Abstract
Purpose:
Oxidative damage is implicated in retinal ganglion cell (RGC) death after optic nerve transection (ONT) and in glaucoma. We analyzed the expression and protective effects of thioredoxins (TRXs), regulators of the cellular reduction-oxidative (redox) state, in RGCs damaged by pharmacologically induced oxidative stress, ONT, and elevated intraocular pressure (IOP).
Methods:
ONT and glaucoma models in the rat were used. The glaucoma model was generated in albino rats by intracameral injection of india ink followed by ab externo laser applications to the pigmented trabecular band. Retrograde labeling of RGCs was performed with dextran tetramethylrhodamine. RGC isolation from rat retinas was performed with magnetic beads coated with Thy-1 monoclonal antibody. Immunoblot analysis, RGC-5 culture and transfection, and cell viability assays were used. Gene delivery was performed with in vivo electroporation.
Results:
Endogenous levels of thioredoxin-2 (TRX2) in RGCs after axotomy and in RGC-5 cells after glutamate/buthionine sulfoximine (BSO) treatment were up-regulated. An increased level of TRX-interacting protein (TXNIP) in the retina was observed 2 and 5 weeks after IOP elevation. TRX1 levels were decreased at 2 weeks and more prominently at 5 weeks after IOP elevation. No change in TRX2 levels in response to IOP elevation was detected. Overexpression of TRX1 and TRX2 in RGC-5 treated with glutamate/BSO increased the cell survival by twofold and threefold 24 and 48 hours after treatment, respectively. Overexpression of these proteins in the retina in vivo increased the survival of RGCs by 35% and 135% at 7 and 14 days after ONT, respectively. In hypertensive eyes, RGC loss was approximately 27% after 5 weeks of IOP elevation compared to controls. TRX1 and TRX2 overexpression preserved approximately 45% and 37% of RGCs, respectively, in the glaucoma model compared to controls.
Conclusion:
Thioredoxin overexpression protects RGCs from death after optic nerve axotomy, in pharmacologically induced oxidative stress in vitro and in an animal model of glaucoma.
INTRODUCTION
Oxidative stress has been implicated in neurodegenerative diseases such as amyotrophic lateral sclerosis and Alzheimer, Parkinson, and Huntington disease and has been proposed to be an important factor in retinal ganglion cell (RGC) death after optic nerve transection (ONT), hypoxia, ischemia, axonal transport disruption, and glaucomatous neurodegeneration.1,2 Cell defense mechanisms against oxidative damage involve superoxide dismutase, the glutathione (GSH) system, and the thioredoxin (TRX) systems.
The TRX system is a ubiquitous thiol-reducing system that includes TRX proteins, TRX-interacting protein (TXNIP), TRX reductase (TRXR), and NADPH. TRX proteins, cytoplasmic TRX1 and mitochondrial TRX2, protect against oxidative damage by scavenging intracellular reactive oxygen species (ROS), which leads to their oxidation. The oxidized TRX can be converted back to its reduced form by TRXR in the presence of NADPH. In addition to protection from oxidative stress, TRX proteins perform a variety of biological functions, including regulation of apoptotic cell death.3 TRX1 negatively regulates the ASK1-JNK/P38 apoptotic pathway by binding and inhibiting the kinase activity of ASK1, which plays an important role in ROS-induced cellular responses.4 Oxidative stress leads to dissociation of TRX1 from the ASK1, allowing ASK1 to form a fully activated complex by recruitment of TRAF2 and TRAF6. TRX2 is an essential regulator of ROS levels in mitochondria. TRX2 anti-apoptotic characteristics are associated with the regulation of the pro-apoptotic BCL-XL level and mitochondrial outer membrane permeability.5 The role of TRX2 in cell survival was demonstrated in TRX2-deficient mice and is characterized by massive apoptosis and early embryonic death.6
TRX activity and expression are negatively regulated by TXNIP. TXNIP directly interacts with the catalytic active center of TRX and inhibits the interaction of TRX with other proteins, including the proliferation-associated gene, or ASK-1, causing cells to be more sensitive to oxidative stress.7
The aim of this study was to analyze the involvement of the TRX protein system in RGC degeneration and to evaluate the neuroprotective effects of TRX1 and TRX2 overexpression after pharmacologic induction of oxidative stress, and in ONT and ocular hypertension rat models.8,9
METHODS
The use of animals for this study was approved by the Animal Research Committee of the University of California, Los Angeles, and was performed in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
To generate the ONT model, the optic nerve of the anesthetized adult male Wistar rat was exposed through a lateral conjunctival incision, and the optic nerve sheath was incised 2 mm longitudinally, starting at a point 3 mm behind the globe. A cross section of the optic nerve was made without damaging the adjacent blood supply. A rat ocular hypertension model was generated as previously described.10 Briefly, anesthetized rats were injected intracamerally with 10 μL of 35% india ink in 0.01M PBS. Five days later, approximately 200 laser burns were delivered ab externo to the pigmented trabecular band at diode laser settings of 532 nm, 200 μm diameter, 150 to 200 mW, and 0.20 second duration. Intraocular pressure (IOP) measurements were monitored once a week in the awake state 1 hour after initiation of the dark phase in a 12-hour light, 12-hour dark cycle.
For the ONT models, retrograde labeling to identify RGCs was performed by placing a small piece of Gelfoam soaked with dextran tetramethylrhodamine (DTMR) against the proximal cut surface of the optic nerve after ONT. In the elevated IOP model, the number of RGCs was determined 5 weeks after IOP elevation by retrograde labeling of these cells with DTMR applied to the proximal cut surface of the axotomized optic nerve 48 hours before the animals were sacrificed. RGCs were counted at 1, 2, and 3 mm from the center of the optic nerve in retinal quadrants under fluorescent microscopy at 200× magnification. RGC isolation from adult rat retinas was performed with magnetic beads coated with Thy-1 monoclonal antibody as described previously.11
Immunoblot analysis was carried out as described previously.12 Briefly, 2 to 5 μg of protein was separated on a 12.5% SDS-polyacrylamide gel and transferred to the polyvinylidene membrane. After blocking with 5% nonfat milk, the membranes were incubated with primary polyclonal antibodies against TXNIP, TRX1, TRX2, or β-actin overnight at 4°C and followed by incubation with peroxidase-conjugated secondary antibodies. The signals were visualized with an ECL Plus Detection Kit and quantified with NIH Image software.
RGC-5 cells were maintained in Dulbecco modified Eagle medium containing 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. EGFP-tagged TRX1 and TRX2 expression plasmid DNAs were introduced into the RGC-5 cells with calcium phosphate-mediated transfection. Cells were seeded on the 96-well plate (5 × 103 cells per well) and treated with 5 mM or 10 mM glutamate and 0.5 mM buthionine sulfoximine (BSO). Twenty-four or 48 hours after treatment, cells were incubated with 10 μL of water-soluble tetrazolium salt-1 solution and the absorbance was measured at 450 nm.
Electroporation (ELP)-mediated gene delivery was performed as described previously.13,14 DNA (4 μL; 10 μg) was injected into the vitreous cavity 0.5 mm posterior to the limbus. ELP parameters were as follows: electric field strength of 6 V/cm, pulse duration of 100 ms, and a stimulation pattern of five pulses at a frequency of one pulse per second. After a 10-minute pause, five more pulses with the same parameters were delivered.
Data are presented as the mean ± standard deviation. Differences among groups were analyzed by one-way ANOVA, followed by the Scheffé or Mann-Whitney test. A probability of P < .05 was considered statistically significant.
RESULTS
EXPRESSION OF TRX1, TRX2, AND TXNIP IN THE RETINA AFTER ONT AND IOP ELEVATION AND IN RGC-5 CELLS WITH INDUCED OXIDATIVE STRESS
Immunohistochemical analysis of TXNIP, TRX1, and TRX2 spatial expression showed a similar distribution of these proteins in the untreated rat retina with the most abundant expression evident in the RGC layer, nerve fiber layer, and inner nuclear layer. The majority of TXNIP-, TRX1- and TRX2-positive cells in the ganglion cell layer colocalized with RGCs.
TRX EXPRESSION IN RGC-5 CELLS IN RESPONSE TO OXIDATIVE STRESS
Oxidative stress in RGC-5 cells was induced by glutamate/BSO treatment. BSO is known to reduce the level of GSH with a consequent increase in ROS and activation of apoptotic pathways, whereas glutamate regulates cellular redox status. The effect of the oxidative stress on the level of TRX expression in RGC-5 cells was determined with immunoblotting. An increase in TRX2 (1.7-fold) and TRX1 (1.4-fold) expression was observed 12 and 18 hours after treatment, respectively.
LEVELS OF TRX PROTEINS AFTER ONT
Significant loss of RGCs was observed starting at day 5 after ONT. By day 7 and by day 14 after ONT, approximately 50% and 90% of RGCs were lost, respectively. The levels of TRX1 and TRX2 in whole retinal extracts were not changed significantly after ONT compared to controls. Since western blot analysis of the whole retinal extract may be not sensitive to detect modulation in TRX expression in RGCs (RGCs constitute a small proportion of retinal cells), TRX1 and TRX2 levels were analyzed in purified RGCs. TRX1 levels were elevated approximately 1.4-fold 7 days after ONT, whereas TRX2 expression was increased approximately 1.3-fold and twofold at 1 and 3 days after ONT, respectively.
THE LEVELS OF TRX PROTEINS AFTER IOP ELEVATION
Increased IOP was sustained for 5 weeks, with a maximum of 32.1 ± 7.7 mm Hg at 1 week. The changes in TRX1, TRX2, and TXNIP expression levels induced by IOP elevation were analyzed in whole retinal extracts with immunoblotting. A ~1.5-fold increase in TXNIP expression was observed in retinas 2 and 5 weeks after IOP elevation compared to controls, whereas TRX1 levels were decreased somewhat at 2 weeks and more prominently at 5 weeks. TRX2 levels were not significantly affected by IOP elevation.
THE EFFECT OF TRX1 AND TRX2 OVEREXPRESSION ON RGC SURVIVAL
EGFP-tagged TRX1- and TRX2-expressing plasmids, pEGFP-C1-TRX1 and pCMV-hTRX2-EGFP,5,15 were used to evaluate the cell protective effect of these proteins in response to glutamate/BSO-induced oxidative stress after ONT and IOP elevation.
TRX1 and TRX2 Overexpression Protects RGC-5 cells Against Oxidative Stress
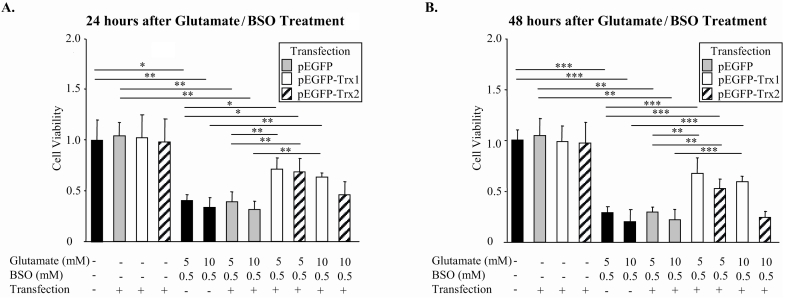
Similar to endogenous TRX proteins, EGFP-tagged TRX1 expression was localized in the cytoplasm, whereas EGFP-tagged TRX2 was colocalized with Mitotracker, indicating its mitochondrial localization. The transfection efficiencies were 67% and 63% for TRX1- and TRX2-expressing plasmids, respectively. To induce dose-dependent oxidative cell death, RGC-5 cells were treated with glutamate/BSO. A significant increase in cell survival was achieved by TRX1 overexpression: approximately twofold 24 hours after exposure to 5 mM or 10 mM glutamate with BSO, 2.5- and 3-fold 48 hours after treatment with 5 mM and 10 mM glutamate/BSO, respectively (Figure 1 A and B). TRX2 overexpression had a significant cell protective effect against 5 mM glutamate with BSO treatment compared to glutamate/BSO-treated nontransfected cells. This effect was not significant when cells were treated with 10 mM glutamate with BSO.
FIGURE 1.
The effect of TRX1 and TRX2 overexpression on RGC-5 survival. Cell protective effect of TRX1 overexpression was observed 24 (A) and 48 (B) hours after treatment with 5.0 or 10.0 mM glutamate and buthionine sulfoximine (BSO) (n = 5–11; *P < .05, **P < .005, ***P < .0005). TRX2 overexpression demonstrated cell protective effects 24 (A) and 48 (B) hours after treatment with 5.0 mM glutamate and BSO (n = 5–11; *P < .05, **P < .005, ***P < .0005).
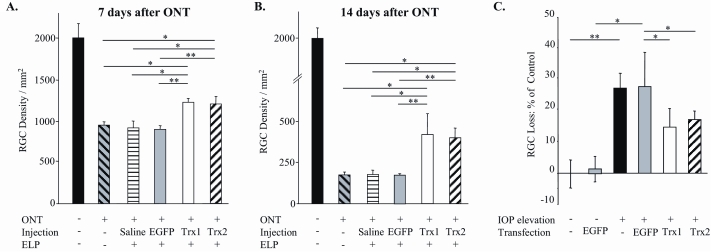
TRX1 and TRX2 Overexpression Increases RGC Survival After ONT. ELP-mediated transfection was used to deliver EGFP-tagged TRX1- and TRX2-expressing plasmids to retinal cells. The RGC transfection efficiency was evaluated by counting EGFP-positive cells colocalized with DTMR-labeled RGCs and the total number of DTMR-labeled RGCs. Approximately 35% of RGCs were transfected with TRX1-EGFP or TRX2-EGFP. The cell protective effect of TRX1 and TRX2 overexpression was evaluated 7 and 14 days after ONT. In 7-day ONT retinas, 1232 ± 43 and 1205 ± 93 RGCs/mm2 were present after TRX1 or TRX2 transfection, respectively, compared to 921 ± 75 cells/mm2 in controls (Figure 2A). In 14-day ONT retinas, 419 ± 125 and 398 ± 58 cells/mm2 remained after TRX1 and TRX2 transfection, respectively, vs 176 ± 27 cells/mm2 in controls (Figure 2B).
FIGURE 2.
The effect of TRX1 and TRX2 overexpression on retinal ganglion cell (RGC) survival after optic nerve transection (ONT) (A and B) and intraocular pressure (IOP) elevation (C). A and B, TRX1 and TRX2 overexpression increased RGC survival by approximately 35% and 135% 1 and 2 weeks after ONT, respectively (n = 4–7; *P < .05, **P < .001). C, Approximately 45% and 37% of RGCs that were destined to die with elevated IOP (compared with controls) were preserved by TRX1 and TRX2 overexpression, respectively (n = 5–14; *P < .05, **P < .005). ELP, electroporation.
TRX1 and TRX2 Overexpression Increases RGC Survival After IOP Elevation. The efficiency of RGC transfection with EGFP-tagged TRX1- and TRX2-expressing plasmids was evaluated as described above by colocalization of EGFP-positive cells with DTMR-labeled RGCs. We observed that RGCs in the nasal retina were consistently more efficiently transfected than in other areas. Therefore, RGCs were counted in the two nasal retinal quadrants. Approximately 44% and 42% of RGCs expressed TRX1-EGFP or TRX2-EGFP, respectively. RGCs constituted approximately 70% of all transfected cells in the GCL. The RGC protective effect of TRX1 and TRX2 overexpression was evaluated 5 weeks after IOP elevation. At this time point the loss of RGCs in nontransfected retinas was approximately 27% compared to controls. RGC loss in EGFP-TRX1 or EGFP-TRX2 transfected retinas was approximately 15% and 17%, respectively, compared to the nontransfected or pEGFP-transfected control eyes (Figure 2C).
DISCUSSION
RGC degeneration after ONT and with glaucoma has been associated with oxidative damage due to increased ROS levels. ROS have direct neurotoxic effects on RGCs and also contribute to secondary degeneration by adversely affecting glial function.16 The current study was initiated to determine the role of TRX proteins, important regulators of the cellular redox state, in protection against ONT- and elevated IOP-induced oxidative RGC injury.
TRX cytoprotective effects were first analyzed in RGC-5 cells treated with glutamate and BSO. Although overexpression of both TRX1 and TRX2 had a cytoprotective effect against oxidative stress induced by these agents, the effect of TRX1 was more potent than that of TRX2. Based on data observed in RGC-5 cells, we analyzed the effect of these proteins on RGC survival after ONT and IOP elevation. ONT shifts the cellular redox state toward oxidation, which may lead to cell death by affecting mitochondrial function or caspase activation.17 The survival of RGCs has been shown to depend on their redox state, and survival was increased by ROS scavengers.18 In our study, TRX1 and TRX2 overexpression increased RGC survival by approximately 35% and 135% at 1 and 2 weeks after axotomy, respectively. A more pronounced effect of TRX proteins at 2 weeks compared to 1 week after ONT may be explained by attenuation of the secondary events associated with increased oxidative damage: RGCs dying early after axotomy may damage neighboring RGCs or lead to activation of microglial cells, which in turn may contribute to secondary RGC degeneration. Induced expression of nitric oxide synthase by injured RGCs and glial cells and subsequent nitric oxide toxicity associated with cellular oxidation has been implicated in RGC death after ONT.19
TRX overexpression also increased RGC survival after IOP elevation. TRX1 and TRX2 preserved approximately 45% and 37% of cells, respectively, compared with controls. We believe that the observed neuroprotective effects of TRX1 and TRX2 could be higher than observed, considering the relatively low efficiency of ELP-mediated RGC transfection with our TRX-expression constructs. Approximately 30% of transfected cells were non-RGCs, including glial cells. Since oxidative stress-induced dysfunction of glial cells has been proposed to play a role in secondary neuronal damage in glaucoma,20 TRX overexpression may decrease the impact of oxidative stress on these cells and thus contribute to RGC survival.
ACKNOWLEDGMENTS
Funding/Support: This study was supported in part by an unrestricted grant from Research to Prevent Blindness, New York, New York. Dr Munemasa was supported by funding from St. Marianna University School of Medicine, Kanagawa, Japan. Dr Piri is supported in part by the Stein Oppenheimer Award, University of California, Los Angeles.
Financial Disclosures: None.
Author Contributions: Design of the study (J.C., Y.M., N.P.); Conduct of the study (Y.M., J.K., N.P.); Collection, analysis and interpretation of data (J.C., Y.M., J.K., N.P.); Preparation and review of the manuscript (J.C., Y.M., J.K., N.P.).
Conformity With Author Information: The use of animals for this study was approved by the Animal Research Committee of the University of California, Los Angeles, and was performed in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
REFERENCES
- 1.Tezel G. Oxidative stress in glaucomatous neurodegeneration: mechanisms and consequences. Prog Retin Eye Res. 2006;25:490–513. doi: 10.1016/j.preteyeres.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar DM, Agarwal N. Oxidative stress in glaucoma: a burden of evidence. J Glaucoma. 2007;16:334–343. doi: 10.1097/01.ijg.0000243480.67532.1b. [DOI] [PubMed] [Google Scholar]
- 3.Masutani H, Ueda S, Yodoi J. The thioredoxin system in retroviral infection and apoptosis. Cell Death Differ. 2005;12:991–998. doi: 10.1038/sj.cdd.4401625. [DOI] [PubMed] [Google Scholar]
- 4.Saitoh M, Nishitoh H, Fujii M, et al. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D, Masutani H, Oka S, et al. Control of mitochondrial outer membrane permeabilization and Bcl-xL levels by thioredoxin 2 in DT40 cells. J Biol Chem. 2006;281:7384–7391. doi: 10.1074/jbc.M509876200. [DOI] [PubMed] [Google Scholar]
- 6.Nonn L, Williams RR, Erickson RP, Powis G. The absence of mitochondrial thioredoxin 2 causes massive apoptosis, exencephaly, and early embryonic lethality in homozygous mice. Mol Cell Biol. 2003;23:916–922. doi: 10.1128/MCB.23.3.916-922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishiyama A, Matsui M, Iwata S, et al. Identification of thioredoxin-binding protein-2/vitamin D(3) up-regulated protein 1 as a negative regulator of thioredoxin function and expression. J Biol Chem. 1999;274:21645–21650. doi: 10.1074/jbc.274.31.21645. [DOI] [PubMed] [Google Scholar]
- 8.Munemasa Y, Kim SH, Ahn JH, et al. Protective effect of thioredoxins 1 and 2 in retinal ganglion cells after optic nerve transection and oxidative stress. Invest Ophthalmol Vis Sci. 2008;49:3535–3543. doi: 10.1167/iovs.08-1716. [DOI] [PubMed] [Google Scholar]
- 9.Munemasa Y, Ahn JH, Kwong JM, et al. Redox proteins thioredoxin 1 and thioredoxin 2 support retinal ganglion cell survival in experimental glaucoma. Gene Ther. 2009;16:17–25. doi: 10.1038/gt.2008.126. [DOI] [PubMed] [Google Scholar]
- 10.Ishii Y, Kwong JM, Caprioli J. Retinal ganglion cell protection with geranylgeranylacetone, a heat shock protein inducer, in a rat glaucoma model. Invest Ophthalmol Vis Sci. 2003;44:1982–1992. [PubMed] [Google Scholar]
- 11.Kwong JM, Lalezary M, Nguyen JK, et al. Co-expression of heat shock transcription factors 1 and 2 in rat retinal ganglion cells. Neurosci Lett. 2006;405:191–195. doi: 10.1016/j.neulet.2006.06.070. [DOI] [PubMed] [Google Scholar]
- 12.Piri N, Song M, Kwong JM, Caprioli J. Modulation of alpha and beta crystallin expression in rat retinas with ocular hypertension-induced ganglion cell degeneration. Brain Res. 2007;1141:1–9. doi: 10.1016/j.brainres.2006.11.095. [DOI] [PubMed] [Google Scholar]
- 13.Dezawa M, Takano M, Negishi H, et al. Gene transfer into retinal ganglion cells by in vivo electroporation: a new approach. Micron. 2002;33:1–6. doi: 10.1016/s0968-4328(01)00002-6. [DOI] [PubMed] [Google Scholar]
- 14.Ishikawa H, Takano M, Matsumoto N, et al. Effect of GDNF gene transfer into axotomized retinal ganglion cells using in vivo electroporation with a contact lens–type electrode. Gene Ther. 2005;12:289–298. doi: 10.1038/sj.gt.3302277. [DOI] [PubMed] [Google Scholar]
- 15.Tanito M, Kwon YW, Kondo N. Cytoprotective effects of geranylgeranylacetone against retinal photooxidative damage. J Neurosci. 2005;25:2396–2404. doi: 10.1523/JNEUROSCI.4866-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thanos S, Mey J, Wild M. Treatment of the adult retina with microglia-suppressing factors retards axotomy-induced neuronal degradation and enhances axonal regeneration in vivo and in vitro. J Neurosci. 1993;13:455–466. doi: 10.1523/JNEUROSCI.13-02-00455.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen SM, Alexejun CN, Levin LA. Amplification of a reactive oxygen species signal in axotomized retinal ganglion cells. Antioxid Redox Signal. 2003;5:629–634. doi: 10.1089/152308603770310293. [DOI] [PubMed] [Google Scholar]
- 18.Geiger LK, Kortuem KR, Alexejun C, Levin LA. Reduced redox state allows prolonged survival of axotomized neonatal retinal ganglion cells. Neuroscience. 2002;109:635–642. doi: 10.1016/s0306-4522(01)00493-6. [DOI] [PubMed] [Google Scholar]
- 19.Koeberle PD, Ball AK. Nitric oxide synthase inhibition delays axonal degeneration and promotes the survival of axotomized retinal ganglion cells. Exp Neurol. 1999;158:366–381. doi: 10.1006/exnr.1999.7113. [DOI] [PubMed] [Google Scholar]
- 20.Tezel G, Wax MB. Glial modulation of retinal ganglion cell death in glaucoma. J Glaucoma. 2003;12:63–68. doi: 10.1097/00061198-200302000-00014. [DOI] [PubMed] [Google Scholar]