Abstract
Purpose:
To compare outcomes across Trabectome, iScience (canaloplasty), trabeculectomy, and aqueous shunts regarding intraocular pressure (IOP), adjunctive medications, and complications after glaucoma-only and combined glaucoma-phacoemulsification surgeries for open-angle glaucomas.
Method:
A literature review compares success rates, complications, efficacy, and limitations of traditional and novel glaucoma surgical procedures.
Results:
Trabectome and canaloplasty provide modest IOP reduction with minimal intraoperative or postoperative complications. Results of Baerveldt glaucoma implant IOP reduction are comparable to trabeculectomy, but typically this shunt requires more postoperative IOP-lowering medication to achieve a success rate comparable to trabeculectomy.
Conclusion:
Trabeculectomy is still the most effective IOP-lowering procedure performed today but continues to have the highest serious complication rates. Trabectome and canaloplasty are reasonable surgical therapy choices for patients in which IOPs in the mid-teens seem adequate.
INTRODUCTION
Trabeculectomy (traditional glaucoma filtering surgery) is frequently accompanied by numerous short- and long-term complications. These include hypotony, bleb leaks, late blebitis, accelerated cataract progression, choroidal effusions and hemorrhage, and prolonged or permanent visual impairment from hypotony maculopathy. These complications are increased when antifibrotics are used; however, without antifibrotics, trabeculectomy has a relatively high short-term failure rate.1–3 Outcomes of primary trabeculectomy with and without antifibrotics have been compared to medical therapy in the Collaborative Initial Glaucoma Treatment Study (CIGTS).4 Results from that trial indicated that primary trabeculectomy with or without antifibrotics was equivalent to medical therapy for open-angle glaucoma through 5 years of follow-up, with intraocular pressures (IOPs) comparable to those after newer surgical procedures, such as the Trabectome and canaloplasty. Although trabeculectomy provided lower IOPs than laser and medical therapy in the CIGTS, the visual field outcomes were judged to be equivalent. The Fluorouracil Filtering Surgery Study and the ongoing Tube vs Trabeculectomy (TVT) study have provided valid data on trabeculectomy outcomes with antifibrotics in eyes with prior cataract surgery or failed prior filters, with IOP outcomes averaging 14 mm Hg, about 25% lower than reported for newer procedures.5,6
Several novel glaucoma procedures have recently been published or presented, including Trabectome (NeoMedix Corporation, Tustin, California), canaloplasty (iScience Interventional, Menlo Park, California), iStent (Glaukos Corporation, Laguna Hills, California), and a gold suprachoroidal shunt (Solx, Inc, Medway, Massachusetts).7 At this time, published literature is available for clinical outcome data only on Trabectome and canaloplasty.7–11 This report aims to contrast the rationales, published procedures performed, success rates, efficacies, surgical complexities, and complications across these two new procedures compared to trabeculectomy and aqueous shunts. We also suggest that ongoing publications should record specific parameters to facilitate future comparisons among these procedures.
METHODS
A literature review of relevant peer-reviewed publications was undertaken. Outcomes among reported cases were tabulated, including success rates, percent IOP lowering, complications, and medication reliance postoperatively for Trabectome, canaloplasty, trabeculectomy, and Baerveldt glaucoma implant surgeries in which at least 1-year follow-up was reported. Minckler and colleagues9 presented the 3-year results of a retrospective Trabectome case series at multiple sites in the United States and a single site in Mexico at the American Ophthalmological Society (AOS) meeting in 2008. These results were reanalyzed for this report to summarize Trabectome outcomes at 1 year. Canaloplasty 1-year interim results were recently published from a prospective, open-label study at 14 clinical sites in the United States and Germany by Lewis and colleagues10,11 and were analyzed for comparative outcomes. The ongoing TVT study results at 1 year were used for comparison of 1-year outcomes of canaloplasty and Trabectome to trabeculectomy and the Baerveldt glaucoma drainage device.6
Statistical analysis for the update of Trabectome outcomes was identical to those previously reported, including survival curves.9
RESULTS
The average preoperative IOP, 12-month postoperative IOP, and number of glaucoma medications at baseline and 12 months following surgery for Trabectome, canaloplasty, trabeculectomy with mitomycin C and Baerveldt glaucoma drainage device are summarized in Table 1. The mean preoperative IOPs for all groups were similar in the mid-20s. The 12-month postoperative IOPs for the Trabectome group and canaloplasty group were comparable at approximately 16 mm Hg, whereas the IOPs of the trabeculectomy and tube groups were significantly lower at approximately 12 mm Hg. The tube group required more medications to maintain this low IOP as compared to the trabeculectomy group.
TABLE 1.
INTRAOCULAR PRESSURE (IOP) AND MEDICATION USE BEFORE AND 12 MONTHS AFTER GLAUCOMA PROCEDURES
| PROCEDURE | N | IOP (mm Hg) | NO. OF MEDICATIONS | ||
|---|---|---|---|---|---|
| PREOP | 12 mo POSTOP | PREOP | 12 mo POSTOP | ||
| Canaloplasty only | 35 | 24.1 ± 3.9 1 | 16.2 ± 3.5 | 1.9 ± 1.0 | 0.6 ± 0.9 |
| Trabectome only | 102 | 25.7 ± 7.7 | 16.1 ± 3.0 | 2.93 ± 1.29 | 1.50 ± 1.27 |
| Trabeculectomy | 105 | 25.6 ± 5.3 | 12.7 ± 5.8 | 3.0 ± 1.2 | 0.5 ± 0.9 |
| Baerveldt implant | 107 | 25.1 ± 5.3 | 12.4 ± 3.9 | 3.2 ± 1.1 | 1.3 ± 1.3 |
The success rates of the 4 procedures at 12 months postoperatively are summarized in Table 2. The literature reviewed for the canaloplasty procedure did not offer a success rate for canaloplasty as a single procedure, but rather included combined canaloplasty and phacoemulsification procedures as well. The highest success rate of the 4 procedures was quoted for the tube shunt, which had a 96% success at 1 year postoperatively. The definition of success varied between the groups and is listed in Table 2.
TABLE 2.
QUALIFIED SUCCESS RATE ONE YEAR POSTOPERATIVELY FOR FOUR GLAUCOMA PROCEDURES*
| PROCEDURE | DEFINITIONS OF QUALIFIED SUCCESS | SUCCESS RATE |
|---|---|---|
| Canaloplasty only | Percentage of patients at or below target values of 21, 18, and 15 mm Hg at 3, 6, and 12 months, respectively | Not reported |
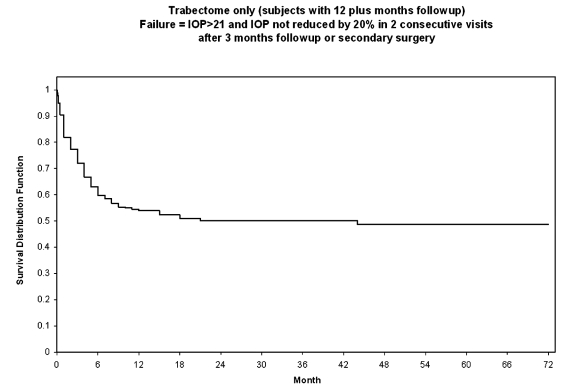
| Trabectome only | No additional glaucoma surgery (1268 of 1415 with no additional glaucoma procedures over 5 years follow-up) (Source: company database 4/7/09) Kaplan-Meier survival analysis at 1 year (Trabectome-only; Figure 5): Failure = IOP >21 or not reduced by 20% below baseline on 2 consecutive visits after 3 months follow-up and no additional glaucoma surgery Kaplan-Meier survival analysis at 1 year (combined Trabectome-phacoemulsification; Figure 6): Failure = IOP > 21 or not reduced by 20% below baseline on 2 consecutive visits after 3 months follow-up and no additional glaucoma surgery |
89.6% 55% 95% |
| Trabeculectomy | IOP <21 mm Hg and >20% below baseline on 2 consecutive follow-up visits, and no additional glaucoma surgery | 86.5% |
| Baerveldt aqueous shunt | IOP <21 mm Hg and >20% below baseline on 2 consecutive follow-up visits, and no additional glaucoma surgery | 96% |
IOP, intraocular pressure.
Patients on or off medications and no additional surgery.
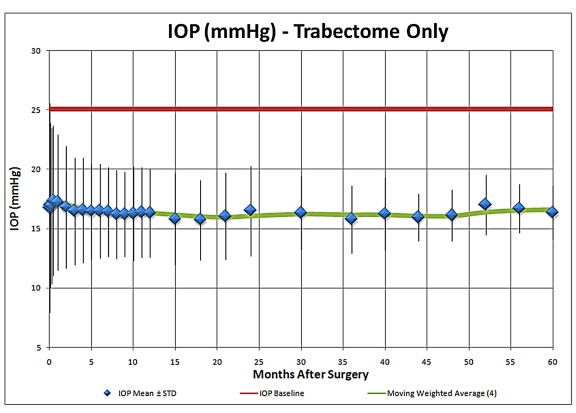
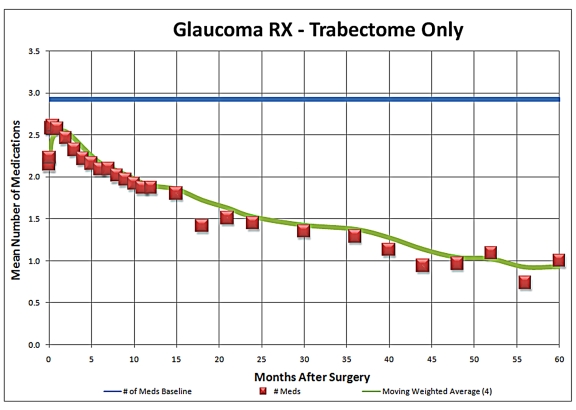
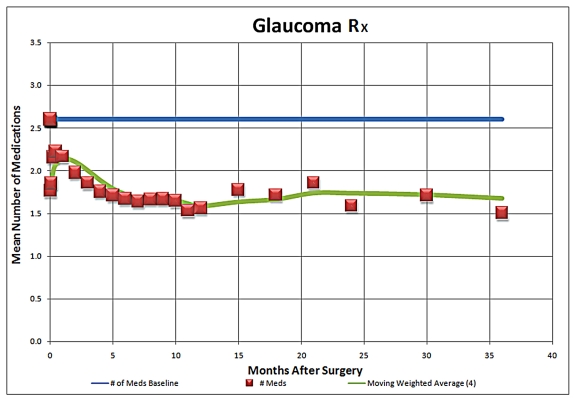
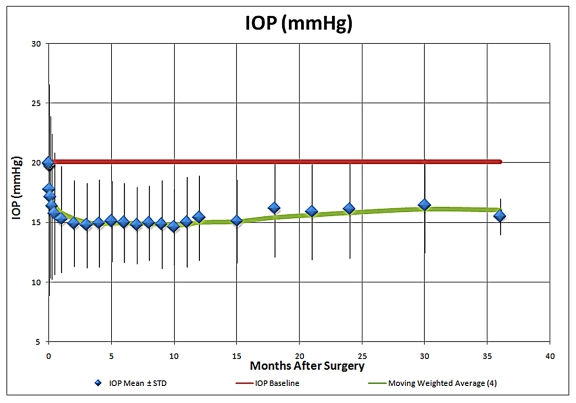
As an update to the Trabectome presentation at the 2008 AOS meeting, the 5-year IOP results compared to mean preoperative IOP for Trabectome-only cases are summarized in Figure 1. This analysis suggests that the IOP-lowering effect of the procedure appears to be sustained at 5 years postoperatively and stabilizes typically in the mid-teens, with the use of fewer IOP-lowering medications, as is illustrated in Figure 2. Average IOP-lowering medication use was reduced from approximately 2.5 medications to approximately one medication at 5 years postoperatively. The medication reliance and IOP-lowering effect of combined Trabectome-phacoemulsification cases also appeared to be sustained at 5 years (Figures 3 and 4).
FIGURE 1.
Intraocular pressure (IOP) during 5-year follow-up in Trabectome-only cases (N = 1287).
FIGURE 2.
Adjunctive medication use during 5-year follow-up in Trabectome-only cases (N = 1287).
FIGURE 3.
Intraocular pressure (IOP) during 5-year follow-up in Trabectome-phacoemulsification cases (N = 687).
FIGURE 4.
Adjunctive medication use during 5-year follow-up in Trabectome-phacoemulsification cases (N = 687)
Kaplan-Meier survival analyses for Trabectome-only surgeries and combined Trabectome-phacoemulsification procedures were performed and are shown in Figures 5 and 6, respectively. For theses analyses, failure was defined as IOP of greater than 21 mm Hg and not reduced by 20% from preoperative levels in two consecutive visits after 3 months of follow-up or secondary glaucoma surgery.
FIGURE 5.
Survival curve for Trabectome-only cases (N = 1287).
FIGURE 6.
Survival curve for combined Trabectome-phacoemulsification cases (N = 687).
TRABECTOME
The Trabectome surgical device was cleared by the US Food and Drug Administration in January 2004 for the treatment of adult and juvenile open-angle glaucoma. The concept is similar in principle to ab interno trabeculotomy, the key difference being that a microelectrocautery device is used to ablate a strip of the trabecular meshwork and inner wall of Schlemm’s canal, thus allowing direct access of aqueous to the collector channels. This theoretically bypasses the main site of resistance to aqueous outflow and reestablishes the natural drainage passageway out of the eye. Briefly, a 1.7-mm clear corneal incision is made temporally, through which the electrosurgical handpiece is inserted and advanced to the nasal angle under gonioscopic visualization. The tip of the device is inserted into Schlemm’s canal through the trabecular meshwork, and the cautery is then activated via a foot pedal. The handpiece is advanced while cautery is activated to ablate an arc of the meshwork, exposing the back wall of Schlemm’s canal, usually for 90° to 120°. A modified Swan-Jacob goniolens (Ocular Instruments Inc, Bellevue, Washington) was developed for this procedure. The handpiece is then removed, and a single 10-0 suture is placed through the incision. This procedure has also been combined with microincisional phacoemulsification, and the IOP-lowering effect of the combined procedure has been previously reported to be 30% at 1 year in a study of 304 consecutive eyes.8,9
The main complication reported in the use of the Trabectome is transient hyphema (79% to 100%). There have been no reports of choroidal effusions, infections, or other permanent visual impairment. The potential advantages of this procedure are that it does not involve manipulation of the conjunctiva, the Tenon capsule, or sclera and therefore preserves the option for subsequent standard filtering surgery if necessary. There is also no risk of bleb-related complications such as leaks, blebitis, endophthalmitis, dellen, and dysesthesia, as there is no bleb formation. Hypotony has been rare, most likely if an inadvertent cyclodialysis occurs. The IOP-lowering effect after several months appears to stabilize in the mid-teens through 5 years of follow-up (n = 10).9 After Trabectome, the majority of eyes require a reduced number of IOP-lowering medications. As a brief update on Trabectome, data now include IOP and medication outcomes on a total of 2,012 surgeries, including 1,228 Trabectome-only (Figures 1 and 2) and 687 combined Trabectome-phacoemulsification surgeries (Figures 3 and 4), continuing to demonstrate that clinically significant IOP and adjuvant medication reliance decrease follow these surgeries. Updated survival analyses for these two groups are also encouraging, especially for the combined cases (Figures 5 and 6).
CANALOPLASTY
Canaloplasty is another new angle surgical procedure that involves conjunctival incision, fashioning a trabeculectomy-like scleral flap and a smaller deeper flap, which is excised to expose Schlemm’s canal.10 A flexible microcatheter with fiber optic illumination at the distal tip is introduced into the canal and advanced for 360° until visible opposite the entrance site. Viscoelastic is continually injected via the catheter lumen and Luer Lock connector, which facilitates controlled infusion of viscoelastic material. A 10-0 Prolene suture is fastened to the distal catheter tip and then pulled back around the canal while the catheter is withdrawn. The suture is then tensioned by hand to distend the canal inward. The superficial flap is then closed watertight, followed by secure closure of the conjunctiva. An ultrasound device can be used to verify tensioning and stretching of the canal, if desired.
Canaloplasty has also been successfully combined with small-incision phacoemulsification. Shingleton and colleagues11 have recently reported IOP lowering from a baseline of 24.4 mm Hg to 13.7 mm Hg with 12-month follow-up in a prospective study of 54 eyes undergoing combination canaloplasty and cataract extraction. Benefits of this procedure over traditional glaucoma surgery include fewer complications, as it is not intended to create a bleb, therefore minimizing bleb-related complications. The most frequent complication reported is hyphema, with an incidence of 3.2%. It should be noted that of 94 subjects enrolled in the study reported by Lewis and colleagues,10 21% of them did not achieve successful 360° canalization, and an additional 12% developed a bleb at some point during the 12-month follow-up period. No bleb-related complications were reported. The reported average postoperative IOPs after canaloplasty or Trabectome at 12-month follow-up were similar.
DISCUSSION
Trabeculectomy enhanced with antimetabolite is the current “gold standard” to which all other surgical procedures are customarily compared. Many studies have repeatedly established that complication rates after trabeculectomy, such as wound leaks, hypotony, and blebitis, are relatively high and frequently associated with permanent visual disability. It has been reported that the risk of late-onset endophthalmitis in mitomycin C–treated trabeculectomies is approximately 1% per patient year.1 Severe loss of central vision has been reported to occur in 6% of eyes after trabeculectomy.2
Tube shunt implants have traditionally been reserved for patients who are deemed at high risk for failure with trabeculectomy, including neovascular or uveitic glaucomas, and patients who had previously failed trabeculectomy. The recently published TVT study has altered the thinking of many glaucoma surgeons, who have generally expressed surprise that the Baerveldt glaucoma implant can result in IOP lowering comparable to trabeculectomy when supplemented with IOP-lowering medications, while maintaining a lower risk profile.3 Nevertheless, both trabeculectomy and tube shunt implants remain labor-intensive in the postoperative period and can be accompanied by serious complications. It is for these reasons that newer devices and procedures are being developed in an attempt to improve patient outcomes.
The utility of these newer technologies remains unclear in the most at-risk patient population with advanced cupping and visual field loss. Both procedures report minimal complication rates, the most common of which is transient hyphema. Canaloplasty is more complex than Trabectome and relies on extensive manipulation of the conjunctiva, sclera, and the Tenon capsule, whereas the Trabectome procedure does not require conjunctival or scleral dissection.
Comparing the reported results of these new procedures across case series is complicated by the lack of a common definition of success or failure and complications. Arbitrary definitions of success may result in misleading comparisons and opportunities for investigators to favorably adjust data analysis to present results that may affect the perceptions of readers. The establishment of a consensus in the glaucoma community on standard definitions of success and failure will greatly aid evaluation of outcomes and minimize the current variable methods by which data are presented. Other desirable issues for ongoing reports on these new angle procedures would include standardized reporting of previous surgeries, such as laser trabeculoplasty, and previous failed filtering operations. Analyses to date indicate that prior laser trabeculoplasty or failed filtering surgery has minimal impact on Trabectome outcomes (S. Vold et al, American Glaucoma Society, San Diego, March 5–8, 2008, poster presentation, and D. Minckler et al, American Glaucoma Society, San Diego, March 5–8, 2008, poster presentation).
In conclusion, trabeculectomy remains the most effective IOP-lowering procedure to date; however, it is accompanied by the highest risk of severe complications. Trabectome and canaloplasty require the use of specialized expensive equipment, implants, or devices that may not be readily available in many parts of the world. Trabeculectomy remains relatively inexpensive and is accessible to any trained ophthalmic surgeon. Current concepts regarding estimating and striving for a pressure goal proportional to the extent of optic nerve damage would generally require lower IOPs in advanced glaucoma than either Trabectome or canaloplasty regularly achieve. However, normalizing IOP may be adequate for many patients. Trabectome has special appeal, as it is less invasive than canaloplasty or trabeculectomy and has no risk of bleb development. Neither Trabectome nor canaloplasty require antifibrotic agents, eliminating the associated risks with mitomycin C and 5-fluorouracil. Both of these new procedures have promise for application early in the glaucoma damage sequence in patients who cannot tolerate or adhere to medications or who fail laser trabeculoplasty.
ACKNOWLEDGMENTS
Funding/Support: Supported in part by the Research to Prevent Blindness Challenge Grant and by grant EY03040 from the National Institutes of Health/National Eye Institute, Bethesda, Maryland.
Financial Disclosures: Dr Mosaed and Dr Minckler are paid consultants for NeoMedix, the patent holder and manufacturer of Trabectome.
Author Contributions: Design and conduct of the study (S.M., D.M.); Collection, management, analysis, and interpretation of data (S.M., D.M., L.D.); Preparation, review, and approval of the manuscript (S.M., D.M).
Conformity With Author Information: Data were collected with local Institutional Review Board approval, in accordance with the Declaration of Helsinki and the Health Insurance Portability and Accountability Act.
REFERENCES
- 1.Greenfield DS, Suñer IJ, Miller MP, Kangas TA, Palmberg PF, Flynn HW., Jr Endophthalmitis after filtering surgery with mitomycin. Arch Ophthalmol. 1996;114:943–949. doi: 10.1001/archopht.1996.01100140151007. [DOI] [PubMed] [Google Scholar]
- 2.Law SK, Nguyen AM, Coleman AL, Caprioli J. Severe loss of central vision in patients with advanced glaucoma undergoing trabeculectomy. Arch Ophthalmol. 2007;125:1044–1055. doi: 10.1001/archopht.125.8.1044. [DOI] [PubMed] [Google Scholar]
- 3.Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC. Surgical complications in the Tube Versus Trabeculectomy Study during the first year of follow-up. Am J Ophthalmol. 2007;143:23–31. doi: 10.1016/j.ajo.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 4.Lichter PR, Musch DC, Gillespie BW, et al. CIGTS Study Group Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108:1943–1953. doi: 10.1016/s0161-6420(01)00873-9. [DOI] [PubMed] [Google Scholar]
- 5.Fluorouracil Filtering Surgery Study Group Five-year follow-up of the Fluorouracil Filtering Surgery Study. Am J Ophthalmol. 1996;121:349–366. doi: 10.1016/s0002-9394(14)70431-3. [DOI] [PubMed] [Google Scholar]
- 6.Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD, Budenz DL. Treatment outcomes in the Tube Versus Trabeculectomy Study after one year of follow-up. Am J Ophthalmol. 2007;143:9–22. doi: 10.1016/j.ajo.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Minckler DS, Hill RA. Use of novel devices for control of intraocular pressure. Exp Eye Res. 2009;88:792–798. doi: 10.1016/j.exer.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Francis BA, Minckler D, Dustin L, et al. Trabectome Study Group Combined cataract extraction and trabeculotomy by the internal approach for coexisting cataract and open-angle glaucoma: initial results. J Cataract Refract Surg. 2008;34:1096–1103. doi: 10.1016/j.jcrs.2008.03.032. [DOI] [PubMed] [Google Scholar]
- 9.Minckler DS, Mosaed S, Dustin L, Francis BA. Trabectome (trabeculectomy-internal approach): additional experience and extended follow-up. Trans Am Ophthalmol Soc. 2008;106:1–12. [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis RA, Von Wolff K, Tretz M, et al. Canaloplasty: circumferential viscodilation and tensioning of Schlemm’s canal using a flexible microcatheter for the treatment of open-angle glaucoma in adults. Interim clinical study analysis. J Cataract Refract Surg. 2007;33:1217–1226. doi: 10.1016/j.jcrs.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 11.Shingleton B, Tetz M, Korber N. Circumferential viscodilation and tensioning of Schlemm canal (canaloplasty) with temporal clear corneal phacoemulsification cataract surgery for open-angle glaucoma and visually significant cataract: one-year results. J Cataract Refract Surg. 2008;34:433–440. doi: 10.1016/j.jcrs.2007.11.029. [DOI] [PubMed] [Google Scholar]