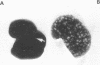
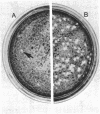
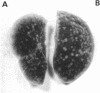
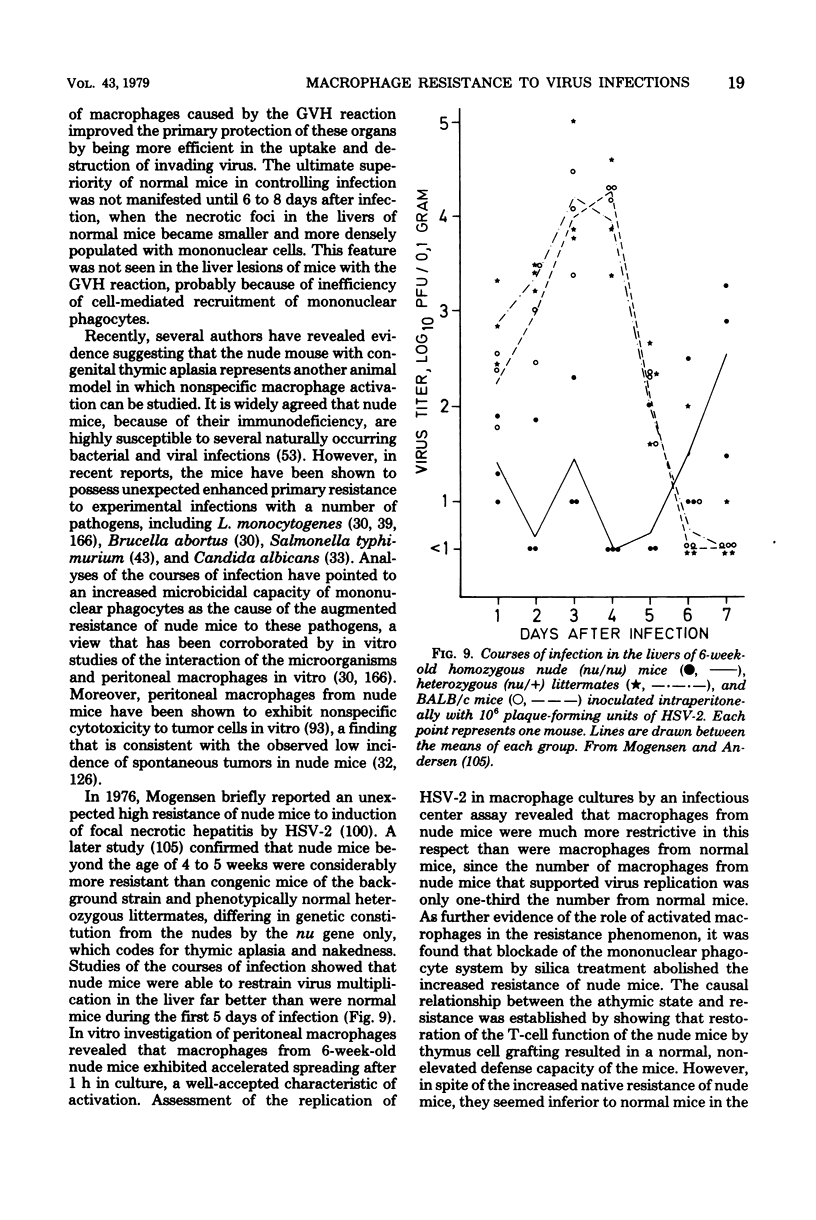
Full text
PDF






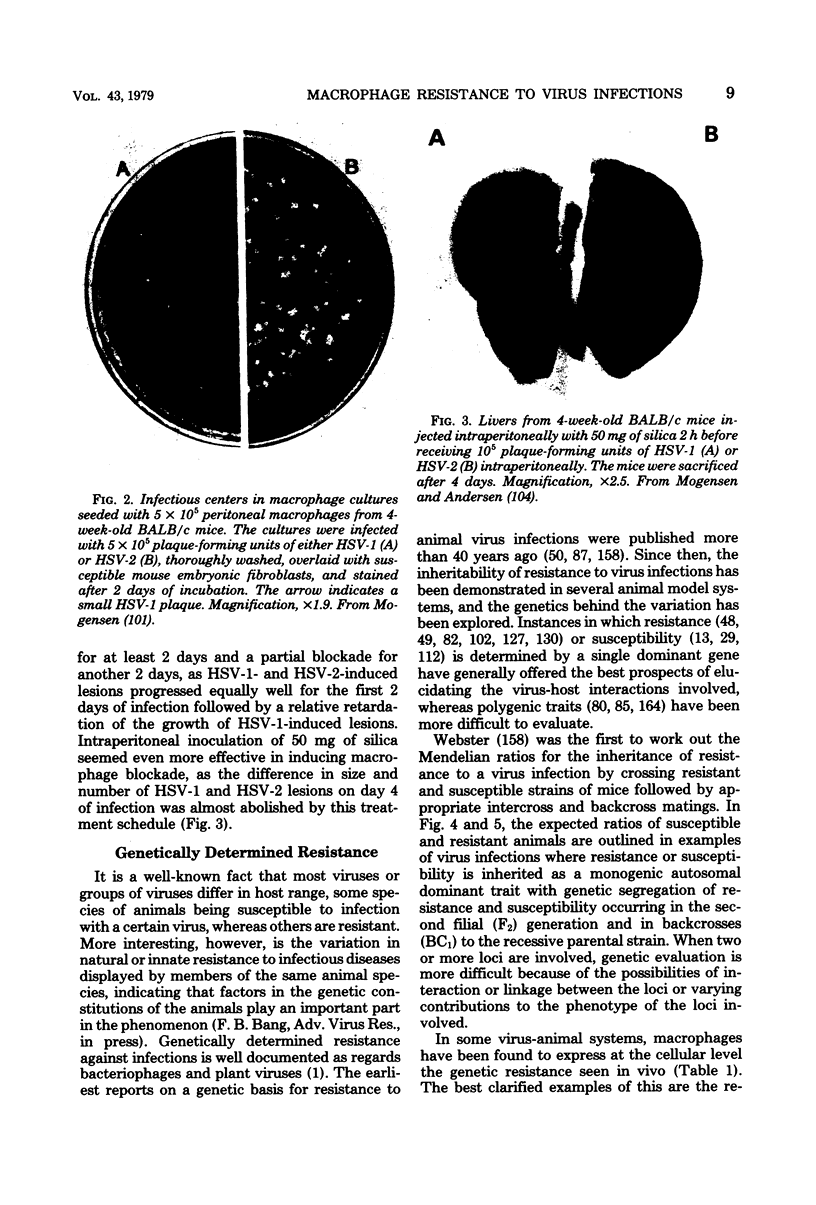
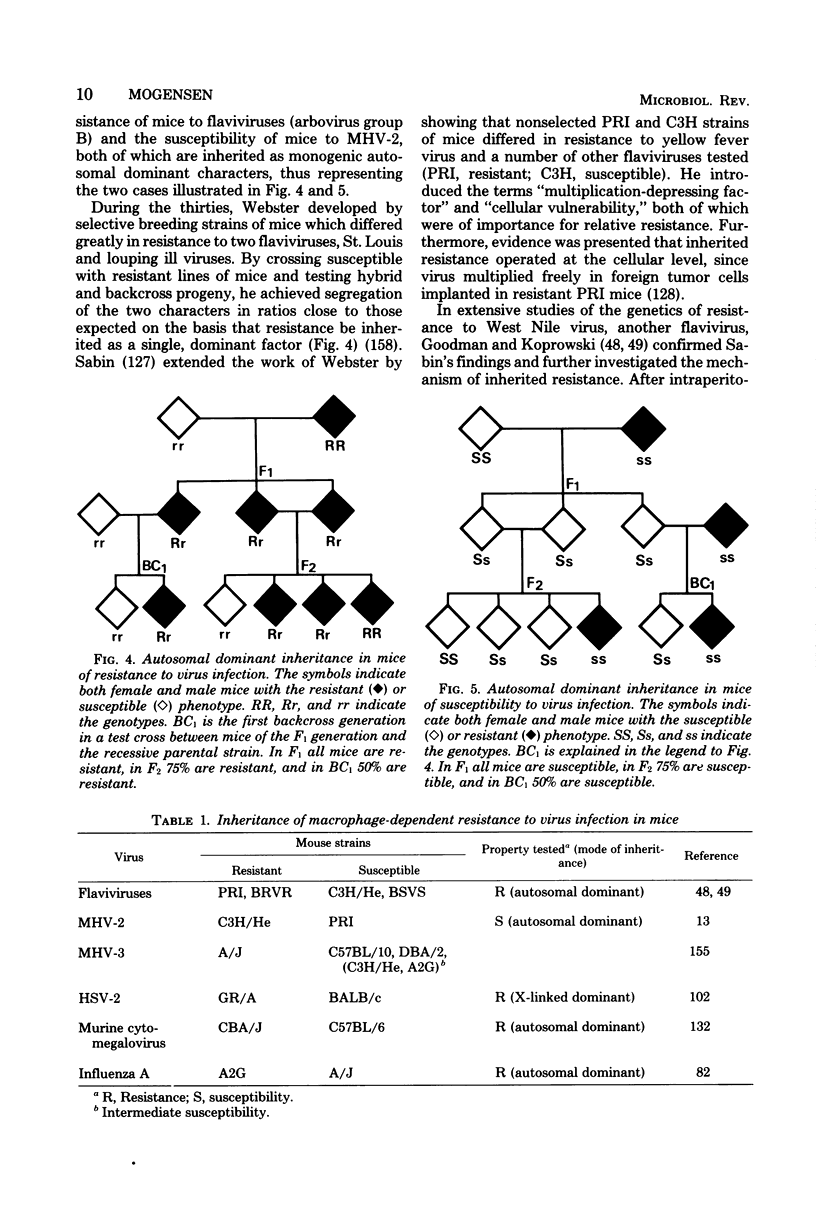


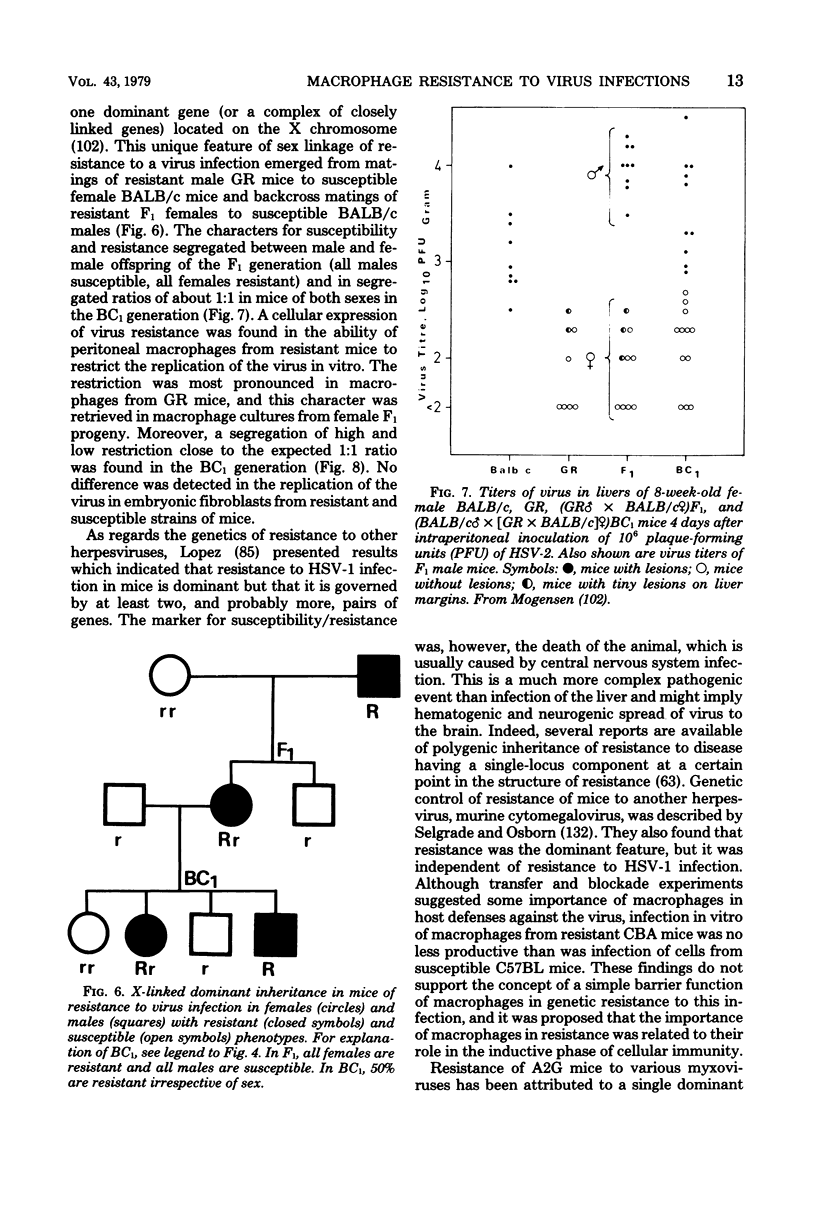
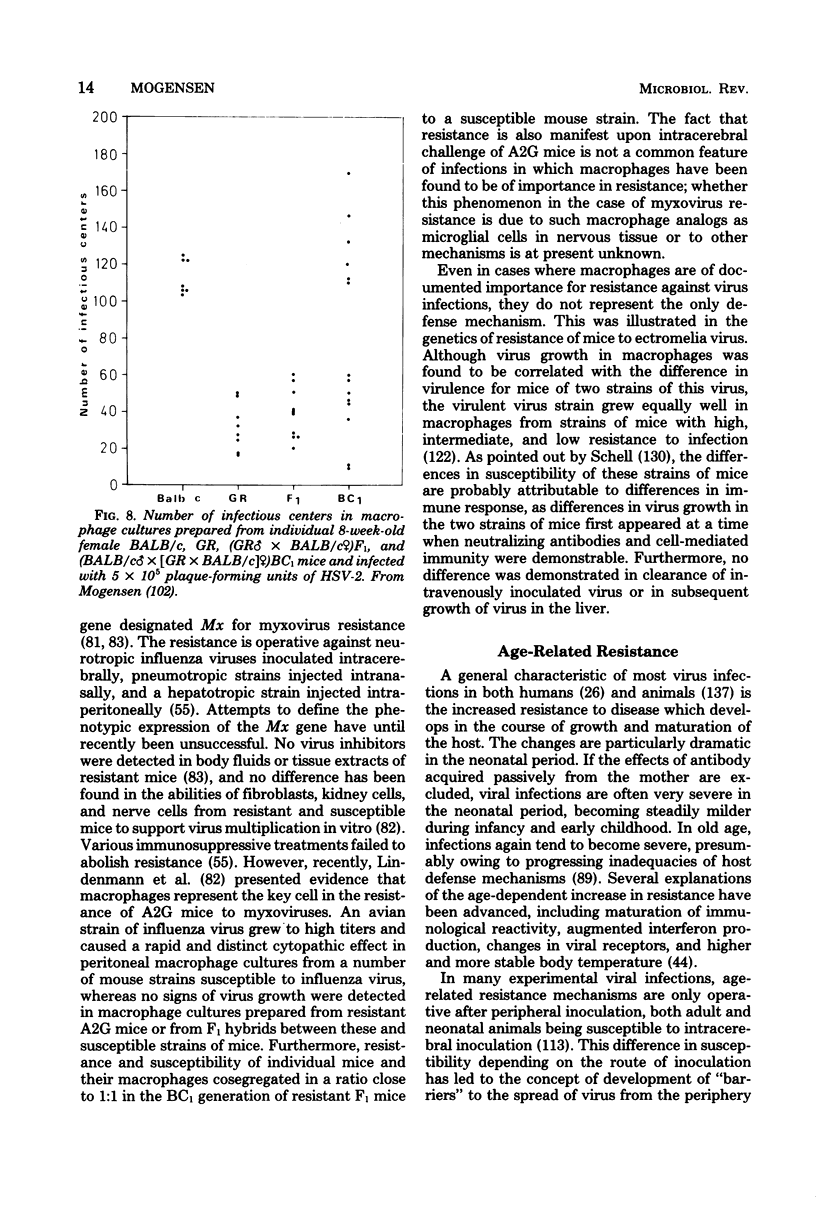











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C. Genetic factors in resistance against virus infections. Arch Gesamte Virusforsch. 1965;17(2):280–294. doi: 10.1007/BF01267912. [DOI] [PubMed] [Google Scholar]
- Allison A. C., Harington J. S., Birbeck M. An examination of the cytotoxic effects of silica on macrophages. J Exp Med. 1966 Aug 1;124(2):141–154. doi: 10.1084/jem.124.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison A. C. Interactions of antibodies, complement components and various cell types in immunity against viruses and pyogenic bacteria. Transplant Rev. 1974;19(0):3–55. doi: 10.1111/j.1600-065x.1974.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Anderson F. D., Ushijima R. N., Larson C. L. Recurrent herpes genitalis. Treatment with Mycobacterium bovis (BCG). Obstet Gynecol. 1974 Jun;43(6):797–805. [PubMed] [Google Scholar]
- BANG F. B., LUTTRELL C. N. Factors in the pathogenesis of virus diseases. Adv Virus Res. 1961;8:199–244. doi: 10.1016/s0065-3527(08)60686-7. [DOI] [PubMed] [Google Scholar]
- BANG F. B., WARWICK A. Macrophages and mouse hepatitis. Virology. 1959 Dec;9:715–717. doi: 10.1016/0042-6822(59)90166-7. [DOI] [PubMed] [Google Scholar]
- BARON S. MECHANISM OF RECOVERY FROM VIRAL INFECTION. Adv Virus Res. 1963;10:39–64. doi: 10.1016/s0065-3527(08)60696-x. [DOI] [PubMed] [Google Scholar]
- BLAESE R. M., MARTINEZ C., GOOD R. A. IMMUNOLOGIC INCOMPETENCE OF IMMUNOLOGICALLY RUNTED ANIMALS. J Exp Med. 1964 Feb 1;119:211–224. doi: 10.1084/jem.119.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUNNER K. T., HUREZ D., McCLUSKEY R. T., BENACERRAF B. Blood clearance of P32-labeled vesicular stomatitis and Newcastle disease viruses by the reticuloendothelial system in mice. J Immunol. 1960 Jul;85:99–105. [PubMed] [Google Scholar]
- Baker M. B., Larson C. L., Ushijima R. N., Anderson F. D. Resistance of female mice to vaginal infection induced by Herpesvirus hominis type 2: effects of immunization with Mycobacterium bovis, intravenous injection of specific Herpesvirus hominis type 2 antiserum, and a combination of these procedures. Infect Immun. 1974 Dec;10(6):1230–1234. doi: 10.1128/iai.10.6.1230-1234.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang F. B., Warwick A. MOUSE MACROPHAGES AS HOST CELLS FOR THE MOUSE HEPATITIS VIRUS AND THE GENETIC BASIS OF THEIR SUSCEPTIBILITY. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1065–1075. doi: 10.1073/pnas.46.8.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett B. Isolation and cultivation in vitro of macrophages from various sources in the mouse. Am J Pathol. 1966 Jan;48(1):165–181. [PMC free article] [PubMed] [Google Scholar]
- Bennett W. E., Cohn Z. A. The isolation and selected properties of blood monocytes. J Exp Med. 1966 Jan 1;123(1):145–160. doi: 10.1084/jem.123.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berken A., Benacerraf B. Properties of antibodies cytophilic for macrophages. J Exp Med. 1966 Jan 1;123(1):119–144. doi: 10.1084/jem.123.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V. Increased antibacterial resistance and immunodepression during graft-versus-host reactions in mice. Transplantation. 1969 Jun;7(6):484–497. doi: 10.1097/00007890-196906000-00005. [DOI] [PubMed] [Google Scholar]
- Blanden R. V. Mechanisms of recovery from a generalized viral infection: mousepox. 3. Regression infectious foci. J Exp Med. 1971 May 1;133(5):1090–1104. doi: 10.1084/jem.133.5.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V. Modification of macrophage function. J Reticuloendothel Soc. 1968 Jun;5(3):179–202. [PubMed] [Google Scholar]
- Blanden R. V. T cell response to viral and bacterial infection. Transplant Rev. 1974;19(0):56–88. doi: 10.1111/j.1600-065x.1974.tb00128.x. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- CHANG S. S., HILDEMANN W. H. INHERITANCE OF SUSCEPTIBILITY TO POLYOMA VIRUS IN MICE. J Natl Cancer Inst. 1964 Aug;33:303–313. [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. MORPHOLOGY, CYTOCHEMISTRY, AND BIOCHEMISTRY. J Exp Med. 1965 Jan 1;121:153–170. doi: 10.1084/jem.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheers C., Waller R. Activated macrophages in congenitally athymic "nude mice" and in lethally irradiate mice. J Immunol. 1975 Sep;115(3):844–847. [PubMed] [Google Scholar]
- Custer R. P., Outzen H. C., Eaton G. J., Prehn R. T. Does the absence of immunologic surveillance affect the tumor incidence in "nude" mice? First recorded spontaneous lymphoma in a "nude" mouse. J Natl Cancer Inst. 1973 Aug;51(2):707–711. [PubMed] [Google Scholar]
- Cutler J. E. Acute systemic candidiasis in normal and congenitally thymic-deficient (nude) mice. J Reticuloendothel Soc. 1976 Feb;19(2):121–124. [PubMed] [Google Scholar]
- Du Buy H. G., Johnson M. L. Studies on the in vivo and in vitro multiplication of the LDH virus of mice. J Exp Med. 1966 Jun 1;123(6):985–998. doi: 10.1084/jem.123.6.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EHRMANN R. L., GEY G. O. The growth of cells on a transparent gel of reconstituted rat-tail collagen. J Natl Cancer Inst. 1956 Jun;16(6):1375–1403. [PubMed] [Google Scholar]
- Emeis J. J., Planqué B. Heterogeneity of cells isolated from rat liver by pronase digestion: ultrastructure, cytochemistry and cell culture. J Reticuloendothel Soc. 1976 Jul;20(1):11–29. [PubMed] [Google Scholar]
- Emmerling P., Finger H., Bockemühl J. Listeria monocytogenes infection in nude mice. Infect Immun. 1975 Aug;12(2):437–439. doi: 10.1128/iai.12.2.437-439.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjuanes L., Cubero I., Viñuela E. Sensitivity of macrophages from different species to African swine fever (ASF) virus. J Gen Virol. 1977 Mar;34(3):455–463. doi: 10.1099/0022-1317-34-3-455. [DOI] [PubMed] [Google Scholar]
- Epstein L. B., Stevens D. A., Merigan T. C. Selective increase in lymphocyte interferon response to vaccinia antigen after revaccination. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2632–2636. doi: 10.1073/pnas.69.9.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauve R. M., Hevin B. Immunostimulation with bacterial phospholipid extracts. Proc Natl Acad Sci U S A. 1974 Feb;71(2):573–577. doi: 10.1073/pnas.71.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauve R. M., Hevin B. Résistance paradoxale des souris thymoprives à l'infection par Listeria monocytogenes et Salmonella typhimurium et action immunostimulante d'un extrait bactérien phospholipidique (EBP). C R Acad Sci Hebd Seances Acad Sci D. 1974 Nov 4;279(19):1603–1605. [PubMed] [Google Scholar]
- GALLILY R., WARWICK A., BANG F. B. EFFECT OF CORTISONE OF GENETIC RESISTANCE TO MOUSE HEPATITIS VIRUS IN VIVO AND IN VITRO. Proc Natl Acad Sci U S A. 1964 Jun;51:1158–1164. doi: 10.1073/pnas.51.6.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODMAN G. T., KOPROWSKI H. Macrophages as a cellular expression of inherited natural resistance. Proc Natl Acad Sci U S A. 1962 Feb;48:160–165. doi: 10.1073/pnas.48.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODMAN G. T., KOPROWSKI H. Study of the mechanism of innate resistance to virus infection. J Cell Comp Physiol. 1962 Jun;59:333–373. doi: 10.1002/jcp.1030590313. [DOI] [PubMed] [Google Scholar]
- Gallily R., Warwick A., Bang F. B. Ontogeny of macrophage resistance to mouse hepatitis in vivo and in vitro. J Exp Med. 1967 Apr 1;125(4):537–548. doi: 10.1084/jem.125.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresser I., Lang D. J. Relationships between viruses and leucocytes. Prog Med Virol. 1966;8:62–130. [PubMed] [Google Scholar]
- Gröschel D., Koprowski H. Development of a virus-resistant inbred mouse strain for the study of innate resistance to Arbo B viruses. Arch Gesamte Virusforsch. 1965;17(3):379–391. doi: 10.1007/BF01241192. [DOI] [PubMed] [Google Scholar]
- Guy-Grand D., Griscelli C., Vassalli P. Peyer's patches, gut IgA plasma cells and thymic function: study in nude mice bearing thymic grafts. J Immunol. 1975 Aug;115(2):361–364. [PubMed] [Google Scholar]
- HOWARD J. G., ROWLEY D., WARDLAW A. C. Investigations on the mechanism of stimulation of non-specific immunity by bacterial lipopolysaccharides. Immunology. 1958 Jul;1(3):181–203. [PMC free article] [PubMed] [Google Scholar]
- HOWARD J. Changes in the activity of the reticulo-endothelial system (RES) following the injection of parental spleen cells into F1 hybrid mice. Br J Exp Pathol. 1961 Feb;42:72–82. [PMC free article] [PubMed] [Google Scholar]
- Haller O., Arnheiter H., Lindenmann J. Genetically determined resistance to infection by hepatotropic influenza A virus in mice: effect of immunosuppression. Infect Immun. 1976 Mar;13(3):844–854. doi: 10.1128/iai.13.3.844-854.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy B., Skutelsky E., Globerson A., Danon D. Ultrastructural differences between macrophages of newborn and adult mice. J Reticuloendothel Soc. 1976 May;19(5):291–299. [PubMed] [Google Scholar]
- Henson D., Smith R. D., Gehrke J. Non-fatal mouse cytomegalovirus hepatitis. Combined morphologic, virologic and immunologic observations. Am J Pathol. 1966 Nov;49(5):871–888. [PMC free article] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Lambert L. H., Jr, Remington J. S. Possible role of macrophage mediated nonspecific cytotoxicity in tumour resistance. Nat New Biol. 1972 Jan 12;235(54):48–50. doi: 10.1038/newbio235048a0. [DOI] [PubMed] [Google Scholar]
- Hirsch M. S., Gary G. W., Jr, Murphy F. A. In vitro and in vivo properties of antimacrophage sera. J Immunol. 1969 Mar;102(3):656–661. [PubMed] [Google Scholar]
- Hirsch M. S., Zisman B., Allison A. C. Macrophages and age-dependent resistance to Herpes simplex virus in mice. J Immunol. 1970 May;104(5):1160–1165. [PubMed] [Google Scholar]
- Hirst R. G., Wallace M. E. Inherited resistance to Corynebacterium kutscheri in mice. Infect Immun. 1976 Aug;14(2):475–482. doi: 10.1128/iai.14.2.475-482.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON R. T. THE PATHOGENESIS OF HERPES VIRUS ENCEPHALITIS. II. A CELLULAR BASIS FOR THE DEVELOPMENT OF RESISTANCE WITH AGE. J Exp Med. 1964 Sep 1;120:359–374. doi: 10.1084/jem.120.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANTOCH M., BANG F. B. Conversion of genetic resistance of mammalian cells to susceptibility to a virus infection. Proc Natl Acad Sci U S A. 1962 Sep 15;48:1553–1559. doi: 10.1073/pnas.48.9.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANTOCH M., WARWICK A., BANG F. B. The cellular nature of genetic susceptibility to a virus. J Exp Med. 1963 May 1;117:781–798. doi: 10.1084/jem.117.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KESSEL R. W., MONACO L., MARCHISIO M. A. THE SPECIFICITY OF THE CYTOTOXIC ACTION OF SILICA--A STUDY IN VITRO. Br J Exp Pathol. 1963 Aug;44:351–364. [PMC free article] [PubMed] [Google Scholar]
- KILBOURNE E. D., HORSFALL F. L., Jr Studies of herpes simplex virus in newborn mice. J Immunol. 1951 Oct;67(4):321–329. [PubMed] [Google Scholar]
- Karnovsky M. L., Lazdins J., Drath D., Harper A. Biochemical characteristics of activated macrophages. Ann N Y Acad Sci. 1975 Jun 13;256:266–274. doi: 10.1111/j.1749-6632.1975.tb36053.x. [DOI] [PubMed] [Google Scholar]
- LINDENMANN J. INHERITANCE OF RESISTANCE TO INFLUENZA VIRUS IN MICE. Proc Soc Exp Biol Med. 1964 Jun;116:506–509. doi: 10.3181/00379727-116-29292. [DOI] [PubMed] [Google Scholar]
- LINDENMANN J., LANE C. A., HOBSON D. THE RESISTANCE OF A2G MICE TO MYXOVIRUSES. J Immunol. 1963 Jun;90:942–951. [PubMed] [Google Scholar]
- LWOFF A. Factors influencing the evolution of viral diseases at the cellular level and in the organism. Bacteriol Rev. 1959 Sep;23(3):109–124. doi: 10.1128/br.23.3.109-124.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson C. L., Ushijima R. N., Baker R. E., Baker M. B., Gillespie C. A. Effect of normal serum and antithymocyte serum on Friend disease in mice. J Natl Cancer Inst. 1972 May;48(5):1403–1407. [PubMed] [Google Scholar]
- Larson C. L., Ushijima R. N., Karim R., Baker M. B., Baker R. E. Herpesvirus hominis type 2 infections in rabbits: effect of prior immunization with attenuated Mycobacterium bovis (BCG) cells. Infect Immun. 1972 Oct;6(4):465–468. doi: 10.1128/iai.6.4.465-468.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavelle G. C., Bang F. B. Influence of type and concentration of sera in vitro on susceptibility of genetically resistant cells to mouse hepatitis virus. J Gen Virol. 1971 Sep;12(3):233–238. doi: 10.1099/0022-1317-12-3-233. [DOI] [PubMed] [Google Scholar]
- Lay W. H., Nussenzweig V. Receptors for complement of leukocytes. J Exp Med. 1968 Nov 1;128(5):991–1009. doi: 10.1084/jem.128.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenmann J., Deuel E., Fanconi S., Haller O. Inborn resistance of mice to myxoviruses: macrophages express phenotype in vitro. J Exp Med. 1978 Feb 1;147(2):531–540. doi: 10.1084/jem.147.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodmell D. L., Niwa A., Hayashi K., Notkins A. L. Prevention of cell-to-cell spread of herpes simplex virus by leukocytes. J Exp Med. 1973 Mar 1;137(3):706–720. doi: 10.1084/jem.137.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez C. Genetics of natural resistance to herpesvirus infections in mice. Nature. 1975 Nov 13;258(5531):152–153. doi: 10.1038/258152a0. [DOI] [PubMed] [Google Scholar]
- Lynch C. J., Hughes T. P. The Inheritance of Susceptibility to Yellow Fever Encephalitis in Mice. Genetics. 1936 Mar;21(2):104–112. doi: 10.1093/genetics/21.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALLUCCI L. OBSERVATIONS ON THE GROWTH OF MOUSE HEPATITIS VIRUS (MHV-3) IN MOUSE MACROPHAGES. Virology. 1965 Jan;25:30–37. doi: 10.1016/0042-6822(65)90248-5. [DOI] [PubMed] [Google Scholar]
- MANNINI A., MEDEARIS D. N., Jr Mouse salivary gland virus infections. Am J Hyg. 1961 May;73:329–343. doi: 10.1093/oxfordjournals.aje.a120192. [DOI] [PubMed] [Google Scholar]
- MIMS C. A. ASPECTS OF THE PATHOGENESIS OF VIRUS DISEASES. Bacteriol Rev. 1964 Mar;28:30–71. doi: 10.1128/br.28.1.30-71.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIMS C. A. An analysis of the toxicity for mice of influenza virus. II. Intravenous toxicity. Br J Exp Pathol. 1960 Dec;41:593–598. [PMC free article] [PubMed] [Google Scholar]
- MYRVIK Q., LEAKE E. S., FARISS B. Studies on pulmonary alveolar macrophages from the normal rabbit: a technique to procure them in a high state of purity. J Immunol. 1961 Feb;86:128–132. [PubMed] [Google Scholar]
- Makinodan T., Perkins E. H., Chen M. G. Immunologicc activity of the aged. Adv Gerontol Res. 1971;3:171–198. [PubMed] [Google Scholar]
- Meltzer M. S. Tumoricidal responses in vitro of peritoneal macrophages from conventionally housed and germ-free nude mice. Cell Immunol. 1976 Mar 1;22(1):176–181. doi: 10.1016/0008-8749(76)90018-6. [DOI] [PubMed] [Google Scholar]
- Merigan T. C. Host defenses against viral disease. N Engl J Med. 1974 Feb 7;290(6):323–329. doi: 10.1056/NEJM197402072900608. [DOI] [PubMed] [Google Scholar]
- Mogensen S. C., Andersen H. K. Effect of silica on the pathogenic distinction between herpes simplex virus type 1 and 2 hepatitis in mice. Infect Immun. 1977 Aug;17(2):274–277. doi: 10.1128/iai.17.2.274-277.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen S. C., Andersen H. K. Role of activated macrophages in resistance of congenitally athymic nude mice to hepatitis induced by herpes simplex virus type 2. Infect Immun. 1978 Mar;19(3):792–798. doi: 10.1128/iai.19.3.792-798.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen S. C. Biological conditions influencing the focal necrotic hepatitis test for differentiation between herpes simplex virus types 1 and 2. Acta Pathol Microbiol Scand B. 1976 Jun;84(3):154–158. [PubMed] [Google Scholar]
- Mogensen S. C. Genetics of macrophage-controlled resistance to hepatitis induced by herpes simplex virus type 2 in mice. Infect Immun. 1977 Aug;17(2):268–273. doi: 10.1128/iai.17.2.268-273.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen S. C. Macrophages and age-dependent resistance to hepatitis induced by herpes simplex virus type 2 im mice. Infect Immun. 1978 Jan;19(1):46–50. doi: 10.1128/iai.19.1.46-50.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen S. C., Teisner B., Andersen H. K. Focal necrotic hepatitis in mice as a biological marker for differentiation of Herpesvirus hominis type 1 and type 2. J Gen Virol. 1974 Oct;25(1):151–155. doi: 10.1099/0022-1317-25-1-151. [DOI] [PubMed] [Google Scholar]
- Mogensen S. Role of macrophages in hepatitis induced by Herpes simplex virus types 1 and 2 in mice. Infect Immun. 1977 Mar;15(3):686–691. doi: 10.1128/iai.15.3.686-691.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morahan P. S., Glasgow L. A., Crane J. L., Jr, Kern E. R. Comparison of antiviral and antitumor activity of activated macrophages. Cell Immunol. 1977 Feb;28(2):404–415. doi: 10.1016/0008-8749(77)90122-8. [DOI] [PubMed] [Google Scholar]
- Nickol A. D., Bonventre P. F. Anomalous high native resistance to athymic mice to bacterial pathogens. Infect Immun. 1977 Dec;18(3):636–645. doi: 10.1128/iai.18.3.636-645.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The mitotic potential of fixed phagocytes in the liver as revealed during the development of cellular immunity. J Exp Med. 1969 Aug 1;130(2):315–326. doi: 10.1084/jem.130.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke E. J., Halstead S. B., Allison A. C., Platts-Mills T. A. Specific lethality of silica for human peripheral blood mononuclear phagocytes, in vitro. J Immunol Methods. 1978;19(2-3):137–151. doi: 10.1016/0022-1759(78)90174-6. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J., Mitchell G. F., McDevitt H. O. Histocompatibility-linked genetic control of disease susceptibility. Murine lymphocytic choriomeningitis virus infection. J Exp Med. 1973 May 1;137(5):1201–1212. doi: 10.1084/jem.137.5.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panijel J., Cayeux P. Immunosuppressive effects of macrophage antiserum. Immunology. 1968 Jun;14(6):769–780. [PMC free article] [PubMed] [Google Scholar]
- ROBERTS J. A. GROWTH OF VIRULENT AND ATTENUATED ECTROMELIA VIRUS IN CULTURED MACROPHAGES FROM NORMAL AND ECTROMELIAIMMUNE MICE. J Immunol. 1964 Jun;92:837–842. [PubMed] [Google Scholar]
- ROBERTS J. A. HISTOPATHOGENESIS OF MOUSEPOX: III. ECTROMELIA VIRULENCE. Br J Exp Pathol. 1963 Oct;44:465–472. [PMC free article] [PubMed] [Google Scholar]
- RUCHMAN I. Virulence of strains of herpes simplex virus for mice. Proc Soc Exp Biol Med. 1954 Aug-Sep;86(4):649–652. doi: 10.3181/00379727-86-21191. [DOI] [PubMed] [Google Scholar]
- Rager-Zisman B., Allison A. C. The role of antibody and host cells in the resistance of mice against infection by coxsackie B-3 virus. J Gen Virol. 1973 Jun;19(3):329–338. doi: 10.1099/0022-1317-19-3-329. [DOI] [PubMed] [Google Scholar]
- Rao G. R., Rawls W. E., Perey D. Y., Tompkins W. A. Macrophage activation in congenitally athymic mice raised under conventional or germ-free conditions. J Reticuloendothel Soc. 1977 Jan;21(1):13–20. [PubMed] [Google Scholar]
- Renis H. E., Eidson E. E., Mathews J., Gray J. E. Pathogenesis of herpes simplex virus types 1 and 2 in mice after various routes of inoculation. Infect Immun. 1976 Aug;14(2):571–578. doi: 10.1128/iai.14.2.571-578.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roser B. The distribution of intravenously injected Kupffer cellsin the mouse. J Reticuloendothel Soc. 1968 Oct;5(5):455–471. [PubMed] [Google Scholar]
- Roser B. The distribution of intravenously injected peritoneal macrophages in the mouse. Aust J Exp Biol Med Sci. 1965 Aug;43(4):553–562. doi: 10.1038/icb.1965.41. [DOI] [PubMed] [Google Scholar]
- Rygaard J., Povlsen C. O. The mouse mutant nude does not develop spontaneous tumours. An argument against immunological surveillance. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Feb;82(1):99–106. doi: 10.1111/j.1699-0463.1974.tb02299.x. [DOI] [PubMed] [Google Scholar]
- SABIN A. B. Genetic factors affecting susceptibility and resistance to virus diseases of the nervous system. Res Publ Assoc Res Nerv Ment Dis. 1954;33:57–66. [PubMed] [Google Scholar]
- SCHELL K. Studies on the innate resistance of mice to infection with mousepox. I. Resistance and antibody production. Aust J Exp Biol Med Sci. 1960 Aug;38:271–288. doi: 10.1038/icb.1960.29. [DOI] [PubMed] [Google Scholar]
- Sabin A. B. Nature of Inherited Resistance to Viruses Affecting the Nervous System. Proc Natl Acad Sci U S A. 1952 Jun;38(6):540–546. doi: 10.1073/pnas.38.6.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz R. M., Woan M. C., Tompkins W. A. Macrophage immunity to vaccina virus: factors affecting macrophage immunity in vitro. J Reticuloendothel Soc. 1974 Jul;16(1):37–47. [PubMed] [Google Scholar]
- Selgrade M. K., Osborn J. E. Role of macrophages in resistance to murine cytomegalovirus. Infect Immun. 1974 Dec;10(6):1383–1390. doi: 10.1128/iai.10.6.1383-1390.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayegani M., Lief F. S., Mudd S. Specific and nonspecific cell-mediated resistance to influenza virus in mice. Infect Immun. 1974 Jun;9(6):991–998. doi: 10.1128/iai.9.6.991-998.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shif I., Bang F. B. In vitro interaction of mouse hepatitis virus and macrophages from genetically resistant mice. I. Adsorption of virus and growth curves. J Exp Med. 1970 Apr 1;131(4):843–850. doi: 10.1084/jem.131.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shif I., Bang F. B. In vitro interaction of mouse hepatitis virus and macrophages from genetically resistant mice. II. Biological characterization of a variant virus MHV (C3H) isolated from stocks of MHV(PRI). J Exp Med. 1970 Apr 1;131(4):851–862. doi: 10.1084/jem.131.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shif I., Bang F. B. Plaque assay for mouse hepatitis virus (MHV-2) on primary macrophage cell cultures. Proc Soc Exp Biol Med. 1966 Mar;121(3):829–831. doi: 10.3181/00379727-121-30899. [DOI] [PubMed] [Google Scholar]
- Smith H. Mechanisms of virus pathogenicity. Bacteriol Rev. 1972 Sep;36(3):291–310. doi: 10.1128/br.36.3.291-310.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr S. E., Allison A. C. Role of T lymphocytes in recovery from murine cytomegalovirus infection. Infect Immun. 1977 Aug;17(2):458–462. doi: 10.1128/iai.17.2.458-462.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr S. E., Visintine A. M., Tomeh M. O., Nahmias A. J. Effects of immunostimulants on resistance of newborn mice to herpes simplex type 2 infection. Proc Soc Exp Biol Med. 1976 May;152(1):57–60. doi: 10.3181/00379727-152-39327. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Restriction of herpes simplex virus by macrophages. An analysis of the cell-virus interaction. J Exp Med. 1971 Jan 1;133(1):19–38. doi: 10.1084/jem.133.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS J. A. Conception du système réticulohistiocytaire; la régulation de l'état hystiocytaire et la spécificité cellulaire. Rev Hematol. 1949;4(4):639–654. [PubMed] [Google Scholar]
- Tegtmeyer P. J., Craighead J. E. Infection of adult mouse macrophages in vitro with cytomegalovirus. Proc Soc Exp Biol Med. 1968 Dec;129(3):690–694. doi: 10.3181/00379727-129-33399. [DOI] [PubMed] [Google Scholar]
- Tosolini F. A., Mims C. A. Effect of murine strain and viral strain on the pathogenesis of lymphocytic choriomeningitis infection and a study of footpad responses. J Infect Dis. 1971 Feb;123(2):134–144. doi: 10.1093/infdis/123.2.134. [DOI] [PubMed] [Google Scholar]
- Turner G. S., Ballard R. Interaction of mouse peritoneal macrophages with fixed rabies virus in vivo and in vitro. J Gen Virol. 1976 Feb;30(2):223–231. doi: 10.1099/0022-1317-30-2-223. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. Properties and some uses of anti-macrophage antibodies. Nature. 1968 Apr 6;218(5136):36–38. doi: 10.1038/218036a0. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Adv Immunol. 1972;15:95–165. doi: 10.1016/s0065-2776(08)60684-7. [DOI] [PubMed] [Google Scholar]
- VAINIO T., GWATKIN R., KOPROWSKI H. Production of interferon by brains of genetically resistant and susceptible mice infected with West Nile virus. Virology. 1961 Jul;14:385–387. doi: 10.1016/0042-6822(61)90328-2. [DOI] [PubMed] [Google Scholar]
- Vahlne A., Blomberg J., Olofsson S., Lycke E. Subtyping of herpes simplex virus. Acta Pathol Microbiol Scand B. 1975 Oct;83(5):506–512. doi: 10.1111/j.1699-0463.1975.tb00131.x. [DOI] [PubMed] [Google Scholar]
- Virelizier J. L., Allison A. C. Correlation of persistent mouse hepatitis virus (MHV-3) infection with its effect on mouse macrophage cultures. Arch Virol. 1976;50(4):279–285. doi: 10.1007/BF01317953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardley R. C., Rouse B. T., Babiuk L. A. The mammary gland of the ox: a convenient source for the repeated collection of neutrophils and macrophages. J Reticuloendothel Soc. 1976 Jan;19(1):29–36. [PubMed] [Google Scholar]
- Weiser W. Y., Bang F. B. Blocking of in vitro and in vivo susceptibility to mouse hepatitis virus. J Exp Med. 1977 Nov 1;146(5):1467–1472. doi: 10.1084/jem.146.5.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser W., Bang F. B. Macrophages genetically resistant to mouse hepatitis virus converted in vitro to susceptible macrophages. J Exp Med. 1976 Mar 1;143(3):690–695. doi: 10.1084/jem.143.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser W., Vellisto I., Bang F. B. Congenic strains of mice susceptible and resistant to mouse hepatitis virus. Proc Soc Exp Biol Med. 1976 Sep;152(4):499–502. doi: 10.3181/00379727-152-39426. [DOI] [PubMed] [Google Scholar]
- Willenborg D. O., Shah K. V., Bang F. B. Effect of cyclophosphamide on the genetic resistance of C 3 H mice to mouse hepatitis virus. Proc Soc Exp Biol Med. 1973 Mar;142(3):762–766. doi: 10.3181/00379727-142-37111. [DOI] [PubMed] [Google Scholar]
- Wirth J. J., Levy M. H., Wheelock E. F. Use of silica to identify host mechanisms involved in suppression of established Friend virus leukemia. J Immunol. 1976 Dec;117(6):2124–2130. [PubMed] [Google Scholar]
- Yoon J. W., Notkins A. L. Virus-induced diabetes mellitus. VI. Genetically determined host differences in the replicating of encephalomyocarditis virus in pancreatic beta cells. J Exp Med. 1976 May 1;143(5):1170–1185. doi: 10.1084/jem.143.5.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZILVERSMIT D. B., BOYD G. A., BRUCER M. The effect of particle size on blood clearance and tissue distribution of radioactive gold colloids. J Lab Clin Med. 1952 Aug;40(2):255–260. [PubMed] [Google Scholar]
- Zinkernagel R. M., Blanden R. V. Macrophage activation in mice lacking thymus-derived (T) cells. Experientia. 1975 May 15;31(5):591–593. doi: 10.1007/BF01932477. [DOI] [PubMed] [Google Scholar]
- Zisman B., Hirsch M. S., Allison A. C. Selective effects of anti-macrophage serum, silica and anti-lymphocyte serum on pathogenesis of herpes virus infection of young adult mice. J Immunol. 1970 May;104(5):1155–1159. [PubMed] [Google Scholar]
- Zisman B., Wheelock E. F., Allison A. C. Role of macrophages and antibody in resistance of mice against yellow fever virus. J Immunol. 1971 Jul;107(1):236–243. [PubMed] [Google Scholar]
- duBuy H. Effect of silica on virus infections in mice and mouse tissue culture. Infect Immun. 1975 May;11(5):996–1002. doi: 10.1128/iai.11.5.996-1002.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Furth R., Cohn Z. A., Hirsch J. G., Humphrey J. H., Spector W. G., Langevoort H. L. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ. 1972;46(6):845–852. [PMC free article] [PubMed] [Google Scholar]
- van der Groen G., Vanden Berghe D. A., Pattyn S. R. Interaction of mouse peritoneal macrophages with different arboviruses in vitro. J Gen Virol. 1977 Feb;34(2):353–361. doi: 10.1099/0022-1317-34-2-353. [DOI] [PubMed] [Google Scholar]