Abstract
Background and Aims
Intraspecific ploidy-level variation is an important aspect of a species' genetic make-up, which may lend insight into its evolutionary history and future potential. The present study explores this phenomenon in a group of eastern Asian Cardamine species.
Methods
Plant material was sampled from 59 localities in Japan and Korea, which were used in karyological (chromosome counting) and flow cytometric analyses. The absolute nuclear DNA content (in pg) was measured using propidium iodide and the relative nuclear DNA content (in arbitrary units) was measured using 4,6-diamidino-2-phenylindole fluorochrome.
Key Results
Substantial cytotype diversity was found, with strikingly different distribution patterns between the species. Two cytotypes were found in C. torrentis sensu lato (4x and 8x, in C. valida and C. torrentis sensu stricto, respectively), which displays a north–south geographical pattern in Japan. Hypotheses regarding their origin and colonization history in the Japanese archipelago are discussed. In Korean C. amaraeiformis, only tetraploids were found, and these populations may in fact belong to C. valida. C. yezoensis was found to harbour as many as six cytotypes in Japan, ranging from hexa- to dodecaploids. Ploidy levels do not show any obvious geographical pattern; populations with mixed ploidy levels, containing two to four cytotypes, are frequently observed throughout the range. C. schinziana, an endemic of Hokkaido, has hexa- and octoploid populations. Previous chromosome records are also revised, showing that they are largely based on misidentified material or misinterpreted names.
Conclusions
Sampling of multiple populations and utilization of the efficient flow cytometric approach allowed the detection of large-scale variation in ploidy levels and genome size variation attributable to aneuploidy. These data will be essential in further phylogenetic and evolutionary studies.
Keywords: Cardamine amaraeiformis, Cardamine schinziana, Cardamine torrentis, Cardamine valida, Cardamine yezoensis, cytogeography, DNA ploidy level, flow cytometry, Japan, Korea, polyploidy
INTRODUCTION
Polyploidy has long been recognized as an important force in plant evolution. It is a widespread phenomenon that has brought evolutionary advantages to many plant groups (Soltis et al., 2004, 2009). The actual frequency of polyploidy in angiosperms is difficult to determine, and although these estimates have varied rather widely from 30–35 to 80 % (reviewed by Hegarty and Hiscock, 2008), it is now accepted that the vast majority of angiosperm lineages are of polyploid origin (Soltis et al., 2009). It has been suggested that roughly 2–4 % of all speciation events in angiosperms may have involved polyploidization, implying that it is a major mode of sympatric speciation (Otto and Whitton, 2000). Considerable attention has been focused on allopolyploid speciation, showing allopolyploids to be dynamic and complex systems with high evolutionary potentials (see, for example, Soltis and Soltis, 2000; Wendel, 2000; Osborn et al., 2003; Hegarty and Hiscock, 2008). Autopolyploidy, in turn, has long been viewed as less frequent and less important, although its role in plant evolution has surely been historically under-estimated (Soltis et al., 2007).
Ploidy variation also exists within species, and it is more frequent than previously anticipated. The occurrence and ubiquity of intraspecific cytotype variation has been documented, especially in recent years, as studies with thorough and intensive sampling that have been greatly enhanced by the use of flow cytometric techniques have accumulated (Kron et al., 2007; most recently see, for example, Schönswetter et al., 2007a; Suda et al., 2007; Kubátová et al., 2008; Kolář et al., 2009). Cytotype variation may be geographically structured, with populations mostly consisting of a single cytotype and some overlap among their distribution areas (Baack, 2004; Pannell et al., 2004; Mráz et al., 2008), but a surprisingly widespread co-occurrence of multiple cytotypes has also been observed within populations (Suda, 2002; Husband, 2004; Suda et al., 2007; Hufft Kao, 2007, 2008; Halverson et al., 2008). Mechanisms by which new cytotypes arise, establish and persist in populations, as well as the ecological and evolutionary consequences of such cytotype coexistence, have still not been well explored and present challenging questions in polyploidy research (Husband, 2004).
The family Brassicaceae is well known for its large variation in chromosome numbers and the frequent occurrence of polyploidy (reviewed in Marhold and Lihová, 2006). One of the most prominent examples is Draba, the largest genus of this family (comprising around 355 species), which shows enormous ploidy level variation and increased speciation and polyploidization rates (Jordon-Thaden and Koch, 2008). Recently, chromosome number data have been reviewed in detail for Cardamine (Kučera et al., 2005; Warwick and Al-Shehbaz, 2006), which includes around 200 species worldwide, representing one of the most species-rich genera in the family. These surveys have shown that around 64 % of the examined species are polyploid and that ploidy variation exists within species (Lihová and Marhold, 2006). Despite the considerable species richness in eastern Asia, few studies of chromosome data exist for this part of the genus distribution range (reported only for eight species, with 12 records altogether; Kučera et al., 2005). Nonetheless, detailed knowledge of chromosome numbers, ploidy level variation and the geographical distribution of multiple cytotypes is crucial for evolutionary studies and phylogenetic inferences, as well as taxonomic revisions in this genus.
The present study focuses on a group of perennial, rhizomatous Cardamine species that typically grow in moist sites near forest streams in Japan, Korea and the Russian Far East. A few chromosome number records (eight in total) have reported diploid to nonaploid levels, indicating polyploidy and also intraspecific variation in ploidy levels (Table 1). There is considerable confusion and conflict with respect to the taxonomy and nomenclature of these species. On the basis of morphometric analyses, and thorough field and herbarium studies, a new taxonomic concept for this group was recently developed (J. Lihová, H. Kudoh and K. Marhold, unpubl. res.), which is followed here. The species examined include C. yezoensis, C. schinziana, C. amaraeiformis and C. torrentis sensu lato (s.l.) [comprising two morphologically very close species C. torrentis sensu stricto (s.s.) and C. valida]. C. yezoensis occurs mainly in Hokkaido, extending to the Akita prefecture of northern Honshu in the south and to the southernmost Sakhalin in the north. Within C. torrentis s.l., C. torrentis s.s. grows on the islands of Kyushu, Shikoku, and southern and central Honshu, while C. valida is found in northern Honshu, Hokkaido and possibly also Sakhalin. C. schinziana is endemic to the Hidaka Mountain Range of Hokkaido. C. amaraeiformis, which closely resembles C. valida and may even be conspecific with it (requiring further research), occurs throughout the Korean Peninsula.
Table 1.
Data on chromosome numbers published for Cardamine yezoensis and C. torrentis s.l. (comprising C. valida and C. torrentis s.s.)
| Taxon | 2n | Locality | Author | Original determination | Note |
|---|---|---|---|---|---|
| C. valida | 32 | Japan, Hokkaido, Tokachi, Meto Spa | Kurosawa, 1981 | C. yezoensis | Only C. valida was found at this locality in 2004 |
| C. valida | 32 | Japan, Hokkaido, Tokachi, Yamada Spa | Kurosawa, 1981 | C. yezoensis | Only C. valida was found at this locality in 2004 |
| C. torrentis s.s. | 56 | Japan, Honshu, Gunma, Yamanohana, Oze | Kurosawa, 1981 | C. torrentis | |
| C. valida? | 32 | Russia, Sakhalin, okr. g. Yuzhno-Sakhalinska, gora Chekhova v sisteme Susunaiskogo khrebta, Probatova N. S., Rudyka E. G. 6294, 1982 (VLA) | Rudyka, 1984 | C. yezoensis | Specimen deposited in VLA was revised (incomplete plants only) |
| C. amara s.l. | 16 | Russia, Sakhalin | Sokolovskaya, 1960 | C. yezoensis | Most likely referring to the same locality and the same chromosome count as the next record |
| C. amara s.l. | 16 | Russia, Sakhalin, Anivskii r-n, okr. pos. Novo-Aleksandrovska, na sklone gory Chekhova, Sokolovskaya A. P. 85, 1957 (LEU) | Probatova and Sokolovskaya, 1988 | C. yezoensis | Specimens deposited in LEU and VLA were revised |
| C. yezoensis | 72 | Japan, [Hokkaido], Chiyogaoka, Higashikagura[-cho] | Nishikawa, 1986 | C. yezoensis | |
| C. yezoensis? | 46-48 | plants from Botanical Garden Edinburgh | Manton, 1932 | C. leucantha prol. yezoensis | No voucher specimen was found in herbaria CGE, LDS and MANCH |
No chromosome numbers were previously reported for C. amaraeiformis or C. schinziana. Revision was based either on voucher specimens or populations occurring in the given localities. C. amara s.l. refers to a widely conceived species complex of C. amara, but the exact taxonomic identity of these plants requires further study.
The present paper explores the polyploidy and cytotype variation of the above-mentioned taxa. The aims were to: (1) screen chromosome numbers, ploidy levels and genome size variations, and revise previously published karyological records; (2) determine geographical patterns of cytotype variation; and (3) explore if multiple cytotypes coexist within populations. A standard method of chromosome counting was applied and DNA ploidy levels were estimated using flow cytometry. The use of flow cytometry has recently revolutionized several fields of biosystematic research (Kron et al., 2007; Doležel et al., 2007a). Compared with the traditional methods of chromosome counting, this approach significantly enhances both large-scale assessments of ploidy level variation and distribution, and detailed within-population screening (e.g. Lihová et al., 2007; Schönswetter et al., 2007a, b; Suda et al., 2007; Hufft Kao, 2008; Kolář et al., 2009).
MATERIALS AND METHODS
Sampling
Material was sampled from 55 localities throughout Japan, covering the whole known distribution area of the studied species, except for their sporadic occurrence in Sakhalin (Appendix and Fig. 1). Sampling also included material from the original localities (or localities in their vicinity) from where Cardamine schinziana, C. torrentis s.s. and C. yezoensis and their synonyms were described (see Appendix). In addition, four localities of C. amaraeiformis were sampled in South Korea. The examined species are perennial, with efficient vegetative propagation by stolons, plant fragments and plantlets generated in leaf axils; they grow on river and stream banks, and alluvial sites. Seed dispersal and vegetative spreading can be enhanced by running water, and thus gene flow appears to be more extensive along the same stream than between different ones (our pers. observ.). Most localities sampled here represent separate, geographically distant populations of different streams. In a few cases (indicated in Appendix), populations were sampled on two disjunct sites along the same stream (separated by hundreds of metres and with an altitudinal difference of 90–280 m), which may be characterized by a higher extent of gene flow. From one to 14 living plants were collected at each site. Plants were collected from distinct patches to avoid collecting clones. The plants were transferred into pots in experimental gardens and used for flow cytometric measurements and chromosome counting. They were cultivated in the gardens of the Institute of Botany, Slovak Academy of Sciences in Bratislava (Slovakia), and the Graduate School of Science of Kobe University (Japan). Voucher specimens were deposited in the SAV and the herbarium of Shoei Junior College, Kobe, Japan, and duplicates were distributed to KYO, MAK and TUS (for acronyms see Holmgren et al., 1990).
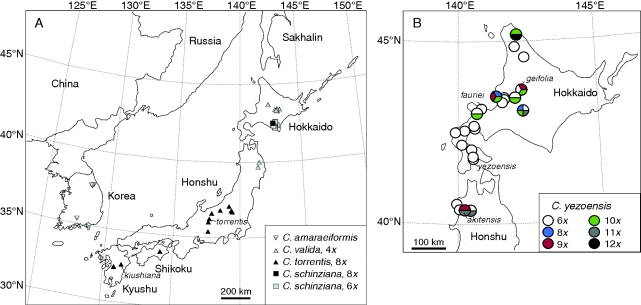
Fig. 1.
(A) Distribution of cytotypes of Cardamine torrentis s.l. (comprising C. torrentis s.s. and C. valida), C. schinziana and C. amaraeiformis in Japan and South Korea. (B) Distribution of cytotypes of C. yezoensis in Hokkaido and northern Honshu. Intra-population cytotype variation is illustrated by two- to four-coloured symbols. Type localities (locus classicus) of relevant names are indicated by species epithets.
Chromosome number counts
Chromosome numbers were counted in metaphase cells of root meristems. Two protocols were followed for the preparation of microscopic slides. (1) Root tips were pre-treated with 0·002 mol dm–3 aqueous solution of 8-hydroxyquinoline for 3–5 h at 4 °C, fixed in a freshly prepared solution of ethanol and acetic acid (3 : 1) for 1–24 h, and stored in 75 % ethanol. Root tips were then hydrolysed in a mixture of concentrated hydrochloric acid and ethanol (1 : 1) for 3–5 min, and rinsed with water. Subsequently, root tips were squashed using a cellophane square, and after the cellophane was removed, slides were stained in a 10 % solution of Giemsa stock solution in Sörensen phosphate buffer for 1 h, and rinsed with water (protocol used by the team in Bratislava). (2) Root tips were pre-treated with cold water at 0 °C for 24 h, fixed in a freshly prepared solution of ethanol and acetic acid (3 : 1) at 5 °C for 1 h, stained in 2 % acetic-orcein for 3–4 d and squashed under a cover glass (protocol used by K. Watanabe in Kobe).
Flow cytometric measurements and estimation of DNA ploidy levels
Flow cytometry was used to measure nuclear DNA content and to assess DNA ploidy levels in the studied populations. DNA ploidy levels were inferred on the basis of DNA content measured in plants with known (here counted) chromosome numbers. Whenever possible, at least one individual per species and ploidy level was analysed for both DNA content and chromosome number (see Appendix). The prefix ‘DNA’ indicates that the ploidy levels were inferred from the measured nuclear DNA content, in some cases also without knowing exact chromosome numbers (Suda et al., 2006). Absolute nuclear DNA content (in pg) was measured using propidium iodide (PI, a DNA-intercalating fluorochrome), and relative nuclear DNA content (in arbitrary units, a.u.) was measured using the AT-selective DAPI (4′,6-diamidino-2-phenylindole) fluorochrome (Doležel et al., 2007b). The latter fluorochrome generally provides better resolution and is more appropriate for assessing intraspecific DNA content variation (Shapiro, 1995). For the absolute DNA content, Lycopersicon esculentum ‘Stupické polní tyčkové rané’ (2C = 1·96 pg, Doležel et al., 1992) and Bellis perennis (Schönswetter et al., 2007b) were used as internal standards. The nuclear DNA content of Bellis was calibrated against Lycopersicon (mean 2C = 3·38 pg, based on three replications on different days). The relative DNA content was measured with L. esculentum as a primary standard and, in order to avoid overlap between sample and standard peaks that appeared in a few cases, Bellis perennis and samples JP 124-8 and JP 78-1 of C. yezoensis and C. schinziana, respectively, were used as secondary standards. The genome size of the secondary standards was calibrated against the primary standard, based on a minimum of three replicates performed on different days.
Tissue from a fresh young leaf of each analysed individual was co-chopped with leaf tissue from the internal standard in 0·5 mL ice-cold Otto I buffer (0·1 m citric acid monohydrate, 0·5 % Tween 20; Otto, 1990) using a razor blade. The resulting suspension of nuclei was filtered through a nylon mesh (42 µm), and after short incubation, 1 mL of staining solution was added to the flow-through fraction. The staining solution consisted of Otto II buffer (0·4 m Na2HPO4.12H2O) supplemented with β-mercaptoethanol (2 µL mL–1), fluorochrome (DAPI at a final concentration of 4 µg mL–1, or PI at 50 µg mL–1), and in case of PI measurements also RNase (at a final concentration of 50 µg mL–1). After incubation for 10 min at room temperature, the fluorescence intensity of 5000 particles (stained nuclei) was measured in Partec PA II flow cytometer (Partec GmbH, Münster, Germany) equipped with an argon ion laser (used for PI measurements) and HBO-100 mercury arc lamp (for DAPI measurements). Flow cytometric histograms were evaluated using Partec FloMax software (v. 2·4d; Partec GmbH).
Nuclear DNA content (in pg and a.u.) was determined from the ratio of G1 peak positions of the sample (analysed individual) and the standard. The coefficient of variation (CV) was calculated for both the internal standard and the sample. For each sample, three independent measurements were performed on different days in order to estimate between-run variation and to guarantee reliability of the results. Differences in the values measured on different days were recorded as the standard errors of the means, and expressed as a percentage of the mean value. Correlation coefficients (both Pearson and non-parametric Spearman coefficient) were also calculated for the values of absolute (PI-based) and relative (DAPI-based) DNA content measured from the same set of plants. To test for differences in the monoploid DNA content, species/ploidy level mean values were compared using the Tukey–Kramer test (Tukey test for unequal sample sizes; Zar, 1999; SAS Institute, 2007). Furthermore, if considerable differences in DNA content values were recorded for samples within (presumably) the same ploidy level, they were tested in simultaneous runs. Histograms with two clearly separated or bifurcated peaks in such cases are regarded as strong evidence for true differences in DNA content (Greilhuber, 2005; Pecinka et al., 2006; Šmarda and Bureš, 2006).
RESULTS
Chromosome number counts
Metaphase chromosomes were counted from a total of 101 plants sampled from 46 populations (Appendix).
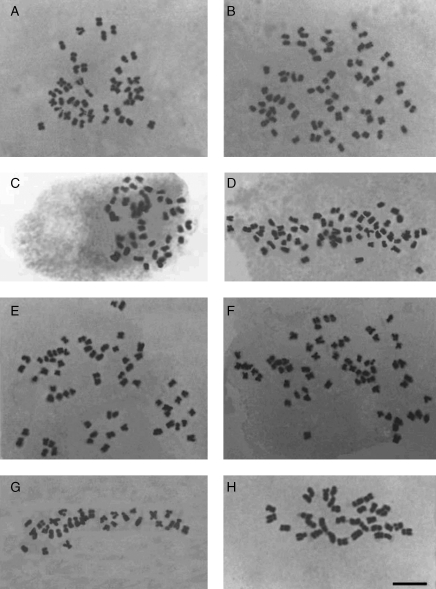
In Cardamine torrentis s.l., tetraploids (2n = 4x = 32, Fig. 2G) and octoploids (2n = 8x = 64, Fig. 2E, F) were found in six (11 plants) and ten (22 plants) populations, respectively. Following the adopted taxonomic treatment, tetraploids correspond to C. valida, while octoploids correspond to C. torrentis s.s. In addition, in two tetraploid populations from Hokkaido, plants with 2n = approx. 33–34 (JP 117) and 2n = 33 (JP 118) were observed. These may represent aneuploids (hypertetraploids) or plants with B chromosomes. The presence of B chromosomes has not previously been confirmed in Cardamine, while aneuploids are commonly reported in some species (e.g. the C. pratensis group, see Lihová and Marhold, 2006). In the absence of definitive evidence, we tentatively consider these plants with supernumerary chromosomes to be aneuploids. In population JP 118, a single hexaploid (2n = 48) individual was also detected, probably originating from an unreduced gamete.
Fig. 2.
Mitotic metaphase chromosomes. (A) Cardamine yezoensis, 2n = 48 (sample JP 46). (B) C. yezoensis, 2n = 72 (sample JP 120). (C) C. schinziana, 2n = 48 (sample 07-811). (D) C. schinziana, 2n = 64 (sample JP 77). (E) C. torrentis s.l., 2n = 64 (= C. torrentis s.s., sample JP 96). (F) C. torrentis s.l., 2n = 64 (= C. torrentis s.s., sample JP 8904). (G) C. torrentis s.l., 2n = 32 (= C. valida, sample JP 116). (H) C. amaraeiformis, 2n = 33 (sample K 3). Scale bar = 5 µm. For details on localities, see Appendix.
In C. amaraeiformis, only the tetraploid (2n = 4x = 32) cytotype was found. In populations K 3 and K 6, (likely) aneuploids with 2n = 33 (Fig. 2H), 2n = approx. 33–34 and 2n = 34 were also observed.
Cardamine yezoensis was found to consist predominantly of hexaploid plants with 2n = 6x = 48 (Fig. 2A). This chromosome number was confirmed in 35 plants originating from 17 populations. Octoploid (2n = 8x = 64), nonaploid (2n = 9x = 72; see Fig. 2B), decaploid (2n = 10x = 80) and dodecaploid (2n = 12x = 96) plants were present at a lower frequency. The sympatric occurrence of two or even three cytotypes was encountered in three populations (JP 56, JP 120 and JP 69, see Appendix).
In C. schinziana, hexaploids (2n = 6x = 48; Fig. 2C) were identified in four populations (seven plants), and in population JP 77 an octoploid individual (2n = 8x = 64; Fig. 2D) was found.
Previously published records of chromosome numbers are listed in Table 1. Revision of voucher specimens (when available) or populations from the given localities revealed that the original determination was often erroneous. The chromosome numbers reported are consistent with the present data, except for a report of heptaploidy (2n = 56) in C. torrentis s.s.
Flow cytometric measurements and estimations of DNA ploidy levels
Relative DNA content (DAPI-based analyses) was measured in 164 plants, and absolute DNA content (PI-based) determined for 155 plants. For most samples (132 individuals originating from 48 populations), both the relative and the absolute values were determined (Appendix). In most cases, flow cytometric analyses yielded high-resolution histograms (see Fig. 3). For DAPI-based measurements, the average CV for the samples was 2·27 % (range 1·33–5·37 %), and the CV of the standards was 2·04 % (range 1·27–4·47 %). For PI-based measurements, the CV of the samples was 4·76 % (range 1·42–9·79 %), and the CV of the standards was 3·63 % (range 1·18–7·98 %). The CV values of PI-based measurements were slightly higher than those proposed by Doležel et al. (2007b); nevertheless, the standard errors of the means of three repeated measurements, expressed as a percentage of the mean value, did not exceed 2 % in PI measurements and 1·5 % in DAPI measurements. This indicates that the data are precise and reliable.
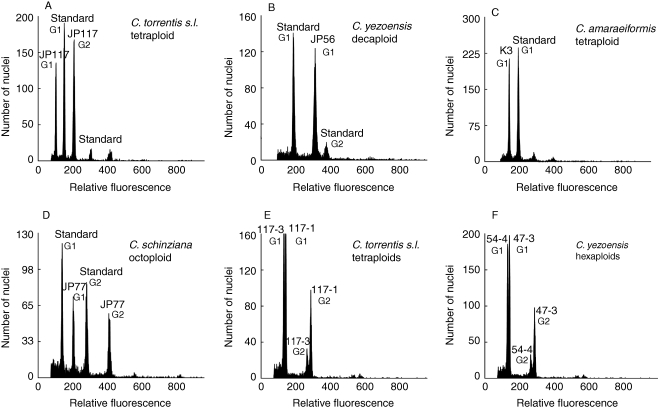
Fig. 3.
(A–D) Flow-cytometric histograms of DAPI-stained nuclei obtained from different cytotypes of the studied Cardamine species and internal standards. Standards: (A,C) Lycopersicum esculentum as a primary internal standard; (B) Bellis perennis as a secondary internal standard; (D) sample JP 78-1 of C. schinziana as a secondary internal standard. Samples: (A) Cardamine torrentis s.l., 2n = 32 (= C. valida, population JP 117); (B) C. yezoensis, 2n = 80 (population JP 56); (C) C. amaraeiformis, 2n = 32 (population K 3); (D) C. schinziana, 2n = 64 (population JP 77). (E,F) Selected flow-cytometric histograms documenting intraspecific divergence in nuclear DNA content within ploidy levels. Two individuals of the same ploidy level but with different fluorescence intensities were analysed simultaneously (with DAPI-stained nuclei). (E) Cardamine torrentis s.l., 2n ≈ 4x (= C. valida, ind. JP 117-3 and JP 117-1: 6·58 % difference); (F) C. yezoensis, 2n ≈ 6x (ind. JP 54-4 and JP 47-3: 8·03 % difference). For details on localities see Appendix.
The correlation between the absolute and relative DNA content values obtained for 132 plants was very high (Pearson r = 0·995, P < 0·0001; Spearman r = 0·946, P < 0·0001).
In several cases, considerable variation in fluorescence intensities was observed, even among individuals of the same ploidy level. Simultaneous analyses of such individuals yielded histograms with clearly separated or bifurcated peaks (Fig. 3E, F) and confirmed that these differences reflected true variation (see also below).
Cardamine torrentis s.l
The absolute DNA content was measured in 51 individuals, and the relative DNA content was assessed in 56 individuals, from a total of 20 populations examined (Appendix, Table 2). The values obtained fell into two distinct classes, which, on the basis of data from karyologically analysed individuals, can be assigned to the tetraploid and octoploid cytotypes, representing C. valida and C. torrentis s.s., respectively (see Appendix, Table 2). All populations were uniform, with no sympatric occurrence of multiple ploidy levels. Within both ploidy levels, considerable variation in DNA content values was found, as detected by both the DAPI and the PI analyses (Table 2). This variation reflects true differences, as shown by histograms obtained from simultaneous runs (Fig. 3E), and can be attributed to the presence of aneuploidy. Indeed, supernumerary chromosomes were karyologically detected at the tetraploid level. Among the tetraploids, the greatest divergence was in populations JP 117 (8 %) and JP 118 (7 %), which both contained aneuploid individuals (Appendix, Fig. 3E). There was also considerable variation at the octoploid level, although this was (with an exception of population JP 96) between (rather than within) populations (Appendix).
Table 2.
Summary results of flow cytometric analyses in Cardamine species, using DAPI (relative DNA content, given in arbitrary units) and PI (absolute DNA content, given in pg) fluorochromes
| (a) Relative DNA content (a.u.) | ||||||
|---|---|---|---|---|---|---|
| Taxon, DNA ploidy level | DNA content: min.–max. (mean) | Intracytotype variation, max./min. (%) | Mean relative DNA content per monoploid genome | CV of samples, min.–max. (mean) (%) | CV of standard, min.–max. (mean) (%) | No. of plants |
| torrentis s.l.: | ||||||
| valida 2n ≈ 4x | 0·651–0·737 (0·687) | 13·21 | 0·172a | 1·83–4·43 (2·582) | 1·52–3·5 (1·994) | 21 |
| torrentis s.s. 2n ≈ 8x | 1·105–1·189 (1·138) | 7·6 | 0·142 | 1·33–3·07 (1·756) | 1·29–4·47 (1·964) | 35 |
| amaraeiformis 2n ≈ 4x | 0·663–0·763 (0·696) | 15·08 | 0·174a | 2·06–4·36 (2·896) | 1·9–3·43 (2·286) | 10 |
| yezoensis 2n ≈ 6x | 0·674–0·785 (0·715) | 16·47 | 0·119c | 1·69–5·37 (2·418) | 1·27–3·12 (1·945) | 60 |
| yezoensis 2n ≈ 6x (excl. JP 135, JP 136) | 0·674–0·759 (0·712) | 12·61 | 0·119c | 1·69–5·37 (2·411) | 1·27–3·12 (1·927) | 57 |
| yezoensis 2n ≈ 8x | 0·842–0·852 (0·847) | 1·19 | 0·106b | 2·41–3·18 (2·708) | 2·12–2·98 (2·485) | 2 |
| yezoensis 2n ≈ 10x | 0·995–1·073 (1·027) | 7·79 | 0·103b | 1·44–3·25 (2·007) | 1·58–3·57 (2·364) | 16 |
| yezoensis 2n ≈ 11x | 1·082–1·137 (1·110) | 5·08 | 0·101b | 1·65–2·6 (2·033) | 1·68–2·71 (2·1) | 5 |
| yezoensis 2n ≈ 12x | – | – | – | – | – | – |
| schinziana 2n ≈ 6x | 0·682–0·755 (0·713) | 10·7 | 0·119c | 1·56–4·13 (2·422) | 1·56–3 (1·963) | 12 |
| schinziana 2n ≈ 8x | 1·014–1·054 (1·031) | 3·88 | 0·129 | 1·58–2·3 (2·011) | 2·11–2·95 (2·61) | 3 |
| (b) Absolute DNA content (pg) | ||||||
| Taxon, DNA ploidy level | DNA content, min.–max. (mean) | Intracytotype variation, max./min. (%) | Mean monoploid genome size | CV of samples, min.–max. (mean) (%) | CV of standard, min.–max. (mean) (%) | No. of plants |
| torrentis s.l.: | ||||||
| valida 2n ≈ 4x | 1·419–1·587 (1·493) | 11·84 | 0·373a | 1·76–9·35 (5·971) | 1·32–5·69 (4·29) | 18 |
| torrentis s.s. 2n ≈ 8x | 2·26–2·469 (2·362) | 9·25 | 0·295 | 2·02–6·19 (4·411) | 1·3–7·03 (4·255) | 33 |
| amaraeiformis 2n ≈ 4x | 1·411–1·64 (1·495) | 16·23 | 0·374a | 1·42–5·36 (2·583) | 1·19–2·85 (1·716) | 10 |
| yezoensis 2n ≈ 6x | 1·394–1·66 (1·482) | 19·08 | 0·247c | 1·86–8·96 (5) | 1·35–7·98 (3·656) | 57 |
| yezoensis 2n ≈ 6x (excl. JP 135, JP 136) | 1·394–1·583 (1·471) | 13·56 | 0·245c | 1·99–8·96 (5·21) | 1·35–7·98 (3·747) | 52 |
| yezoensis 2n ≈ 8x | 1·736–1·786 (1·756) | 2·88 | 0·220b | 2·5–6·12 (3·389) | 1·18–3·11 (2·011) | 3 |
| yezoensis 2n ≈ 10x | 1·97–2·099 (2·051) | 6·55 | 0·205b | 4·4–7·57 (5·6) | 3·19–5·78 (3·808) | 10 |
| yezoensis 2n ≈ 11x | 2·248–2·362 (2·308) | 5·07 | 0·210b | 2·08–5·25 (3·641) | 1·39–4·18 (2·803) | 6 |
| yezoensis 2n ≈ 12x | 2·47–2·528 (2·499) | 2·35 | 0·208b | 1·96–4·57 (3·015) | 1·4–2·66 (1·74) | 2 |
| schinziana 2n ≈ 6x | 1·363–1·565 (1·448) | 14·82 | 0·241c | 1·86–9·79 (4·915) | 1·4–5·61 (3·557) | 14 |
| schinziana 2n ≈ 8x | 2·008–2·081 (2·045) | 3·64 | 0·256c | 4·96–6·48 (5·682) | 3·04–5·41 (4·047) | 2 |
Data are summarized for each ploidy level of a given taxon. For hexaploid C. yezoensis, data are given also without JP 135 and JP 136, which most likely also comprise aneuploids. Means of relative DNA content and monoploid genome size are significantly different at P < 0·05 (Tukey–Kramer test), unless marked by the same lower-case letter.
Cardamine amaraeiformis
Both the absolute and the relative DNA contents were measured in ten plants that were sampled from four South Korean populations (Appendix, Table 2). In all plants, a single DNA-ploidy level was inferred. Based on chromosome counting, this represents tetraploids. The considerable fluorescence divergence, which was as high as 9 % within populations and 16 % in total (Appendix, Table 2), was apparently due to the presence of aneuploids (hypertetraploids). Indeed, two karyologically confirmed aneuploids, with 2n = 34 and 2n = 33–34, had fluorescence values at the upper limit of the measured range.
Cardamine yezoensis
The absolute DNA content was measured in 78 individuals, and the relative DNA content was determined for 83 individuals, which were sampled from 23 populations altogether (Appendix, Table 2). Extensive variation in fluorescence intensities was found both among and within populations, confirming the presence of several cytotypes and their co-occurrence within populations. Interpretation of the resulting histograms was challenging. Five ploidy levels were identified by chromosome counting (2n = 6x, 8x, 9x, 10x and 12x). On the basis of fluorescence intensities, six DNA-ploidy levels (2n ≈ 6x, 8x, 10x, 11x and 12x) were inferred in the following way. For two ploidy levels, the hexaploid and decaploid, several plants were identified with both flow cytometric and karyological data. These were used to determine the nuclear DNA content per monoploid genome (the Cx-value, according to Greilhuber et al., 2005). As a decrease of monoploid genome size with increasing ploidy level is a commonly observed phenomenon (Table 2; see also below), the monoploid genome size of hexaploids was used to infer DNA-ploidy levels for lower fluorescence intensity values, and the monoploid genome size of decaploids was used to interpret the higher values. All of the DNA-ploidy levels inferred in this way were also determined by chromosome counting (Appendix), except for 2n ≈ 11x. DNA-enneaploids (2n ≈ 11x) were found in three populations, JP 133, JP 134 and JP112.
The vast majority of plants of C. yezoensis were DNA-hexaploids (72 % in DAPI-based and 73 % in PI-based measurements), and they were found in 19 populations (Fig. 1). Second most common were DNA-decaploids (19 % in DAPI-based and 12 % in PI-based measurements), which were found in six populations. All other DNA-ploidy levels were relatively rare (Table 2). Co-occurrence of up to four different cytotypes was found in seven populations (Appendix, Fig. 1). All higher ploidy-level cytotypes (2n ≈ 8x and higher) were found exclusively in mixed ploidy-level populations.
There was considerable variation in fluorescence intensity, even within the DNA-hexaploids of C. yezoensis. The divergence in PI-based analyses was 19·08 %, and in DAPI-based analyses was 16·47 %. The variation was illustrated and confirmed by simultaneous analyses that yielded histograms with double peaks (Fig. 3F). Much of this variation was found in two populations (JP 135 and JP 136 from northern Honshu), and when these two are excluded, the divergence decreases to 13–14 % (Table 2), which is comparable with the variation within the cytotypes of C. torrentis s.l. We assume that the higher DNA content values observed in populations JP 135 and 136 may be due to a past or recent hybridization with plants of higher ploidy levels growing in adjacent populations, or possibly within the studied populations (although such individuals were not detected). The variation within higher DNA-ploidy levels was considerably lower, although this may be due to the smaller sample size.
Cardamine schinziana
The absolute DNA content was measured in 16 individuals, and the relative DNA content was determined for 15 individuals, which were sampled from six populations altogether (Appendix, Table 2). Two DNA-ploidy levels were inferred, in accordance with the chromosome counts: DNA-hexaploids and DNA-octoploids. No sympatric intra-population occurrence of both cytotypes was observed. The DNA-hexaploids largely prevailed, and DNA-octoploids were found in only one population, JP 77 (Appendix). In hexaploids, fluorescence divergence higher than expected from the instrumental drift was observed (14·82 % divergence for PI-based and 10·7 % for DAPI-based measurements). We assume that this variation was due to the presence of aneuploids, although aneuploids were not detected by chromosome counting.
Monoploid genome size variation
Table 2 compares the nuclear DNA content calculated per monoploid genome between the species and cytotypes. Between-species comparisons show that C. yezoensis and C. schinziana have similar monoploid genome sizes (at hexaploid levels), as do the tetraploid cytotypes of C. torrentis s.l. and C. amaraeiformis. The latter two species have monoploid genomes that are approx. 1·5-times larger than the former two. In both C. yezoensis and C. torrentis s.l., the monoploid genome size decreases with increasing ploidy level. The means of the sizes of the monoploid genome/relative DNA content per monoploid genome are significantly different between tetraploids and octoploids of C. torrentis s.l., and between hexaploids and higher ploidy levels of C. yezoensis (at P < 0·05, Tukey–Kramer test; Table 2).
Cytotype distribution patterns
The two cytotypes of C. torrentis s.l. displayed clear north–south geographical patterns in their distribution (Fig. 1). The tetraploids (C. valida) were exclusively found in northern territories (in the Iwate prefecture of northern Honshu and in Hokkaido), while the octoploids (C. torrentis s.s.) occurred in central and southern Honshu, and on Shikoku and Kyushu. No overlap between the areas occupied by these two cytotypes was found, nor was any cytotype mixing within populations. It should be noted that the disjunction seen between the southernmost records of the tetraploids in northern Honshu and the northernmost records of the octoploids in central Honshu is real; based on our herbarium and field surveys, there are no populations of C. torrentis s.l. in the border region of central and northern Honshu.
Chromosome counting and flow cytometric screening revealed six ploidy levels in C. yezoensis. Sixteen populations, distributed across the whole species range in Japan, comprised only hexaploids, while multiple cytotypes were found to coexist in the other eight populations sampled. These populations contained plants of two (five populations), three (two populations) or even four (one population) different cytotypes. Cytotypes were scattered across the species range, without any obvious geographical pattern (Fig. 1).
DISCUSSION
Cytotype diversity and distribution patterns
Chromosome number counting and flow cytometric screening of DNA-ploidy levels confirmed polyploidy in the East Asian Cardamine species studied. Broad sampling and the use of the efficient flow cytometric approach allowed us to reveal the large cytotype diversity in these species. Previously, there were only a few chromosome number records available, and most of them were based on misidentified material or referred to misinterpreted names (see Table 1). Indeed, only a single chromosome number record was definitively attributable to C. yezoensis, suggesting nonaploidy (2n = 72, Nishikawa, 1986; see Table 1). However, the intraspecific ploidy variation in C. yezoensis was found to be remarkably high. As many as six different cytotypes, ranging from common hexaploids to more rare dodecaploids, were found. We confirmed tetraploidy in C. torrentis s.l. (2n = 32, Kurosawa, 1981; probably also Rudyka, 1984; Table 1), but recorded no cases of heptaploidy (2n = 56, as reported in Kurosawa, 1981; Table 1). However, previously unreported octoploids (2n = 64) in C. torrentis s.l. were frequently discovered. No chromosome data records were previously available for C. schinziana and C. amaraeformis. The former species was found to exhibit two cytotypes (hexa- and octoploidy), while C. amaraeformis exhibits only a single ploidy level (2n = 32).
The patterns of cytotype distribution were strikingly different between the studied species. Whereas multiple cytotypes were frequently observed in populations of C. yezoensis, populations of C. torrentis s.l. and C. schinziana appeared to be uniform, generally comprising only a single cytotype (although the data on C. schinziana might be biased due to the low number of populations studied). The only exception was that one hexaploid plant of C. torrentis s.l. was found within an otherwise tetraploid population, which probably indicates an occasional event of unreduced gamete production. Furthermore, whereas the two cytotypes of C. torrentis s.l. grow allopatrically, displaying a prominent north–south geographical pattern, no geographical structure was observed for hexaploids and the higher-ploidy cytotypes of C. yezoensis. Hexaploids, the most common cytotype, grow across the entire area sampled, and the higher-ploidy level cytotypes do not appear to form their own populations, but occur in mixed ploidy-level populations. These cytotype-mixed populations, each containing between two and four distinct cytotypes, make up as much as one-third of the examined populations and were found across the entire species range (Fig. 1). Apparently, there is a strong tendency towards an increase of ploidy levels in this species. Although the lowest level, the hexaploid, is stable, the higher levels are not, suggesting a scenario of continued polyploidization via a production of unreduced gametes.
According to theoretical models, the coexistence of multiple cytotypes within populations should be unstable and may represent only a transient stage following the immigration or generation of a divergent cytotype. On the basis of the frequency-dependent process described ar minority cytotype exclusion (Levin, 1975; Felber, 1991), newly generated or immigrated cytotypes are at a disadvantage and will be excluded from the given population, unless some ecological or genetic factors stabilize their persistence. Both theoretical and empirical evidence indicate that increased selfing, vegetative propagation, apomixis, highly restricted pollen and seed dispersal, production of unreduced gametes, phenological shifts, niche divergence or fitness differences may be crucial for maintaining cytotype-mixed populations (Felber, 1991; Thompson & Lumaret, 1992; Maceira et al., 1993; Felber-Girard, et al., 1996; Felber and Bever, 1997; Husband, 2004; Baack, 2005; Hufft Kao, 2007). Which of these factors explain the common and widespread co-occurrence of multiple cytotypes in populations of C. yezoensis remains an open question. Asexual reproduction, either via vegetative propagation or via apomixis, undoubtedly belongs to the strongest factors stabilizing coexistence of multiple cytotypes (Hufft Kao, 2007). Admittedly, efficient vegetative propagation, observed in this species by means of plant fragments, plantlets in leaf axils and stolons, plays a significant role, but more intense sampling is needed to explore the relative frequencies of cytotypes within populations and their fine-scale spatial distribution. Also, data on phenology, fitness and the crossing behaviour of individual cytotypes may shed more light on the mechanisms operating in these populations. Based on the overall spatial patterns, we assume that higher-level cytotypes arise repeatedly within populations and that gene flow between cytotypes may also increase the cytotype complexity. Similar cases have indeed been observed in other Cardamine polyploids, in which interspecific and/or inter-cytotype gene flow has resulted in a nearly continuous series of chromosome numbers, both euploid and aneuploid (C. dentata from northern Europe, Lövkvist, 1956; hybrid swarms of C. pratensis and C. raphanifolia, Lihová et al., 2007), as well as in a hybridogeneous species Ixeris nakazonei from Japan and Taiwan, which comprises six cytotypes (3x to 8x; Denda and Yokota, 2004).
The high intraspecific cytotype diversity observed in C. yezoensis is rarely found in sexually reproducing vascular plants. A few comparable examples include Cardamine pratensis s.s. from the Iberian Peninsula, in which six ploidy levels (2x to 7x) were determined (Lihová et al., 2003); North American C. diphylla, with chromosome numbers spanning from 2n = 78 to 256 (Harriman, 1965); or Senecio carniolicus, which exhibits five ploidy levels (2x to 7x) in the eastern Alps (Suda et al., 2007). Detailed fine-scale screening of S. carniolicus revealed that the cytotypes are not randomly distributed; altitudinal segregation of the cytotypes was indicated, with a wider ecological amplitude for the diploids compared with the hexaploids (Schönswetter et al., 2007a). Such studies illustrate the utility of flow cytometry in detecting intraspecific and intrapopulation variation and emphasize the need for both broad- and fine-scale population screening, which may provide revolutionary insights into the evolutionary dynamics of polyploids.
Origin and biogeography of Cardamine torrentis s.l.
The two cytotypes of Cardamine torrentis s.l. occupied clearly distinct ranges with no contact zone. Such strong spatial segregation might be due to several factors. Baack (2004), for instance, pointed to three non-exclusive hypotheses to explain the apparent cytotype segregation in Ranunculus adoneus: ecological differentiation, also often observed in other species (see, for example, Rothera and Davy, 1986; Felber-Girard et al., 1996); reproductive exclusion (Levin, 1975); and historical factors coupled with dispersal limitation. The area occupied by C. torrentis s.l. spans a broad range of climatic conditions, and environmental variables can be strikingly different between its northernmost and southernmost localities (Yoshino, 1980). Although it can be characterized as a temperate to cold temperate species complex, in southernmost areas (Kyushu, Shikoku, southern Honshu) it is restricted to higher altitudes and/or cold deep valleys (our pers. observ.), and detailed ecological surveys and cultivation experiments will be needed in the future to specify the precise ecological niches of its cytotypes. Nevertheless, inferring from the knowledge of the origin of the flora of Japan, and recent phylogeographical studies from this area, we assume that historical factors, mainly the climatic oscillations and sea-level regression of the Late Pleistocene, may have played a key role in the development of the cytotype distribution pattern.
Although it is has been determined that approximately 40 % of Japanese plant species are endemic, most of the flora of Japan apparently originated on the Asian continent and colonized Japan via northern (Sakhalin, the Kuril Archipelago) or southern (the Korean Peninsula, Ryukyu Isles) routes (Hotta, 1974; Maekawa, 1974; Murata, 2000). We can assume that C. torrentis s.l. or its immediate progenitor originated in the easternmost part of mainland Asia. First, there are no related diploids in Japan, and second, the morphologically and genetically closest relative appears to be diploid C. amara (Carlsen et al., 2009), which is distributed throughout Europe and extends to West Siberia (Doron'kin, 1994; Malyshev et al., 2003). There are, however, diploid chromosome data that have been reported for plants resembling C. amara in Sakhalin (Sokolovskaya, 1960; Probatova and Sokolovskaya, 1988; erroneously determined as C. yezoensis, see Table 1). Alternatively, we can hypothesize that some diploid progenitors of C. torrentis s.l. may have originally occurred in Japan, but have gone extinct during the Pleistocene.
We can hypothesize that the tetraploid cytotype of C. torrentis s.l. arose either through genome doubling (autopolyploidy) or interspecific hybridization (allopolyploidy) with the contribution of C. amara s.l. The climatic oscillations of the Late Pleistocene may have been a key factor in its spread into and establishment within Japan. Sea-level regressions during that time created land bridges in the straits of Soya (between Hokkaido and Sakhalin) and in Tsugaru (between Honshu and Hokkaido), connecting Japan to the Eurasian continent (Minato and Ijiri, 1984). The land connections and climatic cooling probably allowed many plant species from the Asian mainland to spread southward and colonize Japan. This scenario can also be expected for C. torrentis s.l.; the tetraploid probably colonized northern Japan from some northern mainland territories (Sakhalin or other territories of the Russian Far East), and gave rise to octoploid populations of C. torrentis s.l., through either autopolyploidy or allopolyploidy. These octoploids then spread to more southern areas of the Japanese archipelago. During the glacial period(s), the octoploid populations may have been widespread, but during interglacial and postglacial periods, they probably retreated to colder habitats at higher altitudes and the deep, cool valleys where they are currently found. As in other plant species (Cain et al., 2000), apart from land bridges, long-distance dispersal may have played a considerable role in the (post-)glacial spread of C. torrentis s.l.
The distribution pattern seen in the cytotypes of C. torrentis s.l. is not rare among Japanese plants. It coincides with one of the two major phylogeographical patterns identified for Japanese species: differentiation in a north–south direction, with one intraspecific lineage located in Hokkaido, often extending to northern Honshu, and the other inhabiting central Honshu and stretching to Shikoku and Kyushu islands (Tsuda and Ide, 2005; Fujii and Senni, 2006; Okaura et al., 2007; Ikeda et al., 2008; Koga et al., 2008) The distribution of the two haplotype groups was also resolved within the cold-adapted and submerged herbaceous species of Ranunculus subgen. Batrachium. It coincides very well with the distribution ranges of the two cytotypes of C. torrentis s.l. (Koga et al., 2008). Two colonization routes from the Asian continent to Japan, via a southern and a northern land bridge, as well as northeast–southwest orientated mountain ranges of the Kanto and Chubu districts (central/northern Honshu), forming a definite barrier for gene flow, are invoked to explain such congruence in phylogeographical patterns (Fujii and Senni, 2006).
Finally, considering the overall morphological similarity (J. Lihová, H. Kudoh and K. Marhold, unpubl. res.) and the existence of the same monoploid genome size in C. amaraeiformis and tetraploid C. torrentis s.l., we speculate that they have the same polyploid origin and may even belong to the same taxon. The tetraploids were presumably established on the eastern Asian mainland (see above) and may have migrated during the glacial period not only to Japan, but also to north-east China and the Korean Peninsula. Such a migration route was also hypothesized for Asarum sect. Asiasarum (Yamaji et al., 2007). The closest relative of the boreal A. heterotropoides var. heterotropiodes, which is distributed across Hokkaido, the northernmost part of Honshu, southern Sakhalin and the southern Kurils (a distribution similar to that of tetraploid C. torrentis s.l.), is A. versicolor, which is found in central Korea (as C. amaraeiformis).
Nuclear DNA content variation
When multiple individuals were analysed, considerable variation in fluorescence intensity was recorded within several cytotypes of the studied species (Table 2). The intracytotype variation should be considered to be reliable, and we have ensured that it was not caused by artefacts such as instrumental drift or metabolite interference. We are confident in these results for several reasons: (1) reasonably low CV values and standard errors of the means of three repeated measurements for each individual were obtained; (2) analyses based on two different fluorochromes (PI and DAPI) gave consistent and highly correlated data; and (3) simultaneous analyses of individuals of the same cytotype, but with divergent DNA content values, yielded histograms with one bifurcated or two separate peaks (Fig. 3E, F; see Greilhuber, 2005).
At least part of this variation can be explained by aneuploidy, i.e. duplication of one or two chromosomes, which has been confirmed through chromosome counting in tetraploid plants of C. torrentis s.l. and C. amaraeiformis. Although it was not directly observed at higher ploidy levels or in C. yezoensis, DNA content variation due to aneuploidy most likely occurs also here. Furthermore, gene flow across ploidy levels, assumed to occur in C. yezoensis (see above), may result in individuals with irregular chromosome numbers, increasing the complexity of the cytotype variation within populations. This phenomenon has also been observed in other polyploid complexes of Cardamine (Lövkvist, 1956; Lihová et al., 2007). Although many earlier reports on extensive intraspecific DNA content variation were discarded due to experimental errors (Greilhuber, 1998, 2005), there have also been cases where such variation was verified (Pecinka et al., 2006; Šmarda and Bureš, 2006; Suda et al., 2007). Determining the sources of this variation, however, is not always straightforward. An intracytotype divergence of 15·7 % detected in Senecio carniolicus (Suda et al., 2007) was attributed to aneuploidy, although this was not recorded by direct chromosome counting. Šmarda and Bureš (2006) speculated on the role of retrotransposon activities and sequence elimination to explain variation in Festuca pallens (see also Bennetzen et al., 2005).
Pronounced decreases in monoploid genome sizes with increases of ploidy levels were observed in C. torrentis s.l. and also to lesser extent in C. yezoensis (Table 2). Such trends have been reported for several other taxa (Lysák and Doležel, 1998; Hörandl and Greilhuber, 2002; Pecinka et al., 2006) and have been attributed to partial eliminations of repetitive DNA sequences (Greilhuber, 1998; Hörandl and Greilhuber, 2002). Indeed, the genome size of polyploids is often not additive relative to their diploid (or lower ploidy) progenitors, but changes towards a lower or a higher (less frequently) genome size are observed, reflecting the dynamic nature of polyploids (Wendel et al., 2002; Leitch and Bennett, 2004). Despite the ubiquity of genomic processes increasing genome size (mainly polyploidy, gene duplications or amplification of transposable elements), mechanisms suppressing genome expansion are apparently also operating in plants, although they are poorly understood so far (Lysak et al., 2009).
ACKNOWLEDGEMENTS
Our sincere thanks go to Hidetoshi Kato (Tokyo), Cho Seong-ho and Lee Don-hwa (Daegu) for their considerable help with plant sampling in the field, and to Jan Suda and Pavel Trávníček (both Prague) for providing flow cytometric facilities and initial help with the analyses. This work was supported by the Grant Agency for Science, VEGA, Bratislava [grant number 2/6055/26 to J.L.]; the Slovak Research and Development Agency, APVV [grant number RPEU-0003-06 to K.M.]; the Ministry of Education, Youth and Sports of the Czech Republic [grant number 0021620828 to K.M.]; the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, Grant-in-Aid for Scientific Research of Priority Areas ‘Genome Barriers in Plant Reproduction’ [grant number 19043010 to H.K.]; Global COE Program A06 of Kyoto University; and the exchange programme of the Japan Society for the Promotion of Science and Slovak Academy of Sciences.
APPENDIX
Population samples of Cardamine yezoensis, C. torrentis s.l. (comprising C. torrentis s.s. and C. valida), C. amaraeiformis and C. schinziana analysed in this study. Chromosome numbers (2n) are given, followed by the number of plants analysed and the name of the person who performed the count. Flow-cytometric data (FCM measurements) include PI and DAPI data, which refer to 2C values expressed either in pg (absolute DNA content) or in arbitrary units (a.u., relative fluorescence). Data for most populations are arranged in multiple rows; each row gives value(s) for a single plant or several plants of the same ploidy level; the number of analysed plants and intracytotype variation are also indicated, the latter expressed as the max./min. ratio given as a percentage. Analyses referring to cases where the same individual was subjected to both direct chromosome counting and flow-cytometric measurements are also given separately, shown in bold type. Localities are arranged geographically. Superscripts in locality codes indicate populations sampled on two disjunct sites along the same stream. Name abbreviations: HK, Hiroshi Kudoh; JL, Judita Lihová; KM, Karol Marhold; KW, Kuniaki Watanabe; SŠ, Stanislav Španiel.
| Locality code | Locality and collection details | 2n (no. of plants, analysed by) | FCM measurements |
(DNA) Ploidy level | |
|---|---|---|---|---|---|
| PI in pg (no. of plants/variation in %) | DAPI in a.u. (no. of plants/variation in %) | ||||
| Cardamine torrentis Nakai | |||||
| JP 8904 | Kyushu, Kumamoto, Kuma-gun, Itsuki-mura, Kozuru, Shirataki, 480 m, 32°26·706′N, 130°46·583′E, coll. HK 04-468 & KM, 15 Jun. 2004; K. Miyagi & HK M0213, 27 Apr. 2005 | – | 2·412–2·441 (3/1·2 %) | 1·149–1·161 (3/1·04 %) | ≈ 8x |
| 64 (2, KW), Fig. 2F | – | – | 8x | ||
| JP 9004 | Kyushu, Kumamoto, Kuma-gun, Itsuki-mura, Kozuru, Otaki, 687 m, 32°27·982′N, 130°47·433′E, coll. HK 04-469 & KM, 14 Jun. 2004 | – | – | 1·164 (1/–) | ≈ 8x |
| 64 (1, KW) | – | – | 8x | ||
| JP 8504 | Kyushu, Miyazaki, Nishiusuki-gun, Hinokage-cho, Shiitani, 230 m, 32°41·283′N, 131°19·422′E, coll. HK 04-463 & KM, 13 Jun. 2004; K. Miyagi & HK M0209, 26 Apr. 2005 (type locality of C. kiusiana Hara) | – | 2·45 (1/–) | 1·16 (1/–) | ≈8x |
| 64 (1, KW) | – | – | 8x | ||
| JP 8204 | Shikoku, Tokushima, Mima-gun, Kiyadaira-mura, Mt Tsurugisan, Gyojyo, 1700 m, 33°51·422′N, 134°05·878′E, coll. HK 04-454 & KM, 10 Jun. 2004 | – | 2·385–2·432 (3/1·97 %) | 1·153–1·189 (3/3·12 %) | ≈ 8x |
| 64 (4, KW) | – | – | 8x | ||
| JP 95 | Honshu, Gifu, Nakatsugawa-shi, Mt Ena, Kuroisawa-River, 1222 m, 35°24·417′N, 137°35·93′E, coll. HK 04-474, 04-956 & KM, 20 Jun. 2004, 15 Nov. 2004 | – | 2·469 (1/–) | 1·173–1·185 (3/3·13 %) | ≈ 8x |
| 64 (3, KW) | – | – | 8x | ||
| JP 87 | Honshu, Nagano, Azumi-gun, Nagawa-mura, Nomugitoge-Pass, Honzawa-River, 1532 m, 36°02·444′N, 137°36·726′E, coll. HK 03-378, JL & KM, 13 Jul. 1003, HK 04-954 & KM, 15 Nov. 2004 | – | 2·329–2·349 (3/0·86 %) | 1·123–1·139 (3/1·42 %) | ≈ 8x |
| 64 (2, KW) | – | – | 8x | ||
| JP 88 | Honshu, Nagano, Minamizumi-gun, Azumi-mura, Kamikochi, 1540 m, 36°15·654′N, 137°40·707′E, coll. HK 03-380, 03-389, JL & KM, 14 Jul. 2003, HK 04-951, 04-952 & KM, 14 Nov. 2004 (type locality of C. torrentis Nakai) | – | 2·308–2·325 (3/0·74 %) | 1·13–1·14 (3/0·88 %) | ≈ 8x |
| 64 (4, KW) | – | – | 8x | ||
| JP 321 | Honshu, Tochigi, Nikko-shi, Senjyugahama, Toyamazawagawa-River (W of Chyuzenji lake), 1290 m, 36°44·837′N, 139°25·160′E, coll. HK 03-375, JL & KM, 30 Apr. 2002, 6 Jul. 2003 | – | 2·304–2·335 (5/1·35 %) | 1·109–1·127 (5/1·62 %) | ≈ 8x |
| JP 9904 | Honshu, Tochigi, Nikko-shi, Senjyugahara, flood plain along Sotoyamazawa River, 1307 m, 36°45·002′N, 139°24·803′E, coll. KM & HK, 24 Jun. 2004 | – | – | 1·127 (1/–) | ≈ 8x |
| JP 1001 | Honshu, Tochigi, Nikko-shi, Toyamazawagawa-River, 1408 m, 36°45·628′N, 139°24·587′E, coll. HK 04-494 & KM, 24 Jun. 2004, JL, KM & H. Kato, 20 Jun. 2005 | 2·384 | 1·119–1·122 (2/0·27 %) | ≈8x | |
| 64 (1, KW) | 8x | ||||
| JP 86 | Honshu, Nagano, Hakuba village, Sano, Himekawa-Genryu, 750 m, 36°37·926′N, 137°50·897′E, coll. JL, KM & H. Kato, 18 Apr. 2002, 13 Jul. 2003, JL, KM & H. Kato, 18 Jun. 2005 | – | 2·36–2·377 (3/0·72 %) | 1·152–1·154 (3/0·17 %) | ≈ 8x |
| JP 82 | Honshu, Gunma, Tone-gun, Katashina-mura, Lake Marunuma, 1445 m, 36°49·977′N, 139°20·957′E, coll. HK 03-376, JL & KM, 6 Jul. 2003, JL, KM & H. Kato, 19 Jun. 2005 | 2·26–2·344 (3/3·72 %) | 1·117–1·126 (3/0·81 %) | ≈ 8x | |
| JP 96 | Honshu, Nagano, Shimotakai-gun, Kijimadaira-mura, Kayano-taira, Kitadobushitugen, 1574 m, 36°50·972′N, 138°29·545′E, coll. HK 04-479 & KM, 21 Jun. 2004 | – | 2·271–2·461 (7/8·37 %) | 1·105– 1·118 (4/1·18 %) | ≈ 8x |
| 64 (2, KW) | 8x | ||||
| 64 (1, KW), Fig. 2E | 2·271 | 1·118 | 8x | ||
| JP 98 | Honshu, Niigata, Kitauonuma-gun, Yunotani-mura, Ginzandaira, 914 m, 37°07·245′N, 139°09·782′E, coll. HK 04-484 & KM, 22 Jun. 2004 | 64 (1, KW) | 8x | ||
| Cardamine valida (Takeda) Nakai | |||||
| JP 103 | Honshu, Iwate, Shimohei-gun, Iwaizumi-cho, Ohkawa-River, Hitsutori-shitugen, 983 m, 39°42·317′N, 141°27·143′E, coll. HK 04-503 & KM, 27 Jun. 2004 | 1·427–1·429 (2/0·14 %) | 0·657–0·663 (3/0·91 %) | ≈ 4x | |
| 32 (1, SŠ) | 1·429 | 0·657 | 4x | ||
| 32 (2, KW) | 4x | ||||
| JP 102 | Honshu, Iwate, Iwate-gun, Kuzumaki-cho, Mt Sode-yama, Mabeshigawa-River, 915 m, 40°00·418′N, 141°32·277′E, coll. HK 04-501 & KM, 27 Jun. 2004 | 1·453 (1/–) | 0·653–0·654 (2/0·46 %) | ≈ 4x | |
| JP 1142 | Hokkaido, Kato-gun, Kamishihoro-cho, Shiishikaribetsugawa-River, 688 m, 43°19·428′N, 143°02·337′E, coll. HK 04-522 & KM, 03 Jul. 2004 | 1·483–1·494 (3/0·74 %) | 0·69–0·692 (3/0·29 %) | ≈ 4x | |
| JP 1152 | Hokkaido, Kato-gun, Kamishihoro-cho, Shiishikaribetsugawa-River, 969 m, 43°22·447′N, 143°02·247′E, coll. HK 04-524 & KM, 3 Jul. 2004, HK 05-041 JL & KM, 5 Jun. 2005 | 1·419–1·434 (3/1·06 %) | 0·662–0·667 (3/0·76 %) | ≈ 4x | |
| 32 (1, KW) | 4x | ||||
| JP 117 | Hokkaido, Kato-gun, Shikaoi-cho, Shikaribetsu, Yamadaonsen-Spa, 883 m, 43°18·583′N, 143°07·508′E, coll. HK 04-529 & KM, 04 Jul. 2004, HK 05-043 JL & KM, 5 Jun. 2005 | 1·451–1·571 (4/8·27 %) | 0·678–0·734, Fig. 3A, E (5/8·26 %) | ≈ 4x | |
| 32 (1, SŠ) | 1·451 | 0·679 | 4x | ||
| approx. 33–34 (1, SŠ) | 1·571 | 0·734 | 4x | ||
| 32 (3, KW) | 4x | ||||
| JP 130 | Hokkaido, Kato-gun, Shikaoi-cho, Shikaribetsu, Kannoonsen-Spa, 43°20·237 N, 143°03·312 E, coll. HK 05-042 & JL & KM, 07 Jun. 2005 | 32 (1, KW) | 4x | ||
| JP 116 | Hokkaido, Asyoro-gun, Asyoro-cho, Metoonsen-Spa, Nukanangawa-River, 458 m, 43°23·847′N, 143°19·305′E, coll. HK 04-526 & KM, 04 Jul. 2004 | 1·502–1·572 (3/4·66 %) | 0·695–0·724 (3/4·17 %) | ≈ 4x | |
| 32 (1, KW), Fig. 2G | 4x | ||||
| JP 118 | Hokkaido, Kato-gun, Kamishihoro-cho, Horokagawa-River, 774 m, 43°25·79′N, 143°07·493′E, coll. HK 04-531 & KM, 6 Jul. 2004, HK 05-044, JL & KM, 5 Jun. 2005 | 1·489–1·587 (2/6·58 %) | 0·687–0·737 (2/7·28 %) | ≈ 4x | |
| 33 (1, KW) | 4x | ||||
| 48 (1, KW) | 6x | ||||
| 07–817 | Hokkaido, Asyoru-gun, Asyoro-cho, along Kitoushi River, 2 km N of the entrance of the forest road along the river, approx. 510 m, 43°42·066 N, 143°2·300 E, coll. HK 07-817 & KW, 12 Aug. 2007 | 32 (1, KW) | 4x | ||
| Cardamine amaraeiformis Nakai | |||||
| K 3 | Korea, Gangwon-do, Pyeongchang-gun, Yongpyoeng-myeon, Nodong-ri, Mt Geybang, 840 m, 37°42·323′N, 128°29·125′E, coll. HK 05-065, JL & KM, 14 Jun. 2005 | 1·434–1·503 (3/4·81 %) | 0·668–0·7 (3/4·79 %) | ≈ 4x | |
| 32 (1, SŠ) | 1·434 | 0·668, Fig. 3C | 4x | ||
| 33 (1, KW), Fig. 2H | 4x | ||||
| 32 (3, KW) | 4x | ||||
| K 6 | Korea, Kyungsangnam-do, Macheo-myeon, Bakmundong, Mt Chiri, near Ganaeso Pokpo, 930 m, 35°20·529′N, 127°40·988′E, coll. HK 05-081, JL, KM & J.-H. Pak, 16 Jun. 2005 | 1·501–1·640 (3/9·26 %) | 0·696–0·763 (3/9·63 %) | ≈ 4x | |
| 34 (1, SŠ) | 1·640 | 0·755 | 4x | ||
| approx. 33–34 (1, SŠ) | 1·640 | 0·763 | 4x | ||
| 32 (3, KW) | 4x | ||||
| K 5A3 | Korea, Gangwon-do, Pyeongchang-gun, Mt Odaesan National Park, Wolcheong Temple, 950 m, 37°47·564′N, 128°33·661′E, coll. HK 05-070, JL & KM, 15 Jun. 2005 | 1·411–1·528 (2/8·29 %) | 0·663–0·705 (2/5·22 %) | ≈ 4x | |
| 32 (1, SŠ) | 1·411 | 0·67 | 4x | ||
| K 5B3 | Korea, Gangwon-do, Pyeongchang-gun, Mt Odaesan National Park, Wolcheong Temple, 720 m, 37°44·508′N, 128°35·135′E, coll. HK 05-072, JL & KM, 15 Jun. 2005 | 1·416–1·427 (2/0·78 %) | 0·663–0·665 (2/0·3 %) | ≈ 4x | |
| 32 (1, KW) | 4x | ||||
| Cardamine yezoensis Maxim. | |||||
| JP 133 | Honshu, Akita, Kitaakita-gun, Tashiro-machi, S foot of Mt Tashirodake, Usuichizana, 280 m, 40°23·002′N, 140°23·768′E, coll. JL, KM, H. Kato & Takuya Kikuchi, 22 Jun. 2005 (type locality of C. akitensis Mochizuki) | 2·3 (1/–) | 1·082 (1/–) | ≈ 11x | |
| 48 (1, KW) | 6x | ||||
| JP 134 | Honshu, Kitaakita-gun, Fujisato-machi, Daira-kyo, Ittori-bashi (bridge), 240 m, 40°23·748′N, 140°17·812′E, coll. JL, KM, H. Kato & Takuya Kikuchi, 22 Jun. 2005 | 2·329–2·362 (3/1·42 %) | 1·128–1·137 (2/0·8 %) | ≈ 11x | |
| 72 (1, KW) | 9x | ||||
| JP 135 | Honshu, Akita, Yamamoto-gun, Hachimori-machi, Mase-zawa (river), Ichinomata-zawa (a branch of Mase-zawa), 120 m, 40°25·005′N, 140°03·09′E, coll. JL, KM & H. Kato, 23 Jun. 2005 | 1·598–1·602 (2/0·25 %) | 0·762–0·77 (2/1·05 %) | ≈ 6x | |
| 48 (1, KW) | 6x | ||||
| JP 136 | Honshu, Akita, Nishitsugaru-gun, Iwasaki-mura, Juniko, Chojukannonbosatsu, 120 m, 40°33·763′N, 139°58·730′E, coll. JL, KM & H. Kato, 23 Jun. 2005 | 1·529–1·66 (3/8·57 %) | 0·785 (1/–) | ≈ 6x | |
| 48 (1, KW) | 6x | ||||
| JP 110 | Hokkaido, Kamiiso-gun, Kamiiso-cho, Moheji, Yunosawa-river, 96 m, 41°47·762′N, 140°32·405', coll. HK 04-515 & KM, 1 Jul. 2004 (type locality of C. yezoensis Maxim.) | 0·751–0·756 (3/0·67 %) | ≈ 6x | ||
| JP 109 | Hokkaido, Kamiiso-gun, Kamiiso-cho, Moheji, Todagawa-River, 99 m, 41°48·677′N, 140°32·372′E, coll. HK 04-512 & KM, 1 Jul. 2004, HK 05-021, JL & KM, 2 Jun. 2005 (type locality of C. yezoensis Maxim.) | 1·473–1·539 (4/4·48 %) | 0·71–0·743 (3/4·65 %) | ≈6x | |
| 48 (1, KW) | 1·475 | 6x | |||
| JP 111 | Hokkaido, Kayabe-gun, Mori-cho, Shimazakigawa-River, 236 m, 42°03·097′N, 140°29·67′E, coll. HK 04-517 & KM, 2 Jul. 2004, HK 05-025, JL & KM, 2 Jun. 2005 | 1·481 (1/–) | 0·722–0·734 (3/1·66 %) | ≈ 6x | |
| 48 (1, KW) | 6x | ||||
| JP 49 | Hokkaido, Yamakosi-gun, Yakumo-cho, Namarigawa-river, 220 m, 42°10·928′N, 140°07·843′E, coll. HK 03-316, 05-028, JL & KM, 24 Jun. 2003, 2 Jun. 2005 | 1·544–1·567 (3/1·49 %) | 0·743–0·759 (3/2·15 %) | ≈ 6x | |
| 48 (1, KW) | 6x | ||||
| JP 50 | Hokkaido, Setana-gun, Setana-cho, Babagawa-River, Kariba-keikoku, 200 m, 42°31·484′N, 139°54·569′E, coll. HK 03-318, 05-031, JL & KM, 24 Jun. 2003, 3 Jun. 2005 | 1·448–1·478 (3/2·07 %) | 0·715–0·726 (3/1·54 %) | ≈ 6x | |
| 48 (1, KW) | 6x | ||||
| JP 48 | Hokkaido, Yamakosi-gun, Osyamanbe-cho, Futamatagawa-river, 180 m, 42°34·893′N, 140°14·191′E, coll. HK 03-314, 05-033, JL & KM, 23 Jun. 2003, 3 Jun. 2005 | 1·544–1·583 (3/2·53 %) | 0·735–0·739 (3/0·54 %) | ≈ 6x | |
| approx. 47–48 (1, SŠ) | 1·583 | 0·739 | 6x | ||
| 48 (1, KW) | 6x | ||||
| JP 47 | Hokkaido, Abuta-gun, Toyoura-cho, Obokogawa-River, 130 m, 42°38·368′N, 140°34·922′E, coll. HK 03-312, 05-034, JL & KM, 23 Jun. 2003, 3 Jun. 2005 | 1·45–1·543 (3/6·41 %) | 0·716–0·741, Fig. 3F (3/3·49 %) | ≈ 6x | |
| JP 46 | Hokkaido, Isoya-gun, Rankoshi-cho, Shikaribetsugawa-River (a branch of Konbu-River), 165 m, 42°43·003′N, 140°33·655′E, coll. HK 03-309, JL & KM, 23 Jun. 2003 | – | 1·477–1·53 (4/3·59 %) | 0·707–0·728 (4/2·97 %) | ≈ 6x |
| 48 (1, KW), Fig. 2A | 1·508 | 0·711 | 6x | ||
| 48 (1, KW) | 6x | ||||
| JP 44 | Hokkaido, Iwanai-gun, Kyowa-cho, Inaho-pass, Shimatukenaigawa-River, 280 m, 43°03·022′N, 140°40·832′E, coll. HK 03-305, JL & KM, 22 Jun. 2003, HK 04–541 & KM, 6 Jul. 2004 | 1·403–1·512 | 0·687–0·739 | ≈ 6x | |
| 48 (3, KW) | 6x | ||||
| 2·051 (1/–) | 1·013 (1/–) | ≈ 10x | |||
| JP 112 | Hokkaido, Sorachi-gun, Minamifurano-cho, Kanayama. Tonashibetsugawa-River, 435 m, 43°06·872′N, 142°19·308′E, coll. HK 04-518, 04-521 & KM, 2 Jul. 2004, HK 05–052, JL & KM, 7 Jun. 2005 | 2·248–2·256 (2/0·36 %) | 1·1–1·101 (2/0·09 %) | ≈ 11x | |
| 2·052 (1/–) | 1·036 (1/–) | ≈ 10x | |||
| 1·736–1·786 (3/2·88 %) | 0·842–0·852 (2/1·19) | ≈ 8x | |||
| 1·407–1·437 (4/2·13 %) | 0·685–0·69 (2/0·73 %) | ≈ 6x | |||
| 48 (3, KW) | 6x | ||||
| 48 (1, KW) | 1·414 | 0·69 | 6x | ||
| JP 124 | Hokkaido, Otaru, Ranjima, Mochiyazawa-River, 141 m, 43°10·138′N, 140°51·975′E, coll. HK 04-539 & KM, 6 Jul. 2004, HK 05–058, JL & KM, 8 Jun. 2005 (type locality of C. fauriei Franch.) | 1·427–1·513 (4/6·03 %) | 0·674–0·707 (3/4·9 %) | ≈ 6x | |
| 48 (2, KW) | 6x | ||||
| 48 (1, KW) | 1·427 | 6x | |||
| JP 127 | Hokkaido, Atsuta-gun, Atsuta-mura, Kotan, Kotangawa-River, 91 m, 43°23·903′N, 141°30·162′E, coll. HK 04-547 & KM, 7 Jul. 2004, HK 05-057, JL & KM, 8 Jun. 2005 | 1·451 (1/–) | 0·707–0·72 (3/1·84 %) | ≈ 6x | |
| 48 (3, KW) | 6x | ||||
| JP 54 | Hokkaido, Ishikari-gun, Toubetsu-cho, Nibangawa-River, 300 m, 43°28·710′N, 141°39·694′E, coll. HK 03-326, JL & KM, 25 Jun. 2003 | 1·394–1·52 (3/9·04) | 0·675–0·722, Fig. 3F (3/6·96) | ≈ 6x | |
| JP 57 | Hokkaido, Utashinai-shi, Nishiyama, Pankeutashinaigawa-River, 260 m, 43°28·085′N, 142°02·787′E, coll. HK 03-333, 05-053, JL & KM, 26 Jun. 2003, 7 Jun. 2005 | 2·099 (1/–) | 1·024–1·039 (3/1·51 %) | ≈ 10x | |
| 1·397–1·515 (9/8·45 %) | 0·682–0·717 (8/5·13 %) | ≈ 6x | |||
| JP 51 | Hokkaido, Ishikari-gun, Toubetsu-cho, Sanbangawa-River, 210 m, 43°30·260′N, 141°37·172′E, coll. HK 03-320, 05-054, JL & KM, 25 Jun. 2003, 8 Jun. 2005 | 1·456–1·483 (3/1·85 %) | 0·703–0·705 (3/0·28 %) | ≈ 6x | |
| 48 (1, KW) | 6x | ||||
| JP 56 | Hokkaido, Hamamasu-gun, Hamamasu-mura, Okurige, 35 m, 43°30·837′N, 141°22·575′E, coll. HK 03-331, 05-056, JL & KM, 25 Jun. 2003, 8 Jun. 2005 | – | 2·053–2·094 (3/2 %) | 1·014–1·063, Fig. 3B (6/4·83 %) | ≈ 10x |
| 80 (1, JL) | 2·055 | 1·014 | 10x | ||
| 80 (1, SŠ) | 1·063 | 10x | |||
| 64 (2, KW) | 8x | ||||
| 72 (1, KW) | 9x | ||||
| JP 66 | Hokkaido, Nakagawa-gun, Bifuka-cho, Panke, Bifukapankegawa-River, 400 m, 44°34·122′N, 142°24·463′E, coll. HK 03-340, 05-045, JL & KM, 29 Jun. 2003, 6 Jun. 2005 | 48 (2, KW) | 6x | ||
| JP 120 | Hokkaido, Asahikawa-shi, Kami-ufun, along Ufun-gawa River, near the entrance of a forest road, 221 m, 43°40·943′N, 142°19·92′E, coll. HK 04-535 & KM, 5 Jul. 2004, HK 05-049, JL & KM, 7 Jun. 2005 (type locality of C. geifolia Koidz.) | 1·414–1·422 (2/0·57 %) | 0·683–0·69 (2/1·02 %) | ≈6x | |
| – | 1·008–1·073 (2/6·49 %) | ≈ 10x | |||
| 48 (4, KW) | 6x | ||||
| 72 (1, KW), Fig. 2B | 9x | ||||
| JP 67 | Hokkaido, Nakagawa-gun, Nakagawa-cho, Ohtomi, Kunneshirigawa-River, 20 m, 44°51·544′N, 142°4·274′E, coll. HK 03-342, 05-048, JL & KM, 30 Jun. 2003, 6 Jun. 2005 | 1·408–1·425 (2/1·21 %) | 0·68–0·692 (3/1·76 %) | ≈6x | |
| 48 (1, KW) | 1·425 | 0·689 | 6x | ||
| 48 (3, KW) | 6x | ||||
| JP 69 | Hokkaido, Sarufutsu-gun, Sarufutsu-mura, Sarufutsugawa-River, 20 m, 45°10·737′N, 142°8·744′E, coll. HK 03-346, 05-047, JL & KM, 30 Jun. 2003, 06 Jun. 2005 | 1·97–2·058 (4/4·77 %) | 0·995–1·003 (3/0·74 %) | ≈ 10x | |
| 2·47–2·528 (2/2·35) | ≈12x | ||||
| 80 (2, KW) | 10x | ||||
| 96 (1, KW) | 12x | ||||
| Cardamine schinziana O. E. Schulz | |||||
| JP 764 | Hokkaido, Urakawa-gun, Urakawa-cho, Shyumangawa-River, 160 m, 42°15·980′N, 142°56·749′E, coll. HK 03-366, 05-036, JL & KM, 4 Jun. 2005 | 1·363–1·469 (4/7·78 %) | 0·682–0·738 (4/8·21 %) | ≈ 6x | |
| 48 (2, KW) | 6x | ||||
| JP 1284 | Hokkaido, Urakawa-gun, Urakawa-cho, Shyumangawa-River, near the entrance of Shyuman forest road, a pond on private land (Sakuraoka), 70 m, 42°14·430′N, 142°55·980′E, coll. HK, JL & KM, 04 Jun. 2005 | 1·543–1·565 (2/1·43 %) | 0·754–0·755 (2/0·13 %) | ≈ 6x | |
| JP 75 | Hokkaido, Hiroo-gun, Hiroo-cho, Toyonigawa-River, 420 m, 42°22·476′N, 143°03·663′E, coll. HK 03-362, 05-038, JL & KM, 03 Jul. 2003, 04 Jun. 2005 | 1·423–1·493 (6/4·92 %) | 0·689–0·721 (5/4·64 %) | ≈ 6x | |
| 48 (3, KW) | 6x | ||||
| JP 129 | Hokkaido, Kawanishi-gun, Nakasatsunai-mura, upstream of Satsunaigawa river, I-no-sawa (a branch of Satsunaigawa river), 405 m, 42°35·158′N, 142°56·415′E, coll. HK, JL & KM, 04 Jun. 2005 | 1·426 (1/–) | ≈ 6x | ||
| 07–811 | Hokkaido, Urakawa-gun, Urakawa-cho, upstream of Motourakawa river, Bunkaiyama bridge, near the entrance of the forest road Motourakawa-rindo, approx. 120 m, 42°20·016′N, 142°46·766′E, coll. HK 07-811 & KW, 10 Aug. 2007 | 48 (1, KW), Fig. 2C | 6x | ||
| 07–805 | Hokkaido, Kamikawa-gun; Shimizu-cho, Meto, upstream of Meto river, near the entrance of the trail to Mt Meto-dake (Metodake-tozanguchi), approx. 610 m, 42°53·983 N, 142°47·766′E, coll. HK 07-805 & KW, 09 Aug. 2007 | 48 (1, KW) | 6x | ||
| JP 775 | Hokkaido, Shizunai-gun, Shizunai-cho, Shizunaigawa-River, 420 m, 42°30·051′N, 142°44·730′E, coll. HK 03-368, JL & KM, 04 Jul. 2003 | 2·008–2·081 (2/3·64 %) | 1·014–1·054 (3/3·88 %) | ≈ 8x | |
| 64 (1, KW), Fig. 2D | 2·008 | 1·014, Fig. 3D | 8x | ||
| JP 785 | Hokkaido, Shizunai-gun, Shizunai-cho, Shizunaigawa-River, 408 m, 42°27·913′N, 142°44·913′E, coll. HK 03-370, JL & KM, 04 Jul. 2003 | 1·374 (1/–) | 0·69 (1/–) | ≈ 6x | |
LITERATURE CITED
- Baack EJ. Cytotype segregation on regional and microgeographic scales in snow buttercups (Ranunculus adoneus: Ranunculaceae) American Journal of Botany. 2004;91:1783–1788. doi: 10.3732/ajb.91.11.1783. [DOI] [PubMed] [Google Scholar]
- Baack EJ. To succeed globally, disperse locally: effects of local pollen and seed dispersal on tetraploid establishment. Heredity. 2005;94:538–546. doi: 10.1038/sj.hdy.6800656. [DOI] [PubMed] [Google Scholar]
- Bennetzen JJ, Ma J, Devos KM. Mechanisms of recent genome size variation in flowering plants. Annals of Botany. 2005;95:127–132. doi: 10.1093/aob/mci008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain ML, Milligan BG, Strand AE. Long-distance seed dispersal in plant populations. American Journal of Botany. 2000;87:1217–1227. [PubMed] [Google Scholar]
- Carlsen T, Bleeker W, Hurka H, Elven R, Brochmann C. Biogeography and phylogeny of Cardamine (Brassicaceae) Annals of the Missouri Botanical Garden. 2009;96:215–236. [Google Scholar]
- Denda T, Yokota M. Cytogeography of Ixeris nakazonei (Asteraceae, Lactuceae) in the Ryukyu Archipelago of Japan and Taiwan. Journal of Plant Research. 2004;117:3–11. doi: 10.1007/s10265-003-0124-4. [DOI] [PubMed] [Google Scholar]
- Doležel J, Sgorbati S, Lucretti S. Comparison of 3 DNA fluorochromes for flow cytometric estimation of nuclear-DNA content in plants. Physiologia Plantarum. 1992;85:625–631. [Google Scholar]
- Doležel J, Greilhuber J, Suda J, editors. Flow cytometry with plant cells. Weinheim: Willey-VCH Verlag; 2007. [Google Scholar]
- Doležel J, Greilhuber J, Suda J. Estimation of nuclear DNA content in plants using flow cytometry. Nature Protocols. 2007;2:2233–2244. doi: 10.1038/nprot.2007.310. [DOI] [PubMed] [Google Scholar]
- Doron'kin VM. 23. Cardamine L. – Serdechnik. In: Malyshev LI, Peshkova GA, editors. Flora Sibiri 7. Novosibirsk: Nauka; 1994. pp. 78–84. [Google Scholar]
- Felber F. Establishment of a tetraploid cytotype in a diploid population: effect of relative fitness of the cytotypes. Journal of Evolutionary Biology. 1991;4:195–207. [Google Scholar]
- Felber F, Bever JD. Effect of triploid fitness on the coexistence of diploids and tetraploids. Biological Journal of the Linnean Society. 1997;60:95–106. [Google Scholar]
- Felber-Girard M, Felber F, Buttler A. Habitat differentiation in a narrow hybrid zone between diploid and tetraploid Anthoxanthum alpinum. New Phytologist. 1996;133:531–540. [Google Scholar]
- Fujii N, Senni K. Phylogeography of Japanese alpine plants: biogeographic importance of alpine region of Central Honshu in Japan. Taxon. 2006;55:43–52. [Google Scholar]
- Greilhuber J. Intraspecific variation in genome size: a critical reassessment. Annals of Botany. 1998;82(Suppl. A):27–35. [Google Scholar]
- Greilhuber J. Intraspecific variation in genome size in Angiosperms: identifying its existence. Annals of Botany. 2005;95:91–98. doi: 10.1093/aob/mci004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greilhuber J, Doležel J, Lysák M, Bennett MD. The origin, evolution and proposed stabilization of the terms ‘Genome size’, and ‘C-value’ to describe DNA contents. Annals of Botany. 2005;95:255–260. doi: 10.1093/aob/mci019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson K, Heard SB, Nason JD, Stireman JO., III Origins, distribution, and local co-occurrence of polyploid cytotypes in Solidago altissima (Asteraceae) American Journal of Botany. 2008;95:50–58. doi: 10.3732/ajb.95.1.50. [DOI] [PubMed] [Google Scholar]
- Harriman NA. The genus Dentaria (Cruciferae) in eastern North America. 1965 Unpublished PhD dissertation, Vanderbilt University, Nashville. [Google Scholar]
- Hegarty MJ, Hiscock SJ. Genomic clues to the evolutionary success of polyploid plants. Current Biology. 2008;18:R435–R444. doi: 10.1016/j.cub.2008.03.043. [DOI] [PubMed] [Google Scholar]
- Holmgren PK, Holmgren NH, Barnett LC. Index Herbariorum. Regnum Vegetabile. (8th edn) 1990;120:16–93. Part I: The Herbaria of the World Updates available on-line: http://sciweb.nybg.org/science2/IndexHerbariorum.asp . [Google Scholar]
- Hörandl E, Greilhuber J. Diploid and autotetraploid sexuals and their relationships to apomicts in the Ranunculus cassubicus group: insights from DNA content and isozyme variation. Plant Systematics and Evolution. 2002;234:85–100. [Google Scholar]
- Hotta M. History and geography of plants 3. Tokyo: Sanseido (in Japanese); 1974. [Google Scholar]
- Hufft Kao R. Asexuality and the coexistence of cytotypes. New Phytologist. 2007;175:764–772. doi: 10.1111/j.1469-8137.2007.02145.x. [DOI] [PubMed] [Google Scholar]
- Hufft Kao R. Origins and widespread distribution of co-existing polyploids in Arnica cordifolia (Asteraceae) Annals of Botany. 2008;101:145–152. doi: 10.1093/aob/mcm271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husband BC. The role of triploid hybrids in the evolutionary dynamics of mixed-ploidy populations. Biological Journal of the Linnean Society. 2004;82:537–546. [Google Scholar]
- Ikeda H, Senni K, Fujii N, Setoguchi H. Consistent geographic structure among multiple nuclear sequences and cpDNA polymorphisms of Cardamine nipponica Franch. et Savat. (Brassicaceae) Molecular Ecology. 2008;17:3178–3188. doi: 10.1111/j.1365-294X.2008.03821.x. [DOI] [PubMed] [Google Scholar]
- Jordon-Thaden I, Koch MA. Diversity patterns in the genus Draba: a first global perspective. Plant Ecology & Diversity. 2008;1:255–263. [Google Scholar]
- Koga K, Kadono Y, Setoguchi H. Phylogeography of Japanese water crowfoot based on chloroplast DNA haplotypes. Aquatic Botany. 2008;89:1–8. [Google Scholar]
- Kolář F, Štech M, Trávníček P, et al. Towards resolving the Knautia arvensis agg. (Dipsacaceae) puzzle: primary and secondary contact zones and ploidy segregation at landscape and microgeographic scales. Annals of Botany. 2009;103:963–974. doi: 10.1093/aob/mcp016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kron P, Suda J, Husband BC. Applications of flow cytometry to evolutionary and population biology. Annual Review of Ecology and Systematics. 2007;38:847–876. [Google Scholar]
- Kubátová B, Trávníček P, Bastlová D, Čurn V, Jarolímová V, Suda J. DNA ploidy-level variation in native and invasive populations of Lythrum salicaria at a large geographical scale. Journal of Biogeography. 2008;35:167–176. [Google Scholar]
- Kučera J, Valko I, Marhold K. On-line database of the chromosome numbers of the genus Cardamine (Brassicaceae) Biologia (Bratislava) 2005;60:473–476. (database available on-line at http://www.cardamine.sav.sk . [Google Scholar]
- Kurosawa S. Notes on chromosome numbers of Spermathophytes (3) Journal of Japanese Botany. 1981;56 [Google Scholar]
- Leitch IJ, Bennett MD. Genome downsizing in polyploid plants. Biological Journal of the Linnean Society. 2004;82:651–663. [Google Scholar]
- Levin DA. Minority cytotype exclusion in local plant populations. Taxon. 1975;24:35–43. [Google Scholar]
- Lihová J, Marhold K. Phylogenetic and diversity patterns in Cardamine (Brassicaceae) – a genus with conspicuous polyploid and reticulate evolution. In: Sharma AK, Sharma A, editors. Plant genome: biodiversity and evolution, vol. 1C: Phanerogams (Angiosperms – Dicotyledons) Enfield, NH: Science Publishers, Inc; 2006. [Google Scholar]
- Lihová J, Tribsch A, Marhold K. The Cardamine pratensis (Brassicaceae) group in the Iberian Peninsula: taxonomy, polyploidy and distribution. Taxon. 2003;52:783–802. [Google Scholar]
- Lihová J, Kučera J, Perný M, Marhold K. Hybridization between two polyploid Cardamine (Brassicaceae) species in northwestern Spain: discordance between morphological and genetic variation. Annals of Botany. 2007;99:1083–1096. doi: 10.1093/aob/mcm056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lövkvist B. The Cardamine pratensis complex. Outlines of its cytogenetics and taxonomy. Symbolae Botanicae Upsalienses. 1956;14/2:1–131. [Google Scholar]
- Lysák MA, Doležel J. Estimation of nuclear DNA content in Sesleria (Poaceae) Caryologia. 1998;52:123–132. [Google Scholar]
- Lysak MA, Koch MA, Beaulieau JM, Meister A, Leitch IJ. The dynamic ups and downs of genome size evolution in Brassicaceae. Molecular Biology and Evolution. 2009;26:85–98. doi: 10.1093/molbev/msn223. [DOI] [PubMed] [Google Scholar]
- Maceira NO, Jacquard P, Lumaret R. Competition between diploid and derivative autotetraploid Dactylis glomerata L. from Galicia. New Phytologist. 1993;124:321–328. doi: 10.1111/j.1469-8137.1993.tb03822.x. Implications for the establishment of novel polyploid. populations. [DOI] [PubMed] [Google Scholar]
- Maekawa F. Origin and characteristics of Japan's Flora. In: Numata M, editor. The flora and vegetation of Japan. Tokyo: Kodansha & Amsterdam: Elsevier; 1974. pp. 33–86. [Google Scholar]
- Malyshev LI, Peshkova GA, Baikov KC, editors. Flora Sibiri 14 – Dopolneniya i ispravleniya, alfavitnye ukazateli. Novosibirsk: Nauka; 2003. [Google Scholar]
- Manton I. Introduction to the general cytology of the Cruciferae. Annals of Botany. 1932;46:509–556. [Google Scholar]
- Marhold K, Lihová J. Polyploidy, hybridization and reticulate evolution: lessons from the Brassicaceae. Plant Systematics and Evolution. 2006;259:143–174. [Google Scholar]
- Minato M, Ijiri S. The Japanese archipelago. Ed. 3. Tokyo: Iwanami-shoten (in Japanese); 1984. [Google Scholar]
- Mráz P, Šingliarová B, Urfus T, Krahulec F. Cytogeography of Pilosella officinarum (Compositae): altitudinal and longitudinal differences in ploidy level distribution in the Czech Republic and Slovakia and the general pattern in Europe. Annals of Botany. 2008;101:59–71. doi: 10.1093/aob/mcm282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata J. Flora of Japan. In: Iwatsuki K, Kato M, editors. Floristic research (The Botany of Biodiversity I) Tokyo: University of Tokyo Press; 2000. 24–47 (in Japanese) [Google Scholar]
- Nishikawa T. Chromosome counts of flowering plants of Hokkaido (10) Journal of Hokkaido University of Education (Section II B) 1986;37:5–17. [Google Scholar]
- Okaura T, Quang ND, Ubukata M, Harada K. Phylogeographic structure and late Quaternary population history of the Japanese oak Quercus mongolica var. crispula and related species revealed by chloroplast DNA variation. Genes & Genetic Systems. 2007;82:465–477. doi: 10.1266/ggs.82.465. [DOI] [PubMed] [Google Scholar]
- Osborn TC, Pires JCh, Birchler JA, et al. Understanding mechanisms of novel gene expression in polyploids. Trends in Genetics. 2003;19:141–147. doi: 10.1016/s0168-9525(03)00015-5. [DOI] [PubMed] [Google Scholar]
- Otto F. DAPI staining of fixed cells for high-resolution flow cytometry of nuclear DNA. Methods in Cell Biology. 1990;33:105–110. doi: 10.1016/s0091-679x(08)60516-6. [DOI] [PubMed] [Google Scholar]
- Otto SP, Whitton J. Polyploid incidence and evolution. Annual Review of Genetics. 2000;34:401–437. doi: 10.1146/annurev.genet.34.1.401. [DOI] [PubMed] [Google Scholar]
- Pannell JR, Obbard DJ, Buggs RJA. Polyploidy and the sexual system: what can we learn from Mercurialis annua? Biological Journal of the Linnean Society. 2004;82:547–560. [Google Scholar]
- Pecinka A, Suchánková P, Lysak MA, Trávníček B, Doležel J. Nuclear DNA content variation among Central European Koeleria taxa. Annals of Botany. 2006;98:117–122. doi: 10.1093/aob/mcl077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probatova NS, Sokolovskaya AP. Chromosome numbers in vascular plants from Primorye territory, the Amur river basin, North Koryakia, Kamchatka and Sakhalin. Botanicheskii Zhurnal. 1988;73:290–293. (in Russian) [Google Scholar]
- Rothera SL, Davy AJ. Polyploidy and habitat differentiation in Deschampsia caespitosa. New Phytologist. 1986;102:449–467. [Google Scholar]
- Rudyka EG. Chromosome numbers in vascular plants from the southern part of the Soviet Far East. Botanicheskii Zhurnal. 1984;69:1699–1700. (in Russian) [Google Scholar]
- SAS Institute Inc. Cary, NC: SAS Institute; 2007. SAS OnlineDoc®version 9.1.3. (available online http://support.sas.com/onlinedoc/913/docMainpage.jsp. ) [Google Scholar]
- Schönswetter P, Lachmayer M, Lettner Ch., et al. Sympatric diploid and hexaploid cytotypes of Senecio carniolicus (Asteraceae) in the Eastern Alps are separated along an altitudinal gradient. Journal of Plant Research. 2007;120:721–725. doi: 10.1007/s10265-007-0108-x. [DOI] [PubMed] [Google Scholar]
- Schönswetter P, Suda J, Popp M, Weiss-Schneeweiss H, Brochmann Ch. Circumpolar phylogeography of Juncus biglumis (Juncaceae) inferred from AFLP fingerprints, cpDNA sequences, nuclear DNA content and chromosome numbers. Molecular Phylogenetics and Evolution. 2007;42:92–103. doi: 10.1016/j.ympev.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Shapiro HM. Practical flow cytometry. 3rd edn. New York: Wiley-Liss/John Wiley and Sons, Inc; 1995. [Google Scholar]
- Šmarda P, Bureš P. Intraspecific DNA content variability in Festuca pallens on different geographical scales and ploidy levels. Annals of Botany. 2006;98:665–678. doi: 10.1093/aob/mcl150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolovskaya AP. Geograficheskoe rasprostranenie poliploidnykh vidov rastenii (Issledovanie flory o. Sakhalina) Vestnik Leningradskogo Universiteta. 1960;1960(21):42–58. [Google Scholar]
- Soltis DE, Soltis PS, Tate JA. Advances in the study of polyploidy since plant speciation. New Phytologist. 2004;161:173–191. [Google Scholar]
- Soltis DE, Soltis PS, Schemske DW, et al. Autopolyploidy in angiosperms: have we grossly underestimated the number of species? Taxon. 2007;56:13–30. [Google Scholar]
- Soltis DE, Albert VA, Leebens-Mack J, et al. Polyploidy and angiosperm diversification. American Journal of Botany. 2009;96:336–348. doi: 10.3732/ajb.0800079. [DOI] [PubMed] [Google Scholar]
- Soltis PS, Soltis DE. The role of genetic and genomic attributes in the success of polyploids. Proceedings of the National Academy of Sciences ot the United States of America. 2000;97:7051–7057. doi: 10.1073/pnas.97.13.7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda J. Sympatric occurrences of various cytotypes of Vaccinium sect. Oxycoccus (Ericaceae) Nordic Journal of Botany. 2002;22:593–601. [Google Scholar]
- Suda J, Krahulcová A, Trávníček P, Krahulec F. Ploidy level versus DNA ploidy level: an appeal for consistent terminology. Taxon. 2006;55:447–450. [Google Scholar]
- Suda J, Weiss-Schneeweiss H, Tribsch A, Schneeweiss GN, Trávníček P, Schönswetter P. Complex distribution patterns of di-, tetra-, and hexaploid cytotypes in the European high mountain plant Senecio carniolicus (Asteraceae) American Journal of Botany. 2007;94:1391–1401. doi: 10.3732/ajb.94.8.1391. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Lumaret R. The evolutionary dynamics of polyploid plants: origins, establishment and persistence. Trends in Ecology and Evolution. 1992;7:302–307. doi: 10.1016/0169-5347(92)90228-4. [DOI] [PubMed] [Google Scholar]
- Tsuda Y, Ide Y. Wide-range analysis of genetic structure of Betula maximowicziana, a long-lived pioneer tree species and noble hardwood in the cool temperate zone of Japan. Molecular Ecology. 2005;14:3929–3941. doi: 10.1111/j.1365-294X.2005.02715.x. [DOI] [PubMed] [Google Scholar]
- Warwick SI, Al-Shehbaz IA. Brassicaceae: chromosome number index and database on CD-Rom. Plant Systematics and Evolution. 2006;259:237–248. [Google Scholar]
- Wendel JF. Genome evolution in polyploids. Plant Molecular Biology. 2000;42:225–249. [PubMed] [Google Scholar]
- Wendel JF, Cronn RC, Johnston JS, Price HJ. Feast and famine in plant genomes. Genetica. 2002;115:37–47. doi: 10.1023/a:1016020030189. [DOI] [PubMed] [Google Scholar]
- Yamaji H, Fukuda T, Yokoyama J, et al. Reticulate evolution and phylogeography in Asarum sect. Asiasarum (Aristolochiaceae) documented in internal transcribed spacer sequences (ITS) of nuclear ribosomal DNA. Molecular Phylogenetics and Evolution. 2007;44:863–884. doi: 10.1016/j.ympev.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Yoshino MM. Natural regions of Japan. GeoJournal. 1980;4.2:161–172. [Google Scholar]
- Zar JR. Biostatistical analysis. 4th edn. Upper Saddle River, NJ: Prentice Hall; 1999. [Google Scholar]