Abstract
Purpose:
A multicenter, 2-visit, open-label, 4-week study was conducted to determine the acceptability of hydroxypropyl cellulose ophthalmic inserts in adult patients with a history of dry eye syndrome (DES).
Methods:
At visit 1, patients (N = 520) were evaluated, screened by slit-lamp biomicroscopy, and completed the Ocular Surface Disease Index (OSDI), a validated measure of quality of life. Patients were trained in the proper placement and use of hydroxypropyl cellulose ophthalmic inserts and were contacted by telephone on day 3 of the study. At week 4, patients were given a clinical evaluation and completed a second questionnaire. Answers determined changes in symptoms and quality of life. Adverse events were monitored throughout the study.
Results:
Four hundred eighteen patients completed the study and reported significant improvements in discomfort, burning, dryness, grittiness, stinging, and light sensitivity (P = .05) after 4 weeks use of hydroxypropyl cellulose ophthalmic inserts. Significant improvements in clinical signs (keratitis, conjunctival staining, and tear volume) were reported. Contact lens wearers reported significant improvements similar to nonwearers, with a strong trend toward improvement in light sensitivity. Mean OSDI total scores, measuring quality of life, significantly improved by 21.3% (from 41.8 ± 22.38 to 32.9 ± 21.97, P ≤ .0215). The most commonly reported adverse event leading to discontinuation was blurred vision, observed in 8.7% of patients (n = 45). Compliance during the study was good; 41.5% of subjects were fully compliant. Of the 58.5% of subjects who missed doses, the majority (69.4%) missed only one to five.
Conclusions:
Hydroxypropyl cellulose ophthalmic inserts significantly reduced symptoms and clinical signs of moderate to severe DES. They also significantly improved DES in patients wearing contact lenses. Patients experienced a statistically significant improvement in quality of life, as measured by the OSDI, of 21.3%.
INTRODUCTION
Five million Americans are affected by dry eye syndrome (DES), a multifactorial disease of the tears and ocular surface that results in symptoms of discomfort, including burning, itching, foreign body sensation, soreness, dryness, photophobia, redness, and reduced visual acuity.1,2 Approximately one-third of patients seeking treatment from an ophthalmologist have symptoms of DES.2 It affects about 5 million Americans 50 years of age and older, and nearly twice as many women as men. The number of individuals with DES is expected to increase dramatically as the population of older Americans rises in the coming decades.3 Furthermore, 52% of contact lens wearers experience symptoms of DES.
Its impact on patients’ activities of daily living (ADLs) and quality of life is considerable, with many patients experiencing difficulty when reading, watching television, and using a computer.2 In addition to tangible symptoms, intangible decreases in leisure time and social interactions, impaired physical functioning and quality of life, and declines in mental and general health, DES directly impacts costs of care due to increased health care system utilization, and indirectly contributes to lost work time and productivity.3
Often, patients’ symptoms are at odds with the results of clinical tests, and no single repeatable, reliable test is in common use.3 DES itself varies from patient to patient, its symptoms are subjective, and responses to questions about the physical sensations in the eyes may also vary.3 Patients may even present with severe damage to the ocular surface with no or few symptoms of DES.2
Additionally, preservatives such as benzalkonium chloride used in many dry eye therapies cause inflammation of the ocular surface and damage to the corneal and conjunctival epithelium. Effective, preservative-free treatment options are absolutely necessary, particularly for patients with moderate to severe DES.4
Hydroxypropyl cellulose ophthalmic inserts (Lacrisert; Aton Pharma, Lawrenceville, New Jersey) are indicated in patients with moderate to severe DES, including keratoconjunctivitis sicca. They are indicated especially in patients who remain symptomatic after an adequate trial of therapy with artificial tear solutions. They are also indicated for patients with exposure keratitis, decreased corneal sensitivity, and recurrent corneal erosions.5
Each hydroxypropyl cellulose ophthalmic insert is 5 mg of hydroxypropyl cellulose 1.27 mm in diameter by 3.5 mm long. The inserts are placed into the inferior cul-de-sac of each eye beneath the base of the tarsus, not in apposition to the cornea, nor beneath the eyelid at the level of the tarsal plate.5
Hydroxypropyl cellulose ophthalmic inserts act to stabilize the precorneal tear film and prolong tear film breakup time (TFBUT), which is usually accelerated in patients with DES. They also act to lubricate and protect the eye.5
The purpose of this study was to determine the acceptability of hydroxypropyl cellulose ophthalmic inserts in adult patients with a history of moderate to severe DES. It was hypothesized that patients would experience reduced symptoms and signs of DES, along with improvements in their quality of life.
METHODS
STUDY DESIGN
This was a multicenter, open-label study consisting of 2 office visits. At visit 1 (day 0), the following was done: Patients read, signed, and dated an institutional review board–approved, HIPAA-compliant informed consent form. Patient information was collected. Best-corrected visual acuity (BCVA) was measured, slit-lamp biomicroscopy was performed, and a general dry eye evaluation was conducted (specific evaluation procedures were left to the discretion of the individual investigator and included, but were not limited to, fluorescein staining and Schirmer test). Participants completed Patient Questionnaire A, a panel of questions on a numerical/visual analog scale that assessed DES symptoms. Patients were instructed on how to properly place hydroxypropyl cellulose ophthalmic inserts into the cul-de-sac of each eye, which included viewing an instructional video, and the participants each placed one 5-mg insert bilaterally in the presence of the investigator or coordinator after training. Finally, patients were dispensed 58 5-mg hydroxypropyl cellulose ophthalmic inserts and scheduled for visit 2.
As a follow-up to visit 1, patients were contacted by telephone at day 3 (±1 day) for approximately 10 minutes to assess any adverse events and to determine whether hydroxypropyl cellulose ophthalmic inserts were being properly used. Reinstruction on correct use was given if necessary.
On day 28 (±3 days), patients returned for an approximately 1-hour follow-up visit. During this time, any adverse events were reviewed, BCVA was assessed, and slit-lamp biomicroscopy was performed. A general dry eye evaluation was conducted (as in visit 1), and participants completed Patient Questionnaires B and C, a panel of questions on a numerical/visual analog scale that assessed DES symptoms. Investigators completed the Physician Questionnaire at this time prior to exiting patients from the study.
MONITORING OF ADVERSE EVENTS
Adverse events (whether elicited or observed) were monitored throughout the study. All adverse events were promptly reviewed by the relevant investigator for accuracy and completeness and were documented appropriately.
PATIENT INCLUSION AND EXCLUSION CRITERIA
Only subjects who were at least 18 years of age who provided written informed consent and were willing and able to follow all instructions and attend all visits were enrolled in the study. All participating subjects had either a diagnosis of DES in both eyes with a history of intermittent or regular artificial tear use, or a desire to use artificial tears within the past week prior to study initiation.
Subjects were excluded from participation in this study if they were affected by any of the following: had clinically significant blepharitis, meibomian gland dysfunction, or lid margin inflammation and were currently taking systemic or topical medication used to treat any of these diagnoses; had a diagnosis of ongoing ocular infection (bacterial, viral, or fungal), active ocular inflammation (eg, follicular conjunctivitis), or preauricular lymphadenopathy; had laser in situ keratomileusis (LASIK) surgery within 12 months of visit 1; had ocular surgical intervention within 3 months prior to or during the study period; had a systemic disease or uncontrolled medical condition that, in the opinion of the investigator, could interfere with study measurements or subject compliance; used any new dry eye therapies throughout the duration of the trial; were currently taking any systemic medications known to cause ocular drying and had not been on a stable dose within 30 days of visit 1; had a known allergy and/or sensitivity to hydroxypropyl cellulose; had received an investigational drug or device within 30 days of visit 1.
STATISTICAL ANALYSES
Overall comparison of variables was performed by multivariate analysis of variance (MANOVA) using a standard software package for social sciences (SPSS Inc, Chicago, Illinois). After F tests univariate analyses were done using t tests. This enabled the investigators to isolate the strength of impact of the treatment on specific clinical and quality of life outcomes. Confidence intervals (CIs) were reported at 95% to provide a practical structure for reporting such diverse findings with large numbers of variables..
RESULTS
PATIENT CHARACTERISTICS
This study enrolled 520 patients across 49 sites throughout the United States. Of the 520 patients who enrolled in the study, 418 (80.4%) completed through visit 2. The majority (n = 337, 64.8%) of patients participating were women, and 54.6% (n = 284) were 50 years of age or older. Twenty-six patients (5.0%) withdrew consent or were lost to follow-up prior to completion of the study.
CHANGE IN DRY EYE SYNDROME SYMPTOMS
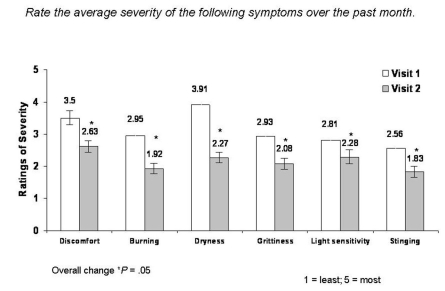
At each visit, patients were asked to rate the average severity of their DES symptoms over the past month (eg, the month prior to visit 1 and the 28 days between visit 1 and visit 2). Patients scored the severity of their symptoms on a numerical/visual analog scale ranging from 1 (least severe) to 5 (most severe). After 1 month of treatment with hydroxypropyl cellulose ophthalmic inserts, significant reductions in the mean severity of DES symptoms, including discomfort, burning, dryness, grittiness, sensitivity to light, and stinging, were reported (Figure 1). Patient-reported severity of discomfort was reduced by 24.9% (mean change −0.87, 95% CI 3.50 ± 1.10 to 2.63 ± 1.31; P = .05), burning improved by 34.9% (mean change −1.03, 95% CI 2.95 ± 1.34 to 1.92 ± 1.21; P = .05), severity of dryness improved by 41.9% (mean change −1.64, 95% CI 3.91 ± 1.05 to 2.27 ± 1.25; P = .05), feeling of grittiness was reduced by 29.0% (mean change −0.85, 95% CI 2.93 ± 1.38 to 2.08 ± 1.25; P = .05), sensitivity to light improved by 18.9% (mean change −0.53, 95% CI 2.81 ± 1.52 to 2.28 ± 1.37; P = .05), and severity of stinging improved by 28.5% (mean change −0.73, 95% CI 2.56 ± 1.31 to 1.83 ± 1.11; P = .05).
FIGURE 1.
Mean change in severity of DES symptoms reported in the past month from visit 1 to visit 2.
CHANGE IN OCULAR SURFACE DISEASE INDEX
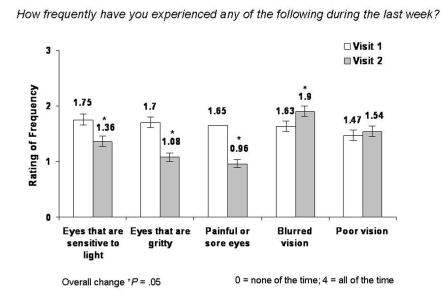
The Ocular Surface Disease Index (OSDI) is a validated instrument that measures patient quality of life. It consists of 12 individual questions separated into 3 categories, including DES symptoms, performance of ADLs, and environmental conditions that contribute to or cause DES. Respondents rank the frequency of occurrence of symptoms, limitations in performing ADLs due to DES, and ocular discomfort over the past week on a numerical/visual analog scale ranging from 0 (none of the time) to 4 (all of the time). The OSDI was incorporated into Patient Questionnaires A and B that patients completed at visit 1 and visit 2. After 1 month of treatment with hydroxypropyl cellulose ophthalmic inserts, significant mean improvement was observed in the majority of OSDI components.
Patients reported significantly fewer occurrences of most DES symptoms scored by the OSDI (Figure 2). Patient-reported occurrence of sensitivity to light was significantly reduced (by 22.3%; mean change −0.39, 95% CI 1.75 ± 1.45 to 1.36 ± 1.32; P = .05), 36.5% fewer incidences of sensations of grittiness were observed (mean change −0.62, 95% CI 1.70 ± 1.27 to 1.08 ± 1.14; P = .05), and significant reductions (41.8%) in occurrence of painful or sore eyes occurred (mean change −0.69, 95% CI 1.65 ± 1.31 to 0.96 ± 1.09; P = .05). Occurrence of blurred vision, the most commonly reported adverse event associated with hydroxypropyl cellulose ophthalmic inserts, increased by 16.6% (mean change 0.27, 95% CI 1.63 ± 1.29 to 1.90 ± 1.32; P = .05). No significant difference was reported for the occurrence of poor vision between visit 1 and visit 2.
FIGURE 2.
Mean change in frequency of DES symptoms from visit 1 to visit 2 as scored by the OSDI.
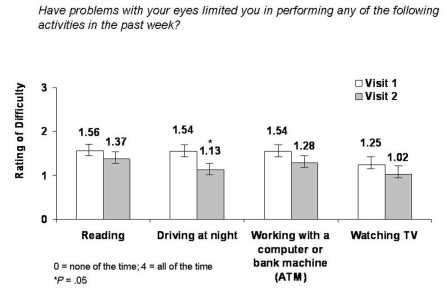
Significant reductions in frequency of difficulty performing daily tasks as measured by the OSDI were also reported (Figure 3). A significant (12.2%) reduction in occurrence of difficulty when reading was reported (mean change −0.19, 95% CI 1.56 ± 1.35 to 1.37 ± 1.28; P = .05). Incidence of difficulty driving at night was significantly reduced (by 26.6%; mean change −0.41, 95% CI 1.54 ± 1.39 to 1.13 ± 1.27; P = .05). Incidence of difficulty working with a computer or automated teller machine (ATM) was significantly reduced (by 16.9%; mean change −0.26, 95% CI 1.54 ± 1.31 to 1.28 ± 1.25; P = .05), and occurrence of difficulty watching television was significantly reduced (by 18.4%; mean change −0.23, 95% CI 1.25 ± 1.22 to 1.02 ± 1.17; P = .05).
FIGURE 3.
Mean change in occurrence of difficulty when performing daily tasks from visit 1 to visit 2 as scored by the OSDI..
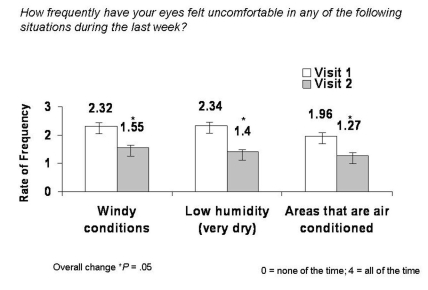
Significant reductions in occurrence of discomfort in certain environmental conditions as measured by the OSDI were also observed (Figure 4). Patients experienced a significant (33.2%) reduction in occurrence of discomfort in windy conditions (mean change −0.77, 95% CI 2.32 ± 1.38 to 1.55 ± 1.41; P = .05), 40.2% fewer incidences of discomfort in areas of low humidity (mean change −0.94, 95% CI 2.34 ± 1.33 to 1.40 ± 1.33; P = .05), and a significant (35.2%) reduction in occurrence of discomfort in areas that are air-conditioned (mean change −0.69, 95% CI 1.96±1.37 to 1.27±1.35; P = .05).
FIGURE 4.
Mean change in frequency of discomfort in various environmental conditions from visit 1 to visit 2 as scored by the OSDI.
After 1 month of therapy with hydroxypropyl cellulose ophthalmic inserts, mean OSDI total scores improved by 21.3% (from 41.8 ± 22.38 at visit 1 to 32.9 ± 21.97 at visit 2; P ≤ .0215).
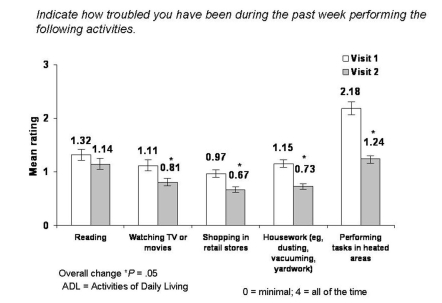
CHANGE IN ACTIVITIES OF DAILY LIVING
At each visit, patients were asked to rate how troubled they had been during the past week in performing daily tasks due to their DES symptoms. Any difficulty was rated on a numerical/visual analog scale ranging from 0 (minimal, slight discomfort when performing a task) to 3 (severe, ability to perform a task is prevented). Significant improvements in patients’ ability to perform ADLs were reported after 1 month of treatment with hydroxypropyl cellulose ophthalmic inserts (Figure 5). Patients experienced a significant (13.6%) reduction in difficulty reading (mean change −0.18, 95% CI 1.32 ± 0.99 to 1.14 ± 1.04; P = .05), a 27.0% improvement in ability to watch television or movies (mean change −0.30, 95% CI 1.11 ± 0.95 to 0.81 ± 0.94; P = .05), a 30.9% reduction in problems while shopping in retail stores (mean change −0.30, 95% CI 0.97 ± 0.97 to 0.67 ± 0.89; P = .05), a 36.5% improvement in ability to perform housework (mean change −0.42, 95% CI 1.15 ± 1.01 to 0.73 ± 0.88; P = .05), and a 43.1% reduction in difficulty performing tasks in heated areas (mean change −0.94, 95% CI 2.18 ± 1.89 to 1.24 ± 1.59; P = .05).
FIGURE 5.
Mean change in difficulty in performing activities of daily living in the past week from visit 1 to visit 2.
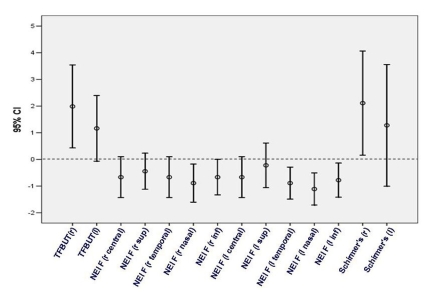
CHANGE IN CLINICAL SIGNS
Investigators conducted a general dry eye evaluation at visit 1 and visit 2 to measure changes in TFBUT, fluorescein staining, and tear volume as measured by the Schirmer test (Figure 6). Mean TFBUT increased bilaterally, reaching statistical significance in the right eye, with a strong trend for improvement observed in the left eye. Mean tear volume also increased bilaterally, reaching significance in the right eye, with a trend toward improvement seen in the left eye. A strong trend toward reduced mean fluorescein staining was observed in the right eye, with staining in the nasal region reaching statistical significance. Mean fluorescein staining in the left eye was reduced significantly in most areas, with a trend for improvement in the central and superior regions.
FIGURE 6.
Mean change in clinical signs from visit 1 to visit 2. NEI F, National Eye Institute fluorescein staining; Schirmer’s, Schirmer’s test of tear volume; TFBUT, tear film breakup time.
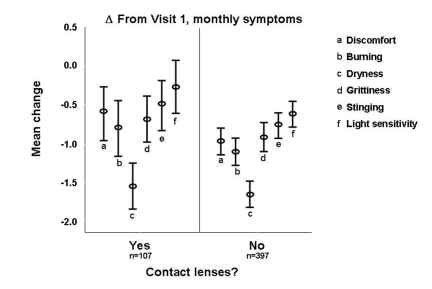
CONTACT LENS WEARERS
Of the 520 patients enrolled in the study, 107 reported that they wore contact lenses throughout the 1-month treatment period. Similar to nonwearers, patients wearing contact lenses experienced significant reductions in the severity of feelings of discomfort, burning, dryness, grittiness, and stinging after 1 month of treatment with hydroxypropyl cellulose ophthalmic inserts. A strong trend for reduction in the severity of sensitivity to light was observed in lens wearers (Figure 7).
FIGURE 7.
Mean change in severity of DES symptoms reported in the past month from visit 1 to visit 2 among contact lens wearers vs nonwearers.
ADHERENCE/COMPLIANCE
Overall, compliance during the study was good; 41.2% (n = 179) of patients for whom this information was available indicated that they did not miss any hydroxypropyl cellulose ophthalmic insert doses. Of the 58.5% of patients (n = 255) who missed doses, the majority (69.4%, n = 177) indicated that they missed only one to five doses.
SAFETY
The most commonly reported adverse event associated with hydroxypropyl cellulose ophthalmic inserts was blurred vision. Blurred vision led to discontinuation in 8.7% of patients (n = 45). Incidence of additional adverse events leading to discontinuation was low—less than 1.0% of patients (Table). One corneal abrasion was reported during this study; however, it was unrelated to treatment with hydroxypropyl cellulose ophthalmic inserts. The abrasion occurred when a participating patient who was attempting to remove a gas-permeable contact lens inadvertently self-inflicted a corneal abrasion. The patient was not using hydroxypropyl cellulose ophthalmic inserts at the time of the incident.
TABLE.
INCIDENCE OF ADVERSE EVENTS AND OTHER CAUSES FOR DISCONTINUATION*
| ADVERSE EVENT | PERCENT (N) |
|---|---|
| Blurred vision | 8.7 % (45) |
| Ocular discomfort | 0.96% (5) |
| Foreign body sensation | 0.96% (5) |
| Ocular stinging/grittiness/ache | 0.58% (3) |
| Ocular irritation | 0.38% (2) |
| Swollen lids | 0.38% (2) |
| Excessive tearing | 0.38% (2) |
| Eyelash crusting/stickiness | 0.38% (2) |
| Other | 0.58% (3) |
| Withdrew consent | 3.5% (18) |
| Lost to follow-up | 1.54% (8) |
| Did not complete visit 2 outcome questions | 1.34% (7) |
One incidence per patient.
DISCUSSION
After only 1 month of treatment with hydroxypropyl cellulose ophthalmic inserts as an adjunct to ongoing therapy, participants in this study experienced significant reductions in the severity of DES symptoms, fewer occurrences of DES symptoms, less difficulty when performing daily tasks, and reduced discomfort in environmental conditions that exacerbate DES symptoms. Investigators reported improvements in clinical signs of DES, including increased TFBUT and tear volume, along with decreased fluorescein staining.
Patients reported a significant and dramatic improvement in overall quality of life as scored by the OSDI. Significant improvements were reported in incidence of sensitivity to light, feelings of grittiness, and painful or sore eyes. No significant changes were reported in occurrence of poor vision. Blurred vision, the most commonly reported adverse event associated with hydroxypropyl cellulose ophthalmic inserts, was reported to occur with increased frequency. This is likely due to the thickened precorneal tear film observed after placement of the inserts. Immediate, transient blurring can be managed by instilling a drop of artificial tears to thin the tear film. Some patients may find that blurred vision is eliminated if hydroxypropyl cellulose ophthalmic inserts are emplaced at night and removed in the morning.
If blurring does not occur immediately after placement but is observed later in the day, patients can remove the inserts and replace them with a new pair. Blurring several hours after placement may be caused by the softened, bleb-shaped insert. Most patients will not experience blurred vision; however, if blurring is observed, it can be easily managed.
Of interest, while the incidence of blurred vision did increase in this analysis, patients reported significant improvement in ADLs that require a high degree of visual acuity, particularly reading, watching television or movies, working with a computer or ATM, and driving at night. It is likely that hydroxypropyl cellulose ophthalmic inserts provide significant enough relief of additional DES symptoms that offset any inconvenience experienced by transient, manageable blurred vision. This is reflected in the significant, dramatic 21.3% improvement in OSDI total score.
A large number of contact lens wearers participated in this study. Lens wearers experienced relief of DES symptoms that was comparable to patients who do not wear contact lenses, with a strong trend toward improvement in sensitivity to light. Additional analyses are under way to determine the activity of hydroxypropyl cellulose ophthalmic inserts in further subsets of patients (ie, those with glaucoma, cataracts, or previous refractive surgery). Once-daily use of hydroxypropyl cellulose ophthalmic inserts is an effective, preservative-free option for the treatment of moderate to severe DES.
ACKNOWLEDGMENTS
Funding/Support: Supported by Aton Pharma Inc, Lawrenceville, New Jersey.
Financial Disclosures: Dr McDonald is a consultant/scientific advisor for Allergan, AMO, Aton Pharma, Bausch & Lomb, and Santen, and receives grant/research support from Allergan, AMO, and Aton Pharma. Dr D’Aversa lectures for Alcon, Allergan, Aton Pharma, and Inspire. Dr Perry is a consultant/advisor for Allergan; receives grant/research support from Alcon, Bausch & Lomb, ISTA, Inspire, and Sirion; and lectures for Allergan, Bausch & Lomb, and Inspire. Dr Wittpenn is a consultant for Allergan. Dr Donnenfeld declares no financial interests. Dr. Nelinson is an advisor for and receives research support from Aton Pharma, and is a consultant for the Lions Eye Institute for Transplant and Research.
Author Contributions: Design of the study (M.M.); Conduct of the study (M.M., G.D., H.P., J.W.); Management, analysis, and interpretation of data (D.N.); Preparation, review, or approval of manuscript (M.M., G.D., H.P., J.W., E.D., D.N.).
Conformity With Author Information: Upon enrollment in the study, patients read, signed, and dated an IRB-approved, HIPAA-compliant informed consent form. This study was conducted in accordance with Institutional Review Board regulations (U.S. 21 CFR Part 56.103). IRB approval of the protocol and informed consent documents was obtained prior to study initiation.
Other Acknowledgments: The authors would like to thank Frederick Parente, PhD, and Norman Nagl, PhD, for assistance with data analysis and editorial support.
REFERENCES
- 1.The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye Workshop (2007) Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 2.Lemp MA. Advances in understanding and managing dry eye disease. Am J Ophthalmol. 2008;146:350–356. doi: 10.1016/j.ajo.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 3.The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye Workshop (2007) Ocul Surf. 2007;5:93–107. doi: 10.1016/s1542-0124(12)70082-4. [DOI] [PubMed] [Google Scholar]
- 4.Management and therapy of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye Workshop (2007) Ocul Surf. 2007;5:163–178. doi: 10.1016/s1542-0124(12)70085-x. [DOI] [PubMed] [Google Scholar]
- 5.Lacrisert [package insert] Lawrenceville, NJ: Aton Pharma Inc; 2007. [Google Scholar]