Abstract
Background
The purpose of this study was to reevaluate the ECG features of Arrhythmogenic right ventricular dysplasia (ARVD). The second objective was to evaluate the sensitivity and specificity of the standard and newly proposed diagnostic ECG markers in the presence of a right bundle branch block (RBBB).
Methods and Results
One hundred patients with ARVD (57 males, age 39 ± 15 years) and 57 controls (21 males, age 40 ± 17 years) were included. Among the 100 patients with ARVD, a complete RBBB (CRBBB) was present in 17 patients and 15 patients had an incomplete RBBB (IRBBB). T wave inversion (TWI) through V3 demonstrated optimal sensitivity and specificity in both ARVD patients without a CRBBB or IRBBB (71% (95% CI, 58%–81%) and 96% (95% CI, 81%–100%) respectively) and in ARVD patients with IRBBB (73% (95% CI, 45%–92%) and 95% (95% CI, 77%–100%) respectively). Between ARVD and controls with a CRBBB the only two parameters which differed was the prevalence of TWI through V4 (59% versus 12% respectively, p < 0.05) and an r’ / s ratio in V1 < 1 (88% versus 14% respectively, p < 0.005). In ARVD with CRBBB the most sensitive and specific parameter was an r’/s ratio < 1.
Conclusion
We evaluated comprehensively the diagnostic value of ECG markers for ARVD. Based on the findings we propose an algorithm, with examination of QRS morphology being the first step, for ECG evaluation of ARVD patients. Definite criteria are then applied to based on the presence of no RBBB, IRBBB and CRBBB to obtain the best diagnostic utility of the ECG.
Keywords: Arrhythmogenic right ventricular dysplasia, Right bundle branch block, Prolonged terminal activation duration, Parietal Block
Clinical Summary.
Diagnosis of ARVD relies on results of diagnostic tests which screen for structural abnormalities of the right ventricle, ventricular arrhythmias, a family history of sudden death or ARVD, as well as ECG abnormalities which have been linked to ARVD. Among the many diagnostic tests which are used to screen patients for ARVD the ECG is of particular importance because of its widespread availability and low cost. In this study, we performed a systematic analysis of the 12-lead ECG in ARVD patients as compared with controls. This is the first study to evaluate the ECG features of ARVD patients based on the presence or absence of a CRBBB or IRBBB. This is of particular importance as IRBBB or CRBBB patterns are commonly observed in patients with ARVD. The results of this study reveal that in the absence of a CRBBB or IRBBB, TWI through V3 is the single ECG parameter which demonstrates the optimal sensitivity and specificity. For patients with an IRBBB pattern, TWI through V3 is also the single ECG parameter which demonstrates the optimal sensitivity and specificity. And third, for patients with a CRBBB pattern, an r’/s ratio of < 1 in V1 was the single ECG parameter which demonstrates the optimal sensitivity and specificity. It is our hope that the findings of our study will be confirmed in other populations of ARVD patients and that at some point in the future the ECG can be relied on with greater confidence as a screening tool for ARVD.
Introduction
Arrhythmogenic right ventricular dysplasia (ARVD) is an inherited cardiomyopathy characterized clinically by right ventricular dysfunction and ventricular arrhythmias of right ventricular (RV) origin 1, 2. The pathologic hallmark of ARVD is fibrofatty replacement of the RV myocardium1, 3, 4. The most common genetic abnormality identified in patients with ARVD is a mutation in one or more desmosomal proteins 5–9.
Marcus and Fontaine initially described the electrocardiographic (ECG) features of ARVD more than 25 years ago1. Subsequently, the Task Force of the Working Group on Myocardial and Pericardial Diseases of the European Society of Cardiology and the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology included several ECG features of the disease in the diagnostic criteria for ARVD10. The ECG paramaters included in these diagnostic criteria include T wave inversion (TWI) in right precordial leads (V2 and V3), a prolonged QRS > 110 msec in the right precordial leads, and the presence of an epsilon wave. Since publication of these criteria, several studies have proposed new ECG markers of ARVD that focus on the presence of evidence for delayed RV activation in the right precordial leads. These include the presence of parietal block11, a delayed S wave upstroke ≥ 55 msec in leads V1 – V312, an increased ratio of the QRS duration in the right versus the left precordial leads13, 14, and prolonged terminal activation duration (TAD) in leads V1–V3 ≥ 55 ms15. One unusual feature of the Task Force criteria for ARVD is that it is unclear as to whether they can be applied to patients with a complete RBBB (CRBBB). The Task Force criteria specifically state that the TWI criteria cannot be applied to ARVD patients with a right bundle branch block (RBBB). But the criteria do not make it clear whether the depolarization criteria of a QRS duration in V1 > 110 msec and/or the presence of an epsilon wave can be used for diagnosis of ARVD in a patient with a RBBB pattern.
The purpose of this study was is to reevaluate the ECG features of ARVD. Particular attention is focused on determining the sensitivity and specificity of the newly proposed diagnostic markers of ARVD that reflect delayed activation of the RV. And a second objective of this study is to describe the ECG features of a series of patients with ARVD who have a RBBB pattern and to evaluate the sensitivity and specificity of the standard and newly proposed ECG markers in the diagnosis of ARVD in the presence of a RBBB pattern.
Methods
Study Population
The study population included 100 patients with ARVD (57 males, age 39 ± 15 years) and 57 controls (21 males, age 40 ± 17). The study populations were participants in the Johns Hopkins ARVD registry. Per routine protocol, all individuals underwent a series of clinical tests to ascertain the fulfillment of the Task Force criteria. ARVD was diagnosed based on the Task Force criteria10. All participants provided written informed consent to participate, and the study protocol was approved by the Johns Hopkins Medicine Institutional Review Board. Each of the 100 patients with ARVD met the Task Force criteria for ARVD. The prevalence of minor and major criteria for ARVD in the 100 patients with ARVD enrolled in this study is summarized in Table 1. None of the patients or controls were receiving antiarrhythmic drugs known to affect the QRS complex at the time of acquisition of the ECG tracings.
TABLE 1.
Clinical Characteristics and Task Force Criteria* of Patients With ARVD
| Characteristics | ARVD without CRBBB/IRB BB (n=68 ) |
ARVD with IRBBB (n= 15) |
ARVD with CRBBB (n= 17) |
ARVD (overall) (n=100) |
|---|---|---|---|---|
| Clinical Characteristics | ||||
| Age | 39 ± 16 | 38 ± 11 | 43 ± 14 | 39 ± 15 |
| Gender (Males)† | 33 (49) | 9 (60) | 15 (88) | 57 (57) |
| Task Force Criteria | ||||
| Family History | ||||
| Family History of ARVD confirmed by Biopsy or Autopsy | 15 (22) | 1 (7) | 1 (6) | 17 (17) |
| Family History of premature sudden death (<35 yrs) due to suspected ARVD | 14 (21) | 1 (7) | 0 (0) | 15 (15) |
| Family History (clinical diagnosis based on present criteria) | 18 (26) | 1 (7) | 2 (18) | 21 (21) |
| ECG depolarization/conduction abnormalities | ||||
| Epsilon waves | 5 (7) | 0 (0) | 0 (0) | 5 (5) |
| Localized QRS duration > 110ms in V1, V2 or V3 † | 48 (71) | 11 (73) | 12 (71) | 48 (48) |
| Late potentials on SAECG (n1= 57, n2= 10, n3= 11) | 42 (74) | 9 (90) | 10 (91) | 61 (78) |
| Repolarization abnormalities | ||||
| Inverted T waves in right precordial leads (V2–V3) above age 12 yrs | 60 (88) | 13 (87) | 15 (88) | 88 (88) |
| Tissue characterization of walls | ||||
| Fibrofatty replacement of myocardium in endomyocardial biopsy. (n1= 27, n2= 2, n3= 4) | 9 (33) | 2 (100) | 2 (50) | 13 (39) |
| Structural or Functional abnormalities | ||||
| Severe dilatation and reduction of RV ejection fraction with mild or no LV involvement † | 10 (15) | 6 (40) | 6 (35) | 22 (22) |
| Localized RV aneurysm | 14 (21) | 2 (13) | 5 (29) | 21 (22) |
| Mild global RV dilatation and/or ejection fraction reduction (localized RV disease) | 40 (59) | 7 (47) | 9 (53) | 56 (56) |
| Arrhythmias | ||||
| Left bundle branch block VT on ECG, Holter, or exercise tolerance test | 46 (68) | 14 (93) | 14 (82) | 74 (74) |
| Sustained VT (n1= 64, n2= 15, n3= 16) | 36 (56) | 11 (73) | 11 (69) | 58 (61) |
| Non sustained VT (n1= 58, n2= 13, n3= 16) | 41 (71) | 12 (92 ) | 13 (81 ) | 66 (76) |
| Frequent premature ventricular contractions (>1000/24 hrs Holter) (n1= 44, n2=10, n3= 9) | 30 (68) | 7 (70) | 5 (56) | 42 (67) |
LV indicates left ventricular. All values are in %
To be diagnosed as ARVD an individual must have 2 major, or 1 major plus 2 minor or 4 minor criteria n1= sample size of ARVD with no IRBBB/CRBBB, n2= sample size of ARVD with IRBBB, n3= sample size of ARVD with CRBBB. (when sample size different from 68, 15 and 17 respectively)
P < 0.05 (difference between groups)
The ECGs from two control populations were also evaluated. The first control population was a series of 27 patients (10 males, age 33 ± 17 years) who were evaluated in the ARVD clinic because of a first or second degree relative with ARVD. Each of these patients underwent comprehensive testing for ARVD including an ECG, SAECG, Holter, MRI, and stress testing. All the diagnostic tests were normal in these patients. The second control group was a series of 30 patients (11 males, age 46 ± 14 years) with a RBBB ECG pattern. Each of the control patients with a RBBB pattern underwent a detailed history and physical examination as well as an echocardiogram. No evidence of cardiovascular disease was identified, other than the RBBB pattern on ECG. Each of the ARVD patients and controls was Caucasian.
ECG Parameters and Definitions
Shown in Table 2 are the definitions and criteria used in this study. These ECG parameters include those that are part of the Task Force Criteria10, those newly described measures of conduction delay12, 15, and also several additional parameters which have been reported in the literature11, 13, 14. The criteria for diagnosis of a CRBBB or Incomplete RBBB (IRBBB) are the 1985 World Health Organization Criteria 16. It is important to note that an IRBBB was defined in this study as QRS width < 120 msec with an R wave peak time in V1 or V2 > 50 msec. This definition was recommended by the World Health Organization/International Society and Federation for Cardiology Task Force Ad Hoc criteria 16 According to these criteria there is no minimal QRS duration for IRBBB. It is also important to note that this pattern (i.e. IRBBB) has been attributed to causes other than conduction delay in the right bundle branch.
TABLE 2.
Definitions of ECG Parameters and Measurements Employed in this Study
| VARIABLES | DEFINITIONS |
|---|---|
| Right precordial T wave inversion through V2 | Inverted T wave in Leads V1 and V2 |
| Right precordial T wave inversion through V3 10 | Inverted T wave in Leads V1,V2 and V3 |
| Right precordial T wave inversion through V4 | Inverted T wave in Leads V1, V2, V3 and V4 |
| T wave inversion in Inferior Leads | Inverted T waves in 2 out of 3 inferior leads |
| Epsilon Wave12 | Distinct waves of small amplitude that occupy the ST segment in the right precordial leads and are distinct from the QRS complex |
| QRS prolongation in right precordial leads 10 | QRSd > 110 msec in lead V1,V2, or V3 |
| Parietal Block 11 | QRSd in lead V1 to V3 that exceeds the QRSd in Lead V6 by > 25 ms |
| Prolonged Terminal activation duration 15 | Longest value in V1–V3 from the nadir of the S wave to the end of all depolarization |
| Localized right precordial QRS prolongation (Increased ratio in the QRSd of the right versus left precordial leads) 13,14 | QRS duration:(V1+V2+V3)/(V4+V5+V6)≥ 1.2 with QRSd ≥ 100 msec in 2 of 3 right precordial leads |
| Complete RBBB 16 | QRSd ≥ 120 ms and : |
| A1: R’ or r’ in V1 or V2 | |
| A2: S duration > R duration in I and V6 | |
| A3: S duration > 40 msec in I and V6 | |
| A4: R peak time > 50 msec in V1 or V2 | |
| a: A1+A2 | |
| b: A1+A3 | |
| c: A4+ (A2orA3) | |
| Incomplete RBBB 16 | QRS <120 msec and R peak time in V1 or V2 > 50 msec |
| R or r prime in V1/V2 | A positive deflection in V1/V2 following an S wave |
| r'/s ratio in V1 | Ratio of amplitude of r' and S wave in lead V1 |
ECG analysis
The 12 lead ECG’s were obtained in the traditional lead positions and recorded at 25mm/sec. All recorded ECGs were scanned using a high resolution digital scanner and imported into the Sigma Scan Pro 5.0 software for further evaluation. To increase the accuracy of measurements, all the ECGs were enlarged × 2 to obtain a format comparable to 50 mm/sec. Digital calipers capable of measuring to within 1ms (horizontal axis) and 0.01 mV (vertical axis) were used to determine the intervals using the software. The intervals were measured in 2 consecutive beats in each lead; the mean value of the two beats was used. When the difference between the two beats was more than 10ms than the mean of three beats was taken. Each ECG was analyzed by two independent readers, each of whom was blinded to the clinical diagnosis of the patient. Both readers summarized their findings for each ECG on a pre-designed paper questionnaire. Differences in the interpretation of the ECG parameters were adjudicated by consensus, and the final diagnosis for each parameter was entered in an electronic database for analysis.
In addition to the analysis of conventional and recently reported ECG markers, we also included a novel ECG marker for use in patients with either a CRBBB or IRBBB. The new parameter which we evaluated is the ratio of the amplitude of r’ to s in lead V1. We developed this parameter in an effort to describe the unique RBBB patterns of patients with and without ARVD. We also evaluated for the first time a novel repolarization parameter which we term inferior TWI. This is defined for purposes of this study as the presence of TWI in ≥ 2 inferior leads.
Data Analysis
Continuous variables were expressed as mean ± SD, and compared with the use of Student’s t test or ANOVA, and categorical variables were expressed as frequency (%) and compared using a Χ2 or Fisher’s exact test. Dichotomous variables were created for each ECG marker either based on clinically applicable cutoffs, or cutoffs that yielded optimal sensitivity and specificity in a receiver operator characteristic (ROC) analysis. For each dichotomous variable, sensitivity and specificity were calculated to estimate the diagnostic utility of the variable. As done traditionally, we classified all ECG parameters into repolarization and depolarization abnormalities. The variables were then grouped together based on traditional Task Force recommendation or based on their individual diagnostic utility. ECG parameters selected for this grouping were based on the original Task Force recommendation as well as the newer parameters that were found to have, in our analysis, sensitivity or specificity >80% for ARVD diagnosis. The diagnostic utility of repolarization and depolarization abnormalities was tested alone and in combination with each other. All analyses were stratified by the QRS morphology, which was classified as no RBBB, IRBBB, and CRBBB. Analyses were performed using STATA statistical software (College Station, TX) and a p value <0.05 was considered statistically significant.
Results
ECG Patterns and Clinical Characteristics in Patients with ARVD and Controls
Among the 100 patients with ARVD, a CRBBB was present in 17 patients and an IRBBB was present in 15 patients. The clinical characteristics of the overall patient population and these three subgroups are shown in Table 1. Patients with a CRBBB pattern were older and more likely to have severe RV dilation and/or reduction of the RV ejection fraction.
ECG Features of ARVD as Compared with Controls
Table 3 presents the prevalence of ECG characteristics in ARVD patients as compared with controls. In order to better define the diagnostic utility of the ECG, we compared each ECG parameter based on whether a CRBBB, IRBBB, or no RBBB pattern was observed.
TABLE 3.
Relative Prevalence of ECG Features of ARVD and Normal Controls with and without RBBB
| VARIABLES | ARVD without IRBBB/ CRBBB (n=68 ) |
ARVD with IRBBB (n= 15 ) |
ARVD with CRBBB (n=17 ) |
Controls without IRBBB/ CRBBB' (n= 27) |
Controls with IRBBB (n= 22) |
Controls with CRBBB (n= 8) |
P* | P† | P‡ |
|---|---|---|---|---|---|---|---|---|---|
| Right precordial T wave inversion through V2 | 57 (84) | 12 (80) | 13 (76) | 3 (11) | 6 (27) | 3 (38) | 0.001 | 0.002 | 0.058 |
| Right precordial T wave inversion through V3 | 48 (71) | 11 (73) | 12 (71) | 1 (4) | 1 (5) | 3 (38) | 0.001 | 0.001 | 0.115 |
| Right precordial T wave inversion through V4 | 35 (51) | 9 (60) | 10 (59) | 1 (4) | 0 (0) | 1 (12) | 0.001 | 0.001 | 0.03 |
| T wave inversion in Inferior leads (2 out of 3) | 28 (41) | 7 (47) | 9 (53) | 2 (7) | 0 (0) | 1 (13) | 0.001 | 0.001 | 0.088 |
| Epsilon Wave | 5 (7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.317 | - | - |
| QRS prolongation in right precordial leads QRS> 110ms | 29 (43) | 12 (80) | 17 (100) | 1 (4) | 2 (9) | 8 (100) | 0.001 | 0.001 | - |
| Parietal Block | 9 (13) | 6 (40) | 8 (47) | 1 (4) | 0 (0) | 4 (50) | 0.172 | 0.03 | 0.891 |
| Prolonged terminal activation duration | 31 (46) | 12 (80) | 17 (100) | 3 (11) | 8 (36) | 7 (88) | 0.002 | 0.009 | 0.137 |
| Increased ratio in QRSd of the right versus left precordial leads | 15 (22) | 6 (40) | 6 (35) | 6 (21) | 2 (9) | 5 (63) | 0.986 | 0.025 | 0.201 |
| r'/s ratio < 1 | - | 13 (93) | 15 (88) | - | 21 (95) | 1 (14) | - | 0.74 | 0.001 |
Values are n (%)
ARVD without IRBBB/CRBBB' vs control without IRBBB/CRBBB pattern
ARVD with IRBBB vs control with IRBBB
ARVD with CRBBB vs control with CRBBB
Characteristics of ARVD in the Absence of an IRBBB or CRBBB Pattern
Shown in Figure 1 is a representative 12 lead ECG obtained from an ARVD patient without a CRBBB or IRBBB pattern. TWI is observed in leads V1 through V5 and an epsilon wave can also be seen in lead V1. Table 3 presents the prevalence of ECG characteristics in ARVD patients in the absence of a CRBBB or IRBBB pattern in comparison to a control population of patients. Six of the nine ECG parameters which we examined were more commonly observed in ARVD patients as compared with controls. The variables which did not differ in frequency between the two groups were: 1) parietal block, 2) an increased ratio in QRSd of the right versus left precordial leads, and 3) epsilon waves. Epsilon waves were observed in only five ARVD patients as compared with no controls, a difference which was not statistically significant likely due to small sample size. Table 4 summarizes the sensitivity and specificity of each of these parameters in distinguishing ARVD patients from controls. Among the ECG parameters which differed in the two groups, the most sensitive parameter was right precordial TWI through V2 (84%) (95% CI, 73%–92%). The most specific parameter was the presence of an epsilon wave (100%) (95% CI, 87%–100%). The parameter which demonstrated optimal sensitivity and specificity was TWI through V3 (71% (95% CI, 58%–81%) and 96% (95% CI, 81%–100%) respectively).
Figure 1.
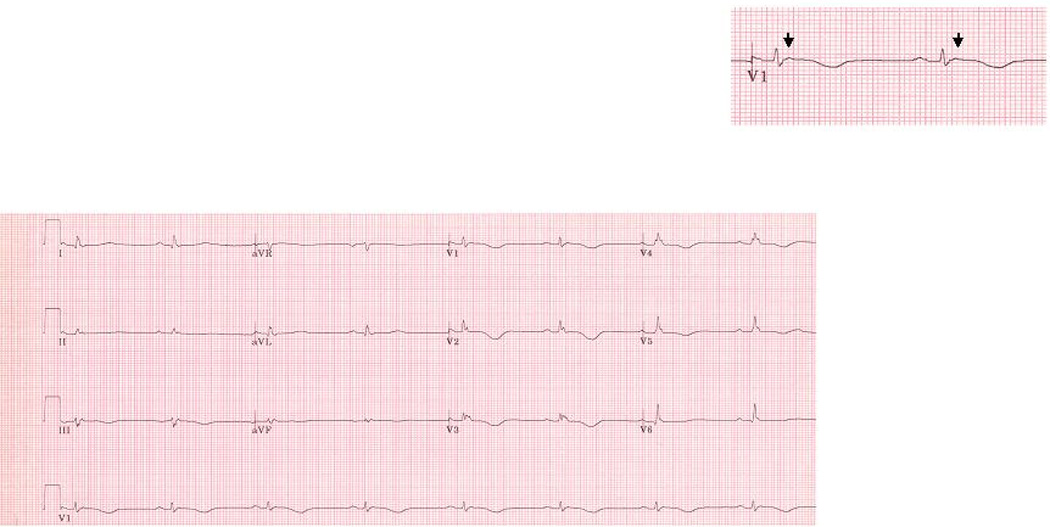
Representative ECG obtained from an ARVD patient without IRBBB or CRBBB. This patient had an advanced form of ARVD. The arrow indicates an Epsilon wave. ECG also illustrates TWI in V1–V5, TAD ≥ 55ms in V1 and QRSd in V3 > 110 ms. This ECG also demonstrates low voltage, which in our experience is an uncommon finding in patients with ARVD and when seen is usually with advanced disease. There was no parietal block or localized right precordial QRS prolongation.
TABLE 4.
Sensitivity and Specificity of ECG Features
| VARIABLES | No RBBB | IRBB | CRBBB | |||
|---|---|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | Sensitivity | Specificity | |
| Repolarization Criteria | ||||||
| Right precordial T wave inversion through V2 | 84 (73–92) | 89 (71–98) | 80 (52–96) | 73 (50–89) | 76 (50–93) | 62 (24–91) |
| Right precordial T wave inversion through V3 | 71 (58–81) | 96 (81–100) | 73 (45–92) | 95 (77–100) | 71 (44–90) | 62 (24–91) |
| Right precordial T wave inversion through V4 | 51 (39–64) | 96 (81–100) | 60 (32–84) | 100 (85–100) | 59 (33–82) | 88 (47–100) |
| T wave inversion in Inferior leads (2 out of 3) | 41 (29–54) | 93 (76–99) | 47 (21–73) | 100 (85–100) | 53 (28–77) | 87 (47–100) |
| Depolarization Criteria | ||||||
| Epsilon Wave | 7 (2–16) | 100 (87–100) | 0 (0–15) | 100 (78–100) | 0 (0–37) | 100 (80–100 |
| QRS prolongation in right precordial leads QRS > 110ms | 43 (31–55) | 96 (81–100) | 80 (52–96) | 91 (71–99) | 100 (80–100) | 0 (0–37) |
| Parietal Block | 13 (6–24) | 96 (81–100) | 40 (16–68) | 91 (70–99) | 47 (23–72) | 50 (16–84) |
| Prolonged terminal activation duration | 46 (33–58) | 89 (71–98) | 80 (52–96) | 64 (41–83) | 100 (80–100) | 12 (0.32–53) |
| Increased ratio in QRSd of the right versus left precordial leads | 22 (13–34) | 79 (58–91) | 40 (16–68) | 91 (71–99) | 35 (14–62) | 37 (9–76) |
| r'/s ratio < 1 | - | - | 93 (66–100) | 5 (0.12–23) | 88 (64–99) | 86 (42–100) |
ECG Characteristics of ARVD in the presence of IRBBB
Figure 2A shows a representative ECG obtained from an ARVD patient with an IRBBB. Figure 2B shows a representative ECG from a control subject with an IRBBB. Several differences in these two ECGs can be appreciated. The major difference is that the ECG obtained from the ARVD patient reveals TWI in leads V1 through V5 whereas the ECG from the normal control reveals TWI only through V2.
Figure 2.
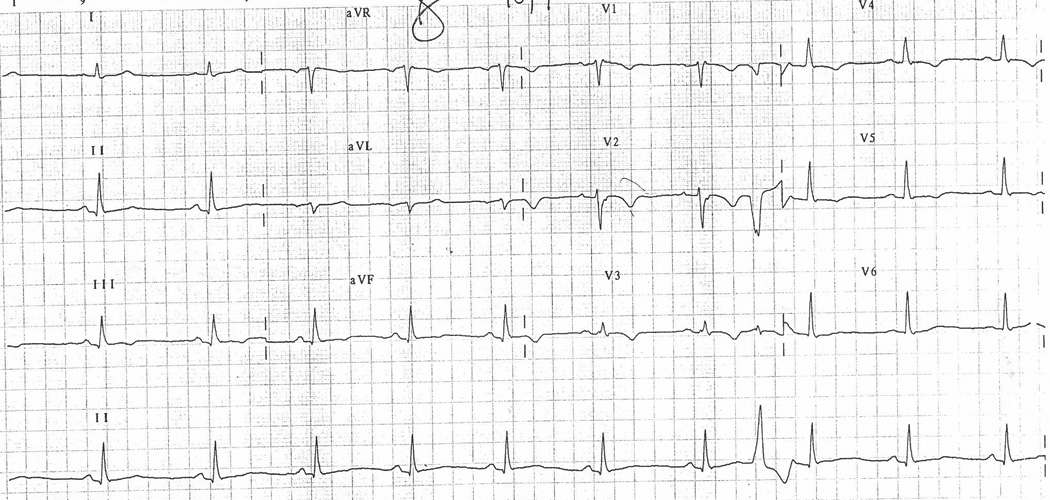
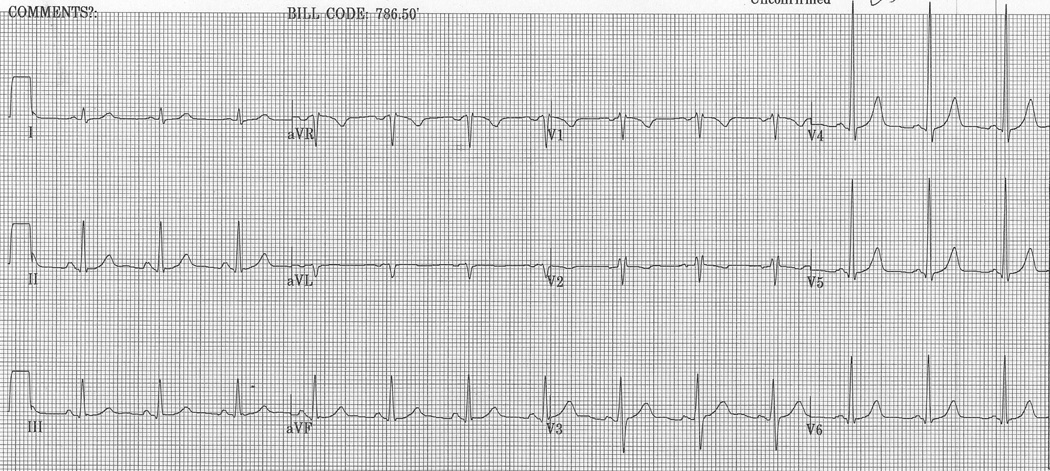
A, Representative ECG obtained from an ARVD patient with IRBBB. ECG illustrates TWI in V1–V5, TAD ≥ 55 ms, QRSd > 110 ms, ratio < 1.2 and PVC.
B, Representative ECG of control with IRBBB illustrating TWI in V1–V2.
Table 3 presents the prevalence of ECG characteristics in ARVD patients with an IRBBB pattern in comparison to a control population with an IRBBB. Nine of the ten ECG parameters which we examined were more commonly observed in ARVD patients as compared with controls. The only parameter which did not differ was our newly proposed parameter for ARVD in the presence of a CRBBB of an r’/s ratio < 1. Table 4 summarizes the sensitivity and specificity of these parameters in distinguishing ARVD patients from controls. Among the ECG parameters which differed in the two groups, the three most sensitive parameters were TWI through V2 (80%) (95% CI, 52%–96%), QRS prolongation > 110 msec (80%) (95% CI, 52%–96%) and TAD ≥ 55 msec (80%) (95% CI, 52%–96%). Three parameters demonstrated 100% specificity; 1) TWI through V4 (100%) (95% CI, 85%–100%), 2) TWI in inferior leads (100%) (95% CI, 85%–100%), 3) the presence of an epsilon wave (100%) (95% CI, 78%–100%).The ECG parameter which demonstrated the optimal sensitivity and specificity was again TWI through V3 (73% (95% CI, 45%–92%) and 95% (95% CI, 77%–100%) respectively).
ECG Characteristics of ARVD and Controls in the Presence of CRBBB
Figure 3A shows a representative ECG obtained from ARVD patients with a CRBBB. Figure 3B shows a representative ECG from control subjects with a CRBBB. Several differences in these two ECGs can be appreciated. First, the ECG obtained from the ARVD patients reveals TWI in leads V1 through V4 whereas the ECG from the patient without ARVD reveals TWI only through V1. Second, the rsr’ pattern in lead V1 differs significantly in the ECG from the ARVD patient demonstrating a wide and low amplitude r’ pattern. The r’/s ratio in ECG obtained from the ARVD patient was 0.76 as compared to infinity in the control patient.
Figure 3.
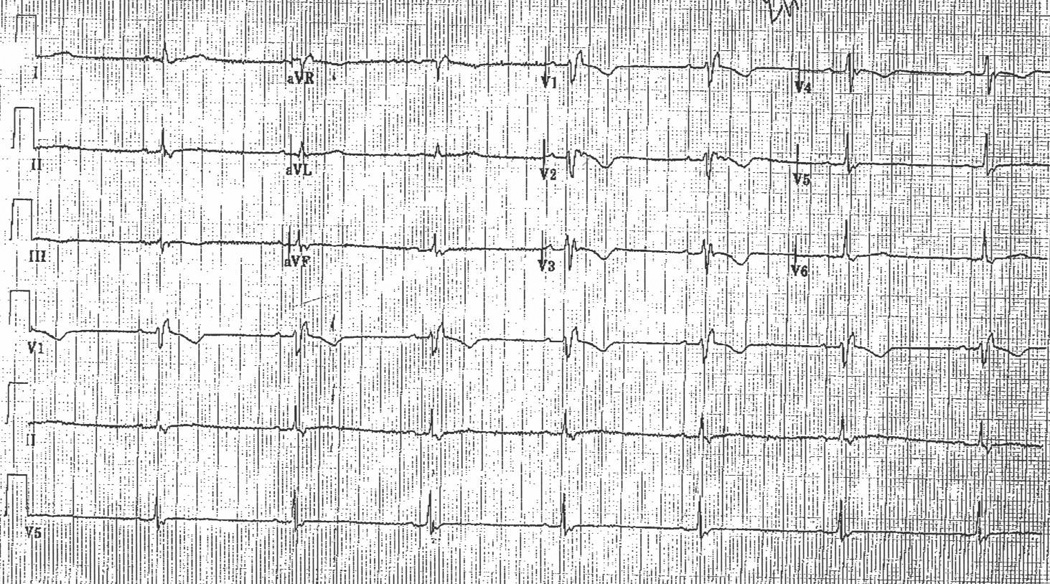
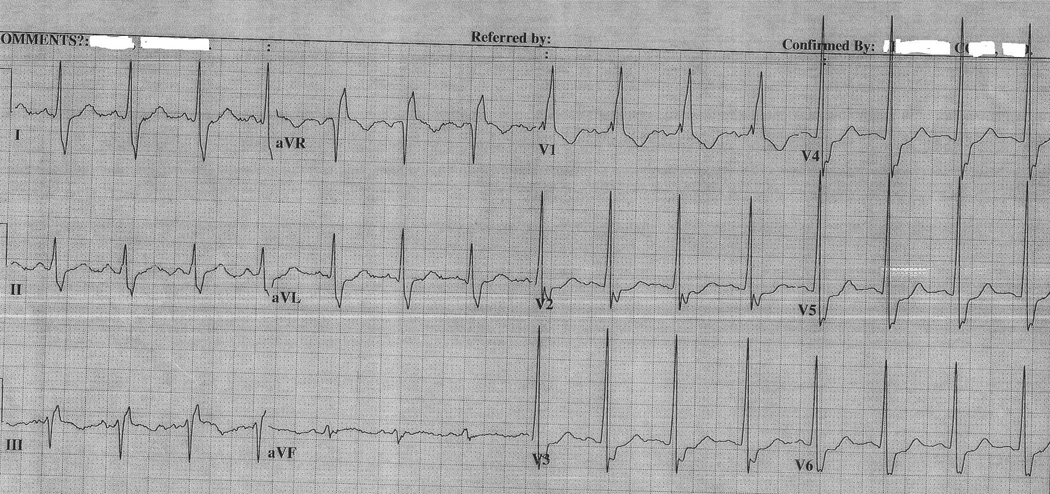
A, Representative ECG obtained from an ARVD patient with CRBBB. It shows TWI in V1–V4 and r’/s ratio <1.
B, Representative ECG of control with CRBBB. It shows TWI in V1 and r’/s >1.
Table 3 presents the prevalence of ECG characteristics in ARVD patients with a CRBBB pattern in comparison to a control population with a CRBBB. The only two parameters which differed in these two groups with a CRBBB pattern was the prevalence of TWI in leads V1 through V4 and an r’ / s ratio < 1 in V1. TWI in V1 through V4 was observed in 59% of ARVD patients as compared with 12 % of controls (p < 0.001). Similarly, an r’/s ratio < 1 in V1 was observed more commonly in ARVD patients than controls (88% versus 14%, p < 0.005). Table 4 summarizes the sensitivity and specificity of each of these parameters in distinguishing ARVD patients from controls. Among the ECG parameters which differed in the two groups, the most sensitive and specific parameter was an r’/s ratio < 1. This parameter also demonstrated optimal sensitivity and specificity (88% (95% CI, 64%–99%) and 86% (95% CI, 42%–100%) respectively).
Diagnostic Value of Combinations of ECG Parameters
One of the goals of our study was to develop ECG criteria for ARVD that can be used in patients with and without a RBBB pattern. To accomplish this we studied the repolarization and depolarization criteria alone and in combination to determine their diagnostic utility in ARVD. For determining the parameters that constitute repolarization and depolarization abnormalities, we relied on prior recommendations as well as the sensitivity/specificity associated with the individual parameter in differentiating ARVD patients from controls. In general, we included all parameters with a specificity or sensitivity > 80% in addition to the previously recommended criteria.
Patients without a CRBBB or IRBBB pattern and patients with an IRBBB had remarkably similar sensitivity and specificity associated with all ECG parameters. Consequently, the repolarization and depolarization variables for these two subgroups included the same parameters. Based on prior recommendation and supported by our present analysis, TWI in leads V1–V3 was considered as a repolarization abnormality in these patients. In addition, TWI in inferior leads was found to have >80% (96% (95% CI, 86%–100%) specificity in this group. Repolarization abnormalities in this group were therefore defined as presence of TWI in the right precordial or inferior leads. A depolarization abnormality in these groups was defined as presence of QRS duration > 110 ms, presence of epsilon wave, and/or TAD. Among patients with no CRBBB/IRBBB, optimal sensitivity and specificity was noted when a combination of depolarization and repolarization was used. In patients with an IRBBB, the optimal sensitivity and specificity were observed by using repolarization alone (Table 5).
TABLE 5.
Prevalence and diagnostic utility of ECG Features Based on Combination of Criteria
| Parameter | Prevalencea | Diagnostic Utilityb | ||
|---|---|---|---|---|
| ARVD (n=100) |
Controls (n=57) |
Sensitivity | Specificity | |
| No RBBB | ||||
| Depolarization Criteria (QRSd > 110 ms, TAD, Epsilon wave)† | 37(54) | 3(11) | 54 (42–67) | 89 (71–98) |
| Repolarization Criteria (TWI thru V3, TWI in Inferior leads (2 out of 3)† | 52(76) | 2(7) | 76 (65–86) | 93 (76–99) |
| (Combination of Repolarization and Depolarization Criteria) | ||||
| 1 Criterion† | 60 (88) | 5 (19) | 88 (78–95) | 81 (62–94) |
| 2 Criteria† | 29 (43) | 0 (0) | 43 (31–55) | 100 (87–100) |
| IRBBB | ||||
| Depolarization Criteria (QRSd > 110 ms, TAD, Epsilon wave)† | 13(87) | 8(36) | 87 (60–98) | 64 (41–83) |
| Repolarization Criteria (TWI thru V3, TWI in Inferior leads (2 out of 3)† | 13(87) | 1(5) | 87 (60–98) | 95 (77–100) |
| (Combination of Repolarization and Depolarization Criteria) | ||||
| 1 Criterion† | 15 (100) | 9 (41) | 100 (78–100) | 59 (36–79) |
| 2 Criteria† | 11 (73) | 0 (0) | 73 (45–92) | 100 (85–100) |
| CRBBB | ||||
| Depolarization Criteria (r'/s amplitude < 1)† | 15(88) | 1(13) | 88 (64–99) | 88 (47–100) |
| Repolarization Criteria (TWI thru V4, TWI in inferior leads (2 out of 3))* | 13(76) | 2(25) | 76 (50–93) | 75 (35–97) |
| (Combination of Repolarization and Depolarization Criteria) | ||||
| 1 Criterion† | 17 (100) | 3 (38) | 100 (80–100) | 63 (24–91) |
| 2 Criteria† | 11 (65) | 0 (0) | 65 (38–86) | 100 (63–100) |
Indicates P<0.01
Indicates P<0.05
Data expressed as frequency (%)
Data expressed as point estimate (95% Confidence Interval)
Previously published criteria are not applicable to patients with CRBBB. In the present analysis, TWI in leads V4 or beyond had a specificity of 88% (95% CI, 47%–100%) in diagnosing ARVD among patients with CRBBB. In addition, TWI in inferior leads was found to have 87% (95% CI, 47%–100%) specificity in this group. Consequently, TWI beyond V4 or in the inferior leads was included as a repolarization abnormality in this subgroup. The only depolarization abnormality that stood out in this subgroup, was r’/s ratio <1 in lead V1. On examining individual repolarization and depolarization abnormalities, and the combination thereof, it was noted that the r’/s ratio by itself yielded a sensitivity of 88% (95% CI, 64%–99%) and a specificity of 88% (95% CI, 42%–100%) (Table 5).
Based on these findings, we propose a clinical algorithm for ECG evaluation of ARVD (Figure 4). Based on this algorithm, we recommend that the QRS morphology be examined as a first step when evaluating a patient for ARVD diagnosis. A fixed set of criteria is then applied to patients based on the presence of no RBBB, IRBBB and CRBBB to obtain the best diagnostic utility of the ECG.
Figure 4.
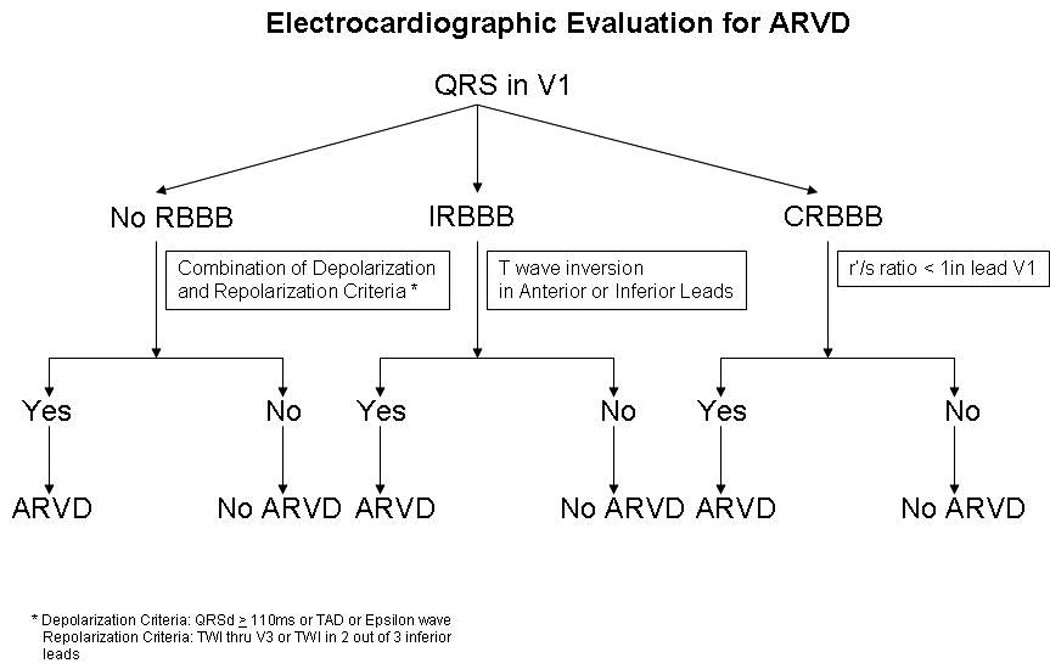
Flow chart summarizing an algorithm that can be used based on the presence or absence of an incomplete or complete RBBB to identify patients with ARVD. *The depolarization criteria used to screen patients with either an incomplete or no RBBB are QRS duration in V1–V3 more than 110 ms, TAD and Epsilon wave. * Repolarization criteria used in the patients with either an Incomplete or no RBBB are TWI V1–V3 and/or TWI in 2 out of the 3 inferior leads. In ARVD patients with CRBBB the depolarization criterion used is r’/s ratio < 1 in lead V1. The repolarization criteria used in ARVD patients with CRBBB are TWI through V4 and/or TWI in 2 out of the three inferior leads.
Discussion
Main Findings
In this study, we performed a systematic analysis of the 12-lead ECG in ARVD patients as compared with controls and investigated the diagnostic value of ECG criteria that are included in the Task Force Criteria, other previously described ECG markers of ARVD including those that were developed as markers of right ventricular conduction delay, and also new markers of ARVD in patients with a RBBB pattern. This is the first study to evaluate the ECG features of ARVD patients based on the presence or absence of a CRBBB or IRBBB. This is of particular importance as IRBBB or CRBBB patterns are commonly observed in patients with ARVD. There are three main findings of this study. First, in the absence of a CRBBB or IRBBB, TWI through V3 is the single ECG parameter which demonstrates the optimal sensitivity and specificity. The use of a simple scoring system that screens for depolarization and/or repolarization criteria improves sensitivity with a slight decrease in specificity. Second, for patients with an IRBBB pattern, TWI through V3 is also the single ECG parameter which demonstrates the optimal sensitivity and specificity. The use of a simple scoring system that screens for depolarization and/or repolarization criteria improves sensitivity with no decrease in specificity. Third, for patients with a CRBBB pattern, an r’/s ratio of < 1 in V1 was the single ECG parameter which demonstrates the optimal sensitivity and specificity. The use of a simple scoring system that screens for depolarization and/or repolarization criteria did not improve sensitivity and specificity as compared with this single parameter alone.
ECG Features of ARVD Identified in the International Task Force Criteria
The Task Force Criteria include three ECG features of the disease in the diagnostic criteria for ARVD10. One of the criteria, which is identified as a minor criteria under the category of repolarization abnormalities, is the presence of TWI in leads V2 and V3 in the absence of a CRBBB. The prevalence of TWI in leads V1–V3 in ARVD patients has been reported to be between 55% and 85% in literature1, 12–14, 17–20 and has been shown to be more common in patients with greater RV dysfunction12, 21. This parameter has not been studied in ARVD patients with a CRBBB in any of the above mentioned studies. The results of our study confirm and extend the findings of these prior studies. Consistent with these prior studies TWI in our study was present in 59 of 83 patients with ARVD who did not have a CRBBB versus 2 of 49 controls giving a sensitivity of 71% (95% CI, 60%–81%) and specificity of 96% (95% CI, 86%–100%). We found that the sensitivity and specificity of this parameter did not differ based on the presence or absence of an IRBBB. A new finding of our study was that TWI in leads V1 – V3 does not differ in ARVD patients with a CRBBB versus a control population of patients with a CRBBB. However, TWI in leads V1 through V4 is more common in ARVD patients with a RBBB pattern than controls. The sensitivity and specificity of this new criterion for ARVD in the presence of a CRBBB was 59% (95% CI, 33%–82%) and 88% (95% CI, 47%–100%) respectively.
The other two criteria which were included as diagnostic criteria in the International Task Force Criteria under the category of depolarization abnormalities, were the presence of an epsilon wave and the presence of a prolonged QRS > 110 msec in the right precordial leads. The presence of either of these parameters was identified as major criteria for ARVD. Prior studies have reported that epsilon waves are present in between 10% and 35% of ARVD patients12–15. This wide difference in the prevalence of epsilon waves likely reflects differences in the severity of ARVD in different studies as well as varying definitions of an epsilon wave. In this study we defined an epsilon wave as a distinct wave of small amplitude that occupies the ST segment in the right precordial leads and is distinct from the QRS complex. In the present study we report an epsilon wave in 5 of 68 ARVD patients (7%). This finding is very similar to that of Cox et al15 who used a definition of epsilon waves virtually identical to that used in the present paper and reported a prevalence of 10%. We did not identify epsilon waves in any of the ARVD patients or controls with a CRBBB pattern.
Perhaps the most controversial and poorly defined diagnostic parameter for ARVD identified in the original Task Force criteria was the presence of a QRSd > 110 msec in the right precordial leads. One of the most confusing aspects of the 1994 criteria10 is that they do not specify whether this parameter can be applied to patients with a CRBBB or IRBBB pattern. Reflecting this uncertainty in the diagnostic criteria is an extremely wide variation from 26% to 75% in the prevalence of this parameter in prior studies13–15. Not surprisingly, the prevalence of a QRSd > 110 msec differed markedly depending on whether there was no IRBBB or CRBBB, an IRBBB pattern, or a CRBBB pattern (43%, 80%, and 100% respectively). Marked differences in the sensitivity and specificity of this criterion were observed depending on the baseline conduction pattern.
Other ECG Markers of ARVD
In addition to the three ECG parameters that have been relied on as part of the Task Force Criteria for ARVD, we have evaluated five other ECG markers of ARVD. Three of these have been reported to be of diagnostic value in prior reports (parietal block, localized right precordial QRS prolongation, and TAD) and two are novel ECG parameter (inferior TWI and r’/s ratio in V1 < 1). In this section of this discussion we will identify each of these parameters in the order of which they were first reported, provide a brief review of prior studies, and then review the results of the current study.
Parietal Block
The concept that the conduction abnormalities observed in patients with ARVD result from parietal block without definite alteration of conduction in the bundle branches was first proposed by Fontaine et al11. He proposed that a QRS duration in leads V1–V3 that exceeds the QRS duration in V6 by 25 msec or greater in the presence of a RBBB block is a marker of parietal block and therefore can be used as a diagnostic criteria for ARVD patients who demonstrate a CRBBB pattern. In the present study we evaluated, for the first time, the prevalence of Parietal block in patients and controls with and without a CRBBB or IRBBB pattern. An important new finding of this study was that the prevalence of Parietal block differed markedly depending on whether there was no IRBBB or CRBBB, an IRBBB pattern, or a CRBBB pattern (13%, 40%, and 47% respectively). Of particular importance was our finding that in the presence of a RBBB this parameter was of no value is distinguishing ARVD patients from controls. We therefore conclude that this parameter is not of diagnostic value in patients being evaluated for ARVD. This likely reflects the error involved in measurement of QRS duration in multiple leads.
Localized Right Precordial QRS Prolongation (Increased ratio)
Peters et al first proposed that localized right precordial QRS prolongation (defined as the ratio of the sum of the QRSd in leads V1–V3 versus V4 – V6 of ≥ 1.2) as an ECG marker for ARVD in 200313. In this initial study he reported that 98% of ARVD patients had localized right precordial QRS prolongation. Importantly, no control population was included in this study. In the present study we evaluated, for the first time, the prevalence of localized right precordial QRS prolongation in patients and controls with and without a CRBBB or IRBBB pattern. An important new finding of this study was that the prevalence of localized right precordial QRS prolongation differed depending on whether there was no IRBBB or CRBBB, an IRBBB, or a CRBBB (22%, 40%, and 47% respectively). We did not find this parameter to be of diagnostic value when compared with a control population of patients with and without a CRBBB or IRBBB pattern.
Prolonged Terminal Activation Duration (TAD)
TAD was first described by Cox et al. as a marker of delayed right ventricular activation15. This parameter is an extension to “delayed S wave upstroke” which was proposed by Nasir et al12. Cox et al reported TAD in 30 of the 42 ARVD patients (71%) versus in one of 27 controls (4%). In the present study we evaluated, for the first time, the prevalence of TAD in patients and controls with and without a CRBBB or IRBBB pattern. An important new finding of this study was that the prevalence of TAD differed depending on whether there was no IRBBB or CRBBB, an IRBBB pattern, or a CRBBB pattern (46%, 80%, and 100% respectively). As in prior studies we found that this parameter was of value in identifying ARVD patients. Importantly, the sensitivity, specificity, and diagnostic value of this parameter was greatest in patients without an IRBBB or CRBBB. TAD was not of diagnostic value in patients with a CRBBB pattern.
T wave Inversion in Inferior leads
One of the novel ECG parameters which we investigated in this study is inferior TWI, which we defined as TWI in two of three inferior ECG leads. We report the presence of inferior TWI in 41%, 47%, and 53% of ARVD versus in 7%, 0% and 13% of control patients with no CRBBB or IRBBB, an IRBBB and a CRBBB respectively. This parameter is an insensitive but specific marker of ARVD in patients without a CRBBB pattern. Because of its diagnostic value, inferior TWI has been included in our ECG scoring system.
r’/s ratio < 1 in Lead V1
The second novel marker of ARVD included in this study is the r’/s ratio < 1 in V1 which is a parameter which we developed for use in ARVD patients with a CRBBB pattern. In the present study we report this parameter to be the best diagnostic marker of ARVD in the setting of a CRBBB pattern with a sensitivity and specificity of 88% (95% CI, 64%–99%) and 86% (95% CI, 42%–100%) respectively.
Diagnostic Value of Combinations of ECG Parameters
One of the goals of our study was to develop ECG criteria for ARVD that can be used in patients with and without a RBBB pattern. To accomplish this we studied the repolarization and depolarization criteria alone and in combination to determine their diagnostic utility in ARVD. The results of our analysis reveal that in the absence of a CRBBB or IRBBB pattern, TWI through V3 is the single ECG parameter which demonstrates the most optimal sensitivity and specificity for identifying patients with ARVD. The use of a simple scoring system that screens for depolarization criteria (QRS duration greater than 110 ms, epsilon wave, and/or TAD) and repolarization criteria (TWI in the anterior of inferior leads) improves sensitivity with a slight decrease in specificity (88% (95% CI, 78%–95%) and 81% (95% CI, 62%–94%) respectively). For patients with an IRBBB pattern, TWI through V3 is also the single ECG parameter which demonstrates the most optimal sensitivity and specificity. The use of a simple scoring system that screens for the depolarization and/or repolarization criteria listed above improves sensitivity with marked decrease in specificity (100% (95% CI, 78%–100%) and 59% (95% CI, 36%–79%) respectively). For this reason our proposed algorithm suggests that repolarization criteria alone be used in screening patients with an IRBBB pattern. And for patients with a CRBBB pattern, an r’/s ratio of < 1 in V1 was the single ECG parameter which demonstrates the most optimal sensitivity and specificity (88% (95% CI, 64%–99%) and 86% (95% CI, 42%–100%) respectively). The use of a simple scoring system that screens for depolarization and/or repolarization criteria did not improve sensitivity and specificity as compared with this single parameter alone. The scoring system needs validation in further studies with larger sample size of patients with IRBBB.
Study Limitations
There are several limitations of this study. First, there is currently no absolute “gold standard” for diagnosis of ARVD. In the absence of a true “gold standard” we and others in the field have relied on the 1994 International Task Force Criteria 10. Although the criteria have proved enormously helpful in standardizing research on ARVD and patients care, they are imperfect and currently undergoing revision. Due to the absence of a true “gold standard” for diagnosis of ARVD it is difficult to estimate the true sensitivity and specificity of any diagnostic test including the ECG. In this study we relied on the 1994 Task Force criteria as “gold standard”. This may have resulted in over-estimation of sensitivity and specificity of the ECG parameters tested, particularly those which are included in the 1994 Task Force criteria. Due to a possible correlation between the ECG parameters not included and those included in the 1994 Task Force criteria, the sensitivity and specificity of such parameters may also have been over-estimated in our analyses. This is a limitation of our study as well as to all other studies which have relied on these criteria. A second limitation of our study is that the control patients with RBBB pattern did not undergo contrast echocardiogram to screen for atrial septal defects. These control patients also did not undergo an MRI. A third limitation of this manuscript is that it is a small study. This is particularly true when considering the new algorithm which we propose when interpreting ECGs which have an IRBBB or CRBBB pattern. In this study we analyzed ECGs from 17 and 15 ARVD patients with a CRBBB and IRBBB pattern respectively. The small sample sizes may have resulted in low precision for estimating sensitivity and specificity (as noted by the wide confidence intervals) in these subgroups. It is clear that the criteria proposed in this manuscript will need prospective validation before they can be relied on to make or exclude the diagnosis of ARVD.
Conclusions
The value of the ECG in the diagnosis of ARVD cannot be underestimated, though definite diagnosis is only possible after a comprehensive evaluation which includes evaluation of the family history, the structure and function of the RV, and screening for arrhythmias. In this study we have critically and comprehensively evaluated the diagnostic value of various ECG markers for ARVD and presented an algorithm for ECG screening for ARVD which is based on the presence or absence of a CRRB or an IRBBB. It is our hope that the findings of our study will be confirmed in other populations of ARVD patients and that at some point in the future the ECG can be relied on with greater confidence as a screening tool for ARVD.
Acknowledgments
Funding Sources
The Johns Hopkins ARVD program is supported by the Bogle Foundation, the Healing Hearts Foundation, the Campanella family, and the Wilmerding Endowment. We also acknowledge funding from the National Heart, Lung, and Blood Institute (1 U01 HL65594-01A1) and (K23HL093350 to HT), NIH grant HL088072, Boston Scientific Corp., St Jude Medical Inc., Medtronic Inc.
Conflict of Interest Disclosures
We acknowledge funding from the National Heart, Lung, and Blood Institute (1 U01 HL65594-01A1) and (K23HL093350 to HT), NIH grant HL088072, Boston Scientific Corp., St Jude Medical Inc., and Medtronic Inc.
References
- 1.Marcus FI, Fontaine GH, Guiraudon G, Frank R, Laurenceau JL, Malergue C, Grosgogeat Y. Right ventricular dysplasia: a report of 24 adult cases. Circulation. 1982;65:384–398. doi: 10.1161/01.cir.65.2.384. [DOI] [PubMed] [Google Scholar]
- 2.Dalal D, Nasir K, Bomma C, Prakasa K, Tandri H, Piccini J, Roguin A, Tichnell C, James C, Russell SD, Judge DP, Abraham T, Spevak PJ, Bluemke DA, Calkins H. Arrhythmogenic right ventricular dysplasia: a United States experience. Circulation. 2005;112:3823–3832. doi: 10.1161/CIRCULATIONAHA.105.542266. [DOI] [PubMed] [Google Scholar]
- 3.Corrado D, Basso C, Thiene G, McKenna WJ, Davies MJ, Fontaliran F, Nava A, Silvestri F, Blomstrom-Lundqvist C, Wlodarska EK, Fontaine G, Camerini F. Spectrum of clinicopathologic manifestations of arrhythmogenic right ventricular cardiomyopathy/dysplasia: a multicenter study. J Am Coll Cardiol. 1997;30:1512–1520. doi: 10.1016/s0735-1097(97)00332-x. [DOI] [PubMed] [Google Scholar]
- 4.Basso C, Thiene G, Corrado D, Angelini A, Nava A, Valente M. Arrhythmogenic right ventricular cardiomyopathy. Dysplasia, dystrophy, or myocarditis? Circulation. 1996;94:983–991. doi: 10.1161/01.cir.94.5.983. [DOI] [PubMed] [Google Scholar]
- 5.Gerull B, Heuser A, Wichter T, Paul M, Basson CT, McDermott DA, Lerman BB, Markowitz SM, Ellinor PT, MacRae CA, Peters S, Grossmann KS, Michely B, Sasse-Klaassen S, Birchmeier W, Dietz R, Breithardt G, Schulze-Bahr E, Thierfelder L. Mutations in the desmosomal protein plakophilin-2 are common in arrhythmogenic right ventricular cardiomyopathy. Nat Genet. 2004;36:1162–1164. doi: 10.1038/ng1461. [DOI] [PubMed] [Google Scholar]
- 6.Rampazzo A, Nava A, Malacrida S, Beffagna G, Bauce B, Rossi V, Zimbello R, Simionati B, Basso C, Thiene G, Towbin JA, Danieli GA. Mutation in human desmoplakin domain binding to plakoglobin causes a dominant form of arrhythmogenic right ventricular cardiomyopathy. Am J Hum Genet. 2002;71:1200–1206. doi: 10.1086/344208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asimaki A, Syrris P, Wichter T, Matthias P, Saffitz JE, McKenna WJ. A novel dominant mutation in plakoglobin causes arrhythmogenic right ventricular cardiomyopathy. Am J Hum Genet. 2007;81:964–973. doi: 10.1086/521633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Awad MM, Dalal D, Cho E, Amat-Alarcon N, James C, Tichnell C, Tucker A, Russell SD, Bluemke DA, Dietz HC, Calkins H, Judge DP. DSG2 mutations contribute to arrhythmogenic right ventricular dysplasia/cardiomyopathy. Am J Hum Genet. 2006;79:136–142. doi: 10.1086/504393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Syrris P, Ward D, Evans A, Asimaki A, Gandjbakhch E, Sen-Chowdhry S, McKenna WJ. Arrhythmogenic right ventricular dysplasia/cardiomyopathy associated with mutations in the desmosomal gene desmocollin-2. Am J Hum Genet. 2006;79:978–984. doi: 10.1086/509122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKenna WJ, Thiene G, Nava A, Fontaliran F, Blomstrom-Lundqvist C, Fontaine G, Camerini F. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology. Br Heart J. 1994;71:215–218. doi: 10.1136/hrt.71.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fontaine G, Fontaliran F, Hebert JL, Chemla D, Zenati O, Lecarpentier Y, Frank R. Arrhythmogenic right ventricular dysplasia. Annu Rev Med. 1999;50:17–35. doi: 10.1146/annurev.med.50.1.17. [DOI] [PubMed] [Google Scholar]
- 12.Nasir K, Bomma C, Tandri H, Roguin A, Dalal D, Prakasa K, Tichnell C, James C, Jspevak P, Marcus F, Calkins H. Electrocardiographic features of arrhythmogenic right ventricular dysplasia/cardiomyopathy according to disease severity: a need to broaden diagnostic criteria. Circulation. 2004;110:1527–1534. doi: 10.1161/01.CIR.0000142293.60725.18. [DOI] [PubMed] [Google Scholar]
- 13.Peters S, Trummel M. Diagnosis of arrhythmogenic right ventricular dysplasia-cardiomyopathy: value of standard ECG revisited. Ann Noninvasive Electrocardiol. 2003;8:238–245. doi: 10.1046/j.1542-474X.2003.08312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peters S, Trummel M, Koehler B, Westermann KU. The value of different electrocardiographic depolarization criteria in the diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Electrocardiol. 2007;40:34–37. doi: 10.1016/j.jelectrocard.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Cox MG, Nelen MR, Wilde AA, Wiesfeld AC, van der Smagt J, Loh P, Cramer MJ, Doevendans PA, van Tintelen JP, de Bakker JM, Hauer RN. Activation delay and VT parameters in arrhythmogenic right ventricular dysplasia/cardiomyopathy: toward improvement of diagnostic ECG criteria. J Cardiovasc Electrophysiol. 2008;19:775–781. doi: 10.1111/j.1540-8167.2008.01140.x. [DOI] [PubMed] [Google Scholar]
- 16.Willems JL, Robles de Medina EO, Bernard R, Coumel P, Fisch C, Krikler D, Mazur NA, Meijler FL, Mogensen L, Moret P, Pisa Z, Rautaharju PM, Surawicz B, Watanabe Y, Wellens HJJ. Criteria for intraventricular conduction disturbances and pre-excitation. World Health Organizational/International Society and Federation for Cardiology Task Force Ad Hoc. J Am Coll Cardiol. 1985;5:1261–1275. doi: 10.1016/s0735-1097(85)80335-1. [DOI] [PubMed] [Google Scholar]
- 17.Hamid MS, Norman M, Quraishi A, Firoozi S, Thaman R, Gimeno JR, Sachdev B, Rowland E, Elliott PM, McKenna WJ. Prospective evaluation of relatives for familial arrhythmogenic right ventricular cardiomyopathy/dysplasia reveals a need to broaden diagnostic criteria. J Am Coll Cardiol. 2002;40:1445–1450. doi: 10.1016/s0735-1097(02)02307-0. [DOI] [PubMed] [Google Scholar]
- 18.Lemola K, Brunckhorst C, Helfenstein U, Oechslin E, Jenni R, Duru F. Predictors of adverse outcome in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy: long term experience of a tertiary care centre. Heart. 2005;91:1167–1172. doi: 10.1136/hrt.2004.038620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nava A, Bauce B, Basso C, Muriago M, Rampazzo A, Villanova C, Daliento L, Buja G, Corrado D, Danieli GA, Thiene G. Clinical profile and long-term follow-up of 37 families with arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol. 2000;36:2226–2233. doi: 10.1016/s0735-1097(00)00997-9. [DOI] [PubMed] [Google Scholar]
- 20.Turrini P, Corrado D, Basso C, Nava A, Bauce B, Thiene G. Dispersion of ventricular depolarization-repolarization: a noninvasive marker for risk stratification in arrhythmogenic right ventricular cardiomyopathy. Circulation. 2001;103:3075–3080. doi: 10.1161/01.cir.103.25.3075. [DOI] [PubMed] [Google Scholar]
- 21.Bauce B, Basso C, Rampazzo A, Beffagna G, Daliento L, Frigo G, Malacrida S, Settimo L, Danieli G, Thiene G, Nava A. Clinical profile of four families with arrhythmogenic right ventricular cardiomyopathy caused by dominant desmoplakin mutations. Eur Heart J. 2005;26:1666–1675. doi: 10.1093/eurheartj/ehi341. [DOI] [PubMed] [Google Scholar]


