Preface
Common cytokine-receptor γ-chain (γc) family cytokines have critical roles in the development, proliferation, survival and differentiation of multiple cell lineages of both the innate and adaptive immune system. In this review, we focus on our current understanding of the distinct and overlapping effects of IL-2, IL-7, IL-9, IL-15, and IL-21, as well as the IL-7-related cytokine TSLP (thymic stromal lymphopoietin), on the survival and proliferation of conventional αβ T cells, γδ T cells, and regulatory T cells. This knowledge potentially allows for the therapeutic manipulation of immune responses for the treatment of cancer, autoimmunity, allergic diseases and immunodeficiency, as well as for vaccine development.
Introduction
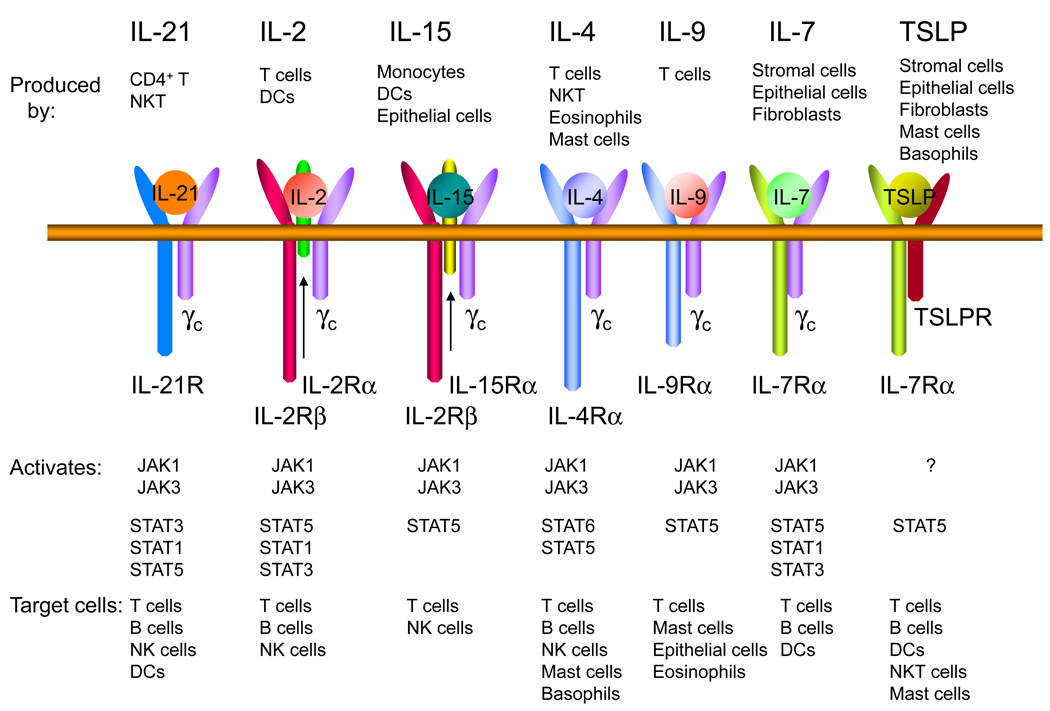
Cytokines are hormones of the immune system that have important functions related to cellular proliferation, differentiation and survival. Type I cytokines have four α-helical bundle structures and include many interleukins, as well as some growth and haematopoietic factors. One important family of type I cytokines is the common cytokine-receptor γ-chain (γc) family, which consists of interleukin-2 (IL-2), IL-4, IL-7, IL-9, IL-15 and IL-21 (FIG. 1), and is so named because the receptors for these cytokines share γc1,2.
Figure 1. Receptors for γc family cytokines and TSLP.
Shown are the receptors for IL-2, IL-4, IL-7, IL-9, IL-15, IL-21, and TSLP. IL-2 and IL-15 are the only two of these cytokines to have three receptor chains. These two cytokines share IL-2Rβ, whereas IL-7 and TSLP share TSLPR, and of the cytokines shown, only TSLP does not share γc. There are three classes of IL-2 receptors, binding IL-2 with low affinity (IL-2Rα alone), intermediate affinity (IL-2Rβ + γc), and high affinity (IL-2Rα + IL-2Rβ + γc); only the high affinity IL-2 receptor is shown in the figure. Each γc family cytokine activates JAK1 and JAK31, whereas TSLP was reported to not activate any JAK kinase25,26. The major STAT proteins activated by these cytokines are shown. STAT5 refers to both STAT5A and STAT5B.
γc was first discovered as a component of the receptor for IL-23, the prototypic member of this family. The IL-2 receptor (IL-2R) consists of three chains, which together form the high-affinity IL-2R (FIG. 1), but which in other combinations bind IL-2 with low affinity (IL-2Rα alone) or intermediate affinity (IL-2Rβ and γc). The structures for some of the receptors for γc family members are known, providing an insight into how different cytokines can each interact with γc4.
The gene encoding γc (IL2RG) is mutated in humans with X-linked severe combined immunodeficiency (X-SCID)5, who lack T cells and NK cells, which indicates that γc is crucial for the development of these cells. However, the finding that the immune defects in patients with X-SCID are much more severe than those of humans or mice lacking IL-2, in which the development of T and NK cells is intact, originally led to the hypothesis and subsequent confirmation that γc was shared by multiple cytokines1.
IL-2 functions as a T cell growth factor, can augment NK cell cytolytic activity, and promotes immunoglobulin production by B cells6. In addition, it contributes to the development of regulatory T (Treg) cells and therefore peripheral T cell tolerance7, as well as regulating the expansion and apoptosis of activated T cells8,9. IL-4 is required for the development and function of T helper 2 (Th2) cells and is therefore regarded as the classical Th2-type cytokine. IL-4 also has an important role in allergy and immunoglobulin class switching10. IL-7 has a central role in the development of T cells in both humans and mice. Indeed, defective IL-7-induced signaling is responsible for the defective T cell development that is observed in patients with XSCID5, as well as in patients with SCID caused by mutations in a signaling molecule downstream of γc, Janus kinase 3 (JAK3),11,12 or by mutations in IL7R13 (Table 1). Interestingly, IL-7 is also required for the development of B cells in mice but it is not necessary for their development in humans as evidenced by normal B cell development in patients with XSCID (IL2RG deficiency), JAK3-deficient-SCID, and IL7R-deficient SCID1. Nevertheless, in an in vitro model, IL-7 can promote the development of human B cells from adult bone marrow hematopoietic stem cells (HSCs), although not from cord blood HSCs14. IL-7 is well known for its potent role as a survival factor15,16. IL-9 is produced by a subset of activated CD4+ T cells17,18 and induces the activation of epithelial cells, B cells, eosinophils, and mast cells19 (FIG. 1). Although IL-9 has been shown to function as a T cell growth factor late during an immune response20, its role in T cell biology remains unclear. IL-15 is essential for the development of NK cells, and it is the defective signaling by IL-15 that results in the failure of NK-cell development in both X-SCID and JAK3-deficient SCID1. IL-15 also has an essential role in CD8+ T cell homeostasis16. IL-21 is the most recently described member of this family2, and it has broad actions that include promoting terminal B-cell differentiation to plasma cells, cooperating with IL-7 or IL-15 to drive the expansion of CD8+ T cell populations, and acting as a pro-apoptotic factor for NK cells and incompletely activated B cells2. IL-21 has also been shown to be an essential mediator of the development of type 1 diabetes21,22 and systemic lupus erythematosus23 in animal models, and to have potent anti-tumor actions2.
Table 1. The basis of defects in Severe Combined Immunodeficiency.
Mutations in IL2RG or JAK3 genes cause defects in T and NK cell development, with functional abnormalities of B cell lineage in human and lack of B cells in mice. IL7R mutations greatly diminish T cell numbers, with normal or even increased number of B and NK cells. IL2RB mutations result in defective NK cell development but do not affect T and B cell development.
| Form of SCID |
Lineage abnormalities |
Causes of defects | References |
|---|---|---|---|
|
X-linked SCID |
TB+NK−. T and NK cells absent; B cells present but non-functional. |
The disease results from mutations in the IL2RG gene. Decreased T cell development is due to defective IL-7- induced signaling. Lack of NK cell development is the result of defective IL-15-induced signaling. Functional B cell abnormalities are due to a lack of T-cell help and defects in IL-4 and IL- 21-induced signaling, as indicated by the pan-hypogammaglobulinemia found in Il4/Il21r double KO mice, which is associated with germinal center abnormalities in these mice57 |
1,5 |
|
JAK3- deficient SCID |
TB+NK−. T and NK cells absent; B cells present but non-functional. |
The disease results from mutations in the JAK3 gene. Abnormalities are due to same reasons as in XSCID. |
11,12 |
|
IL7R- deficient SCID |
TB+NK+−. T cells absent. In humans B cells are present but in Il7r- deficient mice, B cells are absent. |
Defective IL-7-induced signaling with a possible partial contribution from TSLP. Humans with IL7-deficiency have not been reported. Mice with Il7r deficiency have a somewhat more severe T cell phenotype than mice with Il7 deficiency. |
13,42,43 |
|
IL2RB- deficient SCID |
T+B+NK−. NK cells absent. |
Defective IL-15-induced signaling, based on Il15, Il15ra, and Il2rb deficient mice, IL2RB-deficient SCID, and in vitro studies. |
1,146 |
γc family cytokines all signal through the JAK–STAT (signal transducer and activator of transcription) pathway. Interestingly, IL-2, IL-7, IL-9 and IL-15 mainly activate STAT5A and STAT5B (together referred to as STAT5 proteins), whereas IL-4 generally activates STAT6 and IL-21 mainly activates STAT324 (FIG. 1). The activation of different STAT proteins may help to explain the different actions induced by these cytokines.
As partially indicated above, some of the γc family cytokines broadly contribute to lymphocyte homeostasis, which will be a main focus of this review. We also discuss another cytokine that is not a member of the γc family but has overlapping functions with IL-7, known as thymic stromal lymphopoietin (TSLP)25. Whereas the IL-7 receptor contains IL-7Rα (also known as CD127) and γc, the TSLP receptor consists of IL-7Rα and TSLPR, which is closely related to γc26,27 (FIG. 1).
Direct effects of γc family cytokines on T cells
Regulation of naïve and memory αβ T cell homeostasis
γc-deficient mice show a low thymic output of T cells and lymphopaenia, and those T cells that do develop have impaired survival28. IL-7 seems to be the most important γc family cytokine for regulating the homeostasis of naïve and memory T cells29–32 (FIG. 2). In contrast to other γc cytokines, the levels of which increase after immune cell activation, IL-7 is constitutively produced by stromal and epithelial cells in the bone marrow and thymus and by fibroblastic reticular cells in the T-cell zones of secondary lymphoid organs16,33. The availability of IL-7 is regulated both by its production and by its consumption by CD4+ T cells16,34. Thus, diminished numbers of CD4+ T cells in humans are associated with increased levels of IL-734.
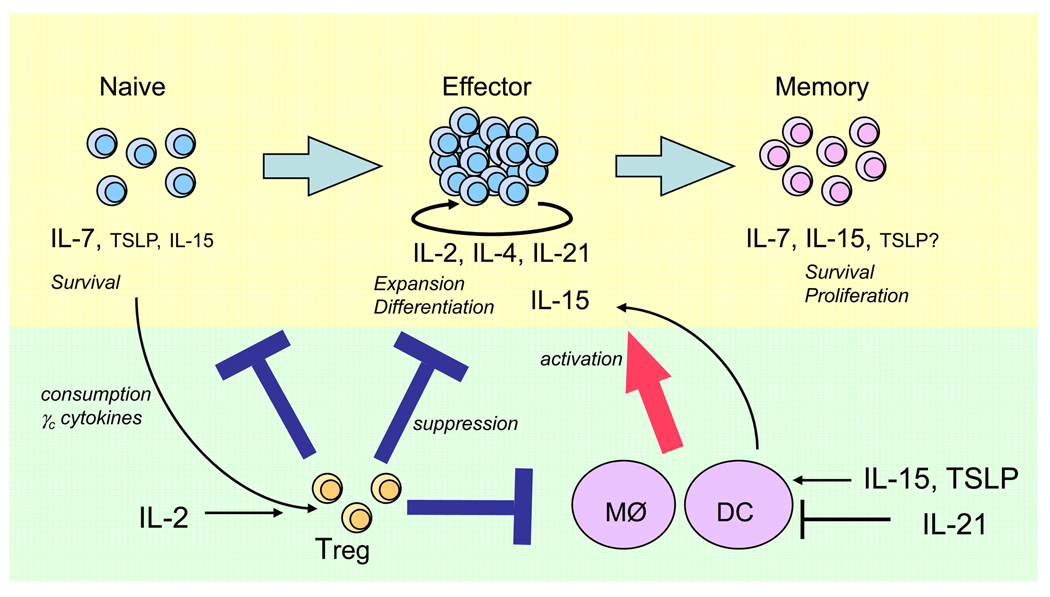
Figure 2. Direct and indirect actions of γc cytokines and TSLP on T cell expansion, homeostasis, and differentiation.
γc cytokines can directly influence the survival, activation, proliferation, and differentiation of T cells (see top half of the figure), as well as indirectly affecting these processes via actions on DCs, macrophages, and Treg cells (bottom half of the figure). Although IL-15 is a critical factor for memory CD8+ T cell homeostasis, it also is responsible for the recovery of naïve CD8+ T cells in lymphopenic conditions. In the absence of IL-7, IL-15 has major effects on the homeostasis of memory CD4+ T cells. Both IL-7 and TSLP are important for the survival of naïve T cells, with IL-7 playing the greater role. The action of TSLP on memory T cells has not been evaluated. IL-2, IL-4 and IL-21 are produced by activated T cells and play essential role in T cell differentiation. In addition, IL-2 and IL-15 can augment expansion of T cells upon antigen-stimulation.
A distinctive feature of IL-7 compared with other γc cytokines relates to the expression of its receptor. Whereas most of the cognate chains for γc cytokines are up-regulated after T-cell receptor (TCR) activation, IL-7Rα is expressed by naïve and memory resting T cells but is down-regulated after TCR activation29,35 (Table 2). This indicates that IL-7 primarily mediates its functions on naïve and memory T cells rather than on activated T cells (see below). IL-2, IL-7 and other pro-survival cytokines can transiently reduce the expression of IL-7Rα35–37, which could decrease IL-7 consumption, thus increasing the availability of IL-7 for other cells that express high levels of IL-7Rα and are poised to receive survival signals in vivo37. Maintaining IL-7Rα expression is dependent, at least in part, on the transcription factor GABP38, and GABP and Gfi-1, respectively, control the up- and down-regulation of IL-7Rα expression39.
Table 2. Expression of receptors for γc family cytokines and TSLP.
The expression of various cytokine receptors in different stages of the T cell immune response. γc is constitutively expressed by T cells. IL-2Rα is rapidly up-regulated in the early stage of T cell activation, thus augmenting the number of high-affinity IL-2Rs. The levels of expression of IL-2Rβ, IL-4Rα, IL-15Rα, IL-21R, and TSLPR are increased after T cell activation, and IL-2Rβ expression is sustained at a high level on CD8+ T memory cells.
|
type of T cells receptor chain |
Naive T cells | Effector T cells | Memory T cells |
|---|---|---|---|
| γc (CD132) | ++ | ++ | ++ |
| IL-2Rα (CD25) | − | +++ | − |
| IL-2Rβ (CD122) | + | +++ | +++ |
| IL-4Rα (CD124) | − | +++ | Nd |
| IL-7Rα (CD127) | ++ | −* | +++ |
| IL-15Rα | + | +++ | +++ |
| IL-21R | + | +++ | Nd |
| TSLPR** | + | ++ | Nd |
Only a few effector T cells express IL-7Rα.
The level of TSLPR expression on T cells is shown relative to expression on dendritic cells. Levels of IL-4Rα, IL-21R, and TSLPR on memory T cells have not been evaluated (nd, not done).
IL-7 uses at least two different mechanisms for supporting T cell homeostasis. First, it promotes survival by activating the pro-survival phosphoinositol 3-kinase/AKT signaling pathway and by increasing the expression of survival factors such as BCL2 and myeloid cell leukemia sequence 1 (MCL-1) whereas it inhibits the expression of pro-apoptotic factors BAX and BAD15,16. Second, it induces the proliferation of naïve and memory T cells in lymphopaenic conditions and of memory T cells but not of naïve T cells under normal physiological conditions29,30,40,41 (FIG. 2).
Although Il7ra−/− and Il7−/− mice each have markedly decreased numbers of T cells, the absence of IL-7 can be partially compensated for by increasing TSLP, which can result in the partial restoration of normal T and B cell numbers42,43. These observations suggested that TSLP might have a role in T cell homeostasis. Indeed, administration of recombinant TSLP to Il2rg−/− mice, which cannot respond to IL-7 or other γc family cytokines, results in a partial increase in CD4+ and CD8+ T cell numbers. Consistent with this, restoration of CD4+ and CD8+ T cells following irradiation is less efficient in Tslpr–/– mice than in irradiated wild-type mice42. Moreover, TSLP promotes the survival of CD8+ T cells in both normal and lymphopaenic conditions44. Interestingly, whereas IL-7 induces proliferation (as well as survival) of naïve CD8+ T cells in lymphopaenic mice, TSLP does not affect the proliferation of these cells44. A possible explanation for this observation is that IL-7, but not TSLP, can activate JAK3 and is a more potent activator of STAT525,26.
IL-15 is another important homeostatic cytokine, which preferentially has activity for memory CD8+ T cells30,45–47 (FIG. 2) that express high levels of IL-2Rβ (CD122), particularly the IL-2Rβhi Ly49+ subset of CD8 memory cells48. Although IL-15 does not play an essential role in the homeostastic expansion of memory CD4+ T cells, in the absence of IL-7 IL-15 was reported to affect the homeostasis of these cells49. Furthermore, depleting CD8+ T cells and NK cells, which are main consumers of IL-15, results in a greater availability of IL-15 and increases the homeostatic proliferation of memory CD4+ T cells49. Although IL-15 is not vital for the development of naïve T cells, Il15−/− mice have reduced numbers of naïve CD8+ T cells and exhibit diminished proliferative rates, likely explaining a slower recovery of adoptively transferred naïve CD8+ T cells in these mice than in wild-type mice40,47,50. Memory CD8+ T cells are dependent on IL-15; interestingly, however, although these cells express high levels of IL-15Rα46 (Table 2), they proliferate better to the administration of an IL-15–IL-15Rα complex than to purified IL-15 alone.51,52. This term refers to a process whereby IL-15 that is bound to IL-15Rα is presented to cells expressing the other IL-15R subunits, IL-2Rβ and γc53. Indeed, under physiological conditions, trans-presentation of IL-15 by IL-15Rα on the surface of other non-T cells is required54, underscoring the importance of this mode of signaling for IL-15.
IL-7 and IL-15 can also function cooperatively with IL-21 to expand CD8+ T cells in vitro55. Although IL-21 alone induces the survival of naïve but not memory mouse CD8+ T cells, in the presence of IL-15 the rate of apoptosis in both populations of CD8+ T cells is markedly reduced and cell proliferation is strongly augmented55. Similarly, the combination of IL-15 and IL-21 increases antigen-independent proliferation of human naïve CD8+ T cells in vitro56, increases the number of CD8+ T cells producing IL-2 and IFNγ and enhances the cytotoxic activity of these cells following TCR activation55,56. Although Il21r−/− mice have normal numbers of peripheral CD8+ T cells57, over-expression of IL-21 increases memory CD8+ T cell numbers58; whether this is an effect of IL-21 or the result of synergistic actions of IL-21 with constitutively produced IL-7 or IL-15 is unclear. These data overall underscore the wide range of actions of various γc family cytokines, particularly IL-7 and IL-15, in naïve and memory T cell homeostasis.
Proliferation and survival of effector T cells
IL-2 is perhaps the earliest cytokine secreted by T cells following TCR stimulation59 and is important in the initiation of Th2 cell differentiation60,61. It is well known that IL-2 can induce the proliferation and survival of TCR-activated human and mouse T cells6,62 and is required for sustained expansion of T cell populations8. Although administration of supra-physiological levels of IL-2 to LCMV-infected mice during the expansion phase of the anti-viral T cell response unexpectedly decreases the numbers of antigen-specific CD4+ T cells63, this might reflect the ability of IL-2 to induce apoptosis of T cells that have been recently activated through their TCR9. Conversely, IL-2 treatment during the contraction phase of the T cell response results in increased survival and accumulation of T cells8,63,64. Overall, the role of IL-2 is multi-faceted due to its complex effects on driving T cell proliferation, promoting the expansion of Treg cells (see later) and mediating activation-induced cell death.
The role of IL-7 and IL-15 in the expansion of effector and memory T cell populations has been widely studied. Immediately after TCR activation, most T cells down-regulate IL-7Rα and up-regulate IL-2Rα, IL-15Rα, and IL-2Rβ expression (Table 2). It was thus predicted that IL-7 is not required for the function of activated T cells29, and IL-2 and/or IL-15, the production of which is also increased by activated DCs65–67, may increase the proliferative rate and/or diminish the contraction of effector T cells64,68,69. Although a selective population of primed T cells re-expresses IL-7Rα70, IL-7 signaling is not essential for the formation of functional memory CD4+ and CD8+ T cells71–74, which could reflect potentially redundant functions of TSLP and IL-7 or instead important actions of other cytokines. Several findings support a possible role for TSLP in the expansion of effector and memory T cell populations: TSLPR expression is increased after TCR activation44,75 (Table 2), TSLP increases the proliferation of TCR-activated CD4+ T cells in vitro42,75, and TSLP production is increased during the acute phase of the immune response to pathogens and allergens76.
Regulation of γδ T cell homeostasis
γδ T cells are a population of T cells that arise from the same precursors as αβ T cells in the thymus. They migrate to the periphery, mostly to epithelial tissues, and have broad immunological actions, which include their production of cytokines and chemokines, cytolytic activity in response to pathogens, regulation of the viability and proliferation of keratinocytes, induction of macrophage and DC responses, and presentation of antigen to T cells77,78. The expression of γc and IL-7Rα are both essential for normal γδ T cell development78. Under lymphopaenic conditions, γδ T cells undergo MHC-independent homeostatic proliferation that requires IL-7 or IL-1579,80. Although αβ and γδ T cells express comparable levels of IL-7Rα and IL-2Rβ79, partial depletion of αβ T cells, NK cells, or γδ T cells themselves significantly enhances the homeostatic proliferation of γδ T cells, revealing that these cells compete for limited quantities of IL-7 and IL-1579–81. Thus, the maintenance of γδ T cells as well as αβ T cells is regulated by IL-7 and IL-15.
The maintenance of Treg cells
Treg cells are a subset of CD4+ T cells that were defined in part by their constitutive expression of the IL-2 receptor α chain (CD25) and the Treg-cell specific transcription factor forkhead box P3 (FOXP3), which controls the development and function of these cells7. Although IL-2 induces the proliferation and clonal expansion of conventional T cells6,62, IL-2 also mediates immune tolerance at least in part through its regulation of Treg cells. IL-2 deficiency is characterized by a reduction in the number and function of Treg cells, and accordingly, leads to lymphoproliferation and autoimmunity7. However, the lack of IL-2, IL-2Rα, or IL-2Rβ (CD122) does not alter FOXP3 expression nor result in a complete loss of Treg cells82–85. By contrast, γc-deficient (Il2rg−/−) mice and Jak3−/− mice, in addition to having very low numbers of T cells, are devoid of FOXP3+ Treg cells83,86.
STAT5 appears to be vital for Treg cells development. STAT5 activation is sufficient to increase the number of CD4+CD25+ Treg cells even in the absence of IL-2 production87 or when there is defective IL-2-induced signaling88, whereas, conversely, deletion of the Stat5a and Stat5b genes is characterized by a dramatic reduction in the number of Treg cells in the thymus and in the periphery86. Correspondingly, a patient with a missense mutation in the human STAT5B gene exhibited diminished numbers of CD4+CD25hi T cells and these cells had a dramatic reduction in FOXP3 expression levels and impaired suppressive activity89. These findings confirm earlier observations indicating that STAT5A and STAT5B are critical factors downstream of IL-2R90–93 and suggest that other γc family cytokines, such as IL-7 and IL-15, which also activate STAT5 proteins, might contribute to Treg cell development and maintenance.
Although deficiency of IL-7 or IL-15 (which both activate STAT5 proteins) does not alter the number of FOXP3+ Treg cells88,94, the absence of IL-7- or IL-15-induced signaling in combination with disrupted IL-2R signaling results in a greater reduction in Treg cells than is observed in mice lacking either IL-2 or IL-2Rα alone88,95. Interestingly, mouse Treg cells express low levels of IL-7Rα94,95, but in contrast to other subsets of T cells that can re-express IL-7Rα after culture in vitro, peripheral Treg cells do not upregulate IL-7Rα expression after culture in vitro94. Nevertheless, the low level of IL-7Rα that is expressed seems to be functional, as IL-7, even in the absence of IL-2-induced signaling, can mediate the survival, although not the proliferation, of mouse Treg cells95. Similarly, most human CD4+CD25+FOXP3+ Treg cells have lower levels of IL-7Rα expression than do CD4+ CD25+FOXP3− T cells96,97. Il7ra−/− mice have a marked reduction in the number of Treg cells in lymphoid tissues and decreased suppressive activity, as compared with Il7−/− mice94. These differences can be explained by the ability of TSLP (which signals through a receptor that contains IL-7Rα) to also mediate the induction of Treg cells94. Thus, IL-2, IL-7, IL-15, and TSLP all contribute to Treg cell development and function.
Although IL-2, IL-4, IL-7, IL-15, and IL-21 can promote the survival of Treg cells and rescue them from apoptosis in vitro, only IL-2 induces their proliferation and clonal expansion98. Consistent with this, peripheral homeostasis of Treg cells in vivo is more dependent on IL-2 than on other γc cytokines7, and neutralizing IL-2 not only reduces the number of mouse Treg cells in the thymus but also prevents their clonal expansion in lymph nodes99. Correspondingly, IL-2 therapy during immune reconstitution after chemotherapy markedly increases the size of the Treg-cell compartment100.
Indirect effects of γc cytokines on T cells
T cell survival and proliferation through DCs
It is well known that γc family cytokines have pleiotropic effects on the immune system and that they can stimulate various populations of cells in addition to T cells, which in turn affect T cell homeostasis. For example, DCs are key players in the activation of the adaptive immune response, and γc family and related cytokines that are induced by pathogens may activate (e.g. IL-15 or TSLP) or inhibit (e.g. IL-7 or IL-21) the function of DCs (FIG. 2).
DCs constitutively express IL-2Rβ and γc66,101,102 and up-regulate the expression of IL-15Rα in response to type I and II interferons (IFNs) and inducers of NF-κB activation, such as ligands for Toll-like receptors (TLRs)65,66. Similar signals promote IL-15 production by DCs and epithelial cells65,66. Therefore, IL-15 has paracrine and autocrine actions on DCs that result in increased survival of mature DCs, up-regulation of co-stimulatory molecules and enhanced presentation of antigen by DCs to CD4+ and CD8+ T cells65–67,103. DCs from aged mice produce less IL-15 that those from young mice, and the functional defects of DCs from aged mice can be reversed by IL-15 treatment104. Thus, reduction of IL-15 production might be a factor that contributes to reduced immunity to pathogens in the elderly. Moreover, infection of humans with hepatitis C virus reduces IFNα-mediated IL-15 production by DCs and therefore decreases the maturation of functional DCs, which has been suggested as a possible mechanism for the poor T-cell immunity against this virus105.
Both IL-4 and TSLP are involved in Th2-cell responses and have essential roles in allergic diseases10,25,76; however, their actions on DCs differ. IL-4 is a survival factor for DCs and in combination with GM-CSF promotes the differentiation of DCs from mouse bone marrow progenitor cells and from human monocytes in vitro148,149. DCs pre-cultured in the presence of IL-4 express a relatively low level of MHC and co-stimulatory molecules, which is indicative of an immature phenotype, and these cells poorly respond to IFNα106. Unlike IL-4, TSLP is not needed for DC differentiation, but as shown by in vitro experiments, it promotes their activation and upregulation of expression of MHC class II and co-stimulatory molecules, including CD80, CD86 and OX40L25. TSLP-activated human DCs support naïve CD4+ T cell homeostasis and induce robust expansion and differentiation of human CD4+ T cells into inflammatory Th2 cells25. In mice, TSLP plays a role in intestine immunity, by inhibiting production of IL-12 by DCs induced by LPS and thereby reducing the number of IFNγ+ CD4+ T cells107. Interestingly, IL-7, which shares IL-7Rα with TSLP, maintains the immature phenotype of DCs and can down-regulate MHC class II expression by mature mouse DCs, correlating with decreased homeostatic proliferation of CD4+ T cells108. IL-21 is not needed for DC differentiation, but pre-treatment of DCs with IL-21 inhibits their maturation in response to TLR stimuli, thus suppressing DC functions, such as antigen presentation and cytokine and chemokine secretion109,110. Because IL-21 is produced by CD4+ T cells following antigen stimulation2 and IL-21-primed DCs have inhibitory effects on T cell responses, the production of IL-21 by T cells may activate a DC-mediated negative loop.
Conventional T cells homeostasis through Treg cells
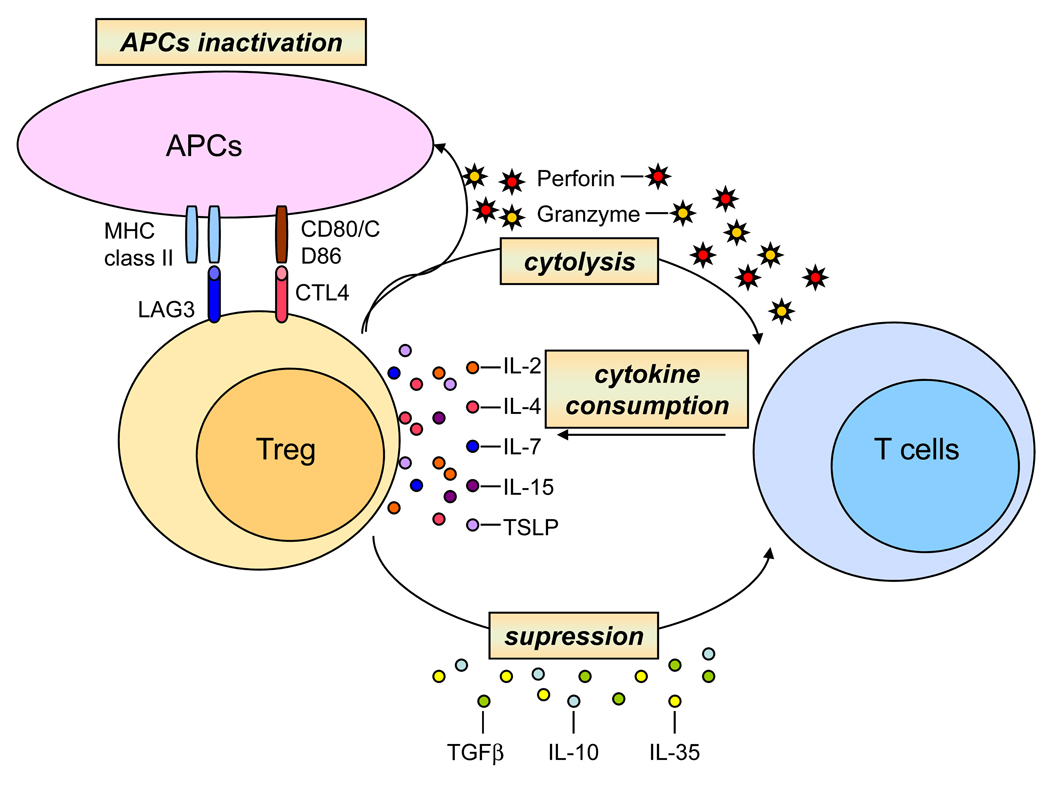
As discussed above, γc cytokines have a key role in the development and maintenance of Treg cells. In turn, these cells inhibit the expansion of auto-reactive T cells, preventing autoimmunity and suppressing the response of conventional T cells to foreign and self-antigens. Several mechanisms have been proposed to explain how Treg cells mediate this suppression (FIG. 3), including their inhibiting of responder T cells by producing suppressive cytokines such as TGFβ, IL-10, and IL-35; inactivating antigen-presenting cells (APCs) through the expression of the inhibitory molecules cytotoxic T-lymphocyte antigen 4 (CTLA4) and lymphocyte-activation gene 3 (LAG3); killing target cells through cytolytic activity; and consuming pro-survival γc cytokines, thus resulting in apoptosis of conventional T cells in vitro98,111. Although it is not yet clear whether this latter cytokine-deprivation mechanism occurs in vivo, Treg cells also induce cytokine-dependent apoptosis of conventional CD4+ T cells in mice98. Note that the mechanisms listed above are not mutually exclusive, so that more than one mechanism might be used.
Figure 3. Mechanisms of T cell regulation by Treg cells.
Treg cells exhibit several mechanisms to suppress activation and expansion of conventional T cells. Treg cells modulate functions of APCs by inhibiting their maturation and blocking of MHC molecules and co-stimulatory molecules (CD80 and CD86) on the surface of APCs and thus attenuating interactions between APCs and T cells. Treg cells might have cytolytic effects on target T cells as well as on APCs. Treg cells suppress activation and proliferation of T cells by their secretion of inhibitory cytokines, such as TGFβ, IL-10, and IL-35 and by consumption of γc cytokines. Deprivation of γc cytokines induces expression of pro-apoptotic proteins and elevates apoptotic rate of conventional T cells.
γc family cytokines and T-cell differentiation
Naive T cells can differentiate during a primary antigen response into several distinct polarized subsets, such as Th1, Th2, Th17, and T follicular helper (TFH) cells. These subsets produce discrete sets of cytokines and chemokines to collectively allow responses to different classes of pathogens. Four γc family cytokines are among the main cytokines that are produced by these polarized cells: IL-2 by Th1 cells, IL-4 and IL-9 by Th2 cells and IL-21 by Th17 and TFH cells as well as Th1 and Th2 cells. These cytokines act on other target cells to direct immune responses, but IL-2, IL-4 and IL-21 also have important roles in the early development of CD4+ T cell subsets.
Th1 cell differentiation
Th1 cell differentiation depends primarily on APC-derived IL-12, which then leads to IFNγ production and increased IL-12Rβ2 expression112. Although IL-2 is the earliest detected cytokine produced by naive CD4+ T cells following TCR stimulation59, and this cytokine is a major product of Th1 cells113, it is not clear whether IL-2 signaling contributes to early commitment to the Th1 cell lineage. IL-21 was reported to be a Th2-type cytokine that had inhibitory effects on Th1 cells114, but IL-21 does not affect the expression of Th1 cell-associated transcription factor T-bet or of IL-12Rβ2 by mouse CD4+ T cells114. Instead, IL-21 can inhibit IFNγ production by naive CD4+ T cells that are undergoing Th1 differentiation by the repressing the expression of the T-box transcription factor Eomesodermin115. It is unclear whether this inhibition of IFNγ by IL-21 has a role in modulating Th1 cell responses in vivo as opposed to promoting Th17 cell differentiation (see below). Interestingly, in human peripheral blood T cells stimulated via the TCR, IL-21 actually induces IFNγ, T-bet and IL-12Rβ2 expression116, which suggests that IL-21 under certain circumstances might promote Th1 cell differentiation.
Th2 cell differentiation
In vitro studies have demonstrated that IL-2 and IL-4 are both required for the efficient induction of Th2 cells. IL-2 is produced early following the activation of naive CD4+ T cells and activates STAT5A and STAT5B to promote increased transcription of the Il4ra gene, thus leading to the enhanced cell surface receptor expression of IL-4Rα and subsequent increased responsiveness to IL-461. IL-2 also induces STAT5 binding to consensus binding sites located within DNase-I hypersensitive sites in the Il4 locus, thus promoting increased accessibility of this locus to the formation of transcriptional complexes60. A genome-wide in vivo analysis revealed that IL-2 via its actions on STAT5 activates not only the Il4ra locus but also the entire Th2 cytokine locus, which includes the Sept8, Kif3a, Il4, Il13, Rad50, Il15, and Irf1 genes. Interestingly, this analysis showed that STAT5A and STAT5B bind first at the Il4ra locus and then at the Th2 locus in vivo, consistent with observation that IL-4 is produced by Th2 cells after they express IL-4Rα61. Thus, IL-2-induced signaling during Th2 cell differentiation results in both increased production of IL-4 and increased responsiveness to IL-4, leading to stabilization of this lineage. Other cytokines that can activate STAT5 proteins including IL-7 and IL-15 were also shown to induce IL-4Rα expression, indicating that multiple STAT5 activators may be able to prime T cells for Th2 cell differentiation61. Although IL-21 also can promote STAT5 activation, IL-21-induced signaling does not affect the efficiency of Th2 cell differentiation in vitro57, which is consistent with its primarily activating STAT3 rather than STAT5. Il21r-deficient mice exhibit reduced responses to Th2 cell-inducing pathogens; however, it is possible that this results from diminished effects of IL-21 on macrophage activation117 rather than IL-21 having direct Th2-related effects.
Th17 cell differentiation
Th17 cell differentiation depends in part on TGFβ, an immunosuppressive cytokine that also has a role in Treg cell differentiation. The presence of either IL-6 or IL-21 during priming with TGFβ event subverts cell differentiation from the FOXP3-directed Treg cell pathway to the Th17 cell pathway through the induction of the orphan nuclear receptor RORγt118–120. Th17 versus Treg cell differentiation is therefore determined by the presence of IL-6 or IL-21.
IL-2 can promote the development of Treg cells, and it inhibits the differentiation of naïve CD4+ T cells into Th17 cells121,122. Accordingly, administration of IL-2 to tumor-bearing mice can diminish the number of IL-17-producing cells while increasing the number of Treg cells123. Correspondingly, Il2−/− mice exhibit a decrease in the number of Treg cells84 and an increase in the production of IL-17121, suggesting that IL-17 producing cells may contribute to the autoimmune disease that develops in Il2−/− mice7. However, Il17−/−Il2−/− double knockout mice develop systemic autoimmune hemolytic anemia to the same extent as Il2−/− mice, suggesting that IL-17 producing cells are not absolutely essential for this disease process and that other potentially redundant cytokines contribute as well124. Although IL-2 inhibits Th17 cell differentiation, it can provide proliferative signals to human Th17 cells, as indicated by the IL-2-induced expansion of these Th17 cells from normal donors and from patients with uveitis or scleritis in vitro125. Interestingly, the inhibitory effects of IL-2 on the Th17 cell lineage is prevented by IL-1, suggesting that the local cytokine profile controls the IL-17+ T cell pool126.
The role of IL-21 in the differentiation of Th17 cells is controversial. In vitro experiments have shown that IL-21 is critical for upregulating IL-23R expression by Th17 cells120. Although IL-23, which is produced by APCs, is an important factor in the differentiation and expansion of Th17 cells and therefore in inflammatory diseases, IL-23R is not expressed by naive CD4+ T cells. IL-21 therefore, promotes the expansion of Th17 cell populations by increasing their responsiveness to IL-23. Although Th17 cell differentiation is reduced in the absence of IL-21 signaling in vitro118–120, the role of IL-21 in Th17 cell development in vivo and in Th17 cell-mediated autoimmune disease is less clear. Specifically, Th17 cell development in the lamina propria of the small intestine can occur in the absence of IL-21-induced signaling127. Moreover, although one study reported that the development of experimental autoimmune encephalomyelitis (EAE) was significantly reduced in IL-21-deficient mice119, two other studies found no difference in the development of EAE in either IL-21- or IL-21R-deficient mice128,129. Thus, although IL-21 can promote the differentiation of Th17 cells, its actions can apparently be subserved by other cytokines, as well, at least in certain circumstances.
TFH cell differentiation
TFH cells are a distinct subset of CD4+ T cells that provide help to B cells within germinal centers during the generation of T cell dependent antibody responses. TFH cells are characterized by the expression of high levels of CXC-chemokine receptor 5 (CXCR5) and the co-stimulatory molecules ICOS and CD40L. TFH cells produce high levels of IL-21130, which can act on B cells in germinal centers and as an autocrine factor for TFH cells. Il21−/− mice exhibit defective germinal center formation as well as reduced levels of TFH cells131. Unlike Th17 cells, which can also produce high levels of IL-212, TFH cells develop independently of RORγt and do not produce IL-17132. Although the differentiation of TFH cells during a normal T cell-dependent antibody response requires IL-21 production, the excessive differentiation of TFH cells that accompanies systemic autoimmunity in sanroque mice is independent of IL-21, suggesting that there are alternative mechanisms for the maintenance and/or expansion of TFH cells within germinal centers in some systemic autoimmune diseases133.
CD8+ T cell differentiation
CD8+ T cells also undergo differentiation into polarized Tc1, Tc2, and Tc17 cell populations, which parallel the CD4+ Th1, Th2, and Th17 cell populations. One distinction is that naive CD8+ T cells produce only minimal levels of IL-2 and no IL-21, so that the source of these cytokines during an immune response must be from either activated CD4+ T cells or other cells, such as NKT cells (which can produce IL-21134). In addition to a role for γc cytokines in the expansion of CD8+ T cell populations, IL-2 and IL-21 have distinct effects on CD8+ T cell differentiation when they are present during TCR priming. The presence of IL-21 during priming leads to the generation of CD28hiCD8+ T cells that can produce IL-2, thus potentially overcoming the requirement for IL-2 from helper CD4+ T cells135. Moreover, whereas priming of tumor-specific CD8+ T cells in vitro in the presence of IL-2 can potently promote their proliferation and enhance their cytolytic activity, priming in the presence of IL-21 was shown to inhibit these processes136. However, when these two populations of cells primed under different conditions were transferred into tumor-bearing mice, the IL-21-primed CD8+ T cells displayed superior anti-tumor immunity and greater secondary clonal expansion and persistence than did the IL-2-primed CD8+ T cells. These differences that persisted in vivo in the absence of further cytokine stimulation were associated with distinctive and persistent gene expression profiles in either IL-2- or IL-21-primed CD8+ T cells136, suggesting the possibility of these cytokines induced epigenetic changes at the time of priming. Thus, distinct γc cytokines exhibit different factors for CD8+ T cell differentiation, with particularly dramatic diversity for IL-2 and IL-21 in priming for anti-tumor effects.
Therapeutic implications
As is evident from the information presented above, γc family cytokines and TSLP have vital roles in regulating numerous immune cell activities, which have been harnessed to modulate immune responses for therapeutic purposes (Table 3). IL-2 is already used in the clinic to expand and maintain CD4+ T cell numbers in patients with HIV infection137,138 and as an anti-cancer agent, and this is showing efficacy in treating some patients with melanoma and renal cell carcinoma139. The related cytokine IL-15, the receptor of which consists of IL-2Rβ, γc and IL-15Rα (FIG. 1), holds promise as an adjuvant for vaccines139. IL-15 preferentially induces the proliferation of CD8+ T cells rather than Treg cells and therefore, is not expected to induce enhanced tolerance in a similar fashion to IL-2 and has stronger effects on NK cell and CTL activity139. IL-7 and TSLP are other potential agents that might be using for expanding the number of T cells in individuals with inherited or acquired immunodeficiency (Table 3). Indeed, the treatment of SIV-infected primates with IL-7 was shown to increase the number of circulating naïve and memory T cells140 and similarly, the administration of IL-7 to humans induces a selective increase in the number of CD4+ and CD8+ T cells but does not affect Treg cell numbers32,141.
Table 3. Effect of diminished versus augmented signaling by γc family cytokines and TSLP.
Aberrant regulation of γc cytokine signals associated with the development of immunodeficiency, autoimmunity, allergic diseases, inflammation, and cancer. Different strategies of manipulating these signals might be used in immunotherapy.
| Disruption of signaling (knock out or blocking/neutralization of receptors or cytokines) |
Enhancing of signaling (overexpression or administration of cytokines) |
|||
|---|---|---|---|---|
| Positive effect | Negative effect | Positive effect | Negative effect | |
| IL-2 | Immunosupression in organ allografts and leukemia/lymphomas (daclizumab)* |
Lymphoproliferative disorders and autoimmunity associated with loss of Treg cells |
Anti-cancer and immunodeficiency (HIV) treatment, increased NK cells, and NK and LAK activity |
Capillary leak syndrome |
| IL-4 | Diminish asthma symptoms, resistance to leishmania |
Defect in Ig class switch, no IgE production, failure to protection against worms |
None | Allergy, atopic dermatits, airway inflammation, pro- fibrotic agent |
| IL-7 | None | Severe combined immunodeficiency disease (SCID), defect in T cell homeostasis |
Immunodeficiency treatment |
Lymphomas, dermatitis, chronic colitis |
| IL-9 | Diminish asthma symptoms, impaired goblet cell hyperplasia |
None | None | Airway inflammation |
| IL-15 | None | Diminish innate immunity associated with loss of NK cells, defective memory CD8+ T cell homeostasis |
Adjuvant with viral vaccine, anti-cancer and immunodeficiency treatment |
Lymphomas |
| IL-21 | Prevent autoimmune diseases (EAE, SLE, diabetes) |
Defect in B cell maturation, with reduced IgG1 and IgG3 production, pan- hypogammaglobulinemia, defect in Th17 differentiation |
Anti-cancer agent | Aberrant CD8+ T cell homeostasis, autoimmunity |
| TSLP | Prevent development of allergic lung inflammation |
Possible relationship to Crohn’s disease |
Increase lymphoid cellularity |
Atopic dermatitis, airway inflammation, inflammatory arthritis |
Daclizumab (Zenapax, anti-Tac) is a humanized anti-IL-2Rα blocking antibody that prevents IL-2 binding to the high affinity IL-2 receptor complex that is expressed on leukemia/lymphomas cells and allograft activated CTLs147.
IL-21 also may have substantial clinical potential (Table 3); its potent anti-tumor effects have been described in animal models with large established tumors, and it is now in Phase II clinical trials for the treatment of humans with cancer2,142. By contrast, blocking IL-21 may prove valuable in treating autoimmune diseases. In this regard, diabetes does not develop in the non-obese diabetic (NOD) mouse model of type 1 diabetes when the animals are crossed to the Il21r−/− background21, and similarly, manifestations of systemic lupus erythematosus no longer develops when BXSB-Yaa mice are crossed to the Il21r−/− background23. These studies underscore the potential role of IL-21 in autoimmunity and suggest that interfering with the action of IL-21 might have therapeutic potential for several autoimmune disorders. Finally, the IL-7-related cytokine TSLP seems to have a role in the development of atopic dermatitis and asthma25,76 and thus perhaps other allergic diseases as well. Blocking of TSLP with a soluble TSLPR-specific antibody has already been shown to protect against the development of pulmonary allergic inflammation in a mouse model143–145. These studies collectively underscore a range of therapeutic roles for γc cytokines and TSLP.
Concluding remarks and future directions
The γc family cytokines have central roles in the regulation of a range of immunological processes. The sharing of γc could represent a mechanism for inducing overlapping actions but it also could be a basis for the ability of this family of cytokines to compete with each other for the recruitment of γc. Additionally, these cytokines can affect signaling by other γc cytokines changing expression of their receptors, creating a system of intricate cross-regulation (for example, IL-2 increases the expression of its own receptor and IL-4Rα but represses the expression of IL-7Rα). The actions of these cytokines have clear clinical relevance, and increasing or decreasing their effects have implications for the treatment of cancer, autoimmunity, allergy and immunodeficiency. Future efforts will be directed not only towards further elucidation of the basic biology of these cytokines, including aspects of gene regulation and signaling, but also towards achieving therapeutic benefits in a range of pathological states.
Acknowledgments
We thank Dr. Jian-Xin Lin for critical comments. This work was supported by the Division of Intramural Research, National Heart, Lung, and Blood Institute, NIH. R.S. and W.J.L are inventors on patents and patent applications related to γc family cytokines and TSLP.
Footnotes
X-linked severe combined immunodeficiency
(XSCID). Recessive inherited disease in which the the X-chromosomally located common cytokine receptor γ-chain an essential component of six cytokine receptors — is mutated. It is a profound immunodeficiency that accounts for approximately half of the cases of SCID and is characterized by an absence of T cells and natual killer cells. B cells are normal in number but are non-functional.
Regulatory T cell
(Treg cell). A specialized type of CD4+ T cell that can suppress the responses of other T cells. These cells provide a crucial mechanism for the maintenance of peripheral T cell tolerance and are characterized by the expression of the α-chain of the interleukin-2 receptor (also known as CD25) and the transcription factor forkhead box P3 (FOXP3).
Systemic lupus erythematosus
(SLE). An autoimmune disease in which autoantibodies that are specific for DNA, RNA or proteins associated with nucleic acids form immune complexes that damage small blood vessels, especially in the kidney. Patients with SLE generally have abnormal B- and T-cell function.
Activation-induced cell death
(AICD). A process in which activated Tcell-receptor-restimulated T cells undergo cell death after engagement of cell-death receptors, such as CD95 or the tumour-necrosis factor receptor (TNFR), or after exposure to reactive oxygen species (ROS).
Interferons
(IFNs). Interferons are proteins with potent antiviral activity that are of particular importance during the early response to pathogens. Type I or viral IFNs include families (α, β and ω) of homologous proteins that interact with a common two-chain receptor (IFNAR1 and IFNAR2), and type II or immune IFN is represented by a single protein (IFNγ) that interacts with a different two-chain receptor (IFNγR1 and IFNγR2).
DNAse-I hypersensitivity
Refers to sites of nuclease sensitivity when nuclei from cells are exposed to limiting concentrations of DNase I. The digested regions of DNA correspond to sites of open DNA, which might be factor-binding sites or areas of altered nucleosome conformation.
Lamina propria
The layer of mucosal tissue directly under the mucosal epithelial cell surface of the gastrointestinal tract, in which effector immune cells for mucosal immunity reside.
Experimental autoimmune encephalomyelitis
(EAE). An experimental model of multiple sclerosis that is induced by immunization of susceptible animals with myelin-derived antigens, such as myelin basic protein, proteolipid protein or myelin oligodendrocyte glycoprotein.
Sanroque mice
An autoimmune strain of mice that carries a loss-of-function mutation in the gene roquin. These mice have a T-cell-mediated systemic-lupus-erythematosus-like syndrome and severe autoimmune diabetes when on a susceptible genetic background.
Non-obese diabetic (NOD) mice
NOD mice spontaneously develop type 1 diabetes mellitus as a result of autoreactive T-cell-mediated destruction of pancreatic beta-islet cells.
References
- 1.Leonard WJ. Cytokines and immunodeficiency diseases. Nat Rev Immunol. 2001;1:200–208. doi: 10.1038/35105066. [DOI] [PubMed] [Google Scholar]
- 2.Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 3.Takeshita T, et al. Cloning of the gamma chain of the human IL-2 receptor. Science. 1992;257:379–382. doi: 10.1126/science.1631559. [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Lupardus P, Laporte SL, Garcia KC. Structural Biology of Shared Cytokine Receptors. Annu Rev Immunol. 2009;27:29–60. doi: 10.1146/annurev.immunol.24.021605.090616.A comprehensive review that details the structures for families of cytokine receptors that contain gp130, γc, or βc, highlights structural similarities and differences, and discusses their abilities to bind ligands and mediate signaling.
- 5.Noguchi M, et al. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73:147–157. doi: 10.1016/0092-8674(93)90167-o.This paper demonstrates that mutations in IL2RG results in X-SCID in humans and thus revealed important roles for γc in T and NK cell development. The authors correctly speculated that γc has major roles beyond the action of IL-2.
- 6.Kim HP, Imbert J, Leonard WJ. Both integrated and differential regulation of components of the IL-2/IL-2 receptor system. Cytokine Growth Factor Rev. 2006;17:349–366. doi: 10.1016/j.cytogfr.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 8.D'Souza WN, Lefrancois L. IL-2 is not required for the initiation of CD8 T cell cycling but sustains expansion. J Immunol. 2003;171:5727–5735. doi: 10.4049/jimmunol.171.11.5727. [DOI] [PubMed] [Google Scholar]
- 9.Lenardo MJ. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature. 1991;353:858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- 10.Holgate ST, Polosa R. Treatment strategies for allergy and asthma. Nat Rev Immunol. 2008;8:218–230. doi: 10.1038/nri2262. [DOI] [PubMed] [Google Scholar]
- 11.Macchi P, et al. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID) Nature. 1995;377:65–68. doi: 10.1038/377065a0. [DOI] [PubMed] [Google Scholar]
- 12.Russell SM, et al. Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science. 1995;270:797–800. doi: 10.1126/science.270.5237.797. [DOI] [PubMed] [Google Scholar]
- 13.Puel A, Ziegler SF, Buckley RH, Leonard WJ. Defective IL7R expression in T(−)B(+)NK(+) severe combined immunodeficiency. Nat Genet. 1998;20:394–397. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- 14.Parrish YK, et al. IL-7 Dependence in human B lymphopoiesis increases during progression of ontogeny from cord blood to bone marrow. J Immunol. 2009;182:4255–4266. doi: 10.4049/jimmunol.0800489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: intelligent design. Nat Rev Immunol. 2007;7:144–154. doi: 10.1038/nri2023. [DOI] [PubMed] [Google Scholar]
- 16.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Veldhoen M, et al. Transforming growth factor-beta 'reprograms' the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 18.Dardalhon V, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(−) effector T cells. Nat Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hauber HP, Bergeron C, Hamid Q. IL-9 in allergic inflammation. Int Arch Allergy Immunol. 2004;134:79–87. doi: 10.1159/000078384. [DOI] [PubMed] [Google Scholar]
- 20.Uyttenhove C, Simpson RJ, Van Snick J. Functional and structural characterization of P40, a mouse glycoprotein with T-cell growth factor activity. Proc Natl Acad Sci U S A. 1988;85:6934–6938. doi: 10.1073/pnas.85.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spolski R, Kashyap M, Robinson C, Yu Z, Leonard WJ. IL-21 signaling is critical for the development of type I diabetes in the NOD mouse. Proc Natl Acad Sci U S A. 2008;105:14028–14033. doi: 10.1073/pnas.0804358105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Datta S, Sarvetnick NE. IL-21 limits peripheral lymphocyte numbers through T cell homeostatic mechanisms. PLoS ONE. 2008;3:e3118. doi: 10.1371/journal.pone.0003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bubier JA, et al. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proc Natl Acad Sci U S A. 2009;106:1518–1523. doi: 10.1073/pnas.0807309106.References 21 – 23 demonstrate a critical role for IL-21 in the pathogenesis of both organ-specific and systemic autoimmune disease.
- 24.Leonard WJ, Spolski R. Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nat Rev Immunol. 2005;5:688–698. doi: 10.1038/nri1688. [DOI] [PubMed] [Google Scholar]
- 25.Liu YJ, et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 26.Pandey A, et al. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat Immunol. 2000;1:59–64. doi: 10.1038/76923. [DOI] [PubMed] [Google Scholar]
- 27.Park LS, et al. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: Formation of a functional heteromeric complex requires interleukin 7 receptor. J Exp Med. 2000;192:659–670. doi: 10.1084/jem.192.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakajima H, Shores EW, Noguchi M, Leonard WJ. The common cytokine receptor gamma chain plays an essential role in regulating lymphoid homeostasis. J Exp Med. 1997;185:189–195. doi: 10.1084/jem.185.2.189.The first paper to show a critical role for γc in lymphocyte homeostasis.
- 29.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 30.Goldrath AW, et al. Cytokine requirements for acute and Basal homeostatic proliferation of naive and memory CD8+ T cells. J Exp Med. 2002;195:1515–1522. doi: 10.1084/jem.20020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seddon B, Tomlinson P, Zamoyska R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat Immunol. 2003;4:680–686. doi: 10.1038/ni946. [DOI] [PubMed] [Google Scholar]
- 32.Sportes C, et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med. 2008;205:1701–1714. doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Link A, et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8:1255–1265. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 34.Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol. 2005;174:6571–6576. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 35.Alves NL, van Leeuwen EM, Derks IA, van Lier RA. Differential regulation of human IL-7 receptor alpha expression by IL-7 and TCR signaling. J Immunol. 2008;180:5201–5210. doi: 10.4049/jimmunol.180.8.5201. [DOI] [PubMed] [Google Scholar]
- 36.Xue HH, et al. IL-2 negatively regulates IL-7 receptor alpha chain expression in activated T lymphocytes. Proc Natl Acad Sci U S A. 2002;99:13759–13764. doi: 10.1073/pnas.212214999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park JH, et al. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21:289–302. doi: 10.1016/j.immuni.2004.07.016.References 36 and 37 demonstrate that IL-2 and IL-7 signals negatively regulate the expression of IL-7Rα.
- 38.Xue HH, et al. GA binding protein regulates interleukin 7 receptor alpha-chain gene expression in T cells. Nat Immunol. 2004;5:1036–1044. doi: 10.1038/ni1117. [DOI] [PubMed] [Google Scholar]
- 39.Chandele A, et al. Formation of IL-7Ralphahigh and IL-7Ralphalow CD8 T cells during infection is regulated by the opposing functions of GABPalpha and Gfi-1. J Immunol. 2008;180:5309–5319. doi: 10.4049/jimmunol.180.8.5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan JT, et al. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci U S A. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Min B, Yamane H, Hu-Li J, Paul WE. Spontaneous and homeostatic proliferation of CD4 T cells are regulated by different mechanisms. J Immunol. 2005;174:6039–6044. doi: 10.4049/jimmunol.174.10.6039. [DOI] [PubMed] [Google Scholar]
- 42.Al-Shami A, et al. A role for thymic stromal lymphopoietin in CD4(+) T cell development. J Exp Med. 2004;200:159–168. doi: 10.1084/jem.20031975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chappaz S, Flueck L, Farr AG, Rolink AG, Finke D. Increased TSLP availability restores T- and B-cell compartments in adult IL-7 deficient mice. Blood. 2007;110:3862–3870. doi: 10.1182/blood-2007-02-074245. [DOI] [PubMed] [Google Scholar]
- 44.Rochman Y, Leonard WJ. The role of thymic stromal lymphopoietin in CD8+ T cell homeostasis. J Immunol. 2008;181:7699–7705. doi: 10.4049/jimmunol.181.11.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 46.Schluns KS, Williams K, Ma A, Zheng XX, Lefrancois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J Immunol. 2002;168:4827–4831. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 47.Berard M, Brandt K, Bulfone-Paus S, Tough DF. IL-15 promotes the survival of naive and memory phenotype CD8+ T cells. J Immunol. 2003;170:5018–5026. doi: 10.4049/jimmunol.170.10.5018. [DOI] [PubMed] [Google Scholar]
- 48.Judge AD, Zhang X, Fujii H, Surh CD, Sprent J. Interleukin 15 controls both proliferation and survival of a subset of memory-phenotype CD8(+) T cells. J Exp Med. 2002;196:935–946. doi: 10.1084/jem.20020772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Purton JF, et al. Antiviral CD4+ memory T cells are IL-15 dependent. J Exp Med. 2007;204:951–961. doi: 10.1084/jem.20061805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sandau MM, Winstead CJ, Jameson SC. IL-15 is required for sustained lymphopenia-driven proliferation and accumulation of CD8 T cells. J Immunol. 2007;179:120–125. doi: 10.4049/jimmunol.179.1.120. [DOI] [PubMed] [Google Scholar]
- 51.Rubinstein MP, et al. Converting IL-15 to a superagonist by binding to soluble IL-15R{alpha} Proc Natl Acad Sci U S A. 2006;103:9166–9171. doi: 10.1073/pnas.0600240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stoklasek TA, Schluns KS, Lefrancois L. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J Immunol. 2006;177:6072–6080. doi: 10.4049/jimmunol.177.9.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6.This paper describes the formation of stable IL-15–IL-15Ra complexes on the cell surface that mediate trans-presentation of IL-15 and provide survival signals for target cells.
- 54.Burkett PR, et al. IL-15R alpha expression on CD8+ T cells is dispensable for T cell memory. Proc Natl Acad Sci U S A. 2003;100:4724–4729. doi: 10.1073/pnas.0737048100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zeng R, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–148. doi: 10.1084/jem.20041057.This paper describes the ability of IL-21 to act synergistically with other γc-cytokines as a proliferative agent in vitro and in vivo during an anti-tumor response.
- 56.Alves NL, Arosa FA, van Lier RA. IL-21 sustains CD28 expression on IL-15-activated human naive CD8+ T cells. J Immunol. 2005;175:755–762. doi: 10.4049/jimmunol.175.2.755. [DOI] [PubMed] [Google Scholar]
- 57.Ozaki K, et al. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 58.Allard EL, et al. Overexpression of IL-21 promotes massive CD8+ memory T cell accumulation. Eur J Immunol. 2007;37:3069–3077. doi: 10.1002/eji.200637017. [DOI] [PubMed] [Google Scholar]
- 59.Sojka DK, Bruniquel D, Schwartz RH, Singh NJ. IL-2 secretion by CD4+ T cells in vivo is rapid, transient, and influenced by TCR-specific competition. J Immunol. 2004;172:6136–6143. doi: 10.4049/jimmunol.172.10.6136. [DOI] [PubMed] [Google Scholar]
- 60.Cote-Sierra J, et al. Interleukin 2 plays a central role in Th2 differentiation. Proc Natl Acad Sci U S A. 2004;101:3880–3885. doi: 10.1073/pnas.0400339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liao W, et al. Priming for T helper type 2 differentiation by interleukin 2-mediated induction of interleukin 4 receptor alpha-chain expression. Nat Immunol. 2008;9:1288–1296. doi: 10.1038/ni.1656.References 60 and 61 show a central role of IL-2 in Th2 cell differentiation. IL-2 activates STAT5 proteins, which bind to the Il4 and Il4ra loci promoting their transcription.
- 62.Morgan DA, Ruscetti FW, Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976;193:1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- 63.Blattman JN, et al. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med. 2003;9:540–547. doi: 10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- 64.Rubinstein MP, et al. IL-7 and IL-15 differentially regulate CD8+ T-cell subsets during contraction of the immune response. Blood. 2008;112:3704–3712. doi: 10.1182/blood-2008-06-160945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mattei F, Schiavoni G, Belardelli F, Tough DF. IL-15 is expressed by dendritic cells in response to type I IFN, double-stranded RNA, or lipopolysaccharide and promotes dendritic cell activation. J Immunol. 2001;167:1179–1187. doi: 10.4049/jimmunol.167.3.1179. [DOI] [PubMed] [Google Scholar]
- 66.Dubois SP, Waldmann TA, Muller JR. Survival adjustment of mature dendritic cells by IL-15. Proc Natl Acad Sci U S A. 2005;102:8662–8667. doi: 10.1073/pnas.0503360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ohteki T, et al. Essential roles of DC-derived IL-15 as a mediator of inflammatory responses in vivo. J Exp Med. 2006;203:2329–2338. doi: 10.1084/jem.20061297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yajima T, et al. IL-15 regulates CD8+ T cell contraction during primary infection. J Immunol. 2006;176:507–515. doi: 10.4049/jimmunol.176.1.507. [DOI] [PubMed] [Google Scholar]
- 69.Oh S, et al. IL-15 as a mediator of CD4+ help for CD8+ T cell longevity and avoidance of TRAIL-mediated apoptosis. Proc Natl Acad Sci U S A. 2008;105:5201–5206. doi: 10.1073/pnas.0801003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaech SM, et al. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 71.Hand TW, Morre M, Kaech SM. Expression of IL-7 receptor alpha is necessary but not sufficient for the formation of memory CD8 T cells during viral infection. Proc Natl Acad Sci U S A. 2007;104:11730–11735. doi: 10.1073/pnas.0705007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haring JS, et al. Constitutive expression of IL-7 receptor alpha does not support increased expansion or prevent contraction of antigen-specific CD4 or CD8 T cells following Listeria monocytogenes infection. J Immunol. 2008;180:2855–2862. doi: 10.4049/jimmunol.180.5.2855. [DOI] [PubMed] [Google Scholar]
- 73.Klonowski KD, Williams KJ, Marzo AL, Lefrancois L. Cutting edge: IL-7-independent regulation of IL-7 receptor alpha expression and memory CD8 T cell development. J Immunol. 2006;177:4247–4251. doi: 10.4049/jimmunol.177.7.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lacombe MH, Hardy MP, Rooney J, Labrecque N. IL-7 receptor expression levels do not identify CD8+ memory T lymphocyte precursors following peptide immunization. J Immunol. 2005;175:4400–4407. doi: 10.4049/jimmunol.175.7.4400. [DOI] [PubMed] [Google Scholar]
- 75.Rochman I, Watanabe N, Arima K, Liu YJ, Leonard WJ. Cutting edge: direct action of thymic stromal lymphopoietin on activated human CD4+ T cells. J Immunol. 2007;178:6720–6724. doi: 10.4049/jimmunol.178.11.6720.This is the first demonstration of a direct effect of TSLP on human T cells. TSLPR expression is increased by activated CD4+ T cells. TSLP binding promotes the activation of STAT5, which induces the upregulation of IL-2Rα expression and thus augments the sensitivity of CD4+ T cells to IL-2.
- 76.Rochman Y, Leonard WJ. Thymic stromal lymphopoietin: a new cytokine in asthma. Curr Opin Pharmacol. 2008;8:249–254. doi: 10.1016/j.coph.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Born WK, Reardon CL, O'Brien RL. The function of gammadelta T cells in innate immunity. Curr Opin Immunol. 2006;18:31–38. doi: 10.1016/j.coi.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 78.Jameson J, Havran WL. Skin gammadelta T-cell functions in homeostasis and wound healing. Immunol Rev. 2007;215:114–122. doi: 10.1111/j.1600-065X.2006.00483.x. [DOI] [PubMed] [Google Scholar]
- 79.Baccala R, et al. Gamma delta T cell homeostasis is controlled by IL-7 and IL-15 together with subset-specific factors. J Immunol. 2005;174:4606–4612. doi: 10.4049/jimmunol.174.8.4606. [DOI] [PubMed] [Google Scholar]
- 80.French JD, Roark CL, Born WK, O'Brien RL. {gamma}{delta} T cell homeostasis is established in competition with {alpha}{beta} T cells and NK cells. Proc Natl Acad Sci U S A. 2005;102:14741–14746. doi: 10.1073/pnas.0507520102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Laky K, Lewis JM, Tigelaar RE, Puddington L. Distinct requirements for IL-7 in development of TCR gamma delta cells during fetal and adult life. J Immunol. 2003;170:4087–4094. doi: 10.4049/jimmunol.170.8.4087. [DOI] [PubMed] [Google Scholar]
- 82.Furtado GC, Curotto de Lafaille MA, Kutchukhidze N, Lafaille JJ. Interleukin 2 signaling is required for CD4(+) regulatory T cell function. J Exp Med. 2002;196:851–857. doi: 10.1084/jem.20020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263.Using Il2−/− or Il2ra−/− mice, the authors show that IL-2 is important for maintaining homeostasis of Treg cells. Although IL-2 was not required for Treg cell development, γc-deficient mice are devoid of FOXP3+ Treg cells. These data were consistent with idea that more than one cytokine contributing to this function and it is now clear that IL-2, IL-7 and TSLP are three such cytokines (see references 94, 95).
- 84.Antony PA, et al. Interleukin-2-dependent mechanisms of tolerance and immunity in vivo. J Immunol. 2006;176:5255–5266. doi: 10.4049/jimmunol.176.9.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bayer AL, Yu A, Malek TR. Function of the IL-2R for thymic and peripheral CD4+CD25+ Foxp3+ T regulatory cells. J Immunol. 2007;178:4062–4071. doi: 10.4049/jimmunol.178.7.4062. [DOI] [PubMed] [Google Scholar]
- 86.Yao Z, et al. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109:4368–4375. doi: 10.1182/blood-2006-11-055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Antov A, Yang L, Vig M, Baltimore D, Van Parijs L. Essential role for STAT5 signaling in CD25+CD4+ regulatory T cell homeostasis and the maintenance of self-tolerance. J Immunol. 2003;171:3435–3441. doi: 10.4049/jimmunol.171.7.3435. [DOI] [PubMed] [Google Scholar]
- 88.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178:280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 89.Cohen AC, et al. Cutting edge: Decreased accumulation and regulatory function of CD4+ CD25(high) T cells in human STAT5b deficiency. J Immunol. 2006;177:2770–2774. doi: 10.4049/jimmunol.177.5.2770. [DOI] [PubMed] [Google Scholar]
- 90.Lin JX, et al. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995;2:331–339. doi: 10.1016/1074-7613(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 91.Lin JX, Mietz J, Modi WS, John S, Leonard WJ. Cloning of human Stat5B. Reconstitution of interleukin-2-induced Stat5A and Stat5B DNA binding activity in COS-7 cells. J Biol Chem. 1996;271:10738–10744. [PubMed] [Google Scholar]
- 92.Nakajima H, et al. An indirect effect of Stat5a in IL-2-induced proliferation: a critical role for Stat5a in IL-2-mediated IL-2 receptor alpha chain induction. Immunity. 1997;7:691–701. doi: 10.1016/s1074-7613(00)80389-1. [DOI] [PubMed] [Google Scholar]
- 93.Imada K, et al. Stat5b is essential for natural killer cell-mediated proliferation and cytolytic activity. J Exp Med. 1998;188:2067–2074. doi: 10.1084/jem.188.11.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mazzucchelli R, et al. Development of regulatory T cells requires IL-7Ralpha stimulation by IL-7 or TSLP. Blood. 2008;112:3283–3292. doi: 10.1182/blood-2008-02-137414.Although mice deficient in IL-7 or TSLPR have relatively normal numbers of Treg cells, combined deletion of IL-7 and TSLPR greatly decreases the number of Treg cells, indicating that both IL-7 and TSLP contribute to Treg cell development.
- 95.Bayer AL, Lee JY, de la Barrera A, Surh CD, Malek TR. A function for IL-7R for CD4+CD25+Foxp3+ T regulatory cells. J Immunol. 2008;181:225–234. doi: 10.4049/jimmunol.181.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu W, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Seddiki N, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pandiyan P, Lenardo MJ. The control of CD4+CD25+Foxp3+ regulatory T cell survival. Biol Direct. 2008;3:6. doi: 10.1186/1745-6150-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bayer AL, Yu A, Adeegbe D, Malek TR. Essential role for interleukin-2 for CD4(+)CD25(+) T regulatory cell development during the neonatal period. J Exp Med. 2005;201:769–777. doi: 10.1084/jem.20041179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang H, et al. Lymphopenia and interleukin-2 therapy alter homeostasis of CD4+CD25+ regulatory T cells. Nat Med. 2005;11:1238–1243. doi: 10.1038/nm1312. [DOI] [PubMed] [Google Scholar]
- 101.Fukao T, Koyasu S. Expression of functional IL-2 receptors on mature splenic dendritic cells. Eur J Immunol. 2000;30:1453–1457. doi: 10.1002/(SICI)1521-4141(200005)30:5<1453::AID-IMMU1453>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 102.Mnasria K, et al. Anti-CD25 antibodies affect cytokine synthesis pattern of human dendritic cells and decrease their ability to prime allogeneic CD4+ T cells. J Leukoc Biol. 2008;84:460–467. doi: 10.1189/jlb.1007712. [DOI] [PubMed] [Google Scholar]
- 103.Combe CL, et al. Lack of IL-15 results in the suboptimal priming of CD4+ T cell response against an intracellular parasite. Proc Natl Acad Sci U S A. 2006;103:6635–6640. doi: 10.1073/pnas.0506180103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moretto MM, Lawlor EM, Khan IA. Aging mice exhibit a functional defect in mucosal dendritic cell response against an intracellular pathogen. J Immunol. 2008;181:7977–7984. doi: 10.4049/jimmunol.181.11.7977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jinushi M, et al. Autocrine/paracrine IL-15 that is required for type I IFN-mediated dendritic cell expression of MHC class I-related chain A and B is impaired in hepatitis C virus infection. J Immunol. 2003;171:5423–5429. doi: 10.4049/jimmunol.171.10.5423. [DOI] [PubMed] [Google Scholar]
- 106.Sriram U, et al. IL-4 suppresses dendritic cell response to type I interferons. J Immunol. 2007;179:6446–6455. doi: 10.4049/jimmunol.179.10.6446. [DOI] [PubMed] [Google Scholar]
- 107.Taylor BC, et al. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J Exp Med. 2009;206:655–667. doi: 10.1084/jem.20081499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guimond M, et al. Interleukin 7 signaling in dendritic cells regulates the homeostatic proliferation and niche size of CD4+ T cells. Nat Immunol. 2009;10:149–157. doi: 10.1038/ni.1695.This article describes a new role of IL-7 in regulating CD4+ T cells expansion. Increased accessibility to IL-7 in lymphopenic condition diminishes homeostatic proliferation of CD4+ T cells by decreasing the expression of MHC class II molecules by IL-7Rα-expressing DCs.
- 109.Brandt K, Bulfone-Paus S, Foster DC, Ruckert R. Interleukin-21 inhibits dendritic cell activation and maturation. Blood. 2003;102:4090–4098. doi: 10.1182/blood-2003-03-0669. [DOI] [PubMed] [Google Scholar]
- 110.Strengell M, Lehtonen A, Matikainen S, Julkunen I. IL-21 enhances SOCS gene expression and inhibits LPS-induced cytokine production in human monocyte-derived dendritic cells. J Leukoc Biol. 2006;79:1279–1285. doi: 10.1189/jlb.0905503. [DOI] [PubMed] [Google Scholar]
- 111.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–758. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 113.Gor DO, Rose NR, Greenspan NS. TH1-TH2: a procrustean paradigm. Nat Immunol. 2003;4:503–505. doi: 10.1038/ni0603-503. [DOI] [PubMed] [Google Scholar]
- 114.Wurster AL, et al. Interleukin 21 is a T helper (Th) cell 2 cytokine that specifically inhibits the differentiation of naive Th cells into interferon gamma-producing Th1 cells. J Exp Med. 2002;196:969–977. doi: 10.1084/jem.20020620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Suto A, Wurster AL, Reiner SL, Grusby MJ. IL-21 inhibits IFN-gamma production in developing Th1 cells through the repression of Eomesodermin expression. J Immunol. 2006;177:3721–3727. doi: 10.4049/jimmunol.177.6.3721. [DOI] [PubMed] [Google Scholar]
- 116.Strengell M, Sareneva T, Foster D, Julkunen I, Matikainen S. IL-21 up-regulates the expression of genes associated with innate immunity and Th1 response. J Immunol. 2002;169:3600–3605. doi: 10.4049/jimmunol.169.7.3600. [DOI] [PubMed] [Google Scholar]
- 117.Pesce J, et al. The IL-21 receptor augments Th2 effector function and alternative macrophage activation. J Clin Invest. 2006;116:2044–2055. doi: 10.1172/JCI27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Korn T, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nurieva R, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 120.Zhou L, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488.References 118–120 describe the role of IL-21 in the development of the Th17 cell lineage and in the inflammatory response.
- 121.Laurence A, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 122.Veldhoen M, Hirota K, Christensen J, O'Garra A, Stockinger B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J Exp Med. 2009;206:43–49. doi: 10.1084/jem.20081438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kryczek I, et al. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol. 2007;178:6730–6733. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- 124.Hoyer KK, Kuswanto WF, Gallo E, Abbas AK. Distinct roles of helper T-cell subsets in a systemic autoimmune disease. Blood. 2009;113:389–395. doi: 10.1182/blood-2008-04-153346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Amadi-Obi A, et al. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 126.Kryczek I, et al. Cutting edge: opposite effects of IL-1 and IL-2 on the regulation of IL-17+ T cell pool IL-1 subverts IL-2-mediated suppression. J Immunol. 2007;179:1423–1426. doi: 10.4049/jimmunol.179.3.1423. [DOI] [PubMed] [Google Scholar]
- 127.Ivanov II, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Coquet JM, Chakravarti S, Smyth MJ, Godfrey DI. Cutting edge: IL-21 is not essential for Th17 differentiation or experimental autoimmune encephalomyelitis. J Immunol. 2008;180:7097–7101. doi: 10.4049/jimmunol.180.11.7097. [DOI] [PubMed] [Google Scholar]
- 129.Sonderegger I, Kisielow J, Meier R, King C, Kopf M. IL-21 and IL-21R are not required for development of Th17 cells and autoimmunity in vivo. Eur J Immunol. 2008;38:1833–1838. doi: 10.1002/eji.200838511. [DOI] [PubMed] [Google Scholar]
- 130.Chtanova T, et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- 131.Vogelzang A, et al. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–137. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 132.Nurieva RI, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Linterman MA, et al. Follicular helper T cells are required for systemic autoimmunity. J Exp Med. 2009;206:561–576. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Coquet JM, et al. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J Immunol. 2007;178:2827–2834. doi: 10.4049/jimmunol.178.5.2827. [DOI] [PubMed] [Google Scholar]
- 135.Li Y, Bleakley M, Yee C. IL-21 influences the frequency, phenotype, and affinity of the antigen-specific CD8 T cell response. J Immunol. 2005;175:2261–2269. doi: 10.4049/jimmunol.175.4.2261. [DOI] [PubMed] [Google Scholar]
- 136.Hinrichs CS, et al. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111:5326–5333. doi: 10.1182/blood-2007-09-113050.This study demonstrated the contrasting effects of IL-2 and IL-21 during the priming CD8+ T cells on an anti-tumor response, indicating that the presence of IL-21 during CD8+ T cell priming results in persistence of the cells in vivo and potent anti-tumor activity.
- 137.Kovacs JA, et al. Induction of prolonged survival of CD4+ T lymphocytes by intermittent IL-2 therapy in HIV-infected patients. J Clin Invest. 2005;115:2139–2148. doi: 10.1172/JCI23196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Porter BO, et al. Inferiority of IL-2 alone versus IL-2 with HAART in maintaining CD4 T cell counts during HAART interruption: a randomized controlled trial. Aids. 2009;23:203–212. doi: 10.1097/QAD.0b013e32831cc114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 140.Beq S, et al. IL-7 induces immunological improvement in SIV-infected rhesus macaques under antiviral therapy. J Immunol. 2006;176:914–922. doi: 10.4049/jimmunol.176.2.914. [DOI] [PubMed] [Google Scholar]
- 141.Rosenberg SA, et al. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J Immunother. 2006;29:313–319. doi: 10.1097/01.cji.0000210386.55951.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Andorsky DJ, Timmerman JM. Interleukin-21: biology and application to cancer therapy. Expert Opin Biol Ther. 2008;8:1295–1307. doi: 10.1517/14712598.8.9.1295. [DOI] [PubMed] [Google Scholar]
- 143.Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J Exp Med. 2005;202:829–839. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhou B, et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 145.Ying S, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005;174:8183–8190. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 146.Gilmour KC, et al. Defective expression of the interleukin-2/interleukin-15 receptor beta subunit leads to a natural killer cell-deficient form of severe combined immunodeficiency. Blood. 2001;98:877–879. doi: 10.1182/blood.v98.3.877. [DOI] [PubMed] [Google Scholar]
- 147.Waldmann TA. Anti-Tac (daclizumab, Zenapax) in the treatment of leukemia, autoimmune diseases, and in the prevention of allograft rejection: a 25-year personal odyssey. J Clin Immunol. 2007;27:1–18. doi: 10.1007/s10875-006-9060-0. [DOI] [PubMed] [Google Scholar]
- 148.Mayordomo JI et al. Bone marrow-derived dendritic cells serve as potent adjuvants for peptide-based antitumor vaccines. Stem Cells. 1997;15:94–103. doi: 10.1002/stem.150094. [DOI] [PubMed] [Google Scholar]
- 149.Thurner B et al. Generation of large numbers of fully mature and stable dendritic cells from leukapheresis products for clinical application. J. Immunol. Methods. 1999;223:1–15. doi: 10.1016/s0022-1759(98)00208-7. [DOI] [PubMed] [Google Scholar]