Abstract
Context: Disorders of adrenal development result in significant morbidity and mortality. However, the molecular basis of human adrenal development, and many forms of disease, is still poorly understood.
Objectives: We evaluated the role of two new candidate genes, CBP/p300-interacting transactivator, with Glu/Asp-rich C-terminal domain, 2 (CITED2), and pre-B-cell leukemia transcription factor 1 (PBX1), in human adrenal development and disease.
Design: CITED2 and PBX1 expression in early human fetal adrenal development was assessed using RT-PCR and in situ hybridization. The regulation of CITED2 and PBX1 by steroidogenic factor-1 (SF-1) and dosage-sensitive sex reversal, adrenal hypoplasia congenital, critical region on the X chromosome, gene-1 (DAX1) was evaluated in NCI-H295R human adrenocortical tumor cells by studying promoter regulation. Finally, mutational analysis of CITED2 and PBX1 was performed in patients with primary adrenal disorders.
Results: CITED2 and PBX1 are expressed in the human fetal adrenal gland during early development. Both genes are activated by SF-1 in a dose-dependent manner in NCI-H295R cells, and, surprisingly, PBX1 is synergistically activated by SF-1 and DAX1. Mutational analysis failed to reveal significant coding sequence changes in individuals with primary adrenal disorders.
Conclusions: CITED2 and PBX1 are likely to be important mediators of adrenal development and function in humans, but mutations in these genes are not common causes of adrenal failure in patients in whom a molecular diagnosis remains unknown. The positive interaction between DAX1 and SF-1 in regulating PBX1 may be an important mechanism in this process.
The transcriptional regulators CITED2 and PBX1 are expressed during adrenal development and are regulated by steroidogenic factor-1, but are not frequently disrupted in adrenal failure.
Adrenal failure can be difficult to diagnose in children and is associated with significant mortality and morbidity. Although 21-hydroxylase deficiency and autoimmune Addison disease remain the most likely diagnoses in early infancy and childhood, respectively, a range of metabolic, infectious, infiltrative/destructive, and developmental etiologies can result in a spectrum of adrenal disorders presenting throughout the pediatric and adolescent years (1). Because some of these conditions have different natural histories, potential associated features, and varying modes of inheritance, making a correct diagnosis and undertaking appropriate treatment and counseling are essential.
The past decade has seen steady progress in our understanding of the molecular basis of adrenal disease, especially in the area of adrenal development and regulation (2,3,4). Several single gene disorders causing “adrenal hypoplasia” have now been reported (2). For example, secondary adrenal hypoplasia can result from pituitary dysfunction or isolated ACTH deficiency (e.g. HESX1, SOX3, LHX3, LHX4, and TPIT). ACTH resistance (“familial glucocorticoid deficiency”) can result from defects in ACTH signaling pathways and related mechanisms (e.g. MC2R, MRAP, and AAAS), and primary adrenal hypoplasia most frequently occurs as an X-linked condition due to deletions or mutations in the orphan nuclear receptor dosage-sensitive sex reversal, adrenal hypoplasia congenita (AHC), critical region on the X chromosome, gene-1 (DAX1) (NR0B1), although rare cases due to defects in other factors (e.g. steroidogenic factor-1 [SF-1, NR5A1]) have been reported (2,5,6,7,8). At present, a molecular diagnosis can be reached in approximately 50% of infants or children presenting with adrenal hypoplasia or resistance, and an increasing number of syndromic associations and “non-classic” variants of adrenal disorders are being described (4,5,7,9,10,11,12,13). However, although substantial progress has been made, a significant proportion of cases of syndromic and nonsyndromic adrenal hypoplasia and related disorders currently remain unexplained.
Two candidate genes emerging as potential causes of primary adrenal hypoplasia from work in mice are the transcriptional regulators CBP/p300-interacting transactivator, with Glu/Asp-rich C-terminal domain, 2 (CITED2) (Mendelian Inheritance in Man 602937), and pre-B-cell leukemia transcription factor 1 (PBX1) (Mendelian Inheritance in Man 176310). Targeted disruption of Cited2 in mice results in adrenal agenesis, neurological defects, and cardiac malformations (14), whereas Pbx1-deficient mice have severe adrenal hypoplasia together with pancreatic dysfunction, impaired gonadal development, and skeletal abnormalities (15). Although both knockout models are embryonic lethal, Cited2 haploinsufficient animals have markedly impaired adrenal development when crossed with either Sf1+/− or Wt1+/− strains (16), and Pbx1 haploinsufficient animals are viable, and have smaller adrenal glands with impaired adrenocortical growth and steroidogenesis (17).
To date, the role of CITED2 and PBX1 in humans is poorly understood, but they are candidate genes for unexplained cases of adrenal hypoplasia with or without associated features. Here, we demonstrate the expression of these genes in human fetal adrenal development, their regulation by SF-1 and DAX1, and mutational analysis of CITED2 and PBX1 in a cohort of patients with primary adrenal disorders.
Materials and Methods
RT-PCR
Human fetal adrenal tissue from 7 and 10 wk gestation was provided by the Medical Research Council/Wellcome Trust funded Human Developmental Biology Resource with Research Ethics Committee approval and informed consent. RNA was extracted using the TRIzol method (Invitrogen Corp., Paisley, UK), and RT-PCR was performed according to the manufacturer’s protocol (35 cycles, Access Quick RT-PCR System; Promega, Southampton, UK; detailed conditions are available on request). Primers for CITED2 were located within exon 2 (forward, 5′-CAGGAAGGTCCCCTCTATGTG-3′, and reverse, 5′-GCGCCGTAGTGTATGTGCTC-3′), and for PBX1 within exon 4 (forward, 5′-GTTCCCGATTTCTGGATGC-3′) and within exon 6 (reverse, 5′-CATGGGCTGACACATTGGTA-3′). Glyceraldehyde-3-phosphate dehydrogenase was used as a positive control.
In situ hybridization
In situ hybridization analysis of CITED2 and PBX1 in early human adrenal tissue was performed with ethical approval through the In House Gene Expression Service of the Human Developmental Biology Resource. Human embryos/fetuses at selected stages were dissected and fixed in 4% paraformaldehyde, then dehydrated and embedded in paraffin wax. Sections of 7 μm were cut using a standard microtome and mounted on Superfrost Plus slides (BDH, Poole, UK). In situ hybridization was performed essentially as described by Wilkinson (18) using digoxigenin 11 incorporated riboprobes generated from a pOTB7 vector containing the 1903-bp full cDNA sequence of CITED2 and a pCR4-TOPO vector containing the 1388-bp full cDNA sequence of PBX1 (both plasmids obtained from the Mammalian Gene Collection/National Institutes of Health, Integrated Molecular Analysis of Gnomes and their Expression identification nos. 3640855 and 8069084, respectively). For antibody detection, slides were incubated with antidigoxigenin antibody conjugated with alkaline phosphatase (diluted 1:1000, containing 2% fetal calf serum). Expression patterns were visualized using the nitro-blue tetrazolium chloride/5-bromo-4-chloro-3′-indolyphosphate p-toluidine salt system (Roche, Welwyn Garden City, UK). Sections were mounted in VectaMount (Vector Laboratories, Burlingame, CA) and analyzed using the Axioplan2 imaging system (Zeiss, Jena, Germany). Sense probes for CITED2 and PBX1 were tested on adjacent sections, and showed no staining above background levels.
Reporter and expression vector construction
Based on previously published mouse data (14,17) and analysis for putative SF-1 binding sites (MatInspector, www.genomatix.de) (19), a 3.3-kb upstream region of the CITED2 promoter and a 910-bp upstream region of the PBX1 promoter were PCR amplified and cloned into a pGL4.10[luc2] luciferase reporter vector (Promega). Expression vectors (pCMX) containing SF-1 [wild-type (WT) and mutant G35E] and DAX1 (WT and mutants R267P, A300P, I439S) cDNAs have been described previously (10,20,21,22).
Transient gene expression assays
Transient gene expression assays were performed in 96-well plates (Techno Plastic Products, Trasadingen, Switzerland) using a NCI-H295R human adrenocortical tumor cell line, Lipofectamine 2000 (Invitrogen), and a dual-luciferase reporter assay system (Promega) with cotransfection of pRLSV40 Renilla luciferase (Promega) as a marker of transfection efficiency.
To analyze the effects of SF-1 on CITED2 and PBX1 regulation, increasing doses of pCMXWT or mutant SF-1 expression vectors (10, 20, 50, and 100 ng/well) were cotransfected with either pGL4.10-CITED2-luc or pGL4.10-PBX1-luc reporters (100 ng/well). Activation of the promoters by SF-1 and DAX1 was studied using 100 ng pCMXWTSF1 together with increasing doses of pCMXWTDAX1 (2, 5, 10, 20, and 50 ng/well). The synergistic effects of DAX1 were evaluated further using DAX1 mutants associated with severe (R267P, A300P) or milder (I439S) phenotypes (50 ng/well) (10,22).
In all studies, cells were lysed 48 h after transfection and luciferase assays performed using a FLUOstar Optima fluorescence microplate reader (BMG Labtech, Aylesbury, UK). All data were standardized for Renilla coexpression. Results are shown as the mean ± sem of at least three independent experiments, each performed in triplicate.
Patient cohort
Direct sequencing of CITED2 and PBX1 was undertaken in a diverse cohort of patients with primary adrenal failure, with or without associated features. In many cases, mutations in several other relevant candidate genes [DAX1 (NR0B1), SF1 (NR5A1), MC2R, AAAS, CYP11A1, STAR, and ACD] had been excluded (supplemental Table 1, which is published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org), and common steroidogenic defects (21-hydroxylase deficiency), autoimmune disorders, and metabolic disorders (e.g. X-linked adrenoleukodystrophy) were not detected. In a cohort from one center, 36 patients (31 males, five females) were analyzed for mutations in CITED2 and PBX1. Additional features present in several patients with more complex phenotypes included gonadal, cardiac, and renal abnormalities, and intrauterine growth restriction or Intrauterine growth retardation, Metaphyseal dysplasia, AHC, and Genital anomalies syndrome features (9) (supplemental Table 1A). In another cohort, PBX1 was analyzed in 20 patients with a predominant ACTH-resistance phenotype (supplemental Table 1B). Finally, CITED2 was sequenced in a group of 15 patients with a predominant adrenal hypoplasia phenotype (supplemental Table 1C). The Human Random Control-1 DNA Panel (British Caucasian) (Human Random Control-1 DNA Panel, European Collection of Cell Cultures, UK) was used as control genomic DNA for the analysis of previously unreported polymorphisms.
Mutational analysis
After institutional board approval and with informed consent, genomic DNA was extracted from peripheral blood lymphocytes, and the entire coding regions of CITED2 (exon 2, three primer pairs) and PBX1 (exons 1–9, nine primer pairs) were amplified by PCR (specific conditions and primer sequences available on request). PCR products were purified by gel extraction (QIAGEN, Crawley, UK) or by using exonuclease I (New England Biolabs, Ipswich, MA)/shrimp alkaline phosphatase (USB, Columbus, OH) and then subjected to direct sequencing using dye terminator sequencing kits (dRhodamine/BigDyev1.1; PE Applied Biosystems Inc., Foster City, CA) in an automated capillary based sequencer (MegaBACE1000; Amersham Biosciences Inc., Piscataway, NJ). Sequencher version 4.1 (Genecodes Corp., Ann Arbor, MI) was used to analyze the data.
Results
CITED2 and PBX1 expression
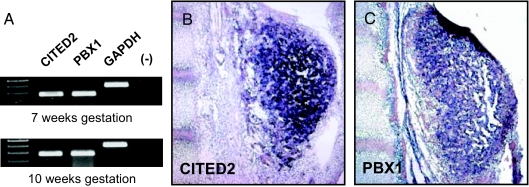
Analysis of CITED2 and PBX1 by RT-PCR showed abundant expression of these genes in RNA derived from 7 and 10-wk human fetal adrenal glands (Fig. 1A). This expression was confirmed by in situ hybridization on human fetal adrenal tissue at Carnegie Stage 20 (7 wk gestation) (Fig. 1, B and C).
Figure 1.
CITED2 and PBX1 expression in human fetal adrenal. A, RT-PCR of CITED2 and PBX1 at 7 and 10 wk gestation. (−), water control. B and C, In situ hybridization of CITED2 and PBX1 in human fetal adrenal tissue at Carnegie Stage 20 (7 wk gestation). GAPDH, Glyceraldehyde-3-phosphate dehydrogenase.
Transcriptional regulation of CITED2 and PBX1
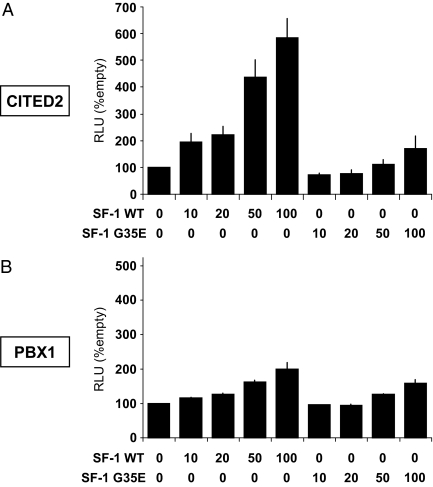
Several putative SF-1 binding sites were identified within the proximal promoter regions of CITED2 (3.3 kb) and PBX1 (910 bp), as has been reported previously in the mouse (12). Cotransfection of SF-1 with reporter constructs containing these regions showed dose-dependent activation of the CITED2 promoter by SF-1 (up to 6-fold) (Fig. 2A), whereas the PBX1 promoter showed 2-fold activation (Fig. 2B).
Figure 2.
Activation of CITED2 (3.3 kb) (A) and PBX1 (910 bp) (B) promoters by WT or mutant (G35E) SF-1 (0–100 ng/well) in NCI-H295R cells. Data shown as mean ± sem of three experiments each performed in triplicate. RLU, Relative light units.
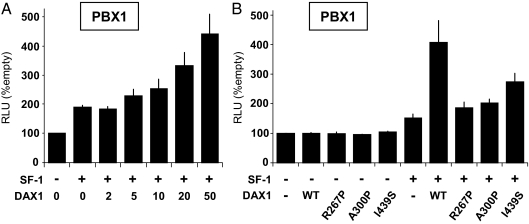
Coexpression of the nuclear receptor DAX1 with SF-1 had only limited effects on regulation of the CITED2 promoter (data not shown). In contrast, synergistic activation was observed when SF-1 and DAX1 were coexpressed with the PBX1 promoter (4-fold higher than empty vector, 2.7- fold greater than SF-1 alone) (Fig. 3). This increased activation was lost when DAX1 mutants associated with severe X-linked adrenal hypoplasia (R267P, A300P) were cotransfected instead of WT cDNA, and partially reduced when the I439S mutant associated with a milder, late-onset form of X-linked adrenal hypoplasia was studied (Fig. 3B).
Figure 3.
A, Cotransfection of increasing doses of WT DAX1 (0–50 ng/well) together with WT SF-1 (100 ng/well) shows a dose-dependent activation of the PBX1 promoter. B, By comparing the effects of WT DAX1 alone (50 ng/well, lane 2), WT SF-1 alone (100 ng/well, lane 6), and WT DAX1 (50 ng/well) and WT SF-1 (100 ng/well) together (lane 7), synergistic activation of the PBX1 promoter is seen. This synergistic activation is attenuated when naturally occurring DAX1 mutants associated with severe (R267P, A300P) or mild (I439S) forms of X-linked adrenal hypoplasia are studied (all 50 ng/well). Data represent the mean ± sem of triplicate experiments (unpaired t tests: WT >R267P, A300P; P < 0.01; I439S >R267P, A300P; P < 0.05). RLU, Relative light units.
Mutational analysis of CITED2 and PBX1
Mutational analysis of CITED2 (n = 51) and PBX1 (n = 56) in individuals with primary adrenal failure failed to reveal significant coding sequence changes. Two polymorphisms were found in CITED2. The previously reported c.21C>A transversion (refSNP rs1131400) was found in 12% of the alleles (consistent with control data). The previously unreported c.*47G>C transversion was present in 2.0% of patient alleles and 2.2% of 186 control alleles. Two polymorphisms were detected in PBX1. The previously described c.61G>A transversion (refSNP rs2275558) was found as a heterozygous change in 26% of patient alleles. The novel c.191 + 37_40delTTTT (intron 1–2) change was present in 17% of patient alleles and in 13% of 128 control alleles.
Discussion
Although significant progress has been made in our identification of several genetic causes of primary adrenal hypoplasia and ACTH resistance syndromes, the underlying etiology remains unknown in a substantial proportion of cases. Recently, the description of adrenal phenotypes associated with deletion of Cited2 or Pbx1 in the mouse has provided potential candidate genes for analysis in patients with disorders of adrenal development and function.
Here, we show that CITED2 and PBX1 are both expressed during the early stages of human fetal adrenal development at a time when the gland is undergoing significant morphological and functional differentiation (3,23) and concordant with expression of SF-1 (24).
Because SF-1 is an important regulator of many target genes involved in adrenal development and function, we hypothesized that SF-1-dependent regulation of CITED2 and PBX1 might occur in humans. Recent studies have shown that Sf-1 can regulate Pbx1 expression in mice (17), and that Pbx1 and Cited2 may in turn mediate Sf1(Nr5a1) expression in in vitro and in vivo systems (16,25). By focusing on a human adrenal cell line, we have shown that SF-1 can strongly activate the human CITED2 promoter. Furthermore, although SF-1 appears to be a relatively weak activator of the minimal promoter of human PBX1, synergistic activation of this promoter by SF-1 and DAX1 was observed in adrenal cells. Although mutations in both DAX1 and SF-1 can result in variable degrees of adrenal insufficiency, it remains enigmatic how these two transcription factors interact during adrenal development and function because most studies have shown that DAX1 can act as a repressor of SF-1-mediated transactivation (22,26,27,28). However, a recent report by Verrijn Stuart et al. (29) has shown activation of the CYP11B1 promoter by SF-1 and DAX1 in an adrenal cell line, and both underexpression and overexpression of Dax1(Nr0b1) have had a detrimental effect on testis development (30,31,32,33,34). Together with the synergistic activation of the PBX1 promoter shown in this report, our findings suggest that DAX1 may also have an activating role during certain stages of development, on specific promoters, or together with cell-specific transcriptional complexes. This mechanism may contribute to the impaired definitive zone development in patients with X-linked AHC because the synergy of DAX1 was attenuated when naturally occurring severe and partial DAX1 mutants were studied.
Given the phenotype of Cited2 and Pbx1 knockout and haploinsufficient mice, together with the human expression and transcriptional data obtained, mutational analysis of CITED2 and PBX1 was undertaken in a cohort of patients with adrenal hypoplasia or adrenal failure phenotypes. A heterogeneous cohort was deliberately chosen because it was possible that associated features could be present (e.g. cardiac defects with CITED2 and skeletal abnormalities with PBX1) (15,35), or that a variable spectrum of milder phenotypes could be seen if severe loss-of-function was not compatible with survival. Although several reported and novel polymorphisms were found, no nonsynonymous mutations in CITED2 and PBX1 were discovered. It is possible that haploinsufficiency or copy number variations of these genes could cause a phenotype in humans, which would not have been detected in our analysis. However, because biallelic variants (heterozygous single nucleotide polymorphisms) were detected in most of the patients studied, haploinsufficiency of CITED2 and/or PBX1 does not appear to be frequent. Therefore, CITED2 and PBX1 are likely to be important mediators of adrenal development and function in humans, but mutations in these genes are unlikely to be a common cause of adrenal hypoplasia or adrenal failure in those patients in whom the etiology remains unknown.
Supplementary Material
Acknowledgments
We thank Larry Jameson, Masafumi Ito, and Ron Evans for plasmids and reagents. We also thank the many pediatricians and physicians who have referred patients. Human embryonic material was provided by the Medical Research Council/Wellcome Trust-funded Human Developmental Biology Resource, Institute of Child Health.
Footnotes
B.F.-d.-S. holds a scholarship from Capes/Brazil (4798066). D.M. is supported by the Biological Network of Rare Diseases, French Health Ministry. A.H. is supported by a grant of the Deutsche Forschungsgemeinschaft, Germany (HU895/3-5). F.B. is supported by a grant of the Landesstiftung Baden-Wurttemberg (P-LS-ASN/5). The Human Developmental Biology Resource receives funding from The Wellcome Trust and Medical Research Council. J.C.A. holds a Wellcome Trust Senior Research Fellowship in Clinical Science (079666). Part of this work was undertaken at Great Ormond Street Hospital/University College London Institute of Child Health which received a proportion of funding from the Department of Health’s National Institute for Health Research Biomedical Research Centres funding scheme.
Disclosure Statement: The authors have nothing to disclose.
First Published Online November 4, 2008
Abbreviations: AHC, Adrenal hypoplasia congenita; CITED2, CBP/p300-interacting transactivator, with Glu/Asp-rich C-terminal domain, 2; DAX1, dosage-sensitive sex reversal, adrenal hypoplasia congenital, critical region on the X chromosome, gene-1; PBX1, pre-B-cell leukemia transcription factor 1; SF-1, steroidogenic factor-1; WT, wild type.
References
- Ten S, New M, Maclaren N 2001 Clinical review 130: Addison’s disease 2001. J Clin Endocrinol Metab 86:2909–2922 [DOI] [PubMed] [Google Scholar]
- Ferraz-de-Souza B, Achermann JC 2008 Disorders of adrenal development. Endocr Dev 13:19–32 [DOI] [PubMed] [Google Scholar]
- Hammer GD, Parker KL, Schimmer BP 2005 Minireview: transcriptional regulation of adrenocortical development. Endocrinology 146:1018–1024 [DOI] [PubMed] [Google Scholar]
- Else T, Hammer GD 2005 Genetic analysis of adrenal absence: agenesis and aplasia. Trends Endocrinol Metab 16:458–468 [DOI] [PubMed] [Google Scholar]
- Metherell LA, Chan LF, Clark AJ 2006 The genetics of ACTH resistance syndromes. Best Pract Res Clin Endocrinol Metab 20:547–560 [DOI] [PubMed] [Google Scholar]
- Muscatelli F, Strom TM, Walker AP, Zanaria E, Recan D, Meindl A, Bardoni B, Guioli S, Zehetner G, Rabl W, Schwarz HP, Kaplan JC, Camerino G, Meitinger T, Monaco AP 1994 Mutations in the DAX-1 gene give rise to both X-linked adrenal hypoplasia congenita and hypogonadotropic hypogonadism. Nature 372:672–676 [DOI] [PubMed] [Google Scholar]
- Lin L, Gu WX, Ozisik G, To WS, Owen CJ, Jameson JL, Achermann JC 2006 Analysis of DAX1 (NR0B1) and steroidogenic factor-1 (NR5A1) in children and adults with primary adrenal failure: ten years’ experience. J Clin Endocrinol Metab 91:3048–3054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biason-Lauber A, Schoenle EJ 2000 Apparently normal ovarian differentiation in a prepubertal girl with transcriptionally inactive steroidogenic factor 1 (NR5A1/SF-1) and adrenocortical insufficiency. Am J Hum Genet 67:1563–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilain E, Le Merrer M, Lecointre C, Desangles F, Kay MA, Maroteaux P, McCabe ER 1999 IMAGe, a new clinical association of intrauterine growth retardation, metaphyseal dysplasia, adrenal hypoplasia congenita, and genital anomalies. J Clin Endocrinol Metab 84:4335–4340 [DOI] [PubMed] [Google Scholar]
- Tabarin A, Achermann JC, Recan D, Bex V, Bertagna X, Christin-Maitre S, Ito M, Jameson JL, Bouchard P 2000 A novel mutation in DAX1 causes delayed-onset adrenal insufficiency and incomplete hypogonadotropic hypogonadism. J Clin Invest 105:321–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh MG, Boyes L, Kingston H, Collins R, Besley GT, Padmakumar B, Ismayl O, Hughes I, Hall CM, Hellerud C, Achermann JC, Clayton PE 2008 Skewed X inactivation is associated with phenotype in a female with adrenal hypoplasia congenita. J Med Genet 45:e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker BY, Lin L, Kim CJ, Raza J, Smith CP, Miller WL, Achermann JC 2006 Nonclassic congenital lipoid adrenal hyperplasia: a new disorder of the steroidogenic acute regulatory protein with very late presentation and normal male genitalia. J Clin Endocrinol Metab 91:4781–4785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Hindmarsh PC, Metherell LA, Alzyoud M, Al-Ali M, Brain CE, Clark AJ, Dattani MT, Achermann JC 2007 Severe loss-of-function mutations in the adrenocorticotropin receptor (ACTHR, MC2R) can be found in patients diagnosed with salt-losing adrenal hypoplasia. Clin Endocrinol (Oxf) 66:205–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamforth SD, Braganca J, Eloranta JJ, Murdoch JN, Marques FI, Kranc KR, Farza H, Henderson DJ, Hurst HC, Bhattacharya S 2001 Cardiac malformations, adrenal agenesis, neural crest defects and exencephaly in mice lacking Cited2, a new Tfap2 co-activator. Nat Genet 29:469–474 [DOI] [PubMed] [Google Scholar]
- Selleri L, Depew MJ, Jacobs Y, Chanda SK, Tsang KY, Cheah KS, Rubenstein JL, O'Gorman S, Cleary ML 2001 Requirement for Pbx1 in skeletal patterning and programming chondrocyte proliferation and differentiation. Development 128:3543–3557 [DOI] [PubMed] [Google Scholar]
- Val P, Martinez-Barbera JP, Swain A 2007 Adrenal development is initiated by Cited2 and Wt1 through modulation of Sf-1 dosage. Development 134:2349–2358 [DOI] [PubMed] [Google Scholar]
- Lichtenauer UD, Duchniewicz M, Kolanczyk M, Hoeflich A, Hahner S, Else T, Bicknell AB, Zemojtel T, Stallings NR, Schulte DM, Kamps MP, Hammer GD, Scheele JS, Beuschlein F 2007 Pre-B-cell transcription factor 1 and steroidogenic factor 1 synergistically regulate adrenocortical growth and steroidogenesis. Endocrinology 148:693–704 [DOI] [PubMed] [Google Scholar]
- Wilkinson DG 1992 In situ hybridization: a practical approach. Oxford, UK: IRL Press at Oxford University Press [Google Scholar]
- Quandt K, Frech K, Karas H, Wingender E, Werner T 1995 MatInd and MatInspector: new fast and versatile tools for detection of consensus matches in nucleotide sequence data. Nucleic Acids Res 23:4878–4884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achermann JC, Ito M, Ito M, Hindmarsh PC, Jameson JL 1999 A mutation in the gene encoding steroidogenic factor-1 causes XY sex reversal and adrenal failure in humans. Nat Genet 22:125–126 [DOI] [PubMed] [Google Scholar]
- Ito M, Achermann JC, Jameson JL 2000 A naturally occurring steroidogenic factor-1 mutation exhibits differential binding and activation of target genes. J Biol Chem 275:31708–31714 [DOI] [PubMed] [Google Scholar]
- Achermann JC, Ito M, Silverman BL, Habiby RL, Pang S, Rosler A, Jameson JL 2001 Missense mutations cluster within the carboxyl-terminal region of DAX-1 and impair transcriptional repression. J Clin Endocrinol Metab 86:3171–3175 [DOI] [PubMed] [Google Scholar]
- Goto M, Piper HK, Marcos J, Wood PJ, Wright S, Postle AD, Cameron IT, Mason JI, Wilson DI, Hanley NA 2006 In humans, early cortisol biosynthesis provides a mechanism to safeguard female sexual development. J Clin Invest 116:953–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley NA, Ball SG, Clement-Jones M, Hagan DM, Strachan T, Lindsay S, Robson S, Ostrer H, Parker KL, Wilson DI 1999 Expression of steroidogenic factor 1 and Wilms’ tumour 1 during early human gonadal development and sex determination. Mech Dev 87:175–180 [DOI] [PubMed] [Google Scholar]
- Zubair M, Ishihara S, Oka S, Okumura K, Morohashi K 2006 Two-step regulation of Ad4BP/SF-1 gene transcription during fetal adrenal development: initiation by a Hox-Pbx1-Prep1 complex and maintenance via autoregulation by Ad4BP/SF-1. Mol Cell Biol 26:4111–4121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Yu R, Jameson JL 1997 DAX-1 inhibits SF-1-mediated transactivation via a carboxy-terminal domain that is deleted in adrenal hypoplasia congenita. Mol Cell Biol 17:1476–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer AK, McCabe ER 2004 Molecular mechanisms of DAX1 action. Mol Genet Metab 83:60–73 [DOI] [PubMed] [Google Scholar]
- Babu PS, Bavers DL, Beuschlein F, Shah S, Jeffs B, Jameson JL, Hammer GD 2002 Interaction between Dax-1 and steroidogenic factor-1 in vivo: increased adrenal responsiveness to ACTH in the absence of Dax-1. Endocrinology 143:665–673 [DOI] [PubMed] [Google Scholar]
- Verrijn Stuart AA, Ozisik G, de Vroede MA, Giltay JC, Sinke RJ, Peterson TJ, Harris RM, Weiss J, Jameson JL 2007 An amino-terminal DAX1 (NROB1) missense mutation associated with isolated mineralocorticoid deficiency. J Clin Endocrinol Metab 92:755–761 [DOI] [PubMed] [Google Scholar]
- Yu RN, Ito M, Saunders TL, Camper SA, Jameson JL 1998 Role of Ahch in gonadal development and gametogenesis. Nat Genet 20:353–357 [DOI] [PubMed] [Google Scholar]
- Jeffs B, Meeks JJ, Ito M, Martinson FA, Matzuk MM, Jameson JL, Russell LD 2001 Blockage of the rete testis and efferent ductules by ectopic Sertoli and Leydig cells causes infertility in Dax1-deficient male mice. Endocrinology 142:4486–4495 [DOI] [PubMed] [Google Scholar]
- Swain A, Zanaria E, Hacker A, Lovell-Badge R, Camerino G 1996 Mouse Dax1 expression is consistent with a role in sex determination as well as in adrenal and hypothalamus function. Nat Genet 12:404–409 [DOI] [PubMed] [Google Scholar]
- Swain A, Narvaez V, Burgoyne P, Camerino G, Lovell-Badge R 1998 Dax1 antagonizes Sry action in mammalian sex determination. Nature 391: 761–767 [DOI] [PubMed] [Google Scholar]
- Barbaro M, Oscarson M, Schoumans J, Staaf J, Ivarsson SA, Wedell A 2007 Isolated 46,XY gonadal dysgenesis in two sisters caused by a Xp21.2 interstitial duplication containing the DAX1 gene. J Clin Endocrinol Metab 92:3305–3313 [DOI] [PubMed] [Google Scholar]
- Sperling S, Grimm CH, Dunkel I, Mebus S, Sperling HP, Ebner A, Galli R, Lehrach H, Fusch C, Berger F, Hammer S 2005 Identification and functional analysis of CITED2 mutations in patients with congenital heart defects. Hum Mutat 26:575–582 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.