The laminins are a secreted family of heterotrimeric molecules essential for basement membrane (BM) formation, structure and function1, 2. Through the study of blistering skin diseases, it is now well established that the α3 subunit of laminins-332, -321 and -311 plays an important role in mediating epidermal-dermal integrity and is essential for the skin to withstand mechanical stresses 3. However, these laminins also regulate cell migration and mechanosignal transduction 4–8. The precise mechanisms involved in cell migration and signaling are not yet fully clarified. This review will provide an overview of the gene, transcripts and protein structures of laminin α3, and we will briefly discuss the proposed functions for the α3 subunit-containing laminins.
LAMA3 gene structure and expression regulation
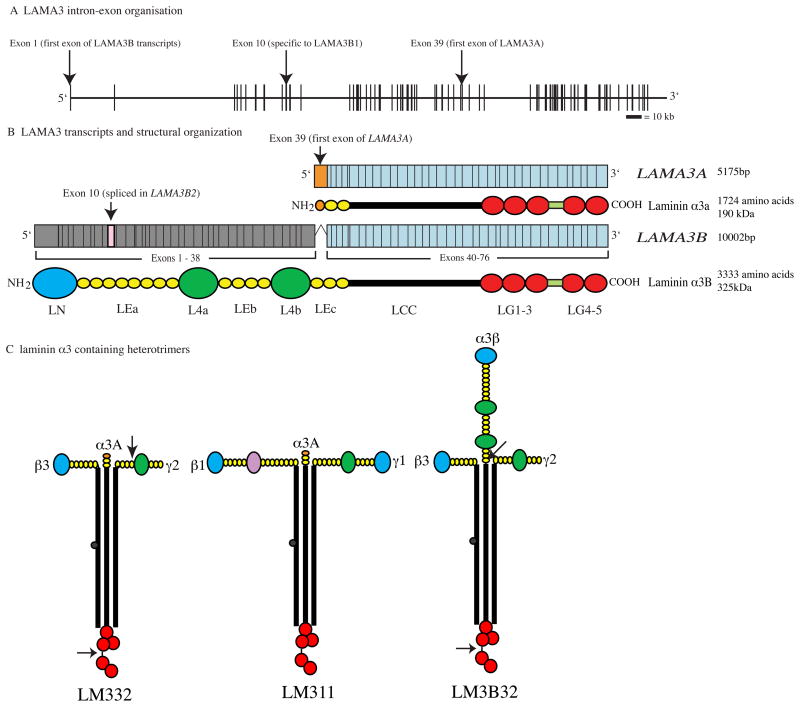
The human LAMA3 gene encodes 76 exons from 318kb of genomic DNA at chromosomal location 18q11.2 (Figure 1A) 9, 10. Isolation of cDNA clones has revealed the presence of two major transcripts: LAMA3A and LAMA3B (Figure 1B). Both of these transcripts share a common 3′ end that includes exons 40 through 76. However, through alternate promoter usage their 5′ ends are markedly different. LAMA3A, encoding laminin α3A, is expressed from a promoter within exon 38 and therefore its protein product is encoded by exons 39–76 (5175 bp open reading frame, encoding 1724 amino acids, calculated molecular weight 190 kDa, Figure 1B). LAMA3B is much longer, consisting of exons 1 to 38 and the common 3′ exons 40–76; exon 39 is skipped (10002 bp open reading frame, encoding 3333 amino acids, calculated molecular weight 366 kDa; Figure 1B) 9–12. In addition, at the message level about 20% of keratinocyte LAMA3B has exon 10 skipped. This shorter isoform has been termed laminin α3B2, whereas the full length transcript encodes laminin α3B1 (Figure 1B)10.
Figure 1.
A) Genomic organization of LAMA3 gene, vertical bars represent exons, horizontal line introns. B) LAMA3A and LAMA3B transcript organization; blue exons – common to both transcripts, orange -specific to LAMA3A, grey – specific to LAMA3B, pink – specific to LAMA3B2, Below each transcript is a diagrammatic representation of the domain architecture; LN – laminin N terminal domain, LE – laminin-type epidermal growth factor-like repeats, L4 – globular domain, LCC – laminin coiled coil domain, LG laminin globular domains. C) Laminin α3 containing heterotrimer structure. Color scheme of conserved domains as in B, with purple - Lβ-laminin β chain globular domain. Arrows in LM332 and LM3B32 indicate processing points discussed in the text.
Testing of the promoter regions for both LAMA3 transcripts reveals them to be responsive to typical epithelial/mesenchymal transcription factors: epidermal growth factor, keratinocyte growth factor, insulin-like growth factor-1, thymosin beta 4, interferon-γ, transforming growth factor (TGF)-α, TGF-β1 and tumour necrosis factor-α 13–17. Both promoters also contain acute phase reactant sequences and interleukin-6 binding sequences, both of which are found in many proteins upregulated at sites of trauma 18.
Through RNase protection assays on total RNA from adult human tissues, Doliana et al have investigated the expression pattern of LAMA3A and LAMA3B 12. Spleen, stomach, kidney, skeletal muscle, pancreas and adrenal gland express similar levels of both transcripts, whereas the salivary gland expresses only LAMA3A. Placenta expresses the highest LAMA3A message, while the uterus expresses the highest LAMA3B message 12. In situ RT-PCR of human embryonic tissues reveals positive staining for LAMA3 (not LAMA3A/B specific) message in developing tubules and developing comma-shaped bodies of the kidney, epithelial cells of the developing lung, in the basal layer of developing skin at gestational week 6.5, and in all layers of the epidermis from gestational week 8 onwards 19.
Laminin α3A/B subunit domain architecture and assembly isoforms
The laminin family of proteins share a common architecture with regions of conserved protein folding 20. Laminins are secreted as heterotrimeric cross-shaped molecules consisting of one α, one β and one γ subunit which assemble intracellularly through a coiled-coil domain termed the LCC (formerly known as domains I and II). In laminin α subunits, this LCC is followed by five globular domains (termed LG1-5). The link between LG3 and 4 is slightly extended in the laminin α3 subunit relative to other laminin α subunits, and is the site of an extracellular processing event (see below) 21–23. In the laminin α3A subunit, the LCC is preceded by a short stretch of rod-like, laminin-type epithelial growth factor-like domains (LE, formerly domain V). In contrast, the amino terminus of laminin α3B subunit is much longer, consisting of a ~250 amino acid laminin N-terminal domain (LN domain, previously domain VI), which has been shown to be important for higher order network formation through co- and self-polymerization 24, 25. The LN domain is followed by three stretches of rod-like LE domains (of 8, 4 and 3 repeats respectively) which are interspersed by two ~250 amino acid globular domains (termed L4a and L4b, previously domain IV, Figure 1B) 20.
The functionality of individual laminin subunits depends not only upon their own domain composition but also upon that of the laminin subunits with which they associate. In terms of laminin α3A subunit, the most abundant and most studied isoform is laminin 332, comprising laminin α3A, β3 and γ2 (LM332, formerly known as laminin 5/kalinin/epiligrin/ladsin, Figure 1C) 21, 26. In addition, laminin α3A associates with laminin β1 and γ1 forming LM311 (laminin 6, k-laminin Figure 1C) and, from co-immunoprecipitation data, with β2 and γ1 to form LM321 (laminin 7, Figure 1C) 2, 21, 27. The expression profile LM332, as expected, roughly matches that of its constituent mRNAs. Immunofluorescence staining for LM332 in adult tissues gives positive results in the BM of glomeruli and tubuli in kidney, the BM of alveoli, bronchioli and bronchi in lung, in the dermal epidermal junction of skin, corneal basement membrane and in the enteric basement membrane zone of the small intestine under the intestinal epithelium 19, 28.
Laminin heterotrimer formation proceeds via a βγ dimer stage and appears to be dependent upon sequences toward the C-terminus of the LCC 30–32. In theory, therefore, the laminin α3B subunit should be capable of associating with the same repertoire of β and γ laminin subunits as α3A. However, although immunohistological analyses has suggested the presence of LM3B11 in the basement membrane of blood vessels 29, only LM3B32 has been studied in any detail to date (Figure 1C). Interestingly, while βγ dimers require α laminin subunit incorporation to drive secretion, there is evidence that laminin α subunits can be secreted independently of trimerization, the functional significance of this observation is yet be established 30.
Human LM332 is secreted as a 460 kDa species that is subsequently processed to a predominant 440 kDa form in keratinocytes maintained in low calcium medium (0.035mM) and to a predominant 400 kDa form in keratinocytes maintained at higher concentrations of calcium (1mM) 33. These size shifts are due to processing of the C-terminus of the α3A subunit which removes LG domains 4 and 5 and converts it from ~190 kDa to 165 kdDa, and processing of the γ2 subunit towards its N-terminus, converting it from a 155 kDa form to105 kDa. 33. LM332 containing the 165 kDa α3A and 105 kDa γ2 processed subunits is sometimes termed mature (matLM332). Processing of the laminin α3B subunit converts it from ~325 kDa to a ~280 kDa mature form 25. An additional minor product of 145 kDa, which is recognized by laminin α3 antibodies, has also been identified in extracts from human amnion, with the secondary processing occurring at the N-terminus, just prior to the LCC 33. Interestingly in vitro studies have demonstrated that laminin α3A in LM311 is processed at a much lower rate than when it is incorporated into LM332, which may be relevant with regards to some of the functional differences between these heterotrimers which we will discuss below 34.
The function of the laminin α3 subunit in cellular adhesion
In epithelial cells LM332 is able to interact with two integrins, α6β4 and α3β1, and thereby is of central importance in the function of the two major forms of dermal-epidermal junctions, namely hemidesmosomes and focal adhesions 35–38.
Hemidesmosome formation
Hemidesmosomes are specialized adhesion structures which provide linkage from LM332 to the intermediate filament cytoskeleton. This linkage is established through the association of the extracellular domains of α6β4 integrin with the laminin α3 subunit and through binding of the intracellular tail of β4 integrin to the plakin molecule plectin (HD1). Plectin, in turn, interacts with the keratin cytoskeleton 39–41. Adhesion is further strengthened through the association with the transmembrane protein, bullous pemphigoid antigen 2 (collagen XVIII), which also interacts with LM332 and by binding to β4 integrin of a second plakin molecule termed BPAG1e (BP230), which acts to strengthen the link to the keratins 42–44
Carboxy terminal processing of laminin α3A may regulate the assembly of hemidesomsomes. Specifically, in tissue culture, only the matrix of cell lines that contain a C-terminally processed form of laminin α3A supports formation of hemidesmosomes 22. Furthermore, treatment with plasmin of an extracellular matrix rich in LM332, but containing an unprocessed α3A laminin subunit that fails to support hemidesmosome formation, results in laminin α3A processing and conversion of that matrix to one which is competent to induce HD assembly 22. In addition to plasmin, all of the Bone Morphogenetic Protein-1 isoenzymes (BMP, Mammalian Tolloid; mTLD, Mammalian tolloid-like1 and –2; mTLL1, mTLL2) have been shown to be capable of processing the laminin α3A subunit to 165 kDa and the laminin γ2 subunit to 105 kDa in vitro 45, 46. The skin of mice deficient for mTLD/BMP1 exhibits defects in hemidesomosomes 46, suggesting the importance of such processing for hemidesmosome formation.
The importance in vivo of laminin α3 in dermal-epidermal adhesion is dramatically exemplified by skin blistering at the dermal-epidermal junction from mutation of the LAMA3 gene in patients with junctional epidermolysis bullosa (JEB), in which hemidesmosomes are either entirely undetectable ultrastructurally or are reduced in number and aberrant (see discussion of JEB in other articles of this issue).
Focal contact formation
In contrast to hemidesmosomes, which provide a link from LM332 to the keratin cytoskeleton, focal adhesions provide a link from LM332 to the actin cytoskeleton through interaction of the laminin α3 subunit with α3β1 integrin 37, 47. α3β1 integrin, in turn, interacts with a number of linker molecules which mediate the association of the actin cytoskeleton with the cell surface 48. Moreover, α3β1 integrin also interacts with molecules involved in signal transduction 37, 49, 50. In cultured epidermal cells, α3β1 integrin is found clustered at the site of focal adhesions 37. In intact skin, focal adhesions are not obvious and are therefore likely transient matrix adhesion points that are assembled by actively moving cells 37. The identification of mutations in FERMT1, a gene encoding a focal contact protein termed Kindlin-1, have recently been demonstrated as pathogenic in another form of epidermolysis bullosa associated with photosensitivity, the Kindler syndrome subtype. These data suggest that the LM332 - α3β1- actin linkage may also be required for maintenance of epithelia-dermal attachment integrity, however, whether the skin fragility of Kindler syndrome is a direct result of loss of LM332-α3β1 integrin linkage or indirect, due to disruption of HD in Kindlin-1 deficient skin, requires further investigation 51–53.
The laminin α3 subunit in cell migration and wound healing
There is considerable evidence that LM332 is an important regulator of cell migration 4,6, 7, 13, 54, 55. Histologically, LM332 is deposited into the provisional BM of healing wound beds within 8 hours of wounding 56. Moreover, in squamous cell carcinoma (SCC) an upregulation of LM332 correlates with poor prognosis as a result of increased metastatic potential 57. The precise mechanisms through which laminins with an α3 subunit regulate cell migration in controversial, particularly with respect to the roles of α3β1 and α6β4 integrin, and the functional significance of LM332 proteolytic processing 4, 58.
Historically, α3β1 integrin has been thought to promote cellular migration, while α6β4 integrin, due to its ability to nucleate hemidesmosome formation, has long been believed to retard migration by promoting stable adhesion 37, 59. However, a recent paper has suggested that the α3β1 integrin-LM332 interaction may actually slow wound healing rates, specifically that α3 integrin deficient keratinocytes migrate with increased velocity and persistence relative to controls 58. Furthermore, there is accumulating data suggesting that α6β4 integrin positively regulates skin cell migration 4, 60, 61.
Processing and regulation of motility and proliferation
The role of proteolytic processing of LM332 in regulating its function requires further clarification. The processed form of LM332 is known to be present in mature, unwounded skin, while the unprocessed form is deposited at the leading edge of acute wounds or in culture equivalents 22, 33, 62. As previously described, laminin α3 subunit processing is required for hemidesmosome formation 63. Similarly, using an antibody to LG4/5 domain of laminin α3, Frank and Carter showed that migrating keratinocytes deposit unprocessed laminin α3 in a linear trail that marks the path of migration 6.
Interestingly, the presence of the released LG4-5 region also seems to aid deposition of LM332 or its incorporation into the basement membrane. Thus, processing could drive a localized increase in LM332 concentration, which in turn may enhance integrin clustering and signaling activities 64, 65. Consistent with this, the level of LM332 deposition in SCCs correlates well with their invasive potential 55, 65, 66. Given that the unprocessed form of the laminin α3A subunit is predominantly found in SCC, while only the mature, processed subunit is present in unwounded skin, the Marinkovich group has generated an antibody specific for the LG4-5 region of the laminin α3A subunit that might specifically target SCC cells therapeutically 66. Indeed, in a mouse model of humanized SCC, treatment with a LG4-5 antibodies induced a significant decrease in tumor volume without causing skin fragility 66.
The C-terminus of the laminin α3 subunit may also activate cell proliferative responses. Function-inhibiting antibodies to the laminin α3 LG domain inhibit proliferation of epithelial cells and decrease the level of p42/p44 MAPK activity 47. Ligation of either of its integrin binding partners may be responsible for initiation of this response. Ligation of α6β4 integrin by LM332 induces phosphorylation of the β4 cytoplasmic domain. The Shc adaptor protein binds to these phosphorylated tyrosines and is subsequently tyrosine phosphorylated. Upon phosphorylation, Shc recruits Grb2 (which is stably associated with the Ras-GTP exhange factor mSOS) and this leads to activation of the Ras-Erk and Rac-Jnk MAPK pathways 60, 67, 68. Similarly, function blocking antibodies to integrin α3 and β1 also block proliferation and MAPK phosphorylation. Further, laminin α3 subunit antibody-induced inhibition of proliferation can be rescued through treatment with β1 activating antibodies, indicating that α3β1 integrin likely mediates signals initiated by LM332 that control growth and drive proliferation 47.
Laminin γ2 subunit processing is also an important regulator of LM332 function. The second stretch of LE repeats in the laminin γ2 subunit has been shown to be capable of interacting with the epidermal growth factor (EGFR) and therefore it has been proposed that the amino terminal processing of the laminin γ2 subunit exposes this region and allows this interaction to occur, thereby triggering cell motility 69.
LM332 deposition
A critical aspect of appropriate cell migration is the ability to move in a polarized manner and this ability is dependent on the exogenous ligand presented to cells; in one study approximately 50% of cultured epithelial cells displayed a polarised phenotype when plated on LM332, compared to only ~11% when plated onto collagen 6. However, the precise way LM332 is deposited, rather than deposition alone, is most important in supporting directed keratinocyte migration 4.
The involvement of both α3β1 integrin and α6β4 integrin in LM332 deposition has been made apparent through analyses of LM332 matrix patterns in keratinocytes deficient in either α3 integrin or β4 integrin 4, 70. Specifically, keratinocytes derived from α3 integrin null mice deposit LM332 into spikes and arrowhead patterns, compared to more diffuse arcs in wild type keratinocytes 70. Furthermore, α3 integrin deficient keratinocytes are unable to reorganize precoated LM332 into ring structures in the same way that wild-type cells do 70. In comparison, migrating human cells deficient in integrin β4 deposit LM332 in circular arrays, as compared to the linear trails deposited by migrating wild-type keratinocytes 4. Moreover, the precise way LM332 is deposited into the matrix is dominant with regards to motile behavior, since plating β4 deficient cells onto the LM332 trails deposited by wild-type cells restores their migration patterns while plating wild type cells onto the circular tracks laid down by β4 deficient cells leads to a circular motility phenotype 4.
Multiple further studies have implicated a role for the actin cytoskeleton in determining the specific arrangement of LM332 in the matrix of cultured keratinocytes. Inhibition of actomyosin contraction in wild-type cells, either through drug treatment or through introduction of dominant negative forms of the Rac, Rho and Cdc42 small GTPases, leads to an aberrant organization of LM332 4, 71. It has also been observed that reorganization of precoated LM332 occurs in regions which have been extended over by filopodia and lamellipodia 70. The different ability of α3β1 and α6β4 integrins to activate RhoGTPase family members plays a role in their mediating deposition of LM332. Specifically, α3 integrin has been implicated in the activation of RhoA, while β4 integrin regulates Rac activity 4, 60, 72, 73. In the case of α6β4 this regulation likely is due to formation of a complex with Rac, since Rac can be co-immunoprecipiated with β4 integrin and the activity level of Rac is decreased in β4 deficient cells 4, 74. Downstream of Rac, activity of the actin severing and remodelling protein cofilin is also reduced in β4 deficient cells 4. Rac and cofilin activity are intrinsically linked to directed migration through their ability to nucleate and drive extension of lamellipodia; therefore, the α6β4 integrin-Rac association may provide a means of spatially restricting this signalling 75, 76. Intriguingly, recent data indicates that the interaction of β4 integrin with Rac, and Rac activition, is dependent upon BPAG1e and further, that BPAG1e knockdown cells show a loss of front-rear polarity (Hamill et al. Mol. Biol. Cell in press). These results are somewhat surprising since both α6β4 integrin and BPAG1e are hemidesmosomal components and have been thought to be primarily involved in stable adhesion rather than migration 43.
Other Laminins with an α3 subunit
1) LM311
To this point the data discussed refers almost exclusively to laminin α3A as part of LM332. However, in various tissues including bronchial epithelial cells laminin α3a associates with β1 and γ1 to form LM311 (laminin 6) 2. Recently, a distinct mechano-signaling function for laminin α3A within this context has been demonstrated using rat primary alveolar endothelial cells (AEC) grown on elastomer membranes and stretched to mimic deformation during breathing. AEC cells secrete a fibrous matrix enriched for LM311, perlecan and nidogen secreted in ‘cable-like’ structures 8. Stretching of AEC cells on this matrix leads to activation of p42/p44 MAPK whilst treatment with function blocking antibodies to laminin α3 decreases MAPK phosphorylation by 40%. Similarly, α-dystroglycan antibody inhibition or shRNA knockdown leads to a ~30% or ~50% decrease respectively whereas antibodies to integrin α3 and β1 has no affect 8. These data implicate LM311 as having a role in stretch-induced signaling, and further, that this signalling involves the cell surface receptor dystroglycan.
In addition to mechanosignalling in the lung, LM311, along with LM321, can be isolated from skin and human amnion basement membrane. Isolated LM311 has cell adhesive and cell migration supporting activities but both of these are significantly less than that observed for LM332 34. Most strikingly in JEB patients with mutations in LAMB3, LM311 is still produced but is unable to provide sufficient adhesive capability to prevent blister formation65. However, from rotary shadowed images of complexes it appears that LM332 and LM311 interact via their short (amino terminal) arms and there could be cooperativity of actions 27, 34. Compared to the other laminin heterotrimers, LM332 is significantly different in that its short arms are much shorter and that both the α3A and γ2 chains lacks the amino terminal LN domain through which other laminins form order network structures (Figure 1B) 25, 77. Association, therefore in the BM of LM332 with LM311 and LM321 may enable the construction of a more cohesive, integrated network of laminins, which may lead to an increased ability to withstand stresses.
2) LM3B32
As described above, a longer form laminin α3 subunit is derived from the LAMA3 gene, laminin α3B, which differs from the laminin α3A subunit in the length of its amino terminus (Figure 1B). The greatest functional significance of this is likely to be the presence of the LN domain, which, may allow self and co-polymerisation with other LN domain containing laminins (similarly LM311/LM321 may also be able to from higher order networks due to the presence of the LN domain containing β1/2 and γ1 chains) 25, 77. Interestingly, one of the few studies that has been carried out of laminin α3B function has demonstrated significantly higher cell adhesion activities and cell migration promoting activities for LM3B32 compared to LM332 78. In addition to the activities of the intact molecule, proteolytic processing of the amino terminus releases a 190kDa fragment which, through interaction with α3β1 integrin, promotes adhesion, migration and proliferation 78. These data present the interesting possibility that two regions of the same protein, separated by 100+nm long rod domain, are capable of stimulating the same processes through ligation of the same integrin. Clearly, further research is required to shed light onto the regulation and transition between the N and C terminal mediated signaling responses of LM3B32.
Conclusions and perspectives
Analyses of blistering skin diseases and epithelial cells in culture have shed considerable light on the functions of the laminin α3 subunit. It is now apparent that the α3 subunit is a multifunctional molecule with roles in adhesion, motility and signaling. We now know much about its processing and its receptor binding, and are beginning to dissect how such processing and interactions regulate behavior of cells in a variety of tissues. Further work is needed on defining how the laminin α3 subunit functions in a tissue context. In addition, we still know very little about some of the functions of those laminin trimers that contain splice variants or proteolytically cleaved versions of the laminin α3 subunit. These represent interesting avenues of future investigation.
Acknowledgments
Work in the Jones lab is supported by by grant RO1 AR054184 (JCRJ) from the NIH
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Amy S. Paller, Email: apaller@northwestern.edu.
Jonathan C.R. Jones, Email: j-jones3@northwestern.edu.
References
- 1.Sugawara K, Tsuruta D, Ishii M, Jones JC, Kobayashi H. Laminin-332 and -511 in skin. Exp Dermatol. 2008 Jun;17(6):473–480. doi: 10.1111/j.1600-0625.2008.00721.x. [DOI] [PubMed] [Google Scholar]
- 2.Marinkovich MP, Lunstrum GP, Keene DR, Burgeson RE. The dermal-epidermal junction of human skin contains a novel laminin variant. J Cell Biol. 1992 Nov;119(3):695–703. doi: 10.1083/jcb.119.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nievers MG, Schaapveld RQ, Sonnenberg A. Biology and function of hemidesmosomes. Matrix Biol. 1999 Feb;18(1):5–17. doi: 10.1016/s0945-053x(98)00003-1. [DOI] [PubMed] [Google Scholar]
- 4.Sehgal BU, DeBiase PJ, Matzno S, et al. Integrin beta4 regulates migratory behavior of keratinocytes by determining laminin-332 organization. J Biol Chem. 2006 Nov 17;281(46):35487–35498. doi: 10.1074/jbc.M606317200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budinger GR, Urich D, DeBiase PJ, et al. Stretch-induced activation of AMP kinase in the lung requires dystroglycan. Am J Respir Cell Mol Biol. 2008 Dec;39(6):666–672. doi: 10.1165/rcmb.2007-0432OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frank DE, Carter WG. Laminin 5 deposition regulates keratinocyte polarization and persistent migration. J Cell Sci. 2004 Mar 15;117(Pt 8):1351–1363. doi: 10.1242/jcs.01003. [DOI] [PubMed] [Google Scholar]
- 7.Kariya Y, Miyazaki K. The basement membrane protein laminin-5 acts as a soluble cell motility factor. Exp Cell Res. 2004 Jul 15;297(2):508–520. doi: 10.1016/j.yexcr.2004.03.044. [DOI] [PubMed] [Google Scholar]
- 8.Jones JC, Lane K, Hopkinson SB, et al. Laminin-6 assembles into multimolecular fibrillar complexes with perlecan and participates in mechanical-signal transduction via a dystroglycan-dependent, integrin-independent mechanism. J Cell Sci. 2005 Jun 15;118(Pt 12):2557–2566. doi: 10.1242/jcs.02395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryan MC, Tizard R, VanDevanter DR, Carter WG. Cloning of the LamA3 gene encoding the alpha 3 chain of the adhesive ligand epiligrin. Expression in wound repair. J Biol Chem. 1994 Sep 9;269(36):22779–22787. [PubMed] [Google Scholar]
- 10.McLean WH, Irvine AD, Hamill KJ, et al. An unusual N-terminal deletion of the laminin alpha3a isoform leads to the chronic granulation tissue disorder laryngo-onycho-cutaneous syndrome. Hum Mol Genet. 2003 Sep 15;12(18):2395–2409. doi: 10.1093/hmg/ddg234. [DOI] [PubMed] [Google Scholar]
- 11.Vidal F, Baudoin C, Miquel C, et al. Cloning of the laminin alpha 3 chain gene (LAMA3) and identification of a homozygous deletion in a patient with Herlitz junctional epidermolysis bullosa. Genomics. 1995 Nov 20;30(2):273–280. doi: 10.1006/geno.1995.9877. [DOI] [PubMed] [Google Scholar]
- 12.Doliana R, Bellina I, Bucciotti F, Mongiat M, Perris R, Colombatti A. The human alpha3b is a ‘full-sized’ laminin chain variant with a more widespread tissue expression than the truncated alpha3a. FEBS Lett. 1997 Nov 3;417(1):65–70. doi: 10.1016/s0014-5793(97)01251-9. [DOI] [PubMed] [Google Scholar]
- 13.Amano S, Akutsu N, Ogura Y, Nishiyama T. Increase of laminin 5 synthesis in human keratinocytes by acute wound fluid, inflammatory cytokines and growth factors, and lysophospholipids. Br J Dermatol. 2004 Nov;151(5):961–970. doi: 10.1111/j.1365-2133.2004.06175.x. [DOI] [PubMed] [Google Scholar]
- 14.Kainulainen T, Hakkinen L, Hamidi S, et al. Laminin-5 expression is independent of the injury and the microenvironment during reepithelialization of wounds. J Histochem Cytochem. 1998 Mar;46(3):353–360. doi: 10.1177/002215549804600309. [DOI] [PubMed] [Google Scholar]
- 15.Korang K, Christiano AM, Uitto J, Mauviel A. Differential cytokine modulation of the genes LAMA3, LAMB3, and LAMC2, encoding the constitutive polypeptides, alpha 3, beta 3, and gamma 2, of human laminin 5 in epidermal keratinocytes. FEBS Lett. 1995 Jul 24;368(3):556–558. doi: 10.1016/0014-5793(95)00740-z. [DOI] [PubMed] [Google Scholar]
- 16.Virolle T, Monthouel MN, Djabari Z, Ortonne JP, Meneguzzi G, Aberdam D. Three activator protein-1-binding sites bound by the Fra-2.JunD complex cooperate for the regulation of murine laminin alpha3A (lama3A) promoter activity by transforming growth factor-beta. J Biol Chem. 1998 Jul 10;273(28):17318–17325. doi: 10.1074/jbc.273.28.17318. [DOI] [PubMed] [Google Scholar]
- 17.Sosne G, Xu L, Prach L, et al. Thymosin beta 4 stimulates laminin-5 production independent of TGF-beta. Exp Cell Res. 2004 Feb 1;293(1):175–183. doi: 10.1016/j.yexcr.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 18.Ferrigno O, Virolle T, Galliano MF, et al. Murine laminin alpha3A and alpha3B isoform chains are generated by usage of two promoters and alternative splicing. J Biol Chem. 1997 Aug 15;272(33):20502–20507. doi: 10.1074/jbc.272.33.20502. [DOI] [PubMed] [Google Scholar]
- 19.Miosge N, Kluge JG, Studzinski A, et al. In situ-RT-PCR and immunohistochemistry for the localisation of the mRNA of the alpha 3 chain of laminin and laminin-5 during human organogenesis. Anat Embryol (Berl) 2002 Oct;205(5–6):355–363. doi: 10.1007/s00429-002-0256-7. [DOI] [PubMed] [Google Scholar]
- 20.Aumailley M, Bruckner-Tuderman L, Carter WG, et al. A simplified laminin nomenclature. Matrix Biol. 2005 Jun 24; doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Marinkovich MP, Verrando P, Keene DR, et al. Basement membrane proteins kalinin and nicein are structurally and immunologically identical. Lab Invest. 1993 Sep;69(3):295–299. [PubMed] [Google Scholar]
- 22.Goldfinger LE, Stack MS, Jones JC. Processing of laminin-5 and its functional consequences: role of plasmin and tissue-type plasminogen activator. J Cell Biol. 1998 Apr 6;141(1):255–265. doi: 10.1083/jcb.141.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsubota Y, Mizushima H, Hirosaki T, Higashi S, Yasumitsu H, Miyazaki K. Isolation and activity of proteolytic fragment of laminin-5 alpha3 chain. Biochem Biophys Res Commun. 2000 Nov 30;278(3):614–620. doi: 10.1006/bbrc.2000.3851. [DOI] [PubMed] [Google Scholar]
- 24.Cheng YS, Champliaud MF, Burgeson RE, Marinkovich MP, Yurchenco PD. Self-assembly of laminin isoforms. J Biol Chem. 1997 Dec 12;272(50):31525–31532. doi: 10.1074/jbc.272.50.31525. [DOI] [PubMed] [Google Scholar]
- 25.Garbe JH, Gohring W, Mann K, Timpl R, Sasaki T. Complete sequence, recombinant analysis and binding to laminins and sulphated ligands of the N-terminal domains of laminin alpha3B and alpha5 chains. Biochem J. 2002 Feb 15;362(Pt 1):213–221. doi: 10.1042/0264-6021:3620213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyazaki K, Kikkawa Y, Nakamura A, Yasumitsu H, Umeda M. A large cell-adhesive scatter factor secreted by human gastric carcinoma cells. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11767–11771. doi: 10.1073/pnas.90.24.11767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Champliaud MF, Lunstrum GP, Rousselle P, Nishiyama T, Keene DR, Burgeson RE. Human amnion contains a novel laminin variant, laminin 7, which like laminin 6, covalently associates with laminin 5 to promote stable epithelial-stromal attachment. J Cell Biol. 1996 Mar;132(6):1189–1198. doi: 10.1083/jcb.132.6.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tuori A, Uusitalo H, Burgeson RE, Terttunen J, Virtanen I. The immunohistochemical composition of the human corneal basement membrane. Cornea. 1996 May;15(3):286–294. doi: 10.1097/00003226-199605000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Kariya Y, Mori T, Yasuda C, et al. Localization of laminin alpha3B chain in vascular and epithelial basement membranes of normal human tissues and its down-regulation in skin cancers. J Mol Histol. 2008 Aug;39(4):435–446. doi: 10.1007/s10735-008-9183-0. [DOI] [PubMed] [Google Scholar]
- 30.Yurchenco PD, Quan Y, Colognato H, et al. The alpha chain of laminin-1 is independently secreted and drives secretion of its beta- and gamma-chain partners. Proc Natl Acad Sci U S A. 1997 Sep 16;94(19):10189–10194. doi: 10.1073/pnas.94.19.10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nomizu M, Utani A, Beck K, Otaka A, Roller PP, Yamada Y. Mechanism of laminin chain assembly into a triple-stranded coiled-coil structure. Biochemistry. 1996 Mar 5;35(9):2885–2893. doi: 10.1021/bi951555n. [DOI] [PubMed] [Google Scholar]
- 32.Utani A, Nomizu M, Timpl R, Roller PP, Yamada Y. Laminin chain assembly. Specific sequences at the C terminus of the long arm are required for the formation of specific double- and triple-stranded coiled-coil structures. J Biol Chem. 1994 Jul 22;269(29):19167–19175. [PubMed] [Google Scholar]
- 33.Marinkovich MP, Lunstrum GP, Burgeson RE. The anchoring filament protein kalinin is synthesized and secreted as a high molecular weight precursor. J Biol Chem. 1992 Sep 5;267(25):17900–17906. [PubMed] [Google Scholar]
- 34.Hirosaki T, Tsubota Y, Kariya Y, Moriyama K, Mizushima H, Miyazaki K. Laminin-6 is activated by proteolytic processing and regulates cellular adhesion and migration differently from laminin-5. J Biol Chem. 2002 Dec 20;277(51):49287–49295. doi: 10.1074/jbc.M111096200. [DOI] [PubMed] [Google Scholar]
- 35.Rousselle P, Lunstrum GP, Keene DR, Burgeson RE. Kalinin: an epithelium-specific basement membrane adhesion molecule that is a component of anchoring filaments. J Cell Biol. 1991 Aug;114(3):567–576. doi: 10.1083/jcb.114.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carter WG, Ryan MC, Gahr PJ. Epiligrin, a new cell adhesion ligand for integrin alpha 3 beta 1 in epithelial basement membranes. Cell. 1991 May 17;65(4):599–610. doi: 10.1016/0092-8674(91)90092-d. [DOI] [PubMed] [Google Scholar]
- 37.Carter WG, Kaur P, Gil SG, Gahr PJ, Wayner EA. Distinct functions for integrins alpha 3 beta 1 in focal adhesions and alpha 6 beta 4/bullous pemphigoid antigen in a new stable anchoring contact (SAC) of keratinocytes: relation to hemidesmosomes. J Cell Biol. 1990 Dec;111(6 Pt 2):3141–3154. doi: 10.1083/jcb.111.6.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zamir E, Geiger B. Components of cell-matrix adhesions. J Cell Sci. 2001 Oct;114(Pt 20):3577–3579. doi: 10.1242/jcs.114.20.3577. [DOI] [PubMed] [Google Scholar]
- 39.Koster J, van Wilpe S, Kuikman I, Litjens SH, Sonnenberg A. Role of binding of plectin to the integrin beta4 subunit in the assembly of hemidesmosomes. Mol Biol Cell. 2004 Mar;15(3):1211–1223. doi: 10.1091/mbc.E03-09-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rousselle P, Aumailley M. Kalinin is more efficient than laminin in promoting adhesion of primary keratinocytes and some other epithelial cells and has a different requirement for integrin receptors. J Cell Biol. 1994 Apr;125(1):205–214. doi: 10.1083/jcb.125.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones JC, Hopkinson SB, Goldfinger LE. Structure and assembly of hemidesmosomes. Bioessays. 1998 Jun;20(6):488–494. doi: 10.1002/(SICI)1521-1878(199806)20:6<488::AID-BIES7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 42.Gagnoux-Palacios L, Gache Y, Ortonne JP, Meneguzzi G. Hemidesmosome assembly assessed by expression of a wild-type integrin beta 4 cDNA in junctional epidermolysis bullosa keratinocytes. Lab Invest. 1997 Nov;77(5):459–468. [PubMed] [Google Scholar]
- 43.Koster J, Geerts D, Favre B, Borradori L, Sonnenberg A. Analysis of the interactions between BP180, BP230, plectin and the integrin alpha6beta4 important for hemidesmosome assembly. J Cell Sci. 2003 Jan 15;116(Pt 2):387–399. doi: 10.1242/jcs.00241. [DOI] [PubMed] [Google Scholar]
- 44.Schaapveld RQ, Borradori L, Geerts D, et al. Hemidesmosome formation is initiated by the beta4 integrin subunit, requires complex formation of beta4 and HD1/plectin, and involves a direct interaction between beta4 and the bullous pemphigoid antigen 180. J Cell Biol. 1998 Jul 13;142(1):271–284. doi: 10.1083/jcb.142.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amano S, Scott IC, Takahara K, et al. Bone morphogenetic protein 1 is an extracellular processing enzyme of the laminin 5 gamma 2 chain. J Biol Chem. 2000 Jul 28;275(30):22728–22735. doi: 10.1074/jbc.M002345200. [DOI] [PubMed] [Google Scholar]
- 46.Veitch DP, Nokelainen P, McGowan KA, et al. Mammalian tolloid metalloproteinase, and not matrix metalloprotease 2 or membrane type 1 metalloprotease, processes laminin-5 in keratinocytes and skin. J Biol Chem. 2003 May 2;278(18):15661–15668. doi: 10.1074/jbc.M210588200. [DOI] [PubMed] [Google Scholar]
- 47.Gonzales M, Haan K, Baker SE, et al. A cell signal pathway involving laminin-5, alpha3beta1 integrin, and mitogen-activated protein kinase can regulate epithelial cell proliferation. Mol Biol Cell. 1999 Feb;10(2):259–270. doi: 10.1091/mbc.10.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zamir E, Geiger B. Molecular complexity and dynamics of cell-matrix adhesions. J Cell Sci. 2001 Oct;114(Pt 20):3583–3590. doi: 10.1242/jcs.114.20.3583. [DOI] [PubMed] [Google Scholar]
- 49.Critchley DR. Focal adhesions - the cytoskeletal connection. Curr Opin Cell Biol. 2000 Feb;12(1):133–139. doi: 10.1016/s0955-0674(99)00067-8. [DOI] [PubMed] [Google Scholar]
- 50.Wozniak MA, Modzelewska K, Kwong L, Keely PJ. Focal adhesion regulation of cell behavior. Biochim Biophys Acta. 2004 Jul 5;1692(2–3):103–119. doi: 10.1016/j.bbamcr.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 51.Ashton GH, McLean WH, South AP, et al. Recurrent mutations in kindlin-1, a novel keratinocyte focal contact protein, in the autosomal recessive skin fragility and photosensitivity disorder, Kindler syndrome. J Invest Dermatol. 2004 Jan;122(1):78–83. doi: 10.1046/j.0022-202X.2003.22136.x. [DOI] [PubMed] [Google Scholar]
- 52.Siegel DH, Ashton GH, Penagos HG, et al. Loss of kindlin-1, a human homolog of the Caenorhabditis elegans actin-extracellular-matrix linker protein UNC-112, causes Kindler syndrome. Am J Hum Genet. 2003 Jul;73(1):174–187. doi: 10.1086/376609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jobard F, Bouadjar B, Caux F, et al. Identification of mutations in a new gene encoding a FERM family protein with a pleckstrin homology domain in Kindler syndrome. Hum Mol Genet. 2003 Apr 15;12(8):925–935. doi: 10.1093/hmg/ddg097. [DOI] [PubMed] [Google Scholar]
- 54.Kinumatsu T, Hashimoto S, Muramatsu T, et al. Involvement of laminin and integrins in adhesion and migration of junctional epithelium cells. J Periodontal Res. 2008 Sep 11; doi: 10.1111/j.1600-0765.2007.01036.x. [DOI] [PubMed] [Google Scholar]
- 55.Baba Y, Iyama KI, Hirashima K, et al. Laminin-332 promotes the invasion of oesophageal squamous cell carcinoma via PI3K activation. Br J Cancer. 2008 Mar 11;98(5):974–980. doi: 10.1038/sj.bjc.6604252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lampe PD, Nguyen BP, Gil S, et al. Cellular interaction of integrin alpha3beta1 with laminin 5 promotes gap junctional communication. J Cell Biol. 1998 Dec 14;143(6):1735–1747. doi: 10.1083/jcb.143.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marinkovich MP. Tumour microenvironment: laminin 332 in squamous-cell carcinoma. Nat Rev Cancer. 2007 May;7(5):370–380. doi: 10.1038/nrc2089. [DOI] [PubMed] [Google Scholar]
- 58.Margadant C, Raymond K, Kreft M, Sachs N, Janssen H, Sonnenberg A. Integrin alpha3beta1 inhibits directional migration and wound re-epithelialization in the skin. J Cell Sci. 2009 Jan 15;122(Pt 2):278–288. doi: 10.1242/jcs.029108. [DOI] [PubMed] [Google Scholar]
- 59.Sonnenberg A, Calafat J, Janssen H, et al. Integrin alpha 6/beta 4 complex is located in hemidesmosomes, suggesting a major role in epidermal cell-basement membrane adhesion. J Cell Biol. 1991 May;113(4):907–917. doi: 10.1083/jcb.113.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mercurio AM, Rabinovitz I, Shaw LM. The alpha 6 beta 4 integrin and epithelial cell migration. Curr Opin Cell Biol. 2001 Oct;13(5):541–545. doi: 10.1016/s0955-0674(00)00249-0. [DOI] [PubMed] [Google Scholar]
- 61.Pullar CE, Baier BS, Kariya Y, et al. beta4 integrin and epidermal growth factor coordinately regulate electric field-mediated directional migration via Rac1. Mol Biol Cell. 2006 Nov;17(11):4925–4935. doi: 10.1091/mbc.E06-05-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aumailley M, El Khal A, Knoss N, Tunggal L. Laminin 5 processing and its integration into the ECM. Matrix Biol. 2003 Mar;22(1):49–54. doi: 10.1016/s0945-053x(03)00013-1. [DOI] [PubMed] [Google Scholar]
- 63.Goldfinger LE, Hopkinson SB, deHart GW, Collawn S, Couchman JR, Jones JC. The alpha3 laminin subunit, alpha6beta4 and alpha3beta1 integrin coordinately regulate wound healing in cultured epithelial cells and in the skin. J Cell Sci. 1999 Aug;112 (Pt 16):2615–2629. doi: 10.1242/jcs.112.16.2615. [DOI] [PubMed] [Google Scholar]
- 64.Schatzmann F, Marlow R, Streuli CH. Integrin signaling and mammary cell function. J Mammary Gland Biol Neoplasia. 2003 Oct;8(4):395–408. doi: 10.1023/B:JOMG.0000017427.14751.8c. [DOI] [PubMed] [Google Scholar]
- 65.Sigle RO, Gil SG, Bhattacharya M, et al. Globular domains 4/5 of the laminin alpha3 chain mediate deposition of precursor laminin 5. J Cell Sci. 2004 Sep 1;117(Pt 19):4481–4494. doi: 10.1242/jcs.01310. [DOI] [PubMed] [Google Scholar]
- 66.Tran M, Rousselle P, Nokelainen P, et al. Targeting a tumor-specific laminin domain critical for human carcinogenesis. Cancer Res. 2008 Apr 15;68(8):2885–2894. doi: 10.1158/0008-5472.CAN-07-6160. [DOI] [PubMed] [Google Scholar]
- 67.Mainiero F, Murgia C, Wary KK, et al. The coupling of alpha6beta4 integrin to Ras-MAP kinase pathways mediated by Shc controls keratinocyte proliferation. Embo J. 1997 May 1;16(9):2365–2375. doi: 10.1093/emboj/16.9.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jonkman MF. Hereditary skin diseases of hemidesmosomes. J Dermatol Sci. 1999 Jun;20(2):103–121. doi: 10.1016/s0923-1811(99)00017-1. [DOI] [PubMed] [Google Scholar]
- 69.Schenk S, Hintermann E, Bilban M, et al. Binding to EGF receptor of a laminin-5 EGF-like fragment liberated during MMP-dependent mammary gland involution. J Cell Biol. 2003 Apr 14;161(1):197–209. doi: 10.1083/jcb.200208145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.deHart GW, Healy KE, Jones JC. The role of alpha3beta1 integrin in determining the supramolecular organization of laminin-5 in the extracellular matrix of keratinocytes. Exp Cell Res. 2003 Feb 1;283(1):67–79. doi: 10.1016/s0014-4827(02)00028-9. [DOI] [PubMed] [Google Scholar]
- 71.DeHart GW, Jones JC. Myosin-mediated cytoskeleton contraction and Rho GTPases regulate laminin-5 matrix assembly. Cell Motil Cytoskeleton. 2004 Feb;57(2):107–117. doi: 10.1002/cm.10161. [DOI] [PubMed] [Google Scholar]
- 72.Rabinovitz I, Mercurio AM. The integrin alpha6beta4 functions in carcinoma cell migration on laminin-1 by mediating the formation and stabilization of actin-containing motility structures. J Cell Biol. 1997 Dec 29;139(7):1873–1884. doi: 10.1083/jcb.139.7.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nguyen BP, Ren XD, Schwartz MA, Carter WG. Ligation of integrin alpha 3beta 1 by laminin 5 at the wound edge activates Rho-dependent adhesion of leading keratinocytes on collagen. J Biol Chem. 2001 Nov 23;276(47):43860–43870. doi: 10.1074/jbc.M103404200. [DOI] [PubMed] [Google Scholar]
- 74.Russell AJ, Fincher EF, Millman L, et al. Alpha 6 beta 4 integrin regulates keratinocyte chemotaxis through differential GTPase activation and antagonism of alpha 3 beta 1 integrin. J Cell Sci. 2003 Sep 1;116(Pt 17):3543–3556. doi: 10.1242/jcs.00663. [DOI] [PubMed] [Google Scholar]
- 75.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995 Apr 7;81(1):53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 76.Yang N, Higuchi O, Ohashi K, et al. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 1998 Jun 25;393(6687):809–812. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- 77.Odenthal U, Haehn S, Tunggal P, et al. Molecular analysis of laminin N-terminal domains mediating self-interactions. J Biol Chem. 2004 Oct 22;279(43):44504–44512. doi: 10.1074/jbc.M402455200. [DOI] [PubMed] [Google Scholar]
- 78.Kariya Y, Yasuda C, Nakashima Y, Ishida K, Tsubota Y, Miyazaki K. Characterization of laminin 5B and NH2-terminal proteolytic fragment of its alpha3B chain: promotion of cellular adhesion, migration, and proliferation. J Biol Chem. 2004 Jun 4;279(23):24774–24784. doi: 10.1074/jbc.M400670200. [DOI] [PubMed] [Google Scholar]