Abstract
Purpose:
The role of T regulatory (Treg) cells in blunting immune response to cancer appears to be significant, but the presence of Treg cells in uveal melanoma has not been extensively examined. We therefore evaluated the presence of tumor-infiltrating Treg cells in uveal melanomas.
Methods:
A retrospective search of Mayo Clinic records from 2000 to 2005 was performed to identify cases of eyes enucleated as a consequence of uveal melanoma. Histologic examination included location of the tumor, presence of emissary canal invasion, direct sclera extension, extraocular extension, cell type and predominant cell type, mitotic figures per 40 high-power fields, lymphocytic tumor invasion, necrosis, microvascular pattern, and presence of CD3, CD4, CD25, and Foxp3cells. Factors obtained by chart review were also evaluated, including clinical size and ultrasound thickness of tumor before enucleation, patient age at time of enucleation, systemic evaluation for metastatic disease both before and after enucleation, monosomy 3, and systemic status at last patient visit.
Results:
Of 42 enucleated eyes, 17 (40.5 %) were found to have lymphocytic infiltrate and 5 (11.9%) were considered positive for the presence of Treg cells (CD3+CD4+CD25+Foxp3+ or CD3+CD4+CD25-Foxp3+). Thus 29.4% (5 of 17) of those with lymphocytic infiltates had Treg cells, and 4 of the 5 with Treg cells had a large lymphocytic infiltrate (>1400 CD3 cells). When using “death due to disease” as the hazard ratio (HR) end point, the HR for presence of CD3 was 5.5 (P = .03) and for clinical size, 1.2 (P = .03). Furthermore, when using “presence of metastasis” as the end point, the HR for presence of CD3 was 3.6 (P = .05) and for clinical size, 1.3 (P = .003).
Conclusion:
Though T lymphocyte infiltration is a bad prognostic indicator, Treg cells are rarely seen in enucleated choroidal melanoma, so their local effect may be limited in contradistinction to other cancers.
INTRODUCTION
Uveal melanoma, the most common primary intraocular malignancy, traditionally has responded poorly to chemotherapy; thus prevention and early detection are hallmarks of therapy.1 Results of the Collaborative Ocular Melanoma Study (COMS) included guidelines to aid in management decisions regarding uveal melanoma.2–5 Some studies have suggested that the presence of tumor-infiltrating lymphocytes is associated with a poor prognosis for uveal melanomas.6,7 In one study,8 tumors containing prominent lymphocytic infiltrate had a higher 15-year mortality when compared to tumors with low lymphocytic infiltrate. Recent studies have evaluated the role of a specific type of cellular immune infiltrate––T regulatory (Treg) cells––which appears to be associated with a worse prognosis in a number of cancers. The role of Treg cells in blunting the body’s immune response to cancerous cells and preventing autoimmune and hypersensitivity reactions has been studied in mouse models and human disease.9–15 Treg cells are considered T cells that are positive for CD4, CD25, and Foxp3.16–18 Foxp3 is generally considered the most reliable marker for Treg cells; however, cells that are CD25+Foxp3− are still considered Treg cells.19 These differences in immunophenotype reinforce the apparent heterogeneity of Treg cells. In cutaneous melanoma, biopsies of lymph nodes that were positive for metastatic disease have shown an increased number of Treg cells when compared to controls.10 In addition, Tregs have been found in the circulation.20,21 The presence of Treg cells in uveal melanoma lymphocytic infiltrates has not been studied. This knowledge may contribute to understanding the role of the host’s immune response in the progression, prognosis, and response to treatment of uveal melanoma.22
The purpose of this study was to demonstrate the presence of tumor-infiltrating Treg cells by means of histologic examination and immunophenotyping of tissue samples from 42 eyes enucleated on account of uveal melanoma.
METHODS
Mayo Clinic Institutional Review Board approval was obtained for this study. A computer-based search of Mayo Clinic patient records from January 2000 to December 2005 was performed to identify all cases of eyes enucleated as a consequence of uveal melanoma. A retrospective review of the patients’ medical records and a histologic review of all specimens were performed. Demographic information from the patients’ charts included patient age, sex, laterality of the enucleated eye (left or right), visual acuity prior to surgery, and follow-up including development of metastases and mortality. The cases were randomly assigned a number to ensure patient confidentiality.
The clinical size of tumor was determined on the basis of the ophthalmic electronic record or prior written record in association with the findings on ultrasound (A-scan and/or B-scan) performed closest to the time of enucleation. In a few cases there was no mention of clinical size in the patient record, and size was then determined by means of drawings and imaging. In all cases ultrasound was performed within 2 months of enucleation. Tumors were categorized according to their location as originating from the posterior choroid, peripheral choroid, or ciliary body. Tumors that straddled the equator were considered to originate from the posterior choroid. One tumor extended from the ciliary body to the peripheral choroid. Any tumor that involved both iris and ciliary body was considered to be a ciliary body tumor.
Systemic evaluation before enucleation consisted of laboratory tests to detect elevated liver enzyme levels (aspartate aminotransferase, alanine aminotransferace, alkaline phosphatase, and gamma–glutamyl transpeptidase), computed tomography (CT), and/or CT/positron emission tomography (PET). If CT scan findings were questionable for metastatic disease, patients underwent liver ultrasound, liver biopsy, or both. In a few cases no preenucleation studies were available in the Mayo Clinic records. Results of chest x-rays and other imaging studies were also reviewed. The presence of liver metastasis was considered metastatic disease. Other organ involvement was considered extensive metastatic disease, because no records showed involvement of other organs without hepatic involvement.
Data on postenucleation systemic evaluation and follow-up were not available for all patients, because some patients ended their care at Mayo after enucleation and elected to have their clinical course followed by their local ophthalmologist or oncologist. Systemic evaluation after enucleation was similar to that done before enucleation. In some cases, where the actual systemic evaluation results were not available, the notes and comments in the ophthalmology records describing the results of the systemic evaluation were substituted for viewing the results of laboratory and imaging tests. The terminology used to describe metastatic disease was the same in preenucleation and postenucleation evaluations.
Last date of follow-up was defined as the last date the patient was seen by or had recorded contact via phone or mail by Mayo healthcare personnel. Each case was assigned to one of the following 6 categories based on follow-up data: alive without metastatic disease (AWOD); alive with metastatic disease (AWD); dead without evidence of metastatic disease (DWOD); dead with evidence of metastatic disease (DWD); lost to follow-up (LTF); and dead metastatic status unknown (DMU). It was assumed that if a patient was found to have metastatic disease and subsequently died, the patient died with metastatic disease. It was also assumed that if a patient’s last systemic workup was negative, the patient was disease-free regardless if dead or alive. The category DMU was used only when a patient was known to have died but no records regarding metastatic evaluation and no comments regarding metastatic disease could be found. The category LTF was used when a patient had not been seen 6 months after enucleation, no record of his or her progress was discovered, and the patient was not known to have died within 6 months of the surgery.
All enucleated eyes had been fixed in neutral buffered formalin for a minimum of 48 hours, submitted to gross examination, and embedded in paraffin for histologic processing. At initial histologic examination, the features recorded for each specimen included the cell type, tumor location, presence of emissary canal invasion, direct scleral extension, extraocular extension, presence of mitotic figures per 40 high-power fields, presence of a tumor-related lymphocytic infiltrate, tumor necrosis, and the type of microvascular pattern.
For this study, immunoperoxidase staining was performed with monoclonal antibodies against CD3 (clone PS1; Novocastra, Newcastle, United Kingdom), CD4 (clone 4B12; Novocastra), CD25 (clone 4C9; Novocastra), and Foxp3 (clone 236A/E7; Abcam/Novus, Cambridge, Massachusetts). All antibodies were applied to 4-μm sections of tissue with standard immunohistochemical techniques, with adequate positive and negative controls.
Cases were reviewed by two of the authors (E.L. and D.R.S.) in the following order: hematoxylin and eosin, control, CD3, CD4, CD25, and Foxp3. Hematoxylin and eosin staining was reviewed for the presence of a tumor-associated lymphocytic infiltrate and any histologic features that were lacking from the surgical pathology reports. CD3 immunostaining was used to confirm the presence of a lymphocytic infiltrate; this was reported as absent, focal (single cluster of lymphocytes), multifocal (multiple clusters of lymphocytes), or diffuse (lymphocytes seen throughout tumor). CD3, CD4, and CD25 stained slides were considered positive if intense membranous staining was seen. Foxp3 was considered positive if intense nuclear staining was seen. Cell counts were obtained by counting individual cells for focal and multifocal infiltrates. For diffuse infiltrates, 3 high-power fields that were representative of the tumor were averaged, then the number of high-power fields with infiltrate was counted and multiplied by the average. The percentage of positive cells for each marker was also determined. Figures 1 and 2 are examples of staining patterns.
FIGURE 1.
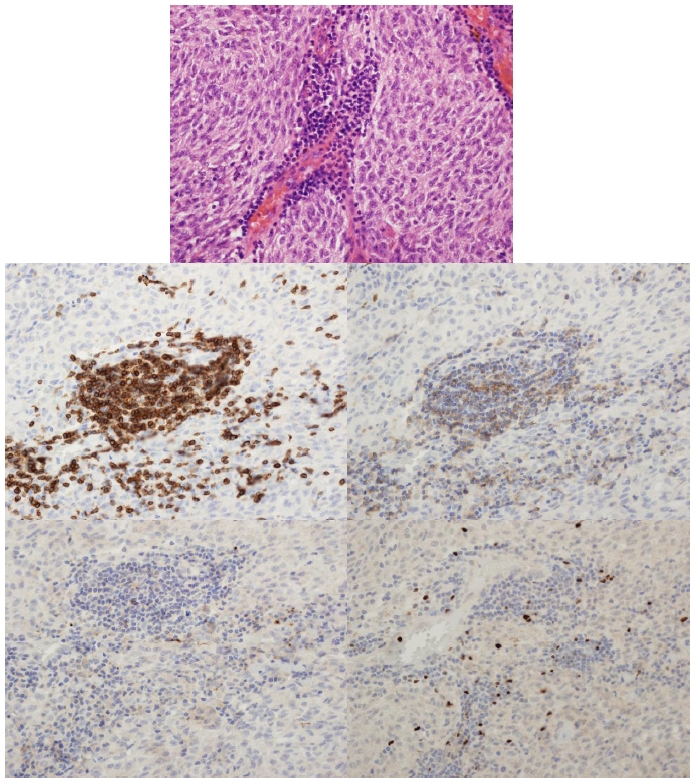
Case 26. Staining patterns (400× magnification): hematoxylin and eosin (top), CD3 immunostain (middle left), CD4 (middle right), CD25 (bottom left), and Foxp3 (bottom right). Note the lack of staining on the CD25 slide.
FIGURE 2.
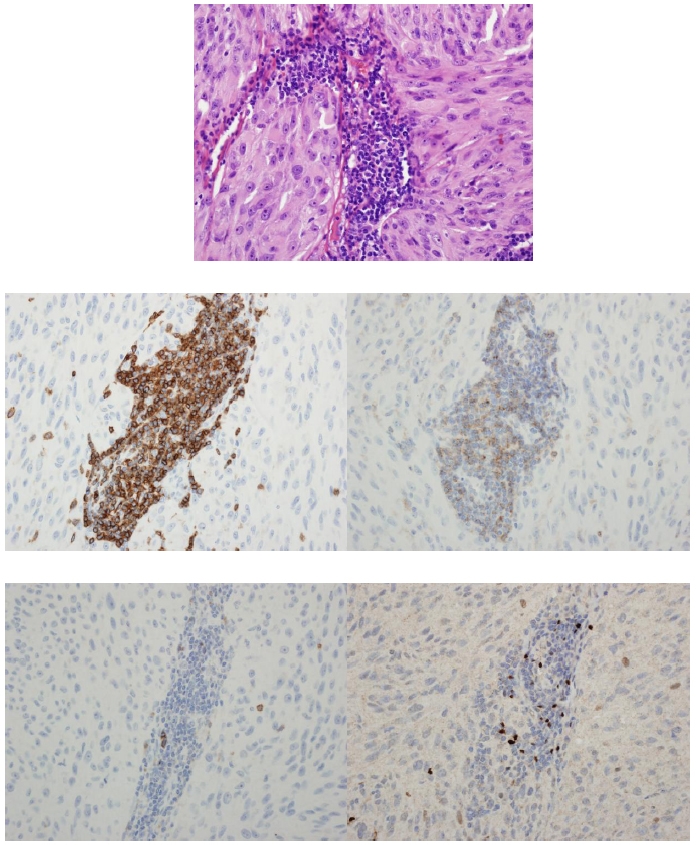
Case 15. Staining patterns (400× magnification): hematoxylin and eosin (top), CD3 immunostain (middle left), CD4 (middle right), Foxp3 (bottom left), and CD25 (bottom right). Note the membranous staining of CD3, CD4, and CD25 as well as the nuclear staining of Foxp3.
One of the authors (E.T.) performed in situ hybridization studies in paraffin-embedded tissue using probes for monosomy 3. Results were reported as positive or negative for monosomy 3 according to the percentage of tumor cells showing this abnormality. The cutoff in our laboratory for a negative result is <23% of cells.
Statistical analysis with chi-square tests was performed to compare groups with categorical variables. Continuous variables were compared between groups using t tests or rank sum tests. Prognosis end points of death and death due to disease or metastasis were estimated using the Kaplan-Meier method. Risk factors for these end points were evaluated using proportional hazard models. Statistical significance was placed at P < .05.
RESULTS
A total of 44 cases of archived paraffin-embedded tissues and glass slides from 44 cases of uveal melanoma at the Mayo Clinic met the criteria. Two of these cases were omitted from the study because of insufficient tissue remaining in the paraffin blocks to perform the additional studies. Of the remaining 42 cases, 20 were left eyes and 22 were right eyes. The specimens were from 21 men and 21 women, whose average age at enucleation was 60.1 years (range, 30–84 years). For men, the average age at enucleation was 65.4 years and for women, 54.9 years.
Of the 42 specimens, tumor-associated lymphocytic infiltrate was found in only 17 (40.5 %). The lymphocytic infiltrate was considered focal in 11 cases, multifocal in 3, and diffuse in 3. It was present in the specimens of 10 of 21 women (48%) and 7 of 21 men (33%); this difference was not statistically significant (P = .34).
The average cell count for CD3+ focal infiltrate was 41.3 (range, 8–84) (see Table). Average cell count for the nonfocal (multifocal or diffuse) CD3 infiltrate was 1214 (range, 204–1680). CD4 positivity was present in 4 of 11 (36%) of the focal group and 4 of 6 (67%) of the nonfocal group. The 2 cases that were positive for CD3 lymphocytic infiltrates, but negative for CD4, were multifocal infiltrates. The average percent of CD3 cells positive for CD4 in the focal group was 31.9% and in the nonfocal group, 17 %. No cells were negative for CD4 and positive for either CD25 or Foxp3. The presence of CD25 was observed in 2 cases with nonfocal infiltrates, and as expected, the percentage of positive CD25 cells was lower than the percentage of positive CD4 cells in these cases (20% CD25+ vs 33% CD4+, and 5% CD25+ vs 7% CD4+). CD25 was not present in any of the cases with focal infiltrates. One case with focal infiltrate had cells positive for Foxp3 (9 total cells, 17.3% of CD3). All 4 cases with nonfocal infiltrates had cells positive for CD4 and also cells positive for Foxp3, with nearly identical percentages. Thus, of the 42 cases, 17 cases had the presence of a tumor-associated lymphocytic infiltrate, and 5 cases were considered positive for the presence of Treg cells (CD3+CD4+CD25+Foxp3+ or CD3+CD4+CD25-Foxp3+). Treg cells were present in 11.9% of all cases studied (5 of 42) and in 29.4% of the tumors that had lymphocytic infiltrate (5 of 17). Of the 5 tumors that were positive for Treg cells, 4 had a large lymphocytic infiltrate (>1400 CD3 cells). There were too few cases with Treg cells to show statistical significance with histologic features.
TABLE.
RESULTS OF IMMUNOSTAINING IN 17 CASES POSITIVE FOR LYMPHOCYTIC INFILTRATE
| CASE NO. | LYMPHOCYTIC INFILTRATION ON HE | CD3 COUNT | CD3 % | CD4 COUNT | CD4 % | CD25 COUNT | CD25 % | Foxp3 COUNT | Foxp3 % |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Focal | 8 | 8 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| 8 | Focal | 45 | 100 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| 9 | Focal | 28 | 100 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| 12 | Focal | 48 | 100 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| 15 | Diffuse and multifocal | 1500 | 100 | 500 | 33.3 | 300 | 20.0 | 500 | 33.3 |
| 18 | Diffuse | 1650 | 100 | 120 | 7.3 | 90 | 5.5 | 100 | 6.1 |
| 19 | Focal | 54 | 100 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| 24 | Multifocal | 204 | 100 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| 25 | Diffuse | 1680 | 100 | 300 | 17.9 | 0 | 0.0 | 290 | 17.3 |
| 26 | Multifocal | 1452 | 100 | 150 | 10.3 | 0 | 0.0 | 150 | 10.3 |
| 27 | Focal | 60 | 100 | 28 | 46.7 | 0 | 0.0 | 0 | 0.0 |
| 28 | Focal | 84 | 100 | 10 | 11.9 | 0 | 0.0 | 0 | 0.0 |
| 30 | Multifocal | 800 | 100 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| 34 | Focal | 22 | 100 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| 38 | Focal | 10 | 100 | 5 | 50.0 | 0 | 0.0 | 0 | 0.0 |
| 41 | Focal | 43 | 100 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| 42 | Focal | 52 | 100 | 10 | 19.2 | 0 | 0.0 | 9 | 17.3 |
HE, hematoxylin and eosin.
When comparing cases with the presence of Treg cells, 2 cases of the Treg-positive tumors were from male and 3 were from female patients. The average age at enucleation for all 42 patients was 60.1 years (range, 30–84 years), whereas the average age at enucleation for patients with Treg-positive tumors was 53.4 years, though this was not statistically significant.
The base dimensions were available or could be calculated for 39 of the 42 eyes. Average base area based on clinical data and measurements was 203 mm2 (range, 36–480 mm2). Average base area for the tumors that were positive for Treg cells was 246.6 mm2 (range, 143–324 mm2). Ultrasound tumor thickness was also available for all patients. Average tumor thickness on ultrasound was 7.7 mm. For Treg-positive tumors, average thickness was 9.7 mm. Average volume for all tumors was 1563 mm3. Average tumor volume for Treg-positive tumors was 2392 mm3. There was a relationship between pathologic size and the presence of CD3 approaching a statistically significant relationship between clinical size and the presence of CD3 cells with larger tumors having CD3 cells present (1983 ± 930 vs 1835 ± 2458; P = .07). The presence of CD4 was significantly related only to clinical size; larger tumors had a greater chance of having CD4-positive cells (2239 ± 539 vs 1807 ± 2183; P = .05). CD25 and Foxp3 relationships were not significant because of the small number of cases with positive cells for these 2 markers.
One patient (case 30) had evidence of metastatic disease before enucleation. This patient had biopsy-proven hepatic and extrahepatic metastasis as well as extraocular extension. On histologic examination, the tumor was mixed-cell type and negative for monosomy 3. There were no Treg cells associated with this tumor. The patient died of metastatic disease
At the time of this data collection, 11 (29%) of the 42 patients were classified as DWD, 20 (47%) as AWOD, 4 (9%) as LTF, 4 (9%) as DWOD, 2 (5%) as DMU, and 1 (2.4%) as AWD. However, the patient classified as AWD had transferred care 2 years previously and could possibly be DWD. Of the cases with Treg-positive cells, 2 were classified as DWD, 2 as AWOD, and one as DWOD. The patient in the DWOD category died of metastatic primary prostate cancer.
Histologically, 13 tumors were spindle-cell type, 22 were mixed-cell type, and 7 were epithelioid. Of the cases with Treg-positive cells, 3 were mixed-cell type, 1 was spindle-cell type, and 1 was epithelioid. Of the mixed-cell type, 9 were classified as AWOD (41%), 7 as DWD (32%), 2 as DWOD (9%), 2 as LTF (9%), 1 as AWD (4%), and 1 as DMU (4%). Of the tumors with spindle-cell type, 8 were classified as AWOD (61%), 2 as DWD (15%), 2 as LTF (15%), and 1 as DWOD (8%). Of the tumors with epithelioid cell type, 3 were classified as AWOD (43%), 2 as DWD (29%), 1 as DMU (14%), and 1 as DWOD (14%). As mentioned, there was no relationship between cell type and the presence of a tumor-associated lymphocytic infiltrate. Extraocular extension was observed in 4 of the 42 cases (9%), including 1 of 5 cases with Treg-positive cells (20%). Two of these patients are AWOD, 1 is DWOD, and 1 is DWD.
Monosomy 3 data was available for 39 cases, and 37 of these tumors showed monosomy 3 by fluorescence in situ hybridization. Three of the 5 Treg-positive tumors had monosomy 3 data; 2 of the 3 (1 spindle-cell type, 1 epithelioid cell type) were positive and 1 was negative (1 mixed-cell type). The patient whose tumor was negative for monosomy 3 was AWOD, and the 2 patients with tumors positive for monosomy 3 were DWD (1 with extrahepatic metastasis).
Twenty-two tumors were located in the posterior choroid (52.4%), 13 involved the ciliary body and peripheral choroid (30.9%), 4 were located in the peripheral choroid (9.5%), and 3 were considered ciliary body tumors (7.1%). Of the 22 patients with posterior choroid tumors, 13 were AWOD (59.1%), 4 were DWD (18.1%), 3 were DWOD (13.6%), 1 was DMU (4.6%), and 1 was LTF (4.6%). Of the 4 patients with peripheral choroid tumors, 1 was DMU (25%), 1 was LTF (25%), 1 was AWOD (25%), and 1 was DWD (25%). Of the 13 patients with tumors located in the ciliary body and peripheral choroid, 4 were AWOD (30.7%), 5 were DWD (38.5%), 2 were LTF (15.4%), 1 was DWOD (7.7%), and 1 was AWD (7.7%).
Overall, the 4-year survival rate was 51% (95% confidence interval [CI], 34%–76%) for all 42 patients in this study. The 4-year survival free of death due to disease was 60% (95% CI, 42%–86%). Survival free of any metastasis at 4 years was 57% (95% CI, 40%–83%).
Using death as an end point, hazard ratios (HRs) for proportional hazard models were calculated for CD3, CD4, CD25, Foxp3, gender, cell type, age, and clinical size. The HR for the presence of tumor-related CD3-positive infiltrates was statistically significant and increased the risk of mortality to 3.0 (P = .04). The HRs for cellular infiltrates positive for CD4, CD25, and Foxp3 were not statistically significant owing to the small numbers of eyes in which these were present (HR, 1.3 [P = .15], 1.8 [P = .68], and 1.3 [P = .66], respectively). Gender, age, pathologic size, and cell type did not show a statistically significant HR. Clinical size (1000-unit increase) had a HR of 1.2 (P = .05). Thus, of the HRs, calculated clinical size and presence of CD3 (lymphocytic infiltrate) were statistically significant, and both were associated with an increased risk of death.
This finding was reinforced when death due to disease was used as the end point for the HR: for presence of CD3 the HR was 5.5 (P = .03) and for clinical size the HR was 1.2 (P = .03). Furthermore, when presence of metastases was used as the end point, the HR for presence of CD3 was 3.6 (P = .05) and the HR for clinical size was 1.3 (P = .003).
DISCUSSION
Our data show that both lymphocytic infiltrate and increased clinical size are associated with a worse prognosis. This finding is consistent with existing literature regarding lymphocytic infiltrate and prognosis.6–8 Also, we noted that Treg cells were not commonly found even in these melanomas that were mainly of the monosomy 3 form, which were the most aggressive. The few that had Treg cells were present primarily in tumors with a large amount of infiltrating CD3-positive cells (80% of Treg-positive tumors were either diffuse or multifocal).
The current understanding of Treg function is prevention of autoimmunity. Tumors are supposed to recruit them to help in down-regulating the immune response to the cancer. However, our data show that with uveal melanomas, Tregs are not common.
One would expect that tumor with Treg infiltrates would tend to grow both faster and larger. It would also be logical to assume that these tumors would present earlier in life than those without Treg+ cells present. In fact, in mouse models, inhibition of Treg cells has been shown to not only aid in tumor regression and improved host response, but also to aid in the prevention of future tumor growth by both direct and indirect means.13 Furthermore, it has recently been hypothesized that if the relative immune compromised state that chemotherapy causes in a host can be counteracted by administration of immune reconstituting agents, the host’s body may have improved response to treatment and be better able to naturally defend against the unwanted invader.23 However, blunting of Treg activity, either by spontaneous mutation or intentional manipulation, is linked to increased autoimmune phenomena, hypersensitivity activity, and IPEX (immunodysregulation, polyendocrinopathy, enteropathy, X-linked) syndrome.12,24–28 In addition, Treg cells have been found in tumors of many cancers.10,13,14,19 In this study, Treg cells were found in 5 of 42 uveal melanomas. This relatively low percentage would tend to point away from the conclusion of a universal mechanism of blunted response via Treg cells at the level of the tumor itself. However, the presence of Treg cells in lymph nodes and in the circulation was not explored in this study because of its retrospective nature, and possibly their presence in those locations is much more universal, as increased percentages of Treg cells have been documented in metastatic melanoma lymph nodes.10
This study had other limitations besides not evaluating the Treg population in lymph nodes and the circulation. This was a retrospective study, and though there was good follow-up, its findings need to be corroborated by larger prospective studies. In addition, of the tumors examined, 5 were considered to be positive for Treg cells. Only two of these tumors stained for CD25, whereas all stained for Foxp3. While multiple reports regarding CD4+CD25+Foxp3− staining cells were found, no reports could be found regarding CD4+CD25-Foxp3+ cells.11,17,19 This finding is probably related to the superiority of Foxp3 when compared to CD25 staining and the difficulty in interpreting the membranous staining because of its similarity to the melanoma pigment. In addition, other studies have used alternative techniques, such as confocal microscopy, for creating merged images for cell counting.19 However, singly immunostained slides, such as the ones used in this study, allow for careful examination of overlapping regions of consecutive or near-consecutive cuts to count cells. Yet, because each stain is located on separate slides, the precision of the percentages reported could be affected, even without cell count being affected. With dual-author review and examination of the number of negatively stained lymphocytes on each slide, the possibility of inaccurate percentages was reduced. Another possible issue is that the small number of tumors that were found to be positive for Treg cells may limit the extrapolation of trends and make it difficult to draw conclusions regarding Treg cells.
In summary, we have corroborated that infiltration of lymphocytes in uveal melanomas is associated with a worse prognosis. In addition, we have shown that infiltration by Treg cells is a rare event even in the tumors with the worst prognosis. Further studies as to why Treg cells are not found in uveal melanomas need to be performed.
ACKNOWLEDGMENTS
Funding/Support: Supported in part by an unrestricted grant from Research to Prevent Blindness Inc, New York, New York, and by NIH grant 1R01CA132734-01A1 from the National Institutes of Health.
Financial Disclosures: None.
Author Contributions: Design and conduct of the study (J.S.P., D.S., R.V.); Collection, management, analysis, and interpretation of the data (E.L., D.S., D.H., E.T.); Preparation, review, or approval of the manuscript (E.L., D.S., R.V., E.T., J.S.P.).
Conformity With Author Information: This study was approved by the Mayo Clinic Institutional Review Board.
REFERENCES
- 1.Markovic SN, Erickson LA, Rao RD, et al. Melanoma Study Group of the Mayo Clinic Cancer Center Malignant melanoma in the 21st century, part 1: epidemiology, risk factors, screening, prevention, and diagnosis. Mayo Clin Proc. 2007;82:364–380. doi: 10.4065/82.3.364. [DOI] [PubMed] [Google Scholar]
- 2.Singh AD, Kivelä T.The collaborative ocular melanoma study Ophthalmol Clin North Am 200518129–142.ix. [DOI] [PubMed] [Google Scholar]
- 3.Diener-West M, Reynolds SM, Agugliaro DJ, et al. Collaborative Ocular Melanoma Study Group Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Arch Ophthalmol. 2005;123:1639–1643. doi: 10.1001/archopht.123.12.1639. [DOI] [PubMed] [Google Scholar]
- 4.Hawkins BS, Collaborative Ocular Melanoma Study Group The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma: IV. Ten-year mortality findings and prognostic factors. COMS report number 24. Am J Ophthalmol. 2004;138:936–951. doi: 10.1016/j.ajo.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Sieving PA. Fifteen years of work: the COMS outcomes for medium-sized choroidal melanoma. Arch Ophthalmol. 2001;119:1067–1068. doi: 10.1001/archopht.119.7.1067. [DOI] [PubMed] [Google Scholar]
- 6.McLean IW, Saraiva VS, Burnier MN., Jr Pathological and prognostic features of uveal melanomas. Can J Ophthalmol. 2004;39:343–350. doi: 10.1016/s0008-4182(04)80004-8. [DOI] [PubMed] [Google Scholar]
- 7.Niederkorn JY, Wang S. Immunology of intraocular tumors. Ocul Immunol Inflamm. 2005;13:105–110. doi: 10.1080/09273940490518586. [DOI] [PubMed] [Google Scholar]
- 8.Singh AD, Shields CL, Shields JA. Prognostic factors in uveal melanoma. Melanoma Res. 2001;11:255–263. doi: 10.1097/00008390-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Barnett BG, Rüter J, Kryczek I, et al. Regulatory T cells: a new frontier in cancer immunotherapy. Adv Exp Med Biol. 2008;622:255–260. doi: 10.1007/978-0-387-68969-2_20. [DOI] [PubMed] [Google Scholar]
- 10.Viguier M, Lemaître F, Verola O, et al. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol. 2004;173:1444–1453. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- 11.Giatromanolaki A, Bates GJ, Koukourakis MI, et al. The presence of tumor-infiltrating FOXP3+ lymphocytes correlates with intratumoral angiogenesis in endometrial cancer Gynecol Oncol 2008110216–221.Epub 2008 Jun 3. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z, Herman AE, Matos M, Mathis D, Benoist C. Where CD4+CD25+ T reg cells impinge on autoimmune diabetes. J Exp Med. 2005;202:1387–1397. doi: 10.1084/jem.20051409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones E, Dahm-Vicker M, Simon AK, et al. Depletion of CD25+ regulatory cells results in suppression of melanoma growth and induction of autoreactivity in mice. Cancer Immunol. 2002;2:1. [PubMed] [Google Scholar]
- 14.Sonabend AM, Rolle CE, Lesniak MS. The role of regulatory T cells in malignant glioma. Anticancer Res. 2008;28:1143–1150. [PubMed] [Google Scholar]
- 15.Beyer M, Schultze JL. Immunoregulatory T cells: role and potential as a target in malignancy. Curr Oncol Rep. 2008;10:130–136. doi: 10.1007/s11912-008-0021-z. [DOI] [PubMed] [Google Scholar]
- 16.Hori S. Rethinking the molecular definition of regulatory T cells. Eur J Immunol. 2008;38:928–930. doi: 10.1002/eji.200838147. [DOI] [PubMed] [Google Scholar]
- 17.Schabowsky RH, Madireddi S, Sharma R, Yolcu ES, Shirwan H. Targeting CD4+CD25+FoxP3+ regulatory T-cells for the augmentation of cancer immunotherapy. Curr Opin Invest Drugs. 2007;8:1002–1008. [PubMed] [Google Scholar]
- 18.Sakaguchi S, Wing K, Miyara M. Regulatory T cells–a brief history and perspective. Eur J Immunol. 2007;37(suppl 1):S116–123. doi: 10.1002/eji.200737593. [DOI] [PubMed] [Google Scholar]
- 19.Siddiqui SA, Frigola X, Bonne-Annee S, et al. Tumor-infiltrating Foxp3−CD4+CD25+ T cells predict poor survival in renal cell carcinoma. Clin Cancer Res. 2007;13:2075–2081. doi: 10.1158/1078-0432.CCR-06-2139. [DOI] [PubMed] [Google Scholar]
- 20.Vence L, Palucka AK, Fay JW, et al. Circulating tumor antigen-specific regulatory T cells in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2007;104:20884–20889. doi: 10.1073/pnas.0710557105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woo EY, Chu CS, Goletz TJ, et al. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–4772. [PubMed] [Google Scholar]
- 22.Sekulic A, Haluska P, Jr, Miller AJ, et al. Melanoma Study Group of the Mayo Clinic Cancer Center Malignant melanoma in the 21st century: the emerging molecular landscape. Mayo Clin Proc. 2008;83:825–846. doi: 10.4065/83.7.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L, Dermawan KT, Jin ML, Xiong SD, Chu YW.Does chemotherapy augment anti-tumor immunotherapy by preferential impairment of regulatory T cells Med Hypotheses 200871802–804.Epub 2008 Aug 8. [DOI] [PubMed] [Google Scholar]
- 24.van der Vliet HJ, Nieuwenhuis EE. IPEX as a result of mutations in FOXP3. Clin Dev Immunol. 2007;2007:89017. doi: 10.1155/2007/89017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. 2008;29:114–126. doi: 10.1016/j.immuni.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Wu K, Bi Y, Sun K, Xia J, Wang Y, Wang C. Suppression of allergic inflammation by allergen-DNA-modified dendritic cells depends on the induction of Foxp3+ Regulatory T cells. Scand J Immunol. 2008;67:140–151. doi: 10.1111/j.1365-3083.2007.02050.x. [DOI] [PubMed] [Google Scholar]
- 27.Kido M, Watanabe N, Okazaki T, et al. Fatal autoimmune hepatitis induced by concurrent loss of naturally arising regulatory T cells and PD-1-mediated signaling Gastroenterology 2008June25[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 28.Brusko TM, Putnam AL, Bluestone JA. Human regulatory T cells: role in autoimmune disease and therapeutic opportunities. Immunol Rev. 2008;223:371–390. doi: 10.1111/j.1600-065X.2008.00637.x. [DOI] [PubMed] [Google Scholar]


