Abstract
Many of the physiological hallmarks associated with neurogenic inflammatory processes in cutaneous tissues are similarly present within orofacial structures. Such attributes include the dependence upon capsaicin-sensitive sensory neurons and the involvement of certain inflammatory mediators derived therein, including calcitonin gene-related peptide (CGRP). However, there are also important differences between the trigeminal and spinal nervous systems, and the potential contributions of neurogenic processes to inflammatory disease within the trigeminal system have yet to be fully elucidated. We present here a model system that affords the ability to study mechanisms regulating the efferent functions of peptidergic terminals that may subserve neurogenic inflammation within the oral cavity. Freshly dissected buccal mucosa tissue from adult, male, Sprague–Dawley rats was placed into chambers and superfused with oxygenated, Krebs buffer. Serial aliquots of the egressing superfusate were acquired and analysed by radioimmunoassay for immunoreactive CGRP (iCGRP). Addition of the selective excitotoxin, capsaicin (10–300 μM), to the superfusion buffer resulted in a significant, concentration-dependent increase in superfusate levels of iCGRP. Similarly, release of iCGRP from the buccal mucosa could also be evoked by a depolarizing concentration of potassium chloride (50 mM) or by the calcium ionophore A23187 (1 μM). The specific, capsaicin receptor antagonist, capsazepine (300 μM), completely abolished the capsaicin-evoked release of iCGRP while having no effect whatsoever on the potassium-evoked release. Moreover, capsaicin-evoked release was dependent upon the presence of extracellular calcium ions and was significantly, though incompletely, attenuated by neonatal capsaicin denervation. Collectively, these data indicate that the evoked neurosecretion of iCGRP in response to capsaicin occurs via a vanilloid receptor-mediated, exocytotic mechanism. The model system described here should greatly facilitate future investigations designed to identify and characterize the stimuli that regulate the release of CGRP or other neurosecretory substances in isolated tissues. This system may also be used to elucidate the role of these mediators in the aetiology of inflammatory processes within the trigeminal field of innervation.
Keywords: exocytosis, in vitro superfusion, neuropeptide, nociceptor, pain, sensory neuron
Introduction
The process whereby the peripheral activation of certain primary sensory neurons can produce a local inflammatory reaction has been termed neurogenic inflammation (Jancsó et al., 1967). In cutaneous tissues, this response manifests primarily as oedema and erythema, resulting from plasma extravasation and vasodilatation, respectively. This so-called ‘wheal and flare reaction’ can be elicited by either chemical or electrical stimulation and is consistent with the original demonstrations of Bayliss (1901). Capsaicin, the potent phlogogenic compound derived from hot peppers of the Capsicum genus, has been used extensively in the characterization of sensory afferent physiology and neurogenic inflammation (for reviews, see Jancsó, 1968 and Holzer, 1991). This is because capsaicin activates one or more cation channels, such as the vanilloid receptor type 1 (e.g. VR1; Caterina et al., 1997), that are selectively expressed by a subpopulation of primary nociceptive neurons, causing the release of a variety of inflammatory mediators.
One of the principal biochemical participants in the neurogenic inflammatory response is calcitonin gene-related peptide (CGRP), which produces vasodilatation. In addition, CGRP can potentiate inflammatory responses to other mediators like substance P (SP), bradykinin and histamine (Gamse & Saria, 1985; Gazelius et al., 1987; Cruwys et al., 1992; Karimian & Ferrell, 1994; Brain et al., 1985, Brain & Williams, 1985). Electrical stimulation of peripheral spinal or trigeminal nerves produces vasodilatation and plasma extravasation due, at least in part, to the release of CGRP (Escott et al., 1995; Gazelius et al., 1987; Holzer, 1988). Taken together, these studies support the concept that peripheral peptidergic fibers contribute to local tissue inflammation via the secretion of neuropeptides such as CGRP. Accordingly, CGRP may serve as a marker for the neurogenic inflammatory process.
Inflammation in regions of the palatal and buccal mucosa, gingiva and periodontium constitutes a major oral health problem, and it has been suggested that there is an important neurogenic component to inflammatory processes in oral mucosa (for review, see Györfi et al., 1992). CGRP-immunoreactive fibers of trigeminal origin have been localized throughout the oropharyngeal mucosal epithelium, including the palatal perivascular submucosa and subepithelium, lamina propria, taste buds and glands (Rodgrigo et al., 1985; Silverman & Kruger, 1989; Kato et al., 1998) as well as in the junctional epithelium of the gingiva (Byers et al., 1987; Nagata et al., 1992), the dental pulp (Uddman et al., 1986) and the submandibular gland (Soinila et al., 1989). In humans, CGRP immunoreactivity in the oral cavity has been localized to varicose neuronal fibers and/or terminals in the subepithelial and perivascular areas of the oral mucosa (Hilliges et al., 1994; Fantini et al., 1995), to the propria and basal epithelium (Luthman et al., 1988) and to buccal mucosa (Ruokonen et al., 1993).
Compared with studies on neurogenic inflammation involving pericutaneous structures within the spinal representation, there is a paucity of similar investigations on orofacial structures within the trigeminal representation. However, electrical stimulation of the trigeminal nerve (Jancsó-Gábor & Szolcsányi, 1972) or topical application of capsaicin onto the oral mucosa (Fazekas et al., 1990, 1991) of the rat has been shown to produce increases in vascular permeability of the gingiva and of the mandibular and alveolar mucosa. Moreover, augmentation in facial skin blood flow (Escott et al., 1995) or Evans blue extravasation (Kerezoudis et al., 1994) following electrical stimulation of the trigeminal ganglion or inferior alveolar nerve (IAN), respectively, was inhibited by pretreatment with the CGRP receptor antagonist CGRP8–37.
Nonetheless, while the above investigations have focused on the histochemical distribution of CGRP and various other mediators in the oral mucosa, as well as on the ability of these substances (e.g. SP) to produce certain sequelae associated with neurogenic inflammatory responses (see Györfi et al., 1993, 1995), relatively little is known about how the secretion of proinflammatory neuropeptides is regulated in orofacial tissues. Indeed, the rationale for investigating such processes is enhanced by the growing list of differences that have been shown to exist between the trigeminal and spinal systems. For example, the innervation of orofacial structures is more dense and demarcated (i.e. less overlapping) than in other tissues, and there are fewer unmyelinated axons in trigeminal (50%) than in spinal (80%) nerves (for review, see Light, 1992). Moreover, whereas during embryogenesis, neurons of the dorsal root ganglia (DRG) derive entirely from the neural crest, those of the trigeminal ganglia have origins in both the neural crest as well as the neurogenic placodes, the physiological significance of which is unknown. From a functional perspective, following ligation of or constriction injury to the sciatic nerve, there is an increase in sympathetic nerve sprouting into the affected DRG; in contrast, similar injuries to the IAN produced no such sympathetic outgrowth into the affected trigeminal ganglion (Bongenhielm et al., 1999). Similarly, there was much less ongoing, ectopic discharge from myelinated and unmyelinated axons in neuromas of infraorbital compared with sciatic nerves (Tal & Devor, 1992). Collectively, these examples demonstrate that there may be substantial differences in the density, morphology, ontogeny and function of trigeminal vs. spinal nociceptors. The purpose of the present studies, therefore, was to develop a model system that would allow for the evaluation of trigeminal CGRP release from peripheral neuronal terminals under basal and stimulated conditions. Portions of this work have been published previously in abstract form (Leong et al., 1997).
Materials and methods
Animals
All experiments were carried out in accordance with protocols approved by the Institutional Animal Care and Use Committees at The University of Texas Health Science Center at San Antonio and the University of Minnesota as well as with the guidelines for the ethical treatment of animals established by the National Institutes of Health.
In vitro Superfusion
The buccal mucosae (both sides of the oral cavity of one animal per chamber, equivalent to approximately 90 mg tissue) from adult, male, Sprague–Dawley rats (Harlan, Madison, WI, USA; 175–250 g) were liberated from the underlying buccinator muscle, dissected and placed in a 1.5-mL superfusion chamber. The tissue was constantly superfused (0.23 mL/min), via a peristaltic pump, with oxygenated, physiologic (95% O2, 5% CO2, pH = 7.4) Krebs buffer containing (in mM): NaCl, 135; KCl, 3.5; MgCl2, 1; NaH2PO4, 1; NaHCO3, 1; CaCl2, 2.5; BSA, 0.1%; dextrose, 3.3; ascorbic acid, 0.1; HEPES, 10 and thiorphan, 16 μM (BACHEM, Bubendorf, Switzerland). The tissue chambers were submerged partially in a waterbath to maintain the intrachamber temperature at 37 °C. Superfusates were collected over 10-min periods for subsequent quantification of immunoreactive CGRP (iCGRP) by radioimmunoassay (RIA).
Experimental Design
To characterize the time course of capsaicin-evoked iCGRP release, immediately following dissection, the tissue was superfused with physiologic Krebs buffer for approximately 70 min, at which time spontaneous rates of iCGRP release had stabilized and then Krebs buffer containing capsaicin (Fluka, Ronkonkoma, NY, USA) was applied at the concentrations indicated in the text and figure legends (also, see below). After 10 min of stimulation by capsaicin, the chambers were again superfused with Krebs buffer alone. The length of the tubing between the reservoir and the chamber was designed so that there would be a 10-min (i.e. one fraction) lag period between the time the drug was added and the time it would reach the chamber. Superfusates were collected for up to 140 min, during which time the tissue maintained its viability as assessed by evoked iCGRP release (data not shown). To characterize the concentration-dependence of capsaicin-evoked iCGRP release, separate groups of tissue chambers were superfused with varying concentrations of capsaicin (10–300 μM). To determine whether capsaicin-evoked iCGRP release was occurring via a receptor-mediated mechanism, capsazepine {N-[2-(4-Chlorophenyl)ethyl]-1,3, 4,5-tetrahydro-7,8-dihydroxy-2H-2-benzazepine- 2-carbothioamide; RBI, Natick, MA, USA}, a competitive capsaicin receptor antagonist, was used. In this case, 300 μM capsazepine was applied for a total of 30 min (three fractions), covering the 10 min periods before, during and after the application of capsaicin. Dependency of capsaicin-evoked iCGRP release on calcium was assessed using a modified Krebs buffer in which the calcium was replaced with 10 mM ethylene glycol tetraacetic acid (EGTA). To evaluate the general ability of other secretagogues to release iCGRP, buccal mucosa tissue was superfused with either potassium chloride (KCl) at a concentration of 50 mM, in the presence or absence of 300 μM capsazepine (to demonstrate the selectivity of its antagonism of the capsaicin-evoked iCGRP release), or with the calcium ionophore A23187 (Sigma Chemical Co., St. Louis, MO) at a concentration of 1 μM. To investigate the effects of selectively lesioning the capsaicin-sensitive neuronal population, animals were injected subcutaneously with 50 mg/kg capsaicin or Tween-80: ethanol: saline vehicle (10: 10: 80) on the second postnatal day, as originally described by Jancsó et al. (1977). Six weeks later, capsaicin denervation was confirmed using the eye wipe test (Hammond & Ruda, 1989), and only capsaicin-treated animals exhibiting a negative response to this test were used. The ability of 100 μM capsaicin to evoke iCGRP release was evaluated in the buccal mucosae from capsaicin-denervated or vehicle-treated animals.
Tissue content
Buccal mucosa was homogenized, suspended in 500 μL of 2 N acetic acid, incubated at 90 °C for 10 min and then sonicated. Following centrifugation, supernatants were lyophilized, resuspended in phosphate buffer and assayed for iCGRP by RIA as described below.
Radioimmunoassay
Following in vitro superfusion or tissue extraction, individual aliquots of the superfusate (2 mL) or tissue extract (500 μL) were incubated with a C-terminally directed anti-CGRP antiserum (kindly donated by Dr Michael Iadarola, NIDCR, NIH, Bethesda, MD, USA). After 48 h, 100 μL of [125I]CGRP28–37 (approximately 20 000–25 000 c.p.m.) and 50 μL of goat anti-rabbit antibody conjugated to ferric beads were added. After another 48 h, bound peptide was separated from free peptide via immunomagnetic separation (PerSeptive Biosystems, Framingham, MA, USA). All incubations were carried out at 4 °C. The minimum detection limit for this assay is approximately 2–3 fmol per tube, with 50% displacement occurring at 10–30 fmol per tube. The intra-assay and interassay variabilities are less than 5% and 12%, respectively. To account for the possibility of any nonspecific effects on the RIA, all drugs used in the superfusion experiments were included, individually or in combination, in separate standard curves for the purposes of data analysis.
Statistical analysis
All data are expressed as mean ± SEM of either iCGRP release (fmol) or net peak iCGRP release (fmol), which was calculated as the arithmetic difference between peak and basal release. Concentration–response data were analysed by analysis of variance (ANOVA) for linear regression to test for a significantly nonzero slope and by nonlinear regression to estimate Emax and EC50 values. Antagonist and calcium dependency data were analysed by a two-way ANOVA for repeated measures, followed by Bonferroni’s post hoc test. Capsaicin denervation data were analysed by a one-tailed, unpaired, Student’s t-test. Statistical significance was accepted at an α value of P < 0.05. All data were analyzed using prism statistical Software (GraphPad Software, Inc., San Diego, USA).
Results
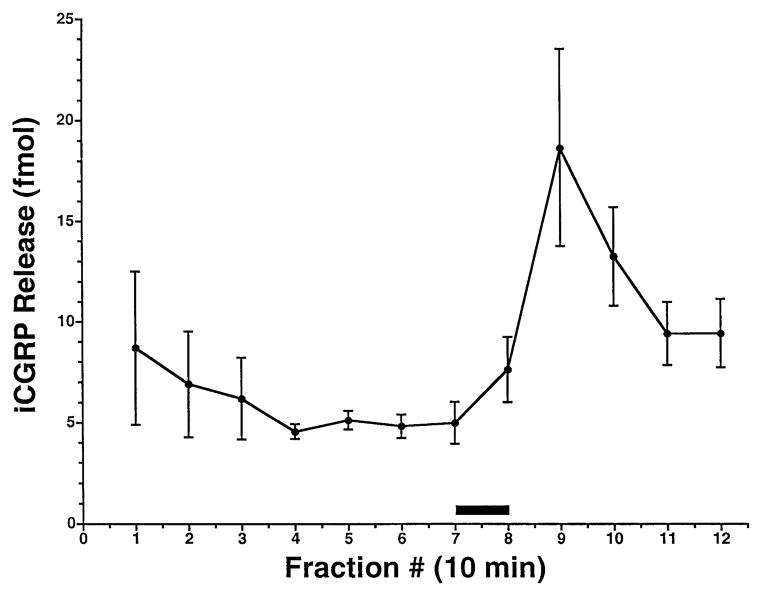
Figure 1 shows a representative time course of basal and capsaicin-evoked iCGRP release from rat buccal mucosa. Basal superfusate levels of iCGRP gradually decreased during the equilibration period and then stabilized at approximately 5 fmol per chamber per 10 min fraction. The inclusion of 100 μM capsaicin in the superfusion buffer for 10 min (i.e. one fraction, indicated by the solid bar), led to a rapid, transient increase in the amount of iCGRP measured in the egressing superfusate, which then returned toward basal levels over the ensuing collection period. Peak, capsaicin-evoked iCGRP concentrations typically occurred during the second fraction following application of capsaicin, and the release evoked by 100 μM capsaicin represented on average a 3.3-fold increase over basal levels (n = 53 tissue chambers). The inter- and intra-assay coefficents of variation for the net peak release of iCGRP evoked by 100 μM capsaicin (n = 9 experiments) were 0.36 and 0.53, respectively. Therefore, unless otherwise indicated, results are hereafter expressed as net peak iCGRP release in fmol per sample, where the sample consists of the total buccal mucosae dissected from the oral cavity of one rat (88.13 ± 2.42 mg; n = 6).
Fig. 1.
Time course of capsaicin-evoked iCGRP release from rat buccal mucosa. Freshly dissected buccal mucosa tissue (both sides of the oral cavity of one rat per chamber) was superfused with physiologic Krebs buffer (pH = 7.4) for 70 min to establish a stable level of basal iCGRP release in the superfusate. Capsaicin (100 μM) was applied to the tissue via the superfusion buffer for 10 min or the equivalent of one fraction (as indicated by the solid bar). Data are expressed as mean ± SEM iCGRP release (fmol, n = 5). Superfusate levels of iCGRP were determined by radioimmunoassay as described.
The total content of iCGRP in buccal mucosa was measured to be 1764.76 ± 118.96 fmol per sample or 20.01 ± 1.16 fmol/mg tissue (n = 6). There was no clear and consistent relationship between tissue weight and either basal or capsaicin-evoked iCGRP release (data not shown), and neither was there a significant correlation between tissue weight and total tissue iCGRP content (r2 = 0.29). Most likely, this lack of correlation reflects the fact that the variability in tissue weights is quite low compared with that of tissue CGRP content (CV = 0.06 and 0.15, respectively), as well as the likelihood that the releasable pool of CGRP is not highly concentrated in the marginal areas of the tissue from which the between-subject variability in tissue weight primarily derives. The cumulative, net capsaicin-evoked iCGRP release (based on the three consecutive fractions immediately following capsaicin application) represented on average 1.9 ± 0.29% of total content.
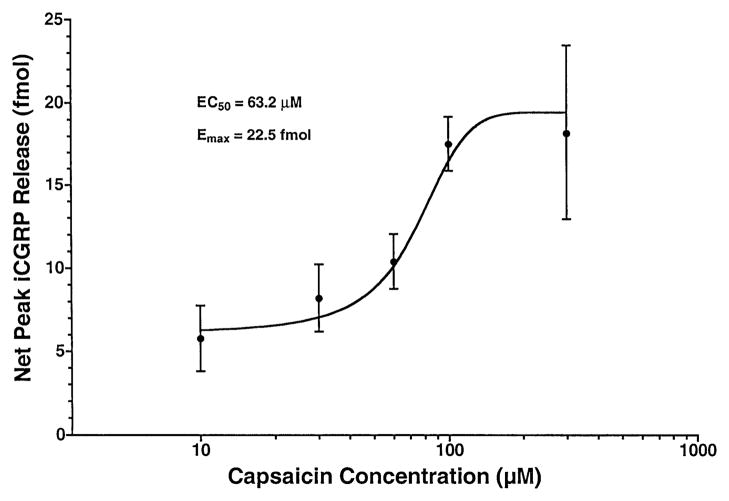
To better characterize the pharmacodynamic properties of the capsaicin-evoked iCGRP release, separate groups of chambers (n = 5–6 per group) were exposed to varying concentrations of capsaicin (10–300 μM) during the same 10 min fraction. Figure 2 demonstrates that, over the indicated range, capsaicin led to a concentration-dependent increase in peak superfusate levels of iCGRP, with the slope of the regression equation describing this relationship being significantly nonzero (F1,3 = 20.12; P < 0.05). The theoretical, maximal, net peak iCGRP release (Emax) elicited by capsaicin was calculated to be 22.5 fmol, and the concentration of capsaicin required to produce 50% of this effect (EC50) was calculated to be 63.2 μM. Concentrations of capsaicin greater than 300 μM could not be tested, because the concentration of ethanol that would be required for solubilization is capable of eliciting iCGRP release (data not shown).
Fig. 2.
Concentration–response relationship of capsaicin-evoked iCGRP release from rat buccal mucosa. Freshly dissected buccal mucosa tissue (both sides of the oral cavity of one rat per chamber) was superfused with physiologic Krebs buffer (pH = 7.4) for 70 min to establish a stable level of basal iCGRP release in the superfusate. Separate groups of chambers (n = 3–6 per group) were superfused with different concentrations of capsaicin (10–300 μM) for 10 min or the equivalent of one fraction. Data are expressed as mean ± SEM net peak iCGRP release (fmol). Analysis of variance for linear regression revealed a significantly nonzero slope (F1,3 = 20.12, P < 0.05). Calculated estimates of the maximal effect (Emax) and the concentration required to produce 50% of this effect (EC50) are indicated.
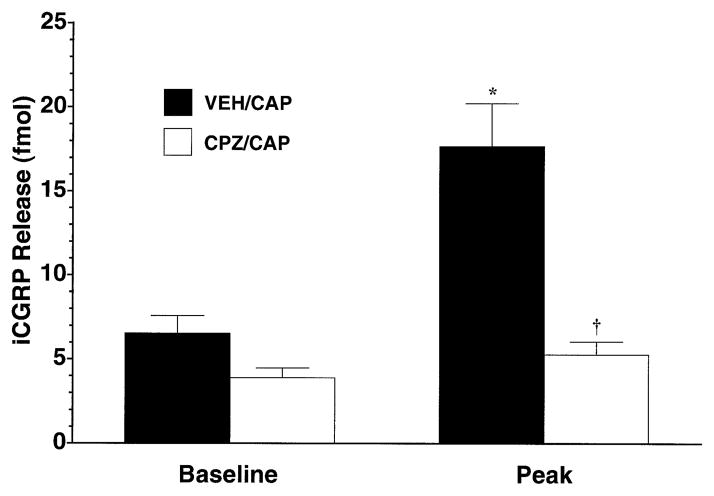
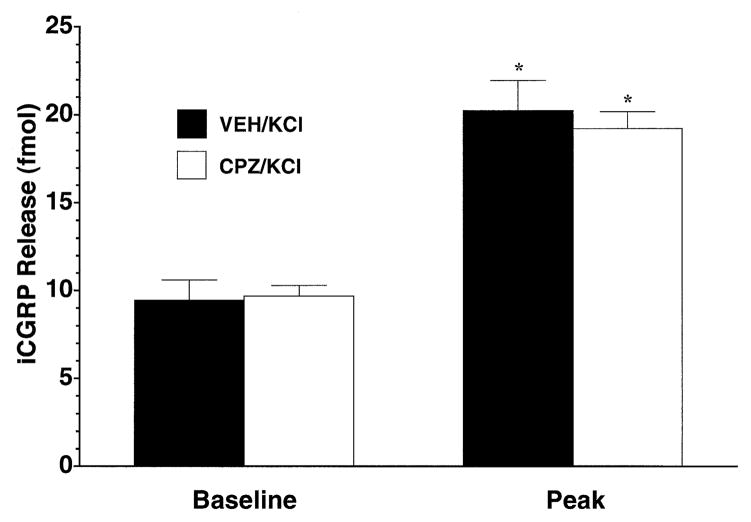
To address the receptor specificity of the observed effects, the ability of capsaicin to increase superfusate levels of iCGRP in the absence or presence of the selective capsaicin receptor antagonist capsazepine was assessed. As demonstrated in Fig. 3, while 100 μM capsaicin led to a significant increase in iCGRP over basal levels (P < 0.001), the inclusion of capsazepine (300 μM) in the three fractions immediately before, during and after capsaicin treatment led to a complete abolition of capsaicin-induced release (P < 0.001). Capsazepine by itself had no significant effect on basal iCGRP levels (baseline, open bar).
Fig. 3.
Antagonism of capsaicin-evoked iCGRP release from rat buccal mucosa by capsazepine. Freshly dissected buccal mucosa tissue (both sides of the oral cavity of one rat per chamber) was superfused with physiologicic Krebs buffer (pH = 7.4) for 70 min to establish a stable level of basal iCGRP release in the superfusate. Separate groups of chambers (n = 5–6 per group) were either superfused with vehicle (solid bars) or 300 μM capsazepine (open bars) for three fractions with 100 μM capsaicin being cosuperfused during the middle fraction. Data are expressed as mean ± SEM iCGRP release (fmol). Analysis of variance revealed significant main effects for fraction number (F1,10 = 27.98, P < 0.0005) and for antagonist pretreatment (F1,10 = 55.42, P < 0.0001) as well as a significant interaction term (F1,10 = 16.94, P < 0.005). Significantly different from *VEH/CAP Baseline or †VEH/CAP Peak (P < 0.001).
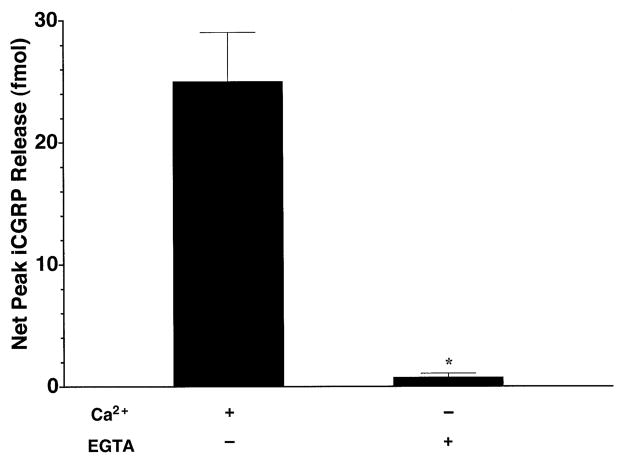
To determine the calcium-dependency of capsaicin-evoked iCGRP release, we evaluated the effect of 100 μM capsaicin on separate groups of tissue using either normal Krebs buffer (containing 2.5 mM CaCl2) or modified Krebs buffer in which the CaCl2 had been replaced with 10 mM EGTA to chelate any remaining calcium in the tissue samples. Figure 4 demonstrates that the substitution of EGTA for CaCl2 in the buffer resulted in a complete attenuation of the capsaicin-evoked iCGRP release (P < 0.001). Removal of calcium ions from the Krebs buffer also resulted in a significant decrease in basal iCGRP release during the first 40 min of superfusion (P < 0.05–0.001, depending on the time point; data not shown).
Fig. 4.
Dependence of capsaicin-evoked iCGRP release from rat buccal mucosa on extracellular calcium. Freshly dissected buccal mucosa tissue (both sides of the oral cavity of one rat per chamber) was superfused with physiologic Krebs buffer (pH = 7.4) or Ca2+-free Krebs buffer containing 10 mM EGTA for 70 min to establish a stable level of basal iCGRP release in the superfusate. Capsaicin (100 μM) was applied to the tissue via the superfusion buffer for 10 min or the equivalent of one fraction in normal Krebs buffer or Ca2+-free Krebs buffer containing 10 mM EGTA, respectively. Data are expressed as mean ± SEM net peak iCGRP release (fmol, n = 5 per group). *Significantly different from normal Krebs (P < 0.001).
To evaluate whether evoked iCGRP release from buccal mucosa is unique to capsaicin or could be mimicked by other secretagogues and to demonstrate the selectivity of the antagonism of the capsaicin-evoked release by capsazepine, tissue was superfused with 50 mM KCl in the presence or absence of capsazepine (300 μM) or with 1 μM A23187, a calcium ionophore. Figure 5 demonstrates that the nonspecific depolarizing stimulus KCl resulted in a robust iCGRP response (P < 0.001), which was comparable in magnitude to that produced by 100 μM capsaicin but which, unlike capsaicin, was not affected by pretreatment with capsazepine. Moreover, the direct transport of calcium across the plasma membrane using a mobile carrier of calcium (A23187) also led to a significant increase in superfusate levels of iCGRP (data not shown). In contrast, the combination of bradykinin and PGE2 (1 μM or 10 μM each) in the presence or absence of histamine and 5-HT (1 μM or 10 μM each) was without effect (data not shown).
Fig. 5.
Effect of KCl on iCGRP release from rat buccal mucosa in the presence or absence of capsazepine. Freshly dissected buccal mucosa tissue (both sides of the oral cavity of one rat per chamber) was superfused with physiologic Krebs buffer (pH = 7.4) for 70 min to establish a stable level of basal iCGRP release in the superfusate. Separate groups of chambers (n = 7 per group) either were superfused with vehicle (solid bars) or 300 μM capsazepine (open bars) for two fractions with 50 mM KCl being cosuperfused during the second fraction. Data are expressed as mean ± SEM iCGRP release (fmol). Analysis of variance revealed significant main effects for fraction number (F1,12 = 87.25, P < 0.0001) but not for drug pretreatment. *Significantly different from respective Baseline value (P < 0.001).
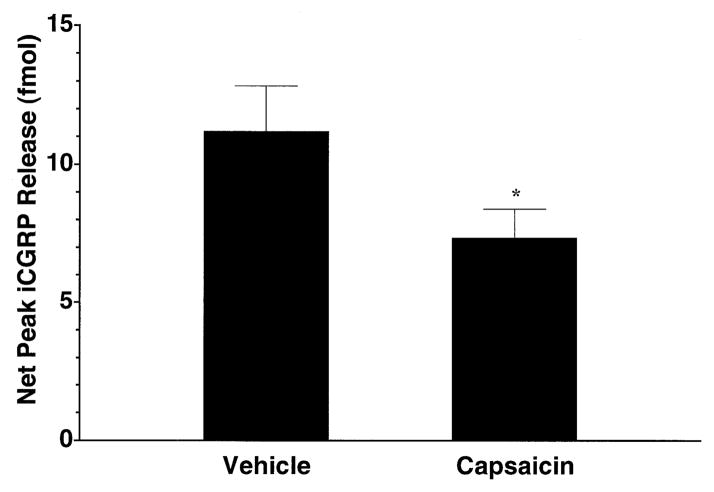
Finally, the effect of neonatal capsaicin denervation on capsaicin-evoked iCGRP release from the buccal mucosa of the adult animals was evaluated. Treatment with 50 mg/kg capsaicin on the second postnatal day resulted in a behavioural tolerance to nociceptive stimulation at six weeks of age, as determined by applying capsaicin onto the conjunctivae. Of all animals evaluated following the capsaicin treatment, 100% exhibited a negative response to this test. As demonstrated in Fig. 6, compared with vehicle-treated animals, neonatal capsaicin treatment led to a significant, 35% reduction in the CGRP neurosecretory response of the buccal mucosa to a 100-μM capsaicin challenge (P < 0.05). The neonatal capsaicin treatment had no effect on basal iCGRP release (data not shown).
Fig. 6.
Effects of neonatal capsaicin denervation on capsaicin-evoked iCGRP release from rat buccal mucosa. Freshly dissected buccal mucosa tissue (both sides of the oral cavity of one rat per chamber) from six-week-old rats treated on the second postnatal day with 50 mg/kg capsaicin or Tween-80: ethanol: saline (10: 10: 80) vehicle was superfused with physiologic Krebs buffer (pH = 7.4) for 70 min to establish a stable level of basal iCGRP release in the superfusate. Capsaicin (100 μM) was applied to the tissue via the superfusion buffer for 10 min or the equivalent of one fraction in normal Krebs buffer. Data are expressed as mean ± SEM net peak iCGRP release (fmol, n = 7–8 per group). *Significantly different from vehicle control (P < 0.05).
Discussion
In the middle part of the 20th century, a group of Hungarian scientists pioneered a series of investigations aimed at understanding the mechanisms subserving neurogenic inflammation, a constellation of processes that are associated with peripheral tissue injury and include erythema, oedema and pain (see Jancsó, 1968). These seminal studies, which extended the earlier works of Goltz (1874), Bayliss (1901), Lewis et al. (1927) and colleagues (for review, see Chapman et al., 1961), broadly exploited the ability of the excitotoxin capsaicin to selectively stimulate a subpopulation of unmyelinated and thinly myelinated, peptidergic, sensory neurons. Capsaicin, 8-methyl-N-vanillyl- 6-nonenamide, is the pungent ingredient in hot chili peppers of the Capsicum genus and selectively activates VR1, a ligand-gated ion channel that is expressed predominantly on the unmyelinated, C-fibre class of nociceptors (Caterina et al., 1997). Here, we report the development of a model for assessing the efferent, neurosecretory activity of this neuronal class within the trigeminal field of innervation, via the quantitative measurement of capsaicin-stimulated iCGRP release from rat buccal muscosa. The present studies demonstrate that capsaicin evokes the release of iCGRP release from this tissue in a concentration-related, capsazepine-reversible and calcium-dependent fashion, consistent with the conclusion that this occurs via a vanilloid receptor-mediated, exocytotic process.
As there is now substantial evidence to support the hypothesis that the aetiology of certain oral inflammatory diseases may have a neurogenic component (for review, see Györfi et al., 1992), this model should provide a useful tool for understanding the mechanisms by which peripheral neurosecretion from trigeminal nociceptors is regulated. Moreover, the current approach realizes important advantages for such investigations. First, by utilizing freshly dissected tissue containing functional neuronal terminals, this measurement of neuropeptide release in vitro avoids systemic effects that are present in vivo and that may otherwise confound or obfuscate the dependent measure of interest. Second, the use of the selective secretagogue capsaicin as the test stimulus provides that only the neuronal terminals which express capsaicin-activated receptor channels, and which are principally involved in the neurogenic inflammatory response, will be targeted. Finally, the oral cavity is particularly susceptible to traumatic challenge from a variety of endogenously or exogenously derived physicochemical and biopathogenic entities, like protons, temperature or endotoxin to name but a few. Thus, the present model should have widespread utility in readily permitting a systematic and quantitative examination of the effects of such factors on the oral mucosa. Indeed, our demonstration of iCGRP release in response to depolarization with high concentrations of potassium, or to direct calcium stimulation using the mobile calcium carrier A23187, indicate the general suitability of this system for studying stimuli in addition to capsaicin.
Similarly, this model would be amenable to investigating neurogenic mechanisms engaged during certain oral inflammatory disease states, like periodontitis associated with tobacco use (for example, see Haber et al., 1993), streptozotocin-induced diabetes (Györfi et al., 1996) or chemotherapy-induced mucositis (for review, see Epstein & Schubert, 1999). Indeed, preliminary studies have evaluated the effects of nicotine and its congeners on iCGRP released from in vitro superfused buccal mucosa (Leong et al., 1997; Dussor et al., 1998). The case of iatrogenic oral inflammation, following treatment with chemotherapeutic agents (e.g. 5-fluorouracil), is intriguing, because capsaicin [contained in a taffy (candy) vehicle] produced a significant decrease in pain among patients suffering from oral mucositis, presumably as a result of nociceptor desensitization (Nadoolman et al., 1994). Moreover, it has been suggested that the CGRP-immunoreactive fibers penetrating the oral mucosal epithelium are involved in wound healing and tissue regeneration (Fazekas et al., 1990; Györfi et al., 1992) and, thus, these processes may be evaluated as well, using CGRP release as a marker of tissue repair.
Analagous model systems for studying peptidergic neurosecretory activity of peripheral terminals have been previously described in the rat paw skin (Kilo et al., 1997) as well as in bovine (Hargreaves et al., 1992; Jackson et al., 1992) and rat (Bowles et al., 1998) dental pulp. However, there is mounting evidence to indicate that there are differences in the neurogenic inflammatory processes not only between the trigeminal and spinal systems but also between various tissues within the oral cavity. For examples, previous studies in the spinal system have shown that as soon as 10 days following transection of the sciatic nerve (Franco-Cereceda et al., 1991) or up to six months following dorsal root ganglionectomy (Li et al., 1997), there is a nearly complete loss of CGRP immunoreactivity in the skin. However, Fazekas et al. (1991) demonstrated that 14 days following IAN transection, there was virtually no change either in the density of CGRP immunoreactivity in the mandibular mucosa or in capsaicin-induced elevations in blood flow, although this may reflect the fact that innervation of the facial, mandibular mucosa is supplied by the buccal nerve and not the IAN.
A primary consideration in the interpretation of the present studies relates to the concentrations of capsaicin (100 μM) and capsazepine (300 μM) that were used to produce the observed effects. However, whereas a maximal effective capsaicin concentration of 300 nM was required to evoke iCGRP release from spinal cord in vitro, 10-fold higher concentrations were required to elicit maximal release in bladder (Donnerer et al., 1992). Indeed, we have shown previously that a 1000-fold higher concentration of capsaicin (300 μM) was required to achieve maximal iCGRP from an in vitro skin preparation (Kilo et al., 1997), which compares favourably to the 100 μM value required here for the buccal mucosa. Similarly, the EC50 of approximately 30 μM in skin is very similar to the value of about 60 μM observed here. While it is possible that such discrepancies reflect pharmacodynamic differences in capsaicin activation of centrally vs. peripherally localized vanilloid receptors, it is more likely such differences derive, at least in part, from pharmacokinetic issues related to the thickness and permeability of the peripheral tissues used compared with spinal cord slices, for example. Morever, these factors would be expected to play a more significant role in the context of lipophilic drugs, like capsaicin and capsazepine, that may be sequestered in the lipid membranes of relatively large pieces of tissue as were used here. Therefore, it is probably the case that the concentration of capsaicin or capsazepine achieved at their site(s) of action in the mucosa is considerably lower than that which initially is applied to the tissue.
Based on the reported literature (for review, see Szallasi & Blumberg, 1999), capsazepine exhibits up to 10-fold lower affinity for [3H]-RTX (resiniferatoxin) binding sites compared with capsaicin, depending on the tissue. Thus, the 3-fold higher concentration of capsazepine used here to block capsaicin-evoked iCGRP release is relatively conservative. On the other hand, it has been shown that capsazepine blocks voltage-gated calcium channels at the same micromolar concentration range as is required to block capsaicin responses (Docherty et al., 1997). However, while the concentrations of capsazepine used in the present studies completely blocked capsaicin-evoked iCGRP release (Fig. 3), they had absolutely no effect on potassium-evoked release (Fig. 5). Moreover, while replacement of calcium ions with EGTA not only completely blocked capsaicin-evoked iCGRP release but also significantly attenuated basal release, capsazepine failed to alter basal release. Taken together, therefore, these lines of evidence make it unlikely that capsazepine is exerting its antagonistic effects on capsaicin-evoked iCGRP release via the nonspecific blockade of calcium channels. Accordingly, while it is prudent to be cautious in reaching firm conclusions based on results obtained with these drugs at these concentrations, nonetheless, the simplest and most probable interpretation of the present results is that capsaicin is acting in buccal mucosa at one or more capsazepine-sensitive vanilloid receptors to release iCGRP.
With regard to studies on the effects of neonatal capsaicin denervation in the spinal system, for example, Kilo et al. (1997) found that this procedure causes a complete abolition of capsaicin-evoked iCGRP release in rat paw skin. However, consistent with the data presented here regarding the incomplete loss of capsaicin sensitivity in adult animals that had been treated with capsaicin as neonates, Rodrigo et al. (1985) observed only a small depletion of CGRP-immunoreactive fibers in the palatal epithelium following neonatal capsaicin treatment and no significant change in extractable CGRP content. Similarly, Wimalawansa (1993) demonstrated that neonatal capsaicin treatment resulted in a partial decrease (approximately 60%) in iCGRP content of trigeminal ganglion or nerve. In any case, the present findings, that neonatal capsaicin treatment significantly, but only partially, reduced the capasaicin-evoked CGRP neurosecretory response in the adult animals, strongly suggest that within orofacial structures and/or the nerves that innervate them, there is a source of CGRP that may be relatively resistant to, or that may substantially recover from ablation by neonatal capsaicin treatment. The extent to which this source comprises non-neuronal cell types that presumably would be capable of regenerating during postnatal development remains to be determined.
In contrast, however, others have found that the identical neonatal capsaicin denervation method used here led to a near total loss of CGRP-immunoreactivity in the mucosa, submucosa and perivascular regions of the tongue (Terenghi et al., 1986b) and in the intra- and subepithelial fibers of the tongue, epiglottis and pharynx (Terenghi et al., 1986a). Likewise, adult capsaicin treatment produced a ‘marked’, nearly complete decrease in CGRP-immunoreactive nerves supplying the junctional epithelium of the maxillary and mandibular molars (Kondo et al., 1995). Finally, neonatal or adult treatment with capsaicin abolished the effects of electrical stimulation of the IAN on gingivo-mucosal blood flow and vascular permeability (Fazekas et al., 1990). Thus, it would appear that the net effect of capsaicin denervation on oral neurogenic inflammatory processes will greatly depend on the parameter under study. Nonetheless, these findings collectively illustrate substantive differences within and between trigeminal and spinal neurogenic mechanisms and indicate a clear need for more comprehensive evaluations in both systems. In particular, it will be important to identify and characterize the various stimuli that can mobilize the distinct stores of CGRP in individual tissues as well as the conditions and mechanisms by which this occurs.
In addition to a role for CGRP in oral neurogenic processes, there is good evidence to suggest the involvement of other participants as well. Accordingly, Barthold et al. (1994) identified a redistribution of SP immunoreactivity around blood vessels and inflammatory cell infiltrates in inflamed human gingival tissue. In addition, Linden et al. (1997) measured an increase in immunoreactive SP and neurokinin A levels in the gingival crevicular fluid of patients with gingivitis or periodontitis. Finally, other studies have also implicated, in these responses, direct as well as indirect actions of histamine and prostaglandins (Kerezoudis et al., 1993; Györfi et al., 1995). Thus, the experimental paradigm described here could be adapted to measure the basal and stimulated release of other mediators for which there are sufficiently specific and sensitive probes.
In conclusion, the present studies validate a new model system for elucidating trigeminal mechanisms of neurogenic inflammatory processes via the in vitro superfusion of rat buccal mucosa. Not only should this approach provide a means to evaluate peripheral neurosecretory activity in normal tissues, but also it will permit investigations on changes that may occur in various disease states or in response to challenges by a variety of chemical, thermal or mechanical stimuli. Ultimately, such studies could lead to the generation of improved therapeutics that would have particular efficacy in the treatment of oral inflammatory disease.
Acknowledgments
This work was supported by NIH grant DA10510 from the National Institute on Drug Abuse, the Smokeless Tobacco Research Council and the Deutsche Forschungsgemeinschaft.
Abbreviations
- CGRP
calcitonin gene-related peptide
- EGTA
ethylene glycol tetraacetic acid
- IAN
inferior alveolar nerve
- iCGRP
immunoreactive CGRP
- RIA
radioimmunoassay
- SP
substance P
- VR1
vanilloid receptor type 1
References
- Barthold PM, Kylstra A, Lawson R. Substance P: an immunohistochemical and biochemical study in human gingival tissues. A role for neurogenic inflammation? J Periodontol. 1994;65:1113–1121. doi: 10.1902/jop.1994.65.12.1113. [DOI] [PubMed] [Google Scholar]
- Bayliss WM. On the origin from the spinal cord of the vaso-dilator fibres of the hindlimb, and on the nature of these fibres. J Physiol (Lond) 1901;26:173–209. doi: 10.1113/jphysiol.1901.sp000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongenhielm U, Boissonade FM, Westermark A, Robinson PP, Fried K. Sympathetic nerve sprouting fails to occur in the trigeminal ganglion after peripheral nerve injury in the rat. Pain. 1999;82:183–288. doi: 10.1016/S0304-3959(99)00064-0. [DOI] [PubMed] [Google Scholar]
- Bowles WR, Oh W, Sabino ML, Harding-Rose C, Hargreaves KM. Development of an in vitro rat dental pulp superfusion model. J Dent Res. 1998;77:160. [Google Scholar]
- Brain SJ, Morris H, MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985;313:54–56. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- Brain S, Williams T. Inflammatory edema induced by synergism between CGRP and mediators of increased vascular permeability. Br J Pharmacol. 1985;86:855–860. doi: 10.1111/j.1476-5381.1985.tb11107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers MR, Mecifi KB, Kimberly CL. Numerous nerves with calcitonin gene-related peptide-like immunoreactivity innervate junctional epithelium of rats. Brain Res. 1987;419:311–314. doi: 10.1016/0006-8993(87)90598-1. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chapman LF, Ramos AO, Goddell H, Wolff HG. Neurohumoral features of afferent fibers in man. Arch Neurol. 1961;4:617–650. doi: 10.1001/archneur.1961.00450120031005. [DOI] [PubMed] [Google Scholar]
- Cruwys SC, Kidd BL, Mapp PI, Walsh DA, Blake DR. The effects of calcitonin gene-related peptide on formation of intra-articular oedema by inflammatory mediators. Br J Pharm. 1992;107:116–119. doi: 10.1111/j.1476-5381.1992.tb14472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty RJ, Yeats JC, Piper AS. Capsazepine block of voltage-activated calcium channels I adult rat dorsal root ganglion neurons in culture. Br J Pharmacol. 1997;121:1461–1467. doi: 10.1038/sj.bjp.0701272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnerer J, Schuligoi R, Amann R. Time-course of capsaicin-evoked release of calcitonin gene-related peptide from rat spinal cord in vitro. Effect of concentration and modulation by Ruthenium Red. Regul Pept. 1992;37:27–37. doi: 10.1016/0167-0115(92)90061-x. [DOI] [PubMed] [Google Scholar]
- Dussor GO, Leong AS, Gracia NB, Hargreaves KM, Arneric SP, Flores CM. Differential effects of neuronal nicotinic receptor agonists on capsaicin-evoked CGRP release from peripheral terminals of primary sensory neurons. Soc Neurosci Abstract. 1998;24:1625. [Google Scholar]
- Epstein JB, Schubert MM. Oral mucositis in myelosuppressive cancer therapy. Oral Surg Oral Med Oral Pathol. 1999;88:273–276. doi: 10.1016/s1079-2104(99)70026-0. [DOI] [PubMed] [Google Scholar]
- Escott KJ, Beattie DT, Connor HE, Brain SD. Trigeminal ganglion stimulation increases facial skin blood flow in the rat: a major role for calcitonin gene-related peptide. Brain Res. 1995;669:93–99. doi: 10.1016/0006-8993(94)01247-f. [DOI] [PubMed] [Google Scholar]
- Fantini F, Giannetti A, Benassi L, Cattaneo V, Magnoni C, Pincelli C. Nerve growth factor receptor and neurochemical markers in human oral mucosa: an immunohistochemical study. Dermatology. 1995;190:186–191. doi: 10.1159/000246682. [DOI] [PubMed] [Google Scholar]
- Fazekas Á, Györfi A, Pósch E, Jacab G, Bártfai Z, Rosivall L. Effect of denervation on the neurogenic inflammation of the rat mandibular mucosa. Naunyn-Schmiedeberg’s Arch Pharmacol. 1991;343:393–398. doi: 10.1007/BF00179044. [DOI] [PubMed] [Google Scholar]
- Fazekas Á, Vindisch K, Pósch E, Györfi A. Experimentally-induced neurogenic inflammationin the rat oral mucosa. J Periodont Res. 1990;25:276–282. doi: 10.1111/j.1600-0765.1990.tb00916.x. [DOI] [PubMed] [Google Scholar]
- Franco-Cereceda A, Rydh M, Lou YP, Dalsgaard CJ, Lundberg JM. Endothelin as a putative sensory neuropeptide in the guinea-pig: different properties in comparison with calcitonin gene-related peptide. Regulatory Peptides. 1991;32:253–265. doi: 10.1016/0167-0115(91)90019-d. [DOI] [PubMed] [Google Scholar]
- Gamse R, Saria A. Potentiation of tachykinin-induced plasma protein extravasation by CGRP. Eur J Pharmacol. 1985;114:61–66. doi: 10.1016/0014-2999(85)90520-5. [DOI] [PubMed] [Google Scholar]
- Gazelius B, Edwall B, Olgart L, Lundberg J, Hokfelt T, Fischer J. Vasodilatory effects and coexistence of CGRP and substance P in sensory nerves of cat dental pulp. Acta Physiol Scand. 1987;130:33–40. doi: 10.1111/j.1748-1716.1987.tb08108.x. [DOI] [PubMed] [Google Scholar]
- Goltz F. Über gefässerweiternde Nerven. Pflueger Arch Ges Physiol. 1874;9:15. [Google Scholar]
- Györfi A, Fazekas Á, Fehér E, Ender F, Rosivall L. Effects of streptozotocin-induced diabetes on neurogenic inflammation of gingivomucosal tissue in rat. J Periodont Res. 1996;31:249–255. doi: 10.1111/j.1600-0765.1996.tb00489.x. [DOI] [PubMed] [Google Scholar]
- Györfi A, Fazekas Á, Irmes F, Jakab G, Süto T, Rosivall L. Role of substance P (SP) in development of symptoms of neurogenic inflammationin the oral mucosa of the rat. J Peridont Res. 1993;28:191–196. doi: 10.1111/j.1600-0765.1993.tb01068.x. [DOI] [PubMed] [Google Scholar]
- Györfi A, Fazekas Á, Irmes F, Rosivall L. Effect of substance P administration on vascular permeability in the rat oral mucosa and sublingual gland. J Peridont Res. 1995;30:181–185. doi: 10.1111/j.1600-0765.1995.tb01271.x. [DOI] [PubMed] [Google Scholar]
- Györfi A, Fazekas Á, Rosivall L. Neurogenic inflammation and the oral mucosa. J Clin Periodontol. 1992;19:731–736. doi: 10.1111/j.1600-051x.1992.tb02162.x. [DOI] [PubMed] [Google Scholar]
- Haber J, Wattles J, Crowby M, Mandel R, Kaunusi J, Kent R. Evidence for smoking as a major risk factor for periodontitis. J Periodontol. 1993;64:16–23. doi: 10.1902/jop.1993.64.1.16. [DOI] [PubMed] [Google Scholar]
- Hammond DL, Ruda MA. Developmental alterations in thermal nociceptive threshold and the distribution of immunoreactive calcitonin gene-related peptide and substance P after neonatal administration of capsaicin in the rat. Neurosci Lett. 1989;97:57–62. doi: 10.1016/0304-3940(89)90139-0. [DOI] [PubMed] [Google Scholar]
- Hargreaves KM, Bowles WR, Garry G. An in vitro method to evaluate regulation of neuropeptide release. J Endodontics. 1992;18:597–600. doi: 10.1016/S0099-2399(06)81329-4. [DOI] [PubMed] [Google Scholar]
- Hilliges M, Hellman M, Ahlström U, Johansson O. Immunohistochemical studies of neurochemical markers in normal human buccal mucosa. Histochemistry. 1994;101:235–244. doi: 10.1007/BF00315910. [DOI] [PubMed] [Google Scholar]
- Holzer P. Local effector function of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988;24:739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- Holzer P. Capsaicin as a tool for studying neuron functions. In: Costa M, Surrenti C, Gorini S, Maggi CA, Meli A, editors. Sensory Nerves & Neuroeptides in Gastroenterology. Plenum Press; New York: 1991. [Google Scholar]
- Jackson DL, Garry M, Engelstad M, Geier H, Hargreaves KM. An in vitro method to evaluate neuropeptide secretion from dental pulp. J Dent Res. 1992;71:178. [Google Scholar]
- Jancsó N. Desensitization with capsaicin and related acylamides as a tool for studying the function of pain receptors. In: Lim RKS, Armstrong D, Pardo EG, editors. Pharmacology of Pain; Proceedings of the Third International Pharmacological Meeting; Oxford: Pergamon Press Ltd; 1968. pp. 33–55. [Google Scholar]
- Jancsó N, Jancsó-Gábor A, Szolcsányi J. Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. Br J Pharmacol. 1967;31:138–151. doi: 10.1111/j.1476-5381.1967.tb01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancsó G, Kiraly E, Jancsó-Gábor A. Pharmacologically induced selective degeration of chemosensitive primary sensory neurons. Nature. 1977;270:741–743. doi: 10.1038/270741a0. [DOI] [PubMed] [Google Scholar]
- Jancsó-Gábor A, Szolcsányi J. Neurogenic inflammatory responses. J Dent Res. 1972;51 (Suppl):264–269. doi: 10.1177/00220345720510020901. [DOI] [PubMed] [Google Scholar]
- Karimian M, Ferrell WR. Plasma protein extravasation into the rat knee joint induced by calcitonin gene-related peptide. Neurosci Lett. 1994;166:39–42. doi: 10.1016/0304-3940(94)90835-4. [DOI] [PubMed] [Google Scholar]
- Kato J, Uddman R, Sundler F, Kurisu K. Immunohistochemical study of the innervation of the boundary area of the hard and soft palates of the rat. Acta Anat. 1998;163:92–98. doi: 10.1159/000046488. [DOI] [PubMed] [Google Scholar]
- Kerezoudis NP, Olgart L, Edwall L. Evans blue extravasation in rat dental pulp and oral tissues induced by electrical stimulation of the inferior alveolar nerve. Arch Oral Biol. 1993;10:893–901. doi: 10.1016/0003-9969(93)90099-8. [DOI] [PubMed] [Google Scholar]
- Kerezoudis NP, Olgart L, Edwall L. Involvement of substance P but not nitric oxide or calcitonin gene-related peptide in neurogenic plasma extravasation in rat incisor pulp and lip. Arch Oral Biol. 1994;39:769–774. doi: 10.1016/0003-9969(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Kilo S, Harding-Rose C, Hargreaves KM, Flores CM. Peripheral CGRP release as a marker for neurogenic inflammation: a model system for the study of neuropeptide secretion in rat paw skin. Pain. 1997;73:201–207. doi: 10.1016/S0304-3959(97)00108-5. [DOI] [PubMed] [Google Scholar]
- Kondo T, Kido MA, Kiyoshima T, Yamaza T, Tanaka T. An immunohistochemical and monastral blue-vascular labelling study on the involvement of capsaicin-sensitive sensory innervation of the junctional ipithelium in nurogenic plasma extravasation in the rat gingiva. Arch Oral Biol. 1995;10:931–940. doi: 10.1016/0003-9969(95)00060-3. [DOI] [PubMed] [Google Scholar]
- Leong AS, Kilo S, Hargreaves KM, Flores CM. Modulation of capsaicin-evoked neuropeptide release by nicotine in the rat buccal musocsa. Soc Neurosci Abstr. 1997;23:874. [Google Scholar]
- Lewis T, Harris KE, Grant RT. Influence of the cutaneous nerves on various reaction of the cutaneous vessels. Heart. 1927;14:1. [Google Scholar]
- Li Y, Hsieh ST, Chien HF, Zhang X, McArthur JC, Griffin JW. Sensory and motor denervation influence epidermal thickness in rat foot and glabrous skin. Exp Neurol. 1997;147:452–462. doi: 10.1006/exnr.1997.6624. [DOI] [PubMed] [Google Scholar]
- Light AR. The initial processing of pain and its descending control: spinal and trigeminal systems. In: Gildenberg PL, editor. Pain and Headache. Vol. 12. Karger; Basel, New York: 1992. pp. 51–74. [Google Scholar]
- Linden GJ, McKinnell J, Shaw C, Lundy FT. Substance P and neurokinin A in gingival crevicular fluid in periodontal health and disease. J Clin Periodontol. 1997;24:799–803. doi: 10.1111/j.1600-051x.1997.tb01192.x. [DOI] [PubMed] [Google Scholar]
- Luthman J, Johansson O, Ahlström U, Kvint S. Immunohistochemical studies of the neurochemical markers, CGRP, enkephalin, galanin, γ-MSH, NPY, PHI, proctolin, PTH, somatostatin, SP, VIP, tyrosine hydroxylase and neurofilament in nerves and cells of the human attached gingiva. Arch Oral Biol. 1988;33:149–158. doi: 10.1016/0003-9969(88)90039-8. [DOI] [PubMed] [Google Scholar]
- Nadoolman W, Duffy VB, Berger AM, Bartoshuk LM. Successive desensitization: a low pain/high dose technique for oral capsaicin delivery. Chem Senses. 1994;19:494. [Google Scholar]
- Nagata E, Kondo T, Ayasaka N, Nakata M, Tanaka T. Immunohistochemical study of nerve fibres with substance P- or calcitonin gene-related peptide-like immunoreactivity in the junctional epithelium of developing rats. Arch Oral Biol. 1992;37:655–662. doi: 10.1016/0003-9969(92)90128-u. [DOI] [PubMed] [Google Scholar]
- Rodrigo J, Polak JM, Terenghi G, Cervantes C, Ghatei MA, Mulderry PK, Bloom SR. Calcitonin gene-related peptide (CGRP) - immunoreacctive sensory and motor nerves of the mammalian palate. Histochemistry. 1985;82:67–74. doi: 10.1007/BF00502092. [DOI] [PubMed] [Google Scholar]
- Ruokonen H, Hietanen J, Malmström M, Sane J, Häyrinen-Immonen R, Hukkanen M, Konttinen YT. Peripheral nerves and mast cells in normal buccal mucosa. J Oral Pathol Med. 1993;22:30–34. doi: 10.1111/j.1600-0714.1993.tb00116.x. [DOI] [PubMed] [Google Scholar]
- Silverman JD, Kruger L. Calcitonin gene-related peptide immunoreactive innervation of the rat head with emphasis on specialized sensory structures. J Comp Neurol. 1989;280:303–330. doi: 10.1002/cne.902800211. [DOI] [PubMed] [Google Scholar]
- Soinila J, Salo A, Unsitalo H, Yanaihara N, Happole O. CGRP-immunoreactive sensory nerve fibers in the submandibular gland of the rat. Histochemistry. 1989;91:455–460. doi: 10.1007/BF00492515. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:160–211. [PubMed] [Google Scholar]
- Tal M, Devor M. Ectopic discharge in injured nerves: comparison of trigeminal and somatic afferents. Brain Res. 1992;579:148–151. doi: 10.1016/0006-8993(92)90753-v. [DOI] [PubMed] [Google Scholar]
- Terenghi G, Polak JM, Rodrigo J, Mulderry PK, Bloom SR. Calcitonin gene-related peptide-immunoreactive nerves in the tonjue, epiglottis and pharynx of the rat: occurrence, distribution and origin. Brain Res. 1986a;365:1–14. doi: 10.1016/0006-8993(86)90716-x. [DOI] [PubMed] [Google Scholar]
- Terenghi G, Zhang SQ, Under WG, Polak JM. Morphological changes of sensory CGRP-immunoreactive and sympathetic nerves in peripheral tissues following chronic denervation. Histochemistry. 1986b;86:89–95. doi: 10.1007/BF00492350. [DOI] [PubMed] [Google Scholar]
- Uddman R, Grunditz T, Sundler F. Calcitonin gene-related peptide: a sensory transmitter in dental pulps? Scand J Dent Res. 1986;94:219–224. doi: 10.1111/j.1600-0722.1986.tb01756.x. [DOI] [PubMed] [Google Scholar]
- Wimalawansa SJ. The effects of neonatal capsaicin on plasma levels and tissue contents of CGRP. Peptides. 1993;14:247–252. doi: 10.1016/0196-9781(93)90037-h. [DOI] [PubMed] [Google Scholar]