Abstract
Background and Aims
Previous molecular phylogenetic studies disagree with the informal generic-level taxonomic groups based on morphology. In this study morphological characters in the caesalpinioid clade Detarieae are evaluated within a phylogenetic framework as a means of better understanding phylogenetic relationships and morphological evolution.
Methods
Morphological characters were observed and scored for representative species of Detarieae focusing on the resin-producing genera. Phylogenetic analyses were carried out with morphological characters alone and then combined with DNA sequences.
Key Results
Despite a high level of homoplasy, morphological data support several clades corresponding to those recovered in molecular phylogenetic analyses. The more strongly supported clades are each defined by at least one morphological synapomorphy. Several characters (e.g. apetaly) previously used to define informal generic groups evolved several times independently, leading to the differences observed with the molecular phylogenetic analyses. Although floral evolution is complex in Detarieae some patterns are recovered.
Conclusions
New informal taxonomic groupings are proposed based on the present findings. Floral evolution in the diverse Detarieae clade is characterized by a repeated tendency toward zygomorphy through the reduction of lateral petals and toward complete loss of petals.
Key words: Caesalpinioideae, Detarieae, floral evolution, Leguminosae, morphology, phylogeny, resins, taxonomy
INTRODUCTION
Various resin-producing Caesalpinioideae belong to the large tropical tribe Detarieae as defined by Mackinder (2005) and Bruneau et al. (2000, 2001), which comprises the previously recognized tribes Amherstieae Benth. emend. Léonard and Detarieae DC. Of the 84 genera in Detarieae, 14 (147–149 species) produce terpenes that frequently make up resins (shown in bold in Tables 1 and 2). These terpenes are principally bicyclic diterpenes (in some genera the diterpenes are both bi- and tricyclic) and sesquiterpenes. The resin-producing genera were previously placed in different generic groups (the Brownea, Crudia, Detarium, Hymenaea and Hymenostegia groups) of tribe Detarieae sensu Cowan and Polhill (1981b). However, several molecular phylogenetic studies (Bruneau et al., 2000, 2001, 2008; Fougère-Danezan et al., 2003, 2007) have suggested that the terpene-producing genera occurred in only two clades: the Prioria clade, with five of the six genera producing terpenes, and the Detarieae sensu stricto (s.s.) clade, with nine of the 16 genera producing terpenes. The latter study (Fougère-Danezan et al., 2007) resolved the resin-producing Detarieae as monophyletic, albeit with moderate clade support. Few other members of Fabaceae produce terpenes. Langenheim (1981, 2003) noted that diterpenes produced by species of Detarieae are similar to each other, but differ from those produced in other groups. It thus appears that diterpenes and particularly bicyclic diterpenes are the most characteristic terpenes of the resin-producing Detarieae.
Table 1.
Summary of the phylogenetic analyses of morphological characters, combined analyses of morphological and plastid characters (MC) and combined analyses of morphological, plastid and nuclear data (MCN)
| Clade | Morphology: n = 1101; L = 653; CI = 0·19; RI = 0·69 | Plastid: n = 20 000*; L = 1098; CI = 0·53; RI = 0·81 | Nuclear (ITS): n = 2; L = 2285; CI = 0·35; RI = 0·58 | Combined (MC): n = 4560; L = 1687; CI = 0·38; RI = 0·73 | Combined (MCN): n = 4; L = 3622; CI = 0·37; RI = 0·62 |
|---|---|---|---|---|---|
| Resin-producing Detarieae | – | J65 | J54 | – | – |
| Prioria s.l. clade: | B2 (plus Brandzeia and Neoapaloxylon) | J96 | – | B6 | – |
|
Colophospermum Hardwickia |
B9 | J100 | J100 | B46 | B88 |
|
Prioria s.s. clade: Gossweilerodendron Kingiodendron Oxystigma Prioria |
B2 | J < 50 | J < 50 | B3 | B10 |
| Detarieae s.s. clade | – | J98 (without Daniellia) J60 (with Daniellia) |
– | B5 (without Daniellia) – (with Daniellia) |
B4 (without Daniellia) – (with Daniellia) |
|
Hymenaea clade: Guibourtia Hymenaea Peltogyne |
B1 | J99 | J98 | B8 | B17 |
| Eperua s.l. clade: | – | J96 | – | B3 | B3 |
|
Augouardia Stemonocoleus |
– | J99 | J93 | B6 | B14 |
|
Eperua s.s. clade: Eperua Eurypetalum |
B1 (plus Daniellia) | J99 | – | B6 | B9 |
|
Detarium clade: Baikiaea Tessmannia Sindoropsis Detarium Copaifera Pseudosindora Sindora |
B1 | J97 | J85 | B3 | B8 |
Molecular results are from Fougère-Danezan et al. (2007). Values preceded by ‘J’ indicate jackknife support, and values preceded by ‘B’ indicate decay indices. n is the number of trees recovered for each analysis and the asterisk indicates that the maximum number of trees was reached. Genera that have been recorded as producing terpenes are in bold. For other taxa it is not known whether they have been tested and lack resins or whether they never have been tested for the presence of resins.
Table 2.
New groupings and their diagnostic characters
| Tribe Detarieaes.l. | Style bent on the abaxial side of the flower |
| Amyloids in seeds | |
| Stipule insertion intrapetiolar | |
| I. Sub-tribe Detariinae | Bicyclic diterpenes |
| 1. Daniellia group:Brandzeia, Daniellia, Neoapaloxylon | Molecular characters only |
| 2. Prioria group | Only one ovule per ovary |
| No amyloids in seeds | |
| No ‘Zwischenkörper‘ | |
| a. Prioria sub-group: Gossweilerodendron, Kingiodendron, Oxystigma, Prioria | Cotyledons remaining in seed |
| Hypogeal germination | |
| Axial canals diffuse | |
| Stigma non-papillose, usually attenuate | |
| b. Hardwickia sub-group: Colophospermum, Hardwickia | Bifoliolate leaves |
| Strongly asymmetrical leaflets | |
| Actinodromous primary venation | |
| Peltate stigma | |
| Pantoporate pollen | |
| Rachis extension | |
| Haploid number of chromosomes n = 17 | |
| Pollen with reticulate ornamentation | |
| 3. Hymenaea group:Guibourtia, Hymenaea, Peltogyne, | Bifoliolate leaves |
| Strongly asymmetrical leaflets | |
| Primary nerve close to the distal margin of the leaflet | |
| Stipule insertion lateral | |
| 4. Eperuagroup | Molecular characters only |
| a. Eperua sub-group: Eperua, Eurypetalum | Only one big developed petal and four vestigial |
| b. Stemonocoleus sub-group: Augouardia, Stemonocoleus | Fertile stamens less than ten |
| 5. Detarium group:Baikiaea, Copaifera, Detarium, Gilletiodendron, Hylodendron, Pseudosindora, Sindora, Sindoropsis, Tessmannia | Distichous flower arrangement in inflorescence |
| ‘Sub-valvate sepals’ | |
| Axial canals tangentially organized |
Barnebydendron, Goniorrhachis and Schotia are not included because their positions are not well resolved. Genera that have been recorded as producing terpenes are in bold. For other taxa it is not known whether they have been tested and lack resins or whether they never have been tested for the presence of resins.
The resin-producing Detarieae are diverse morphologically. Members of this group are mostly unarmed trees or in rare instances shrubs with compound or rarely unifoliolate leaves. Their flowers are diverse in size and structure (Cowan and Polhill, 1981b; Mackinder, 2005). These may be small and apetalous or large and showy, with floral variations occurring in all whorls. The two adaxial sepals are often fused. All five petals can be present (e.g. Schotia), all can be absent (e.g. Prioria), or some can be absent or reduced and some present (e.g. Eperua). Stamens can be free (e.g. Prioria) or connate (e.g. Eperua) and although most species have ten fertile stamens, some have more (Colophospermum mopane) or fewer (e.g. Stemonocoleus). Some species with fewer than ten fertile stamens have staminodes with sterile anthers (e.g. Sindora) or without anthers (Augouardia letestui). The ovary consists of a single carpel (as in most Fabaceae) that is stipitate (e.g. Schotia) or not (e.g. Kingiodendron), and when present the stipe can be central (e.g. Prioria) or fused to the adaxial side of the hypanthium (e.g. Schotia).
Because of the great morphological diversity in Detarieae in general and in the resin-producing Detarieae in particular, the taxonomy of this tribe is problematic (cf. Cowan and Polhill, 1981a, b versus Breteler, 1995) and warrants further study. Moreover, recent molecular studies (Bruneau et al., 2000, 2001, 2008; Fougère-Danezan et al., 2003, 2007) do not support the traditional classification (tribe delimitation and generic groupings; Cowan and Polhill, 1981a, b) based on morphology. Following the results of molecular analyses, the most recent classification of Fabaceae (Lewis et al., 2005) treats Detarieae (Mackinder, 2005) as a single tribe, but no taxonomic groupings are proposed within the tribe. This reflects our current understanding of the group because few attempts have been made to use morphology in a phylogenetic context, in order to better define taxonomic groups. The arguments put forward by Luckow and Bruneau (1997) in favour of the inclusion of ecological characters could also be applied to the inclusion of morphological characters in phylogenetic analyses (see also Jenner, 2004; Wiens, 2004).
The aims of this study are to evaluate the utility of morphological characters, to determine morphological synapomorphies for clades (allowing us to propose new generic groupings) and to understand better the complex floral evolution in this group. To address these issues, we analysed morphological data both alone and in combination with molecular data within a phylogenetic framework focusing on the resin-producing Detarieae.
MATERIALS AND METHODS
Sampling
At least one species from each genus belonging to the Detarieae s.s. and Prioria clades (resin-producing Detarieae) were included in the study. Herbarium specimens from 90 species (40 genera) were selected including three outgroups and nine place-holders for the Amherstieae clade (Appendix 1).
Methods
Herbarium specimens were observed using a binocular microscope and, when possible, 1–3 flowers were dissected after rehydratation (using warm water or water with 10 % glycerin). From these observations, 75 morphological characters describing vegetative morphology, inflorescence structure and floral morphology were coded and scored (Appendix 2). The methods for preparing twig wood samples to document vestured pits (our only anatomical character) are described in Herendeen (2000). Using information from the literature, 13 characters describing pollen grains (Banks and Klitgaard, 2000; Banks, 2003; Banks et al., 2003), wood anatomy (Gasson et al., 2003), seedlings (Léonard, 1957, 1994; Watson and Dallwitz, 1993), fruits (Gunn, 1991) and seeds (Léonard, 1957; Kooiman, 1960; Gunn, 1991) were added. Because the species selected in those different studies and the present study were not always the same, when no variation was observed in a genus all the species of that genus were considered as having the same character state. When variation was observed, the species for which that character was not observed was scored as missing for this character.
Analyses
The morphological matrix (see Supplementary Data, available online) was analysed with PAUP* 4.0b10 (Swofford, 2002). All characters were considered as unordered. The morphological data were also combined with the molecular data of Fougère-Danezan et al. (2007) in a concatenated matrix. For all analyses, we used the heuristic search algorithm with tree bisection reconnection (TBR), MULTREES and steepest descent in effect. An initial set of trees was obtained via random stepwise addition (1000 replicates) with 10–50 trees retained per replicate. Those trees were then used as starting trees for a full heuristic search using TBR to search for additional optimal trees, retaining a maximum of 10 000 trees. All the values of consistency indices are calculated without the autapomorphies. Bremer support values (Bremer, 1988, 1994) were generated using autodecay 5·0 (Eriksson, 2001). Values <3 were considered as low (clade poorly supported), values from 3 to 5 as moderate, and values >6 were considered to indicate strong clade support.
The partition homogeneity test or incongruence length difference test (ILD test; Farris et al., 1995) as implemented in PAUP* was used to test for incongruence among the different data sets. A threshold α = 0·01 was used as recommended by Cunningham (1997).
The evolution of corolla symmetry (i.e. characters 52, 53 and 54 taken together) was reconstructed on one of the most-parsimonious trees using the parsimony criterion in Mesquite 2·6 (Maddison and Maddison, 2009). Because internal transcribed spacer (ITS) sequences for several taxa were missing, the results of the morphology and plastid data (MC), with more complete taxon sampling, rather than the results of the combined analysis of all three data sets (MCN) were used to examine floral evolution. We chose one of the most-parsimonious trees from MC analysis where Brandzeia is sister to Daniellia because it is the relationship obtained with MCN analysis.
RESULTS
Analysis of the morphological data yielded 1101 trees (length = 653; CI = 0·19; RI = 0·70; Table 1). Bremer support values were usually low (data not shown). Results of the ILD test indicated a slightly significant incongruence between the morphological data and the plastid DNA data (P = 0·01) and between the morphological data and the plastid plus ITS data set (P = 0·01). These results are not highly significant and the use of ILD as an indicator of data set combinability has been questioned (see Yoder et al., 2001). The main difference between the morphological and plastid data sets relates to the position of Brandzeia. Removing this genus improved the result of the ILD test and increased the resolution at the base of the tree without other modification in the relationships. It was thus decideded to combine the morphology and molecular data for analysis. The analysis of morphology and plastid data yielded 4560 trees (MC; length = 1687; CI = 0·38; RI = 0·73; Table 1; Fig. 1). A combined analysis of all three data sets yielded four most-parsimonious trees (MCN; length = 3622; CI = 0·37; RI = 0·62; Table 1).
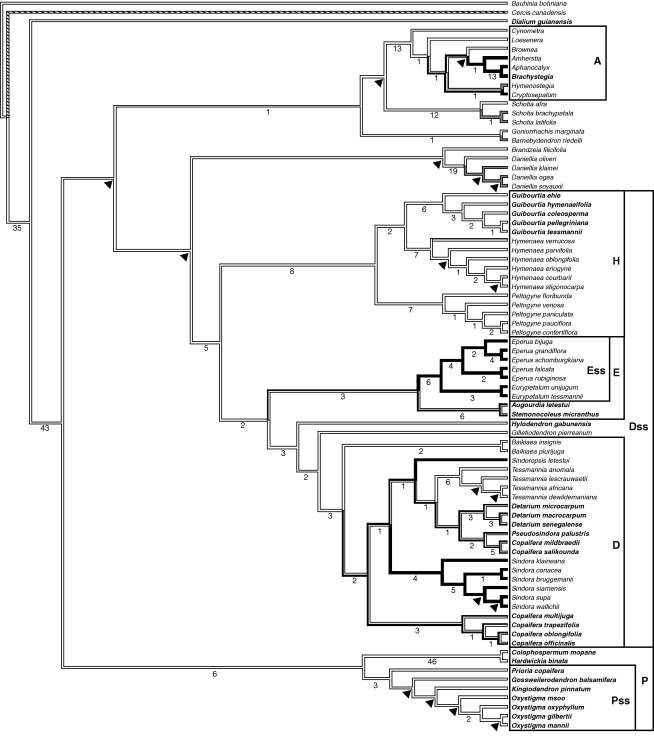
Fig. 1.
One of the 4560 most-parsimonious trees obtained in the combined analysis of morphological and plastid data (L = 1687, CI = 0·38, RI = 0·73). Letters in the frames indicate the names of clades (A, Amherstieae clade; D, Detarium clade; Dss, Detarieae s.s. clade; E, Eperua s.l. clade; Ess, Eperua s.s. clade; H, Hymenaea clade; P, Prioria s.l. clade; and Pss, Prioria s.s. clade). Numbers under the branches indicate decay indices. Arrows indicate clades that are not supported in the strict consensus tree. The branches are shaded according to the reconstruction by Mesquite of the ancestral state for corolla pattern: white for five developed petals, hatched for ‘Cercis’ pattern (applicable only to Cercis), grey for three developed petals and black for one developed petal (species in bold are the apetalous species coded as non-applicable for this character).
The results of these analyses are summarized and compared with our molecular study (Fougère-Danezan et al., 2007) in Table 1. Several groups were resolved as monophyletic in both the morphological analysis and the combined data analyses (MC and MCN). Although the resin-producing Detarieae clade was recovered in the molecular analyses (Fougère-Danezan et al., 2007), neither the morphological nor the combined analyses resolved this group as monophyletic. The Prioria sensu lato (s.l.) clade was strongly supported in the MC combined analysis, and, although also supported in the morphological analysis, it included the genera Brandzeia and Neoapaloxylon. This clade was strongly supported in previous analyses of plastid DNA but was not recovered in the ITS analyses because the genera Colophospermum and Hardwickia were placed elsewhere (Fougère-Danezan et al., 2007). The Prioria s.s. clade was resolved as monophyletic in all analyses but was poorly supported, except in the combined analyses where it was moderately to strongly supported. The Detarieae s.s. clade was resolved as monophyletic in the plastid DNA analyses (Fougère-Danezan et al., 2007) and here in the combined analyses, but not in the morphological analysis. The inclusion of the genus Daniellia in this clade was not well supported. The Hymenaea clade was always resolved as monophyletic and usually strongly supported (except in the morphological analysis where all support values were low). The Eperua s.l. clade was resolved as monophyletic in the combined analyses only (with moderate support), whereas the Eperua s.s. clade was resolved as monophyletic in both the morphological and combined analyses, but with strong support only in the latter. The Detarium clade was always resolved as monophyletic. It was strongly supported in the previous molecular analyses, and moderately to well supported in the combined analyses presented here. The positions of two genera, Gilletiodendron and Hylodendron, were ambiguous. As in our previous molecular analyses, in both the morphological and combined analyses Gilletiodendron was resolved as sister to the Detarium clade with poor support, whereas Hylodendron was sister to this entire clade (MC combined analysis), sister to Gilletiodendron (MCN combined analysis) or sister to Stemonocoleus (morphology alone).
DISCUSSION
The Detarieae clade has been recognized as monophyletic with strong support for some time (Bruneau et al., 2001), but morphological synapomorphies have yet to be clearly identified (although some characters, such as intrapetiolar stipules, are present in most Detarieae). Our analyses point to several morphological characters that are useful at different phylogenetic levels for defining clades within the resin-producing Detarieae. Characters from the corolla (e.g. characters 52–54) can be useful synapomorphies for closely related genera but are problematic to use at the level of the resin-producing Detarieae as a whole because of convergent evolution (especially apetaly). Apetaly has been used as a primary character to define the Crudia group (Cowan and Polhill, 1981b), but members of this generic group are scattered in our analyses, suggesting that loss of petals has arisen several times (six times) in the resin-producing Detarieae (Fig. 1). Some vegetative characters, such as secondary venation (characters 23 and 24), are also synapomorphies for closely related genera or for certain clades (e.g. the Hymenaea and Detarium clades). Some of the characters previously used for developing classifications have proved to be good synapomorphies in our analyses, such as inflorescence and sepal characters (e.g. characters 32 and 48 for the Detarium clade). In addition, it was found that ovary characters are particularly useful synapomorphies for the Prioria s.l. and s.s. clades (characters 74 and 75), for closely related genera (character 70; Eperua s.l. in part), and even for genera (character 75; Guibourtia). Characters from fruits and seeds (characters 77, 87–89) are also useful to support clades (Prioria s.l. and Eperua s.s.) and at the generic and intrageneric levels. Pollen and wood anatomy characters (characters 78–80, 82) support clades and resolve relationships among closely related genera, as do seedling characters (characters 83–85), which Léonard (1957, 1994) studied in detail and used as a basis for his substantial taxonomic revisions.
Morphological synapomorphies in Detarieae
The resin-producing Detarieae are not resolved as monophyletic in all analyses and, other than their ability to produce bicyclic diterpenes (Fougère-Danezan et al., 2007), no clear synapomorphies are known for this group (see sub-tribe Detariinae; Table 2). However, within the resin-producing Detarieae, most of the clades recognized in our molecular analyses can also be diagnosed by morphological synapomorphies. These characters are used as a basis for proposing new informal taxonomic groups for the resin-producing Detarieae (Table 2).
The Prioria s.l. clade is defined by having only one ovule per ovary. This state also occurs in Cynometra mannii and Guibourtia arnoldiana, but these species belong to genera usually having two ovules per ovary. A proximal wing is frequently present (Brandzeia, Gossweilerodendron, Hardwickia, Neoapaloxylon and some species of Kingiodendron and Oxystigma) even if not present in all the species of the clade (Prioria never has a wing). A similar wing is also observed in the monotypic genus Hylodendron (Detarium clade). In the Prioria s.l. clade, flowers are generally apetalous, except in the monotypic genus Brandzeia, which has five petals. Within the clade, some characters are shared with members of the Prioria s.s. clade and the two monotypic genera Colophospermum and Hardwickia, whereas other characters are shared with members of the Prioria s.s. clade and the genera Brandzeia and Neoapaloxylon. Colophospermum and Hardwickia share with the Prioria s.s. clade the absence of amyloids in seeds, present in all other Detarieae examined (Kooiman, 1960). In addition, Colophospermum and members of the Prioria s.s. clade do not have ‘Zwischenkörper’ (a state unknown for Hardwickia), which have been observed in all other Detarieae and in Cercis (Banks, 2003). ‘Zwischenkörper’ (character 78; see Appendix 2) are pectic structures associated with the pores of pollen (Banks, 2003). Brandzeia and Neoapaloxylon share an attenuate stigma with all members of the Prioria s.s. clade, except Kingiodendron which has a crateriform stigma. Brandzeia and Neoapaloxylon are quite distinct from members of the Prioria s.s. clade by having more numerous smaller leaflets and larger flowers, which in Brandzeia have petals. The presence or absence of amyloids and ‘Zwischenkörper’ is unknown in those two genera.
The species of the Prioria s.s. clade are roughly similar, with multifoliolate leaves possessing a small number of leaflets and small apetalous flowers in dense racemes. In all these species, the cotyledons remain in the seed during germination, which is hypogeal rather than epigeal, typical of other Detarieae and the outgroup taxa (Gasson et al., 2003). In addition, axial canals in the wood are diffuse, except in Prioria where they are tangentially organized (Gasson et al., 2003).
Breteler (1999) recently revised the genus Prioria to include all species of the Prioria s.s. clade. Among the members of this clade, Kingiodendron is easily distinguished by its crateriform stigma and unisexual flowers (Table 3). The genus Gossweilerodendron has only four sepals (it is difficult to know whether this is due to fusion of the two adaxial sepals or whether only four sepals are initiated), whereas all other genera in this clade have five sepals. The genus Prioria has flat cotyledons, whereas they are ruminate or canaliculate in Kingiodendron and Oxystigma, and plano-convex in Gossweilerodendron. The polymorphic genus Oxystigma lacks any distinct unifying character (Table 3). An exhaustive species-level sampling of the Prioria s.s. clade and further examination of the morphological data are necessary, but, given the morphological diversity encountered in this clade, our results to date do not contradict the proposal that these four genera be united under the single genus Prioria. For the moment, we simply consider these four genera as being at least in the same sub-group (Prioria sub-group; see Table 2).
Table 3.
Morphological and anatomical characters that distinguish genera in each of the main clades of the resin-producing Detarieae
|
Colophospermum and Hardwickia | ||
|---|---|---|
| Character | Colophospermum mopane | Hardwickia binata |
| Sepal number | Four | Five |
| Stamen number | 20–25 | 10 |
| Disk | Present | Absent |
| Anther surface | Smooth | Verrucose |
| Fruit shape | Asymmetrical, reniform | Symmetrical |
| Radicle position | Lateral | Terminal |
| Wing on seed | Present | Absent |
| Resin vesicle on seed | Present | Absent |
| Aspect of the seed after soaking | Mucilaginous | Non-mucilaginous |
|
Prioria s.s. clade | ||||
|---|---|---|---|---|
| Character | Gossweilerodendron | Kingiodendron | Oxystigma | Prioria |
| Sepal number | Four | Five | Five | Five |
| Stigma shape | Attenuate | Crateriform | Attenuate | Attenuate |
| Hermaphroditic or unisexual flowers | Flowers all bisexual | At least some flowers unisexual | Flowers all bisexual | Flowers all bisexual |
| Cotyledons | Plano-convex | Ruminate | Ruminate or canaliculate | Flat |
|
Hymeanaea clade | |||
|---|---|---|---|
| Character | Guibourtia | Hymenaea | Peltogyne |
| Petal number | None | Five | Five |
| Sepal margins | Thin, imbricate | Thin, not imbricate | Thin, not imbricate |
| Ovule number | Two rarely one (G. arnoldiana) | Numerous | Numerous |
| Axial canals | Present, traumatic | Absent | Absent |
| Endocarp | Thin | Fleshy | Thin |
| Ovary stipe | Central | Adnate to the adaxial side of the hypanthium | Central |
| Crateriform glands | Present | Present | Absent |
|
Eperua s.s. clade | ||
|---|---|---|
| Character | Eperua | Eurypetalum |
| Stamen sheath | Long | Short |
| Flower size | Large | Small |
| Ovule number | Numerous, rarely two (E. grandiflora) | Two |
| Secondary venation | Intramarginal vein | Brochidodromous |
| Mucronate leaflets | Present | Absent |
| Twisted petiolules | Absent | Present |
|
Detarium clade | |||||||
|---|---|---|---|---|---|---|---|
| Character | Baikiaea | Copaifera | Detarium | Pseudosindora | Sindoropsis | Sindora | Tessmannia |
| Petal number | Five | None | None | None | One | Three or five | Five |
| Vestigial petals | None | NA | NA | NA | None | Two or four | None |
| Stamen fusion | Nine fused | All free | All free | All free | Nine fused | Nine fused | Nine fused |
| Staminodes | None | None | None | None | None | Eight | None |
| Anther shape | Rectangular | Rectangular | Square | Rectangular | Rectangular | Rectangular | Rectangular |
| Mucronate leaflets | Absent | Present | Absent | Absent | Absent | Absent | Absent |
| Ovary stipe | Central | Central | Absent | Adnate to the adaxial side of the hypanthium | Absent | Central | Central |
| Stipule fusion | Present | Absent | Absent | Absent | Absent | Absent | Absent |
| Marginal vein | Present | Present | Present | Present | Present | Present | Absent |
The two genera of the Hardwickia clade, Colophospermum and Hardwickia, share bifoliolate leaves (also occurring in the Hymenaea clade, Aphanocalyx cynometroides and Eurypetalum unijugum), asymmetrical leaflets with an actinodromous primary venation (also in Aphanocalyx, Bauhinia and Cercis), a peltate stigma and pantoporate pollen (Table 3). They also lack axial canals, unlike other members of the Prioria clade (Banks and Gasson, 2000). Other characters shared by these two monospecific genera, but not included in our matrix for practical reasons, include a rachis extension beyond the insertion point of the leaflets (Herendeen, 2000), a haploid chromosome number of 17 (Goldblatt, 1981; Breteler et al., 1997; vs. n = 12 or 11 in other studied Detarieae), pollen grains with a reticulate ornamentation (Banks and Klitgaard, 2000), seeds with ruminate cotyledons and wood with a similar anatomy (Breteler et al., 1997; Banks and Gasson, 2000). Breteler et al. (1997) suggested their unification because of these shared characters, but Léonard (1999) insisted on keeping them separate, arguing that they are clearly distinct regarding characters of generic importance. Indeed, Colophospermum has been interpreted as having four sepals, whereas Hardwickia has five, resulting in a difference in calyx structure (alternate for Colophospermum and imbricate for Hardwickia). However, an ontogenetic study of Colophospermum by Krüger et al. (1999) showed that the two lateral lobes of the perianth are probably bracteoles, not sepals (Fig. 2). Similarly, the structure of the flower of Hardwickia suggests that the two lateral lobes of the perianth are also likely to be bracteoles (Fig. 2). The differences in flower structure thus appear to be the result of a similar event: the bracteoles becoming larger and closer to the calyx and the loss of the sepals contiguous to the bracteoles. These two genera also differ in stamen number, presence or absence of a staminal disk, anther surface texture, shape and dehiscence of the fruit, and seed morphology (see Léonard, 1999, and Table 3). Therefore, although the unification of the two genera is possible, there remains a question of rank. They are, however, close relatives and are considered to be in the same sub-group (Hardwickia sub-group; see Table 2).
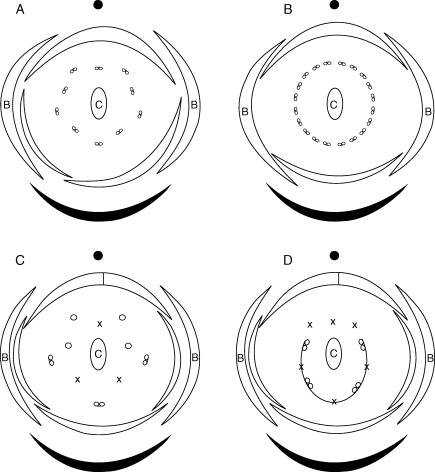
Fig. 2.
Floral diagrams for four apetalous species (A) Hardwickia binata, (B) Colophospermum mopane, (C) Augouardia letestui and (D) Stemonocoleus micranthus. The ellipse marked with a ‘C’ is the single carpel. Parts marked with a ‘B’ are bracteoles. Empty circles indicate staminodes, and crosses indicate putative positions for missing stamens.
As detailed above, members of this clade share several characters with the Prioria s.s. clade. The plastid data emphasize a close relationship with the Prioria s.s. clade, but the ITS data are more ambiguous. This conflict has been interpreted as an indication of a possible hybrid origin of Colophospermum and Hardwickia (Fougère-Danezan et al., 2007). Moreover, the long branch observed in the molecular analyses and the large number of morphological apomorphies characteristic of this lineage suggest an accelerated rate of evolution. The relatively high chromosome number (n = 17) suggests a triploid origin for this lineage, which may be associated with a hybridization event involving the Prioria s.s. clade lineage. As observed in other groups (Levin, 1983; Wendel, 2000), the apparently accelerated evolution could be the result of a polyploidization event (possibly an allopolyploidization event).
The genus Brandzeia (and its close relative Neoapaloxylon, sampled in the morphological data set only and thus absent in Fig. 1) is resolved in the morphological data analysis as a close relative of the Prioria s.s. clade, despite several non-exclusive characters being shared with the genus Daniellia. Although molecular data suggest a close relationship between Brandzeia and Daniellia, several characters shared by Brandzeia and members of the Prioria s.s. clade are linked with wind dispersal (one ovule per ovary, fruit indehiscent with a proximal wing) and could thus be the result of convergence. Moreover, several characters among those scored from the literature are missing for Brandzeia and Neoapaloxylon. We thus consider Brandzeia and Neoapaloxylon as being in the Daniellia group rather than in the Prioria group (Table 2).
The boundaries of the Detarieae s.s. clade are not well established, and its only synapomorphy is the protruding apertures of the pollen. This clade is difficult to define morphologically because of its high degree of variability.
Within Detarieae s.s., members of the Hymenaea clade have more or less regular flowers with five petals (Hymenaea and Peltogyne) or without any petals (Guibourtia; see Table 3 for more characters). This clade is better defined by vegetative characters, such as bifoliolate leaves (shared with Colophospermum, Hardwickia, Aphanocalyx cynometroides and Eurypetalum unijugum). The leaflets are asymmetrical (often curved) with pinnate secondary venation and the primary vein close to the distal margin. The insertion of the stipules is lateral (also occurring in Hardwickia) rather than intrapetiolar, as seen in other Detarieae. The genera Hymenaea and Peltogyne were included in the same generic group by Cowan and Polhill (1981b), but Guibourtia was included in the apetalous Crudia group even though morphologically this genus is otherwise similar to members of the Hymenaea clade. The three genera of the strongly supported Hymenaea clade are thus considered to belong to the same group (Hymenaea group; Table 2).
There is no morphological synapomorphy for the Eperua s.l. clade because the two monotypic genera Augouardia and Stemonocoleus are unique and do not seem to share characters with any other genus. These two genera have apetalous flowers with a reduction in the number of fertile stamens, but the patterns are different (Fig. 2). The genus Augouardia has three fertile stamens facing the abaxial sepals and four staminodes on the adaxial side, and all the androecium parts are free. The genus Stemonocoleus has four fertile stamens fused in a sheath on the abaxial side, but in alternate positions relative to the sepals. Among the resin-producing Detarieae, a reduction in number of fertile stamens also occurs in the genus Sindora and in some species of Eperua. However, in the genus Sindora, the two fertile stamens are on the adaxial side (the others are reduced to staminodes), and in the genus Eperua, five sterile stamens alternate with five fertile stamens. The lower number of fertile stamens observed in Augouardia and Stemonocoleus, with fertile stamens on the abaxial side only, therefore appears to be homologous in the two genera, despite differences in fusion and number of stamens. Those two genera are also included in the Eperua group, but in a different sub-group (Stemonocoleus sub-group; Table 2).
The genera Eperua and Eurypetalum (Eperua s.s. clade) are undoubtedly closely related. The two genera have a similar flower structure, with nine of the ten stamens abaxially fused in a sheath, one large petal (larger than the calyx lobes) and four vestigial ones. Sindora klaineana has almost the same flower structure, but the only developed petal is smaller (comparable with calyx lobes). Despite their similarity in floral morphology, Eperua and Eurypetalum have been considered to belong to different generic groups (Cowan and Polhill, 1981b). Eperua was placed in the Brownea group, although it lacks the main character defining this group (connate bracteoles) and shares other characters with other groups within Detarieae. Recent phylogenetic analyses have shown that the genus does not belong to the Brownea clade, which includes eight of the ten genera of the Brownea group (Bruneau et al., 2000, 2001, 2008; Herendeen et al., 2003). Moreover, Eperua lacks the main character defining the clade (woody lip on the placental suture on each valve of the fruit; Redden and Herendeen, 2006). Eurypetalum was placed in the Hymenostegia group, although it lacks one of the main characters of the group (well developed bracteoles; Cowan and Polhill, 1981b). The only character supporting its inclusion is the presence of twisting fruit valves, a character shared by Eperua and Eurypetalum. Their similarity may have been unnoticed because Eurypetalum is exclusively African, whereas Eperua is exclusively American. In addition, the two genera differ by a number of other characters such as the length of the stamen sheath, size of the flowers, number of ovules per ovary, secondary venation, presence of a mucro on the leaflets and presence of twisted petiolules (Table 3). These two genera are treated here as members of the same sub-group (Eperua sub-group; Table 2).
Among our well supported clades, the Detarium clade is the most variable morphologically, even though it contains only members of the Detarium group as defined by Cowan and Polhill (1981b) and excludes one genus (Goniorrhachis), which had been included in the group. Some members of this clade have simple flowers that are more or less regular and apetalous (Copaifera, Detarium and Pseudosindora), whereas others have more or less regular flowers with five petals and nine of the ten stamens fused in a sheath (Baikiaea and Tessmannia; Table 3). However, in a few genera, the flowers are irregular with only one petal developed and with two (Asian Sindora) to four (African S. klaineana) vestigial petals or without vestigial petals (Sindoropsis letestui), and with nine stamens of the ten fused in a sheath, but with either ten fertile stamens (Sindoropsis letestui) or two fertile stamens and eight staminodes (Sindora). Nevertheless, members of this clade share several characters. The flowers are distichous in the inflorescence (also occurring in Gilletiodendron and Hylodendron and in the distantly related Goniorrhachis), except in Pseudosindora (sometimes included in Copaifera), which is reported to have spirally inserted flowers (Symington, 1944); however, the specimen observed in the present study was not well enough preserved for this character to be scored. The sepals are imbricate and the overlapped margins have a different texture (character 48; also occurring in Guibourtia and the closely related genera Gilletiodendron and Hylodendron). Those two characters were used by Cowan and Polhill (1981b) to define the Detarium group, although the latter was described as ‘sub-valvate sepals’. The wood shows, in most of the species of this clade, some tangentially organized axial canals (also occurring in Brandzeia, Cryptosepalum, Daniellia, Eperua and Prioria). In contrast, the genus Baikiaea has diffuse axial canals (as do the closely related genera Gilletiodendron and Hylodendron). Exinous bridges over the centre of the pollen aperture occur in four of the seven genera (Copaifera, Pseudosindora, Sindora and Sindoropsis) and in the closely related genus Hylodendron. Within the Detarium clade, intergeneric relationships remain poorly resolved, but each genus is well defined by morphology (Table 3) and combined data, except Copaifera which is not resolved as monophyletic in the combined analyses. We thus consider the members of Detarium clade as belonging to the same group (Detarium group) without defining any sub-groups (Table 2).
Floral evolution
Although few ontogenetic studies have been carried out on resin-producing Detarieae, several apetalous species of the Amherstieae clade were studied by Tucker (2000a, b; 2001b), and one apetalous species, Colophospermum, of the resin-producing Detarieae was studied by Krüger et al. (1999). Within the Amherstieae clade, in Crudia some petal primordia are initiated but do not develop (Tucker, 2001b), in Brachystegia all five petals are initiated but none develop (Tucker, 2000a), and in Saraca it seems that five petals are initiated but four petal primordia develop later into stamens and one does not develop (Tucker, 2000b). In contrast, in Colophospermum, the petals are not initiated (Krüger et al., 1999). It remains to be determined whether all apetalous resin-producing Detarieae have this same developmental pattern, with absence of petals being the result of non-initiation of petal primordia.
Among the species having flowers with petals, several have fewer than five developed petals. These generally follow two patterns: either three petals are developed on the adaxial side and the abaxial ones are reduced (smaller or vestigial), or the median adaxial petal is developed and the four others are vestigial or absent (initiated or not). In the resin-producing Detarieae, the first pattern (3 + 2) occurs more often in early diverging lineages (Fig. 1) such as Barnebydendron, Daniellia and Schotia, and even in some individuals of Hymenaea verrucosa (not observed, but see Lee and Langenheim, 1975), whereas the second pattern (1 + 4) predominates in later diverging lineages (Eperua, Eurypetalum, Sindora and Sindoropsis). It is thus hypothesized that repeated shifts occur from actinomorphy to zygomorphy through the reduction of lateral petals (the abaxial ones being more labile) in the resin-producing Detarieae. Reversal to an actinomorphic corolla is possible, and complete petal loss is frequent (six losses in resin-producing Detarieae; Fig. 1). This complex pattern of floral evolution may be associated with adaptation to a wide array of pollinators (Lewis et al., 2000), but pollinators are known only for few species of this tribe.
Floral ontogeny depends not only on the interactions of the well-known A, B, C, D and E class genes encoding MADS box transcription factors (Theißen, 2001; Theißen and Saedler, 2001), but also on the interactions between those genes and other genes in a complex genetic network (e.g. see Irish, 2008 for petal organogenesis in Arabidopsis). Moreover, several genes have been identified as being involved in this complex network of interactions to establish zygomorphy in Antirrhinum (Luo et al., 1996, 1999; Almeida et al., 1997; Galego and Almeida, 2002; Cubas, 2004), and orthologues of one of those genes have been discovered in papilionoid legumes (Citerne et al., 2006).
According to Irish (2008), some genes appear to play a role in the formation of petal primordia and their later development (e.g. RBE), whereas others appear to act on petal growth (e.g. JAG and ANT), although it is not clear when the latter genes start acting. If the same genetic processes apply to legumes, this would strengthen the hypothesis that apetalous flowers with initiated petals (e.g. Brachystegia, Crudia) are not homologous to apetalous flowers without initiated petals (e.g. Colophospermum) as suggested by Tucker (2001b, 2003). Unfortunately, it is impossible to test whether the taxa with petal primordia give rise to the taxa lacking petal primordia because only four apetalous genera in Detarieae have been studied from an ontogenetic point of view. Tucker (2000d) invoked the ‘suppression’ of regulatory genes to explain changes of floral patterns, but recent improvements in our understanding of the genetic basis of floral development suggest otherwise. Small changes in cis-regulatory elements and trans-regulatory regions may lead to dramatic effects on the expression (in time and place) of a developmental gene, which will in turn affect the expression of other genes in the developmental regulatory network (Lynch and Wagner, 2008). Several causes can thus lead to the same result (i.e. lack of expression of a developmental gene at a specific time and place) and, as such, structural homology in floral characters could be the result of genetic non-homology. This could explain why similar developmental patterns occur repeatedly during the evolution of the resin-producing Detarieae.
CONCLUSIONS
Morphology appears to be more in agreement with molecular data than was previously thought on the basis of traditional classifications (Cowan and Polhill, 1981b). Despite a high level of homoplasy, several characters are useful for reconstructing phylogenetic relationships. Among the most promising characters, those from the ovary, fruit and seed should be further investigated.
Although some of the relationships within Detarieae remain poorly resolved, the classification of the tribe should be modified to take into account the groupings suggested by our analyses (Table 2). While the more strongly supported groups are probably correct (sub-tribe Amherstiinae, Hymenaea group, Detarium group), some others may need further modifications.
SUPPLEMENTARY DATA
Supplementary Material
ACKNOWLEDGEMENTS
We thank the curators at BR, L, MT, P, US and WAG for providing access to collections. We also thank Michael Fay and two anonymous reviewers for their useful comments. M.F.-D thanks Marie-Pierre Gauthier and Vincent Manzanilla for their help with herbarium material. This study was funded by grants from the Natural Sciences and Engineering Research Council of Canada to A.B., by a National Science Foundation (USA) grant to P.S.H., A.B. and G.P. Lewis, and by a fellowship from the International Council for Canadian Studies to M.F.-D. Funding from the Open Laboratory of Biodiversity Conservation and Ecology of Chengdu Institute of Biology, Chinese Academy of Sciences (CAS), to Li-Bing Zhang and a CAS Research Fellowship for International Young Researchers provided support to M.F.-D. during the writing.
APPENDIX 1
List of specimens used to score morphological characters.
| Amherstia nobilis Wall., M.C. Carlson 513 (F), s.c. 596 (L), Calcutta Botanical Garden s.n. (L), M. Fougère-Danezan & M. N. B. Jumaai 24 (MT), M. Fougère-Danezan & M.N.B. Jumaai 29 (MT) |
| Aphanocalyx cynometroides Oliv., J. Louis 13815 (K), J. Louis 3814 (K), Le Testu 8418 (P), F.J. Breteler 12868 (WAG) |
| Augouardia letestui Pellegr., G. Mcpherson 15493 (MO), G. Mcpherson 16282 (MO), G. Le Testu 2228 (BM), A.J.M. Leeuwenberg & J.G.M. Persoon 13651 (P) |
| Baikiaea insignis Benth., J. Louis 3464 (US, K), P.L. Comanor 1172 (F), A. Hladik 1872a (P), N. Hallé 2859 (P) |
| Baikiaea plurijuga Harms, B. de Winter 3648 (P), N.B. Zimba et al. 928 (P) |
| Barnebydendron riedelii (Tul.) J.H. Kirkbr., B. Krukoff 5651 (F, MO) |
| Bauhinia bohniana L. Chen, G. Forrest s.n. (US), J.F. Rock 9045 (US) |
| Bikinia letestui (Pellegr.) Wieringa, X.M. van der Burgt 600 (WAG) |
| Brachystegia bussei Harms, Y.S. Abeid et al. 1434 (MO), B.D. Burtt 5004 (US), C.G. Trapnell 1807 (P), H.J. Schlieben 5898 (P), P.S. Herendeen & F. Mbago 20-XII-97-2 (US), P.S. Herendeen & F. Mbago 20-XII-97-3 (US), F.J. Breteler 11859 (WAG) |
| Brownea grandiceps Jacq., M. Valverde 358 (US), T. Plowman 13718 (F), B.J. Pienaar 218 (WAG) |
| Cercis canadensis L., T. Burckhardt s.n. (US), A.E. Porsild 23473 (MT) |
| Colophospermum mopane (J. Kirk ex Benth.) J. Léonard, F. White 10081 (MO), H.M. Biegel 791 (K), H.D.V. Prendergast HDVP 618 (K), J.M. de Aguiar Macedo 4859 (K), N.K.B. Robson 956 (K), B. de Winter 3066 (P), A. de Menezes 3661 (P), O. Azancot de Menezes 1403 (P), R. Dechamps et al. 1193 (BR) |
| Copaifera coriacea Mart., R.M. Harley 19147 (P), G. Gardner 2090 (P) |
| Copaifera guianensis Desf., R.A.A. Oldeman B.2594 (P), B. Maguire 24015 (P), J.-J. de Granville et al. 8166 (BR) |
| Copifera langsdorfii Desf., W.R. Anderson 10037 (P), H.S. Irwin et al. 18767 (P), R.M. Harley et al. 16854 (P), H.S. Irwin & T.R. Soderstrom 6898 (WAG) |
| Copaifera mildbraedii Harms, R Letouzey 10725 (P), R. Letouzey 12154 (P), D. Kenfack 999 (WAG), F.J. Breteler et al. 13149 (WAG) |
| Copaifera multijuga Hayne, A. Ducke 16910 (P), G.T. Prance et al. 14208 (P), G.T. Prance et al. 20723 (WAG) |
| Copaifera oblongifolia Mart., H.S. Irwin et al. 9500 (P), H.S. Irwin et al. 17508 (P), H.S. Irwin & T.R. Soderstrom 6050 (BR) |
| Copaifera officinalis L., J. de Bruijn 1152 (P, WAG), J. Léonard 36 (BR) |
| Copaifera salikounda Heckel, K.R. Mayer 204 (US), L. Ake Assi 15797 (MO), J. de Koning 6970 (WAG), F.J. Breteler 13383 (WAG) |
| Copaifera trapezifolia Hayne, M. Hunger s.n. (P), A. Glaziou s.n. (P), P.R. Reitz 5·711 (BR) |
| Cryptosepalum tetraphyllum (Hook. f.) Benth., F.J. Morton and Gledhill SL1036 (K), K. Morton & D. Gledhill 1036 (WAG) |
| Cynometra mannii Oliv., W.C. Thompson s.n. (K), G. McPherson 16856 (WAG) |
| Daniellia klainei Pierre ex A. Chev., A.P. Thomson 12 (K), J.M. Reitsma 1414 (NY), Hombert 572 (BR), F.J. Breteler 7735 (WAG) |
| Daniellia ogea (Harms) Rolfe ex Holland, P. Adames 561 (K), C.L.M. van Eijnatten 1341 (WAG), J. Léonard s.n. (BR), A.G. Voorhoeve 735 (BR) |
| Daniellia oliveri (Rolfe) Hutch. & Dalziel, W.J.J.O. de Wilde 625 (K), W.R. Elliott 14 (K), J. Ellenberger 1053 (P), W.J.J.O. de Wilde et al. 4963 (P) |
| Daniellia soyauxii (Harms) Rolfe, J.J. Wieringa & R.M.A.P. Haegens 2614 (WAG) |
| Detarium macrocarpum Harms, A.J.M. Leeuwenberg 9030 (MO), G.A. Zenker 452 (US), C. Tisserant 286 (P), F.J. Breteler 12528 (WAG) |
| Detarium microcarpum Guill. & Perr., C.C.H. Jongkind & C.M.J. Nieuwenhuis 2985 (WAG), J. Raynal & A. Raynal 5324 (P) |
| Detarium senegalense J.F. Gmel., G.P. Cooper 401 (F), J.F. Gmel 57·8·1 (K), W.E. Broadway 6917 (F), s.c. 1904 (P), C.R.A. Bambey 606 (P) |
| Dialium guianense (Aubl.) Sandwith, B.V. Rabelo et al. 3118 (US), D. Neill 6986 (F), N. Zamora 1451 (F), R. Romero Castaneda 4789 (US), T.B. Croat 20619 (F), W. Palacios 1364 (US) |
| Eperua bijuga Mart. ex Benth., A. Ducke 16927 (P), R.L. Froes & G.A. Black 27281 (P) |
| Eperua falcata Aubl., S.S. Tillett & C.L. Tillett 45791 (K), H. Jimenénez-Saa 14358 (P), S.A. Mori et al. 21517 (P), M.F. Prevost 1723 (BR) |
| Eperua grandiflora (Aubl.) Benth., W. Hahn 3744 (WAG) |
| Eperua rubiginosa Miq., C. Farney 1929 (K), D.B. Fanshawe 4840 (K), M. Hoff 6767 (P), H.S. Irwin et al. 57579 (P), M.J. Jansen-Jacobs et al. 1437 (P) |
| Eperua schomburgkiana Benth., G.T. Prance et al. 4977 (P), R.H. Schomburgk 517 (P) |
| Eurypetalum tessmannii Harms, G. McPherson 16216 (US), A.M. Louis & F.J. Breteler 696 (BR), A.R. Walker s.n. (P) |
| Eurypetalum unijugum Harms, R. Letouzey 9787 (P), G. Zenker 581 (P) |
| Gilletiodendron pierreanum J. Léonard, J.J.F.E. de Wilde 693 (MO), J.J. Wieringa & R.M.A.P. Haegens 2289 (WAG), G. McPherson 16758 (BR) |
| Goniorrhachis marginata Taub., G. & M. Hatschbach et al. 61982 (MO, BR), G.P. Lewis et al. 1976 (K), G.P. Lewis et al. 1909 (K), G.P. Lewis & S.M.M. de Andrade 1994 (BR) |
| Gossweilerodendron balsamiferum (Vermoesen) Harms, G.P. Lewis 2430 (K), R. Forressaint 214 (K), F.J. Breteler 10601 (WAG), J.D. Kennedy 554 (P), C. Maudoux 988 (BR) |
| Guibourtia arnoldiana (De Wild. & T. Durand) J. Léonard, J. Wagemans1346 (BR) |
| Guibourtia coleosperma (Benth.) J. Léonard, N.B. Zimba et al. 830 (WAG), O. Azancot de Menezes 889 (P) |
| Guibourtia demeusei (Harms) J. Léonard, A. Corbisier 1479 (US), J. Leonard 428 (K), J. Louis 9889 (K), L. White 1095 (WAG), R. Letouzey 10593 (P) |
| Guibourtia ehie (A. Chev.) J. Léonard, C. Vigne 4916 (K), G. McPherson 15444 (WAG) |
| Guibourtia hymenaeifolia (Moric.) J. Léonard, L. Bernardi 18946 (P) |
| Guibourtia pellegriniana J. Léonard, T. Levry 33591 (K), F. Dowsett-Lemaire 1578 (BR), G. McPherson 17057 (BR) |
| Guibourtia tessmannii (Harms) J. Léonard, G.M.P.C. Le Testu 9555 (P), L. White 1536 (WAG) |
| Hardwickia binata Roxb., M. Anderson 28 (P), V. Jacquemont 237 (P) |
| Hylodendron gabunense Taub., J. Olorunfemi FHI 43934 (K), J. Olorunfemi FHI 30693 (K), J.J.F.E. de Wilde 8214 (MO), Gauchotte 1791 (P), A.M. Louis & F.J. Breteler 464 (P) |
| Hymenaea courbaril L., A.C. Smith 3109 (US), J. Saunders 603B (F), M.J. Jansen-Jacobs 1365 (US), G. E. Schatz 764 (MO), M.F. Prévost 3737 (P), D. Plouvier 21 (BR), W. Milliken & R. Miller 822 (BR), F.J. Breteler 4268 (WAG) |
| Hymenaea eriogyne Benth., R.M. Harley 19016 (P) |
| Hymenaea oblongifolia Huber, J. Schunke V. 2129 (US), R. E. Schultes & I. Cabrera 14817 (K), B.A. Krukoff 6323 (BR) |
| Hymenaea parvifolia Huber, G.T. Prance & T.D. Pennington 1914 (P), B.A. Krukoff 6250 (BR), W. Milliken & R. Miller 771 (BR) |
| Hymenaea stigonocarpa Mart. ex Hayne var. pubescens Benth., R.M. Harley 19831 (P), T.M. Pedersen 11122 (L) |
| Hymenaea stigonocarpa Mart. ex Hayne var. stigonocarpa, R.M. Harley 18644 (P) |
| Hymenaea verrucosa Gaertn., G.E. Schatz et al. 2345 (US), J.L. Zarucchi et al. 7419 (US), P.S. Herendeen & F. Mbago 11-XII-97–3 (US), R.B. & A.J. Faden 77–771 (US), J.L. Zarucchi et al. 7419J (BR), A. Gomes e Sousa 3370 (BR) |
| Hymenostegia floribunda (Benth.) Harms, G. McPherson 15843 (MO, WAG), J.M. & B. Reitsma 1410 (MO) |
| Kingiodendron alternifolium (Elmer) Merr. & Rolfe, E.F. Solevin 27392 (P), M.D. Sulit 6432 (BR) |
| Kingiodendron pinnatum (Roxb. ex DC.) Harms, D.H. Nicolson et al. HFP 2871 (US), A. Kostermans 28130 (L, P), Calcuta Botanical Garden s.n. (L) |
| Kingiodendron platycarpum B.L. Burtt, A.C. Smith 7549 (L), A.C. Smith 8185 (P) |
| Loesenera kalantha Harms, A.G. Voorhoeve 961 (MO), G.P. Cooper 461 (F), A. de Gire 303 (WAG) |
| Neoapaloxylon madagascariense (Drake) Rauschert, D.K. Harder et al. 1681 (P), F. Chauvet 98 (P) |
| Oxystigma buchholzii Harms, S.R.F.K. Bena 1609 (P), R. Letouzey 11916 (P), J. Léonard 229 (P) |
| Oxystigma gilbertii J. Léonard, J. Dubois 195 (BR), J. Dubois 96 (BR), J.J.F.E. de Wilde 8375 (WAG) |
| Oxystigma mannii (Baill.) Harms, C. Doumenge 307 (MO), R. Letouzey 14933 (P), D.W. Thomas 2355 (P), D.W. Thomas 2353 (P) |
| Oxystigma msoo Harms, L.L. Bancroft s.n. (K), P.S. Herendeen & F. Mbago 18-XII-97–1 (US), R.B. & A.J. Faden 74/1265 (K), Kisena & Shabani 538 (WAG), Ruffo & Kmari 2253 (WAG) |
| Oxystigma oxyphyllum (Harms) J. Léonard, J.L.P. Louis 977 (P), C. Davio 50 (BR), C. Wilks 2563 (BR), F.J. Breteler et al. 11264 (WAG) |
| Peltogyne confertiflora (Mart. ex Hayne) Benth., E.P. Heringer 16633 (US), E.P. Heringer & F. Eiten 14102 (US), E.P. Heringer 13147 (K), H.S. Irwin et al. 31466 (US), H.S. Irwin et al. 212119 (WAG) |
| Peltogyne floribunda (Kunth) Pittier, M.J. Jansen-Jacobs et al. 2711 (P), M.J. Jansen-Jacobs et al. 2652 (P) |
| Peltogyne paniculata Benth. subsp. pubescens (Benth.) M.F. Silva, M.J. Jansen-Jacobs et al. 2646 (P), J.J. Wurdack & L.S. Adderley 43505 (P), R.A.A. Oldeman 1095 (P) |
| Peltogyne pauciflora Benth., R.M. Harley et al. 16400 (P), R.M. Harley et al. 16146 (P) |
| Peltogyne venosa (Vahl) Benth. subsp. densiflora (Spruce ex Benth.) M.F. Silva, J. Thiel 687 (P), G.T. Prance et al. 15205 (P) |
| Prioria copaifera Griseb., R. Foster 927 (F), T. B. Croat 6860 (F), P. Warner 498 (P) |
| Pseudosindora palustris Sym., J. Léonard s.n. (BR), Ashton & Kaling 5690 (WAG), Tahir 12268 (L) |
| Schotia afra (L.) Thunb., H.S. Gentry & A.S. Barclay 18896 (US), C.G.G.J. van Steenis 23890 (L), J.F. Drège s.n. (P) |
| Schotia brachypetala Sond., B.M. Browning et al. 42 (MO), H.J. Schlieben 7245 (F), J.L. Sidney 3458 (US), T. Muller 677 (K), G. Dehn 40239 (P), Willd 4122 (BR), D. Zunguze et al. 610 (BR) |
| Schotia latifolia Jacq., L.E. Codd 9778 (K), R.D.A. Bayliss 8397 (US, BR), R.D.A. Bayliss 6952 (US), P. McOwan 77 (P), Codd 38 (BR) |
| Sindora bruggemanii de Wit, Teysmann 3697 (A), J.J.F.E. de Wilde 3967 (WAG), H.C.D. de Wit IB77 (L) |
| Sindora coriacea (Baker) Prain, S. Phusomsaeng 223 (L), Ang Khoon Cheng 27819 (L) |
| Sindora klaineana Pierre ex Pellegrin, G. McPherson 16828 (BR), F.J. Breteler et al. 11403 (WAG), P. Sita 3643 (P) |
| Sindora siamensis Teysm. ex Miq., D.D. Soejarto et al. 6006 (L), J.F. Maxwell 76–185 (L), J.F. Maxwell 92–375 (L) |
| Sindora supa Merr., M. Curran 10653 (P), M. Ramos 13230 (P), M.D. Sulit 5669 (L) |
| Sindora wallichii Benth., Ambriansyah & Z. Arifin 609 (L), Ambriansyah & Z. Arifin 427 (L) |
| Sindoropsis letestui (Pellegr.) J. Léonard, G. Le Testu 2237 (BM, BR), G. McPherson 13720 (F), G. McPherson 16302 (MO, US, BR), F.J. Breteler 12143 (WAG) |
| Stemonocoleus micranthus Harms, D.J. Harris et al. 1067 (MO), G. Le Testu 7968 (BM), R.A.A. Oldeman 695 (K, P), R.A.A. Oldeman 383 (K, P, BR), F.J. Breteler (BR) |
| Tessmannia africana Harms, G. McPherson 16311 (MO), J. Louis 9230 (US), F.J. Breteler et al. 13322 (WAG), H. Butler 1364 (BR), J.J.F.E. de Wilde 7716 (P), R. Letouzey 13569 (P) |
| Tessmannia anomala Harms, T. B. Hart 1160 (MO), C. Wilks 2702 (BR), J. Louis 9790 (P) |
| Tessmannia dewildemaniana Harms, J. Léonard 4686 (P), J.M. Reitsma 2930 (WAG), F.J. Breteler et al.14618 (WAG) |
| Tessmannia lescrauwaetii Harms, Flamigni 9521 (K), G. Le Testu 8562 (BR) |
APPENDIX 2
Morphological characters used. All multistate characters are treated as unordered. Some states may not appear in the matrix (Supplementary Data, available online) but have been kept to ease comparison with other studies on Caesalpinioideae.
| 1 | Vestured pits: absent (0), present (1). |
| 2 | Compound leaf structure: multifoliolate (0), bifoliolate (1), unifoliolate (2). Characters 3 and 4 have been considered as independent because some imparipinnate leaves have leaflets with an opposite insertion, whereas others have leaflets with an alternate insertion. |
| 3 | Pinnation type: imparipinnate (0), paripinnate (1), distal leaflet sub-terminal (2). Leaves with a sub-terminal leaflet exhibit an extension of the rachis beyond the distal-most leaflet, which is inserted on one side of the rachis. |
| 4 | Leaflet insertion: opposite or sub-opposite (0), alternate (1), variable among leaves on a branch (2). Minor variation in placement of leaflets especially near base and apex of leaf is disregarded; the character is judged in the central portion of the leaf. Characters 5 and 6 describe leaflet symmetry. |
| 5 | Primary vein: straight (0), curved (1). |
| 6 | Primary vein position: central (0), near distal margin (1), near proximal margin (2). |
| 7 | Rachis (and/or petiole) grooved adaxially: absent (0), present (1). This character can be difficult to interpret in taxa in which the rachis is prone to collapse on drying. The groove is usually less pronounced or interrupted at leaflet attachment points. |
| 8 | Rachis (and/or petiole) winged between leaflets pairs: absent (0), present (1). |
| 9 | Leaflet shape: ovate or obovate, lamina larger on one section of the leaflet only (0), oblong, lamina larger on one part of the leaflet centred on the middle of this one (1). |
| 10 | Leaflet base: acute (0), obtuse to truncate (1), cordate (2), oblique (3). |
| 11 | Leaflet base: equal (0), unequal, one of the two margins of the lamina attach lower than the other on the petiolule (1). |
| 12 | Leaflet apex emarginate or retuse: absent (0), present (1). |
| 13 | Leaflet apex mucronate: absent (0), present (1). Glands are surrounded by a raised rim and show a pore or a concavity in their centre. |
| 14 | Apical gland: absent (0), present (1). These glands are located on the primary vein on the apex or immediately below. Several species have a bulge in this position but lack a pore; these are considered to lack this character. Other species have this gland but not on every leaflet and have been considered as having it. |
| 15 | Marginal gland: absent (0), present (1). These glands are located on the leaflet margin on the basal part of the leaflets. |
| 16 | Crater-like glands on abaxial lamina: absent (0), present (1). These structures are different from the gland dots (which do not show any raised rim) and have sometimes been referred to as ‘domatia’ in the literature. Crater-like glands are often located on secondary veins or veins of less importance. Their location is consistent within a species. |
| 17 | Leaflets petiolulate: petiolulate (0), sessile to sub-sessile (petiolule <1 mm long) (1). |
| 18 | Twisted petiolules: absent (0), present (1). The twisted impression is given by a ridge coming from the distal margin of the lamina to the base of the petiolule on the proximal side. |
| 19 | Stipule form: scale-like (0), foliose (1). Stipules are considered as foliose when they have a leaf-like lamina and several conspicuous veins that are often branching. |
| 20 | Stipule base: straight (0), auriculate (1), cordate (2). |
| 21 | Stipule insertion: lateral (0), intrapetiolar (1). Intrapetiolar stipules are inserted obliquely such that the proximal edge is between the petiole base and the axillary bud. |
| 22 | Stipule pairs connate basally (intrapetiolar fusion): absent (0), present (1). |
| 23 | Secondary venation: brochidodromous (0), semi-craspedodromous (1), intramarginal vein (2), cladodromous to craspedodromous (3). For a description of the different states see the Manual of leaf architecture (Leaf Architecture Working Group, 1999). |
| 24 | Leaflet marginal vein: absent (0), present (1). |
| 25 | Primary venation: pinnate, a single well-defined primary vein present (0), actinodromous, several veins of equal thickness radiate from the base of the lamina (1). |
| 26 | Basal acrodromous vein: absent (0), present (1). An acrodromous vein is a secondary vein on the proximal side having an ascendant trajectory from the primary vein and then showing a sudden inflexion toward the margin of the leaflet. |
| 27 | Primary vein continuous to the apex: present (0), absent, ramifies and is lost before reaching the apex (1). |
| 28 | Trichomes on rachis and/or petiole: absent (0), straight (1), uncinate (2). |
| 29 | Trichomes on peduncles and/or pedicels: absent (0), straight (1), uncinate (2). |
| 30 | Inflorescence structure: indeterminate (raceme, spike, panicle, head) (0), determinate (cymose) (1), flowers solitary (2). |
| 31 | Inflorescence structure: simple (0), compound (1). |
| 32 | Flower arrangement in inflorescence: spiral (0), distichous (1). |
| 33 | Pedicel: present (0), absent (1). |
| 34 | Pedicel jointed: absent (0), present (1). The pedicel shows a pronounced weak point at a consistent position and the unfertilized flowers fall down leaving a piece of the pedicel always of the same size on the peduncle. |
| 35 | Bracteoles: caducous (0), persistent up to anthesis (1). |
| 36 | Bracteoles enclosing late flower bud: absent (0), present (1). Bracteoles are as big as the bud and enclose the bud up to anthesis. |
| 37 | Bracteoles aestivation: distant (0), valvate (1), imbricate (2), adaxial surfaces touching (3). |
| 38 | Bracteoles position on pedicel (observed on late buds or flowers right after anthesis): low to middle (0), high, attached at the base of the calyx or the hypanthium (1). |
| 39 | Bracteoles fusion: free (0), connate at least at base (1). |
| 40 | Flower rotated in development, pedicel twisted: absent (0), present (1). |
| 41 | Sexuality: perfect (flowers all bisexual) (0), staminate flowers present (1). Among the species observed here, the species of the genus Kingiodendron are the only ones not having perfect sexuality (flowers all bisexual). Breteler (1999) also recorded Eurypetalum tessmannii as having this state but we have not been able to see any differences between the flowers observed for this species. Breteler (1999) recorded the species of the genus Kingiodendron as having both staminate and bisexual flowers (on the same individual), but Verdcourt (1979) considered Kingiodendron pinnatum as having functionally unisexual flowers (male or female). Our observations seem to confirm Verdcourt's statement, but more specimens should be observed. |
| 42 | Hypanthium: absent (0), present (1). |
| 43 | Hypanthium shape: cup shaped (0), tubular (1). Studies on floral ontogeny (Tucker, 2000a, b, c, 2001a, b, 2002a, b, 2003) demonstrate that in Sindora, Schotia and a couple of species of the Amherstieae clade five sepals are initiated but that the two adaxial sepals fuse late in ontogeny, resulting in four apparent sepals. It is highly probable that all the Detarieae s.l. with four sepals undergo this kind of phenomenon. Indeed, several species of the Amherstieae clade have a bilobed or bifurcate adaxial sepal which could be the result of an incomplete fusion. Moreover, the sepal supposed to be the result of the fusion is always in the median adaxial position, which corresponds to the position of the two lateral adaxial sepals in other Fabaceae. When the floral ontogeny is unknown, species having a bigger median adaxial sepal have been considered as having undergone a fusion of the two adaxial sepals. For three genera (Hardwickia, Colophospermum and Gossweilerodendron) of the resin-producing Detarieae, this character has been considered as unknown because Hardwickia and Colophospermum have fewer than four sepals, making any hypothesis of homology difficult, and Gossweilerodendron has four sepals of equal size. |
| 44 | Sepal number at initiation: five (0), four (1), three (2), two (3), one (4). |
| 45 | Fusion of the two adaxial sepals: absent (0), present (1). |
| 46 | Fusion of others sepals: free to base of flower or hypanthium (0), connate at least in basal portion excluding hypanthium (1). |
| 47 | Calyx aestivation: quincuncial (0), valvate (1), distant (2). |
| 48 | Sepal thickness: uniform (0), narrow thin margin, bent or foiled (the covered sepal shows a foil followed by a thin margin with a different texture, the margin of the covering sepal is imbricate in this foil) (1), wide thin margin never foiled (2). |
| 49 | Calyx base gibbous on one side (asymmetrical): absent (0), present (1). |
| 50 | Outer surface of sepal lobe bearing simple hairs: absent (0), present (1). |
| 51 | Inner surface of sepal lobe bearing simple hairs or bristles: absent (0), present (1). |
| 52 | Petal number at anthesis, including vestigial petals: five (0), four (1), three (2), two (3), one (4), zero (5). |
| 53 | Size of the median petal blade compared with lateral ones: uniform (0), median bigger than the others (1), median smaller than the others (2). |
| 54 | Size of the lateral petals: uniform, all vestigial or all developed (0), the abaxial ones smaller than adaxial ones (1), the abaxial ones larger than the adaxial ones (2). |
| 55 | Petal aestivation in late bud: imbricate ascending, standard innermost (0), imbricate descending, standard outermost (1), valvate (2). |
| 56 | Petal clawed: absent (0), median petal clawed (1), four lateral petals clawed (2), all petals clawed (3). |
| 57 | Androecium type, total number of parts including staminodes: diplostemonous (0), haplostemonous (1), less than haplostemonous (2), more than diplostemonous (3). |
| 58 | Staminodes: absent (0), present (1). |
| 59 | Filament connation: all connate in a tube (0), all connate in a sheath (open on one side) (1), nine connate and one free on the adaxial side (2), all free (3). |
| 60 | Filament relative length as compared with the perianth at anthesis: shorter or equal to the perianth (0), longer than the perianth (1). |
| 61 | Simple hairs on filament: absent (0), present (1). |
| 62 | Anther attachment: basifixed (0), dorsifixed (1). |
| 63 | Fertile anther size: uniform (0), dimorphic or heteromorphic (1). |
| 64 | Simple hairs on anther: absent (0), present (1). |
| 65 | Anther base markedly sagittate: absent (0), present (1). |
| 66 | Anther dehiscence orientation: introrse (0), latrorse (1), extrorse (2). |
| 67 | Prolongation of anther connective: absent (0), distal prolongation (1), proximal prolongation (2). |
| 68 | Anther shape: square (0), rectangular (1). |
| 69 | Simple hairs on gynoecium: absent (0), present (1). |
| 70 | Stipitate ovary: stipe absent (0), stipe present and central (1), stipe present and adnate to the adaxial side of the hypanthium or receptacle (2). |
| 71 | Style at anthesis: slender, as long or longer than ovary (0), short and stout, clearly shorter than ovary at anthesis (1), absent (2), short but not stout (3). |
| 72 | Style: curved, bent or coiled abaxially (0), curved, bent or coiled adaxially (1). |
| 73 | Style adaxial groove: absent (0), present (1). |
| 74 | Stigma shape: truncate, stigma non-papillose (0), peltate (1), crateriform (2), tubular (3), funnel shaped (4), capitate, more or less bulging but always papillose (5), attenuate (6). |
| 75 | Ovule number: consistently one (0), consistently two (1), numerous (always more than two) (2). |
| 76 | Fruit wing: absent (0), placental vascularized wing (1), non-vascularized placental wing (2), vascularized wing on both sutures (3), distal wing (4), proximal wing (5). |
| 77 | Amyloids in seed (Kooiman, 1960): absent (0), present (1). |
| 78 | ‘Zwischenkörper’ on pollen: absent (0), present (1). ‘Zwischenkörper’ are pectic structures associated with the pores of pollen and are detected by a positive reaction to Alcian blue (Banks, 2003). |
| 79 | Exinous projection over the centre of the pollen aperture (Banks and Klitgaard, 2000; Banks, 2003; Banks et al., 2003): absent (0), present (1). |
| 80 | Protruding aperture on pollen (Banks and Klitgaard, 2000; Banks, 2003; Banks et al., 2003): absent (0), present (1). |
| 81 | Pollen (Banks and Klitgaard, 2000; Banks, 2003; Banks et al., 2003): triporate or tricolpate (0), pantoporate (1). |
| 82 | Axial canals in the wood (Gasson et al., 2003): absent (0), diffuse (1), tangentially organized (2), traumatic (3). |
| 83 | Germination (Léonard, 1957, 1994; Watson and Dallwitz, 1993): epigeal (0), hypogeal (1). |
| 84 | Appendices on the collar of seedling (Léonard, 1957, 1994): absent (0), present (1). |
| 85 | Cotyledons on seedling (Léonard, 1957, 1994): spread at ground level (0), spread above ground (1), remain in the seed at ground level (2). |
| 86 | Two first leaves (Léonard, 1957, 1994): alternate (0), opposite (1). |
| 87 | Fruit (Gunn, 1991): indehiscent (0), dehiscent on both sutures (1), dehiscent on both sutures with valves coiled (2), dehiscent at apex only (3), dehiscent on ventral suture only (4). |
| 88 | Endocarp (Gunn, 1991): thin (0), thick, fleshy (1). |
| 89 | Aril (Léonard, 1957, Gunn, 1991): absent (0), present (1). |
LITERATURE CITED
- Almeida J, Rocheta M, Galego L. Genetic control of flower shape in Antirrhinum majus. Development. 1997;124:1387–1392. doi: 10.1242/dev.124.7.1387. [DOI] [PubMed] [Google Scholar]
- Banks H. Structure of pollen apertures in the Detarieae sensu stricto (Leguminosae: Caesalpinioideae), with particular reference to underlying structures (Zwischenkörper) Annals of Botany. 2003;92:425–435. doi: 10.1093/aob/mcg155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks H, Gasson PE. Pollen morphology and wood anatomy of the Crudia group (Leguminosae, Caesalpinioideae, Detarieae) Botanical Journal of the Linnean Society. 2000;134:19–59. [Google Scholar]
- Banks H, Klitgaard BB. Palynological contribution to the systematics of detarioid legumes (Leguminosae: Caesalpinioideae) In: Herendeen P, Bruneau A, editors. Advances in legume systematics, Part 9. Kew: Royal Botanic Gardens; 2000. pp. 79–106. [Google Scholar]
- Banks H, Klitgaard BB, Lewis GP, Crane PR, Bruneau A. Pollen and the systematic of tribes Caesalpinieae and Cassieae (Caesalpinioideae: Leguminosae) In: Klitgaard BB, Bruneau A, editors. Advances in legume systematics, Part 10. Kew: Royal Botanic Gardens; 2003. pp. 95–122. [Google Scholar]
- Bremer K. The limits of amino acid sequence data in angiosperm phylogenetic reconstruction. Evolution. 1988;42:795–803. doi: 10.1111/j.1558-5646.1988.tb02497.x. [DOI] [PubMed] [Google Scholar]
- Bremer K. Branch support and tree stability. Cladistics. 1994;10:295–304. [Google Scholar]
- Breteler FJ. The boundary between Amherstieae and Detarieae (Caesalpinioideae) In: Crisp MD, Doyle JJ, editors. Advances in legume systematics, Part 7. Kew: Royal Botanic Gardens; 1995. pp. 53–61. [Google Scholar]
- Breteler FJ. A revision of Prioria, including Gossweilerodendron, Kingiodendron, Oxystigma, and Pterygopodium (Leguminoseae-Caesalpinioideae-Detarieae) with emphasis on Africa. Wageningen Agricultural Papers. 1999;99:1–61. [Google Scholar]
- Breteler FJ, Ferguson IK, Gasson PE, Welle BJHt. Colophospermum reduced to Hardwickia (Leguminoseae-Caesalpinioideae) Adansonia série 3. 1997;19:279–291. [Google Scholar]
- Bruneau A, Breteler FJ, Wieringa JJ, Gervais GYF, Forest F. Phylogenetic relationships in tribes Macrolobieae and Detarieae as inferred from chloroplast trnL intron sequences. In: Herendeen P, Bruneau A, editors. Advances in legume systematics, Part 9. Kew: Royal Botanic Gardens; 2000. pp. 121–149. [Google Scholar]
- Bruneau A, Forest F, Herendeen P, Klitgaard BB, Lewis GP. Phylogenetic relationships in the Caesalpinioideae (Leguminosae) as inferred from the chloroplast trnL intron sequences. Systematic Botany. 2001;26:487–514. [Google Scholar]
- Bruneau A, Mercure M, Lewis GP, Herendeen PS. Phylogenetic patterns and diversification in the caesalpinioid legumes. Botany. 2008;86:697–718. [Google Scholar]
- Citerne HL, Pennington RT, Cronk QCB. An apparent reversal in floral symmetry in the legume Cadia is a homeotic transformation. Proceedings of the National Academy of Sciences, USA. 2006;103:12017–12020. doi: 10.1073/pnas.0600986103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan RS, Polhill RM. Amherstieae Benth. emend. J. Léon. (1957) In: Polhill RM, Raven PH, editors. Advances in legume systematics, Part 1. Kew: Royal Botanic Gardens; 1981a. pp. 135–142. [Google Scholar]
- Cowan RS, Polhill RM. Detarieae DC. (1957) In: Polhill RM, Raven PH, editors. Advances in legume systematics, Part 1. Kew: Royal Botanic Gardens; 1981b. pp. 117–134. [Google Scholar]
- Cubas P. Floral zygomorphy, the recurring evolution of a successful trait. Bioessays. 2004;26:1175–1184. doi: 10.1002/bies.20119. [DOI] [PubMed] [Google Scholar]
- Cunningham CW. Can three incongruence tests predict when data should be combined? Molecular Biology and Evolution. 1997;14:733–740. doi: 10.1093/oxfordjournals.molbev.a025813. [DOI] [PubMed] [Google Scholar]
- Eriksson T. 2001 AutoDecay, version 5.0. Stockholm: Bergius Foundation, Royal Swedish Academy of Sciences. [Google Scholar]
- Farris JS, Källersjö M, Kluge AG, Bult C. Testing significance of incongruence. Cladistics. 1995;10:315–319. [Google Scholar]
- Fougère-Danezan M, Maumont S, Bruneau A. Phylogenetic relationships in resin-producing Detarieae inferred from molecular data and preliminary results for a biogeographic hypothesis. In: Klitgaard BB, Bruneau A, editors. Advances in legume systematics, Part 10. Kew: Royal Botanic Gardens; 2003. pp. 161–180. [Google Scholar]
- Fougère-Danezan M, Maumont S, Bruneau A. Relationships among resin-producing Detarieae s.l. (Leguminosae) as inferred by molecular data. Systematic Botany. 2007;32:748–761. [Google Scholar]
- Galego L, Almeida J. Role of DIVARICATA in the control of dorsoventral asymmetry in Antirrhinum flowers. Genes and Development. 2002;16:880–891. doi: 10.1101/gad.221002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasson P, Trafford C, Matthews B. Wood anatomy of Caesalpinioideae. In: Klitgaard BB, Bruneau A, editors. Advances in legume systematics, Part 10. Kew: Royal Botanic Gardens; 2003. pp. 63–93. [Google Scholar]
- Goldblatt P. Cytology and the phylogeny of Leguminosae. In: Polhill RM, Raven PM, editors. Advances in legume sytematics, Part 2. Kew: Royal Botanic Gardens; 1981. pp. 427–463. [Google Scholar]
- Gunn CR. 1991 Fruits and seeds of genera in the subfamily Caesalpinioideae (Fabaceae). USDA Technical Bulletin No. 1755. [Google Scholar]
- Herendeen PS. Structural evolution in the Caesalpinioideae (Leguminosae) In: Herendeen PS, Bruneau A, editors. Advances in legume systematics, Part 9. Kew: Royal Botanic Gardens; 2000. pp. 45–64. [Google Scholar]
- Herendeen P, Lewis GP, Bruneau A. Floral morphology in caesalpinioid legumes: testing the monophyly of the ‘Umtiza clade. International Journal of Plant Sciences. 2003;164:393–407. [Google Scholar]
- Irish VF. The Arabidopsis petal: a model for plant organogenesis. Trends in Plant Science. 2008;13:430–436. doi: 10.1016/j.tplants.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Jenner RA. Accepting partnership by submission? Morphological phylogenetics in a molecular millennium. Systematic Biology. 2004;53:333–342. doi: 10.1080/10635150490423962. [DOI] [PubMed] [Google Scholar]
- Kooiman P. On the occurrence of amyloids in plant seeds. Acta Botanica Neerlandica. 1960;9:208–219. [Google Scholar]
- Krüger H, Tiedt LR, Wessels DCJ. Botanical Journal of the Linnean Society. Vol. 131. Caesalpinioideae: Detarieae; 1999. Floral development in the legume tree Colophospermum mopane; pp. 223–233. [Google Scholar]
- Langenheim JH. Terpenoids in the Leguminosae. In: Polhill RM, Raven PH, editors. Advances in legume systematics, Part 2. Kew: Royal Botanic Gardens; 1981. pp. 627–656. [Google Scholar]
- Langenheim JH. 2003 Plant resins: chemistry, evolution, ecology, and ethnobotany. Portland, OR: Timber Press. [Google Scholar]
- Lee Y-T, Langenheim JH. 1975 Systematics of the genus Hymenaea L. (Leguminosae, Caesalpinioideae, Detarieae). University of California Publications in Botany, Vol. 69. University of California Press. [Google Scholar]
- Leaf Architecture Working Group. 1999 Manual of leaf architecture: morphological description and categorization of dicotyledonous and net-veined monocotyledonous angiosperms. Washington, DC: Smithsonian Institution. [Google Scholar]
- Léonard J. Genera des Cynometreae et des Amherstieae africaines (Leguminosae-Caesalpinoideae). Essai de blastogénie appliquée à la systématique. Mémoires de l'Académie Royale de Belgique. Classe des Sciences. 1957;30:1–314. [Google Scholar]
- Léonard J. Nouveaux apports de la blastogénie à la délimitation générique des Caesalpiniaceae africaines (Detarieae et Amherstieae) Bulletin du Jardin Botanique National de Belgique. 1994;63:357–395. [Google Scholar]
- Léonard J. Colophospermum n'est pas synonyme d'Hardwickia (Caesalpiniaceae). Conclusion d'une méthode objective de travail. Bulletin du Jardin Botanique National de Belgique. 1999;67:21–43. [Google Scholar]
- Levin DA. Polyploidy and novelty in flowering plants. American Naturalist. 1983;122:1–25. [Google Scholar]
- Lewis GP, Schrire BD, Mackinder B, Lock M. 2005 Legumes of the world. Kew: Royal Botanic Gardens. [Google Scholar]
- Lewis GP, Simpson BB, Neff JL. Progress in understanding the reproductive biology of the Caesalpinioideae (Leguminosae) In: Herendeen PS, Bruneau A, editors. Advances in legume sytematics, Part 9. Kew: Royal Botanic Gardens; 2000. pp. 65–78. [Google Scholar]
- Luckow M, Bruneau A. Circularity and independence in phylogenetic tests of ecological hypotheses. Cladistics. 1997;13:145–151. doi: 10.1111/j.1096-0031.1997.tb00248.x. [DOI] [PubMed] [Google Scholar]
- Luo D, Carpenter R, Vincent C, Copsey L, Coen E. Origin of floral asymmetry in Antirrhinum. Nature. 1996;383:794–799. doi: 10.1038/383794a0. [DOI] [PubMed] [Google Scholar]
- Luo D, Carpenter R, Copsey L, Vincent C, Clark J, Coen E. Control of organ asymmetry in flowers of Antirrhinum. Cell. 1999;99:367–376. doi: 10.1016/s0092-8674(00)81523-8. [DOI] [PubMed] [Google Scholar]
- Lynch VJ, Wagner GP. Resurrecting the role of transcription factor change in developmental evolution. Evolution. 2008;62:2131–2154. doi: 10.1111/j.1558-5646.2008.00440.x. [DOI] [PubMed] [Google Scholar]
- MacKinder B. Detarieae. In: Lewis GP, Schrire BD, Mackinder B, Lock M, editors. Legumes of the world. Kew: Royal Botanic Gardens; 2005. pp. 68–109. [Google Scholar]
- Maddison WP, Maddison DR. 2009 Mesquite: a modular system for evolutionary analysis. Version 2.6. http://mesquiteproject.org . [Google Scholar]
- Redden KM, Herendeen PS. Morphology and phylogenetic analysis of Paloue and related genera in the Brownea clade (Detarieae, Caesalpinioideae) International Journal of Plant Science. 2006;167:1229–1246. [Google Scholar]
- Swofford DL. 2002 PAUP*: Phylogenetic Analysis Using Parsimony (*and other methods), version 4.0beta10. Sunderland, PA: Sinauer Associates. [Google Scholar]
- Symington CF. Pseudosindora palustris. Proceedings of the Linnean Society. 1944;155:285–288. [Google Scholar]
- Theißen G. Development of floral organ identity: stories from the MADS house. Current Opinion in Plant Biology. 2001;4:75–85. doi: 10.1016/s1369-5266(00)00139-4. [DOI] [PubMed] [Google Scholar]
- Theißen G, Saedler H. Floral quartets. Nature. 2001;409:469–471. doi: 10.1038/35054172. [DOI] [PubMed] [Google Scholar]
- Tucker SC. Evolutionary loss of sepals and/or petals in detarioid legume taxa Aphanocalyx, Brachystegia, and Monopetalanthus (Leguminosae: Caesalpinioideae) American Journal of Botany. 2000a;87:608–624. [PubMed] [Google Scholar]
- Tucker SC. Floral development and homeosis in Saraca (Leguminosae: Caesalpinioideae: Detarieae) International Journal of Plant Sciences. 2000b;161:537–549. [Google Scholar]
- Tucker SC. Floral development in tribe Detarieae (Leguminosae: Caesalpinioideae): Amherstia, Brownea, and Tamarindus. American Journal of Botany. 2000c;87:1385–1407. [PubMed] [Google Scholar]
- Tucker SC. Organ loss in detarioid and other leguminous flowers, and the possibility of saltatory evolution. In: Herendeen P, Bruneau A, editors. Advances in legume sytematics, Part 9. Kew: Royal Botanic Gardens; 2000d. pp. 107–120. [Google Scholar]
- Tucker SC. Floral development in Schotia and Cynometra (Leguminosae: Casalpinioideae: Detarieae) American Journal of Botany. 2001a;88:1164–1180. [PubMed] [Google Scholar]
- Tucker SC. The ontogenetic basis for missing petals in Crudia (Leguminosae: Caesalpinioideae: Detarieae) International Journal of Plant Sciences. 2001b;162:83–89. [Google Scholar]
- Tucker SC. Comparative floral ontogeny in Detarieae (Leguminosae: Caesalpinioideae). 2. Zygomorphic taxa with petal and stamen suppression. American Journal of Botany. 2002a;89:888–907. doi: 10.3732/ajb.89.6.888. [DOI] [PubMed] [Google Scholar]
- Tucker SC. Comparative floral ontogeny in Detarieae (Leguminosae: Caesalpinioideae). I. Radially symmetrical taxa lacking organ suppression. American Journal of Botany. 2002b;89:875–887. doi: 10.3732/ajb.89.6.875. [DOI] [PubMed] [Google Scholar]
- Tucker SC. Comparative floral ontogeny in Detarieae (Leguminosae: Caesalpinioideae). III. Adaxially initiated whorls in Julbernardia and Sindora. International Journal of Plant Sciences. 2003;164:275–286. [Google Scholar]
- Verdcourt B. A manual of New Guinea legumes. Botany Bulletin. 1979;11:93–98. [Google Scholar]
- Watson L, Dallwitz MJ. 1993 onwards.The genera of Leguminosae – Caesalpinioideae and Swartzieae: description, identification, and information retrieval. In English and French; French translation by E. Chenin. http://delta-intkey.com , accessed 22 March 2009. [Google Scholar]
- Wendel JF. Genome evolution in polyploids. Plant Molecular Biology. 2000;42:225–249. [PubMed] [Google Scholar]
- Wiens JJ. The role of morphological data in phylogeny reconstruction. Systematic Biology. 2004;53:653–661. doi: 10.1080/10635150490472959. [DOI] [PubMed] [Google Scholar]
- Yoder AD, Irwin JA, Payseur BA. Failure of the ILD to determine data combinability for slow loris phylogeny. Systematic Biology. 2001;50:408–424. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.