Abstract
Purpose:
We report the results of injection of bupivacaine (BUP) and botulinum toxin (BT) into agonist and antagonist muscles, respectively, to treat horizontal strabismus.
Methods:
We treated both horizontal muscles of 7 patients with comitant horizontal strabismus, 2 patients with partial lateral rectus (LR) paralysis, and one elderly myopic patient with acquired esotropia, injecting the agonist muscle with BUP in concentrations of 0.75% to 3.0% and volumes of 3.0 to 5.0 mL, and the antagonist with BT in about half the usual therapeutic dose to prevent it from stretching the BUP-treated muscle during its regeneration following BUP myotoxicity. We reinjected BT in one patient who had an inadequate response from the initial BT dose.
Results:
The 7 comitant patients were corrected (on average) 19.7 prism diopters (Δ), from 28.3Δ to 8.6Δ, at 193 days after injection. Muscle volume increase after BUP injection was 5.8% at 158 days. One LR palsy patient without LR atrophy was changed 55Δ; the other, with LR atrophy, was corrected 4Δ. Two patients had transient vertical deviations from the BT injection. The myopic patient with esotropia was unchanged.
Conclusions:
Injections of BUP and BT corrected 7 patients with comitant horizontal strabismus an average of 19.7Δ, about double the correction reported from BUP injection alone. BUP-injected muscles increased size by 5.8%. Of 2 patients with LR weakness, one without LR atrophy was changed by 55Δ, but another with LR atrophy was corrected only 4Δ.
INTRODUCTION
In addition to having an anesthetic effect, injection of the aminoamide anesthetic bupivacaine (BUP) into the fast muscle fibers, such as those of the extraocular muscles, damages the muscle fibers within minutes, probably by allowing excess Ca2+ ions to enter the cytoplasm from the sarcoplasmic reticulum. This results in separation of the muscle fiber sarcomeres at the Z band.1 The damaged fibers and myocytes are then removed by macrophages, leaving the cell membranes, nerves, and blood vessels intact.2 Within a few hours of exposure to BUP, autocrine growth factor molecules such as insulin-like growth factor 1 and mechano growth factor are released from the damaged area. These molecules activate satellite cells that proliferate over the next 10 to 20 days to form new muscle fibers and myocytes to replace the damaged muscle.3,4 In extraocular muscles, proliferation continues on to build a muscle having greater contractile strength, intrinsic elastic stiffness, and size than before, with consequent effects on eye alignment.5 These biomechanical alterations are not achievable with strabismus surgery. When this muscle response occurs inadvertently after retrobulbar anesthetic injection for eye surgery, misalignment or strabismus is produced.6,7 We are successfully harnessing this muscle response to improve eye alignment and correct strabismus.8,9
We believe that yet another property of extraocular muscles can be manipulated with BUP: the BUP-injected muscle will regenerate to the length at which it is held during the process of regeneration. In the present study, to further increase strabismus correction, we sought to shorten the agonist muscle during regeneration by paralyzing its antagonist with a small dose of botulinum toxin (BT). That induces the BUP-injected muscle to regenerate to a shorter length than if the antagonist were allowed to stretch it out during the time of regeneration.
METHODS
Under a protocol approved by the Smith-Kettlewell Institutional Review Board, we treated 10 patients with injections of both BUP and BT. Seven of these patients had large comitant horizontal deviations or were previously unresponsive to injection of a single drug, either BUP or BT. We also injected the muscles of 2 patients with lateral rectus (LR) paresis in a similar fashion and those of one patient with esotropia from high myopia and a displaced LR muscle who asked to be treated in an attempt to avoid surgery. The averages reported are for the 7 comitant patients and do not include the data from these 3 patients. Using needles insulated except at their tips to record the EMG signal and thus determine the position of the needle tip, we injected BUP in concentrations of 0.75% to 3.0% and volumes of 3.0 to 5.0 mL into the medial rectus in exotropia or the LR in esotropia. Earlier magnetic resonance imaging (MRI) studies showed that BUP diffuses poorly along the muscle,6 so we attempted to fill the whole muscle by injecting most of the BUP in the posterior third and the remainder in the middle of the muscle, allowing some BUP to move anteriorly along the needle track. Simultaneously, we injected the antagonist muscle with BT, using small doses to create mild paresis lasting under a month. Patient 6 had little paralytic effect from the initial BT injection and received a second injection. Examination by standard clinical methods and by MR imaging of the eye muscles was done before injection, immediately afterwards to determine the site of injection, and at intervals thereafter to determine eye alignment, comitance, and eye muscle size changes, as described earlier.5
RESULTS
The Table summarizes the patient details and the results. On average, the comitant patients were corrected 19.7Δ at 193 days after injection. Figures 1 through 3 show the data for eye muscle sizes and eye alignment after injection. Figure 4 shows the stages of events after injection for patient 3. In patient 8, whose LR had 30 gm of active force before injection (normal, 70 gm on the fellow LR) and whose LR was not atrophic, the treatment altered alignment by 55Δ, resulting in an overcorrection requiring surgical recession of the LR at 4 months after injection. At that surgery, the LR active force measured 35 gm and showed stiffness of passive rotation to adduction about twice that of the LR on the normal eye. This small increase in active force is well below that required to change alignment by 45Δ and implies that a reduction in length or increased stiffness of the LR, or both, were responsible for much of the alignment change. Treatment was ineffective for patient 9, who had a severely atrophic LR muscle, and for patient 10, who had high myopia, esotropia, and a displaced LR. Two patients developed a transient hypertropia from medial rectus injection of BT, and two patients developed significant swelling and discomfort for a few days after BUP injections that had a strong weakening effect on the injected muscle.
TABLE.
SUMMARY OF PATIENT DATA*
| PATIENT NO. | BUP | BT | DEVIATION | ||||
|---|---|---|---|---|---|---|---|
| CONC (%) | VOL (ML) | UNITS | FOLLOW-UP (days) | INITIAL DEV (Δ) | FINAL DEV (Δ) | CHANGE (Δ) | |
| 1 | 0.75 | 4.5 | 1 | 146 | 18 | 10 | 8 |
| 2 | 3.0 | 4 | 1.5 | 88 | 14 | 2 | 12 |
| 3 | 3.0 | 3 | 3 | 355 | 16 | 0 | 16 |
| 4 | 0.75 | 4 | 2 | 286 | 20 | 0 | 20 |
| 5 | 0.75 | 4 | 5 | 247 | 40 | 18 | 22 |
| 6 | 3.0 | 4.5 | 7.5 | 160 | 40 | 0 | 40 |
| 7 | 0.75 | 4.5 | 4 | 70 | 50 | 30 | 20 |
| 8 | 1.5 | 3.0 | 2.5 | 56 | 10 | −45 | 55 |
| 9 | 0.75 | 5 | 1 | 44 | 10 | 6 | 4 |
| 10 | 0.75 | 4.5 | 3 | 110 | 30 | 30 | 0 |
BT, botulinum toxin; BUP, bupivacaine.
Patients 1 through 7 had comitant horizontal strabismus. Patients 8 and 9 had lateral rectus paresis, and patient 10 had high myopia and a displaced lateral rectus muscle.
FIGURE 1.
Changes in deviation over time in 7 patients injected with bupivacaine and botulinum toxin. PD, prism diopters.
FIGURE 3.
Changes in the size of the muscles injected with botulinum toxin.
FIGURE 4.
Case 3. Top row, Preinjection, 16Δ exotropia. Second row, 30 minutes after injection, with paralysis of the left medial rectus (LMR). Third row, 13 days after injection, 16Δ exotropia, with paralysis of both the LMR and left lateral rectus. Bottom row, 355 days after injection, eyes are straight.
CASE REPORTS
Case 2
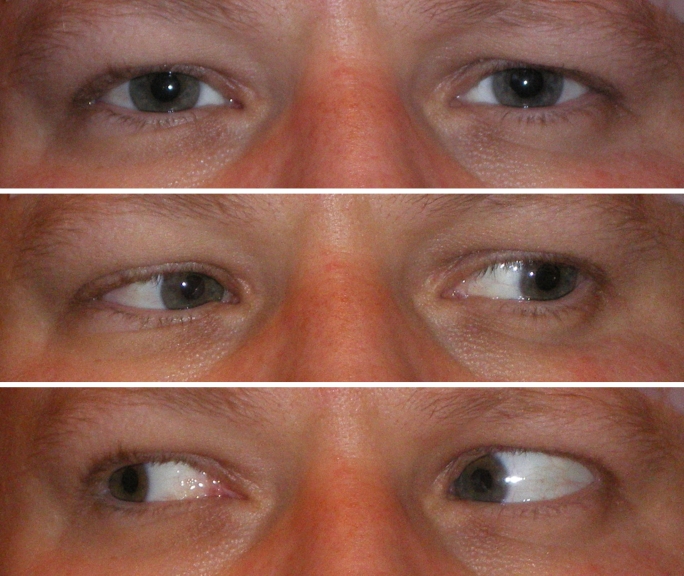
A 35-year-old man had diplopia and difficulty with binocular fusion from 14Δ residual esotropia following surgery for 30Δ esotropia. A first injection of BUP into the right lateral rectus (RLR) markedly weakened abduction and increased the esotropia to 25Δ. On day 76, abduction had fully recovered and the esotropia had returned to 14Δ in all gaze directions. Injection of BUP into the RLR was done again, adding 1.5 units of BT to the right medial rectus (RMR) (see Table). Twenty-one days after this second injection, the eye was straight. Both abduction and adduction were moderately reduced in amplitude, representing moderate paresis of both injected muscles. Thereafter, motility was gradually regained, and on day 88 the eye was 2Δ esotropic in the primary position. MRI scan showed the RLR muscle substantially enlarged in the posterior area. In gaze to the right, the strong RLR retracts the right eye 1 to 2 mm and causes 6Δ exotropia (Figures 5 and 6).
FIGURE 5.
Case 2. Eye rotations 88 days after injection. Bottom photo of patient during right gaze shows retraction and exotropia.
FIGURE 6.
Case 2. Magnetic resonance coronal plane image 88 days after injection, showing local enlargement of right lateral rectus injected with bupivacaine..
Case 6
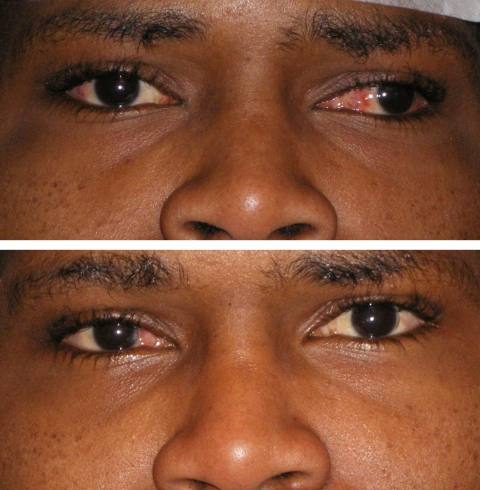
A 26-year-old man had been operated on for 90Δ exotropia by 12-mm recession of each LR and 8-mm resection of the left medial rectus (LMR). A residual 40Δ exotropia remained. The RMR was injected with BUP and the RLR with BT. Five days later adduction was fully paralyzed by the BUP injection and the eye was even further exotropic. The medial conjunctiva was swollen and red for several days. The RLR was only minimally paralyzed by the BT dose used; it was injected with additional BT on day 5, paralyzing the muscle and reducing the exotropia. On day 29, both RLR and RMR were regaining function and the eye was aligned. Full rotations and straight alignment were present on day 160, when the patient was last seen. The RMR is clearly larger than the LMR. Mild swelling of the right eye remains (Figure 7).
FIGURE 7.
Case 6. Top, Before injection, the left eye is still healing from prior surgery. Bottom, 160 days after injection, the eyes are straight.
Case 8
A 62-year-old woman had a pontine hemorrhage in 1994, resulting in a partial RLR paresis. Three horizontal muscle procedures had been done, leaving her with 14Δ esotropia, increasing to 25Δ in right gaze due to abduction weakness. She declined surgical intervention on the left eye. Measured isometric RLR active force was 30 gm, but the RLR was not atrophic on MRI studies. Injection of the RLR with 3.0 mL of 1.5% BUP was done. Seventy days after this injection, the esotropia had improved only to 10Δ esotropia, and eye rotations had returned to the preinjection level. A second injection of 3.0 mL of 1.5% BUP was then done to the RLR with the addition of 2.5 units of BT to the RMR. On day after the second injection, the eye was 10Δ exotropic and there was −3 abduction and −3 adduction, representing moderate paresis of both injected muscles. On day 56, the eye had moved to a position of 45Δ exotropia, where it remained until postinjection day 113. Adduction was limited, but adduction saccades appeared sharp and of normal speed. The patient asked then that the eye be straightened, and a 6.5-mm recession of the RLR was done. At that surgery, the measured RLR isometric active force was 35 gm, only a slight increase above the initial 30-gm force. The RLR stiffness by traction test, however, was estimated to be twice that of the normal left lateral rectus. The RMR isometric active force was 55 gm, within normal limits. The eye measured 5Δ exophoria on postoperative day 7, with a further reduction to zero on postoperative day 138. There is single binocular vision over a useful area.
DISCUSSION
The alignment correction achieved using BUP plus BT was twice what was reported with BUP injection alone.6 Since BT alone has been found ineffective in changing eye alignment in the low doses used, we believe that it acted through the mechanism of temporary paresis of the antagonist, preventing stretch of the agonist as the latter was being rebuilt. The average muscle size increase of 5.8% was very similar to the 6.2% we found from use of BUP alone.5 The decrease in size of the muscles injected by BT is not unexpected but has not been reported for eye muscles. This must play some part in the alignment change, and both alignment and muscle sizes will be monitored further. Cases 2 and 8 show that eye position during regeneration greatly influences the outcome of BUP injection. Each case received minimal effect after BUP alone, but much larger effect after injection of both BUP and BT. Case 6 shows that the BT paralysis must be sufficient to disable the antagonist RLR and prevent it from stretching the RMR muscle injected with BUP. While the total BT fully paralyzed the LR, a correction of 40Δ from a single injection of BT alone would be extraordinary, so we credit the enlargement of the medial rectus as the major factor correcting this deviation.
The specific changes induced in these muscles by BUP are unclear. In treatment of strabismus, strengthening, shortening, and stiffening of the muscle would all be beneficial changes. An increase in myofibrils within the muscle cells would increase size, strength, and stiffness. Intercellular deposition of noncontracting molecules has been postulated to account for the increase in size and stiffness without concomitant increase in force production after BUP injection in rat muscles.10,11 This would likely apply to case 8, in which a large angular correction and increased stiffness of the BUP-injected LR without much increase in muscle strength, suggesting a role for this mechanism as part of the action of BUP injection. In our earlier reports on injecting BUP alone in horizontal strabismus, the stability of the alignment changes and the persistence of muscle enlargement up to 500 days were striking.8,9 Stability seems to be present in these patients, too, although follow-up time is shorter. Orbital inflammation from the muscle damage was the likely cause of the inflammation in case 6. While not a serious or limiting factor in these cases, swelling from injection of multiple muscles or of even one muscle in a compromised orbit with thyroid eye disease might compress the optic nerve.
Case 8 shows that a large correction can be obtained even in a muscle that is partly paralyzed, so long as it is not atrophic. Further, it shows that muscle shortening and stiffening, both desirable in stabilizing strabismus deviations, are major components of the muscle change in addition to muscle strengthening. Patient 9, who had LR paresis with atrophy, received little clinical benefit or change in size of the BUP-injected LR. Perhaps there is a relative absence of satellite cells as a part of the atrophy,12 or perhaps there is a lack of muscle tissue to be injured and thus produce the signaling autocrine proteins. This leaves open the possibility that signaling molecules like mechano growth factor13 given directly, rather than indirectly through the effects of BUP on myofibers, might be useful for these patients. Both clinical and laboratory studies are under way to answer many of these questions.
FIGURE 2.
Changes in the size of the muscles injected with bupivacaine.
ACKNOWLEDGMENTS
Funding Support: This study was supported by the Pacific Vision Foundation and by the S. T. Reeves Foundation.
Financial Disclosures: None.
Author Contributions: Design of the study (A.B.S., J.M.M.); Conduct of the study (A.B.S., J.M.M.); Data analysis (A.B.S., J.M.M., K.R.S.); Preparation, review, or approval of the manuscript (A.B.S., J.M.M., K.R.S.).
Conformity With Author Information: This study was approved by and carried out under the supervision of the Institutional Review Board of the Smith-Kettlewell Eye Research Institute.
Other Acknowledgments: Vanitha Sankaranarayanan, MS, provided important help in data analysis. The authors thank the patients for their willing participation in this study. We thank InSight Health Corporation for their cooperation in supporting this study.
REFERENCES
- 1.Bradley WG. Muscle fiber splitting. In: Mauro A, editor. Muscle Regeneration. New York: Raven Press; 1979. pp. 215–232. [Google Scholar]
- 2.Hall-Craggs EC. Survival of satellite cells following exposure to the local anesthetic bupivacaine (Marcaine) Cell Tissue Res. 1980;209:131–135. doi: 10.1007/BF00219929. [DOI] [PubMed] [Google Scholar]
- 3.Hill M, Wernig A, Goldspink G. Muscle satellite cell activation during local tissue injury and repair. J Anat. 2003;203:89–99. doi: 10.1046/j.1469-7580.2003.00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson BC, Christiansen SP, Grandt S, Grange RW, McLoon LK. Increased extraocular muscle strength with direct injection of insulin-like growth factor-I. Invest Ophthalmol Vis Sci. 2006;47:2461–2467. doi: 10.1167/iovs.05-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott AB, Miller JM, Shieh KR. Bupivacaine injection of the lateral rectus muscle to treat esotropia. J AAPOS. 2009;13:119–122. doi: 10.1016/j.jaapos.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 6.Capo H, Roth E, Johnson T, Munoz M, Siatkowski RM. Vertical strabismus after cataract surgery. Ophthalmology. 1996;103:918–921. doi: 10.1016/s0161-6420(96)30587-3. [DOI] [PubMed] [Google Scholar]
- 7.Hamed LM, Mancuso A. Inferior rectus muscle contracture syndrome after retrobulbar anesthesia. Ophthalmology. 1991;98:1506–1512. doi: 10.1016/s0161-6420(91)32097-9. [DOI] [PubMed] [Google Scholar]
- 8.Scott AB, Alexander DE, Miller JM. Bupivacaine injection of eye muscles to treat strabismus. Br J Ophthalmol. 2007;91:146–148. doi: 10.1136/bjo.2006.110619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott AB, Alexander DE, Miller JM. Bupivacaine injection enlarges eye muscles. Transactions of the 31st European Strabismological Association; Mykonos, Greece. 22–25 May 2007; pp. 177–180. [Google Scholar]
- 10.Rosenblatt JD. A time course study of the isometric contractile properties of rat extensor digitorum longus muscle injected with bupivacaine. Comp Biochem Physiol Comp Physiol. 1992;101:361–367. doi: 10.1016/0300-9629(92)90547-4. [DOI] [PubMed] [Google Scholar]
- 11.Plant DR, Beitzel F, Lynch GS. Length-tension relationships are altered in regenerating muscles of the rat after bupivacaine injection. J Appl Physiol. 2005;98:1998–2003. doi: 10.1152/japplphysiol.01381.2004. [DOI] [PubMed] [Google Scholar]
- 12.Rodrigues Ade C, Schmalbruch H. Satellite cells and myonuclei in long-term denervated rat muscles. Anat Rec. 1995;243:430–437. doi: 10.1002/ar.1092430405. [DOI] [PubMed] [Google Scholar]
- 13.Goldspink G. Changes in muscle mass and phenotype and the expression of autocrine and systemic growth factors by muscle in response to stretch and overload. J Anat. 1999;194:323–334. doi: 10.1046/j.1469-7580.1999.19430323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]