Abstract
Purpose:
To evaluate the feasibility of developing a novel mini drug pump for ophthalmic use.
Methods:
Using principles of microelectromechanical systems engineering, a mini drug pump was fabricated. The pumping mechanism is based on electrolysis, and the pump includes a drug refill port as well as a check valve to control drug delivery. Drug pumps were tested first on the benchtop and then after implantation in rabbits. For the latter, we implanted 4 elliptical (9.9 × 7.7 × 1.8 mm) non-electrically active pumps into 4 rabbits. The procedure is similar to implantation of a glaucoma seton. To determine the ability to refill and also the patency of the cannula, at intervals of 4 to 6 weeks after implantation, we accessed the drug reservoir with a transconjunctival needle and delivered approximately as low as 1 μL of trypan blue solution (0.06%) into the anterior chamber. Animals were followed up by slit-lamp examination, photography, and fluorescein angiography.
Results:
Benchtop testing showed 2.0 μL/min delivery when using 0.4 mW of power for electrolysis. One-way valves showed reliable opening pressures of 470 mm Hg. All implanted devices refilled at 4- to 6-week intervals for 4 to 6 months. No infection was seen. No devices extruded. No filtering bleb formed over the implant.
Conclusions:
A prototype ocular mini drug pump was built, implanted, and refilled. Such a platform needs more testing to determine the long-term biocompatibility of an electrically controlled implanted pump. Testing with various pharmacologic agents is needed to determine its ultimate potential for ophthalmic use.
INTRODUCTION
Chronic ocular diseases such as glaucoma, uveitis, and age-related macular degeneration are the leading causes of blindness around the world.1 Frequent pharmacologic intervention plays a major role in the management of these diseases.2,3 Successful treatment of an ocular disease requires optimal intraocular concentration of drugs for a sufficient period of time.4 Some of the limitations of current methods of ocular drug delivery include physiologic and anatomic barriers, potential side effects, and poor patient compliance with the drug regimen.5,6 For example, despite the fact that eye drops account for 90% of currently accessible ophthalmic formulations, problems such as rapid drug loss in the tear meniscus and by overflow, toxic effects on ocular surfaces, anatomical constraints, and low intraocular bioavailability (less than 10%) limit the efficacy of such a topical route.7
Systemic medications face constraints similar to those of topical medications. The high systemic doses needed to achieve adequate intraocular therapeutic levels, after passing through the blood-retinal barrier, may result in undesired systemic side effects.1,8 After the US Food and Drug Administration (FDA) approved ranibizumab injection (Lucentis; Genentech, South San Francisco, California) as a treatment for neovascular age-related macular degeneration, intravitreal injections have dramatically increased, becoming one of the most common ophthalmic procedures.9,10 Patient discomfort and potential complications such as intraocular pressure (IOP) elevation, cataract formation, retinal detachment, vitreous hemorrhage, and endophthalmitis are challenges associated with this treatment modality.11 Because of the limitations associated with the current methods of drug delivery (systemic, topical, and intraocular injections), attention has turned to the development of newer systems for delivery of ophthalmic solutions. These include the FDA-approved fluocinolone acetonide intravitreal implant (Retisert; Bausch & Lomb, Rochester, New York) for uveitis and ongoing clinical trials with a host of other biodegradable or nonbiodegradable platforms.3,12 However, a limitation of these drug delivery platforms is that they are eluting. However, these systems provide continuous drug delivery at a set dosage and can be stopped only by explanting the device. Herein, we demonstrated the feasibility of developing a novel programmable ophthalmic mini drug pump to customize drug delivery for each patient. Moreover, the pump is refillable while implanted and hence can deliver a variety of drugs for many years.
METHODS
DEVICE DESCRIPTION
Manually and electrically controlled mini drug pumps were designed, fabricated, and tested using principles of microelectromechanical systems (MEMS) engineering.12,13 The manually and electrically controlled systems share a common layout, including a refillable drug reservoir and a transscleral cannula. The reservoir is implanted subconjunctivally, whereas the cannula is inserted through an incision into either the anterior or posterior segment. Dimensions for this mini drug pump were selected such that the device is easily implanted and stores enough drug to last several months without needing a refill. Biocompatible materials (silicone rubber, Parylene C, and platinum) were used to construct the prototypes.
The manually controlled pump includes a check valve (a one-way valve) to control drug delivery. The pressure-sensitive check valve is located at the tip of the cannula. It opens only when the internal reservoir pressure exceeds the check valve cracking pressure. The valve consists of an orifice sealed against a valve seat. Beyond the cracking pressure, the orifice lifts away from the valve seat, creating a flow path. Once driving pressure is removed, the orifice seals against the valve seat again to prevent back flow into the device. The valve opening and closing is linear from 470 to 2250 mm Hg, and then it reaches a steady-state value. A new valve has been designed for integration with the electrically controlled pump and also includes a high-pressure shut-off feature (ie, a bandpass configuration) to protect against transient pressure spikes.
Once the drug is depleted, the reservoir can be refilled with the same or a different drug. Both pump prototypes are refilled through the silicone rubber reservoir wall; a refill site is not specified. Surgical shams and next-generation pump prototypes include a designated refill port. The ability to refill with a 30-gauge needle while implanted is a novel characteristic of our device that is achieved by the resealing capability of silicone rubber. Repeated ability to refill enables prolonged use of the device for several years. Specifically, silicone rubber membranes perforated up to 24 times in the same location were leak-tight even when subjected to a pressure gradient (230 mm Hg).12,13 The concept of an ocular drug delivery device and its subconjunctival placement is illustrated in Figure 1.
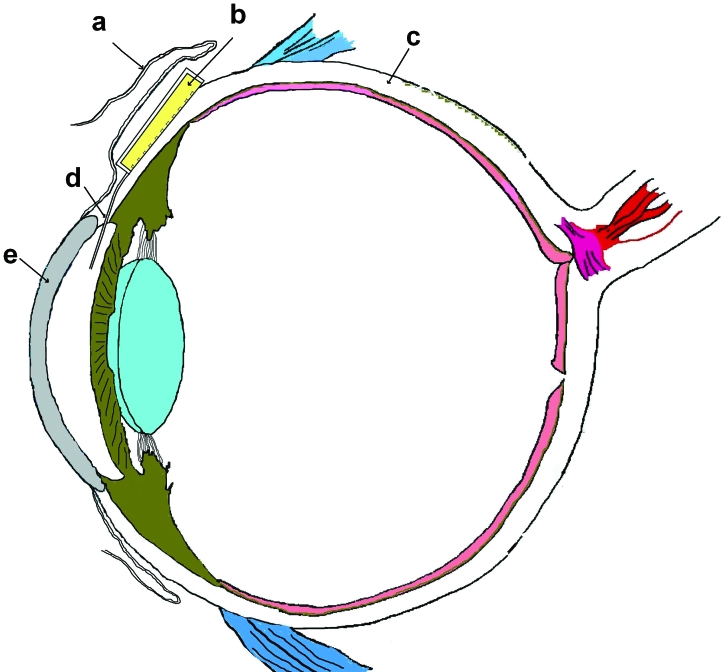
FIGURE 1.
Concept of a refillable ocular mini drug pump showing conjunctiva (a), drug reservoir (b), sclera (c), cannula (d), and cornea (e).
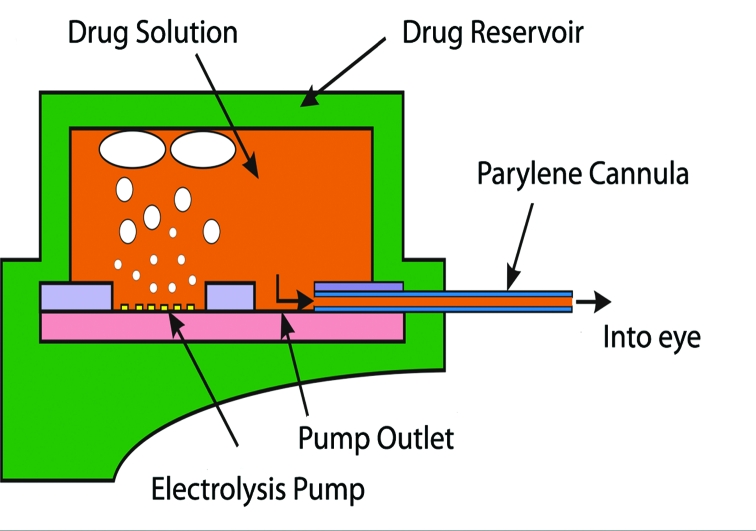
The first prototype mini pumps were designed to dispense drugs into the anterior chamber both manually and electrically, but electrolysis will be the only pumping mechanism used in the next generation of implanted devices. Implanted batteries or wireless inductive power transfer can be used to drive electrolysis. Electrolysis results in the electrochemically induced phase change of water to hydrogen, and oxygen gas generates pressure in the reservoir, forcing the drug through the cannula (Figure 2). Although voltage control is possible, current control is preferred for its direct correlation to the generated gas volume and the fluid flow rate.12 Either continuous or pulsatile delivery into the eye is achieved simply by adjusting the applied current. For example, a pulse of current with a specific magnitude and duration allows a bolus to be delivered; repeated application of this current waveform results in repeated bolus delivery. The length of operation required to deliver a specific dose is determined by both the magnitude and duration of the applied current, which is correlated to the flow rate. For continuous delivery, a constant current is applied to the actuator. Dosing continues until the drug is depleted.
FIGURE 2.
Gas bubble evolution resulting from electrolysis in drug reservoir of packaged electrically controlled device.
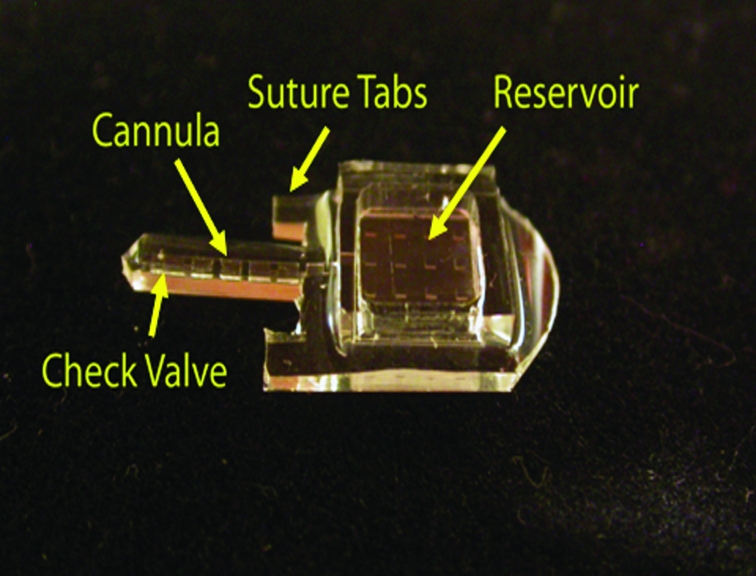
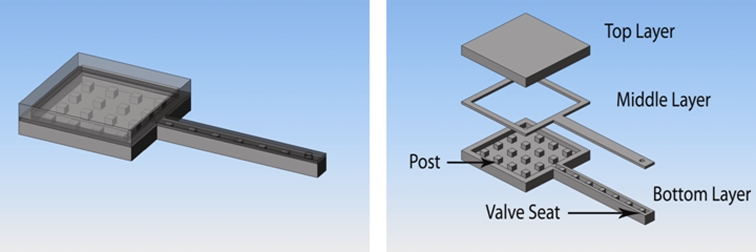
The first-generation manually controlled mini pumps integrate a refillable reservoir (7 × 7 × 1.58 mm), suture tabs, a cannula (1 × 10 mm), and a check valve located at the end of the cannula (Figure 3). The manually controlled pump device consists of three joined pieces of silicone rubber. The top layer defines the chamber for the refillable drug reservoir. The middle layer defines the delivery tube and check valve orifice. The bottom layer forms the base of the device outlining the refillable chamber, delivery tube, and suture tabs. This layer contains posts and the valve seat for the check valve (Figure 4). The individual layers are bonded together after a surface treatment with oxygen plasma for 1 minute. The bonds are reinforced by encapsulation with a thin layer of silicone rubber. The bonded silicone appears homogeneous, and no joined interfaces are visible.
FIGURE 3.
Non-electrically controlled mini drug pump.
FIGURE 4.
Schematic model of first generation manually controlled mini drug pump. 3-D rendered image of the device (left). An exploded view showing the individual layers (right).
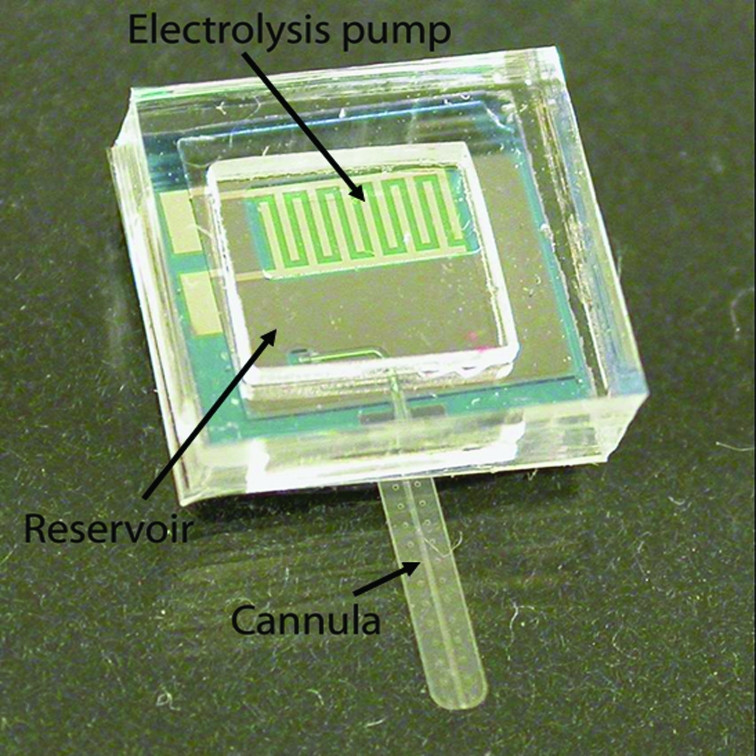
The first electrically active drug pump is similar in layout to the mechanically operated one. It includes an electrolysis pump (a pair of Pt interdigitated electrodes) on a silicon base. The cannula, which is fabricated with two layers of Parylene C to form an embedded micro channel, is also integrated into the base (Figure 5).
FIGURE 5.
Packaged electrically controlled mini drug pump.
BENCHTOP TESTING AND SURGICAL IMPLANTATION
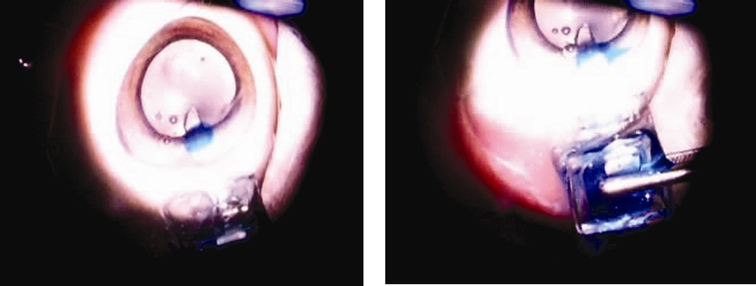
Benchtop and then both ex vivo and in vivo experiments were performed on the mini pumps. To investigate the performance of the electrolysis pump in the electrically controlled pump, experiments examining continuous delivery, bolus delivery, pump efficiency, gas recombination, and back pressure were conducted.12 Preliminary ex vivo surgical modeling in enucleated pig eyes (Siera for Medical Science, Los Angeles, California) was performed, with both manually and electrically controlled pumps, in order to evaluate the effects of surgical handling on device integrity, feasibility of surgical technique, and dispensation of trypan blue solution (0.06%; DORC International BV, The Netherlands) into the anterior chamber. The cannula of the manually controlled drug pump was inserted in the anterior chamber through a ~1.75-mm-wide superior scleral tunnel, 2.5 mm posterior to limbus. The drug delivery tube width measured 1 mm wide to allow a sutureless closure after intraocular insertion. The reservoir was depressed with a blunt forceps, and the check valve opened above the cracking pressure (62 kPa or 470 mm Hg), allowing trypan blue solution to be dispensed from the reservoir into the eye (Figure 6). A normally closed valve design prevented backflow of fluids from the eye into the device. In the electrically controlled pump, the device was packaged and connected to an external power supply with electrical leads. After verification of operation before implantation, the cannula was inserted into the anterior chamber through a 1.5-mm superior temporal limbal incision, followed by application of the current (0.5 mA) to initiate electrolysis. The electrochemically induced phase change of water to hydrogen and oxygen gases elevated the pressure within the reservoir, forcing trypan blue solution through the cannula into the eye. Both devices were repeatedly dispensed and refilled via 30-gauge needles (Hamilton Company, Reno, Nevada) through the side wall.
FIGURE 6.
Trypan blue solution dispensation into anterior chamber in non-electrically controlled mini drug pump after manual pressure with blunt forceps.
For in vivo testing, drug dispensation through both manually and electrically controlled pumps was confirmed with phenylephrine solution (1.5% and 10%, respectively; Akorn, Inc, Buffalo Grove, Illinois) in rabbit eyes. All animal experiments were conducted in accordance with the Association for Research in Vision and Ophthalmology statement for the use of animals in ophthalmic and vision research and were approved by the Institutional Animal Care and Use Committee of the University of Southern California. The higher concentration of phenylephrine was used in the electrically controlled pump to show pupillary dilation according to the calculated flow rate (2.79 ± 0.04 μL/min) in the testing time period. Surgical procedures were identical for both devices. Partial peritomy and fixing of the suture tabs to sclera with 6–0 Vicryl sutures (Ethicon, Inc, Johnson & Johnson Company, Somerville, New Jersey) were performed before manual pump implantation. Under controlled lighting, the pupillary diameter of both eyes was measured in the horizontal and vertical planes to establish the baseline. By gently pressing the reservoir or applying current (0.2 mA) in the manually or electrically controlled pumps, respectively, phenylephrine solution was dispensed into the anterior chamber. Changes in pupillary diameter were recorded every minute for 5 minutes. The left eye was used as a control, with the same dose of phenylephrine introduced by intraocular injection.
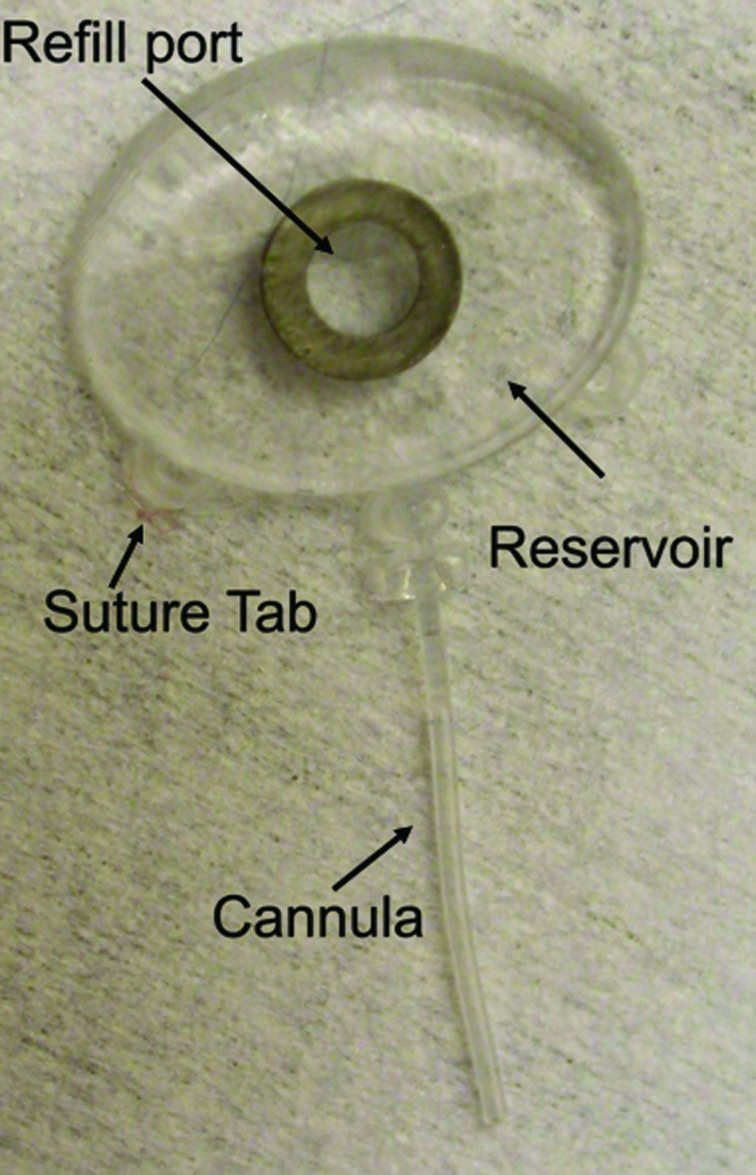
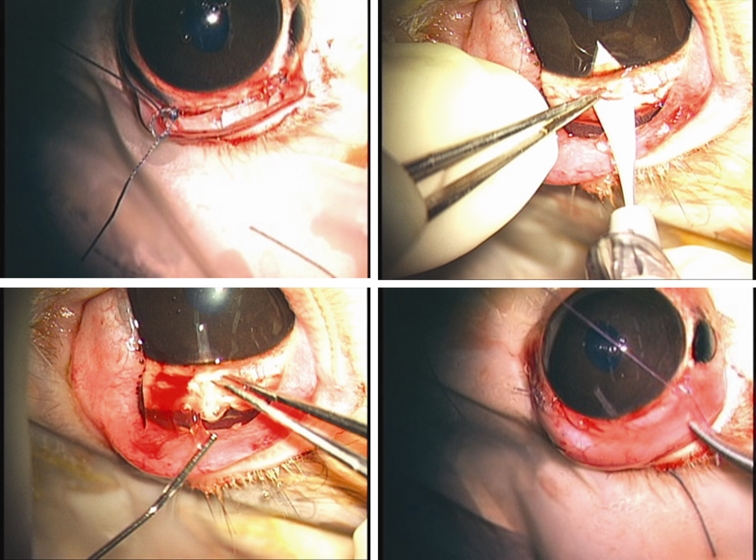
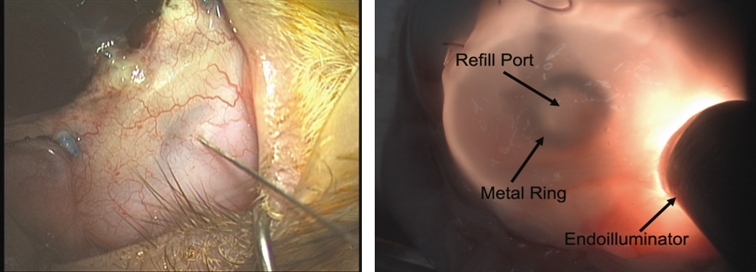
Work is in progress to develop a second-generation electrically active pump for both anterior and posterior segment drug dispensation. To optimize the design, a sham device was fabricated to provide a surgical model for refining the surgical procedure and evaluating the feasibility of refilling procedures. These devices, consisting of an elliptical reservoir with a refill port, a cannula, and suture tabs, have exterior dimensions matching the second-generation pumps (9.9 mm × 7.7 mm × 1.8 mm). Models were fabricated from silicon rubber (Figure 7). The bottom layer of the reservoir includes a sheet of polyetheretherketone (PEEK), which prevents accidental perforation of the globe during refilling (not shown in Figure 7). We implanted sham devices in four rabbits. With the animals under general anesthesia and with due sterility, partial peritomy was performed. A 2-mm-wide scleral tunnel was made in the superior temporal quadrant, 2 mm posterior to the limbus. The tube was inserted into the anterior chamber after fixation of the suture tabs to the sclera with 6–0 Mersiline (Ethicon, Inc, Johnson & Johnson Company) (Figure 8, top left and right, bottom left); if leakage was observed around the tube, interrupted 8–0 Vicryl sutures were used to stop the leakage. Once the device was fixed to the eye, the conjunctiva was appropriately closed with 8–0 Vicryl sutures, making sure the device was completely covered by conjunctiva (Figure 8, bottom right). At the end of surgery, the drug reservoir was refilled via a transconjunctival injection, using a 30-gauge needle to deliver approximately as low as 1 μL of trypan blue ophthalmic solution into the anterior chamber. Topical antibiotic ointment (neomycin-bacitracin-polymyxin; Bausch & Lomb) was instilled in the fornix. To determine the ability to refill the device and the patency of the cannula and drug reservoir, at intervals of 4 to 6 weeks after implantation, refilling and dispensation of dye solution was repeated on a monthly basis for 4 to 6 months. Transillumination was used to confirm refill and to locate the metal refill ring (Figure 9).
FIGURE 7.
Sham mini drug pump.
FIGURE 8.
Surgical implantation of sham devices in rabbits. Top left, Suturing of suture tabs to sclera. Top right, Opening of anterior chamber through scleral tunnel. Bottom left, Insertion of cannula into anterior chamber. Bottom right, Suturing of conjunctiva.
FIGURE 9.
Refilling of sham devices. Left, Transconjunctival refilling of the device using a 30-gauge needle. Right, Localizing of refill ring by transillumination.
FOLLOW-UP EXAMINATIONS
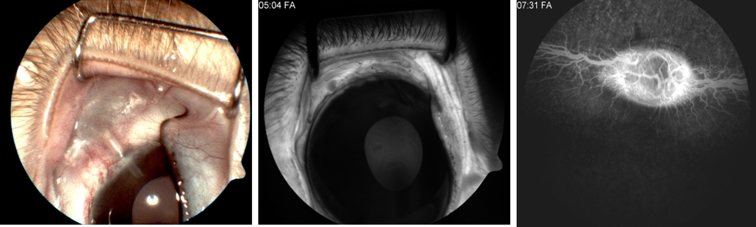
Animals were followed up by slit-lamp examination for anterior chamber evaluation and indirect ophthalmoscope examination for the posterior segment every week. Intraocular pressure was checked with a Tono-Pen (Medtronic, Inc) under topical anesthesia. Diagnostic examinations included monthly anterior segment color photography and fluorescein angiography (FF 450 Plus; Carl Zeiss Meditec, Inc, Dublin, California) under general anesthesia (Figure 10).
FIGURE 10.
Color photography (left) and fluorescein angiography (FA) of the anterior (middle) and posterior (right) segments in a representative rabbit at 6 months.
HISTOPATHOLOGY
At the end of the last follow-up examination, the rabbits were euthanized by intracardiac injection of 2 mL of pentobarbital (Beuthanasia-D; Schering-Plough Animal Health, Omaha, Nebraska). The operative eye and the contralateral eye were enucleated. Each cornea was excised and sectioned into quarters. Each quarter was sectioned into two halves. One half was immersed in Davidson’s fixative solution before paraffin embedding.14 Cross sections (5 μm thick) were stained with hematoxylin and eosin for light microscopy evaluation. The other half was immersed in Karnovsky fixative to be evaluated by scanning electron microscopy for any endothelial damage. After removal of residual fixative and dehydration through a graded series of ethanol solutions, the specimens were sputter coated with a thin layer of silver.15 The prepared specimens were examined using a JEOL JSM-6390 scanning electron microscope (Japan). At least three knowledgeable observers reviewed all slides by masked assessment.
RESULTS
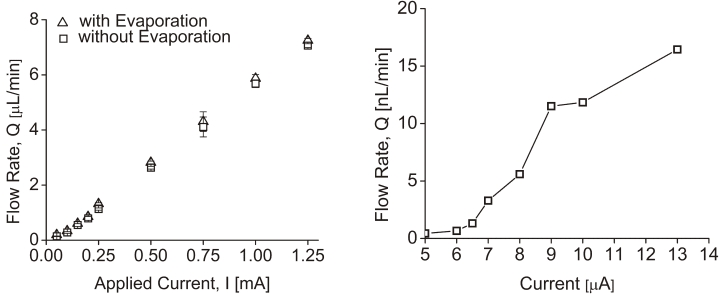
Bolus delivery of trypan blue solution was demonstrated in benchtop experiments and enucleated porcine eyes with the manually controlled surgical models. Bolus and continuous dispensation (pL/min-μL/min) suited for ocular drug delivery, with a fixed rate, was also demonstrated with the electrically controlled device. Benchtop testing showed 2.0 μL/min delivery using 0.4 mW of power for electrolysis. Flow rate was conveniently adjusted by varying the applied current (from 5 μA to 1.25 mA) (Figure 11).
FIGURE 11.
Pump testing in electrically controlled mini drug pump. Left, Current-controlled flow rate after evaporation compensation. Right, Low flow rate operation of the pump. (Reprinted with permission from Elsevier.12)
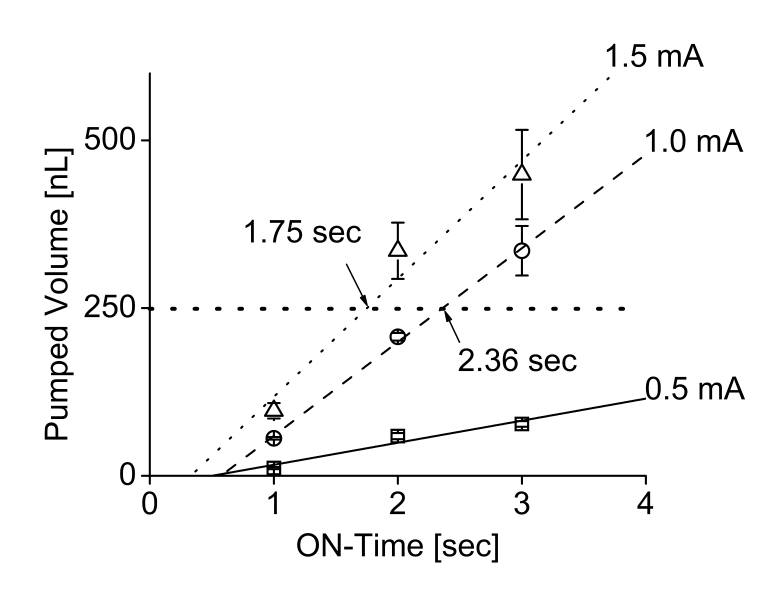
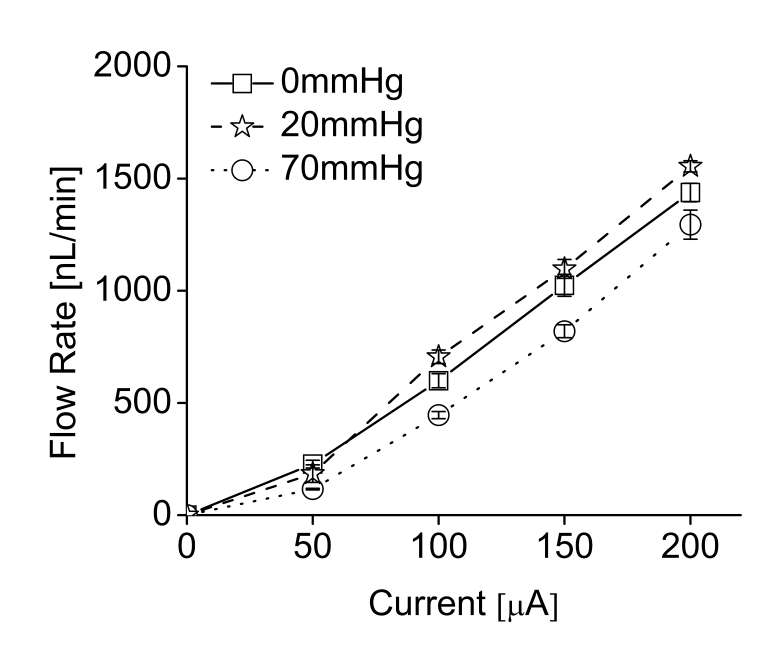
The flow rate ranges obtained experimentally were 438 pL/min at 5 μA to 7 μL/min for 1.25 mA.12 Both data sets were corrected to compensate for the evaporation of fluid during testing. Flow rates below 2 μL/min are recommended for ocular drug delivery. This is consistent with the natural secretion rate of aqueous humor from ciliary body in adults (2.4 ± 0.6 μL/min).12,16 In bolus delivery, if the desired dosing regimen requires 250 nL per dose, this volume can be dispensed by driving the pump for a short duration that is associated with magnitude of the applied current. For example, a 1.0-mA driving current will dispense 250 nL in 2.36 seconds and, for 1.5-mA current, the pulse time can be set as 1.75 seconds (Figure 12).12 The mini ophthalmic pump was able to supply sufficient drug flow (up to 1500 nL/min) against normal and abnormal IOPs. Flow rates varied less than 30% over normal IOP ranges from 5 to 22 mm Hg (mean in humans of 15.5 ± 2.6 mm Hg)16 and abnormal IOPs (0 and 70 mm Hg) (Figure 13).12
FIGURE 12.
Bolus delivery of 250-nL doses using current pulses in electrically controlled mini drug pump. (Reprinted with permission from Elsevier.12)
FIGURE 13.
Flow rate changes in different intraocular pressures in electrically controlled mini drug pump. (Reprinted with permission from Elsevier.12)
Repeated dispensations were demonstrated successfully in both groups. One-way check valves showed reliable opening pressures of 470 mm Hg. Such a high opening pressure would allow refilling of the reservoir without the risk of opening the valve. There was no backflow of fluids from the eye into the device. Reliability of the drug reservoir following multiple refills was confirmed in benchtop experiments, ex vivo (porcine eyes) and in vivo (rabbits). There was no functional damage to the reservoir after multiple refills. In both manually and electrically controlled devices, delivery of 25 μL of phenylephrine solution (1.5% and 10%, respectively) was performed in rabbit eyes and resulted in a real-time pupillary dilation (1.5 mm after 5 minutes). The phenylephrine solution was oxidized, and its color changed to light brown after application of the current for electrolysis; however, activity of discolored drug was verified in a separate control experiment.
Surgical procedures were minimally invasive and well tolerated. The devices were implanted in about 60 minutes. All implanted devices were biocompatible and well tolerated during the 6-month follow-up period. Devices were refilled at 4- to 6-week intervals for a period of 4 to 6 months. Transconjunctival refilling was performed in less than 1 minute without any complications. Anterior chamber depth and IOP were normal in all implanted eyes compared to contralateral eyes, after the surgery and after the monthly refilling. There was no retinal or optical disc damage on indirect ophthalmoscopy examination or fluorescein angiography (Figure 10, right). There was neither leakage around the insertion sites nor filtering bleb formation over the implants. There was no backflow from the anterior chamber into the reservoir. No cornea, iris, or lens damage was seen in slit-lamp examination, anterior color photography, or fluorescein angiography (Figure 10, left, middle). No infection or adverse events were observed. No devices extruded. No occlusions of cannula were noted.
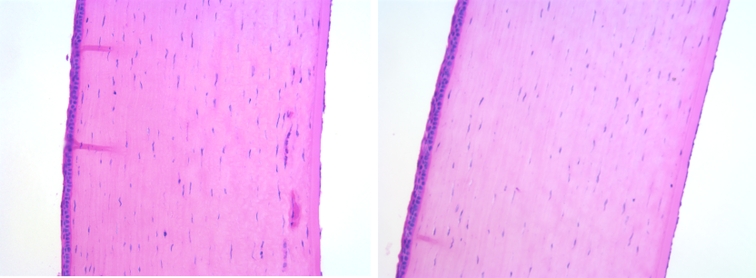
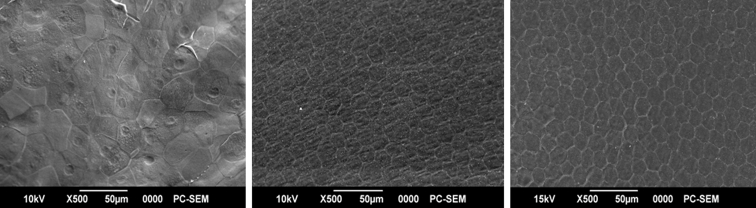
Light microscopic examination of the superior temporal quadrant of cornea after staining with hematoxylin and eosin showed endothelial cell loss close to the tube site (Figure 14). The mean final corneal thickness was unchanged statistically in implanted vs control eyes (mean central corneal thickness in implanted eyes was 0.42 ± 0.02 mm vs 0.41 ± 0.02 mm in control; mean peripheral corneal thickness in implanted eyes was 0.49 ± 0.03 mm vs 0.51 ± 0.02 mm in control). No evidence of corneal edema was seen, and epithelial integrity was uniform. However, examination of the endothelial side of the cornea by scanning electron microscopy revealed changes in density, size, structure, and morphology of endothelial cells in all quadrants of the cornea after 6 months. The mean endothelial cell density in the superior temporal quadrant in implanted eyes was 1348 ± 231 cells/mm2 compared to 4511 ± 177 cells/mm2 in control eyes and 2603 ± 214 cells/mm2 in central cornea vs 4305 ± 202 cells/mm2 in control eyes. The endothelial cell changes improved significantly in one rabbit in which we ceased the refill and dispensation of trypan blue solution at 4 months after surgery (Figure 15). The mean endothelial cell density in the superior temporal quadrant was 4161 ± 278 cells/mm2 compared to 4806 ± 159 cells/mm2 in a control eye and 4326 ± 244 cells/mm2 in central cornea vs 4551 ± 126 cells/mm2 in a control eye after 6 months.
FIGURE 14.
Examination of cornea by light microscopy. Left, Implanted eye with some endothelial cell loss close to the tube site. Right, Normal contralateral eye (hematoxylin-eosin, ×20).
FIGURE 15.
Examination of corneal endothelium by scanning electron microscopy (SEM) after implantation of sham devices for 6 months. Left, Implanted eye. Middle, Normal control eye. Right, Implanted eye after discontinuation of trypan blue solution refilling at 4 months after surgery (white bar = 50 μm).
DISCUSSION
Precise and targeted drug therapy is critical to the management of chronic intraocular diseases such as uveitis, glaucoma, and age-related macular degeneration.2,3 Current routes of ocular treatment have limited efficacy.5,6 Topical and oral therapies have a number of disadvantages resulting from physiological barriers that limit the efficacy of treatment and from adverse systemic side effects caused by larger dose application.5–8,17 Intraocular injections and intravitreal sustained release implants are the current therapy of choice for a variety of ocular disorders,18 since they achieve therapeutic concentrations while bypassing the physiological barriers.19–21 However, the repeated intraocular injections required for long-term therapy are associated with low patient compliance as well as complications such as hemorrhage, retinal detachment, and cataract.11 Sustained-release implants (biodegradable or nonbiodegradable) allow continuous drug release over an extended period.2,3 These devices contain a fixed concentration of drug specially formulated for release at a fixed rate, but after implantation, there is no way to change the drug, its concentration, or its release rate. Nor can these devices be refilled. Once the drug is expended, the implant must be surgically removed and replaced. Sustained-release implants are limited by safety concerns and lack of precision in the erosion rate.4 Of the three types of drug delivery systems (biodegradable or nonbiodegradable implants, implantable pump systems, and atypical implantable systems),22 those that are categorized in either the first or the third group have had limited application. No implantable pump systems for ocular drug delivery have been developed to date because of the inability to make them small or low power.23
The use of MEMS technology allows integration of highly functional electronic and fluidic systems in biomedical platforms for biomedical applications.24–26 The advantages of MEMS fabrication for producing miniaturized and efficient drug delivery systems have already been realized for delivery of insulin and for delivery of bioactive compounds to neural tissues.27,28 MEMS fabrication allows the device to be miniaturized to facilitate surgical implantation and uses materials with a proven biocompatible track record.29,30 To our knowledge, this is the first implantable ocular MEMS mini drug pump that is refillable, enables long-term use, and possesses broad drug compatibility. Furthermore, the device contains a flow control valve and a transscleral cannula for directed delivery in either the anterior or posterior segment while minimizing dose volume and increasing drug bioavailability. These advantages can increase patient comfort and reduce the complications observed with intraocular injections. The system integrates electrolysis pumping for automated and programmable dosing; thus drug delivery can be changed in the future by software, and the loss of efficacy related to poor patient compliance will be minimized. Creation and validation of a prototype were our initial goals. We have not specifically focused on pharmacokinetics of the drugs in this study. Drug stability within an in vivo reservoir depends on drug type; large drug moieties such as antibodies would be expected to precipitate out of solution sooner than smaller drug molecules such as beta blockers. Pump performance and efficiency were demonstrated via pulsatile and continuous delivery in different ranges of back pressure (in the form of IOP) in benchtop testing; however, precise control of delivered volume will be confirmed in vivo after valve integration into the second-generation device. For long-term use and to prevent electrolysis-generated gases from escaping into the eye during delivery, the electrolysis pump and drug reservoir will be separated in second-generation devices. They will be mechanically coupled through a flexible diaphragm to allow pumping. This design prevents oxidation of the drug and its undesirable local pH effects as observed in our phenylephrine solution dispensation experiments.
Other concerns may arise during and after surgical implantation, and these must be considered. Infection may develop, but we did not see this in our animal trial despite monthly transconjunctival refilling. Also, in general, the infection rate of other drug pumps in the body, such as the insulin pump, has been reported with a range of 0.06 to 0.27 infection events per patient per year.31,32 Similar to the preparation for an intraocular injection, the fornix should be sterilized with a drop of 5% povidone-iodine, and aseptic technique should be practiced during refilling of the pump to reduce the risk of any infection. If the check valves become clogged, the elevated pressure generated by electrolysis should easily purge the valve open. Similar to glaucoma seton, proper placement of the tube in the anterior chamber is important to reduce any traumatic damage to the corneal endothelium, iris, pupillary margin, and lens. Careful placement of the tube into the posterior segment will prevent complications such as lens damage and retinal detachment. Corneal endothelial cell damage is one of the adverse complications of glaucoma drainage device implantation such as Ahmed glaucoma valve.33,34 Our histological examinations in implanted eyes showed similar results, although the mechanism is not fully understood. Corneal endothelial cell density was previously assessed after Ahmed glaucoma valve implantation, and mean percentage cell loss from baseline was reported to range from 3.5 % at 1 month to 10.5% at 12 months after surgery.33 Trauma to the endothelium reduces cell density, increases cell size, and disrupts the normal morphological pattern.33 Continued small movements of the tube element relative to the cornea, intraoperative and early postoperative damage caused by macroscopic contact of tube with the cornea in association with poor positioning of a long tube, and anterior chamber shallowing are among the most common causes of this damage.34 These findings indicate that particular care should be taken during the fabrication and implantation of the device to provide a shorter beveled tube with a smoother tip to decrease endothelial damage. In addition to the above hypothesis, as van Dooren and his coworkers35 demonstrated, trypan blue (0.06%) toxicity could be another cause of corneal endothelial cell damage in our study. Generalized endothelial changes in all quadrants of the cornea after monthly refilling and dispensation of trypan blue solution for 6 months and improving of endothelial cells to almost normal shape, size, and density in one of the rabbits (in which we stopped the refilling 4 months after surgery) support this hypothesis as well.36–39 We are undertaking more animal implantations without trypan blue application to further evaluate the degree to which corneal changes were mechanically related to the anterior chamber tube vs chemical toxicity due to trypan blue solution.
In conclusion, a prototype mini drug pump for the eye was built, implanted, and refilled while in place. It provides a platform for more effective drug treatment for chronic ocular diseases because of controlled, programmable drug delivery. Such a platform with integrated valves and electrolysis pump in second-generation devices needs to be tested in vivo to determine its long-term biocompatibility and to test its use with various pharmacologic agents to determine its ultimate potential for ophthalmic use.
ACKNOWLEDGMENTS
Funding/Support: Supported by grants R21EY018490 and Core Grant EY03040 from the National Institutes of Health and grant EEC 0310723 from the National Science Foundation.
Financial Disclosures: Dr Humayun and Dr Varma are consultants for and have equity in Replenish Inc. All authors except Dr Varma stand to get royalty through University of Southern California patents licensed to Replenish Inc.
Author contributions: Design of the study (E.M., M.S.H., R.L., P-Y.L., S.S., R.V.); Conduct of the study (S.S., R.L., P-Y.L.); Management (E.M., M.S.H., R.L., P-Y.L., S.S., R.V.); Preparation, review, or approval of the manuscript (S.S., E.M., M.S.H., R.L., P-Y.L.).
Conformity With Author Information: This study received Institutional Animal Care and Use Committee approval at the University of Southern California.
REFERENCES
- 1.Geroski DH, Edelhauser HF. Drug delivery for posterior segment eye disease. Invest Ophthalmol Vis Sci. 2000;41:961–964. [PubMed] [Google Scholar]
- 2.Hoyng PF, van Beek LM. Pharmacological therapy for glaucoma: a review. Drugs. 2000;59:411–434. doi: 10.2165/00003495-200059030-00003. [DOI] [PubMed] [Google Scholar]
- 3.Hsu J. Drug delivery methods for posterior segment disease. Curr Opin Ophthalmol. 2007;18:235–239. doi: 10.1097/ICU.0b013e3281108000. [DOI] [PubMed] [Google Scholar]
- 4.Shell JW. Ophthalmic drug delivery systems. Surv Ophthalmol. 1984;29:117–128. doi: 10.1016/0039-6257(84)90168-1. [DOI] [PubMed] [Google Scholar]
- 5.Davies NM. Biopharmaceutical considerations in topical ocular drug delivery. Clin Exp Pharmacol Physiol. 2000;27:558–562. doi: 10.1046/j.1440-1681.2000.03288.x. [DOI] [PubMed] [Google Scholar]
- 6.Sultana Y, Jain R, Aqil M, Ali A. Review of ocular drug delivery. Curr Drug Deliv. 2006;3:207–217. doi: 10.2174/156720106776359186. [DOI] [PubMed] [Google Scholar]
- 7.Bourlais CL, Acar L, Zia H, Sado PA, Needham T, Leverge R. Ophthalmic drug delivery systems—recent advances. Prog Retin Eye Res. 1998;17:33–58. doi: 10.1016/s1350-9462(97)00002-5. [DOI] [PubMed] [Google Scholar]
- 8.Metrikin DC, Anand R. Intravitreal drug administration with depot devices. Curr Opin Ophthalmol. 1994;5:21–29. doi: 10.1097/00055735-199406000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Ho QT, Kuo CJ. Vascular endothelial growth factor: biology and therapeutic applications. Int J Biochem Cell Biol. 2007;39:1349–1357. doi: 10.1016/j.biocel.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lloyd R, Harris J, Wadhwa S, Chambers W. Food and Drug Administration approval process for ophthalmic drugs in the US. Curr Opin Ophthalmol. 2008;19:190–194. doi: 10.1097/ICU.0b013e3282f97fa1. [DOI] [PubMed] [Google Scholar]
- 11.Ozkiris A, Erkilic K. Complications of intravitreal injection of triamcinolone acetonide. Can J Ophthalmol. 2005;40:63–68. doi: 10.1016/S0008-4182(05)80119-X. [DOI] [PubMed] [Google Scholar]
- 12.Li P-Y, Shih J, Lo R, et al. An electrochemical intraocular drug delivery device. Sensors Actuators A Phys. 2008;143:41–48. [Google Scholar]
- 13.Lo R, Li P-Y, Saati S, Agrawal R, Humayun MS, Meng E. A refillable microfabricated drug delivery device for treatment of ocular diseases. Lab Chip. 2008;8:1027–1030. doi: 10.1039/b804690e. [DOI] [PubMed] [Google Scholar]
- 14.Agrawal RN, He S, Spee C, Cui JZ, Ryan SJ, Hinton DR. In vivo models of proliferative vitreoretinopathy. Nat Protoc. 2007;2:67–77. doi: 10.1038/nprot.2007.4. [DOI] [PubMed] [Google Scholar]
- 15.Lattanzio FA, Jr, Sheppard JD, Jr, Allen RC, Baynham S, Samuel P, Samudre S. Do injections of 5-fluorouracil after trabeculectomy have toxic effects on the anterior segment? J Ocul Pharmacol Ther. 2005;21:223–235. doi: 10.1089/jop.2005.21.223. [DOI] [PubMed] [Google Scholar]
- 16.Ethier CR, Johnson M, Ruberti J. Ocular biomechanics and biotransport. Annu Rev Biomed Eng. 2004;6:249–273. doi: 10.1146/annurev.bioeng.6.040803.140055. [DOI] [PubMed] [Google Scholar]
- 17.Alward WL. Medical management of glaucoma. N Engl J Med. 1998;339:1298–1307. doi: 10.1056/NEJM199810293391808. [DOI] [PubMed] [Google Scholar]
- 18.Lad EM, Moshfeghi DM. Minimizing the risk of endophthalmitis following intravitreal injections. Compr Ophthalmol Update. 2006;7:277–284. discussion 285–276. [PubMed] [Google Scholar]
- 19.Avery RL, Pearlman J, Pieramici DJ, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113:1695, e1691–1615. doi: 10.1016/j.ophtha.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 20.Bashshur ZF, Bazarbachi A, Schakal A, Haddad ZA, El Haibi CP, Noureddin BN. Intravitreal bevacizumab for the management of choroidal neovascularization in age-related macular degeneration. Am J Ophthalmol. 2006;142:1–9. doi: 10.1016/j.ajo.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 21.Costa RA, Jorge R, Calucci D, Melo LA, Jr, Cardillo JA, Scott IU. Intravitreal bevacizumab (avastin) for central and hemicentral retinal vein occlusions: IBeVO study. Retina. 2007;27:141–149. doi: 10.1097/IAE.0b013e31802eff83. [DOI] [PubMed] [Google Scholar]
- 22.Dash AK, Cudworth GC., 2nd Therapeutic applications of implantable drug delivery systems. J Pharmacol Toxicol Methods. 1998;40:1–12. doi: 10.1016/s1056-8719(98)00027-6. [DOI] [PubMed] [Google Scholar]
- 23.Ambati J, Gragoudas ES, Miller JW, et al. Transscleral delivery of bioactive protein to the choroid and retina. Invest Ophthalmol Vis Sci. 2000;41:1186–1191. [PubMed] [Google Scholar]
- 24.van den Berg A, Lammerink TSJ. Micro total analysis systems: microfluidic aspects, integration concept and applications. Topics Curr Chem. 1997;194:21–49. [Google Scholar]
- 25.Shoji S. Fluids for sensor systems. Topics Curr Chem. 1998;194:163–188. [Google Scholar]
- 26.Vilkner T, Janasek D, Manz A. Micro total analysis systems: recent developments. Analyt Chem. 2004;76:3373–3385. doi: 10.1021/ac040063q. [DOI] [PubMed] [Google Scholar]
- 27.Razzacki SZ, Thwar PK, Yang M, Ugaz VM, Burns MA. Integrated microsystems for controlled drug delivery. Adv Drug Deliv Rev. 2004;56:185–198. doi: 10.1016/j.addr.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 28.Richards Grayson AC, Scheidt Shawgo R, Li Y, Cima MJ. Electronic MEMS for triggered delivery. Adv Drug Deliv Rev. 2004;56:173–184. doi: 10.1016/j.addr.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 29.Rodger DC, Wieland JD, Humayun MS, Tai YC. Scalable high lead-count parylene package for retinal prostheses. Sens Actuators B Chem. 2006;117:107–114. [Google Scholar]
- 30.Wu HK, Huang B, Zare RN. Construction of microfluidic chips using polydimethylsiloxane for adhesive bonding. Lab Chip. 2005;5:1393–1398. doi: 10.1039/b510494g. [DOI] [PubMed] [Google Scholar]
- 31.Phillip M, Battelino T, Rodriguez H, Danne T, Kaufman F. Use of insulin pump therapy in the pediatric age-group: consensus statement from the European Society for Paediatric Endocrinology, the Lawson Wilkins Pediatric Endocrine Society, and the International Society for Pediatric and Adolescent Diabetes, endorsed by the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2007;30:1653–1662. doi: 10.2337/dc07-9922. [DOI] [PubMed] [Google Scholar]
- 32.Ronn B, Mathiesen ER, Vang L, Lorup B, Deckert T. Evaluation of insulin pump treatment under routine conditions. Diabetes Res Clin Pract. 1987;3:191–196. doi: 10.1016/s0168-8227(87)80038-4. [DOI] [PubMed] [Google Scholar]
- 33.Kim CS, Yim JH, Lee EK, Lee NH. Changes in corneal endothelial cell density and morphology after Ahmed glaucoma valve implantation during the first year of follow up. Clin Experiment Ophthalmol. 2008;36:142–147. doi: 10.1111/j.1442-9071.2008.01683.x. [DOI] [PubMed] [Google Scholar]
- 34.Lim KS. Corneal endothelial cell damage from glaucoma drainage device materials. Cornea. 2003;22:352–354. doi: 10.1097/00003226-200305000-00013. [DOI] [PubMed] [Google Scholar]
- 35.van Dooren BT, Beekhuis WH, Pels E. Biocompatibility of trypan blue with human corneal cells. Arch Ophthalmol. 2004;122:736–742. doi: 10.1001/archopht.122.5.736. [DOI] [PubMed] [Google Scholar]
- 36.Gloor B, Gloor ML, Merz-Hill M, Marshall J, Meszaros J, Daicker B. Wound healing of the corneal posterior surface in animal experiments. Klin Monatsbl Augenheilkd. 1986;188:225–230. doi: 10.1055/s-2008-1050617. [DOI] [PubMed] [Google Scholar]
- 37.Ichijima H, Petroll WM, Jester JV, et al. In vivo confocal microscopic studies of endothelial wound healing in rabbit cornea. Cornea. 1993;12:369–378. doi: 10.1097/00003226-199309000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Olsen EG, Davanger M. The healing of rabbit corneal endothelium. Acta Ophthalmol (Copenh) 1984;62:796–807. doi: 10.1111/j.1755-3768.1984.tb05808.x. [DOI] [PubMed] [Google Scholar]
- 39.Van Horn DL, Sendele DD, Seideman S, Buco PJ. Regenerative capacity of the corneal endothelium in rabbit and cat. Invest Ophthalmol Vis Sci. 1977;16:597–613. [PubMed] [Google Scholar]