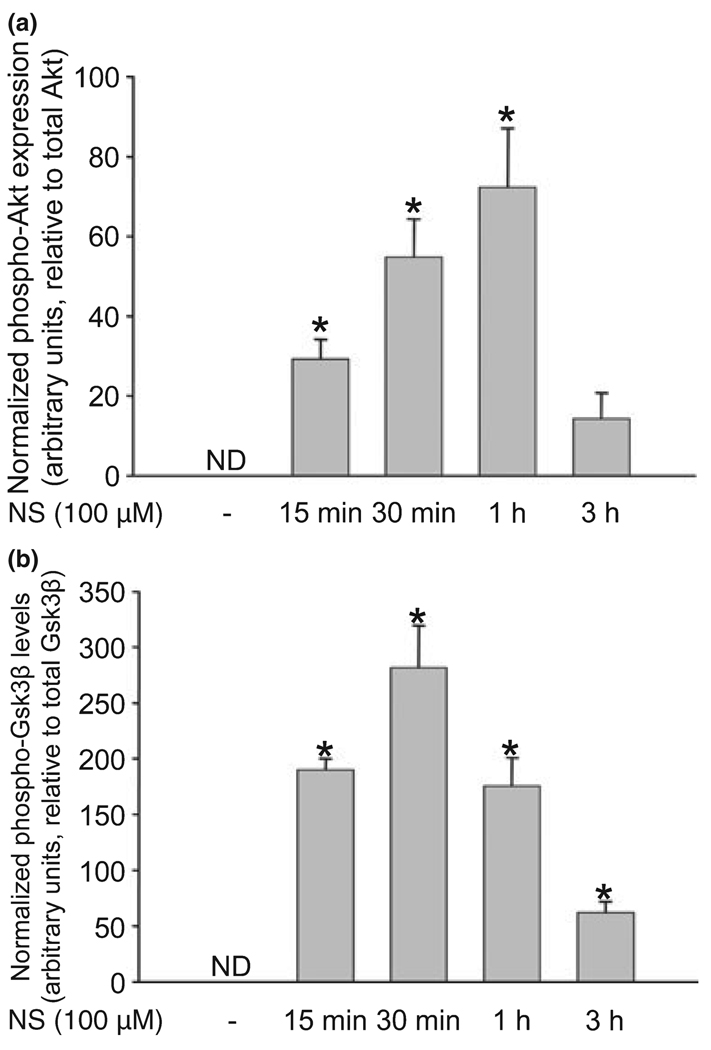
NS1619 induced rapid activation of Akt and inhibition of Gsk3β
Western blot analysis showed undetectable levels of the phosphorylated forms of Akt (Fig. 5a) and Gsk3β (Fig. 5b) in untreated control neurons. Treatment with 100 µM NS1619 resulted in almost immediate phosphorylation of both proteins. While the highest expression of phosphorylated Akt was measured 1 h after the initiation of NS1619 treatment, Gsk3β phosphorylation peaked at 30 min. In both cases, the level of the phosphorylated proteins approached baseline within 3 h. NS1619 had no effect on the expression of phosphorylated protein kinase C (data not shown).
Fig. 5.
NS1619 (NS) induced the phosphorylation of Akt and glycogen synthase kinase 3 β (Gsk3β). Neuronal cultures in 35 mm dishes were treated with NS1619 (100 µM) for 15 min, 30 min, 1 h, and 3 h after which proteins were extracted and subjected to western blot analysis for total and phosphorylated Akt (a), and Gsk3β (b). The levels of phosphorylated forms were normalized to the total amount of the same protein. Expression of the phosphorylated form of proteins in the untreated control groups was not detectable (ND). *Significant difference (p < 0.05) compared to the normalized protein level of untreated control. Data are expressed as mean ± SEM; n = 4.
Chronic NS1619 treatment induced sustained mitochondrial depolarization and mild but significant depletion of cellular ATP
Three-day treatment with increasing doses of NS1619 resulted in dose-dependent mitochondrial depolarization which was still detectable 24 h after the last treatment, at the time of the cell death paradigms (Fig. 6a). Additionally, co-treatment with K+ channel blockers (IbTx, 1 µM; ChTx, 1 µM; Pax, 4 µM; 4AP, 1 mM) had no effect on NS1619-induced sustained mitochondrial depolarization (Fig. 6b).
Fig. 6.
Three-day treatment with NS1619 (NS) induced sustained mitochondrial depolarization and mild ATP depletion. Cultured neurons were treated with different doses of NS (a) or with 100 µM NS in the presence of K+ channel antagonists iberiotoxin (IbTx, 1 µM), charybdotoxin (ChTx, 1 µM), paxilline (Pax, 4 µM), or 4-aminopyridine (4AP, 1 mM) (b) for 6 h daily on three consecutive days. Mitochondrial membrane potential was measured 24 h after the last treatment using tetramethylrhodamine ethyl ester (TMRE) and a fluorescent microplate reader. *Significant difference (p < 0.05) compared to untreated control cultures. (c) Twenty four hours after treatment with NS (50 µM, 100 µM) on three consecutive days for 6 h daily the cells were exposed to hydrogen peroxide (80 µM) for 6 h, glutamate (200 µM) for 1 h, or combined oxygen and glucose deprivation (OGD) for 3 h. ATP content was measured immediately after the termination of the toxic challenges. *Significant difference (p < 0.05) compared to the untreated control group which was not exposed to any of the insults. #Significant difference (p < 0.05) compared to the unexposed control with the same treatment. All data are expressed as mean ± SEM; cells from two individual cultures; n = 8–16.
Treatment for 3 days with NS1619 (50 µM, 100 µM) mildly reduced the ATP content of neurons (Fig. 6c). Six-hour exposure to hydrogen peroxide (80 µM) did not change ATP levels in neurons. In contrast, exposing cells to 60 min of glutamate excitotoxicity (200 µM) resulted in robust ATP depletion. Similarly, 180 min exposure to OGD caused almost complete loss of ATP.
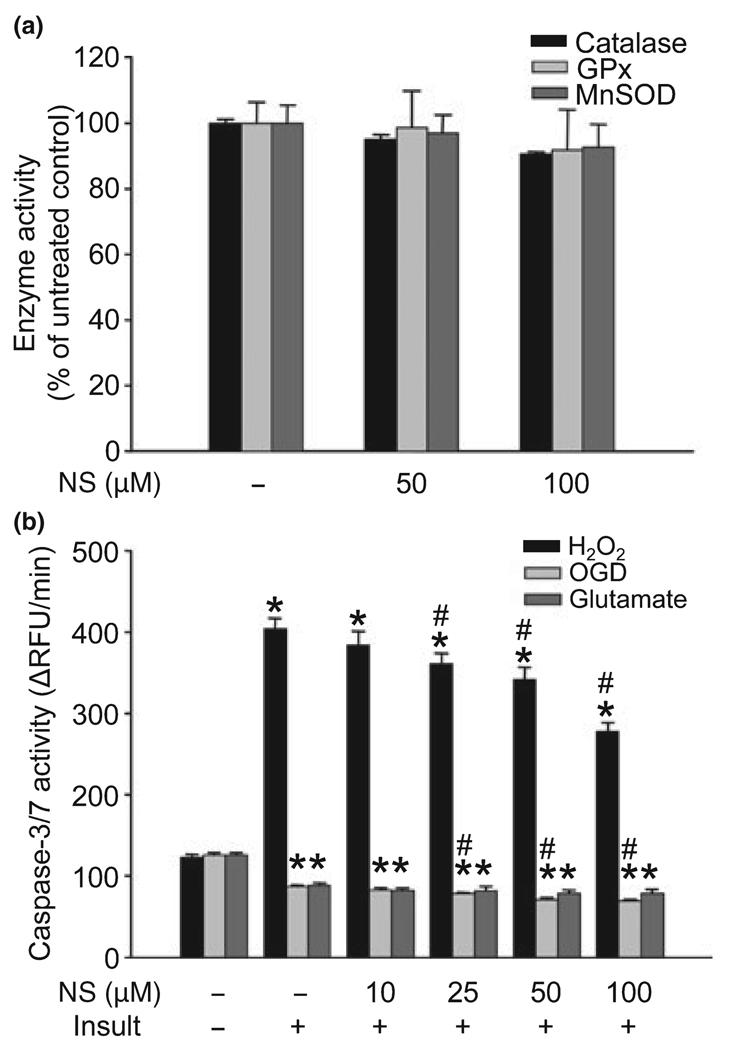
Chronic treatment with NS1619 did not affect the activity of cellular antioxidants, but reduced caspase-3/7 activation upon exposure to hydrogen peroxide
Three-day treatment of cultured neurons with the most effective doses of NS1619 (50 and 100 µM) had little effect on the activity of catalase, GPx, and MnSOD (Fig. 7a). On the other hand, NS1619-treatment effectively decreased the activity of caspases 3 and 7 in resting cells (caspase-3/7 activity in relative fluorescent unit change/min: untreated control, 117.24 ± 3.06; NS1619 10 µM, 121.29 ± 3.37; NS1619 25 µM, 118.28 ± 2.12; NS1619 50 µM, 100.15 ± 1.73*; NS1619 100 µM, 93.11 ± 1.87*, *p < 0.05 vs. untreated control; data expressed as mean ± SEM, n = 8–16). Exposure to H2O2 (80 µM) for 6 h resulted in an approximate four-fold increase in caspase-3/7 activity which was significantly and dose-dependently reduced by NS1619 treatment (Fig. 7b). In contrast, OGD and glutamate excitotoxicity caused a considerable decline in the activity of caspases which was further reduced by NS1619 in the OGD paradigm (Fig. 7b).
Fig. 7.
NS1619 (NS) did not change the activity of catalase, glutathione peroxidase (GPx), and manganese superoxide dismutase (MnSOD) but inhibited the activation of caspase-3/7. Cortical neuronal cultures were treated with NS1619 (50, 100 µM) for 6 h daily on three consecutive days then on the fourth day enzymatic activity of catalase, GPx, and MnSOD was measured using commercially available kits (a). Enzyme activity of the untreated control cultures was considered 100%. Data presented as mean ± SEM, n = 11–16. To evaluate caspase-3/7 activity (b), neuronal cultures were treated with NS1619 (10–100 µM) for 6 h daily on three consecutive days then 24 h after the last treatment cultures were exposed to 80 µM H2O2 for 6 h, oxygen and glucose deprivation (OGD) for 180 min, or 200 µM glutamate for 60 min. Caspase activity measurement was initiated immediately after the termination of toxic insults using a commercially available kit and a fluorescent microplate reader. Fluorescent values were recorded every 5 min for 60 min, and then the change in relative fluorescent units/minute (ΔRFU/min) was calculated. *Significant difference (p < 0.05) compared to the caspase-3/7 activity of untreated control which was not exposed to any of the toxic insults (leftmost triplet of bars). #Significant difference (p < 0.05) compared to untreated cultures exposed to the same insult. Data presented as mean ± SEM; n = 8–16.
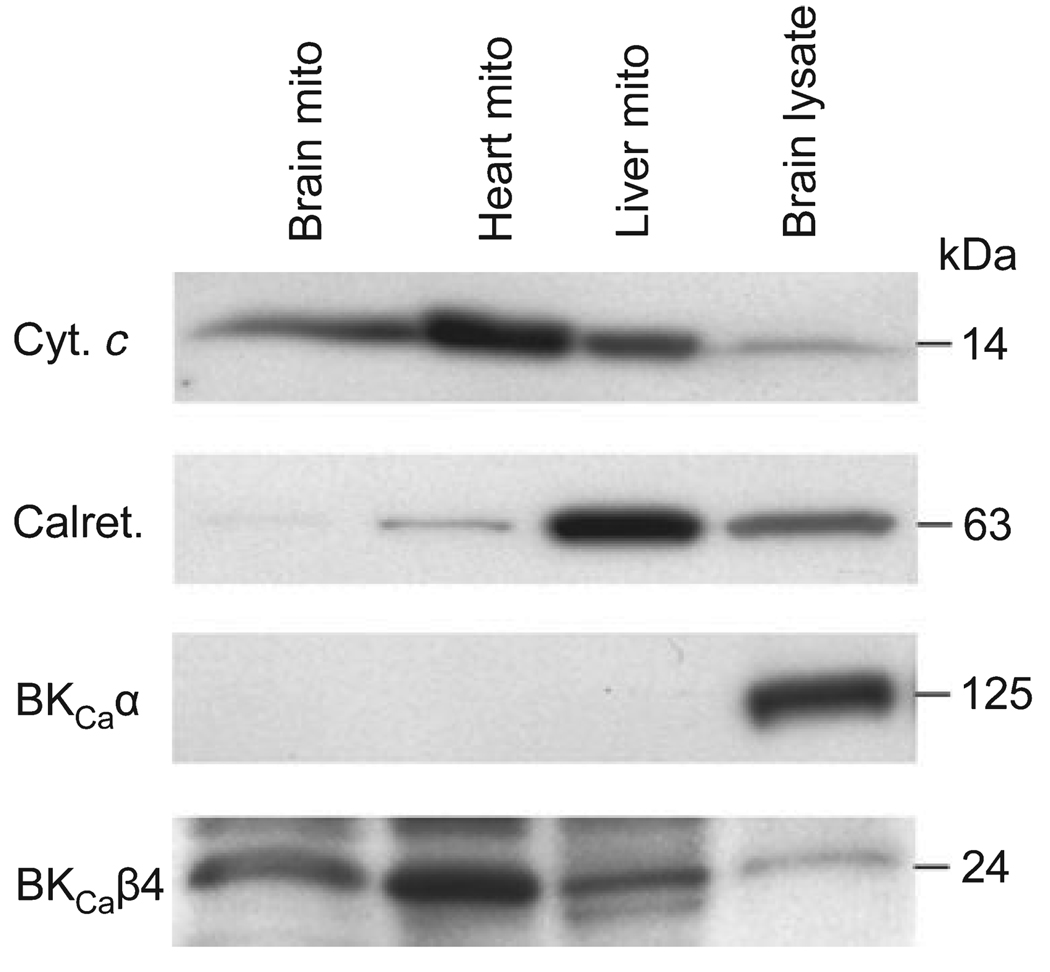
Expression of BKCaα and BKCaβ4 in mitochondria
The expression of BKCaα and BKCaβ4 was examined in mitochondria isolated from the rat brain, heart, and mitochondria rich preparations from the rat liver, as well as in rat brain lysate using western blotting (Fig. 8). The purity of mitochondrial samples was assessed using antibodies against the mitochondrial-specific protein, cytochrome c, and the endoplasmic reticulum-specific calreticulin. Very little contamination with calreticulin was found in the brain and heart samples. Nevertheless, none of the mitochondria enriched preparations expressed BKCaα. Brain lysate, on the other hand, expressed high levels of the examined protein. In contrast, BKCaβ4 could be found in each mitochondrial preparation. Furthermore, we found higher expression in the mitochondrial fractions than in brain lysate.
Fig. 8.
Expression of α and β4 subunits of the large conductance Ca2+ activated K+ (BKCa) channel in mitochondrial preparations. Mitochondria were isolated from the brain (Brain mito), heart (Heart mito), and mitochondria rich preparations from the liver (Liver mito) of male Sprague–Dawley rats. Samples were subjected to western blot analysis for the BKCa channel α (BKCaα), and β4 (BKCaβ4) subunits. Rat brain lysate was used as a positive control. The purity of preparations was tested using antibodies directed against the mitochondria-specific cytochrome c (Cyt. c) and the endoplasmic reticulum-specific calreticulin (Calret.).
Discussion
Our study is the first to demonstrate neuronal PC with the BKCa channel agonist NS1619. We also showed that (i) the neuroprotection could not be counteracted using BKCa or KV channel blockers; on the other hand, the PI3K inhibitor Wrt, and the superoxide dismutase mimetic M40401 completely antagonized the delayed PC effect of NS1619; (ii) NS1619 depolarized mitochondria and increased ROS generation; (iii) the BKCa channel opener activated the PI3K – Akt – Gsk3β signaling axis, and inhibited the activation of caspases 3 and 7; and finally (iv) we could not verify the existence of the α subunit of the BKCa channel, which is the essential pore forming subunit, in rat mitochondria.
The BKCa channel was first identified in the plasma membrane of red blood cells (Gardos 1958). Later, the channel was shown to be present in mitochondria of a glioma cell line (Siemen et al. 1999), in heart mitochondria (Xu et al. 2002), and recently it has been also found in mitochondria of the brain (Douglas et al. 2006). The cardioprotective effect of mitoBKCa channels was first suggested in the heart of guinea pigs (Xu et al. 2002). In that study, NS1619 induced cardioprotection to an extent similar to that elicited by mitochondrial ATP sensitive K+ channel openers. Moreover, the protection was independent of the vasodilator effect of the drug and could be antagonized by the BKCa channel blocker Pax (Xu et al. 2002). Notably, neither NS1619 nor Pax are mitoBKCa specific. Furthermore, no pharmacological agents specific to these putative channels are currently known. Therefore, caution must be exerted when interpreting the PC effect of NS1619 as a pure action on mitoBKCa channels. It has also been reported that BKCa channel openers inhibit mitochondrial function because of inhibition of Complex I of mitochondrial respiratory chain in human glioma cells (Debska et al. 2003) and in isolated cardiac mitochondria (Kicinska and Szewczyk 2004). Moreover, NS1619 was shown to inhibit L-type Ca2+ and KV channels (Edwards et al. 1994; Holland et al. 1996; Park et al. 2007). Finally, NS1619 also induces Ca2+ release from intracellular pools (Yamamura et al. 2001; Korper et al. 2003). These studies suggest that characteristics unrelated to BKCa channels may also contribute to the beneficial effects of NS1619 via mitoBKCa-independent activation of cytoprotective signal transduction pathways.
In our experiments, NS1619 effectively induced delayed neuronal PC against various toxic stimuli. Treatment of neurons for 3 days proved to be more effective than a single-day protocol; therefore, our experiments were performed using the 3-day regimen. Previously we showed a similar improvement in the protection with 3-day paradigms using other pharmacological PC agents (Kis et al. 2003; Gaspar et al. 2007). In the present study, we demonstrated dose-dependent neuroprotection with NS1619 against exogenous hydrogen peroxide toxicity, OGD, and glutamate excitotoxicity, although protection against the latter was much less effective. To address the difference seen in the protection against OGD and glutamate, we tested the involvement of NMDA receptors in the mediation of these cytotoxic paradigms using a specific antagonist and found that under our experimental conditions glutamate excitotoxicity was almost exclusively dependent on NMDA receptor activation while NMDA receptors played only minor role in OGD-induced cell death.
The protective effect of NS1619 could not be antagonized with BKCa channel inhibitors. These results suggest that the activation of cell membrane BKCa channels is not required to initiate PC by NS1619. While IbTx and ChTx are polypeptides, Pax is reportedly lipophilic (Knaus et al. 1994), thus it is expected to cross cell membranes and might also act on the putative mitoBKCa channels in intact cells. Despite this, no inhibition of the protective effect of NS1619 was found by this mycotoxin. On the other hand, elimination of superoxide anion during the initiation of PC using M40401 completely antagonized the neuroprotection. Our results are in agreement with those reported by Stowe et al. (2006) which showed that cardiac PC with NS1619 is also dependent on superoxide generation and removal of this radical in the initiating phase of PC eliminated the cardioprotection. In addition, we found that the PI3K inhibitor Wrt was similarly effective in the inhibition of protection. This suggests that the early activation of the PI3K signaling cascade is essential for the development of delayed neuronal PC.
To identify the initiating events that ultimately led to the development of the preconditioned phenotype, we examined the immediate effects of NS1619. The compound depolarized mitochondria and induced dose-dependent ROS generation in both cultured neurons and isolated mitochondria. However, these effects could not be antagonized with K+ channel blockers. On the other hand, these antagonists blocked the cell membrane hyperpolarization. Previously we showed that 10–20 µM NS1619 induced significant hyperpolarization of cultured neurons in PBS (Gaspar et al. 2007; Mayanagi et al. 2007). Moreover, doses of NS1619 between 10–30 µM were reported to induce PC in the heart (Xu et al. 2002; Wang et al. 2004; Sato et al. 2005). In the present study, higher concentrations of the drug were needed to elicit similar effects. The fact that these experiments were performed in B27-supplemented cell culture media and albumin-containing MIB instead of in a protein-free buffer may explain this discrepancy, as binding to proteins in the medium may reduce the availability of the drug.
Next, we examined the effects of NS1619 on signal transduction pathways. Our western blots showed rapid phosphorylation of two core components of the PI3K pathway, Akt and Gsk3β. The PI3K pathway is a pro-survival signaling cascade, its role in PC was first suggested by Tong et al. (2000). Several constituents of the pathway are ROS-sensitive (Otani 2004; Sugden and Clerk 2006) and their activation leads to the phosphorylation of Akt. Akt (also known as protein kinase B) is a serine/threonine kinase which acts as a master switch integrating signals from both inside and outside the cell and regulates a variety of cellular processes including inhibition of apoptosis, control of cell proliferation, and metabolism with implications in the pathomechanism of diseases such as cancer and diabetes (for reviews see Engelman et al. 2006; Manning and Cantley 2007). Activation of Akt by phosphorylation was demonstrated in several PC and ischemia/reperfusion models (Tong et al. 2000; Yano et al. 2001; Kamada et al. 2007). Among many others, active Akt phosphorylates Gsk3β, caspase-9, and Bad. Gsk3β is a constitutively active serine/threonine kinase and its phosphorylation at serine-9 results in inactivation. Among the substrates of Gsk3β, several metabolic and signaling proteins, structural proteins, and transcription factors have been identified (Grimes and Jope 2001) and numerous studies have linked Gsk3β to Alzheimer’s disease and bipolar disorder (reviewed in Doble and Woodgett 2003). Inhibition of Gsk3β was also demonstrated in neuroprotective studies (Chin et al. 2005; Liang and Chuang 2007). These reports support our findings regarding the central role of the PI3K signaling cascade in the neuroprotective effect of NS1619.
To identify the effectors of NS1619-induced PC, we performed activity assays on several important cellular antioxidant systems, but found no change. On the other hand, we demonstrated that 3-day treatment with NS1619 caused sustained mitochondrial depolarization and a parallel mild ATP depletion which is compatible with the reported inhibitory effect on respiratory complex I (Debska et al. 2003; Kicinska and Szewczyk 2004). Exposure of neurons to exogenous hydrogen peroxide resulted in no further change in cellular ATP content. In contrast, OGD and glutamate excitotoxicity caused robust ATP depletion. Furthermore, H2O2 induced the activation of caspases 3 and 7 which was effectively antagonized by NS1619. The effector caspases 3 and 7 are downstream targets of caspase 9 which is inhibited by active Akt (Manning and Cantley 2007). In contrast, no caspase activation was observed after OGD and glutamate excitotoxicity. These results may support the findings of a previous report from our laboratory showing that in our experimental setting OGD and glutamate exposure resulted predominantly in necrosis (Kis et al. 2003).
The large conductance Ca2+ activated K+ channels consist of four pore-forming α subunits and may have modulatory β subunits; the latter has four known isoforms. Type II BKCa channels, expressed in the brain, are rendered less sensitive to IbTx and ChTx by the presence of BKCaβ4 (Reinhart et al. 1989; Meera et al. 2000). We performed western blot analysis on isolated mitochondria and mitochondria enriched samples to search for BKCaα and BKCaβ4 using commercially available antibodies. We confirmed the presence of the modulatory subunit in our samples. However, our western blots unequivocally showed that the BKCaα subunit which was highly abundant in the brain was not detectable in mitochondria. Thus, our findings neither reject nor support the existence of a functioning BKCa channel in mitochondria. BKCaα is encoded by a single gene but undergoes extensive alternative splicing (Fury et al. 2002) which might explain why our antibody picked up a strong signal in whole brain lysate but did not show any expression of a putative splice variant in the mitochondrial preparations. On the other hand, our results clearly indicate that the delayed neuronal PC effect of NS1619 is not related to this putative mitochondrial BKCa channel as the mitochondrial actions of the compound could not be antagonized using specific channel antagonists, although the doses of the blockers we used were equal to or higher than those reported to inhibit K+ currents through BKCa channels composed of BKCaα and BKCaβ4 (Meera et al. 2000). This finding is in agreement with a recent report which showed that the mitochondrial effects of NS1619 were independent of mitoBKCa specific K+ transport (Cancherini et al. 2007). Furthermore, in our study, none of the BKCa channel inhibitors could antagonize the neuroprotection. These results also demonstrate that the action of NS1619 on the cell surface BKCa channel is not an initiating event of delayed PC.
In summary, this is the first study to demonstrate the PC effect of NS1619 in neurons. The neuroprotection is independent of both cell membrane and mitochondrial BKCa channels, but is probably the result of the inhibition of the mitochondrial electron transport chain, generation of ROS, and activation of the PI3K signaling pathway. This cascade in turn results in the activation of cytoprotective mechanisms including the inhibition of caspases, and ultimately, protects neurons against various toxic insults.
Acknowledgments
The authors gratefully thank Nancy Busija, M.A., for editing the manuscript. This work was supported by the National Institutes of Health Grants (HL-030260, HL-065380, and HL-077731), Y. F. Wu Research and Education Fund, WFUSM Venture Fund, and K. G. Phillips Fund for the Prevention and Treatment of Heart Disease, and WFUSM Interm Funding for D. W. Busija and by the Hungarian Science Research Fund (OTKA K63401, K68976, and IN69967).
Abbreviations used
- 4AP
4-aminopyridine
- BKCa
large conductance Ca2+ activated potassium (channel)
- BKCaα
α subunit of BKCa channel
- BKCaβ
β subunit of BKCa channel
- ChTx
charybdotoxin
- di-8-ANEPPS
4-{2-[6-(dioctylamino)-2-naphthalenyl]ethenyl}-1-(3-sulfopropyl)-pyridinium
- EBSS
Earle’s balanced salt solution
- FM
feeding medium
- GPx
glutathione peroxidase
- Gsk
glycogen synthase kinase
- HEt
hydroethidine
- IbTx
iberiotoxin
- KV
voltage sensitive potassium (channel)
- MIB
mitochondrial isolation buffer
- mitoBKCa
mitochondrial BKCa (channel)
- MnSOD
manganese superoxide dismutase
- NS1619
1,3-dihydro-1-[2-hydroxy-5-(trifluoromethyl)phenyl]-5-(trifluoromethyl)-2H-benzimidazol-2-one
- OGD
oxygen and glucose deprivation
- Pax
paxilline
- PBS
phosphate-buffered saline
- PC
preconditioning
- PI3K
phosphoinositide 3-kinase
- ROS
reactive oxygen species
- TMRE
tetramethylrhodamine ethyl ester
- Wrt
wortmannin
References
- Busija DW, Lacza Z, Rajapakse N, Shimizu K, Kis B, Bari F, Domoki F, Horiguchi T. Targeting mitochondrial ATP-sensitive potassium channels–a novel approach to neuroprotection. Brain Res. Brain Res. Rev. 2004;46:282–294. doi: 10.1016/j.brainresrev.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Cancherini DV, Queliconi BB, Kowaltowski AJ. Pharmacological and physiological stimuli do not promote Ca(2+)-sensitive K+ channel activity in isolated heart mitochondria. Cardiovasc. Res. 2007;73:720–728. doi: 10.1016/j.cardiores.2006.11.035. [DOI] [PubMed] [Google Scholar]
- Cao CM, Xia Q, Gao Q, Chen M, Wong TM. Calcium-activated potassium channel triggers cardioprotection of ischemic preconditioning. J. Pharmacol. Exp. Ther. 2005;312:644–650. doi: 10.1124/jpet.104.074476. [DOI] [PubMed] [Google Scholar]
- Cheney JA, Weisser JD, Bareyre FM, Laurer HL, Saatman KE, Raghupathi R, Gribkoff V, Starrett JE, Jr, McIntosh TK. The maxi-K channel opener BMS-204352 attenuates regional cerebral edema and neurologic motor impairment after experimental brain injury. J. Cereb. Blood Flow Metab. 2001;21:396–403. doi: 10.1097/00004647-200104000-00008. [DOI] [PubMed] [Google Scholar]
- Chin PC, Majdzadeh N, D’Mello SR. Inhibition of GSK3beta is a common event in neuroprotection by different survival factors. Brain Res. Mol. Brain Res. 2005;137:193–201. doi: 10.1016/j.molbrainres.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Mazzon E, Dugo L, Caputi AP, Aston K, Riley DP, Salvemini D. Protective effects of a new stable, highly active SOD mimetic, M40401 in splanchnic artery occlusion and reperfusion. Br. J. Pharmacol. 2001;132:19–29. doi: 10.1038/sj.bjp.0703775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debska G, Kicinska A, Dobrucki J, Dworakowska B, Nurowska E, Skalska J, Dolowy K, Szewczyk A. Large-conductance K+ channel openers NS1619 and NS004 as inhibitors of mitochondrial function in glioma cells. Biochem. Pharmacol. 2003;65:1827–1834. doi: 10.1016/s0006-2952(03)00180-1. [DOI] [PubMed] [Google Scholar]
- Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J. Cell Sci. 2003;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas RM, Lai JC, Bian S, Cummins L, Moczydlowski E, Haddad GG. The calcium-sensitive large-conductance potassium channel (BK/MAXI K) is present in the inner mitochondrial membrane of rat brain. Neuroscience. 2006;139:1249–1261. doi: 10.1016/j.neuroscience.2006.01.061. [DOI] [PubMed] [Google Scholar]
- Edwards G, Niederste-Hollenberg A, Schneider J, Noack T, Weston AH. Ion channel modulation by NS 1619, the putative BKCa channel opener, in vascular smooth muscle. Br. J. Pharmacol. 1994;113:1538–1547. doi: 10.1111/j.1476-5381.1994.tb17171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- Fury M, Marx SO, Marks AR. Molecular Biology: the study of splicing and dicing. Sci. STKE 2002. 2002:PE12. doi: 10.1126/stke.2002.123.pe12. [DOI] [PubMed] [Google Scholar]
- Gardos G. The function of calcium in the potassium permeability of human erythrocytes. Biochim. Biophys. Acta. 1958;30:653–654. doi: 10.1016/0006-3002(58)90124-0. [DOI] [PubMed] [Google Scholar]
- Gaspar T, Kis B, Snipes JA, Lenzser G, Mayanagi K, Bari F, Busija DW. Transient glucose and amino acid deprivation induces delayed preconditioning in cultured rat cortical neurons. J. Neurochem. 2006;98:555–565. doi: 10.1111/j.1471-4159.2006.03899.x. [DOI] [PubMed] [Google Scholar]
- Gaspar T, Kis B, Snipes JA, Lenzser G, Mayanagi K, Bari F, Busija DW. Neuronal preconditioning with the antianginal drug, bepridil. J. Neurochem. 2007;102:595–608. doi: 10.1111/j.1471-4159.2007.04501.x. [DOI] [PubMed] [Google Scholar]
- Goldberg MP, Choi DW. Combined oxygen and glucose deprivation in cortical cell culture: calcium-dependent and calcium-independent mechanisms of neuronal injury. J. Neurosci. 1993;13:3510–3524. doi: 10.1523/JNEUROSCI.13-08-03510.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog. Neurobiol. 2001;65:391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- Hepp S, Gerich FJ, Muller M. Sulfhydryl oxidation reduces hippocampal susceptibility to hypoxia-induced spreading depression by activating BK channels. J. Neurophysiol. 2005;94:1091–1103. doi: 10.1152/jn.00291.2005. [DOI] [PubMed] [Google Scholar]
- Holland M, Langton PD, Standen NB, Boyle JP. Effects of the BKCa channel activator, NS1619, on rat cerebral artery smooth muscle. Br. J. Pharmacol. 1996;117:119–129. doi: 10.1111/j.1476-5381.1996.tb15163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue I, Nagase H, Kishi K, Higuti T. ATP-sensitive K+ channel in the mitochondrial inner membrane. Nature. 1991;352:244–247. doi: 10.1038/352244a0. [DOI] [PubMed] [Google Scholar]
- Kamada H, Nito C, Endo H, Chan PH. Bad as a converging signaling molecule between survival PI3-K/Akt and death JNK in neurons after transient focal cerebral ischemia in rats. J. Cereb. Blood Flow Metab. 2007;27:521–533. doi: 10.1038/sj.jcbfm.9600367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katakam PV, Jordan JE, Snipes JA, Tulbert CD, Miller AW, Busija DW. Myocardial preconditioning against ischemia-reperfusion injury is abolished in Zucker obese rats with insulin resistance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R920–R926. doi: 10.1152/ajpregu.00520.2006. [DOI] [PubMed] [Google Scholar]
- Kicinska A, Szewczyk A. Large-conductance potassium cation channel opener NS1619 inhibits cardiac mitochondria respiratory chain. Toxicol. Mech. Methods. 2004;14:59–61. doi: 10.1080/15376520490257482. [DOI] [PubMed] [Google Scholar]
- Kis B, Rajapakse NC, Snipes JA, Nagy K, Horiguchi T, Busija DW. Diazoxide induces delayed pre-conditioning in cultured rat cortical neurons. J. Neurochem. 2003;87:969–980. doi: 10.1046/j.1471-4159.2003.02072.x. [DOI] [PubMed] [Google Scholar]
- Knaus HG, McManus OB, Lee SH, et al. Tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. Biochemistry. 1994;33:5819–5828. doi: 10.1021/bi00185a021. [DOI] [PubMed] [Google Scholar]
- Korper S, Nolte F, Rojewski MT, Thiel E, Schrezenmeier H. The K+ channel openers diazoxide and NS1619 induce depolarization of mitochondria and have differential effects on cell Ca2+ in CD34+ cell line KG-1a. Exp. Hematol. 2003;31:815–823. doi: 10.1016/s0301-472x(03)00199-1. [DOI] [PubMed] [Google Scholar]
- Liang MH, Chuang DM. Regulation and function of glycogen synthase kinase-3 isoforms in neuronal survival. J. Biol. Chem. 2007;282:3904–3917. doi: 10.1074/jbc.M605178200. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Wang H, Michaelis EK. Developmental expression, compartmentalization, and possible role in excitotoxicity of a putative NMDA receptor protein in cultured hippocampal neurons. Brain Res. 1991;565:94–108. doi: 10.1016/0006-8993(91)91740-r. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Zhang Y, Bose S. Growth factors prevent mitochondrial dysfunction, loss of calcium homeostasis, and cell injury, but not ATP depletion in hippocampal neurons deprived of glucose. Exp. Neurol. 1993;121:1–13. doi: 10.1006/exnr.1993.1066. [DOI] [PubMed] [Google Scholar]
- Mayanagi K, Gaspar T, Katakam PV, Kis B, Busija DW. The mitochondrial K(ATP) channel opener BMS-191095 reduces neuronal damage after transient focal cerebral ischemia in rats. J. Cereb. Blood Flow Metab. 2007;27:348–355. doi: 10.1038/sj.jcbfm.9600345. [DOI] [PubMed] [Google Scholar]
- Meera P, Wallner M, Toro L. A neuronal beta subunit (KCNMB4) makes the large conductance, voltage- and Ca2+- activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc. Natl Acad. Sci. USA. 2000;97:5562–5567. doi: 10.1073/pnas.100118597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen SP, Munch E, Moldt P, Drejer J. Selective activation of Ca(2+)-dependent K+ channels by novel benzimidazolone. Eur. J. Pharmacol. 1994;251:53–59. doi: 10.1016/0014-2999(94)90442-1. [DOI] [PubMed] [Google Scholar]
- Otani H. Reactive oxygen species as mediators of signal transduction in ischemic preconditioning. Antioxid. Redox Signal. 2004;6:449–469. doi: 10.1089/152308604322899521. [DOI] [PubMed] [Google Scholar]
- Park WS, Kang SH, Son YK, Kim N, Ko JH, Kim HK, Ko EA, Kim CD, Han J. The mitochondrial Ca2+-activated K+ channel activator, NS 1619 inhibits L-type Ca2+ channels in rat ventricular myocytes. Biochem. Biophys. Res. Commun. 2007;362:31–36. doi: 10.1016/j.bbrc.2007.07.057. [DOI] [PubMed] [Google Scholar]
- Rajapakse N, Shimizu K, Payne M, Busija D. Isolation and characterization of intact mitochondria from neonatal rat brain. Brain Res. Brain Res. Protoc. 2001;8:176–183. doi: 10.1016/s1385-299x(01)00108-8. [DOI] [PubMed] [Google Scholar]
- Reinhart PH, Chung S, Levitan IB. A family of calcium-dependent potassium channels from rat brain. Neuron. 1989;2:1031–1041. doi: 10.1016/0896-6273(89)90227-4. [DOI] [PubMed] [Google Scholar]
- Runden-Pran E, Haug FM, Storm JF, Ottersen OP. BK channel activity determines the extent of cell degeneration after oxygen and glucose deprivation: a study in organotypical hippocampal slice cultures. Neuroscience. 2002;112:277–288. doi: 10.1016/s0306-4522(02)00092-1. [DOI] [PubMed] [Google Scholar]
- Salvemini D, Wang ZQ, Zweier JL, Samouilov A, Macarthur H, Misko TP, Currie MG, Cuzzocrea S, Sikorski JA, Riley DP. A nonpeptidyl mimic of superoxide dismutase with therapeutic activity in rats. Science. 1999;286:304–306. doi: 10.1126/science.286.5438.304. [DOI] [PubMed] [Google Scholar]
- Sato T, Saito T, Saegusa N, Nakaya H. Mitochondrial Ca2+-activated K+ channels in cardiac myocytes: a mechanism of the cardioprotective effect and modulation by protein kinase A. Circulation. 2005;111:198–203. doi: 10.1161/01.CIR.0000151099.15706.B1. [DOI] [PubMed] [Google Scholar]
- Shintani Y, Node K, Asanuma H, et al. Opening of Ca2+-activated K+ channels is involved in ischemic preconditioning in canine hearts. J. Mol. Cell. Cardiol. 2004;37:1213–1218. doi: 10.1016/j.yjmcc.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Siemen D, Loupatatzis C, Borecky J, Gulbins E, Lang F. Ca2+-activated K channel of the BK-type in the inner mitochondrial membrane of a human glioma cell line. Biochem. Biophys. Res. Commun. 1999;257:549–554. doi: 10.1006/bbrc.1999.0496. [DOI] [PubMed] [Google Scholar]
- Stowe DF, Aldakkak M, Camara AK, Riess ML, Heinen A, Varadarajan SG, Jiang MT. Cardiac mitochondrial preconditioning by Big Ca2 + -sensitive K+ channel opening requires superoxide radical generation. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H434–H440. doi: 10.1152/ajpheart.00763.2005. [DOI] [PubMed] [Google Scholar]
- Sugden PH, Clerk A. Oxidative stress and growth-regulating intracellular signaling pathways in cardiac myocytes. Antioxid. Redox Signal. 2006;8:2111–2124. doi: 10.1089/ars.2006.8.2111. [DOI] [PubMed] [Google Scholar]
- Tong H, Chen W, Steenbergen C, Murphy E. Ischemic preconditioning activates phosphatidylinositol-3-kinase upstream of protein kinase C. Circ. Res. 2000;87:309–315. doi: 10.1161/01.res.87.4.309. [DOI] [PubMed] [Google Scholar]
- Vergara C, Latorre R, Marrion NV, Adelman JP. Calcium-activated potassium channels. Curr. Opin. Neurobiol. 1998;8:321–329. doi: 10.1016/s0959-4388(98)80056-1. [DOI] [PubMed] [Google Scholar]
- Wang X, Yin C, Xi L, Kukreja RC. Opening of Ca2+- activated K+ channels triggers early and delayed preconditioning against I/R injury independent of NOS in mice. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H2070–H2077. doi: 10.1152/ajpheart.00431.2004. [DOI] [PubMed] [Google Scholar]
- Xu W, Liu Y, Wang S, McDonald T, Van Eyk JE, Sidor A, O’Rourke B. Cytoprotective role of Ca2+-activated K+ channels in the cardiac inner mitochondrial membrane. Science. 2002;298:1029–1033. doi: 10.1126/science.1074360. [DOI] [PubMed] [Google Scholar]
- Yamamura H, Ohi Y, Muraki K, Watanabe M, Imaizumi Y. BK channel activation by NS-1619 is partially mediated by intracellular Ca2+ release in smooth muscle cells of porcine coronary artery. Br. J. Pharmacol. 2001;132:828–834. doi: 10.1038/sj.bjp.0703885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano S, Morioka M, Fukunaga K, Kawano T, Hara T, Kai Y, Hamada J, Miyamoto E, Ushio Y. Activation of Akt/protein kinase B contributes to induction of ischemic tolerance in the CA1 subfield of gerbil hippocampus. J. Cereb. Blood Flow Metab. 2001;21:351–360. doi: 10.1097/00004647-200104000-00004. [DOI] [PubMed] [Google Scholar]