Abstract
Background and Aims
The root meristem of the Arabidopsis thaliana mature embryo is a highly organized structure in which individual cell shape and size must be regulated in co-ordination with the surrounding cells. The objective of this study was to determine the role of the AUX1 LAX family of auxin import carriers during the establishment of the embryonic root cell pattern.
Methods
The radicle apex of single and multiple aux1 lax mutant mature embryos was used to evaluate the effect of this gene family upon embryonic root organization and root cap size, cell number and cell size.
Key Results
It was demonstrated here that mutations within the AUX1 LAX family are associated with changes in cell pattern establishment in the embryonic quiescent centre and columella. aux1 lax mutants have a larger radicle root cap than the wild type and this is associated with a significant increase in the root-cap cell number, average cell size, or both. Extreme disorganization of the radicle apex was observed among quadruple aux1 lax1 lax2 lax3 mutant embryos, but not in single aux1 null or in lax1, lax2 and lax3 single mutants, indicating redundancy within the AUX1 LAX family.
Conclusions
It was determined that the AUX1 LAX family of auxin influx facilitators participates in the establishment of cell pattern within the apex of the embryonic root in a gene-redundant fashion. It was demonstrated that aux1 lax mutants are affected in cell proliferation and cell growth within the radicle tip. Thus AUX1 LAX auxin importers emerge as new players in morphogenetic processes involved in patterning during embryonic root formation.
Key words: AUX1 LAX genes, auxin, Arabidopsis thaliana, embryogenesis, meristem, radicle development, cell pattern establishment
INTRODUCTION
Cellular organization of the root apex has been widely studied as a model for understanding both plant development and evolution. Angiosperm evolution is associated with a diversification of root apical meristem (RAM) organization (Groot et al., 2004; Hemish and Seago, 2008); which is developmentally programmed throughout ontogenesis (Baum et al., 2002; Hemisch and Seago, 2008) and, at least during the early post-germination period, is directly dependent on how the radicle apex in the mature embryo is organized (Heimsch and Seago, 2008). Out of all the angiosperms, perhaps the best-studied species is Arabidopsis thaliana with its highly conserved organization of the radicle apex (Dolan et al., 1993; Scheres et al., 1994; Wenzel and Rost, 2001; Shishkova et al., 2008). Young roots in A. thaliana and other members of the Brassicacceae have closed apical meristem organization with three tiers or cell layers of initials, which are distinct for the stele, cortex–endodermis, root cap/protoderm and columella; all of them surrounding a group of central, relatively quiescent cells (quiescent centre, QC). In this work, arabidopsis was used as a model to understand the role of auxin cellular influx carriers during the establishment of root patterned cell proliferation during embryogenesis.
Arabidopsis thaliana embryogenesis involves a highly stereotyped sequence of cell divisions which has been characterized in detail by anatomical analysis and confirmed by lineage analysis (Dolan et al., 1993; Jurgens and Mayer, 1994; Scheres et al., 1994). The embryonic root (radicle) meristem specification process is first evident with the division of the hypophyseal cell at the basal pole of the globular embryo, considering the embryonic base as the pole attached to the maternal tissue and the apex located at the opposite pole (Friml et al., 2006). By early heart stage, the QC cells are established and they are already surrounded by the cells recruited as meristem initials. During torpedo and bent-cotyledon stages the derivatives of the hypophyseal cell keep dividing until they form five layers, the uppermost being relatively mitotically inactive (the QC), and the four basal layers forming the columella root cap (Dolan et al., 1993; Jurgens and Mayer, 1994; Scheres et al., 1994). In this paper, the focus is on the development of the basal pole of the mature embryo which includes the ‘apex of the mature radicle’. Therefore, from here on this term is used as the embryonic root apex constitutes the prospective RAM and root cap.
The arabidopsis embryonic RAM requires active transport of the signalling molecule auxin. This implies auxin relocation from its original source in order to establish a morphogenetic field in which gene expression in individual cells is modified in response to the auxin signal (Bhalearo and Bennett, 2003; Friml, 2003; Benkova et al., 2009). Unlike animal morphogens, auxin embryonic signalling requires dynamic transportation. This has been extensively analysed for the PIN-FORMED (PIN) family of auxin importers and for phosphoglycoprotein (PGP), which together define independent and complementary auxin transport mechanisms (Bandyopadhyay et al., 2007; Vieten et al., 2007; Mravec et al., 2008).
Specification of a single-cell-layered QC tier is a process that is dependent on dynamic auxin transport via the PIN family members PIN1, PIN4 and PIN7. At the early globular stage, protein localization of PIN1 and PIN7 is actively directed to the basal membranes of provascular cells and hypophysis, respectively (apical and basal cell membranes refer to their relative position in the whole embryo, and not relative to the root apex; Friml et al., 2006). At this stage, PIN4 starts its expression non-polarly at the hypophysis plasma membrane and at the basal membrane of the most apical suspensor cell. This PIN distribution is necessary to establish an auxin maximum gradient towards the basal end of the embryo (Steinmann et al., 1999; Aida et al., 2002; Friml et al., 2003; Petrasek et al., 2006; Dhonukshe et al., 2008; Kleine-Vehn et al., 2008). Genetic control of PIN expression and localization involve the transcription or RNA-processing control of the RS domain protein MERISTEM-DEFECTIVE (MD) (Casson et al., 2009) and intracellular relocalization via the membrane-associated ARF GEF protein EMB30/GNOM (Bush et al., 1996; Steinmann et al., 1999; Donaldson and Jackson, 2000; Friml et al., 2003; Geldner et al., 2003; Jurgens and Geldner, 2007; Kleine-Vehn et al., 2008; Tanaka et al., 2009) and the RAB5 endocytic pathway (Dhonukshe et al., 2008). The resulting auxin maximum indirectly activates the auxin response factor MONOPTEROS (MP/ARF5) via degradation of the AUX/IAA family member BODENLOS (BDL/IAA12) (Hamann et al., 1999, 2002, Weijers et al., 2006; Ploense et al., 2009). In a regulatory feedback loop, auxin-downstream activation of transcription factor activity is correlated with PIN1 accumulation at the basal plasma membrane of the provascular cells, promoting auxin flow into the hypophysis (Weijers et al., 2006). This auxin-directed flow is necessary, but not sufficient, for hypophysis specification. A still undetermined mobile signal downstream of MONOPTEROS (MP) is also required non-cell-autonomously for QC specification. MP activation is also required for the correct expression pattern of the AP2 class putative transcription factors PLETHORA1 (PLT1) and PLETHORA2 (PLT2) to the basal end of the globular embryo (Aida et al., 2004). The putative transcription factors of the GRAS family, SCARECROW (SCR) and SHORT ROOT (SHR) (Di Laurenzio et al., 1996; Bolle, 2004), are also required for localization and specification of QC cells (Sabatini et al., 2003; Aida et al., 2004). SCR is required non-cell-autonomously to promote SHR expression, and together they are involved in the specification of single-cell layers including the QC and one layer of the endodermis. SHR expression defines which PLT1- and PLT2-expressing cells acquire QC identity. Together, PLT1, PLT2, SCR and SHR are necessary to establish cell patterning of the embryonic RAM in which a single layer of QC cells is surrounded by initial cells capable of producing all apical root tissues (Aida et al., 2004).
Despite the well-documented participation of the PIN auxin efflux carriers during embryonic patterning, the role of dynamic auxin influx during this process remains unclear. Auxin cellular influx via AUX1 (AUXIN RESISTANT1) and its close homologues LAX1, LAX2 and LAX3 (LIKE AUXIN RESISTANT), which are transmembrane proteins with highly conserved permease activity, participate in many post-embryonic developmental processes such as gravitropism, phyllotaxis and lateral root formation (Marchant et al., 1999, 2002; Parry et al., 2001; Reinhardt et al., 2003; Swarup et al., 2005, 2008; Dubrovsky et al., 2006; Bainbridge et al., 2008; Laskowski et al., 2008). AUX1 was first characterized by Bennett et al. (1996) and its activity as an influx carrier with high affinity to auxin was demonstrated by its heterologous expression in Xenopus oocytes (Yang et al., 2006).
PIN1, PIN2 and AUX1 intracellular targeting requires internalization by endocytosis and targeting for degradation into lumenal vesicles of multivesicular bodies required for transport (ESCRT) machinery. Recently, Spitzer et al. (2009) analysed embryos in which PIN1, PIN2 and AUX1 are mislocalized to the vacuolar membrane in double-mutant embryos for the ESCRT-related CHARGED MULTIVESICULAR BODY PROTEIN/CHROMATIN MODIFYING PROTEIN1A (CHMP1A) and CHMP1B proteins. These embryos show limited polar differentiation and abnormal bilateral symmetry (Spitzer et al., 2009).
In this study, a novel function for the AUX1 LAX family of auxin influx carriers during the establishment of patterned cell proliferation was identified during development of the embryonic basal end. All aux1 lax single and multiple mutant lines contained embryos in which aberrant cell proliferation involved abnormal cell size, cell number or both. Furthermore, the aux1 missense alleles had defects in root cap cell pattern, which were also observed in the quadruple mutants. Interestingly, this latter effect was not associated with aux1 null alleles, or with lax1, lax2 or lax3 single mutant lines. Because the quadruple aux1 lax1 lax2 lax3 mutant line analysed in this study contains an aux1 null mutation, the results indicate that members of the AUX1 LAX family are required redundantly for establishment of correct cell organization in the radicle apex of arabidopsis.
MATERIALS AND METHODS
Plant growth conditions
For adult plant growth, seeds were soaked in sterile water at 4 °C for 3 d prior to being sown. Seeds were sown individually in P40 trays with F2 compost (John Innes), treated with Intercept. They were placed either in a greenhouse or in a Fisons cabinet with 16-h days (daytime conditions of 22 °C, 65 % humidity, 100 µmol light; night conditions of 17 °C, 22 % humidity). Seeds from dried plants were collected for mature embryo analysis.
Plant material
The wild-type (wt) accessions used during this project were Columbia (Col-0), Landsberg erecta (Ler), Wassilwskija (WS) RLD and C24. All of these lines were obtained from The European Arabidopsis Stock Centre (NASC). The following missense and null aux1 alleles were analysed as homozygotes (AT2G38120): the missense allele in Col-0 was aux1-7 (Pickett et al., 1990); the missense alleles in WS were aux1-104, aux1-105, aux1-111, aux1-112, aux1-113, aux1-114, aux1-116, aux1-117, aux1-11 and aux1-120 (Swarup et al., 2004); the null alleles in Col-0 were aux1-21 and aux1-22 (Roman et al., 1995); and the null allele in Ler was wav5-33 (Okada andShimura, 1990). Also previously identified insertion mutants for lax1 (AT5G01240), lax2 (AT2G21050) and lax3 (AT1G77690) (Swarup et al., 2008) were analysed, as well a quadruple homozygous mutant line for aux1-21 lax1 lax2 lax3 (Bainbridge et al., 2008).
Embryonic cell wall staining
Mature embryos were soaked in dH2O overnight. Seed coats were removed under a dissecting microscope using fine forceps and a 250-μm tungsten tip needle. Embryos dissected from the seed coat were transferred to 1·5-mL Eppendorf tubes containing 200–500 µL of fixative (50 % v/v methanol, 10 % v/v acetic acid). Embryos were stored overnight or up to 1 week in fixative at 4 °C. Fixative was removed and the sample was washed twice with water. The water was removed and the embryos incubated at room temperature for 30–45 min in 400 µL of 1 % periodic acid. Periodic acid was removed and the embryos were rinsed three times with water. Then, the water was removed and the embryos were incubated for 2 h in 200 µL of Schiff's reagent (1·9 g sodium metabisulfite, 97 mL water, 3 mL 5 n HCl) and 20 µL of 1 mg mL−1 propidium iodide. Embryos were rinsed with water and transferred to a drop of chloral hydrate (80 g chloral hydrate in 30 mL water) on a microscope slide. Excess chloral hydrate was removed using a P2 Gilson pipette, and the embryos were mounted in Hoyer's solution (30 g gum arabic, 200 g chloral hydrate, 20 g glycerol, 50 mL water). Mounted embryos were covered with cover slips of No. 1 thickness. Mountings were left to harden overnight before confocal imaging.
Microscopy and image analysis
Confocal microscopy was performed using a Zeiss LSM510 META upright confocal microscope. Propidium iodide was excited at 543 nm from the HeNE (1 mW) laser and collected through a 560LP emission filter. Optical transverse reconstructions were made from longitudinal optical sections using Zeiss LSM Image Browser software and Amira® (Indeed Visual Concepts, GmbH, Berlin) Root cap areas were measured using Zeiss AxioVision software. Finally, images were processed using Photoshop.
RESULTS
Radicle apical organization as a model of arabidopsis stereotypic cell architecture establishment
The organization of embryonic root apex in A. thaliana has so far been described in Col-0 genetic background showing a reproducible, relatively simple, and well-defined cell pattern (Dolan et al., 1993; Scheres et al.,1994). RAM organization in this accession is widely considered to be typical for A. thaliana. The organization of the radicle tip in Col-0 wt embryos was used as a reference model to evaluate the effect of mutations in the AUX1 LAX gene family on the embryonic root phenotype.
In order to characterize the cell architecture in the median longitudinal section of both wt and mutant radicle apex, high resolution optical confocal sections of the embryonic root tip were produced. Mature embryos were processed with a modified protocol for staining cell walls with propidium iodide using a periodic acid–pseudo-Schiff reaction (see Materials and methods). The fine resolution in the z-axis provided by confocal microscopy made it possible to discriminate between the cell walls from overlapping cell layers and cell walls from ectopic divisions within a single optical section. Furthermore, the possibility of creating transverse reconstructions from stacks of longitudinal sections made it possible to relate each cell from a given optical section with a specific cell file, thus making it possible to ensure that tissue boundaries were determined as accurately as possible using only cell positional information.
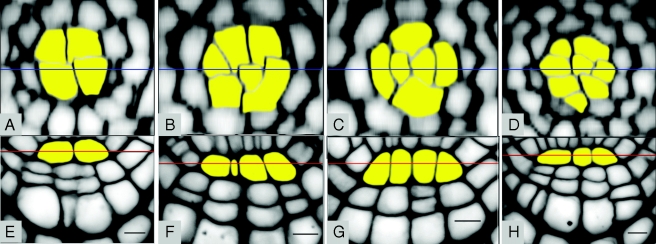
One visual landmark that we used to assess the embryonic RAM cell patterning was the QC organization. In Col-0, a group of two to four QC cells are observed in the median longitudinal optical section of mature wt embryos (Fig. 1E–H). Optical reconstructions of transverse sections of the QC (Fig. 1A–D) reveal that the four QC cells derived from the upper hypophyseal daughter cell during early globular stage (Scheres et al., 1994) can keep dividing, albeit slowly, to form a four to eight-celled QC in the mature embryo. The direction of these cell divisions is highly regulated, resulting mostly in anticlinal cell walls (i.e. cell walls which are perpendicular to the nearest root surface; Shishkova et al., 2008). Only one in 88 Col-0 embryos analysed during this study had a periclinal division within the QC (not shown).
Fig. 1.
Cellular organization of the radicle QC in Col-0 mature embryos. Stacks of longitudinal optical sections of Col-0 wt embryonic root apex were used to analyse the pattern of QC cells (yellow) in transversal reconstructed sections (A–D) and median longitudinal sections of this region (E–H). (A, E), (B, F), (C, G) and (D, H) pairs show each two optical sections from the same embryo. Transverse lines indicate the position of the corresponding perpendicular section, showing that the two images correspond to median longitudinal and transversal sections (respectively) of the QC. These images show a four-to-eight-cell variation in the QC (A–D), which is reflected in a two-to-four-cell variation in the median longitudinal section (E–H). Scale bars = 10 µm.
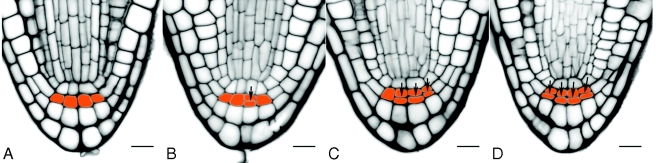
Columella cells are located basally to the QC (Fig. 2). Typically, as in the median longitudinal section shown in Fig. 2D, the columella is neatly organized into four columns and three tiers (T1, T2 and T3). The columella tier T1 is composed of initial cells that, during late embryogenesis, have undergone periclinal divisions. In mature embryos, some T1 cells have divided but not elongated (see examples in Fig. 2B–D). In 43 % of the Col-0 mature embryos analysed (n = 88), T1 consisted of eight cells, four of which were unelongated initials and the other four being their derivatives (as in Fig. 2D). Because in the rest of the embryos T1 was composed of both divided and undivided cells but both types were maintained positionally within the same tier (as in Figs 2A–C), in this study, T1 was considered to be one-to-two-cell layered.
Fig. 2.
Cellular organization of the columella in Col-0 mature embryos. The columella Tier 1 (T1), shown in orange, is located basally adjacent to the QC, and is composed of cells which by the mature embryo stage were in different stages of expansion and periclinal division. (A–D) Wild-type variation in the number of T1 cells that have completed periclinal divisions within T1. This columella tier can comprise a single-celled layer with fully elongated initials (A), a double-celled layer composed of four initials and their daughter cells (D), or any intermediate cell organization (B, C). The progeny of T1 cells that resulted from their periclinal divisions form the basal layers of mature columella cells, typically organized into four columns and two layers by the mature embryo stage. Arrowheads indicate the periclinal cell wall within T1. Scale bars = 10 µm
Compared with the typical Col-0 wt root cap organization, a wide range of variation was observed, both in the other accessions studied (Ler, Ws and RLD) and in the aux1 lax mutant embryos (in Col-0, Ler, Ws and RLD accessions). In order to quantify and interpret this variability, four qualitative criteria regarding cellular organization (described below) were identified that could be analysed in the median longitudinal sections of each embryo. Both wt and aux1-related variations were determined by evaluating each individual feature and assigning a non-parametric value for any given embryo. In Fig. 3, examples of the variability observed for each feature are shown both in the wt and the aux1 lax lines evaluated. The four features analysed were the following.
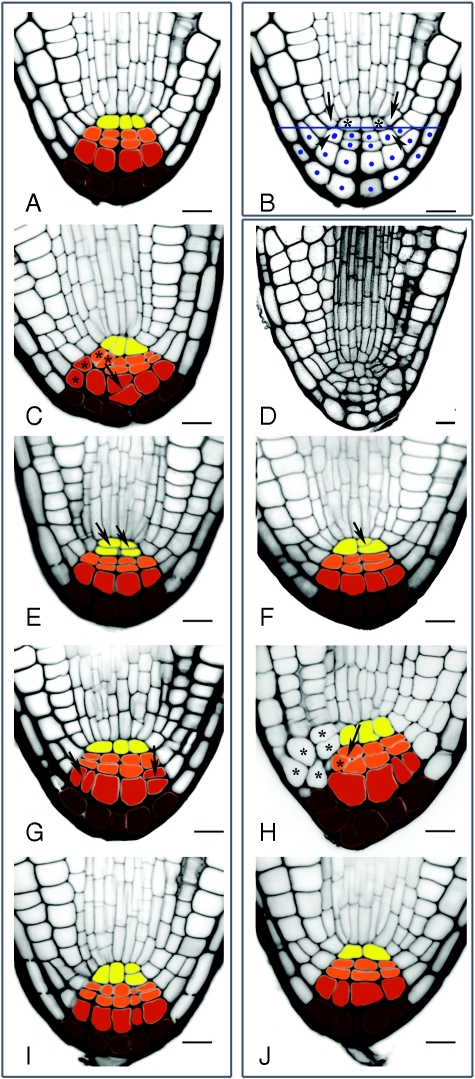
Fig. 3.
Parameters used for evaluation of the radicle apical organization and variations observed in wt and aux1 embryos. (A) QC and columella organization of the embryonic root median longitudinal section as described in Scheres et al. (1994): QC single-cell tier (yellow), columella tiers T1 (orange), T2 (red) and T3 (brown). (B) Criteria applied to delimit the root cap area analysed to estimate root cap size, root cap cell number and root cap cell area. The upper limit of the measured area was established by a transverse straight line intersecting the two meeting points between QC (asterisks), columella initials (arrowheads) and cortex–endodermis initials (arrows). Cells that were at least 50 % inside this area (dots) were included in the cell number and cell area analyses. Variations in radicle apical organization in different accessions and aux1 alleles are shown on (C–J) to illustrate the four traits evaluated. The left panel shows apices of the embryonic roots of various wt accessions (A, C, E, G, I) and the right panel shows those of aux1 embryos (D, F, H, J). (C, D) Embryos with abnormal radicle cap organization into columns and tiers: (C) wt embryo with ectopic cell division within T2 (arrow) and minor deviations from cell organization into columns and tiers (asterisks); (D) aux1 embryo in which the patterned cell organization is lost, resulting in tier boundaries undefined within the radicle apex; cells are not colour-coded as the pattern is not clearly defined. (E, F) Ectopic cell divisions within the QC (arrows). (G, H) Ectopic cell divisions within T1 and T2. (G) Wild-type embryo with ectopic divisions in T2 (arrows). (H) aux1 embryo with ectopic division in T1 (arrow). In the latter embryo columella boundaries are unclear and the surrounding cells have abnormal elongation patterns (compare cells with asterisks in H with equivalent lateral root cap cells in G). (I, J) Median longitudinal section showing deviations from the four-cell-column structure in T1 and T2. Accession backgrounds: (A, B, D, G), Col-0; (C, I), Ler; (E, F, H), Ws; (J), RLD. Scale bars = 10 µm.
(i) Degree of organization of the mature embryonic root apex into columns and tiers
Every wt embryo examined from the Col-0 and Ws ecotype (n = 98) showed a clear organization of the embryonic root cap into columns and tiers (Figs 2 and 3A). However, these embryos showed variability in the number of columella cell columns and in the orientation of cell divisions within the QC and columella tiers. In a minority of wt embryos from the Ler and Rld ecotypes (two out of 20), there was some degree of disorganization of root cap cells (compare C, E, G and I with A in Fig. 3). This effect was also seen in a minority of aux1 single mutant embryos (15 out of 249), and in these mutant embryos the disorganization was more severe (compare D with A in Fig. 3). In the aux1 embryos that exhibit this extreme root cap phenotype it is difficult to identify QC and columella boundaries based on cell position (exemplified in Fig. 3D).
(ii) Deviation from the organization of QC cells as a single-cell layer
With only one exception, all of the Col-0 wt embryos analysed (n = 88) showed exclusively anticlinal divisions within the QC (Figs 1 and 3A). However, variations were observed in the positioning of QC cell walls within embryos from different accessions (as in Fig. 3E). Although ectopic divisions within the QC were observed in both wt and aux1 embryos (Fig. 3E and 3F, respectively), it was impossible to establish a boundary between QC, columella T1, and other cell types in aux1 embryos with the more extreme phenotypes, suggesting abnormal cell proliferation and/or aberrant cell specification (Fig. 3D).
(iii) Deviation from the organization of columella cells within T1 and T2 into single-cell layers
In Col-0 wt embryos, cell lineage within the median longitudinal sections of TI and T2 can typically be traced to the four columella initials, which have undergone synchronized periclinal divisions resulting in single-celled T2 and T3 layers. However, this synchronization was not always tight, especially among embryos from RLD (Fig. 3G) and Ler (not shown) accessions. While minor deviations from this trait still maintain the overall T1 and T2 patterned cell organization, some stronger variations can compromise the establishment of columella boundaries (Fig. 3H).
(iv) Deviation from the organization of columella cells into four columns
In order to evaluate columella organization into cell columns, it was determined how many embryos from each sample did not have T1 and T2 cells aligned into four columns in the median longitudinal section (see examples in Fig. 3I and J). T3 cells were excluded from this analysis because a wide range of wt variation in the organization of these cells was observed (data not shown). Out of the four qualitative criteria evaluated, this is the most variable among wt embryos. However, variations in this trait may not reflect variation in the actual number of columns, but rather variation in cell size or cell number resulting in variation in the number of columns observed in the median longitudinal optical section. It was reasoned, therefore, that aux1 embryos in which T1 and T2 were not organized into four columns were associated with lines in which root cap size and cell proliferation were altered.
The frequency at which embryos showed variations in these four parameters was quantified within each line. Because each parameter was assessed independently, the sum of abnormalities quantified can be higher than the total number of embryos analysed for each line, or than the number of embryos showing deviations from the wt cell pattern.
Radicle apex cell pattern is variable between different arabidopsis accessions
In order to interpret the aux1 lax effect upon the root tip cell pattern, first it was evaluated whether the Col-0 radicle apex organization was conserved in other accessions including Ler, RLD and Ws (Table 1). While Col-0 and Ws are quite similar to the root cap architecture described initially for Col-0 embryos (Scheres et al., 1994), a higher degree of variability was observed in Ler and RLD embryos for all of the traits evaluated. Chi-squared analyses between the four accessions indicate that the number of embryos with ectopic divisions in the QC has the highest variability between accessions (P < 0·0005). The proportion of embryos with variable root basal organization into columns and tiers, ectopic cell divisions in T1 and T2, as well as with deviations from the columella four-column arrangement, also varies significantly (P < 0·05 for each trait). Examples of wt embryonic variation in root cap cell organization are shown in Fig. 3A, C, E, G and J.
Table 1.
Number of wt embryos with variations in radicle apex cell organization
| Accession | Root apex not organized into columns and tiers | Ectopic cell divisions in the QC | Ectopic cell divisions in T1 and T2 | T1 and T2 do not form four columns |
|---|---|---|---|---|
| Col-0 | 0/88 | 1/88 | 1/88 | 19/88 |
| Ler | 1/10 | 4/10 | 2/10 | 5/10 |
| RLD | 1/10 | 2/10 | 1/10 | 5/10 |
| WS | 0/10 | 2/10 | 0/10 | 0/10 |
| P | * | ** | * | * |
Significant differences between distributions in classes were determined using chi-squared test: *P < 0·05; **P < 0·0005.
In summary, significant differences between accessions in the number of embryos showing the typical Col-0 wt radicle apex cell pattern were observed. This wt variation indicates that, while the highly organized root cap from the Col-0 accession reflects tight embryonic regulation of patterned cell division and cell growth, faultless regulation is not essential for root cap development. These results also show that the interpretation of embryonic root apex phenotypes must take into account wt variation in the parental ecotypes. Accordingly, in this study, each of the aux1 lax lines was compared only with its respective parental accession.
Mutations in aux1 affect embryonic root cap organization, size, cell number and area
In order to characterize the effect of the aux1 mutation on root cap architecture a total of 141 single mutant embryos from different aux1 alleles were analysed. These embryos were divided into two groups, missense and null alleles, according to the nature of the aux1 mutation. To facilitate the analysis, random samples of embryos from different lines which shared both the ecotype and the genetic effect of their mutations (e.g. missense or null mutations) were pooled in the same sample. Examples of radicle apex phenotypes observed in aux1 embryos are shown in Fig. 3D, F, H and J.
Missense mutations in aux1 can result in abnormal embryonic apical root cell pattern
To assess the effect of missense aux1 mutations on embryonic root architecture embryos were analysed from lines representing 11 different alleles. All of these lines carry missense point mutations whose positions and effects on seedling root gravitropism had been analysed previously by Swarup et al. (2004). First, it was investigated whether aux1 embryonic defects correlated with specific point mutations within the AUX1 sequence, but significant differences between missense alleles from the same accession background were not found (data not shown). Thus, in order to increase the statistical robustness of this study, the embryo samples were pooled by accession background and only samples from the two accessions with less intrinsic wt variation, Col-0 and Ws, were analysed (see Table 1).
The missense allele that was analysed in the Col-0 background was aux1-7 (n = 21; Pickett et al., 1990). Ten aux1 missense alleles in the Ws background (n =10 for each) were also included: aux1-104, aux1-105, aux1-111, aux1-112, aux1-113, aux1-114, aux1-116, aux1-117, aux1-11 and aux1-120 (Swarup et al., 2004). The embryonic root apex characterization for these two samples is summarized in Table 2.
Table 2.
Number of wt and aux1 embryos with variations in radicle apex cell organization
| Accession | Alleles included in the sample | Root apex not organized into columns and tiers | Ectopic cell divisions in the QC | Ectopic cell divisions in T1 and T2 | T1 and T2 do not form four columns | |
|---|---|---|---|---|---|---|
| wt | Col-0 | wt | 0/88 | 1/88 | 1/88 | 19/88 |
| WS | wt | 0/10 | 2/10 | 0/10 | 0/10 | |
| missense aux1 embryos | Col-0 | aux1-7 | 8/21** | 6/21*** | 8/21*** | 16/21** |
| WS | aux1-104, aux1-105, aux1-111, aux1-112 aux1-113, aux1-114, aux1-116, aux1-117, aux1-118, aux1-120 | 3/100 | 15/100* | 9/100 | 34/100* | |
| null aux1 embryos | Col-0 | aux1-21, aux1-22 | 0/20 | 0/20 | 0/20 | 9/20* |
| aux/lax single mutant embryos | Col-0 | aux1-21 | 0/10 | 0/10 | 0/10 | 5/10* |
| Col-0 | lax1 | 0/10 | 0/10 | 0/10 | 2/10 | |
| Col-0 | lax2 | 0/10 | 0/10 | 0/10 | 2/10 | |
| Col-0 | lax3 | 0/10 | 0/10 | 1/10 | 0/10 | |
| aux/lax quadruple mutant embryos | Col-0 | aux1-21 lax1 lax2 lax3 | 4/68* | 4/68* | 7/68* | 26/68* |
Significant differences between distributions in classes were determined using chi-squared test by comparing each sample with the respective wt accession: *P < 0·05; **P < 0·005; ***P < 0·0005; ns, not significant.
In both the Col-0 and Ws aux1 missense samples embryos were found, albeit a minority, in which root apex cells were not organized into columns and layers (38 % and 3 % for aux1 lines in Col-0 and Ws backgrounds, respectively). An example of this phenotype is shown in Fig. 3D. The number of embryos in which we observed this abnormality was only significantly higher in the aux1 sample in the Col-0 background (Col-0 n =88; aux1 in Col-0 background n =21, chi-squared P < 0·0005; Ws n =10; aux1 in Ws background n =100, chi-squared P > 0·05). However, disorganization of the apex was not observed, either in Col-0 or in Ws wt embryos, which suggests that this is likely to be an aux1-related phenotype with low expressivity.
In the aux1 missense sample, a significant increase was also identified in the number of embryos with ectopic QC divisions (see example in Figs 3E and IF; Col-0 wt vs. aux1 missense in Col-0 background: chi squared P < 0·0005; WS wt vs. aux1 missense in Ws background, chi-squared P < 0·05) and in the number of embryos in which T1 and T2 cells were not aligned into four columns (see example in Fig. 3J, Col-0 wt vs. aux1 missense in Col-0 background, chi squared P < 0·005; WS wt vs. aux1 missense in Ws background, chi-squared test P < 0·05). A significant increase in the number of embryos with ectopic cell divisions within T1 and T2 was only observed in the aux1 sample in the Col-0 background (chi-squared P < 0·0005).
As proposed above, aux1-related variation in the number of cell columns visible within T1 and T2 indicates an effect upon the overall number of cells in the radicle apex. In order to analyse whether AUX1 is involved in the regulation of cell proliferation in the embryonic root tip, a region within the root cap median longitudinal section was established which could be delimited by visible landmarks both in the wt and in the aux1 mutant embryos. In some embryos with extreme aux1 phenotypes, the boundaries of the QC and columella are not clear (see above). However, even in these embryos it is possible to identify the cortex–endodermis initials positioned at each side of the QC, and these cells were used as landmarks to delimit a root cap area to be analysed in all embryos (Fig. 3B). In order to establish an upper limit that would be comparable in all the analysed embryos, even in those in which the boundaries of the columella are not clear, the two points were used where QC, columella and cortex–endodermis initials meet to position a straight line across the root cap (Fig. 3B). To analyse embryonic root cap cell numbers, all cells visible within this delimited area were counted. Cells that were partially inside the selected area were included if at least half of the visible cell was within this area. Mean cell size was estimated by dividing the number of cells within the selected root cap region by the measured area of this region. The area selected for quantitative analysis includes cells belonging to different tissues, ensuring that any observed effects would reflect the whole radicle tip rather than only one specific cell type. An alternative strategy could have involved analysing only cells from one specific cell type (i.e. cells with less inherent size variability), but this would not have been possible in embryos in which abnormal cell pattern makes it impossible to identify cell types using only positional information.
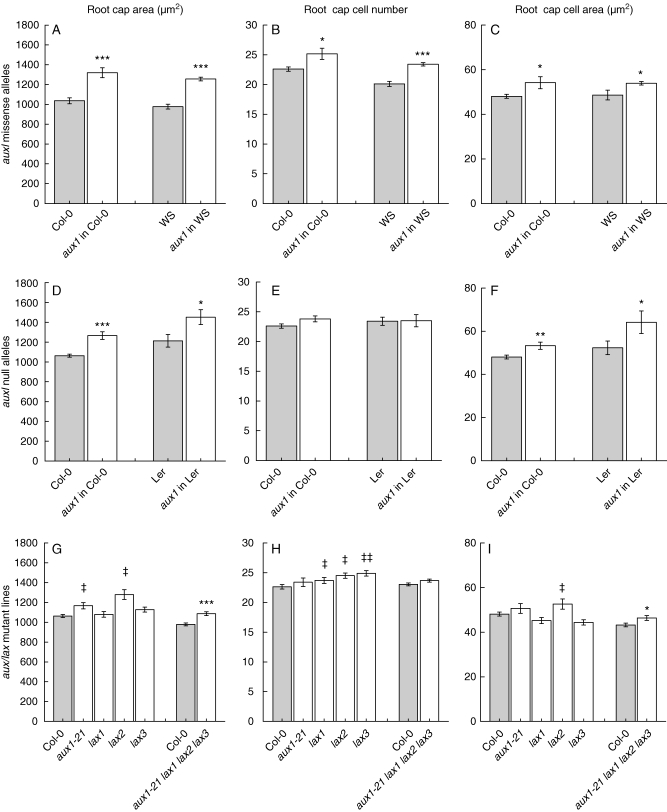
Quantitative analysis of the embryonic root cap effect associated with missense aux1 mutations (Fig. 4A–C) indicated that the root cap areas (n = 21 and n = 100, respectively, for aux1 alleles in Col-0 and Ws ecotypes) were significantly larger than their wt controls (n = 38 and n = 10, respectively, for wt Col-0 and Ws) (t-test P < 0·0005, both ecotypes). This increase in root cap size is correlated with an increase in the number of cells (t-test P < 0·05 and P < 0·0005, respectively), and with an increase in the average area of the cells (t-test P < 0·05, both ecotypes). These results are consistent with our hypothesis that an increase in the number of embryos lacking columella T1 and T2 organized into four columns (as seen in median longitudinal section) is an indicative feature of abnormal root cap size and cell number.
Fig. 4.
aux1 lax effect on embryonic root cap size and cell proliferation. In all cases, n varies between 10 and 100. Shaded bars represent the respective wt accession with which each group was compared. Error bars show the s.e.: *, P < 0·05 t-test; **, P < 0·005 t-test; ***, P < 0·0005 t -test; ‡, P < 0·05 Tukey's post hoc test; ‡‡, P < 0·005 Tukey's post hoc test.
In summary, the aux1 missense mutations affected root cap cell pattern, orientation of divisions in cell tiers, including QC, T1 and T2, and the number of cell columns in the columella that are aligned in the median longitudinal section. These mutations were also responsible for increased root cap size, and this effect was correlated with increases in the number and size of cells within this zone.
aux1 mutations that result in nonsense-mediated mRNA degradation have a subtle effect on embryonic root cap cell pattern
The single mutants for aux1 missense alleles provided evidence of the participation of AUX1 during the establishment of embryonic root architecture. However, this effect was subtle and it was expected that a stronger effect would be found in aux1 null alleles. To investigate this further, a group of previously characterized aux1 null alleles including aux1-21 and aux1-22 (Col-0) (Roman et al., 1995; Swarup et al., 2004) and wav5-33 (Ler) (Okada and Shimura, 1990) was included. These lines carry point mutations that result in premature stop codons, resulting in nonsense mediated mRNA degradation (Marchant and Bennett, 1998). It was reasoned that the lack of aux1 expression might result in a higher penetrance of the phenotype lacking root cap cell organization, and would help in elucidating the role of AUX1 in cell proliferation. Embryos were grouped in two samples according to their respective Col-0 and Ler accession backgrounds. The Col-0 aux1 sample included the alleles aux1-21 and aux1-22 (n = 10 each) and the Ler aux1 sample included the wav5-33 line (n = 10).
Contrary to expectations, the Col-0 background null aux1 embryos developed a far more organized columella than their missense counterparts (Table 2). One hundred per cent of embryos showed columella organization into columns and layers without ectopic divisions within the QC and columella tiers. The only significant qualitative difference between these embryos and the corresponding Col-0 wt controls was an increase in the number of embryos in which columella cells were not organized into four columns (16 % for Col-0 and 45 % for aux1 lines in Col-0 background) (Col-0 n = 38; aux1 in Col-0 background n = 20, chi-squared test P < 0·05). This effect on root cap cell pattern has been shown to be correlated with variations in root cap size and the size or number of cells within the root tip, rather than an aux1 effect upon cell organization (see above). It was not possible to draw conclusions about the effect of the aux1 mutation on root cap organization for the null allele in Ler because of the high variability of the wt control, combined with the small available sample size (not shown). However, quantitative analysis of the root cap from these two groups (i.e. null alleles in Col-0 and Ler) is consistent with the aux1-related effect observed in the null allele in Col-0 (Fig. 4D–F) as shown below.
As anticipated, quantitative evaluation of the columella phenotype for the null alleles in the Col-0 and Ler backgrounds (Fig. 4D–F) showed that the observed variation in root cap organization is correlated with an increase in the measured root cap area (t-test P < 0·0005 and P < 0·05 for the null alleles in Col-0 and Ler, respectively). Furthermore, within the measured area, only the individual cell area was significantly increased (t-test P < 0·005 and P < 0·05 for each group, respectively), while the number of cells was not significantly affected (t-test P > 0·05, both groups), supporting the hypothesis that the altered number of cell columns observed in the median longitudinal sections of T1 and T2 indicated variations in the alignment of cell files, in this case due to increased cell sizes.
In summary, the root cap cell patterning of aux1 null alleles does not appear to be significantly affected. These embryos differ from their wt counterparts only in root cap size and in root cap mean cell area, and not in root cap cell number.
Taken together, the analyses of the aux1 missense alleles and the aux1 null alleles indicate that AUX1 is involved in the establishment of a patterned embryonic root cap and that mutations in the aux1 gene result in variations in root cap size, cell number and area. Interestingly, missense aux1 mutations result in a more pronounced effect on root cap cell organization than null aux1 mutations in which the mRNA is subjected to nonsense-mediated degradation. It is possible that the absence of AUX1 triggers the redundant function of other related proteins, partially rescuing the aux1 mutant phenotype. Therefore, embryos carrying single and multiple mutations in the close homologues aux1, lax1, lax2 and lax3 were examined.
Members of the AUX1 LAX family participate in the establishment of embryonic root cap organization in a gene-redundant fashion
The comparison of the effects of mutations in AUX1 in lines carrying missense alleles or null alleles suggested gene redundancy. In order to determine whether the AUX1 LAX family members have a redundant effect on radicle root cap organization, the organization of cells in the embryonic basal root was evaluated in lines carrying single mutations in aux1-21 (null), lax1, lax2 and lax3 (Swarup et al., 2008), as well as in a quadruple aux1-21 lax1 lax2 lax3 mutant line (Bainbridge et al., 2008). All of these lines were generated in the Col-0 background.
As discussed above, the typical aux1-21 embryonic root cap is highly organized (Table 2), and this is also the case for all the lax1, lax2 and lax3 single mutant embryos (Table 2; n = 10 for each line). None of these embryos had undefined columns or layers within the columella, or ectopic cell divisions within the QC and columella T1 and T2 (except one out of ten lax3 embryos containing an ectopic cell division in T2). Variability in the number of columns observed in T1 and T2 was found in the wt control and in the mutant lines, but there was no significant difference among them (chi-squared test P > 0·05).
The evaluation of cell organization in the radicle apex of the quadruple mutant (aux1-21 lax1 lax2 lax3) embryos is summarized in Table 2. The Col-0 and aux1-21 lax1 lax2 lax3 samples included the pooled embryos from two independent experiments. When compared with the Col-0 wt sample (n = 88), a significant increase in the number of quadruple mutant (aux1-21 lax1 lax2 lax3) embryos (four out of 68) in which the organization of the QC and columella pattern was lost (chi squared test P < 0·05) were found. There was also a significant increase in the number of embryos with deviations from the Col-0 wt cell organization in all the other qualitative parameters assessed, including more embryos with ectopic cell divisions in the QC and columella tiers (T1 and T2), and abnormal numbers of cell columns within T1 and T2 (chi-squared test P < 0·05 for each parameter evaluated). The ectopic cell divisions observed only in the multiple line (i.e. not present in the single mutant aux1 lax embryos or in the null aux1-21 embryos) (total n = 40) resemble the aux1-related effects observed in embryos with the missense allele in Col-0 (n = 21). These results are consistent with the hypothesis of a gene-redundant effect between the AUX1 LAX family on embryonic root cap cell pattern, which is favoured in the null, rather than the missense aux1 single mutant alleles.
Quantitative analysis of the aux1 lax single mutant embryos, shown in Fig. 4G–I, indicated that the mutations in aux1-21 and lax2 genes result in an increased root cap area (Tukey's post hoc test P < 0·05). All of the lax1, lax2 and lax3 single mutant lines showed significantly more cells in this area (Tukey's post hoc test for lax1 and lax2 lines, P < 0·05; for lax3 line, P < 0·005). However, only in the lax2 line was this effect correlated with a significant increase in average cell area (Tukey's post hoc test P < 0·05). Analysis of the quadruple mutant line (aux1-21 lax1 lax2 lax3) indicated that these embryos had significantly larger root caps (t-test P < 0·0005) which correlated with significantly larger cell areas (t-test P < 0·05), but not with an increase in cell number (t-test P > 0·05). These results indicate variation in the effects of single members of the AUX1 LAX family on the embryonic root cap. They also indicate that when all the aux1-21, lax1, lax2 and lax3 are mutated in the same line, the effects on the root cap are stronger than in the single mutants for these alleles and similar to those observed for the missense alleles.
DISCUSSION
Auxin displays some morphogen characteristics during plant developmental programmes because it establishes positional information crucial for pattern formation (Bhalearo and Bennett, 2003; Benkova et al., 2009). Active distribution of this plant hormone during embryogenesis is a key regulator during embryonic root development. Previous studies have focused mainly on the dynamic expression of the PIN family and on the antagonistic participation of its members with PGP in the process of establishing auxin gradients that provide cells with positional information to establish the embryonic shoot and root apical meristems (Steinmann et al., 1999; Aida et al., 2002; Friml et al., 2003; Blilou et al., 2005; kshe et al., 2008; Mravec et al., 2008). Here it is demonstrated that mutants of AUX1 LAX gene family display abnormal phenotypes in the embryonic root organization, which provides evidence that carrier-mediated auxin influx is required for the establishment of the cell pattern in the radicle apex. The results indicate that mutations within this protein family can result in abnormal embryonic QC and columella cell organization. aux1 lax mutant lines also have embryos with increased cell proliferation resulting in larger root caps, a feature that correlates with significant increases in columella cell size, cell number or both. Therefore, novel evidence is provided for the requirement of active auxin influx, in addition to the distribution of auxin directed via the PIN and PGP proteins, in order to control the relationship between cell division and cell expansion, as well as directional orientation of cell growth and division during embryonic root meristem establishment.
Active auxin influx is required during embryonic root apical cell pattern establishment
So far, the characterized mutations affecting embryonic root cap pattern either impact on QC positioning by disrupting establishment of the hypophysis or by affecting proliferation of specific subsets of initials. In the case of the aux1 lax mutant embryos, the RAM is positioned correctly (as interpreted by cell position), but cell proliferation, as well as cell wall positioning, is abnormal in the QC, columella initials and columella differentiated cells (i.e. derivatives of columella initials formed during late embryogenesis). Although attention was focused only on these cell types, the presence of abnormally shaped lateral root cap cells (exemplified in Fig. 1F) suggests that the mutant phenotype was not restricted to these cell types. QC position and cell proliferation are regulated by a root apical auxin maximum established by regulated temporal and spatial expression of the PIN proteins (Sabatini et al., 1999; Steinmann et al., 1999; Aida et al., 2002; Friml et al., 2003; Blilou et al., 2005). The auxin-dependent process necessary for correct hypophysis specification and establishment of the QC position requires the concerted action of the GRAS family transcription factors SHR and SCR (Sabatini et al., 2003; Aida et al., 2004) and non-cell autonomous signals regulated by the interaction of the auxin-responsive transcription factors MP/ARF5 and BDL/IAA12 (Hamann et al., 2002; Hardtke et al., 2004; Weijers et al., 2006; Ploense et al., 2009). Stem cell niche maintenance involves action of RBR and WOX5 downstream of SCR (Wildwater et al., 2005; Sakar et al., 2007; Song et al., 2008) in parallel with members of the AP2 family of transcription factors PLT1, PLT2, PLT3 and BBM (Aida et al., 2004; Blilou et al., 2005; Galinha et al., 2007). However, once the QC is established, the mechanism responsible for cell cycle regulation in these cells, which are characterized by lower proliferative activity than the surrounding initials, is not yet understood. QC cell division orientation is correlated with post-embryonic maintenance of closed and open organization of the root meristem (Groot et al., 2004). Orientation of the cell plate is better understood in the root cap/protoderm initials, where the nuclear NAC domain proteins FEZ and SMB antagonistically interact to regulate alternation between formative and proliferative divisions and to regulate root cap cell proliferation (Willemsen et al., 2008). The present analysis demonstrates that transverse cell divisions within the upper descendants of the hypophysis are rare in Col-0 wt embryos but they are relatively common in embryos from other accessions, especially in Ler background, where overall organization of the columella cells is variable. aux1 missense mutant lines in Col-0 background and aux1 lax1 lax2 lax3 quadruple mutants (also in Col-0) include embryos that develop QC cells with periclinal and/or oblique ell divisions, implicating auxin influx in the regulation of orientation of cell division.
Previous analysis showed that aux1-22 mRNA is subjected to nonsense-mediated degradation (Marchant and Bennett, 1998), while the missense aux1 alleles produce loss of function or partial loss of function aux1 proteins. Furthermore, the Col-0 allele analysed (aux1-7) has been demonstrated to be translated and transported properly to its wt cellular location during post-embryonic root development (Swarup et al., 2004). Contrary to expectations, the null aux1 mutants (including aux1-22), did not have the ectopic cell divisions within the QC and columella that were present in the missense aux1 embryos. However, when a quadruple mutant line containing the null aux1-22 in a lax1 lax2 lax3 background was analysed these ectopic cell divisions were found. These results indicate that the AUX1 LAX genes can influence cell patterning redundantly in the embryonic root apex. A possible implication of the present results is that the redundant rescue of the aux1 phenotype by the LAX1, LAX2 and LAX3 gene products requires substantially lower levels of aux1 mutant protein than the levels observed in aux1-7, which could indicate that aux1-7 interferes with LAX protein function or that lack of AUX1 triggers ectopic expression of the LAX homologues. The expression of AUX1 LAX gene family during embryogenesis has not been characterized, and its analysis is necessary to understand further the functional relationships within this family during embryonic root cell pattern establishment. However, these results are consistent with a redundant role for the AUX1 LAX proteins, previously demonstrated during the stabilization of shoot phyllotactic pattern (Bainbridge et al., 2008).
The AUX1 LAX influx facilitators participate in the regulation of root-cap cell proliferation
Embryos from all of the lines analysed, including aux1 missense and null alleles, lax1, lax2 and lax3 single mutants and aux1 lax1 lax2 lax3 quadruple mutant, had significantly larger root caps than the wt controls (Fig. 4G). This aux1 lax effect was correlated with an increase in columella cell size, columella cell number or both, providing evidence that auxin influx via the AUX1 LAX carriers participate in the regulation of embryonic root cap cell proliferation. Recently, studies of BBM target genes provided evidence of a pathway relating auxin-regulated master regulator activity to a complex network of developmental pathways associated with cell proliferation and growth (Passarinho et al., 2008), but the direct effect of auxin on cell expansion during embryogenesis has not yet been addressed. However, experiments in tobacco leaves and tobacco BY2 cells have demonstrated that auxin induces cell expansion in a dose-dependent manner by inducing ion flux modifications through the plasma membrane, hence modifying activity of H+ transporters (Napier et al., 2002; Chen et al., 2001a, b; Rober-Kleber et al., 2003). This modifies cell wall extensibility by regulating apoplastic acidity (Funke and Edelmann, 2000; Vissenberg et al., 2001; Hager, 2003; Rober-Kleber et al., 2003; Li et al., 2005). In tobacco cells, localized auxin has also been shown to induce microtubule reorientation in order to promote cell elongation at the opposite side of the cell (Vissenberg et al., 2001). Another auxin-related mechanism to control cell volume acts by regulating the duration of G1 and G2 cell cycle phases. Auxin acts at two checkpoints controlling the transitions to S and M phases. Studies on synchronized tobacco cells have demonstrated that by blocking ABP1 (auxin binding protein 1) activity at the S checkpoint, cells grow more than the untreated controls because the G2 phase is extended. On the contrary, ABP1 inactivation at the M checkpoint results in inhibition of post-mitotic cell expansion and de-synchronization of cell growth with cell division (Chen et al., 2001a, b; David et al., 2007). It is possible that an impairment in auxin influx via the AUX1 LAX carriers results in a temporal increase in the free auxin available in the apoplastic space, which in turn induces cell expansion. Interestingly, in the null alleles which maintain wt QC and columella cell organization, an increase in root cap area is correlated only with an increase in root cap cell number, whereas in the missense and mutant alleles (in which some of the embryos had ectopic QC and columella cell divisions), an increase in root cap area is correlated both with an increase in root cap cell number and with root cap cell area. These results suggest that the co-ordination of embryonic root cap cell expansion and cell division may be regulated by two independent mechanisms. While a reduction in auxin influx could result in extra apoplastic free auxin capable of inducing cell expansion in all the aux1 missense and aux1 null lines, the redundant effect of the LAX homologues could be sufficient to maintain wt cell division plate orientation of QC and columella cells.
Carrier-driven auxin influx is required during radicle meristem development
There is no previous evidence for the requirement of carrier-driven auxin influx during embryogenesis. According to the chemiosmotic model, protonated auxin can permeate freely into cells (Kramer and Bennett, 2006), but it is deprotonated in the cytoplasm making necessary the local permeabilization of the membrane by PIN and PGP (Chen et al., 1998; Luschnig et al., 1998; Petrasek et al., 2003, 2006; Geisler et al., 2005; Bouchard et al., 2006; Blakeslee et al., 2007, Grieneisen et al., 2007) to facilitate auxin efflux. During developmental processes where steep auxin gradients are necessary, auxin influx mediated by the AUX1 LAX family is necessary to prevent passive diffusion of the gradients generated via auxin carrier-driven efflux (Kramer, 2004, 2008; Swarup et al., 2005; Kramer and Bennett, 2006). Active auxin influx by members of the AUX1 LAX family has been proven to be required during post-embryonic developmental events such as root gravitropic response, lateral root emergence and phyllotaxis (Swarup et al., 2005, 2008; Bainbridge et al., 2008). Here, evidence is presented for a novel participation of the AUX1 LAX family of auxin influx carriers that are required, in addition to polar efflux facilitators, during embryonic root development. We propose a possible scenario in which rapid auxin influx mediated by the AUX1 LAX proteins limits the availability of free apoplastic auxin, thereby inhibiting uneven induction of columella cell expansion. Simultaneously, the AUX1 LAX family members stabilize redundantly the auxin gradients required for correct orientation of cell division within the QC and columella cells and inhibit cell division in the QC and the columella daughter cells. Future characterization of the embryonic expression patterns of the AUX1 LAX members and their genetic interaction with the rest of auxin-dependent patterning machinery will be important for the understanding of processes such as de novo meristem establishment and the co-ordination between cell division and cell expansion required for root cap cell pattern establishment.
ACKNOWLEDGEMENTS
We thank Joseph Dubrovsky (Instituto de Biotecnología, UNAM, Mexico) for feedback on the early manuscript, and Ottoline Leyser (University of York, UK) for valuable input during the course of the research. We acknowledge Jim Haseloff (University of Cambridge, UK), Carol Wenzel (University of York, UK, now Simon Fraser University, Canada) and Jo Marrison (University of York) for their major contributions to the development of the embryonic cell wall staining technique. We thank Peter O'Toole and Karen Chance (Technology Facility, University of York, UK) for confocal microscopy advice and Petra Stirnberg (University of York) for kindly providing us with mutagenized Col-0 seed. We also thank anonymous referees for discussing the manuscript. This work was supported by Consejo Nacional de Ciencia y Tecnología (CONACyT), Mexico (Y.U-Ch fellowship) and a Marie Curie Intra-European Fellowship within the 7th European Community Framework Programme (B.P.).
LITERATURE CITED
- Aida M, Vernoux T, Furutani M, Traas J, Tasaka M. Roles of PIN-FORMED1 and MONOPTEROS in pattern formation of the apical region of the Arabidopsis embryo. Development. 2002;129:3965–3974. doi: 10.1242/dev.129.17.3965. [DOI] [PubMed] [Google Scholar]
- Aida M, Beis D, Heidstra R, et al. The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell. 2004;119:109–120. doi: 10.1016/j.cell.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Bainbridge K, Guyomarc'h S, Bayer E, et al. Auxin influx carriers stabilize phyllotactic patterning. Genes and Development. 2008;22:810–823. doi: 10.1101/gad.462608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay A, Blakeslee JJ, Lee OR, et al. Interactions of PIN and PGP auxin transport mechanisms. Biochemical Society Transactions. 2007;35:137–141. doi: 10.1042/BST0350137. [DOI] [PubMed] [Google Scholar]
- Baum SF, Dubrovsky JG, Rost TL. Apical organisation and maturation of the cortex and vascular cylinder in Arabidopsis thaliana (Brassicaceae) roots. American Journal of Botany. 2002;89:908–920. doi: 10.3732/ajb.89.6.908. [DOI] [PubMed] [Google Scholar]
- Benkova E, Ivanchenko MG, Friml J, Shishkova S, Dubrovsky JG. A morphogenetic trigger: is there an emerging concept in plant developmental biology? Trends in Plant Science. 2009;14:189–193. doi: 10.1016/j.tplants.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Bennett MJ, Marchant A, Green HG, et al. Arabidopsis AUX1 gene: a permease-like regulator of root gravitropism. Science. 1996;273:948–950. doi: 10.1126/science.273.5277.948. [DOI] [PubMed] [Google Scholar]
- Bhalearo RP, Bennett MJ. The case for morphogens in plants. Nature Cell Biology. 2003;5:939–943. doi: 10.1038/ncb1103-939. [DOI] [PubMed] [Google Scholar]
- Blakeslee JJ, Bandyopadhyay A, Lee OR. Interactions among PIN-FORMED and P-glycoprotein auxin transporters in Arabidopsis. The Plant Cell. 2007;19:131–147. doi: 10.1105/tpc.106.040782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blilou I, Xu J, Wildwater M, et al. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 2005;433:39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- Bolle C. The role of GRAS proteins in plant signal transduction and development. Planta. 2004;218:683–692. doi: 10.1007/s00425-004-1203-z. [DOI] [PubMed] [Google Scholar]
- Bouchard R, Bailly A, Blakeslee JJ, et al. Immunophilin-like TWISTED DWARF1 modulates auxin efflux activities of Arabidopsis P-glycoproteins. Journal of Biological Chemistry. 2006;281:30603–30612. doi: 10.1074/jbc.M604604200. [DOI] [PubMed] [Google Scholar]
- Busch M, Mayer U, Jürgens G. Molecular analysis of the Arabidopsis pattern formation of gene GNOM: gene structure and intragenic complementation. Molecular General Genetics. 1996;250:681–691. doi: 10.1007/BF02172979. [DOI] [PubMed] [Google Scholar]
- Casson SA, Topping JF, Lindsey K. MERISTEM-DEFECTIVE, an RS domain protein, is required for the correct meristem patterning and function in Arabidopsis. The Plant Journal. 2009;57:857–869. doi: 10.1111/j.1365-313X.2008.03738.x. [DOI] [PubMed] [Google Scholar]
- Chen JG, Shimomura S, Sitbon F, Sandberg G, Jones AM. The role of auxin-binding protein 1 in the expansion of tobacco leaf cells. The Plant Journal. 2001a;28:607–617. doi: 10.1046/j.1365-313x.2001.01152.x. [DOI] [PubMed] [Google Scholar]
- Chen JG, Ullah H, Young JC, Sussman MR, Jones AM. ABP1 is required for organized cell elongation and division in Arabidopsis embryogenesis. Genes and Development. 2001b;15:902–911. doi: 10.1101/gad.866201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Hilson P, Sedbrook J, Rosen E, Caspar T, Masson P. The Arabidopsis thaliana AGRAVITROPIC 1 gene encodes a component of the polar-auxin-transport efflux carrier. Proceedings of the National Academy of Sciences of the USA. 1998;95:15112–15117. doi: 10.1073/pnas.95.25.15112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David KM, Couch D, Braun N, et al. The auxin-binding protein 1 is essential for the control of cell cycle. The Plant Journal. 2007;50:197–206. doi: 10.1111/j.1365-313X.2007.03038.x. [DOI] [PubMed] [Google Scholar]
- Dhonukshe P, Tanaka H, Goh T, et al. Generation of cell polarity in plants links endocytosis, auxin distribution and cell fate decisions. Nature. 2008;456:962–966. doi: 10.1038/nature07409. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Di Laurenzio L, Wysocka-Diller J, Malamy J, et al. The SCARECROW gene regulates an asymmetric cell division that is essential for generating the radial organisation of the Arabidopsis root. Cell. 1996;86:423–433. doi: 10.1016/s0092-8674(00)80115-4. [DOI] [PubMed] [Google Scholar]
- Dolan L, Janmaat K, Willemsen V, et al. Cellular organisation of the Arabidopsis thaliana root. Development. 1993;119:71–84. doi: 10.1242/dev.119.1.71. [DOI] [PubMed] [Google Scholar]
- Donaldson JG, Jackson CL. Regulators and effectors of the ARF GTPases. Current Opinion in Cell Biology. 2000;12:475–482. doi: 10.1016/s0955-0674(00)00119-8. [DOI] [PubMed] [Google Scholar]
- Dubrovsky JG, Gambetta GA, Hernandez-Barrera A, Shishkova S, Gonzalez I. Lateral root initiation in Arabidopsis: developmental window, spatial patterning, density and predictability. Annals of Botany. 2006;97:903–915. doi: 10.1093/aob/mcj604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J. Auxin transport – shaping the plant. Current Opinion in Plant Biology. 2003;6:7–12. doi: 10.1016/s1369526602000031. [DOI] [PubMed] [Google Scholar]
- Friml J, Vieten A, Sauer M, et al. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature. 2003;426:147–153. doi: 10.1038/nature02085. [DOI] [PubMed] [Google Scholar]
- Friml J, Benfey P, Benková E, et al. Apical-basal polarity: why plant cells don't stand on their heads. Trends in Plant Science. 2006;11:12–14. doi: 10.1016/j.tplants.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Funke M, Edelmann HG. Auxin-dependent cell wall depositions in the epidermal periplasmic space of graviresponding nodes of Tradescantia fluminensis. Journal of Experimental Botany. 2000;51:579–586. doi: 10.1093/jexbot/51.344.579. [DOI] [PubMed] [Google Scholar]
- Galinha C, Hofhuis H, Luijten M, et al. PLETHORA proteins as dose-dependent master regulators of Arabidopsis root development. Nature. 2007;449:1053–1057. doi: 10.1038/nature06206. [DOI] [PubMed] [Google Scholar]
- Geisler M, Blakeslee JJ, Bouchard R, et al. Cellular efflux of auxin catalyzed by the Arabidopsis MDR/PGP transporter AtPGP1. The Plant Journal. 2005;44:179–194. doi: 10.1111/j.1365-313X.2005.02519.x. [DOI] [PubMed] [Google Scholar]
- Geldner N, Anders N, Wolters H, et al. The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell. 2003;112:219–230. doi: 10.1016/s0092-8674(03)00003-5. [DOI] [PubMed] [Google Scholar]
- Grieneisen VA, Xu J, Maree AFM, Hogeweg P, Scheres B. Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature. 2007;449:1008–1013. doi: 10.1038/nature06215. [DOI] [PubMed] [Google Scholar]
- Groot EP, Doyle JA, Nichol SA, Rost TL. Phylogenetic distribution and evolution of root apical meristem organisation in dicotyledonous angiosperms. International Journal of Plant Sciences. 2004;165:97–105. [Google Scholar]
- Hager A. Role of the plasma membrane H+-ATPase in auxin-induced elongation growth: historical and new aspects. Journal of Plant Research. 2003;116:483–505. doi: 10.1007/s10265-003-0110-x. [DOI] [PubMed] [Google Scholar]
- Hamann T, Benkova E, Bäure I, Kientz M, Jürgens G. The Arabidopsis BODENLOS gene encodes an auxin response protein inhibiting MONOPTEROS-mediated embryo patterning. Genes and Development. 2002;16:1610–1615. doi: 10.1101/gad.229402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann T, Mayer U, Jurgens G. The auxin-insensitive bodenlos mutation affects primary root formation and apical-basal patterning in the Arabidopsis embryo. Development. 1999;126:1387–1395. doi: 10.1242/dev.126.7.1387. [DOI] [PubMed] [Google Scholar]
- Hardtke CS, Ckurshumova W, Vidaurre DP. Overlapping and non-redundant functions of the Arabidopsis auxin response factors MONOPTEROS and NONPHOTOTROPIC HYPOCOTYL 4. Development. 2004;131:1089–1100. doi: 10.1242/dev.00925. [DOI] [PubMed] [Google Scholar]
- Heimsch C, Seago JL., Jr Organisation of the root apical meristem in angiosperms. American Journal of Botany. 2008;95:1–21. doi: 10.3732/ajb.95.1.1. [DOI] [PubMed] [Google Scholar]
- Jurgens G, Geldner N. The high road and the low road: trafficking choices in plants. Cell. 2007;130:977–979. doi: 10.1016/j.cell.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Jurgens G, Mayer U. London: Wolfe Publishers; 1994. pp. 7–21. Arabidopsis. In: Bard JBL. ed. Embryos: colour atlas of development. [Google Scholar]
- Kleine-Vehn J, Leitner J, Zwiewka M, et al. Differential degradation of PIN2 auxin efflux carrier by retromer-dependent vacuolar targeting. Proceedings of the National Academy of Sciences of the USA. 2008;105:17812–17817. doi: 10.1073/pnas.0808073105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer E. PIN and AUX/LAX proteins: their role in auxin accumulation. Trends in Plant Science. 2004;9:578–582. doi: 10.1016/j.tplants.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Kramer EM. Computer models of auxin transport: a review and commentary. Journal of Experimental Botany. 2008;59:45–53. doi: 10.1093/jxb/erm060. [DOI] [PubMed] [Google Scholar]
- Kramer E, Bennett MJ. Auxin transport: a field in flux. Trends in Plant Science. 2006;11:382–386. doi: 10.1016/j.tplants.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Laskowski M, Grieneisen VA, Hofhuis H, et al. PLoS Biology. 2008;6 doi: 10.1371/journal.pbio.0060307. Root system architecture from coupling cell shape to auxin transport. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Yang H, Peer WA, et al. Arabidopsis H+-PPase AVP1 regulates auxin-mediated organ development. Science. 2005;310:121–125. doi: 10.1126/science.1115711. [DOI] [PubMed] [Google Scholar]
- Luschnig C, Gaxiola RA, Grisafi P, Fink GR. EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes and Development. 1998;12:2175–2187. doi: 10.1101/gad.12.14.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant A, Bennett MJ. The Arabidopsis AUX1 gene: a model system to study mRNA processing in plants. Plant Molecular Biology. 1998;36:463–471. doi: 10.1023/a:1005961303167. [DOI] [PubMed] [Google Scholar]
- Marchant A, Kargul J, May S. AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. EMBO Journal. 1999;15:2066–2073. doi: 10.1093/emboj/18.8.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant A, Bhalerao R, Casimiro I, et al. AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. The Plant Cell. 2002;14:589–597. doi: 10.1105/tpc.010354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mravec J, Kubes M, Bielach A, et al. Interaction of PIN and PGP transport mechanisms in auxin distribution-dependent development. Development. 2008;135:3345–3354. doi: 10.1242/dev.021071. [DOI] [PubMed] [Google Scholar]
- Napier RM, David KM, Perrot-Rechenmann C. A short history of auxin-binding proteins. Plant Molecular Biology. 2002;49:339–348. [PubMed] [Google Scholar]
- Okada K, Shimura Y. Reversible root tip rotation in Arabidopsis seedlings induced by obstacle-touching stimulus. Science. 1990;250:274–276. doi: 10.1126/science.250.4978.274. [DOI] [PubMed] [Google Scholar]
- Parry G, Marchant A, May S. Quick on the uptake: characterization of a family of plant auxin influx carriers. Journal of Plant Growth Regulation. 2001;20:217–225. [Google Scholar]
- Passarinho P, Ketelaar T, Xing M. BABY BOOM target genes provide diverse entry points into cell proliferation and cell growth pathways. Plant Molecular Biology. 2008;68:225–237. doi: 10.1007/s11103-008-9364-y. [DOI] [PubMed] [Google Scholar]
- Petrasek J, Cerna A, Schwarzerova K, Elckner M, Morris DA, Zazimalova E. Do phytotropins inhibit auxin efflux by impairing vesicle traffic? Plant Physiology. 2003;131:254–263. doi: 10.1104/pp.012740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrasek J, Mravec J, Bouchard R, et al. PIN proteins perform a rate-limiting function in cellular auxin efflux. Science. 2006;312:914–918. doi: 10.1126/science.1123542. [DOI] [PubMed] [Google Scholar]
- Pickett FB, Wilson AK, Estelle M. The aux1 mutation of Arabidopsis confers both auxin and ethylene resistance. Plant Physiology. 1990;94:1462–1466. doi: 10.1104/pp.94.3.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploense SE, Wu MF, Nagpal P, Reed JW. A gain-of-function mutation in IAA18 alters Arabidopsis embryonic apical patterning. Development. 2009;136:1509–1517. doi: 10.1242/dev.025932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt D, Pesce ER, Stieger P, et al. Regulation of phyllotaxis by polar auxin transport. Nature. 2003;426:255–260. doi: 10.1038/nature02081. [DOI] [PubMed] [Google Scholar]
- Rober-Kleber N, Albrechtova JTP, Fleig S, et al. Plasma membrane H+-ATPase is involved in auxin-mediated cell elongation during wheat embryo development. Plant Physiology. 2003;131:1302–1312. doi: 10.1104/pp.013466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman G, Lubarsky B, Kieber JJ, Rothenberg M, Ecker JR. Genetic analysis of ethylene signal transduction in Arabidopsis thaliana: five novel mutant loci integrated into a stress response pathway. Genetics. 1995;139:1393–1409. doi: 10.1093/genetics/139.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini S, Beis D, Wolkenfelt H, et al. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell. 1999;99:463–472. doi: 10.1016/s0092-8674(00)81535-4. [DOI] [PubMed] [Google Scholar]
- Sabatini S, Heidstra R, Wildwater M, Scheres B. SCARECROW is involved in positioning the stem cell niche in the Arabidopsis root meristem. Genes & Development. 2003;17:354–358. doi: 10.1101/gad.252503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar AK, Luijten M, Miyashima S, et al. Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature. 2007;446:811–814. doi: 10.1038/nature05703. [DOI] [PubMed] [Google Scholar]
- Scheres B, Wolkenfelt H, Willemsen V. Embryonic origin of the Arabidopsis primary root and root meristem initials. Development. 1994;120:2475–2487. [Google Scholar]
- Shishkova S, Rost T, Dubrovsky JG. Determinate root growth and meristem maintenance in angiosperms. Annals of Botany. 2008;101:319–340. doi: 10.1093/aob/mcm251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SK, Hofhuis H, Lee MM, Clark SE. Key divisions in the early Arabidopsis embryo require POL and PLL1 phosphatases to establish the root stem cell organizer and vascular axis. Developmental Cell. 2008;15:98–109. doi: 10.1016/j.devcel.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer C, Reyes FC, Buono R, Sliwinski MK, Haas TJ, Otegui MS. The ESCRT-related CHMP1A and B proteins mediate multivesicular body sorting of auxin carriers in Arabidopsis and are required for plant development. The Plant Cell. 2009;21:749–766. doi: 10.1105/tpc.108.064865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann T, Geldner N, Grebe M, et al. Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF. Science. 1999;286:316–318. doi: 10.1126/science.286.5438.316. [DOI] [PubMed] [Google Scholar]
- Swarup R, Kargul J, Marchant A. Structure–function analysis of the presumptive Arabidopsis auxin permease AUX1. The Plant Cell. 2004;16:3069–3083. doi: 10.1105/tpc.104.024737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup R, Kramer EM, Perry P, et al. Root gravitropism requires lateral root cap and epidermal cells for transport and response to a mobile auxin signal. Nature Cell Biology. 2005;7:1057–1065. doi: 10.1038/ncb1316. [DOI] [PubMed] [Google Scholar]
- Swarup K, Benková E, Swarup R, et al. The auxin influx carrier LAX3 promotes lateral root emergence. Nature Cell Biology. 2008;10:946–954. doi: 10.1038/ncb1754. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Kitakura S, De Rycke R, De Groodt R, Friml J. Fluorescence imaging-based screen identifies ARF GEF component of early endosomal trafficking. Current Biology. 2009;19:391–397. doi: 10.1016/j.cub.2009.01.057. [DOI] [PubMed] [Google Scholar]
- Vieten A, Sauer M, Brewer PB, Friml J. Molecular and cellular aspects of auxin-transport-mediated development. Trends in Plant Science. 2007;12:160–168. doi: 10.1016/j.tplants.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Vissenberg K, Feijó JA, Weisenseel MH, Verbelen JP. Ion fluxes, auxin and the induction of elongation growth in Nicotiana tabacum cells. Journal of Experimental Botany. 2001;52:2161–2167. doi: 10.1093/jexbot/52.364.2161. [DOI] [PubMed] [Google Scholar]
- Weijers D, Schlereth A, Ehrismann JS, Schwank G, Kientz M, Jürgens G. Auxin triggers transient local signalling for cell specification in Arabidopsis embryogenesis. Developmental Cell. 2006;10:265–270. doi: 10.1016/j.devcel.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Wenzel C, Rost TL. Cell division patterns of the protoderm and root cap in the ‘closed’ root apical meristem of Arabidopsis thaliana. Protoplasma. 2001;218:203–213. doi: 10.1007/BF01306609. [DOI] [PubMed] [Google Scholar]
- Wildwater M, Campilho A, Perez-Perez JM, et al. The RETINOBLASTOMA-RELATED gene regulates stem cell maintenance in Arabidopsis roots. Cell. 2005;123:1337–1349. doi: 10.1016/j.cell.2005.09.042. [DOI] [PubMed] [Google Scholar]
- Willemsen V, Bauch M, Bennett T, et al. The NAC domain transcription factors FEZ and SOMBRERO control the orientation of cell division plane in Arabidopsis root stem cells. Developmental Cell. 2008;15:913–922. doi: 10.1016/j.devcel.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Yang Y, Hammes UZ, Taylor CG, Schachtman DP, Nielsen E. High-affinity auxin transport by the aux1 influx carrier protein. Current Biology. 2006;16:1123–1127. doi: 10.1016/j.cub.2006.04.029. [DOI] [PubMed] [Google Scholar]