Abstract
Objective
To receive and collate reports of death or major complications of transfusion of blood or components.
Design
Haematologists were invited confidentially to report deaths and major complications after blood transfusion during October 1996 to September 1998.
Setting
Hospitals in United Kingdom and Ireland.
Subjects
Patients who died or experienced serious complications, as defined below, associated with transfusion of red cells, platelets, fresh frozen plasma, or cryoprecipitate.
Main outcome measures
Death, “wrong” blood transfused to patient, acute and delayed transfusion reactions, transfusion related acute lung injury, transfusion associated graft versus host disease, post-transfusion purpura, and infection transmitted by transfusion. Circumstances relating to these cases and relative frequency of complications.
Results
Over 24 months, 366 cases were reported, of which 191 (52%) were “wrong blood to patient” episodes. Analysis of these revealed multiple errors of identification, often beginning when blood was collected from the blood bank. There were 22 deaths from all causes, including three from ABO incompatibility. There were 12 infections: four bacterial (one fatal), seven viral, and one fatal case of malaria. During the second 12 months, 164/424 hospitals (39%) submitted a “nil to report” return.
Conclusions
Transfusion is now extremely safe, but vigilance is needed to ensure correct identification of blood and patient. Staff education should include awareness of ABO incompatibility and bacterial contamination as causes of life threatening reactions to blood.
Key messages
Blood transfusion, while extremely safe, has several potentially fatal hazards
All staff handling blood should be aware of the importance of correct identity of sample, patient, and blood bag at all stages
Resources should be directed to evaluation of methods for improving identification of patients
Acute fever or collapse during or after transfusion may be due to ABO incompatibility or bacterial contamination
Microbiological complications of transfusion accounted for a minor component of all reports
Introduction
The current incidence of major complications due to blood transfusion is unknown. Until 1996 blood transfusion was not covered by either a confidential inquiry or the yellow card system of the Committee of Safety of Medicines. Perception of transfusion safety focuses on the diminishing risk of viral transmission, while the risk of ABO incompatible transfusion due to errors in blood or patient identification remains a threat.1,2 To analyse the residual risks of transfusion, a confidential voluntary reporting system for major transfusion events—serious hazards of transfusion (SHOT)—affiliated to the Royal College of Pathologists was launched in 1996. We have summarised the main findings from its first two annual reports.
Methods for case ascertainment
In November 1996 haematologists in the United Kingdom and Ireland were invited on a voluntary confidential basis to inform SHOT of deaths and major adverse events in seven categories (see results) associated with the transfusion of red cells, platelets, fresh frozen plasma, or cryoprecipitate. The SHOT launch was publicised at professional conferences and by an editorial in the BMJ.3 Suspected cases of post-transfusion infection were reported to local blood centres, and cases confirmed after donor investigation as related to transfusion were collated by the National Blood Authority/Public Health Laboratory Service Communicable Disease Surveillance Centre.
Incidents other than infections reported to the SHOT office were analysed with a questionnaire then entered on a secure database without identifiers. During the second year hospitals could submit a “nil to report” return card. We have analysed data relating to incidents that occurred between 1 October 1996 and 30 September 1998.
Results
Overview
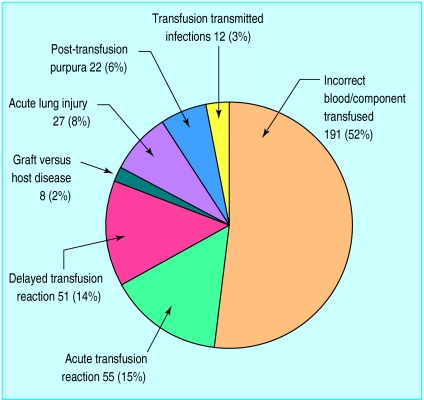
Of 424 eligible hospitals, 94 submitted 169 reports during the first year, with 112 hospitals submitting 197 reports during the second year, an increase of 16.5%. “Nil to report” cards, introduced in the second year, were submitted by 164 hospitals (39%), bringing overall participation to 65%. Reports included 191 incidents of incorrect blood transfused and 12 infections transmitted by transfusion (figure). Of 341 analysed cases, there were 22 deaths and 81 cases of major morbidity, with at least one death in every category (table).
Incorrect blood or component transfused
Of the 191 reported episodes in which a patient was transfused with a wrong blood component, 62 were ABO incompatible transfusions, leading to three deaths, 25 cases requiring intensive care, and six cases of potential rhesus D sensitisation in young female patients. The errors in 177 analysed cases of incorrect transfusion generally consisted of a sequence of one to seven failures to detect incorrect identity of blood or patient, leading to transfusion to the wrong patient (one error—103 cases; two errors—40 cases; three errors—26 cases; four errors—2 cases; six errors—5 cases; seven errors—1 case). First errors occurred at all stages of the process: during the request for blood or sampling of the patient, or both (33), including two incidents (one fatal) of transposition of blood grouping samples at the patient’s bedside, and in the transfusion laboratory (59). Collection of the wrong blood from the blood bank refrigerator was the major source of primary error (61), with blood frequently taken without a formal identity check against the patient’s case record. The bedside check failed to detect discrepancy in blood or patient identity in a total of 80 cases, despite being carried out by two people (one always a qualified nurse or doctor). In 20 incidents, the patient had no identity wrist band.
Immunological interactions between donor and patient
Immunological interactions were reported in five categories (acute and delayed reactions, post-transfusion purpura, transfusion associated graft versus host disease, and transfusion related acute lung injury). These cases were unpredictable and generally did not represent poor practice. In two cases, however, haemolytic antibodies were missed because of non-compliance with current guidelines.4 Occasionally, relevant information was not available because the blood bank computer could not be accessed.
Five reports were received of a hypotensive reaction to components passed through a bedside leucocyte filter.5 Of eight cases of transfusion associated graft versus host disease, one was a premature neonate with an unusual form of immunodeficiency and four patients had B cell lymphoid malignancy, not currently an indication for prevention of transfusion associated graft versus host disease by the use of γ irradiated blood components.
Transfusion transmitted infection
Twelve of 60 suspected cases (20%) were confirmed to be related to the transfusion. Six cases (one hepatitis A infection, one malaria, four bacteraemias) were due to infections for which no testing of donations is performed, while five cases (three hepatitis B, one hepatitis C, one HIV) were due to donations from repeat donors during marker negative “window periods” after recent infection. A further newly reported case of infection with hepatitis C virus was in a patient who received a transfusion before the introduction of donor screening in 1991. The HIV transmission involved components from one donor transfused to three different recipients. The three non-fatal bacterial incidents involved red cells contaminated with Serratia liquefaciens, platelets containing Escherichia coli, and leucocyte depleted platelets containing Bacillus cereus, also grown from the donor’s arm. Fatal septicaemia due to Staphylococcus aureus occurred in one platelet recipient. S aureus was also isolated from the donor’s skin and nose.
The other fatality caused by infection was due to cerebral malaria after transmission of Plasmodium falciparum from a donor who lived in a malarious area as a child and who had revisited a (different) malarious area within the previous 4 years. This has already led to amendments to selection criteria for donors.
Key recommendations and feedback
Major findings and the recommendations arising from them are summarised in the box. Detailed annual reports were sent to all haematologists,6,7 with a summary to trusts’ chief executives and blood bank managers. Seventy nine hospitals had already reviewed procedures for blood handling and staff training after the incident reported. The SHOT findings were highlighted at a transfusion seminar organised by the United Kingdom chief medical officers, and participation in SHOT was recommended in a health service circular to trusts.8
Summary of main findings and recommendations
| Finding | Recommendation |
| Prelabelled cross match sample tubes are a source of misidentity | Current BCSH guideline recommends labelling at the bedside after the sample is drawn |
| Request for blood may omit special requirements, especially over the telephone | Request systems must ensure that all requirements are met and that responsibilities of ward and blood bank staff are clear |
| Previous transfusion information was not always used in decision making | Previous transfusion records should be available at all times and blood groups checked with current results |
| Blood bank errors in grouping, cross matching, and labelling were found | Blood banks should review procedures for adherence to national guidelines and ensure ongoing staff training |
| The most important primary cause of error was in collecting blood from the blood bank | Hospitals should review current procedures and define minimum identification requirements. Novel IT systems merit evaluation |
| Lack of patient wristbands or other identifiers was a source of wrong blood being transfused | Procedures to ensure correct patient identification are particularly important in theatre and for outpatient transfusions |
| Earlier errors are not being detected at the final bedside check, even if two qualified staff check the blood | BCSH will produce a national guideline to cover this issue; hospitals will be responsible for implementation and staff training |
| Local systems for staff training and oversight of transfusion practice are variable | Every hospital should have a transfusion committee with responsibility for all matters related to transfusions |
| Several organisations are responsible for decision making in transfusion safety | A unified approach to setting priorities for transfusion safety would ensure best use of resources |
| Investigation of transfusion reactions was variable and may have led to underestimation of bacterial transmission | Standardised protocols for joint investigation of suspected bacterial sepsis and immunological reactions between hospitals and blood centres would be helpful |
Discussion
Voluntary reporting of serious complications of transfusion has its limitations—we may be seeing only the tip of the iceberg, viral infections may present years after acquisition, and there are no denominators from which to calculate hazard incidence. The incidence of ABO incompatible transfusions reported here (62 in 2 years), however, is not significantly different from that seen in the French haemovigilance system (58 in 3 years), to which reporting is mandatory.9 In the second year, SHOT implemented a nil to report card; this revealed that the 65% of participating hospitals handled 70% of all red cells issued in the United Kingdom. SHOT findings therefore do seem representative of transfusion practice.
The two annual reports have provided the first detailed analysis of transfusion errors in the United Kingdom, an approach already recommended in the United States.10 Following defined procedures for blood handling11 and regular staff training are crucial; bedside ABO grouping has a high error rate12 and is not recommended by SHOT, although it is mandated in France. Medical and nursing staff must be aware of the possibility of ABO incompatibility or bacterial infection in a shocked recipient of transfusion, while errors in identification will be minimised by procedural training for porters and phlebotomists and by forthcoming guidelines for blood handling and administration from the British Committee for Standards in Haematology. Infections transmitted by transfusion were relatively rare, a finding consistent with the calculated low residual viral risk,13 now overtaken by the frequency of bacterial contamination of platelet concentrates.14 SHOT data provide mixed messages: the risk:benefit ratio of appropriate transfusion is high compared with other risks in life,15 but safety can still be improved. The United Kingdom lacks a unified body to take an overview of all aspects of blood safety, sometimes making it difficult to practise “aligning effort with risks.”16 Technological advances such as viral genomic detection and inactivation may be mandated by regulatory authorities, but prevention of transfusion error requires local managerial commitment, “process re-engineering,”17 and an active hospital transfusion committee. Hopefully the concept of clinical governance will focus resources in this important area.
Table.
Morbidity and mortality related to transfusions in fully analysed cases (n=341)
| Detail | Total | Incorrect component transfused | Major acute transfusion reaction | Major delayed transfusion reaction | Post- transfusion purpura | Graft versus host disease | Transfusion related acute lung injury | Transfusion transmitted infections |
|---|---|---|---|---|---|---|---|---|
| Death attributed to transfusion | 22 | 3 | 1 | 3 | 1 | 8 | 4 | 2 |
| Major morbidity* | 84 | 32† | 2 | 16 | 5 | 0 | 19 | 10 |
| Minor/no morbidity | 235 | 141 | 47 | 31 | 16 | 0 | 0 | 0 |
| Total | 341 | 176 | 50 | 50 | 22 | 8 | 23 | 12 |
Admission to intensive care or ventilation, or both; dialysis or renal dysfunction, or both; major haemorrhage; jaundice including intravascular haemolysis; persistent viral infection; acute symptomatic confirmed infection.
Includes six cases of potential rhesus sensitisation in young women/girls.
Figure.
Overview of 366 cases for which initial report forms were received
Footnotes
Funding: UK Transfusion Services, Republic of Ireland Transfusion Service, British Society for Haematology, British Transfusion Society.
Competing interests: None declared.
References
- 1.Sazama K. Reports of 355 transfusion-associated deaths: 1976 through 1985. Transfusion. 1990;30:583–590. doi: 10.1046/j.1537-2995.1990.30790385515.x. [DOI] [PubMed] [Google Scholar]
- 2.McClelland DBL, Phillips P. Errors in blood transfusion in Britain: survey of hospital haematology departments. BMJ. 1994;308:1205–1206. doi: 10.1136/bmj.308.6938.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williamson LM, Heptonstall J, Soldan K. A SHOT in the arm for safer blood transfusion. BMJ. 1996;313:1221–1222. doi: 10.1136/bmj.313.7067.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.BCSH Blood Transfusion Task Force. Guidelines for pre-transfusion compatibility procedures in blood transfusion laboratories. Transfusion Medicine. 1996;6:273–283. [PubMed] [Google Scholar]
- 5.Shiba MK, Tadokoron K, Sawanobori M, Nakajima K, Suzuki K, Juji T. Activation of the contact system by filtration of platelet concentrates with a negatively charged white cell-removal filter and measurement of venous blood bradykinin level in patients who received the filtered platelets. Transfusion. 1997;37:457–462. doi: 10.1046/j.1537-2995.1997.37597293873.x. [DOI] [PubMed] [Google Scholar]
- 6.Williamson LM, Lowe S, Love EM, Cohen H, Soldan K, McClelland DBL, et al. SHOT annual report 1996-1997. Manchester: SHOT Office, Manchester Blood Centre; 1998. [Google Scholar]
- 7.Williamson LM, Lowe S, Love EM, Cohen H, Soldan K, McClelland DBL, et al. SHOT annual report 1997-1998. Manchester: SHOT Office, Manchester Blood Centre; 1999. [Google Scholar]
- 8.NHS Executive. Better blood transfusion. London: NHS Executive; 1999. (Circular HSC 1998/99.) [Google Scholar]
- 9.Centre National d’Hémovigilance. L’ hémovigilance actualites et dossiers: lettre d’information de l’Agence Française du sang en collaboration avec le Centre National d’Hémovigilance. Avril 1995 and Mars 1996. Paris: Agence Française du Sang; 1999. [Google Scholar]
- 10.Kaplan HS, Battles JB, Van der Schaaf TW, Shea CE, Mercer SQ. Identification and classification of the causes of events in transfusion medicine. Transfusion. 1998;38:1071–1081. doi: 10.1046/j.1537-2995.1998.38111299056319.x. [DOI] [PubMed] [Google Scholar]
- 11.Lumadue JA, Boyd JS, Ness PM. Adherence to a strict specimen-labelling policy decreases the incidence of erroneous blood grouping of blood bank specimens. Transfusion. 1997;37:1169–1172. doi: 10.1046/j.1537-2995.1997.37111298088047.x. [DOI] [PubMed] [Google Scholar]
- 12.Ingrand P, Surer-Pierres N, Houssay D, Salmi LR. Reliability of the pretransfusion bedside compatibility test: association with transfusion practice and training. Transfusion. 1998;98:1030–1036. doi: 10.1046/j.1537-2995.1998.38111299056312.x. [DOI] [PubMed] [Google Scholar]
- 13.Schreiber GB, Busch MP, Kleinman SH, Korelitz JJ. The risk of transfusion-transmitted viral infections. N Engl J Med. 1996;334:1685–1690. doi: 10.1056/NEJM199606273342601. [DOI] [PubMed] [Google Scholar]
- 14.Blajchman MA, Ali AM, Richardson HL. Bacterial contamination of cellular blood components. Vox Sang. 1994;67:25–34. doi: 10.1111/j.1423-0410.1994.tb04540.x. [DOI] [PubMed] [Google Scholar]
- 15.Calman KC. Cancer: science and society and the communication of risk. BMJ. 1996;313:799–802. doi: 10.1136/bmj.313.7060.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.AuBuchon JP, Kruskall MS. Transfusion safety; realigning efforts with risks. Transfusion. 1997;37:1211–1216. doi: 10.1046/j.1537-2995.1997.37111298088055.x. [DOI] [PubMed] [Google Scholar]
- 17.McClelland DBL. Treating a sick process. Transfusion. 1998;38:999–1103. doi: 10.1046/j.1537-2995.1998.38111299056306.x. [DOI] [PubMed] [Google Scholar]