Abstract
Background and Aims
Previous studies indicate that the size-controlling capacity of peach rootstocks is associated with reductions of scion water potential during mid-day that are caused by the reduced hydraulic conductance of the rootstock. Thus, shoot growth appears to be reduced by decreases in stem water potential. The aim of this study was to investigate the mechanism of reduced hydraulic conductance in size-controlling peach rootstocks.
Methods
Anatomical measurements (diameter and frequency) of xylem vessels were determined in shoots, trunks and roots of three contrasting peach rootstocks grown as trees, each with different size-controlling characteristics: ‘Nemaguard’ (vigorous), ‘P30-135’ (intermediate vigour) and ‘K146-43’ (substantially dwarfing). Based on anatomical measurements, the theoretical axial xylem conductance of each tissue type and rootstock genotype was calculated via the Poiseuille–Hagen law.
Key Results
Larger vessel dimensions were found in the vigorous rootstock (‘Nemaguard’) than in the most dwarfing one (‘K146-43’) whereas vessels of ‘P30-135’ had intermediate dimensions. The density of vessels per xylem area in ‘Nemaguard’ was also less than in ‘P30-135’and ‘K146-43’. These characteristics resulted in different estimated hydraulic conductance among rootstocks: ‘Nemaguard’ had higher theoretical values followed by ‘P30-135’ and ‘K146-43’.
Conclusions
These data indicate that phenotypic differences in xylem anatomical characteristics of rootstock genotypes appear to influence hydraulic conductance capacity directly, and therefore may be the main determinant of dwarfing in these peach rootstocks.
Key words: Prunus, rootstock, vessel diameter, hydraulic conductance, dwarfing, xylem anatomy, Poiseuille–Hagen
INTRODUCTION
Most fruit tree species are propagated by grafting onto rootstocks selected for specific edaphic conditions, tolerance to pests or diseases, control of scion vigour and/or inducing early production (Webster, 1995). Seleznyova et al. (2008) described the effect of dwarfing apple rootstocks on shoot development, pointing out that in trees grafted on dwarfing rootstocks, the annual number of vegetative extension shoots decreased and the annual number of floral shoots increased.
The physiological explanation of these phenomena has been debated by many authors in the past 70 years (Webster, 1995). Some studies on apple (Olien and Lakso, 1986; Cohen and Naor, 2002) and peach rootstocks (Weibel et al., 2003) showed that specific rootstocks influence shoot growth rate and stem water potential. The pattern of stem water potential occurring during the afternoon strongly affects shoot growth rates in the field (Berman and DeJong, 1997). Basile et al. (2003), who followed stem water potential and shoot growth rate during single days and over a growing season on some of the same peach rootstocks characterized by Weibel et al. (2003), found a strong correlation between stem water potential and shoot growth over a day. Vegetative growth also has been correlated with cumulative water potential differences during the first half of a growing season (Basile et al., 2003). In addition, differences in stem water potential have been causally related to differences in relative shoot growth rate among peach trees on different rootstocks (Solari et al., 2006a).
Stem water potential is strongly influenced by stem hydraulic conductance (Tyree and Sperry, 1988). Low hydraulic conductance can also limit stomatal conductance and therefore lower photosynthetic assimilation. Stomatal closure is the primary means for trees to prevent cavitation in xylem vessels during periods of high potential evapotranspiration (Jones and Sutherland, 1991). Thus, stem or root hydraulic conductance influence stem water potential and can govern growth potential. Hydraulic conductance per unit leaf area has been reported to be less in dwarfing peach rootstocks than in vigorous rootstocks and directly linked to reductions in rootstock hydraulic conductance of genotypes that had reduced shoot extension growth rates (Solari et al., 2006b; Solari and DeJong, 2006). Thus, rootstock hydraulic conductance has been directly related to tree vigour in peach trees. This has provided one explanation for how rootstocks can influence the scion growth in peach trees: hydraulic conductance influences stem water potential (Tyree and Sperry, 1988), which, as demonstrated by Berman and DeJong (1997), Basile et al. (2003) and Solari and DeJong (2006), is a driving variable of shoot extension.
Axial stem hydraulic conductance is a function of anatomical characteristics of the xylem, especially vessel dimensions (Vercambre et al., 2002). Conductances through a capillary tube are theoretically described by the Poiseuille–Hagen equation (Ewers and Fisher, 1989; Tyree and Ewers, 1991) where the flow is given by the summation of vessel or tracheid lumen diameters, each raised to the fourth power. The Poiseuille–Hagen law has also been used to calculate theoretical hydraulic xylem conductance of tree stems (Ewers and Fisher, 1989).
Stem hydraulic conductance in trees is related to xylem vessel numbers and diameters (Gibson et al., 1985). Vessel diameter appears to be highly correlated with hydraulic conductance because of the importance of vessel diameter in the Poiseuille–Hagen equation (Ewers and Fisher, 1989). Sweet cherry dwarf rootstocks appear to have larger numbers of vessels but smaller vessel diameters than vigorous rootstocks (Olmstead et al., 2006; Goncalves et al., 2007).
The aim of the present study was to test the hypothesis that xylem vessel characteristics are correlated with dwarfing characteristics of peach rootstocks in which xylem hydraulic conductance appears to be a main cause of dwarfing (Solari et al., 2006b, c; Solari and DeJong, 2006). If true, this would indicate that the number and the diameter of xylem vessels are important factors that influence dwarfing potential of specific rootstock genotypes and these characteristics could become valuable selection criteria for future rootstock improvement.
MATERIALS AND METHODS
The rootstocks used in this experiment were representative of three different vigour classes: high (Prunus persica L. Batsch × Prunus davidiana hybrid, ‘Nemaguard’, seed-propagated), intermediate (Prunus salicina Lindl. × Prunus persica L. Batsch hybrid, ‘P30-135’, vegetatively propagated) and low (Prunus salicina Lindl. × Prunus persica L. Batsch hybrid, ‘K146-43’, vegetatively propagated). Commercially, ‘P30-135’ and ‘K146-43’ are sold as Controller 9TM and Controller 5TM, respectively. To test whether the xylem vessel characteristics analysed were characteristic of specific genotypes and consistent across tissue origins, the xylem from three different parts of the tree were analysed, namely roots, trunk and shoots. Root and trunk xylem samples were collected from an experimental orchard of the University of California located in the Kearney Agricultural Center, Parlier, CA. Trees used for obtaining root and trunk samples were all grafted with the scion cultivar ‘O'Henry’. All trees were 7 years old, trained to a perpendicular V (DeJong et al., 1995) and received normal horticultural care. During May, 2009, three woody root segments, approx. 5·5 mm in diameter, were sampled from each of five trees on each rootstock. Root samples were collected from a distance of about 20 cm from the base of the trunk from three different positions around the base of each tree. During the same period samples of trunk xylem tissue (approx. 1·5 cm long × 0·5 cm wide × 0·25 cm deep) were extracted from the rootstock trunk below the graft union using a wood chisel. A few days later, shoot samples were collected from the same rootstock genotypes that were grown as grafted trees on ‘Nemaguard’ rootstock for the purpose of growing shoots for vegetative propagation, in experimental orchards on the University of California Davis campus in Davis, CA. Three shoots with a basal diameter of approx. 4·5 mm were collected from each of five trees per rootstock. All samples were immediately placed in plastic bags on ice and subsequently stored at 0 °C until sectioned.
Samples were fresh sectioned with a manual microtome at 150 µm of thickness to obtain two cross-sections from each field sample. The sections were stained with Toluidine-Blue-O to increase visual contrast. Photographs of the cross-sections were taken with a camera (Model Lei 750, Leica, Wetzlar, Germany) mounted on a light microscope (Eclipse E 600, Nikon, Tokyo, Japan). Images were then acquired with DEI-750D software (Optronics, Goleta, CA, USA). Three photographs were made from each cross-section slide: the first at 4× magnification to measure the thickness of xylem tissue, and the other two at 10× to calculate vessel density and dimensions considering two randomly selected fields of view of xylem tissue.
Vessels were measured and counted in frequency classes, as described by Solla and Gil (2002), using a computer graphics program (GIMP, freeware; www.gimp.org) to paste a ruled grid at the same magnification onto photographs of vessels. The frequency classes were established at intervals of 30 µm for trunk and root vessels, and at 15 µm for shoot vessels.
Theoretical hydraulic conductance (kh; kg m MPa−1 s−1) was calculated with the modified Hagen–Poisseuille's law described by Tyree and Ewers (1991):
where d is the radius of the vessel in metres, ρ is the fluid density (assumed to be 1000 kg m−3 or equal to that of water at 20 °C) and η is the viscosity (assumed to be 1 × 10−9 MPs·s, or equal to that of water at 20 °C).
A weighted mean (Wm) diameter was calculated as mean class diameter per total number of vessel products, divided by the total number of measurements:
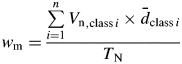 |
where Vn is the number of vessels in each class, d̄ is the mean diameter in each class and TN is the total number of vessels per visual field.
Statistical analyses of the data were performed with SAS statistical software (SAS Institute, Cary, NC, USA). Treatments were analysed by one-way ANOVA with significance level set at 0·05. Means were separated by Tukey's w-procedure at P = 0·05 (Sokal and Rohlf, 1969). For shoot and root measurements, two randomly chosen visual fields from each of two sections of each of three shoots or roots were determined for each of five trees to calculate a grand mean with n = 5. For trunk values, the grand mean values (n = 5) were calculated using data from two randomly chosen visual fields in each of four sections from each of five trees.
RESULTS
Total xylem thickness of the trunk was not evaluated because trunk xylem anatomical analysis was determined on ‘patches’ extracted from the outer layers of xylem tissue from trunks. However, shoot and root xylem thickness relative to their respective diameter did not significantly differ among rootstocks (data not shown).
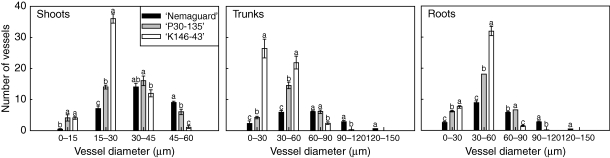
The distribution of shoot xylem vessels in different size classes differed depending on the rootstock genotype. ‘Nemaguard’ (vigorous) shoots had a high number of vessels in the 45–60-μm class and a low number of vessels in the lower diameter classes (Fig. 1). The most frequent diameter class for this genotype was 30–45 µm. ‘P30-135’ (intermediate vigour) had a higher number of vessels in the median diameter size classes (15–30 and 30–45 µm) and lower numbers in the low- and high-diameter size classes (0–15 and 45–60 µm). ‘K146-43’ (substantially dwarfing) had a higher number of vessels in the 15–30-μm class and low numbers in the 0–15- and 30–45-μm classes. For this genotype there were very few vessels with large diameters.
Fig. 1.
Frequency distributions of xylem vessel sizes per visual field in shoots, trunks and roots of ‘Nemaguard’, ‘P30-135’ and ‘K146-43’ rootstock genotypes. For shoots and roots each value is the mean of two visual fields from two sections per three shoots or roots from each of five trees ± s.e. (n = 5). For trunks each value is the mean of two visual fields from four sections of one sample from each of five trees ± s.e. (n = 5). Means with different lower-case letters are significantly different at P < 0·05 (Tukey test).
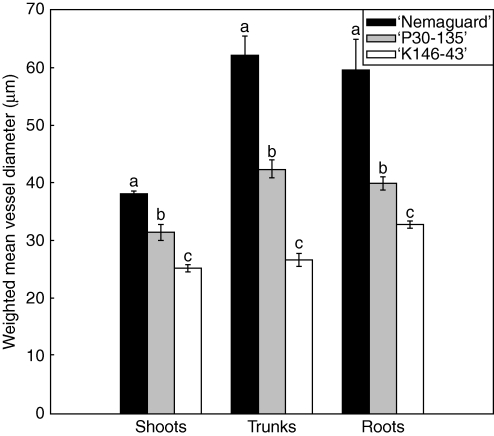
The total number of vessels per visual field in shoots also varied with genotype (Table 1). ‘K146-43’ had more vessels per field than ‘P30-135’ (P < 0·001) and ‘P30-135’ had more vessels per visual field than ‘Nemaguard’ (P < 0·001). The weighted mean vessel dimension (Wm) of shoot xylem was highest in ‘Nemaguard’ followed by ‘P30-135’ and ‘K146-43’ (Fig. 2).
Table 1.
Number of xylem vessels per visual field in shoots (n = 5), trunks (n = 5) and roots (n = 5) of ‘Nemaguard’, ‘P30-135’ and ‘K146-43’ rootstock genotypes
| Rootstock | Shoot | Trunks | Roots |
|---|---|---|---|
| ‘Nemaguard’ | 30·80 ± 0·68c | 17·97 ± 1·39c | 20·15 ± 0·80c |
| ‘P30-135’ | 39·66 ± 1·71b | 25·07 ± 0·98b | 30·75 ± 0·27b |
| ‘K146-43’ | 53·47 ± 0·85a | 50·50 ± 2·11a | 40·55 ± 1·69a |
For shoots each value is the mean of two visual fields from two sections per three shoots from five trees ± s.e. (n = 5). For trunks each value is the mean of two visual fields from four sections from five trees ± s.e. (n = 5). For roots each value is the mean of two visual fields from two sections per three roots from five trees ± s.e. (n = 5). Means with different lower-case letters are significantly different at P < 0·05 (Tukey test).
Fig. 2.
Weighted mean xylem vessel diameters in shoots, trunks and roots of ‘Nemaguard’, ‘P30-135’ and ‘K146-43’ rootstock genotypes. For shoots and roots each value is the mean of two visual fields from two sections per three shoots or roots from each of five trees ± s.e. (n = 5). For trunks each value is the mean of two visual fields from four sections of one sample from each of five trees ± s.e. (n = 5). Means with different lower-case letters are significantly different at P < 0·05 (Tukey test).
Xylem vessel dimensions in trunk xylem also varied depending on the rootstock genotype. ‘Nemaguard’ had high numbers of vessels in the two medium size classes (30–60 and 60–90 µm) and lower numbers in the other classes (Fig. 1). The relatively high number of vessels in the largest dimension class (120–150 µm) was characteristic of this rootstock. ‘P30-135’ had a high number of vessels in the 30–60-μm class with few in the other classes and no vessels in the largest size class. ‘K146-43’ had the highest number of vessels in the 0–30-μm class, but high numbers of vessels were also present in the 30–60-μm class. No vessels were detected in the two largest size classes (90–120 and 120–150 µm).
As found in shoots, in trunk xylem the number of xylem vessels per field varied with rootstock (Table 1). ‘K146-43’ had more vessels than ‘P30-135’ (P < 0·001) and ‘P30-135’ had more than ‘Nemaguard’ (P < 0·001). Wm was higher in ‘Nemaguard’ than in ‘P30-135’ (P < 0·001) and it was higher in ‘P30-135’ than in ‘K146-43’ (P < 0·001).
Xylem vessel characteristics of roots also differed depending on the rootstock genotype, similar to shoots and trunks (Fig. 1). ‘Nemaguard’ had a high number of vessels in the two medium size classes (30–60 and 60–90 µm) while the other classes had lower numbers. Again, ‘Nemaguard’ had some vessels in the larger size classes (90–120 and 120–150 µm). ‘P30-135’ had high numbers of vessels in the 30–60- and 60–90-μm classes and lower numbers in other classes and no vessels in the upper two size classes. ‘K146-43’ had the highest number of vessels in the 30–60-μm class, high numbers of vessels in the 0–30-μm class, very low numbers of vessels in the 60–90-μm class and no vessels in the two largest classes (90–120 and 120–150 µm).
As found in shoots and trunks, the number of xylem vessels per field in roots varied with the rootstock genotype (Table 1). ‘K146-43’ had more vessels than ‘P30-135’ (P < 0·001) and ‘P30-135’ had more vessels than ‘Nemaguard’ (P < 0·001). Wm was higher in ‘Nemaguard’ than in ‘P30-135’ (P < 0·001) and it was higher in ‘P30-135’ than in ‘K146-43’ (P < 0·001).
Wm in all three measured organs (shoots, trunks and roots) varied consistently among rootstock genotypes (Fig. 2). ‘Nemaguard’ had the largest weighted mean vessel dimensions in all organs followed by ‘P30-135’ and ‘K146-43’. In ‘Nemaguard’ and ‘P30-135’ there were no differences between root and trunk weighted mean vessel sizes but there were differences between the weighted mean xylem vessel diameters of organs and shoots for these two cultivars. By contrast, in ‘K146-43’ there were differences between weighted mean vessel diameters of roots and trunks, but not between trunks and shoots.
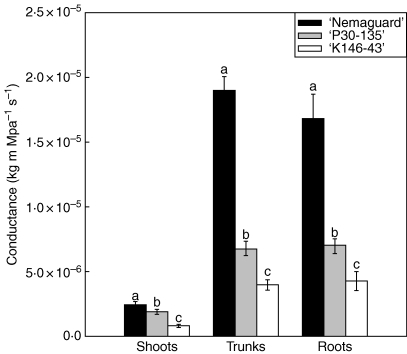
Conductance per visual field calculated using the modified Poiseuille–Hagen equation (Tyree and Ewers, 1991) varied depending on the rootstock genotypes (Fig. 3). ‘Nemaguard’ had the highest calculated conductance in all the organs tested, followed by ‘P30-135’ and then ‘K146-43’.
Fig. 3.
Calculated axial hydraulic conductance per visual field in shoots, trunks and roots of ‘Nemaguard’, ‘P30-135’ and ‘K146-43’ rootstock genotypes. For shoots and roots each value is the mean of two visual fields from two sections per three shoots or roots from each of five trees ± s.e. (n = 5). For trunks each value is the mean of two visual fields from four sections of one sample from each of five trees ± s.e. (n = 5). Means with different lower-case letters are significantly different at P < 0·05 (Tukey test).
DISCUSSION
Xylem vessel numbers and dimensions varied among rootstock genotypes in accordance with the vigour of trees when these rootstocks are used with commercial peach scion genotypes. In all the organs tested there were clear differences in vessel diameter size class distributions. ‘Nemaguard’, the most vigorous rootstock, had larger xylem vessels than ‘P30-135’, the semi-dwarfing rootstock, and ‘K146-43’, the more dwarfing rootstock, respectively (Weibel et al., 2003). Weighted mean vessel diameters of the vigorous rootstock (‘Nemaguard’) were also larger than the semi-dwarfing and dwarfing rootstocks (‘P30-135’ and ‘K146-43’, respectively). This characteristic was the inverse of the number of vessels per visual field (greater in ‘K146-43’ than ‘P30-135’ and ‘Nemaguard’). Thus, the most vigorous rootstock had fewer xylem vessels but with larger diameters than the dwarfing rootstocks, which had more xylem vessels but with smaller diameters. The vessel diameter results are similar to those reported by Goncalves et al. (2007) and Olmstead et al. (2006) for sweet cherry rootstocks. However, contrary to cherries the density of vessels per xylem cross-sectional area in the peach rootstocks also varied.
The potential effect of differences in xylem characteristics on hydraulic conductance were reflected in the theoretical conductance calculated with the Poiseuille–Hagen law. There were clear differences among rootstock genotypes corresponding to previously reported vigour characteristics and measured hydraulic conductances given by Solari et al. (2006b). The scale of theoretical hydraulic conductances in the present study also corresponded to that reported by Solari et al. (2006b) obtained by empirical measurements of stem hydraulic conductance.
The variation in xylem dimensions in the shoots of different genotypes, even though these rootstock genotypes were all grafted onto vigorous rootstocks, indicates that the reductions of vessel diameters of the dwarfing genotypes are probably due to specific characteristics driven by genetic factors. Further studies are necessary to understand these characteristics better.
Therefore, the number and the diameter of xylem vessels appear to be key factors that influence dwarfing potential of specific rootstock genotypes by influencing hydraulic conductance. Reduced hydraulic conductance, as demonstrated by Solari et al. (2006b) and Solari and DeJong (2006), can cause reductions in stem water potential during mid-day hours (Basile et al., 2003; Solari et al., 2006a). This lower water potential is the basis for decreases in shoot growth (Berman and DeJong, 1997; Basile et al., 2003; Solari et al., 2006a). Thus, xylem vessel characteristics appear to be important factors related to the dwarfing capacity of graft-compatible peach rootstocks. Furthermore, the theoretical calculation of xylem hydraulic conductance based on vessel numbers and dimensions per unit of xylem area may be an effective means to estimate the dwarfing capacity of specific genotypes. At the practical level, xylem anatomical measurements may be useful indicators for predicting rootstock vigour inducing characteristics during early stages of plant development in rootstock breeding/development programmes.
ACKNOWLEDGEMENTS
We thank Dr V. Polito for use of anatomical laboratory equipment, Ms K. Pinney for setting up the microscopic equipment and related software and Dr P. Proietti for making this collaboration possible.
LITERATURE CITED
- Basile B, Marsal J, DeJong TM. Daily shoots extension growth of peach trees growing on rootstocks that reduce scion growth to daily dynamics of stem water potential. Tree Physiology. 2003;23:695–704. doi: 10.1093/treephys/23.10.695. [DOI] [PubMed] [Google Scholar]
- Berman ME, DeJong TM. Diurnal patterns of stem ex tension growth in peach (Prunus persica): temperature and fluctuations in water status determine growth rate. Physiologia Plantarum. 1997;100:361–370. [Google Scholar]
- Cohen S, Naor A. The effect of three rootstocks on water use, canopy conductance and hydraulic parameters of apple trees and predicting canopy from hydraulic conductances. Plant Cell and Environment. 2002;25:17–28. [Google Scholar]
- DeJong TM, Day KR, Doyle JF, Johnson RS. The Kearney Agricultural Center perpendicular ‘V’ (KAC-V) orchard system for peaches and nectarines. HortTechnology. 1995;4:362–367. [Google Scholar]
- Ewers FW, Fisher JB. Techniques for measuring vessel lengths and diameters in stems of woody plants. American Journal of Botany. 1989;76:645–656. [Google Scholar]
- Gibson AC, Calkin HW, Nobel PS. 1985. pp. 293–302. Hydraulic conductance and xylem structure in tracheid-bearing plants. International Association of Wood Anatomy Bulletinn.s. 6.
- Goncalves B, Correia CM, Silva AP, et al. Variation in xylem structure and function in roots and stems of scion–rootstock combinations of sweet cherry tree (Prunus avium L.) Trees. 2007;21:121–130. [Google Scholar]
- Jones HG, Sutherland RA. Stomatal control of xylem embolism. Plant Cell and Environment. 1991;14:607–612. [Google Scholar]
- Olien WC, Lakso AN. Effect of rootstock on apple (Malus domestica) tree water relations. Physiologia Plantarum. 1986;67:421–430. [Google Scholar]
- Olmstead MA, Lang NS, Ewers FW, Owens SA. Xylem vessel anatomy of sweet cherries grafted onto dwarfing and non dwarfing rootstocks. Journal of American Society of Horticultural Science. 2006;131:577–585. [Google Scholar]
- Seleznyova AN, Tustin DS, Thorp TG. Apple dwarfing rootstocks and interstocks affect the type of growth units produced during the annual growth cycle: precocious transition to flowering affects the composition and vigour of annual shoots. Annals of Botany. 2008;101:679–687. doi: 10.1093/aob/mcn007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. 1969. Biometry. San Francisco: WH Freeman and Co.
- Solari LI, DeJong TM. The effect of root pressurization on water relations, shoot growth, and leaf gas exchanges of peach (Prunus persica) trees on rootstocks with differing growth potential and hydraulic conductance. Journal of Experimental Botany. 2006;57:1981–1989. doi: 10.1093/jxb/erj145. [DOI] [PubMed] [Google Scholar]
- Solari LI, Johnson RS, DeJong TM. Relationship of water status to vegetative growth and leaf gas exchange of peach (Prunus persica) trees on different rootstocks. Tree Physiology. 2006a;26:1333–1341. doi: 10.1093/treephys/26.10.1333. [DOI] [PubMed] [Google Scholar]
- Solari LI, Johnson RS, DeJong TM. Hydraulic conductance characteristics of peach (Prunus persica) trees on different rootstocks are related to biomass production and distribution. Tree Physiology. 2006b;26:1343–1350. doi: 10.1093/treephys/26.10.1343. [DOI] [PubMed] [Google Scholar]
- Solari LI, Pernice F, DeJong TM. The relationship of hydraulic conductance to root system characteristics of peach (Prunus persica ) rootstocks. Physiologia Plantarum. 2006c;128:324–333. [Google Scholar]
- Solla A, Gil L. Xylem vessel diameter as a factor in resistance of Ulmus minor to Ophiostoma novo-ulmi. Forest Pathology. 2002;32:123–134. [Google Scholar]
- Tyree MT, Ewers FW. New Phytologist. 1991;119:345–360. The hydraulic architecture of trees and other woody plants. [Google Scholar]
- Tyree MT, Sperry JS. Do woody plants operate near the point of catastrophic xylem dysfunction caused by dynamic water stress? Plant Physiology. 1988;88:574–580. doi: 10.1104/pp.88.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercambre G, Doussan C, Pages L, Habib R, Pierret A. Influence of xylem development on axial hydraulic conductance within Prunus root systems. Trees. 2002;16:479–487. [Google Scholar]
- Webster AD. Rootstock and interstock effects on deciduous fruit tree vigour, precocity, and yield productivity. New Zealand Journal of Crop and Horticultural Science. 1995;23:373–382. [Google Scholar]
- Weibel A, Johnson RS, DeJong TM. Comparative vegetative growth responses of two peach cultivars grown on size-controlling versus standard rootstocks. Journal of American Society of Horticultural Science. 2003;128:463–471. [Google Scholar]