Abstract
The proper functioning of the pathways that are involved in the sensing and management of nutrients is central to metabolic homeostasis and is therefore among the most fundamental requirements for survival. Metabolic systems are integrated with pathogen-sensing and immune responses, and these pathways are evolutionarily conserved. This close functional and molecular integration of the immune and metabolic systems is emerging as a crucial homeostatic mechanism, the dysfunction of which underlies many chronic metabolic diseases, including type 2 diabetes and atherosclerosis. In this Review we provide an overview of several important networks that sense and manage nutrients and discuss how they integrate with immune and inflammatory pathways to influence the physiological and pathological metabolic states in the body.
The integration of metabolism and immunity (or of nutrient- and pathogen-sensing pathways) can be traced back to an evolutionary need for survival, which resulted in the co-development of the organ systems and signalling pathways that mediate these two processes1. The pressure to survive would have favoured energy efficiency and storage to prepare for times of food deprivation and for mounting a potent immune response to defend the host against infectious agents. However, the initiation and maintenance of immunity is a metabolically costly endeavour and cannot operate efficiently under conditions of energy deficit2,3. For example, fever is associated with a 7–13% increase in caloric energy consumption per 1°C increase in body temperature, the energy expenditure of which is estimated to equate to 9.4×106 joules; this is approximately the energy cost of a 70 kg person walking 45 km4,5. Sepsis can increase the human metabolic rate by 30–60%6. Furthermore, the production and maintenance of phagocytes during infection is thought to result in an energy consumption of approximately 7.9×105 joules4.
It is also clear that starvation and malnutrition can impair immune function; a total reduction in body fat has been shown to result in a decrease in the energy that is available for immune responses in rodents7. In addition, conditions that trigger an immune response during starvation can severely reduce the survival of insects8. Therefore, immune defence is subject to a trade-off between other energy-demanding processes, such as reproduction, thermoregulation and lactation. Interestingly, energy surplus (which is typical of individuals who are obese or suffer from metabolic syndrome) can also impair immune responses and induce chronic inflammation (see later). Therefore, a balanced energy flux and maintainance of favourable metabolic homeostasis are required for the proper functioning of the immune system.
These processes may have been optimized through the close coordination and co-evolution of metabolic and immune responses, and of the organs that are involved in these processes. Evidence supporting such a developmental history can be found in lower organisms, such as Drosophila melanogaster, in which immune and metabolic responses are controlled by the same organ, the fat body9. In addition, tissues that are important in metabolism are thought to have an evolutionary potential to mediate inflammatory responses. The association between metabolism and inflammation is also evident in tissues of higher organisms, for example the liver and adipose tissue, where immune effector cells, such as Kupffer cells and macrophages, are found alongside hepatocytes and adipocytes, respectively1. Interestingly, lymph nodes are also embedded in adipose tissue, perhaps to have a competitive advantage over other tissues in meeting excessive energy demands at times of immune stress10. In addition, the perinodal adipose tissue, which is located around the lymph nodes, might influence local immune responses owing to its high polyunsaturated fatty-acid content, which could provide nutrients and soluble mediators that are needed for the responses, and the presence of dendritic cells11. Remodelling of the adipose tissue can also accompany certain inflammatory processes, for example, the development of panniculitis during inflammatory bowel disease12.
Despite the evidence suggesting that the immune and metabolic systems need to colocalize to maintain metabolic homeostasis during an immune response, energy can be transported efficiently throughout the body by the circulatory system, which questions a requirement for local energy supplies. However, most infections can suppress the host’s appetite, possibly by inducing the synthesis of leptin (an adipocyte-derived hormone and cytokine), which suggests that local sources of energy and nutrients are more important during an immune response13. Nevertheless, many of these observations have not been supported by experimental evidence and therefore their physiological significance is still unclear.
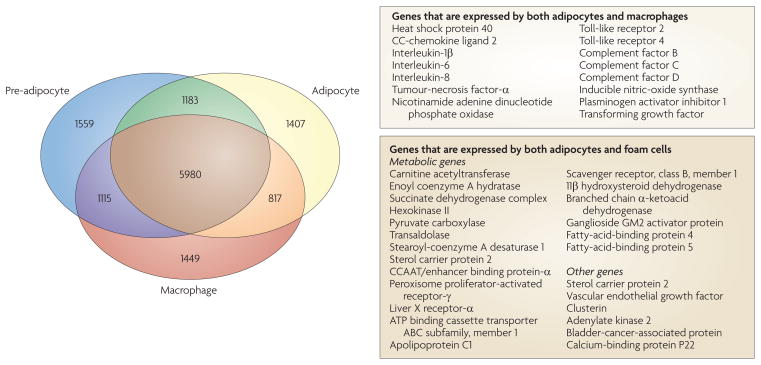
Cells that are involved in metabolic and immune responses also show evidence of coordination and co-evolution. More specifically, macrophages and adipocytes are closely related and share many functions; for example, they both secrete cytokines and can be activated by pathogen-associated components, such as lipopolysaccharide (LPS)14. In addition, phagocytosis and the expression of membrane-bound NADPH oxidases, which are characteristics of macrophages, are traits that have also been attributed to adipocytes15. Indeed, pre-adipocytes have been shown to transdifferentiate into macrophages, and transcriptional profiling has suggested that macrophages and pre-adipocytes are genetically related15,16. Moreover, there is an extensive genetic and functional overlap between fully differentiated adipocytes and macrophages that have transformed into atherogenic foam cells, particularly in terms of metabolic genes (FIG. 1).
Figure 1. molecular characteristics shared between adipocytes and macrophages in physiological conditions and metabolic disease states.
An extensive transcriptional signature is common to adipocytes and macrophages. The Venn diagram shows the number of genes that are expressed preferentially or approximately equally by pre-adipocytes, adipocytes and macrophages. Inflammatory genes that are expressed by both macrophages and adipocytes are also listed, as are adipocyte-specific metabolic and other genes that are upregulated during the transformation of macrophages into foam cells14–16,114,115.
It can be envisioned that a threat to the delicate balance between immune and metabolic responses, such as can be induced by chronic nutrient deficiency or a continuous energy surplus, can transform this intimate, long-lasting and productive interaction into a pathological relationship (in this Review, we focus on overnutrition and not on malnutrition). Exposure to excess amounts of nutrients and energy is a modern phenomenon that has been caused by changes in dietary patterns and lifestyle worldwide. These changes are associated with an increase in the incidence of chronic metabolic diseases, such as obesity, type 2 diabetes, fatty liver disease and atherosclerosis, as well as asthma and some cancers1. Under these energy-rich conditions, the ancient inflammatory potential of metabolically important tissues can be reactivated; the adipose tissue of obese individuals has in fact been shown to produce higher levels of the pro-inflammatory cytokine tumour-necrosis factor (TNF) and other pro-inflammatory factors17,18.
Chronic inflammation, particularly when it occurs in metabolically important organs such as the liver and adipose tissue, has a crucial role in the development of many chronic metabolic diseases, such as diabetes, fatty liver disease and cardiovascular disease17. It is important to recognize that this response does not resemble classic inflammation and perhaps could be considered as an aberrant form of immunity that is triggered by nutrients or other intrinsic cues, and has been referred to as meta-inflammation or para-inflammation1,19. In fact, many branches of the immune response are defective in obese individuals, including the activity of neutrophils, natural killer cells and T cells13,20. Nevertheless, it is important to explore the general mechanisms that integrate the immune response with systemic metabolic homeostasis and to identify ways to exploit these pathways for the treatment of chronic metabolic diseases.
Linking metabolism and inflammation
Insulin is the main anabolic hormone in mammals and is essential for metabolic homeostasis. Binding of insulin to the insulin receptor triggers the tyrosine phosphorylation of its cellular substrates, such as the insulin receptor substrate (IRS) family of proteins21. These signalling events and molecules are crucial for mediating many of the metabolic effects of insulin17,21,22, but are inhibited during conditions of stress and inflammation through modifications, such as serine phosphorylation, that are mediated by intracellular regulatory pathways. This inhibition has also been observed in individuals that are obese and/or suffer from insulin resistance and type 2 diabetes1. The modifications that impair the action of insulin can be triggered by cytokines, such as TNF, indicating that immune mediators can have a crucial regulatory role in systemic glucose homeostasis23–25. Although several inflammatory pathways have been shown to contribute to metabolic dysregulation at several levels, modulation of insulin signalling is perhaps the most crucial, as it is a highly conserved and dominant metabolic pathway in nutrient and energy homeostasis.
The identification of the link between inflammation and insulin signalling also proved to be a productive discovery platform for exploring the links between immune responses and metabolic control26–30. In addition to cytokines, many of the inflammatory signalling pathways that inhibit insulin-receptor signalling are directly triggered by nutrients, such as circulating lipids. Other inflammatory pathways are induced by organelle stress owing to nutrient overload and processing defects and result in metabolic stress. In both cases, activation of kinases, such as JUN N-terminal kinase (JNK; also known as MAPK8) and IκB kinase-β (IKKβ), leads to the serine phosphorylation of IRS1, the disruption of the insulin signalling pathway and altered metabolic responses1 (FIG. 2). Disruption of the insulin signalling pathway in this manner can also be mediated by extracellular-signal-regulated kinase (ERK), ribosomal protein S6 kinase (S6K; also known as RPS6KB1), mammalian target of rapamycin (mTOR; also known as FRAP1), protein kinase C and glycogen synthase kinase 3β, all of which can be activated by immune signalling pathways (see later), although the exact nature of the modifications and the metabolic outcomes in each scenario are still not completely understood22. It is probable that many other immune signalling pathways and proteins will be linked to altered metabolic responses.
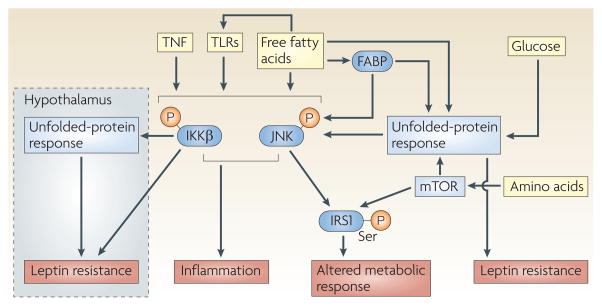
Figure 2. nutrient sensing and inflammation.
The signalling pathways that sense and respond to three basic nutrients, free fatty acids, glucose and amino acids, are depicted. Many pathways that monitor nutrient availability, such as those of mammalian target of rapamycin (mTOR), insulin and nuclear transcription factors (not shown), and the unfolded-protein response (UPR) in the endoplasmic reticulum (ER), closely interact with each other. They also interact with inflammatory pathways, particularly those that are induced through the activation of IκB kinase-β (IKKβ) and JUN N-terminal kinase (JNK). Leptin resistance can be induced through ER stress and through the activation of the IKK pathway when overnutrition is detected by the hypothalamus116,117. In addition to these intracellular points of crosstalk, nutrients may directly induce inflammation through pathogen-sensing receptors, such as the activation of Toll-like receptor (TLR) signalling by free fatty acids. Dysregulation of the crosstalk between the pathways that are shown here can culminate in altered metabolic responses, inflammation and leptin resistance. IκB, inhibitor of nuclear factor-κB; IRS1, insulin receptor substrate 1; FABP, fatty-acid binding protein; TNF, tumour-necrosis factor.
Moreover, signalling pathways that are traditionally considered metabolic can also affect the immune response. For example, activation of nuclear receptors, such as peroxisome-proliferator-activated receptors (PPARs) and liver X receptors (LXRs), can suppress inflammatory pathways17,31. Moreover, several metabolic hormones, such as leptin, resistin and adiponectin, also possess immunological activity13. The mechanism of action and biological significance of these molecular factors have been extensively analyzed in the published literature, so in this Review we focus on the most recent findings on the potential regulation of inflammation in the context of metabolic diseases1.
Organelle stress, inflammation and metabolism
In search of the key signalling pathways that link metabolism, inflammation and insulin action, studies from our laboratory have shown that obesity leads to the activation of JNK in metabolically active sites such as the liver, muscle and adipose tissue28,32. JNK is activated in response to various stress signals, including pro-inflammatory cytokines, free fatty acids, reactive oxygen species (ROS), pathogens and pathogen-associated components, and many of these stress signals also result in the inhibition of insulin signalling. The induction of JNK phosphorylation can increase cytokine production and inflammation and mediates insulin resistance through the serine phosphorylation of IRS proteins (FIG. 2). In mouse models, activation of JNK has a central role in the development of metabolic diseases: JNK1-deficient mice are protected from insulin resistance and the development of type 2 diabetes in both dietary and genetic mouse models of obesity, and experimental activation of JNK is sufficient to induce diabetes in mice28,33,34.
The activation of JNK mediates the serine phosphorylation of IRS1 in conditions of obesity, resulting in defective insulin signalling and inflammation in adipose and liver tissue35. Although JNK has an important role in the inflammatory responses that are mediated by myeloid cells, the effects of JNK activation on glucose metabolism and insulin signalling seem to occur predominantly in cells with primary metabolic function, such as adipocytes and hepatocytes36,37. These observations also indicate that parenchymal cells, such as adipocytes, have a role in the initiation and maintenance of the inflammatory responses that are triggered in conditions of obesity and in the subsequent deterioration of metabolic homeostasis.
It is not clear whether inflammation, metabolic products, such as lipids, or other mechanisms initiate the activation of JNK in conditions of obesity, or whether the activation of JNK precedes inflammation for the induction of the inflammatory signalling pathways that give rise to insulin resistance. Therefore, understanding what the primary insulting factor is for the induction of insulin resistance is very important19. Recent studies have suggested that metabolic stress is sensed inside the cell by organelles, and the resulting dysfunction of organelles then triggers a network of stress-induced signals that disrupt metabolic homeostasis. One such organelle, the endoplasmic reticulum (ER), has been shown to integrate inflammatory and stress signals with the metabolic status of the cell, disruption of which can result in diseases such as type 2 diabetes32.
ER stress and inflammation
The ER has an important role in protein processing and lipid metabolism. An accumulation of unfolded proteins in the ER (known as ER stress) as well as hypoxia, infections, toxins, nutrient overload or energy deprivation can trigger a protective response, known as the unfolded-protein response (UPR). The UPR is mediated by three different stress-sensing pathways that are initiated by three transmembrane proteins which are located in the ER: pancreatic ER kinase (PERK; also known as EIF2AK3), inositol-requiring kinase 1 (IRE1; also known as ERN1) and activating transcription factor 6 (ATF6)1,38,39 (FIG. 3). Activation of PERK leads to the phosphorylation of eukaryotic translation initiation factor 2α (eIF2α) and inhibition of translation45,46. In addition to its kinase activity, which leads to autophosphorylation, IRE1 also possesses endoribonuclease activity that splices X-box binding protein 1 (XBP1) mRNA; this results in the production of the active transcription factor XBP1s40–42. The ATF6-mediated branch of the UPR cooperates with IRE1 by upregulating the expression of XBP1 mRNA43. The expression and activation of XBP1s, as well as the production of active ATF6 and its translocation to the nucleus, where it acts as a transcription factor, leads to a complex transcriptional programme that has a central role in the UPR. This programme includes the upregulation of ER-resident chaperone proteins, which promote protein folding, and the production of components of the protein-degradation apparatus that assist in the re-establishment of ER homeostasis39,44. In addition to these protective responses and stimulation of ER synthesis, these UPR pathways can also induce important inflammatory signals. If ER homeostasis is not restored, the ER activates apoptotic pathways to initiate cell death45.
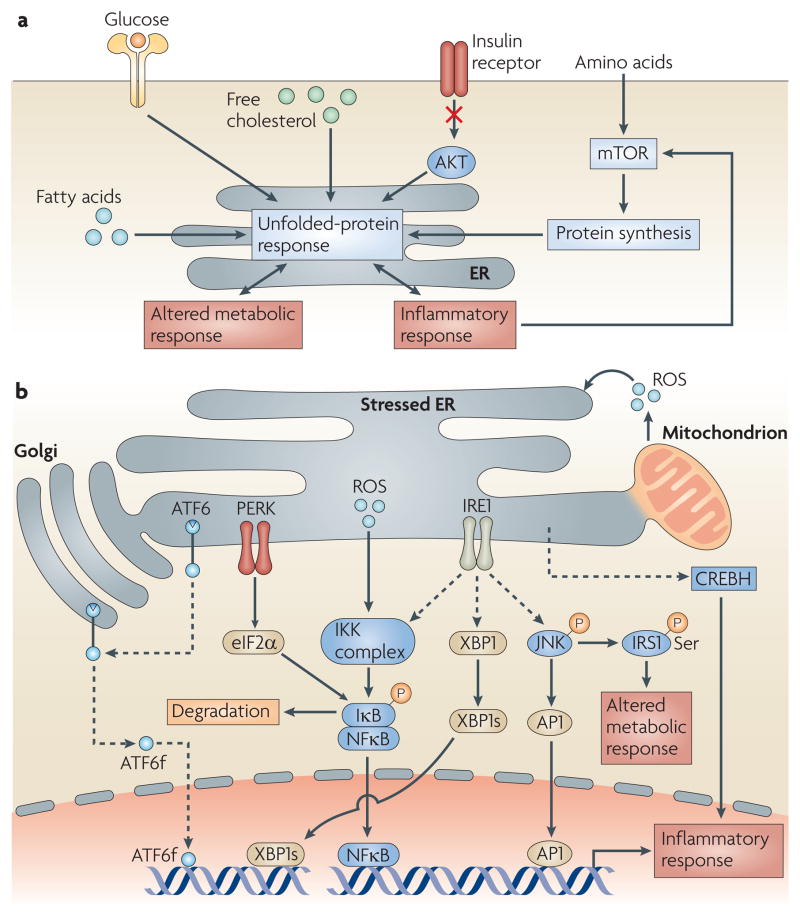
Figure 3. The unfolded-protein response, nutrient sensing and inflammation.
a. The endoplasmic reticulum (ER) is an important organelle that responds to multiple nutrient-associated signals, such as those induced by fatty acids, glucose, free cholesterol, insulin and amino acids. The unfolded-protein response (UPR) is induced in response to ER stress and activates stress-response pathways (not shown) and inflammatory signalling pathways that can result in altered metabolic and inflammatory responses and consequently in metabolic disease. The inflammatory response can enhance the UPR and further activate mammalian target of rapamycin (mTOR). b. The UPR is mediated by three different pathways that are initiated by three transmembrane proteins that are located in the ER — activating transcription factor 6 (ATF6), pancreatic ER kinase (PERK) and inositol-requiring kinase 1 (IRE1). ER stress is linked to inflammation through the activation of the JUN N-terminal kinase (JNK) and the IκB kinase (IKK)–nuclear factor-κB (NFκB) pathways, and through cyclic-AMP-responsive-element-binding protein H (CREBH) activation by the UPR. These pathways result in the induction of an inflammatory response. Activation of JNK can also serine phosphorylate insulin receptor substrate 1 (IRS1), resulting in altered metabolic responses. Key organelles for cellular metabolism, such as the ER, Golgi, mitochondria and peroxisomes (not shown) are connected through an endomembrane network, which provides functional continuity between organelles that can therefore share functional information in the form of lipids and proteins at specific contact sites. This functional and molecular integration between the organelles can mediate the spread of stress from one organelle to the other, resulting in exacerbation of inflammation and cytotoxicity during chronic metabolic stress conditions such as obesity and dyslipidaemia. AP1, activator protein 1; ATF6f, ATF6 fragment; eIF2α, eukaryotic translation initiation factor 2α; IκB, inhibitor of NFκB; XBP1s, spliced X-box binding protein 1.
ER stress is linked with inflammation through several mechanisms. First, IRE1-mediated activation of JNK induces the expression of pro-inflammatory genes by directly influencing the transcription factor activator protein 1 (AP1)42 (FIG. 3). Consistent with this, JNK1-deficient mice have reduced expression levels of TNF and interleukin-6 in high-fat-diet-induced obesity28. This JNK1-mediated pro-inflammatory response, in addition to JNK1-mediated serine phosphorylation of IRS1, can contribute to obesity-induced insulin resistance. Indeed, inhibition of JNK by chemical or peptide inhibitors can reverse ER-stress-induced insulin resistance in cells (discussed later)32.
A second mechanism involves the activation of the IKK–NFκB (nuclear factor-κB) signalling pathway by both PERK and IRE146,47. Activation of PERK triggers the degradation of inhibitor of NFκB (IκB), which allows for the translocation of NFκB into the nucleus and the activation of pro-inflammatory genes46,47. IRE1 associates with the IKK complex through a TNF-receptor-associated factor 2 (TRAF2)-dependent mechanism, resulting again in the degradation of IκB. When NFκB is activated in response to ER stress that is triggered by either viruses or chemical stressors, a potent pro-inflammatory response is induced that is characterized by the production of enzymes, such as cyclooxygenase-2 (COX2), and other inflammatory mediators46,47.
Third, ER stress leads to the cleavage and activation of the transcription factor cyclic-AMP-responsive-element-binding protein H (CREBH), which induces the production of the acute-phase proteins C-reactive protein (CRP) and serum amyloid P-component (SAP), particularly in the liver48. The extent to which these mechanisms contribute to inflammation in physiological conditions, or whether they are involved at all, remains to be determined.
A fourth mechanism that links ER stress to inflammation involves ROS, which are abundantly produced by the ER during conditions of stress, and lead to oxidative damage and the activation of many stress and inflammatory signalling cascades49. An important role for oxidative stress in obesity-associated insulin resistance has been described50. In chronic metabolic disease, stress is evident in many organelles, and the mitochondria in particular have a well-accepted role in the production of ROS in conditions of obesity50. However, organelles are connected through an endomembrane system that allows for the exchange of lipids and proteins, which suggests that stress signals can spread through these cellular subcompartments1,51 (FIG. 3). Therefore, once a chronic disease process has been initiated, it may not be possible to restrict cellular dysfunction to a single organelle. Consequently, dysfunction of the ER could strongly affect the function of mitochondria. Alternatively, defects in mitochondrial function could contribute to ER stress.
So, organelle stress and inflammation both contribute to the development of obesity-associated insulin resistance and chronic metabolic diseases; however, it remains to be determined which of these processes comes first. Future studies in which the intermediate genes in both UPR and inflammatory pathways are chemically or genetically targeted should be instrumental in delineating the order of events. Regardless, therapeutic targeting of organelle dysfunction offers new opportunities for the management of chronic metabolic diseases.
Nutrient sensing and the UPR
ER stress and the UPR are inextricably linked with the nutrient status of cells. In fact, the UPR pathway was first identified by the discovery of a set of glucose-regulated proteins (GRPs) that are induced by glucose deprivation, which suggests that the ER has an important role in nutrient sensing52. Furthermore, UPR signalling, particularly the PERK-mediated branch of this response, has a role in the maintenance of glucose homeostasis, and activation of the UPR is required for withstanding glucose or energy fluctuations in cells, especially pancreatic β-cells and plasma cells53,54. Autophosphorylation of IRE1 can be directly induced by acute glucose stimulation, and UPR-induced transcriptional programmes are also directly linked to the synthesis and breakdown of glucose55. As sustained activation of IRE1 by chronic exposure to high concentrations of glucose or other metabolic signals will engage both the JNK and IKK–NFκB signalling pathways, this branch of the ER stress response is an important integration point between inflammatory and metabolic signals44 (FIG. 3).
The ER is also important in the metabolism of lipids, especially phospholipids and cholesterol, and can monitor their intracellular status. More specifically, cholesterol sensing is initiated at the ER membrane through the transcription factor sterol-regulatory-element-binding protein (SREBP)44. Moreover, there are established links between the UPR and lipid synthesis and breakdown, and saturated fatty acids can trigger ER-stress responses in liver cells, cardiomyocytes and macrophages56,57. Although the exact mechanisms that link lipid sensing and metabolism to ER stress are yet to be fully defined, the outcome of the UPR following lipid sensing is crucial for metabolic homeostasis32. Identification of the molecular links between intra-cellular fatty-acid status and ER-stress responses remains an important but unaddressed question.
As part of its role in integrating nutrient-sensing pathways with insulin signalling and survival pathways, the ER stress responses are also linked to the mTOR pathway58, which regulates several processes in the cell, including energy metabolism. In the absence of the genes that encode components of the tuberous sclerosis complex (TSC), which is a heterodimer of TSC1 (also known as hamartin) and TSC2 (also known as tuberin), the mTOR pathway becomes hyperactivated, resulting in uncontrolled protein synthesis, increased ER stress and UPR activation, including JNK activation58 (FIG. 4). In TSC-deficient mouse embryonic fibroblasts treatment with the chemical chaperone 4-phenyl butyric acid, (which is involved in protein folding) or the induction of exogenous expression of active XBP1s (which is involved in the UPR) was shown to relieve ER stress. This resulted in reduced JNK activity, increased insulin signalling and protection against glucose-deprivation-induced apoptosis58. As the mTOR pathway connects nutrient sensing and immune responses, direct links between mTOR activity and ER stress suggest that the ER has a unique role in coordinating metabolism and immunity.
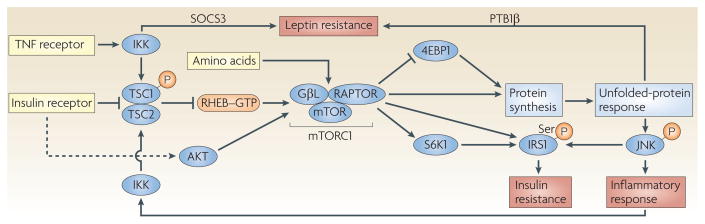
Figure 4. The mammalian target of rapamycin pathway, amino-acid sensing and inflammation.
Mammalian target of rapamycin (mTOR) is an important checkpoint kinase that transmits signals related to amino-acid sufficiency and protein synthesis. A hyperactive mTOR pathway has been associated with increased signalling induced by the unfolded-protein response (UPR) and with the activation of JUN N-terminal kinase (JNK), which can lead to increased inflammation and insulin resistance through serine phosphorylation of insulin receptor substrate 1 (IRS1). Inflammation can further activate mTOR, through IκB kinase (IKK)-mediated phosphorylation of tuberous sclerosis complex 1 (TSC1), as can the UPR through the induction of activating transcription factor 6 (ATF6; not shown). This leads to a vicious inflammatory cycle in metabolically stressed cells. The UPR and IKK activation can also lead to leptin resistance through the induction of polypyrimidine tract-binding protein 1β (PTB1β) and suppressor of cytokine signalling 3 (SOCS3), respectively. 4EBP1, eukaryotic translation-initiation factor 4E-binding protein 1; GβL, G-protein β-subunit-like protein; IκB, inhibitor of nuclear factor-κB; mTORC2, mTOR complex 2; RAPTOR, regulatory associated protein of mTOR; RHEB, RAS homology enriched in brain; S6K1, ribosomal protein S6 kinase 1; TNF, tumour-necrosis factor.
Indeed, ER stress can be triggered by nutrient accumulation in immune cells. For example, in macrophages, accumulation of free cholesterol leads to apoptosis through the activation of the ER stress response and JNK59, and this has also been observed in macrophages that infiltrate atherosclerotic plaques60. Furthermore, macrophages from insulin-receptor-deficient mice show increased susceptibility to apoptosis that is triggered by ER stress61. Taken together, these findings suggest that a reciprocal regulation between the ER-stress and insulin-signalling pathways could establish a vicious cycle in cells, providing a mechanism to explain the interdependence of insulin resistance and atherosclerosis.
Activation of the UPR is also important for the development of other immune cells, such as dendritic cells and lymphocytes, and for the expansion of the ER during plasma-cell differentiation39. However, it remains to be determined how the function of these cell types may be altered through the activation of UPR during conditions of obesity.
The UPR and metabolic diseases
ER stress has been shown to have an important role in the pathogenesis of metabolic disease. More specifically, increased ER stress responses in the liver and adipose tissue of obese mice are known to have a role in the development of systemic insulin resistance and type 2 diabetes. Furthermore, promotion of ER stress through genetic haploinsufficiency of the Xbp1 gene triggers obesity and insulin resistance, whereas alleviation of ER stress by treatment with chaperone proteins is protective against metabolic deterioration in obese animals32,35. Other studies have also shown that compromising ER function through the manipulation of chaperone proteins can modulate the systemic action of insulin1. ER stress leads to insulin resistance, at least in part through the serine phosphorylation of IRS1 by IRE1-activated JNK1 (REF. 32). An interesting recent study also demonstrated a role for ER stress in leptin resistance at the cellular level62. These and other findings highlight the importance of the integration of nutrient and inflammatory responses in metabolic homeostasis, and the ways in which dysfunction of the ER could affect this integration and possibly result in chronic metabolic disease32,59,63,64. Importantly, the ER in immune cells may also serve as the site where cellular responses to pathogens and pathogen-induced signalling pathways are integrated (BOX 1). Therefore, an intriguing speculation is that if pathogens can affect ER homeostasis and the ER is crucial in metabolic disease, disruption of ER homeostasis by pathogens could provide an ‘infectious’ aetiology for chronic metabolic diseases, such as type 2 diabetes and atherosclerosis (discussed later).
Box 1. Microorganisms engage the ER metabolic pathways while evading an immune response.
Viruses use the endoplasmic reticulum (ER) membranes for translation, replication and budding of viral particles. During infection with herpes simplex virus (HSV), accumulation of viral proteins in the ER triggers a stress response through the activation of pancreatic ER kinase (PERK) and the induction of phosphorylation of eukaryotic translation-initiation factor 2α (eIF2α). Phosphorylation of eIF2α leads to the general inhibition of translation, resulting in reduced production of viral proteins. Interestingly, HSV can evade this host defence mechanism by selectively dephosphorylating eIF2α. eIF2α kinases, such as PKR (IFN-inducible dsRNA-dependent protein kinase), can also respond to pathogens and engage inflammatory pathways through the activation of JUN N-terminal kinase (JNK). Whether PKR also contributes to metabolic homeostasis remains an important but unanswered question. Furthermore, virus-mediated ER stress may result in the disruption of protein folding and lead to an accumulation of MHC class I molecules in the lumen and a subsequent reduction in cell-surface MHC class I expression, thereby compromising antigen presentation. In addition, vesicular transport from the ER to the Golgi apparatus can be blocked by viruses, interfering with the production of immune mediators and with the secretion of antibodies101,108.
Many pathogens interact with the ER to subvert its functions and evade host immune surveillance but ER-mediated mechanisms can also preserve host defence. Although hiding and replicating in autophagosomes is beneficial for viruses, in the case of infection with Mycobacterium tuberculosis, the induction of autophagy (potentially through a contribution by the ER) has been shown to be an effective host defence mechanism101,103. Furthermore, induction of the unfolded-protein response by viruses can be both beneficial for the pathogen (owing to the induction of chaperone-assisted viral-protein folding) and detrimental (owing to the activation of PERK and the disruption of viral-protein translation)101,108. The ways in which pathogens engage ER-associated metabolic pathways may provide information on the mechanisms by which pathogens affect, most often in a detrimental manner, the metabolic homeostasis of the host, and may help to identify common therapeutic targets for both infectious and metabolic diseases.
The mTOR pathway and nutrient sensing
Survival during conditions of starvation is dependant on cellular proteins being degraded to release essential amino acids that can be used for the synthesis of new proteins. The mTOR pathway lies at the core of this amino-acid-sensing pathway (FIG. 4). It not only senses nutrient availability and intracellular energy status, but also integrates this information with extracellular stimuli such as insulin and growth factors, which dictate cellular metabolism and growth patterns. Therefore, the mTOR pathway regulates numerous processes within a cell, including cell cycle, cell size, cellular growth, energy metabolism, translation initiation, ribosome biogenesis, transcription, autophagy and immune responses65.
The serine/threonine kinase mTOR can form two distinct complexes (mTORCs), mTORC1 (with G-protein β-subunit-like (GβL) protein and regulatory associated protein of mTOR (RAPTOR)) and mTORC2 (with GβL protein and rapamycin-insensitive companion of TOR (RICTOR))65,66. The mTORC1 complex is regulated by nutrients and AMP and is inhibited by the drug rapamycin65. Activation of the mTORC1 complex leads to the phosphorylation of S6K1 and of eIF4E-binding protein 1 (4EBP1), and the latter leads to the induction of protein synthesis (FIG. 4). The regulation and function of the mTORC2 complex is not yet well understood65,66.
The mTOR pathway transduces diverse nutritional, hormonal and environmental signals. Its activation is regulated by upstream signalling and regulatory complexes, by nucleocytoplasmic shuttling, by its binding partners, by lipid second messengers (such as phosphatidic acid) and by transcriptional regulation during cell differentiation and hypertrophy66. In addition, mTOR is negatively regulated by TSC66 (FIG. 4).
Several studies have suggested that hyperactivation of the mTOR signalling pathway may be an important component of the pathologies that underlie metabolic syndromes. Indeed, continuous activation of mTORC1 in TSC-deficient cells leads to the suppression of insulin-receptor signalling through many mechanisms, including the activation of S6K1 and the activation of JNK through the induction of the UPR58,67. Furthermore, obesity is associated with increased mTOR activity, and deficiency of the downstream mediator S6K1 can protect mice against age-induced and high-fat-diet-induced weight gain and insulin resistance24,68. Despite these beneficial changes, S6K1-deficient mice also exhibit compromised islet function as a result of a selective reduction in pancreatic β-cell mass68. Therefore, activation of S6K1, and possibly mTOR, provides an interesting example in which a deficiency in growth signals can lead to divergent effects at the organismal and cellular levels: that is, an anti-diabetogenic effect on metabolic tissues and a diabetogenic effect owing to underdeveloped pancreatic β-cells.
Another connection between the mTOR pathway and metabolism is seen in lower organisms, such as D. melanogaster, in which TOR signalling in the fat body modulates insulin signalling and growth in peripheral tissues69. Also, the action of mTOR in the central nervous system of rats can affect metabolism through the co-regulation of appetite and body weight70. Most recently, and as mentioned earlier, we established a link between mTOR activity and ER stress, which is an important mechanism that contributes to insulin resistance and cell viability in TSC-deficient mice58. Interestingly, in a recent study IKKβ, activated by the TNF receptor, was shown to directly phosphorylate and inhibit TSC1, resulting in constitutive mTOR activation71. Taken together, these observations, made in the context of tumour angiogenesis, demonstrate how a major inflammatory signal such as IKKβ can establish a link between nutrient-sensing and ER homeostasis pathways. Indeed, such a link is directly shown in the hypothalamus, where activation of IKKβ leads to ER stress and leptin resistance116,117.
Aside from being integral to metabolism, amino acids and amino-acid sensing pathways (such as the mTOR pathway) are also required for immunity. There is a high demand for amino acids as substrates for energy production and as synthetic precursors for proliferating immune cells, as well as for the production of immune mediators, such as cytokines and antibodies, during infection. The high demand for amino acids is met by an increased catabolism of muscle proteins. Amino acids such as glutamine, arginine and branched amino acids are preferentially used during infection. The importance of these substrates for immune function would suggest that an intracellular sensor, such as mTORC1, is required to monitor their presence and metabolism in the cell65. Indeed, activation of mTORC1 promotes protein synthesis, glycolytic metabolism and T-cell proliferation in response to the cytokine interleukin-2 (REF. 118).
Metabolism of a single amino acid, such as metabolism of tryptophan by dendritic cells or L-arginine by myeloid-derived suppressor cells (MDSCs), can be an independent determinant in T-cell responses. Tryptophan is an essential amino acid that is preferentially acquired from the diet and is catabolized by indolamine 2,3-dioxygenase (IDO)-expressing macrophages and dendritic cells. IDO is a potent immunomodulator and inhibits the proliferation of T cells, intracellular pathogens and cancer cells through the catabolism of tryptophan. The depletion of tryptophan is sensed by eIF2αK4 (also known as GCN2) in T cells, and this leads to the phosphorylation of eIF2α and the inhibition of protein translation72. By contrast, L-arginine is a conditional essential amino acid that is only required in situations in which L-arginine metabolism is altered, such as trauma and cancer. MDSCs that enter lymphoid organs or the peripheral tissue are activated by T cells and subsequently block T-cell proliferation. Blocking T-cell proliferation involves altering L-arginine metabolism in MDSCs through two key enzymes: inducible nitric-oxide synthase (iNOS), which generates nitric oxide, and arginase, which degrades arginine. Depletion of L-arginine through the induction of arginase inhibits iNOS expression and restricts the proliferation of immune cells in the local environment through the activation of eIF2αK4 (REF. 73). In a protein-rich diet eIF2αK4 also has an important role in mediating fatty liver disease74. Collectively, these examples show that amino acids and the amino-acid sensitive pathways have a role in the initiation of an immune response.
These nutrient-sensing pathways can also be hijacked by pathogens for their proliferation and survival. For example, viruses such as encephalomyocarditis virus and vesicular stomatitis virus inhibit cap-dependent translation in the host by inducing the dephosphorylation of 4EBP1 (REFS 75,76). As a result, viral mRNA can be translated through the cap-independent internal ribosomal entry site of their mRNA. As translational control is emerging as an important regulator of glucose and energy metabolism, some of the intriguing links between the mTOR pathway and viral infection may be related to the emergence of metabolic diseases.
Lipid mediators and inflammation
The Toll-like receptor family and metabolism
Lipids can activate members of the innate immune Toll-like receptor (TLR) family77 (FIG. 2). These are evolutionarily ancient pattern-recognition receptors that facilitate the detection of pathogens. There are at least 12 family members that recognize various ligands: lipoproteins and glycolipids are recognized by TLR2, double-stranded RNA by TLR3, lipopolysaccharide by TLR4 and bacterial CpG-containing DNA by TLR9. TLR activation triggers a potent immune response against infectious agents that includes the production of cytokines and chemokines and the upregulation of co-stimulatory molecules that contribute to the induction of adaptive immune responses19,78. Interesting new evidence indicates that TLRs can also respond to nutritional lipids and might thereby have a role in the pathogenesis of obesity-associated insulin resistance77,79,80 (FIG. 5).
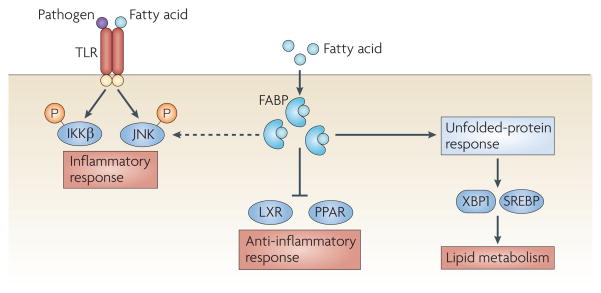
Figure 5. lipid-sensing pathways and inflammation.
Increased amounts of fatty acids can directly induce Toll-like receptors (TLRs) and lipid sensors or transmit stress signals following binding to intracellular lipid chaperones known as fatty-acid binding proteins (FABPs). Activation of TLRs induces the activation of IκB kinase (IKK) and JUN N-terminal kinase (JNK). JNK can also be activated as a result of endoplasmic reticulum (ER) stress inside the cell (not shown). The ER responds to increased levels of lipotoxic fatty acids and free cholesterol by activating the unfolded-protein response. Activation of two transcription factors that are associated with the ER, X-box binding protein 1 (XBP1s) and sterol-regulatory-element-binding protein (SREBP), can modify intracellular lipid metabolism. Furthermore, fatty acids can directly serve as both activating and inhibiting ligands for nuclear receptors, such as peroxisome-proliferator-activated receptors (PPARs) and liver X receptors (LXRs), and affect inflammation.
The recognition of fatty acids by TLR4 can induce the production of pro-inflammatory cytokines in macrophages77, and activation of the TLRs that are expressed by adipocytes can result in NFκB-driven pro-inflammatory responses81. In addition, activation of both TLR2 and TLR4 can mediate fatty-acid-induced activation of JNK in the adipose tissue of obese individuals19. In obese humans, expression of both TLR2 and TLR4 in the adipose tissue is increased82,83. Recent studies showed that TLR4-deficient mice and C3H/HeJ mice (which have a loss of function mutation in TLR4) are partially protected from fat-induced inflammation and insulin resistance77,80,84,85. A selective role for TLR4 in sensing and promoting the obesigenic and pro-inflammatory effects of saturated fats on adipose tissue has also been proposed80. These results suggest that the adipose tissue may be a dynamic contributor to inflammation that develops during conditions of obesity through the activation of TLR-mediated inflammatory pathways.
TLRs may also represent a potential bridge between lipid metabolism and innate immune responses. This became evident when defective TLR signalling was linked to protection from atherosclerosis in individuals who concurrently suffer from hypercholesterolaemia86,87. Macrophages infiltrating both mouse and human atherosclerotic lesions express TLR4 (REF. 86), and the activation of TLR signalling pathways was found to be necessary for the recruitment and activation of other immune cells in the vascular lesions87. TLR signalling pathways were linked to plaque destabilization and to the promotion of advanced atherosclerosis in mice87. Although several human polymorphisms in the TLR4 gene have been associated with protection from atherosclerosis, it is not yet clear whether a similar role for TLRs exists in human cardiovascular or other metabolic diseases88.
Lipid chaperones
Fatty acids need to interact with lipid chaperones or fatty-acid binding proteins (FABPs) to traffic inside the cells. The mammalian FABP family consists of nine cytoplasmic proteins, the size of which ranges between 14 and 15 kDa89. Through a narrow ligand- binding groove, FABPs reversibly bind long-chain fatty acids and other bioactive lipids. Importantly, FABPs, especially those that are expressed by adipocytes and macrophages (FABP4 and FABP5, respectively) have key roles in regulating systemic metabolism and are important mediators of metabolic syndromes in mice89. Moreover, a genetic variant of FABP4 in humans, which leads to FABP4 haploinsufficiency, is associated with decreased risk of type 2 diabetes and cardiovascular disease90.
The importance of FABPs in metabolism has been appreciated for a long time, but their mechanisms of action have remained elusive. However, recent studies have provided several insights; for example, FABPs can translocate to the nucleus and interact with nuclear receptors, such as members of the PPAR family, in a ligand-dependent manner91(FIG. 5). Binding to the ligand does not induce an activated form of FABP but might stabilize the pre-existing protein. Furthermore, binding to ligands can influence the subcellular localization of FABPs as well as their function92. Interestingly, FABP-mediated lipid trafficking may have important implications in the regulation of differentiation and/or survival of cancer cells93. The biological consequences of ligand trafficking by FABPs in immune and metabolic cells remain to be determined.
In addition to having a role in metabolism, FABPs are involved in inflammation. More specifically, metabolically induced pro-inflammatory responses are suppressed in the absence of FABP4. Furthermore, LPS-stimulated cytokine and chemokine secretion, as well as iNOS and COX2 production, were inhibited in FABP4-deficient macrophages, in part owing to the reduced responsiveness of the IKK–NFκB pathway94. Pathways with anti-inflammatory action, such as those that are mediated by PPARγ and LXRα, are significantly upregulated in the absence of FABP4, potentially contributing to the immunological and metabolic functions of FABPs in macrophages94 (FIG. 5). These findings indicate that lipid chaperones could be the point where nutritional and inflammatory pathways converge in adipocytes and macrophages, and potentially other metabolic and inflammatory cells. Consistent with this, macrophages become resistant to inflammation that is induced by saturated fatty acids in the absence of lipid chaperones (E.E. and G.S.H., unpublished observations). Further studies are needed to identify the detailed molecular mechanisms underlying the role of FABPs in resistance to lipotoxicity.
STAMPs
Following the discovery of the inflammatory nature of metabolic diseases, a crucial matter in the field was identifying the regulatory pathways that protect the cells against inflammatory damage caused by physiological fluctuations in nutrient exposure. Recent work from our laboratory showed that STAMP2 (six transmembrane protein of prostate 2; also known as STEAP4) protects adipocytes from metabolically induced inflammation30. STAMP2 belongs to the STEAP (six transmembrane epithelial antigen of the prostate) family of transmembrane proteins that have ferrireductase and cupric-reductase activity95. STAMP2 was also identified by a genome-wide search for molecules that respond to nutritional, metabolic and inflammatory signals in obese TNF-deficient mice. Intriguingly, STAMP2 was found to be selectively induced in the visceral adipose tissues of lean mice in response to feeding30. However, this nutritional regulatory pattern was lost in mouse models of obesity. Similarly to mice, the expression of STAMP2 in obese humans was found to be selectively induced in the visceral adipose tissue96. However, it remains to be determined whether the nutritional regulation of STAMP2 is disrupted in obese humans in the same manner as in mouse models of obesity. Addressing this question is crucial but also challenging, as the nutritional regulation of STAMP2 expression occurs in the visceral adipose tissue.
These observations led to the hypothesis that exposure to nutrients might selectively mount a protective response against inflammation through the regulation of STAMP2. Consistent with this, loss of STAMP2 function in both cultured adipocytes and mice resulted in defective nutrient management and an exaggerated inflammatory response to nutrient challenge occurring in adipocytes. STAMP2-deficient mice exhibit the main signs of a metabolic syndrome (decreased systemic insulin sensitivity, increased inflammatory responses and macrophage infiltration of the visceral adipose tissue, dyslipidaemia and fatty liver disease) even under standard dietary conditions and in the absence of obesity30. This suggests that STAMP2 has an important physiological role in linking nutrient signals with inflammation, which might be vital for the maintenance of metabolic homeostasis. Understanding the structure, regulation and trafficking of STAMP molecules in adipocytes and other metabolically important cells and organs will provide important insights into the molecular networks and signals that control this aspect of the integration and coordination of metabolic and immune signals.
Exploitation of metabolism and inflammation
Although the effect of the close proximity of the immune system and metabolic pathways has only recently been appreciated in metabolic diseases, the intimate relationship between metabolism and inflammation has long been exploited by microorganisms. Some of these links may have a role in the development of metabolic diseases and present new platforms for research in the fields of metabolism and infection.
Chronic infectious diseases can disrupt systemic metabolism and insulin action97,98. One of the infection strategies that is used by some microorganisms involves targeting glucose transporters; for example, human T-cell leukaemia virus uses glucose transporter 1 (GLUT1) to enter host cells99. Most pathogens can also trigger baseline glucose transport in host cells through the regulation of GLUT1, presumably to meet the energy demands of their replication and synthetic phases100. Alternatively, many viruses, such as hepatitis C virus, parasites, such as Toxoplasma gondii, and bacteria, such as Brucella spp., interact with the ER, which allows them to access the metabolic pathways that are needed for their proliferation101. In addition, Mycobacterium tuberculosis infection induces the expansion of the ER and Golgi apparatus, and probably triggers adaptive immune responses through these organelles102. During the course of infection, M. tuberculosis hides in an autophagosome that is derived from components of the ER membrane and heavily relies on lipid-signalling metabolic pathways for its survival and replication103 (BOX 1). An intriguing similarity between M. tuberculosis infection and metabolic disease is the promotion of phospholipid accumulation in infected macrophages, which is similar to that seen in pro-atherogenic foam cells104,105. These observations suggest that the identification of metabolic pathways that are targeted by M. tuberculosis may aid our understanding of the pathogenesis of cardiovascular disease and, therefore, may pinpoint potential treatments for it, as these metabolic pathways may also be altered during the formation of pro-atherogenic foam cells.
One can even speculate that metabolic conditions that are advantageous for the protection of the host against pathogens might have been subject to natural selection and might therefore now contribute to the susceptibility of a high percentage of the human population to metabolic disease (BOX 2). For example, malaria infection is strongly influenced by the hyperglycaemic environment. The parasite that causes malaria can modify the metabolism of the host by generating glucose-lowering by-products, such as inositol phosphoglycans, that are released through the hydrolysis of membrane-bound glycosyl phosphatidylinositols106. Intriguingly, the anti-malarial drug chloroquine can target ataxia telangiectasia mutated (ATM) kinase, an insulin-responsive kinase, and can thereby reduce atherosclerosis and improve systemic glucose homeostasis in mouse models of obesity and insulin resistance107. In summary, pathogens have evolved mechanisms to achieve their goal of accessing metabolic pathways for survival while evading host immune responses. However, it is also possible that some of these pathogen-derived factors are involved in initiating metabolic diseases (BOX 2).
Box 2. Pathogen-derived factors may initiate metabolic diseases.
Although low-grade inflammation is a known pathological component of obesity, the triggering factor (or factors) has yet to be identified. Recent evidence suggests that gut microbiota may have a role in the initiation of obesity and insulin resistance109–112. It has been shown that moderate increases in the plasma concentration of lipopolysaccharide (LPS) accompany the metabolic dysregulation that occurs in high-fat-diet-induced obesity109. Furthermore, the increased plasma levels of LPS correlate with the ratio of Gram-negative and Gram-positive bacteria, and deletion of CD14, which is part of the receptor for LPS, leads to resistance to metabolic diseases that are induced by subcutaneous injection of LPS in mice109. Using antibiotics that can alter the composition of gut microbiota, it is possible to reduce inflammation and improve sensitivity to insulin. Gut microbiota have been linked to the regulation of body weight and energy homeostasis; however, the molecular mechanisms involved have yet to be described. For example, mice kept in germ-free conditions were resistant to high-fat-diet-induced obesity and displayed better metabolic parameters110,111. When colonized by gut microbiota from conventional mice, their weight increased substantially and they developed insulin resistance when kept on a high-fat diet110. Collectively, these data indicate that gut microbiota may have an important role in the induction of chronic inflammation that is associated with metabolic diseases, and have stimulated interest in studying the role of probiotics in immune and metabolic disorders113.
Concluding remarks
The discovery of the intricate links between metabolic homeostasis and inflammatory responses has been exciting and puzzling. Studies have shown that metabolic and inflammatory pathways can converge at many levels, including at the level of cell-surface receptors, intracellular chaperones or nuclear receptors. These molecular sites allow for the coordination between the nutrient-sensing pathways and the immune response in order to maintain homeostasis under diverse metabolic and immune conditions. Evidence also shows that proper protection of nutrient-sensing and immune pathways by molecules such as STAMP is required for homeostasis. Interestingly, microorganisms have exploited the links between metabolic and immune pathways, and understanding the mechanisms by which they achieve this could be an area of extensive research in the field of metabolism. In addition, the understanding and treatment of chronic metabolic diseases, as well as insight into the convergence of nutrient- and pathogen-signalling pathways, may help to manage the infectious agents that are most proficient at exploiting the host metabolic system for their benefit.
Acknowledgments
This work is supported in part by grants from National Institutes of Health (DK52539, DK64360, HL65405), and American Diabetes Association (I03RA41) to G.S.H. E.E. is supported by a Ruth Kirschstein National Research Award. We thank the members of the Hotamisligil laboratory for discussions, comments and research contributions that lead to maturation of this review. We are especially grateful to A. Onur for help with the figures, S. Hummasti for discussion and editing, H. Xu for assistance with analysis of microarray data and R. Foote for technical assistance with the manuscript. We regret that we could not cite or review the scientific contributions of many others owing to space limitations.
- Sepsis
A systemic response to severe bacterial infections that are generally caused by Gram-negative bacterial endotoxins. Sepsis induces a hyperactive and out-of-balance network of pro-inflammatory cytokines, affecting vascular permeability, cardiac function and metabolic balance, and ultimately leads to tissue necrosis, multiple-organ failure and death
- Metabolic syndrome
A cluster of conditions, such as hypertension, hyperinsulinaemia, hypercholesteraemia and abdominal obesity, that occur together, increasing the risk of heart disease, stroke and diabetes
- Adipose tissue
Connective tissue with a network of blood vessels in which fat is stored and the cells are distended by droplets of fat
- Foam cells
Macrophages that localize to sites of early-stage inflammation in the vessel wall, which subsequently ingest oxidized low-density lipoprotein and slowly become overloaded with lipids. They are called foam cells because they have numerous cytoplasmic vesicles that contain cholesterol and other lipids. Foam cells eventually die and attract more macrophages, and further propagate inflammation in the vessel wall
- Atherosclerosis
A chronic disorder of the arterial wall that is characterized by damage to the endothelium, which gradually induces deposits of cholesterol, cellular debris, calcium and other substances and ultimately triggers local inflammation. These deposits finally lead to plaque formation and arterial stiffness
- Anabolic hormone
A hormone that is involved in the synthesis of macromolecules from simpler intermediates
- Insulin resistance
The reduced sensitivity of the body’s insulin-dependent processes (such as glucose uptake and lipolysis) to insulin. insulin resistance is typical of type 2 diabetes but often occurs in the absence of diabetes
- Mammalian target of rapamycin
(mTOR). A conserved serine/threonine protein kinase that regulates cell growth and metabolism, as well as cytokine and growth-factor expression, in response to environmental cues. mTOR receives stimulatory signals from RAS and phosphoinositide 3-kinase downstream of growth factors and nutrients, such as amino acids, glucose and oxygen
- Peroxisome-proliferator-activated receptors
Nuclear receptors that participate in the regulation of cellular metabolism and differentiation
- Unfolded-protein response
An adaptive response that increases the ability of the endoplasmic reticulum to fold and translocate proteins, decreases the synthesis of proteins, coordinates stress and antioxidant responses, and can result in the arrest of the cell cycle and apoptosis
- Chaperone protein
A protein that assists the folding of newly synthesized proteins into a particular three-dimensional conformation by binding and stabilizing folding intermediates
- Acute-phase proteins
A group of proteins, including C-reactive protein, serum amyloid A, fibrinogen and α1-acid glycoprotein, that are secreted into the blood in increased or decreased quantities by hepatocytes in response to trauma, inflammation or disease. These proteins can be inhibitors or mediators of inflammatory processes
- Plasma cells
Terminally differentiated quiescent B cells that develop from plasmablasts and are characterized by their capacity to secrete large amounts of antibodies
- Atherosclerotic plaque
A lesion that consists of a fibrotic cap surrounding a lipid-rich core. The lesion is the site of inflammation, lipid accumulation and cell death
- Leptin resistance
Reduced sensitivity to the effects of leptin, which is a hormone that is produced by fat cells and has a key role in regulating energy intake and energy expenditure
- Rapamycin
An immunosuppressive drug that, in contrast to calcineurin inhibitors, does not prevent T-cell activation but blocks interleukin-2-mediated clonal expansion by blocking mTOR
- Myeloid-derived suppressor cells
(MDSCs). A population of cells that consists of mature and immature myeloid cells. MDSCs are generated and/or activated during an inflammatory immune response and negatively affect T cells through direct interactions and secreted components, which leads to the impairment of T-cell function
- Essential amino acid
An amino acid that cannot be synthesized by cells and must be obtained through the diet
Footnotes
Competing interests statement
The author(s) declare(s) competing financial interests: see web version for details.
References
- 1.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 2.Demas GE, Chefer V, Talan MI, Nelson RJ. Metabolic costs of mounting an antigen-stimulated immune response in adult and aged C57BL/56J mice. Am J Physiol. 1997;273:R1631–R1637. doi: 10.1152/ajpregu.1997.273.5.R1631. [DOI] [PubMed] [Google Scholar]
- 3.Marti A, Marcos A, Martinez JA. Obesity and immune function relationships. Obes Rev. 2001;2:131–140. doi: 10.1046/j.1467-789x.2001.00025.x. [DOI] [PubMed] [Google Scholar]
- 4.Romanyukha AA, Rudnev SG, Sidorov IA. Energy cost of infection burden: an approach to understanding the dynamics of host-pathogen interactions. J Theor Biol. 2006;241:1–13. doi: 10.1016/j.jtbi.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Browning RC, Kram R. Energetic cost and preferred speed of walking in obese vs. normal weight women. Obes Res. 2005;13:891–899. doi: 10.1038/oby.2005.103. [DOI] [PubMed] [Google Scholar]
- 6.Maier SF, Watkins LR, Fleshner M. Psychoneuroimmunology. The interface between behavior, brain, and immunity. Am Psychol. 1994;49:1004–1017. doi: 10.1037//0003-066x.49.12.1004. [DOI] [PubMed] [Google Scholar]
- 7.Demas GE, Drazen DL, Nelson RJ. Reductions in total body fat decrease humoral immunity. Proc Biol Sci. 2003;270:905–911. doi: 10.1098/rspb.2003.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moret Y, Schmid-Hempel P. Survival for immunity: the price of immune system activation for bumblebee workers. Science. 2000;290:1166–1168. doi: 10.1126/science.290.5494.1166. This study shows that immune activation is costly and subject to an energy trade-off during conditions of starvation, leading to the reduced survival of insects. [DOI] [PubMed] [Google Scholar]
- 9.Leclerc V, Reichhart JM. The immune response of Drosophila melanogaster. Immunol Rev. 2004;198:59–71. doi: 10.1111/j.0105-2896.2004.0130.x. [DOI] [PubMed] [Google Scholar]
- 10.Pond CM, Mattacks CA. The source of fatty acids incorporated into proliferating lymphoid cells in immune-stimulated lymph nodes. Br J Nutr. 2003;89:375–383. doi: 10.1079/BJN2002784. [DOI] [PubMed] [Google Scholar]
- 11.Knight SC. Specialized perinodal fat fuels and fashions immunity. Immunity. 2008;28:135–138. doi: 10.1016/j.immuni.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Karagiannides I, Pothoulakis C. Obesity, innate immunity and gut inflammation. Curr Opin Gastroenterol. 2007;23:661–666. doi: 10.1097/MOG.0b013e3282c8c8d3. [DOI] [PubMed] [Google Scholar]
- 13.Lago R, Gomez R, Lago F, Gomez-Reino J, Gualillo O. Leptin beyond body weight regulation — current concepts concerning its role in immune function and inflammation. Cell Immunol. 2008;252:139–145. doi: 10.1016/j.cellimm.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Chung S, et al. Preadipocytes mediate lipopolysaccharide-induced inflammation and insulin resistance in primary cultures of newly differentiated human adipocytes. Endocrinology. 2006;147:5340–5351. doi: 10.1210/en.2006-0536. [DOI] [PubMed] [Google Scholar]
- 15.Charriere G, et al. Preadipocyte conversion to macrophage. Evidence of plasticity. J Biol Chem. 2003;278:9850–9855. doi: 10.1074/jbc.M210811200. [DOI] [PubMed] [Google Scholar]
- 16.Khazen W, et al. Expression of macrophage-selective markers in human and rodent adipocytes. FEBS Lett. 2005;579:5631–5634. doi: 10.1016/j.febslet.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 17.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-α function. Nature. 1997;389:610–614. doi: 10.1038/39335. Using genetic models, this study showed for the first time that inflammation and increased cytokine levels in conditions of obesity have a causal role in the development of insulin resistance. [DOI] [PubMed] [Google Scholar]
- 19.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 20.Chandra RK. Immune response in overnutrition. Cancer Res. 1981;41:3795–3796. [PubMed] [Google Scholar]
- 21.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nature Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 22.Zick Y. Ser/Thr phosphorylation of IRS proteins: a molecular basis for insulin resistance. Sci STKE 2005. 2005:pe4. doi: 10.1126/stke.2682005pe4. [DOI] [PubMed] [Google Scholar]
- 23.Gao Z, et al. Serine phosphorylation of insulin receptor substrate 1 by inhibitor κB kinase complex. J Biol Chem. 2002;277:48115–48121. doi: 10.1074/jbc.M209459200. [DOI] [PubMed] [Google Scholar]
- 24.Um SH, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 25.Hotamisligil GS, et al. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNFα- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. This study showed that the pro-inflammatory cytokine TNF is induced in adipose tissue in conditions of obesity and that TNF contributes to metabolic disease. [DOI] [PubMed] [Google Scholar]
- 26.Xu H, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 28.Hirosumi J, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 29.Arkan MC, et al. IKK-β links inflammation to obesity-induced insulin resistance. Nature Med. 2005;11:191–198. doi: 10.1038/nm1185. References 28 and 29 show that well-known inflammatory pathways are linked with the development of insulin resistance in obesity. [DOI] [PubMed] [Google Scholar]
- 30.Wellen KE, et al. Coordinated regulation of nutrient and inflammatory responses by STAMP2 is essential for metabolic homeostasis. Cell. 2007;129:537–548. doi: 10.1016/j.cell.2007.02.049. This study shows that proper regulation of metabolic and immune pathways by proteins such as STAMP2 promotes metabolic health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bensinger SJ, et al. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell. 2008;134:97–111. doi: 10.1016/j.cell.2008.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozcan U, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 33.Kaneto H, et al. Possible novel therapy for diabetes with cell-permeable JNK-inhibitory peptide. Nature Med. 2004;10:1128–1132. doi: 10.1038/nm1111. [DOI] [PubMed] [Google Scholar]
- 34.Nakatani Y, et al. Modulation of the JNK pathway in liver affects insulin resistance status. J Biol Chem. 2004;279:45803–45809. doi: 10.1074/jbc.M406963200. [DOI] [PubMed] [Google Scholar]
- 35.Ozcan U, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Solinas G, et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 2007;6:386–397. doi: 10.1016/j.cmet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 37.Vallerie SN, Furuhashi M, Fucho R, Hotamisligil GS. A predominant role for parenchymal c-Jun amino terminal kinase (JNK) in the regulation of systemic insulin sensitivity. PLoS ONE. 2008;3:e3151. doi: 10.1371/journal.pone.0003151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 39.Todd DJ, Lee AH, Glimcher LH. The endoplasmic reticulum stress response in immunity and autoimmunity. Nature Rev Immunol. 2008;8:663–674. doi: 10.1038/nri2359. [DOI] [PubMed] [Google Scholar]
- 40.Cox JS, Shamu CE, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993;73:1197–1206. doi: 10.1016/0092-8674(93)90648-a. [DOI] [PubMed] [Google Scholar]
- 41.Mori K, Ma W, Gething MJ, Sambrook J. A transmembrane protein with a CDC2+/CDC28-related kinase activity is required for signaling from the ER to the nucleus. Cell. 1993;74:743–756. doi: 10.1016/0092-8674(93)90521-q. [DOI] [PubMed] [Google Scholar]
- 42.Urano F, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. This paper demonstrated that ER stress is coupled to the activation of the kinase JNK. [DOI] [PubMed] [Google Scholar]
- 43.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 44.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nature Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 45.Gregor MF, Hotamisligil GS. Thematic review series: adipocyte biology. Adipocyte stress: the endoplasmic reticulum and metabolic disease. J Lipid Res. 2007;48:1905–1914. doi: 10.1194/jlr.R700007-JLR200. [DOI] [PubMed] [Google Scholar]
- 46.Deng J, et al. Translational repression mediates activation of nuclear factor κB by phosphorylated translation initiation factor 2. Mol Cell Biol. 2004;24:10161–10168. doi: 10.1128/MCB.24.23.10161-10168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factor α links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1α-mediated NF-κB activation and down-regulation of TRAF2 expression. Mol Cell Biol. 2006;26:3071–3084. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang K, et al. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124:587–599. doi: 10.1016/j.cell.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 49.Xue X, et al. Tumor necrosis factor α (TNFα) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR counteracts ROS accumulation by TNFα. J Biol Chem. 2005;280:33917–33925. doi: 10.1074/jbc.M505818200. [DOI] [PubMed] [Google Scholar]
- 50.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 51.Levine T, Loewen C. Inter-organelle membrane contact sites: through a glass, darkly. Curr Opin Cell Biol. 2006;18:371–378. doi: 10.1016/j.ceb.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 52.Pouyssegur J, Shiu RP, Pastan I. Induction of two transformation-sensitive membrane polypeptides in normal fibroblasts by a block in glycoprotein synthesis or glucose deprivation. Cell. 1977;11:941–947. doi: 10.1016/0092-8674(77)90305-1. [DOI] [PubMed] [Google Scholar]
- 53.Scheuner D, et al. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7:1165–1176. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 54.Harding HP, et al. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7:1153–1163. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 55.Lipson KL, et al. Regulation of insulin biosynthesis in pancreatic β cells by an endoplasmic reticulum-resident protein kinase IRE1. Cell Metab. 2006;4:245–254. doi: 10.1016/j.cmet.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 56.Ota T, Gayet C, Ginsberg HN. Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. J Clin Invest. 2008;118:316–332. doi: 10.1172/JCI32752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Borradaile NM, et al. A critical role for eukaryotic elongation factor 1A-1 in lipotoxic cell death. Mol Biol Cell. 2006;17:770–778. doi: 10.1091/mbc.E05-08-0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ozcan U, et al. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol Cell. 2008;29:541–551. doi: 10.1016/j.molcel.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feng B, et al. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nature Cell Biol. 2003;5:781–792. doi: 10.1038/ncb1035. In this study, the authors show that ER stress has an important role in macrophage cell death that is caused by free-cholesterol loading. [DOI] [PubMed] [Google Scholar]
- 60.Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol. 2005;25:2255–2264. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- 61.Han S, et al. Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell Metab. 2006;3:257–266. doi: 10.1016/j.cmet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 62.Hosoi T, et al. Endoplasmic reticulum stress induces leptin resistance. Mol Pharmacol. 2008 August 28; doi: 10.1124/mol.108.050070. [DOI] [PubMed] [Google Scholar]
- 63.Myoishi M, et al. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation. 2007;116:1226–1233. doi: 10.1161/CIRCULATIONAHA.106.682054. [DOI] [PubMed] [Google Scholar]
- 64.Ozawa K, et al. The endoplasmic reticulum chaperone improves insulin resistance in type 2 diabetes. Diabetes. 2005;54:657–663. doi: 10.2337/diabetes.54.3.657. [DOI] [PubMed] [Google Scholar]
- 65.Reiling JH, Sabatini DM. Stress and mTORture signaling. Oncogene. 2006;25:6373–6383. doi: 10.1038/sj.onc.1209889. [DOI] [PubMed] [Google Scholar]
- 66.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harrington LS, et al. The TSC1–2 tumor suppressor controls insulin–PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166:213–223. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pende M, et al. Hypoinsulinaemia, glucose intolerance and diminished β-cell size in S6K1-deficient mice. Nature. 2000;408:994–997. doi: 10.1038/35050135. [DOI] [PubMed] [Google Scholar]
- 69.Colombani J, et al. A nutrient sensor mechanism controls Drosophila growth. Cell. 2003;114:739–749. doi: 10.1016/s0092-8674(03)00713-x. [DOI] [PubMed] [Google Scholar]
- 70.Cota D, et al. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 71.Lee DF, et al. IKKβ suppression of TSC1 links inflammation and tumorangiogenesis via the mTOR pathway. Cell. 2007;130:440–455. doi: 10.1016/j.cell.2007.05.058. This is an interesting paper showing that a well- known inflammatory pathway can directly intercept an important nutrient pathway. This study documents a molecular link between amino-acid monitoring and regulation of lipid metabolism in the liver. [DOI] [PubMed] [Google Scholar]
- 72.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nature Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 73.Rodriguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol Rev. 2008;222:180–191. doi: 10.1111/j.1600-065X.2008.00608.x. References 72 and 73 both provide an insightful review of how the metabolism of a single amino acid (L-arginine) can directly affect immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guo F, Cavener DR. The GCN2 eIF2α kinase regulates fatty-acid homeostasis in the liver during deprivation of an essential amino acid. Cell Metab. 2007;5:103–114. doi: 10.1016/j.cmet.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 75.Gingras AC, Svitkin Y, Belsham GJ, Pause A, Sonenberg N. Activation of the translational suppressor 4E-BP1 following infection with encephalomyocarditis virus and poliovirus. Proc Natl Acad Sci USA. 1996;93:5578–5583. doi: 10.1073/pnas.93.11.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Connor JH, Lyles DS. Vesicular stomatitis virus infection alters the eIF4F translation initiation complex and causes dephosphorylation of the eIF4E binding protein 4E-BP1. J Virol. 2002;76:10177–10187. doi: 10.1128/JVI.76.20.10177-10187.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shi H, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Medzhitov R. Toll-like receptors and innate immunity. Nature Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 79.Tsukumo DM, et al. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56:1986–1998. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- 80.Davis JE, Gabler NK, Walker-Daniels J, Spurlock ME. Tlr-4 deficiency selectively protects against obesity induced by diets high in saturated fat. Obesity (Silver Spring) 2008;16:1248–1255. doi: 10.1038/oby.2008.210. [DOI] [PubMed] [Google Scholar]
- 81.Ajuwon KM, Spurlock ME. Palmitate activates the NF-κB transcription factor and induces IL-6 and TNFα expression in 3T3-L1 adipocytes. J Nutr. 2005;135:1841–1846. doi: 10.1093/jn/135.8.1841. [DOI] [PubMed] [Google Scholar]
- 82.Ghanim H, et al. Acute modulation of Toll-like receptors by insulin. Diabetes Care. 2008;31:1827–1831. doi: 10.2337/dc08-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vitseva OI, et al. Inducible Toll-like receptor and NF-κB regulatory pathway expression in human adipose tissue. Obesity (Silver Spring) 2008;16:932–937. doi: 10.1038/oby.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Song MJ, Kim KH, Yoon JM, Kim JB. Activation of Toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochem Biophys Res Commun. 2006;346:739–745. doi: 10.1016/j.bbrc.2006.05.170. [DOI] [PubMed] [Google Scholar]
- 85.Suganami T, et al. Attenuation of obesity-induced adipose tissue inflammation in C3H/HeJ mice carrying a Toll-like receptor 4 mutation. Biochem Biophys Res Commun. 2007;354:45–49. doi: 10.1016/j.bbrc.2006.12.190. [DOI] [PubMed] [Google Scholar]
- 86.Xu XH, et al. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation. 2001;104:3103–3108. doi: 10.1161/hc5001.100631. [DOI] [PubMed] [Google Scholar]
- 87.Michelsen KS, et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci USA. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kiechl S, et al. Toll-like receptor 4 polymorphisms and atherogenesis. N Engl J Med. 2002;347:185–192. doi: 10.1056/NEJMoa012673. [DOI] [PubMed] [Google Scholar]
- 89.Erbay E, Cao H, Hotamisligil GS. Adipocyte/macrophage fatty acid binding proteins in metabolic syndrome. Curr Atheroscler Rep. 2007;9:222–229. doi: 10.1007/s11883-007-0023-6. [DOI] [PubMed] [Google Scholar]
- 90.Tuncman G, et al. A genetic variant at the fatty acid-binding protein aP2 locus reduces the risk for hypertriglyceridemia, type 2 diabetes, and cardiovascular disease. Proc Natl Acad Sci USA. 2006;103:6970–6975. doi: 10.1073/pnas.0602178103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tan NS, et al. Selective cooperation between fatty acid binding proteins and peroxisome proliferator-activated receptors in regulating transcription. Mol Cell Biol. 2002;22:5114–5127. doi: 10.1128/MCB.22.14.5114-5127.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ayers SD, Nedrow KL, Gillilan RE, Noy N. Continuous nucleocytoplasmic shuttling underlies transcriptional activation of PPARγ by FABP4. Biochemistry. 2007;46:6744–6752. doi: 10.1021/bi700047a. [DOI] [PubMed] [Google Scholar]
- 93.Schug TT, Berry DC, Shaw NS, Travis SN, Noy N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell. 2007;129:723–733. doi: 10.1016/j.cell.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Makowski L, Brittingham KC, Reynolds JM, Suttles J, Hotamisligil GS. The fatty acid-binding protein, aP2, coordinates macrophage cholesterol trafficking and inflammatory activity. Macrophage expression of aP2 impacts peroxisome proliferator-activated receptor γ and IκB kinase activities. J Biol Chem. 2005;280:12888–12895. doi: 10.1074/jbc.M413788200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Knutson MD. Steap proteins: implications for iron and copper metabolism. Nutr Rev. 2007;65:335–340. doi: 10.1111/j.1753-4887.2007.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 96.Arner P, et al. Expression of six transmembrane protein of prostate 2 in human adipose tissue associates with adiposity and insulin resistance. J Clin Endocrinol Metab. 2008;93:2249–2254. doi: 10.1210/jc.2008-0206. [DOI] [PubMed] [Google Scholar]
- 97.Bloom JD. Glucose intolerance in pulmonary tuberculosis. Am Rev Respir Dis. 1969;100:38–41. doi: 10.1164/arrd.1969.100.1.38. [DOI] [PubMed] [Google Scholar]
- 98.Neuschwander-Tetri BA. Hepatitis C virus-induced insulin resistance: not all genotypes are the same. Gastroenterology. 2008;134:619–622. doi: 10.1053/j.gastro.2007.12.041. [DOI] [PubMed] [Google Scholar]
- 99.Manel N, et al. The ubiquitous glucose transporter GLUT-1 is a receptor for HTLV. Cell. 2003;115:449–459. doi: 10.1016/s0092-8674(03)00881-x. [DOI] [PubMed] [Google Scholar]
- 100.Calder PC, Dimitriadis G, Newsholme P. Glucose metabolism in lymphoid and inflammatory cells and tissues. Curr Opin Clin Nutr Metab Care. 2007;10:531–540. doi: 10.1097/MCO.0b013e3281e72ad4. [DOI] [PubMed] [Google Scholar]
- 101.Roy CR, Salcedo SP, Gorvel JP. Pathogen–endoplasmic-reticulum interactions: in through the out door. Nature Rev Immunol. 2006;6:136–147. doi: 10.1038/nri1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Adams DO. The structure of mononuclear phagocytes differentiating in vivo. I. Sequential fine and histologic studies of the effect of BacillusCalmette-Guerin (BCG) Am J Pathol. 1974;76:17–48. [PMC free article] [PubMed] [Google Scholar]
- 103.Russell DG. Phagosomes, fatty acids and tuberculosis. Nature Cell Biol. 2003;5:776–778. doi: 10.1038/ncb0903-776. [DOI] [PubMed] [Google Scholar]
- 104.Beatty WL, et al. Trafficking and release of mycobacterial lipids from infected macrophages. Traffic. 2000;1:235–247. doi: 10.1034/j.1600-0854.2000.010306.x. [DOI] [PubMed] [Google Scholar]
- 105.Shiratori Y, Okwu AK, Tabas I. Free cholesterol loading of macrophages stimulates phosphatidylcholine biosynthesis and up-regulation of CTP: phosphocholine cytidylyltransferase. J Biol Chem. 1994;269:11337–11348. [PubMed] [Google Scholar]
- 106.Elased KM, et al. Reversal of type 2 diabetes in mice by products of malaria parasites. II Role of inositol phosphoglycans (IPGs) Mol Genet Metab. 2001;73:248–258. doi: 10.1006/mgme.2001.3186. [DOI] [PubMed] [Google Scholar]
- 107.Schneider JG, et al. ATM-dependent suppression of stress signaling reduces vascular disease in metabolic syndrome. Cell Metab. 2006;4:377–389. doi: 10.1016/j.cmet.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 108.Clemens MJ. Translational control in virus-infected cells: models for cellular stress responses. Semin Cell Dev Biol. 2005;16:13–20. doi: 10.1016/j.semcdb.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 109.Cani PD, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 110.Backhed F, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. This study examines the potential effect of microbial flora on energy stores. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ley RE, et al. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Turnbaugh PJ, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 113.Wolowczuk I, et al. Feeding our immune system: impact on metabolism. Clin Dev Immunol. 2008;2008:639–803. doi: 10.1155/2008/639803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cho HJ, et al. Induction of dendritic cell-like phenotype in macrophages during foam cell formation. Physiol Genomics. 2007;29:149–160. doi: 10.1152/physiolgenomics.00051.2006. [DOI] [PubMed] [Google Scholar]
- 115.Ross SE, et al. Microarray analyses during adipogenesis: understanding the effects of Wnt signaling on adipogenesis and the roles of liver X receptor α in adipocyte metabolism. Mol Cell Biol. 2002;22:5989–5999. doi: 10.1128/MCB.22.16.5989-5999.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang X, et al. Hypothalamic IKKβ/NFκB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yang L, Hotamisligil GS. Stressing the brain, fattening the body. Cell. 2008;135:20–22. doi: 10.1016/j.cell.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 118.Sinclair LV, et al. Phospatidylinositol-3-OH kinase and nutrients sensing mTOR pathways control T lymphocyte trafficking. Nature Immunol. 2008;5:513–521. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]