Abstract
Background and Aims
Initial release height and settling speed of diaspores are biologically controlled components which are key to modelling wind dispersal. Most Sphagnum (peat moss) species have explosive spore liberation. In this study, how capsule and spore sizes affect the height to which spores are propelled were measured, and how spore size and spore number of discharged particles relate to settling speed in the aspherical Sphagnum spores.
Methods
Spore discharge and spore cloud development were filmed in a closed chamber (nine species). Measurements were taken from snapshots at three stages of cloud development. Settling speed of spores (14 species) and clusters were timed in a glass tube.
Key Results
The maximum discharge speed measured was 3·6 m s−1. Spores reached a maximum height of 20 cm (average: 15 cm) above the capsule. The cloud dimensions at all stages were related positively to capsule size (R2 = 0·58–0·65). Thus species with large shoots (because they have large capsules) have a dispersal advantage. Half of the spores were released as singles and the rest as clusters (usually two to four spores). Single spores settled at 0·84–1·86 cm s−1, about 52 % slower than expected for spherical spores with the same diameters. Settling speed displayed a positive curvilinear relationship with spore size, close to predictions by Stokes' law for spherical spores with 68 % of the actual diameters. Light-coloured spores settled slower than dark spores. Settling speed of spore clusters agrees with earlier studies. Effective spore discharge and small, slowly settling spores appear particularly important for species in forested habitats.
Conclusions
The spore discharge heights in Sphagnum are among the greatest for small, wind-dispersed propagules. The discharge heights and the slow settling of spores affect dispersal distances positively and may help to explain the wide distribution of most boreal Sphagnum species.
Keywords: Bryophyte, explosive capsules, long-distance dispersal, peatland, peat moss, settling speed, Sphagnum, spore, spore capsule, Sweden, terminal velocity, wind dispersal
INTRODUCTION
Numerous plants and fungi exhibit violent diaspore release mechanisms which promote dispersal (Ingold, 1965; Howe and Smallwood, 1982). The largest ballistic release distances are demonstrated in dicotyledons and fungi, in which relatively large seeds or spore clusters may be thrown several metres. Among fungi, species growing on dung exhibit the largest discharge distances, with a positive relationship between spore size and distance (Buller, 1909, 1933; Ingold, 1965). These dung-inhabiting species do not rely on wind as a secondary dispersal vector. Instead they attach to vegetation and become ingested by grazing animals before completion of their life-cycles. However, violent discharge occurs also among a large proportion of fungi and pteridophytes that rely on wind dispersal of spores, where discharge mainly ensures that spores leave the sporangia, and the distances are generally in the range of 100 µm to a few centimetres (reviewed by Ingold, 1965). Also, violent pollen discharge has been demonstrated in pollen catapults (Edwards et al., 2005). In the thalloid liverwort Conocephalum, sperms are violently discharged in small droplets, reaching a maximum height of 15 cm (Cavers, 1903; Shimamura et al., 2008).
Peat mosses posses a unique and well-known mechanism of violent spore discharge, mentioned first by Linnaeus (Linne, 1771, p. 506: ‘Sphagn. palustre. Antheræ dissiliunt cum sono et crepitu. Angerstein.’), in which spores are ejected from the spore capsule with a sharp noise audible at a distance of several metres, termed the ‘air-gun mechanism’ (Nawaschin, 1897; Ingold, 1965). Nawaschin (1897) reported that the spores were shot to a distance of up to 10 cm, based on traces caused by capsules dehiscing between herbarium sheets, while Ingold (1965) reported a height of 15 cm or more (without mentioning circumstances or species). In bryophytes, violent spore release is also present among liverworts (Ingold, 1965).
Sphagnum is a good study system for dispersal because it has a wide range of capsule and spore sizes, and the species vary widely in their habitats (openness, microtopographic position above the water-table, water chemistry; Rydin et al., 1999) with varying dispersal conditions. Larger spores (>30 µm diameter) are found in species growing predominantly in open habitats, whereas smaller spores are found in species growing in both swamp forests and open habitats. Large capsules are found in species with large capitula irrespective of habitat (Sundberg and Rydin, 1998). In a short-distance dispersal experiment, including six Sphagnum species, dispersal (out of the capsule and beyond the sampled radius of 3·2 m) was positively related to capsule size but also negatively to spore size (Sundberg, 2005).
Dispersal, notably long-distance dispersal, has recently regained attention with the development of mechanistic wind-dispersal models that incorporate the effects of vertical turbulence (e.g. Nathan et al., 2002; Tackenberg, 2003; Soons et al., 2004; Aylor and Boehm, 2006; Kuparinen et al., 2007). Wind-dispersal models incorporate both mechanical traits, such as horizontal and vertical wind components, and biological traits, such as initial release height and settling speed of particles (pollen, seeds, spores, asexual propagules). Lately, modelling of airborne particle transport has become rather close to real events, but the importance of the events at particle release and factors affecting deposition has often been neglected (Greene, 2005; Kuparinen, 2006; Skarpaas et al., 2006).
Settling speed of smooth spherical particles in the diameter range 1–100 µm, including a majority of spores and pollen, are predicted by Stokes' law (Gregory, 1973). Settling speeds of non-spherical particles are less predictable and often have to be tested experimentally, but McCubbin (1944) presented a simplification for predicting settling speed of spherical and ellipsoid particles. Spores generally have densities exceeding that of water, with an average of 1·1 g cm−3 (reviewed by Gregory, 1973). Settling speed of bryophyte spores has previously been recorded only for Polytrichum sp., with a settling speed of 0·23 cm s−1 and a relatively high density of 1·53 g cm−3 (Zeleny and McKeehan, 1910, cited in Gregory, 1973). Spores and pollen are often released and deposited as clusters of various numbers of particles, and incorporating their settling speeds in dispersal models should be considered (Ferrandino and Aylor, 1984; Di-Giovanni et al., 1995).
The aims of the present study were: (a) to document and characterize spore release and the development of the spore cloud, and to examine the average and maximum release height of Sphagnum spores in relation to capsule and spore size, spore colour and habitat openness; and (b) to determine settling speeds of the aspherical Sphagnum spores in relation to spore size, spore colour and to clusters of spores. The study forms a basis for mechanistic modelling of dispersal.
MATERIALS AND METHODS
Characteristics of Sphagnum capsules and spores
The mature Sphagnum spore capsule is yellowish to dark brown, ovoid to spherical in shape when moist (Sundberg and Rydin, 1998; Sundberg, 2000). Capsules range in horizontal diameter from 1·2 mm to 2·3 mm among the species examined (Table 1; range among extreme capsules in the genus: 0·70–2·95 mm), corresponding to an 8-fold difference in inter-specific capsule volume (75-fold between extremes). The capsule is raised on an erect, fleshy pseudopodium (made up of gametophytic tissue, in contrast to the dry, sporophytic seta in the majority of bryophytes) that normally is 5–20 mm but may reach a length of 60 mm in the extreme (in partly inundated, fast-growing S. squarrosum; personal observation). The capsule thus reaches 0–20 mm above the moss surface, facing vertically, when discharge occurs.
Table 1.
Summary of the 14 Sphagnum species studied, their sections (subgenera), main habitats in (east-)central Sweden, average capsule, spore diameters and inclusion in tests (number of samples)
| Test (number of samples) |
||||||
|---|---|---|---|---|---|---|
| Species and section* | Habitat† | Capsule diameter‡ (mm) | Spore diameter§ (μm) | Spore cloud | Spores per particle | Settling speed |
| Sect. Sphagnum | ||||||
| S. centrale | S | 2·33 | 27·6 | 5 | 2 | 2 |
| S. papillosum | P | 2·19 | 33·8 | 3 | 2 | 2 |
| Sect. Rigida | ||||||
| S. compactum | P | 1·59 | 31·6 | 2 | ||
| S. strictum | P | 1·60 | 31·2 | 1 | ||
| Sect. Subsecunda | ||||||
| S. contortum | R | 1·60 | 29·1 | 1 | ||
| Sect. Cuspidata | ||||||
| S. cuspidatum | B | 1·68 | 36·9 | 2 | 2 | |
| S. fallax | P | 1·61 | 27·1 | 2 | 1 | |
| S. lindbergii | B | 2·04 | 32·5 | 4 | 2 | |
| S. tenellum | B | 1·18 | 35·7 | 2 | 1 | |
| Sect. Acutifolia | ||||||
| S. fimbriatum | S | 1·85 | 27·0 | 6 | 2 | 1 |
| S. subnitens | R | 1·66 | 28·4 | 2 | 2 | 2 |
| S. warnstorfii | R | 1·59 | 23·1 | 2 | 1 | |
| Sect. Squarrosa | ||||||
| S. squarrosum | S | 2·16 | 25·8 | 3 | 1 | |
| S. teres | R | 1·70 | 23·4 | 1 | 2 | |
† All habitats are largely open except for the swamp forests: B, bog; P, poor fen; R, rich fen; S, swamp forest.
* From Flatberg (2002); † Rydin et al. (1999) and Sundberg et al. (2006); ‡§ Sundberg and Rydin (1998), Sundberg (2000) and this study.
The bottom portion of the capsule comprises an air-filled cavity, underlying the spore sac. The capsule wall consists of a firm epidermis, four or five cells thick, delimited by a circular line of weakness along the operculum rim (Paton and Pearce, 1957; Ingold, 1965; Duckett et al., 2009). A mature, drying capsule contracts longitudinally and attains a cylindrical shape (Fig. 1). Between 100 and over 1000 thin-walled pseudo-stomata (about 30–35 µm wide and 35–40 µm long) are required for water loss and contraction of the capsule midsection – pseudo-stomata are absent around the operculum and in the basal portion of the capsule (Paton and Pearce, 1957; Andrews, 1961; Duckett et al., 2009). When the capsule contracts, pressure accumulates within the air-filled cavity, eventually amounting to about 500 kPa (5 atm; Nawaschin, 1897), corresponding to the pressure in large lorry tyres. Finally the capsule bursts at the operculum rim and the spores are discharged (illustrated by Nawaschin, 1897; Ingold, 1965; Schofield, 1985). Spores are dispersed during dry, often sunny conditions from late June to September in boreal regions, with different periods for different species and habitats (species of open, wet habitats are early; species of hummocks and forests are late dispersers), and with an apparent diurnal peak around noon (Sundberg, 2002, 2005; Fenton and Bergeron, 2006).
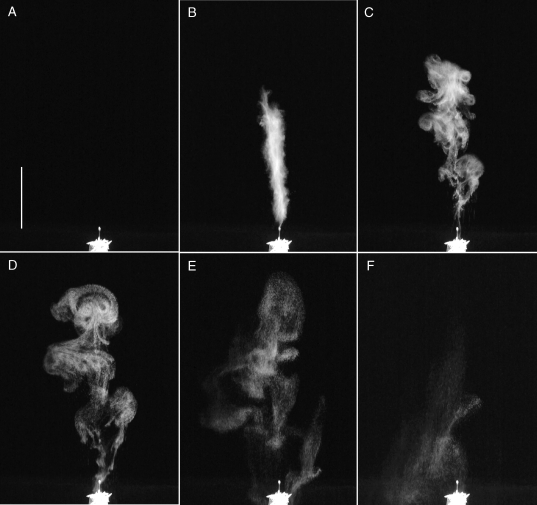
Fig. 1.
A shoot of Sphagnum centrale bearing three spore capsules: mature, spherical capsule (left), dry dehisced capsule lacking operculum (middle), and dry, cylindrical (‘loaded’) capsule seconds before spore discharge (right).
The Sphagnum spore is often described as tetrahedral in shape with a distinct trilete Y-shaped mark on the proximal face (Cao and Vitt, 1986), as opposed to the majority of bryophyte spores which are spherical. However, a Sphagnum spore viewed from above is more circular than triangular (see Cao and Vitt, 1986), and viewed laterally is more of an ellipse than an isosceles triangle (personal observations), altogether resembling an oblate spheroid – these simplifications are important when modelling settling speeds. Sphagnum spores are generally various shades of brown. A few species in section Cuspidata have bright yellow spores (mainly S. balticum, S. fallax and S. tenellum; while S. lindbergii has intermediately light-coloured spores) that tend to float and to be more long-lived than dark spores, possibly because they contain more lipids (Sundberg and Rydin, 1998, 2000; cf. Duckett and Ligrone, 1992). Spore diameters range from 22 µm to 45 µm among species (Cao and Vitt, 1986). Spore number per capsule ranges from 18 500 to 240 000 among species, with a strong positive relationship between capsule to spore-size ratio and spore number (Sundberg and Rydin, 1998; Sundberg, 2000).
Study material
Shoots with mature, undehisced spore capsules of the Sphagnum species studied (Table 1) were collected in late July to early September, 2007, 2008 and 2009 from peatlands in the province of Uppland, east-central Sweden, situated in the northern part of the boreo-nemoral vegetation zone. Capsule-bearing shoots of S. strictum were collected near Malung, in the south-western part of the province of Dalarna (boreal zone), west-central Sweden. Between collection and use in the experiments, the specimens were stored in closed containers in a dark refrigerator at 4 °C (capsules remain fully explosive for about 3 weeks when stored in this way – thereafter they tend to lose the ability; personal observation). Species nomenclature follows Hallingbäck et al. (2006), and section affinity follows Flatberg (2002).
Spore cloud development and characterization
Spore discharge and spore cloud development were recorded by high-definition camcorders, Sony HDR-SR1 (30 frames s−1) and HDR-SR12E (25 frames s−1), set at their widest angle (corresponding to a 40-mm camera lens). To minimize disturbing turbulence, spores were filmed in a closed wooden chamber (depth × height × width: 1·7 × 0·8 × 0·6 m – the excessive depth of the chamber to ensure a deep, dark background without reflections), painted black inside. The chamber was placed in a room with an ambient temperature of about 25 °C. Although no measurements of air humidity inside the chamber were taken, filming was restricted to dry-weather days (outdoor relative air humidity spanned 33–62 %). Film sequences were shot through a 40-mm-diameter circular hole with the camcorder mounted at a height of 13–20 cm above the capsule top at a horizontal distance of 0·8 m from the capsule. Capsule-bearing shoots, for which capsule diameters (D) had been measured with a calliper when the capsules were still moist and spherical, were mounted in a water-filled test tube with the vertically erect capsule protruding above the tube. Shoots were positioned inside the chamber, via a side hatch, when a capsule had attained a characteristic cylindrical ‘soon-to-discharge’ shape after 1–10 h in dry air. Capsule discharge occurred between 1 and 30 min after positioning in the chamber. Target illumination was provided by two spreading beams from a fibre optic light through an acrylic glass-covered hole (60 mm diameter) in the chamber roof. The beams did not cause any apparent turbulence inside the chamber that affected the spore clouds.
Snapshots (JPEG images), showing the most interesting sequences were timed in relation to the discharge event and saved (using video-editing software Sony Vegas Movie Studio Platinum 8.0 and 9.0b) for further measurement: (a) spike height (hspike), i.e. cloud height immediately after the moment of active discharge (the second discharge frame was used, because the first frame, at 0·03–0·04 s, occasionally caught only a glimpse of the incipient discharge); (b) centroid height (hcen; approximation of the height the average spore reached above the capsule top, taken from the snapshot at tcen when approximately as many spores were ascending as descending within the cloud – this was established through back-and-forth, frame-by-frame viewing); (c) peak height (hpeak – the maximum height of the cloud summit above the capsule top after discharge); and (d) cloud width (wcen; from the same snapshot as centroid height). To analyse cloud shape, the width of each cloud was measured at ten evenly distributed positions along the cloud at tcen. Centroid position was estimated with Lispix public domain software (version 2008-06-19; Bright and Milans, 2000) from highly contrasted spore-cloud contour images. Centroid estimates did not take variations in intra-cloud spore concentration into account (the upper half of the cloud appeared be to slightly denser).
A total of 28 spore-discharge sequences were obtained in nine Sphagnum species from both open mires and swamp forests, representing four taxonomic sections and covering ranges in capsule and spore diameters of 1·35–2·60 mm and 23–36·5 µm, respectively (Table 1). Capsule diameter is the maximum horizontal diameter of an erect capsule (Sundberg and Rydin, 1998). Recordings took place on four days between 28 July and 2 August, 3–8 d after field collection. Spore diameters were obtained from the settling speed experiment (see below).
The size distribution of discharged particles (as single spores or as clusters with various numbers of spores) was examined in two capsules each for six species (covering three sections; Table 1). Spores from each dehisced capsule were trapped on four microscope slides (76 × 26 mm), positioned at right angles below and around the spore capsule at the bottom of the film chamber. The slides were then examined in a 1·28-mm-wide and 76-mm-long sweep with a microscope at ×100 magnification. Between 909 and 4880 spores per capsule were examined.
Measurement of settling speed
Measurement of settling speed was made by timing the rate of spore fall inside a vertically oriented, transparent glass tube, a ‘fall tower’ (Gregory, 1973; Ferrandino and Aylor, 1984; Aylor, 2002), 1·5 m long with an inner diameter of 20 mm. The spore mass from a capsule was placed in a central depression of a thin aluminium-foil diaphragm, with a central pinhole of about 0·25 mm, covering the top of the glass tube. A microscope slide (26 × 76 mm) covered the bottom of the settling tube. Measurements were made in a darkened room at about 22 °C, where the settling tube was illuminated from below by a beam of fibre-optic light, centred at and almost parallel to the tube, originating 0·25 m below the tube. The light's power source was positioned around 0·5 m away from the settling tube. Air stability inside the tube was confirmed several times with a puff from an incense stick, between settling speed measurements (Aylor, 2002).
Spore capsules were put individually into empty 12-mL glass vials, up to 2 weeks after collection, where they dehisced while the vial lid was slightly ajar. After spore discharge, the capsule and the operculum were removed and the vial lid was closed, for storage of the spores in the vial at room temperature until use. Spore settling speed was obtained 1–500 d after capsule dehiscence. Settling speed was timed with a digital stopwatch along 1·0 m of the tube, starting 0·25 m from the top and terminating 0·25 m from the bottom. Spores were triggered to fall through the diaphragm pinhole by gently tapping the sides of the tube. Often single spores and clusters containing various numbers of spores were released simultaneously. The number of spores in each timed particle was checked with a dissecting microscope. Solitary single spores were used for obtaining the average and variation in settling speed, whereas the middle spore in small groups of single spores were used only to obtain the average speed. At least 20 readings were obtained for each capsule, including 8–31 observations of solitary spores. Finally, spore diameters of 20 randomly selected spores were measured with a microscope at ×400 magnification.
Settling speeds were obtained from a total of 21 capsules, one or two capsules each of 14 Sphagnum species, representing all six European taxonomic sections (Table 1).
Statistical analysis
Relationships between spore diameter (d), spore colour (sdark, scored as either 1 or 0 for dark spores) and settling speed were tested using t-tests, linear and curvilinear regressions. For testing the relationships between spore cloud dimensions and time, versus capsule diameter (D) and its square (D2), spore diameter, spore colour and habitat openness (sforest, scored as either 1 or 0 for swamp forest), stepwise regressions (α set at 0·15 for entering and removing predictors in the models) were used. Adjusted R2 was used to assess which models to select. Tests were performed with the statistical software Minitab (version 15, 2008; Minitab Inc., State College, PA, USA).
Spore settling speed (vs) was related to Stokes' law and to McCubbin's formula for spherical and ellipsoid particles (McCubbin, 1944):
| 1 |
where speed (vs) is in millimetres per second, and spore dimensions are in micrometres. Relative settling speed of spore clusters was compared with the relationship derived by Ferrandino and Aylor (1984):
| 2 |
where vsN is the relative settling speed in relation to single particles, and N is the number of particles in the cluster.
RESULTS
Spore cloud development and characterization
Spore discharge started as a rather narrow spike in the first two frames (representing 0·04–0·08 s) of each footage, reaching 43–150 mm above the capsule (68 % of the peak height; range 51–87 %; Fig. 2). The maximum calculated speed of discharge was 3·6 m s−1, based on the distance travelled within a video frame. The cloud then gradually, and much more slowly, expanded upwards and sideways. Almost immediately, spores or clusters at the bottom of the cloud (which had lower initial speed and never reached far up) started to descend while those further up were still ascending (see video in Supplementary data, available online).
Fig. 2.
Explosive spore discharge and development of the spore cloud from a Sphagnum fimbriatum capsule with a diameter of 2·15 mm containing approx. 213 000 spores (calculated from formula in Sundberg, 2000). The pictures show snapshots (25 frames s−1) from video footage of: (A) the capsule on its shoot in a test tube in the frame before discharge; (B) the ‘spike’ at the moment of explosive spore discharge from the first frame; (C) the spore cloud after 0·24 s – note that the lowest part of the cloud has already started to settle while the upper parts are expanding upwards and sideways; (D) the centroid maximum at tcen = 0·72 s, when as much of the cloud is settling as is rising, used for obtaining a proxy of hcen, the maximum height of the average spore (in the ‘centre’ of the cloud); (E) peak height (hpeak) at tpeak = 2·44 s, when the cloud summit reaches its maximum (168 mm above the capsule top in this case) – all spores below the summit are now settling; (F) the cloud after 5·0 s, when all spores are descending and a large proportion has already settled while the remaining cloud is becoming difficult to observe because of a diminishing spore concentration. The vertical white scale bar in (A) represents 50 mm. (See also the video provided in Supplementary data, available online.)
Cloud centroid height (hcen) was reached at about tcen = 0·74 s after discharge (range: 0·33–1·57 s), and higher clouds took longer to reach hcen (linear regression; tcen (s) = 0·128 + 0·00727 × hcen (mm); n = 27; R2 = 0·35; P = 0·001; Fig. 2). Centroid height (hcen) ranged from 32 to 118 mm (average: 84 mm) and was thus 56 % of the peak height. Height of the cloud summit at tcen was 93 % (range: 82–99 %) of hpeak. Cloud width (wcen) at tcen ranged from 31 to 81 mm (average: 56 mm). The 3D-shape of the average cloud at tcen resembles a candle light bulb (Fig. 3). The peak (hpeak) was reached about 1·48 s after discharge (range: 0·52–2·47 s), twice as long as hcen. Higher clouds reached their maximum later. Peak height ranged from 57 to 200 mm (average: 151 mm) above the capsule (particles such as operculae and large spore clusters were ejected up to 267 mm). At tpeak, all spores below the cloud summit were descending (Fig. 2). Cloud width increased by 21 % during the 0·74 s from tcen to tpeak and expanded slowly thereafter until the cloud disintegrated by settling. At about 11 s after discharge (range: 4·7–19·3 s), no spores were visible above the capsule top on the films (solitary diluted spores were probably not captured by the camera). Settling time was positively related to hpeak and negatively related to spore diameter (n = 26; R2 = 0·52; P < 0·001 and P = 0·02, respectively).
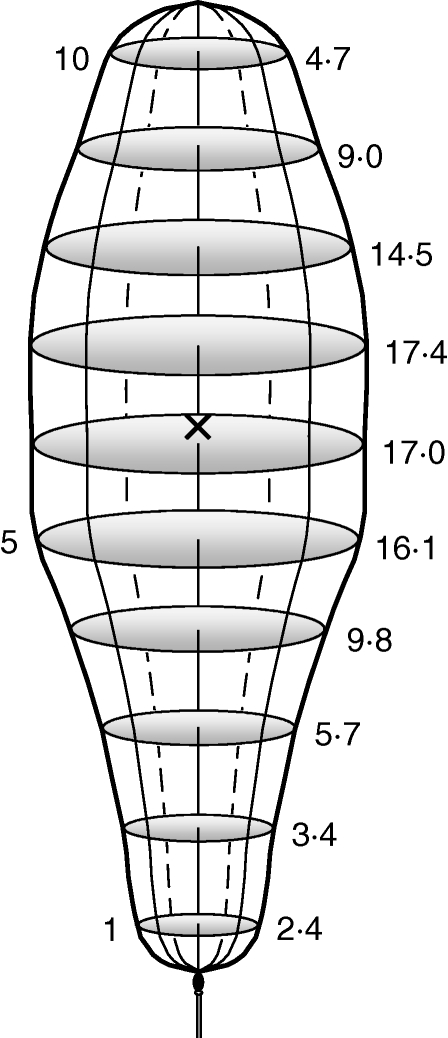
Fig. 3.
The 3D shape of the model Sphagnum spore cloud at the moment (tcen) when as many spores are ascending as descending, with all the normal irregularities and protrusions averaged away. Numbers to the right represent the estimated percentage of spores encountered at each of the ten evenly distributed positions (numbers on the left) along the cloud. The cross indicates the weighted average position (at 56·4 % of the distance from cloud base to summit) of spores, according to the cloud volume.
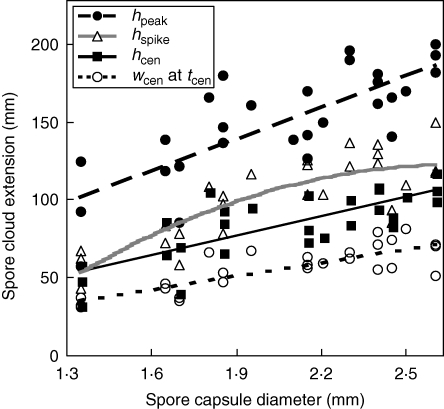
All spore cloud variables (hspike, hcen, wcen and hpeak) were strongly positively related to capsule diameter (D), with R2 ranging from 0·58 to 0·65 (P < 0·001; Fig. 4 and Table 2). Spore diameter (d; negative relationships) and the square of capsule diameter (D2; negative relationships) slightly improved R2 (and adjusted R2) in three of the regressions, as did spore colour (sdark; positive relationship for dark spores) and habitat (sforest; positive relationship for swamp forest species) in one case each, with R2 increasing to between 0·65 and 0·78 (Table 2).
Fig. 4.
Sphagnum spore cloud extensions after discharge in relation to capsule diameter: closed circles and long-dashed line (regression line) represent observations on peak height (hpeak) of the spore cloud; open triangles and grey curved line represent height of the spike (hspike) just after discharge; closed squares and unbroken line represent height of the centroid (the average spore maximum; hcen); open circles and short-dashed line represent horizontal width of the spore cloud (wcen) at the moment of centroid maximum (tcen). The lines are based on the simple models in Table 2, except for hspike that also includes the quadratic relationship to capsule diameter (the only term that showed improved adjusted R2 when including the quadratic directly after the linear relationship).
Table 2.
Best-fit stepwise regression models explaining extensions of the spore cloud (in mm), and simple models including only the linear relationship to capsule diameter as a predictor, at different post-release stages in Sphagnum (n = 27, except for hspike with n = 28)
| Cloud extension variable | Model | Cumulative R2 of predictors† (%) | Simple linear model |
|---|---|---|---|
| Spike height, hspike | −221·1 + 268·9 × D − 54·2 × D2 + 13·6 × sforest | 62·7***; 67·0*; 72·9* | −9·1 + 53·7 × D |
| Centroid height, hcen | −69·9 + 158·3 × D − 1·8 × d + 11·5 × sdark − 29 × D2 | 58·2***; 70·8**; 74·4*; 77·8(*) | −2·2 + 41·8 × D |
| Peak height, hpeak | 63·5 + 70·0 × D − 2·0 × d | 60·5***; 64·7(*) | 8·5 + 69·0 × D |
| Width at tcen, wcen | −43·1 + 95·6 × D − 0·9 × d − 17·0 × D2 | 65·3***; 69·7(*); 72·4(*) | −3·6 + 28·9 × D |
D, Capsule diameter (mm); sforest, swamp forest species (1/0; dummy variable); d, spore diameter (μm); sdark, dark spores (1/0; dummy variable).
***, P < 0·001; **, 0·001 < P < 0·01; *, 0·01 < P < 0·05; (*), 0·05 < P < 0·15.
† Cumulative R2 is the additive explanation of each of the introduced predictors, in the order added to the model. All added predictors resulted in higher model adjusted R2.
The shape of spore clouds varied in that large capsules had clouds that were significantly wider at the ‘waist’ than small capsules (up to 53 % and 31 % broader at positions 5 and 6, respectively; cf. Fig. 3; linear regressions; n = 27; R2 = 0·248 and 0·144; P = 0·008 and 0·05, respectively), and consequently small capsules had clouds that were straighter from bottom to top. Thus, capsule diameter did not affect the weighted average height position (hw) along the spore cloud. Clouds with small spores were wider in the upper parts and consequently narrower in the lower parts (linear regression; hw = 7·17 – 0·0439 × d; n = 27; R2 = 0·277; P = 0·005).
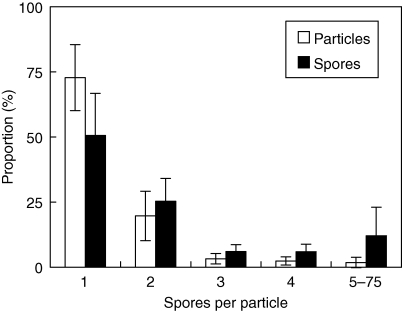
Of the spore particles in the clouds from the 12 capsules examined, single spores made up 73 ± 13 % (standard deviation; range among capsules: 43–89 %), clusters of two spores 20 ± 10 % (range: 9–43 %), and larger clusters the remaining 7 % (Fig. 5). Thus 51 ± 16 % (range: 20–74 %) of the spores were dispersed as singles, 25 ± 9 % (range: 15–43 %) as doubles, 6 ± 3 % as clusters of both three and four spores, and 12 ± 11 % as clusters containing 5–75 spores (range: 0–33 %; Fig. 5). There was no relationship between the proportion of spore particle sizes (large intraspecific variation) and any of the biological variables in Table 1.
Fig. 5.
The proportion of particles and spores, as single spores or clusters (containing various numbers of spores), deposited after discharge from 12 capsules of six Sphagnum species. Larger particles (clusters) have higher settling speeds and should affect deposition patterns. The class ‘5–75’ is the sum of all the clusters containing between 5 and 75 spores. Error bars show the s.d. among capsules.
Settling speed of spores
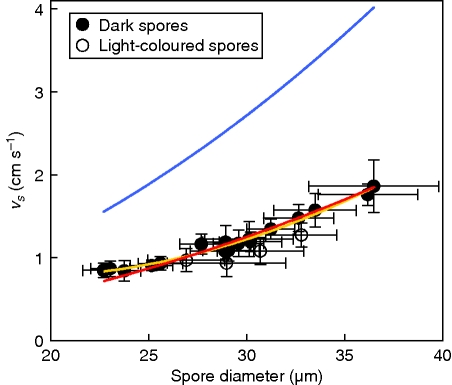
Sphagnum spores settled at speeds ranging from 0·84 to 1·86 cm s−1 among the 21 capsules of 14 species tested. Settling speed (vs, expressed in centimetres per second) showed a strong second-order curvilinear relationship to spore diameter (d, expressed in micrometres; Fig. 6). Also, there was an indication that dark spores (n = 17) settled slightly faster than brightly coloured ones (n = 4: S. fallax, S. lindbergii, S. tenellum; t-test on the residuals: P = 0·030; Fig. 6). The relationship between settling speed and spore diameter was described by the equation: vs = 2·68 – 0·176 × d + 0·00417 × d2 (n = 21; R2 = 0·926; P < 0·001). Using only dark spores, the relationship became: vs = 1·76 – 0·112 × d + 0·00314 × d2 (n = 17; R2 = 0·980; P < 0·001; Fig. 6). The relationship became similar when running the analysis on individual spores from a subset of eight capsules from six of the dark-spored species, excluding among others the species (S. cuspidatum) with the largest spores (vs = 1·08 – 0·0660 × d + 0·00234 × d2; n = 159; R2 = 0·891; P < 0·001). Settling speed was 46–58 % less than expected from spherical spores with the same density as water, according to Stokes' law. Settling speed was thus close to that expected for spherical spores having 68 % of the observed diameters, and was similarly close to the prediction of McCubbin's formula for ellipsoid spores (eqn 1), while levelling out faster towards smaller diameters than expected (outside the 95 % CI for diameters less than about 24 µm; Fig. 6). Spore diameters in the tested capsules ranged from 22·7 to 36·5 µm (individual spores: 19–43 µm), and spore height constituted generally 56·1 % (range in individual spores: 51–64 %) of the diameter (based on 16 spores each of S. fimbriatum and S. papillosum).
Fig. 6.
Settling speed (vs) of the aspherical Sphagnum spores in relation to spore diameter, and the second-order polynomial regression (yellow curve) that gives the best fit for capsules with dark spores. For comparison, the theoretical settling speeds, according to Stokes' law, for spherical particles with the same density as water and with the given diameter (upper blue curve), and for spherical particles with 68 % of the given diameter (lower red curve), are presented. Error bars show the s.d.
Timed spores did not differ in size from randomly selected spores. Spore diameters in these dry spores were on average 2·8 % smaller (t-test; P = 0·046) than in spores measured in water solution among the 12 species for which comparable data were available.
Spore clusters containing two (n = 83; from 19 capsules) and three spores (n = 10; from 8 capsules) showed settling speeds 38 ± 6 % (95 % CI) and 79 ± 26 % higher than for single spores, respectively.
DISCUSSION
Spore cloud development
The violent spore release in Sphagnum clearly works as a very efficient way to increase initial release height, which is at least one order of magnitude higher than the capsule protrusion above the moss surface. This must be important as peat mosses do not possess other mechanisms aiding passive spore liberation, such as a long and thin setae (sensitive to air movement; occasionally twisting), hanging capsules or peristome teeth found in peristomate mosses. Setae are normally a few centimetres long but may reach 10–12 cm in widespread taxa such as Meesia spp., Paludella squarrosa and Polytrichum spp. (Grout, 1936, 1940; Nyholm, 1958, 1969). The explosive spore discharge in Sphagnum means that spores at the top of the cloud will be exposed to higher wind speeds and turbulence than those further down, and that a majority of the spores are dispersed simultaneously (84–95 %, depending on species; Sundberg, 2005). In contrast, the mainly passive liberation mechanism in peristomate mosses probably means a more temporal distribution of spores. Many liverworts also exhibit violent spore release. Because the liverwort capsules are smaller, the mechanism differs (release by means of hygroscopically twisting elaters and longitudinal splitting of the capsule) and the direction of release is both upwards and laterally, the discharge distance is probably much smaller than in Sphagnum – in the case of Cephalozia bicuspidata ‘several millimetres’ (Ingold, 1965). Apparently, most Sphagnum species have superior initial release height of spores in comparison with other ground-living bryophytes. Whether that also results in more effective dispersal remains to be established, because spore release must be put in relation to the prevailing (close-to-ground) wind conditions.
It is tempting to suggest that the relatively limited geographical distribution (mainly south-eastern North America) of species in the morphologically atypical clade within section Subsecunda, including Sphagnum cyclophyllum, S. microphyllum, S. pylaesii and S. macrophyllum, is partly a result of ineffective spore release. They have small, thin-walled spore capsules (borne on short pseudopodia), with large operculae, having no or few pseudostomata and consequently no contraction of the capsule wall – thus lacking discrete, explosive spore discharge or other known features that promote dispersal (Andrews 1960, 1961; Shaw et al. 2004). All 14 boreal species in the present study are widely distributed, with circumpolar or amphi-Atlantic distribution patterns, and four species also occur in the southern hemisphere (Daniels and Eddy, 1990). This signifies that violent spore discharge is indeed an important and functional factor promoting long-distance dispersal. A recent study indicates ongoing trans-Atlantic migration of boreal sphagna (Szövényi et al., 2008).
The strong relationship between capsule size and release height confirms the results from a field experiment, in which Sphagnum species with large capsules were found to have more efficient dispersal than those with small capsules. This was found both in terms of the proportion of spores being dispersed further than the sampled radius of 3·2 m (almost twice as high in the species with the largest capsules, S. riparium, as compared with the species with smallest capsules, S. tenellum) and in the proportion of spores being discharged from the capsules (95 % in S. riparium vs. 84 % in S. tenellum; Sundberg, 2005). Thus species with large shoots (which are generally the ones having large capsules) have a dispersal advantage.
The negative effect of spore diameter (although a rather weak effect) on release height is surprising, because heavier particles should be expected to be ejected farther (distance proportional to the square of the diameter; Buller, 1909). A reasonable explanation is that the latter part of the spore cloud expansion is not so much affected by the actual spore ejection (because drag sets in quickly) but rather by eddies in the puff of air from the capsule, and that the speed of rising is rather small such that it exceeds the settling speeds of small spores but to a lesser extent than that of large spores. An additional possibility is that species with large spores may have less-well developed discharge – all large-spored species grow predominantly in open conditions with more turbulence promoting dispersal (thus selection pressure for higher discharge is reduced) whereas many small-spored species grow also in tree-covered habitats, usually swamp forests, with less wind (Sundberg and Rydin, 1998). The positive influence of being a swamp forest species on the height of the spike (hspike; Table 2) supports this. Swamp forest species are also over-represented as colonizers (in open habitats) of land-uplift islands in the Baltic Sea (Sundberg et al., 2006).
The highest measured speed at which Sphagnum spores were discharged (3·6 m s−1) is in the same order of magnitude as the maximum speed of petal opening in Cornus canadensis (6·7 m s−1; Edwards et al., 2005). The discharge speed for Sphagnum is probably a minimum value because during the recorded 0·033-s snapshot, retardation due to drag should have already set in, which is confirmed by the much shorter distance travelled in consecutive frames. Maximum speed of release should be tested with a high-speed camera.
Settling speed of spores
The slow settling velocities, about 48 % of the expected from the horizontal diameters (20–45 µm), means that Sphagnum spores have aerodynamic diameters of smooth spheres (the shape of most bryophyte spores) covering the range 15–31 µm. Peat mosses have been classified as large-spored bryophytes, based on spore diameters, but in terms of expected settling speed and dispersability their spores encompass both small and large spores (the arbitrary threshold diameter is 20 µm between the two classes; During, 1992). It appears that non-spherical spores are better adapted for wind-dispersal than spherical spores because they have a higher volume to settling speed ratio (in Sphagnum, the volume is equal to spherical spores with approx. 82 % of a given diameter). That light-coloured spores settled more slowly is likely to be a result of their lower density, which is in line with the difficulty of achieving their suspension in water, and their enhanced longevity, probably because they contain more lipids than dark spores (Sundberg and Rydin, 1998, 2000; cf. Duckett and Ligrone, 1992). Thus, light-coloured spores appear to have densities close to water, whereas dark spores have slightly higher densities.
There is only a minute discrepancy in intraspecific spore diameters between spores measured suspended in water solution (Sundberg and Rydin, 1998; Sundberg, 2000) and the dry ones in the present study (average 2·8 % smaller). In the former studies, it was noted that suspended spores tended to expand and change shape from heating by the microscope lamp, so perhaps there is no real size difference at all. Furthermore, at the moment of dehiscence, sporophyte water loss is over 70 % of the fresh weight, and only 15 % higher than the dry weight (Duckett et al., 2009), which indicates that spores are released in a relatively dry state. Altogether, these facts suggest that Sphagnum spores do not change settling speed during the course of dispersal, which will make dispersal modelling easier. The Sphagnum spore thus contrasts some pollen (Aylor, 2002) and spores of fungi (Buller, 1922) and myxomycetes (Tesmer and Schnittler, 2007) that change shape and diameter considerably and, at least in the case of pollen, also settling speed.
The relative settling velocities of spore clusters (two or three spores) were consistent with the relationship presented by Ferrandino and Aylor (1984), as their predicted values (+41·5 % and +75·4 %, respectively; eqn 2) were well within the 95 % confidence limits of the clusters in the present study. The high proportion of clusters (containing half of the discharged spores), probably explains why the spore clouds settled faster than would have been expected from the settling speed of single spores alone (Figs 2 and 5; video in Supplementary data, available online). Clusters should have an impact on deposition patterns and become over-represented close to the source. Thus, this may partly explain the unexpectedly steep modelled slopes of the deposition curves for four of the six Sphagnum species in the experiment by Sundberg (2005), in which 33–76 % of the spores escaped explanation even when the curves were extrapolated to infinity.
This study is the first to document and quantify the dynamics of spore discharge in Sphagnum, despite it being a well-known phenomenon since the days of Linnaeus. The spore discharge heights are possibly the largest among primarily wind-dispersed propagules in relation to the size of the discharge units. The results show that most sphagna have attributes that promote efficient dispersal, such as a relatively high initial release height and a low settling speed of spores. Larger capsules (occurring in species with large capitula) and smaller spores clearly promote dispersal over short distances, but their significance for longer distances is unclear. In a study of colonization by Sphagnum on land-uplift islands up to 40 km from the mainland, total spore output on the mainland was the primary variable predicting colonization efficiency among species (Sundberg et al., 2006). Here it is difficult to separate the relative roles of capsule and spore size as they co-vary with spore output, which also depends on species abundance and capsule density. The results of the present study have improved prediction of dispersal distances and colonization probabilities by making it possible to mechanistically model dispersal of Sphagnum spores in a reliable way.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of a video sequence (12 s) that shows the spore discharge event, development of the spore cloud and settling of spores from a Sphagnum papillosum capsule (diameter 2·4 mm) filmed in the chamber. The capsule contained approx. 172 000 relatively large spores (diameter approx. 33 µm).
Supplementary Material
ACKNOWLEDGEMENTS
I thank Gustaf Granath and Håkan Rydin for valuable comments on the text, Scott Spellerberg for revising the English, Stefan Björklund for help building the film chamber, Anders Ljungberg for the glass tube, Jonas Josefsson and Anders Rydberg for help with data extraction, and Urban Gunnarsson and Karolin Ring for help with spore capsule collection. This work was supported by Formas (grant number 215-2006-1062).
LITERATURE CITED
- Andrews AL. Notes on North American Sphagnum. XII. Sphagnum cyclophyllum. Bryologist. 1960;63:229–234. [Google Scholar]
- Andrews AL. Notes on North American Sphagnum. XIII. Sphagnum pylaesii. Bryologist. 1961;64:208–214. [Google Scholar]
- Aylor DE. Settling speed of corn (Zea mays) pollen. Aerosol Science. 2002;33:1601–1607. [Google Scholar]
- Aylor DE, Boehm MT. Quantifying aerial concentrations of maize pollen in the atmospheric surface layer using remote-piloted airplanes and Lagrangian stochastic modelling. Journal of Applied Meteorology. 2006;45:1003–1015. [Google Scholar]
- Bright DS, Milans KG. Lispix: a public domain scientific image analysis program for the PC and Macintosh. 2000 Abstract for the Microscopy & Microanalysis Annual Conference, Philadelphia, August 2000. [Google Scholar]
- Buller AHR. Researches on fungi. I. London: Longmans; 1909. [Google Scholar]
- Buller AHR. Researches on fungi. II. London: Longmans; 1922. [Google Scholar]
- Buller AHR. Researches on fungi. V. London: Longmans; 1933. [Google Scholar]
- Cao T, Vitt DH. Spore surface structure of Sphagnum. Nowa Hedwigia. 1986;43:191–220. [Google Scholar]
- Cavers F. Explosive discharge of antherozoids in Fegatella conica. Annals of Botany. 1903;17:270–274. [Google Scholar]
- Daniels RE, Eddy A. Handbook of European Sphagna. 2nd edn. London: HMSO; 1990. [Google Scholar]
- Di-Giovanni F, Kevan PG, Nasr ME. The variability in settling velocities of some pollen and spores. Grana. 1995;34:39–44. [Google Scholar]
- Duckett JG, Ligrone R. A survey of diaspore liberation mechanisms and germination patterns in mosses. Journal of Bryology. 1992;17:335–354. [Google Scholar]
- Duckett JG, Pressel S, P'ng KMY, Renzaglia KS. Exploding a myth: the capsule dehiscence mechanism and the function of pseudostomata in Sphagnum. New Phytologist. 2009;183:1053–1063. doi: 10.1111/j.1469-8137.2009.02905.x. [DOI] [PubMed] [Google Scholar]
- During HJ. Ecological classification of bryophytes and lichens. In: Bates JW, Farmer AM, editors. Bryophytes and lichens in a changing environment. Oxford: Clarendon Press; 1992. pp. 1–31. [Google Scholar]
- Edwards J, Whitaker D, Klionsky S, Laskowski MJ. A record-breaking pollen catapult. Nature. 2005;435 doi: 10.1038/435164a. 164. [DOI] [PubMed] [Google Scholar]
- Fenton NJ, Bergeron Y. Sphagnum spore availability in boreal forests. Bryologist. 2006;109:173–181. [Google Scholar]
- Ferrandino FJ, Aylor DE. Settling speed of clusters of spores. Phytopathology. 1984;74:969–972. [Google Scholar]
- Flatberg KI. The Norwegian Sphagna: a field colour guide. 2002 Norwegian University of Science and Technology, Vitenskapsmuseet Rapport Botanisk Serie 2002-1: 1–44 + 54 plates (available at: http://www.ntnu.no/vmuseet/botavd/g_bot_rapp/rapp_bot_2002-1.pdf. ) [Google Scholar]
- Greene DF. The role of abscission in long-distance seed dispersal by the wind. Ecology. 2005;86:3105–3110. [Google Scholar]
- Gregory PH. The microbiology of the atmosphere. 2nd edn. London: Leonard Hill; 1973. [Google Scholar]
- Grout AJ. Moss flora of North America north of Mexico. Vol. 1. Newfane: published by the author; 1936. [Google Scholar]
- Grout AJ. Moss flora of North America north of Mexico. Vol. 3. Newfane: published by the author; 1940. [Google Scholar]
- Hallingbäck T, Hedenäs L, Weibull H. Ny checklista för Sveriges mossor [New checklist of Swedish bryophytes] Svensk Botanisk Tidskrift. 2006;100:96–148. [Google Scholar]
- Howe HF, Smallwood J. Ecology of seed dispersal. Annual Review of Ecology and Systematics. 1982;13:201–228. [Google Scholar]
- Ingold CT. Spore liberation. Oxford: Clarendon Press; 1965. [Google Scholar]
- Kuparinen A. Mechanistic models for wind dispersal. Trends in Plant Science. 2006;11:296–301. doi: 10.1016/j.tplants.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Kuparinen A, Markkanen T, Riikonen H, Vesala T. Modeling air-mediated dispersal of spores, pollen and seeds in forested areas. Ecological Modelling. 2007;208:177–188. [Google Scholar]
- Linne CA. Mantissa plantarum altera. 1771 Stockholm. Available at: http://gallica.bnf.fr/ark:/12148/bpt6k966180 . [Google Scholar]
- McCubbin WA. Relation of spore dimensions to their rate of fall. Phytopathology. 1944;34:230–234. [Google Scholar]
- Nathan R, Katul GG, Horn HS, et al. Mechanisms of long-distance dispersal of seeds by wind. Nature. 2002;418:409–413. doi: 10.1038/nature00844. [DOI] [PubMed] [Google Scholar]
- Nawaschin S. Ueber der Sporenausschleuderung bei der Torfmoosen. Flora. 1897;83:151–159. [Google Scholar]
- Nyholm E. Illustrated moss flora of Fennoscandia. Lund: Swedish Natural Science Research Council; 1958. Fasc. 3. [Google Scholar]
- Nyholm E. Illustrated moss flora of Fennoscandia. Lund: Swedish Natural Science Research Council; 1969. Fasc. 6. [Google Scholar]
- Paton JA, Pearce JV. The occurrence, structure and functions of the stomata in British bryophytes. Transactions of the British Bryological Society. 1957;3:228–259. [Google Scholar]
- Rydin H, Sjörs H, Löfroth M. Mires. Acta Phytogeographica Suecica. 1999;84:91–112. [Google Scholar]
- Schofield WB. Introduction to bryology. New York, NY: Macmillan; 1985. [Google Scholar]
- Shaw J, Cox CJ, Boles SB. Phylogenetic relationships among Sphagnum sections: Hemitheca, Isocladus, and Subsecunda. Bryologist. 2004;107:189–196. [Google Scholar]
- Shimamura M, Yamaguchi T, Deguchi H. Airborne sperm of Conocephalum conicum (Conocephalaceae) Journal of Plant Research. 2008;121:69–71. doi: 10.1007/s10265-007-0128-6. [DOI] [PubMed] [Google Scholar]
- Skarpaas O, Auhl R, Shea K. Environmental variability and the initiation of dispersal: turbulence strongly increases seed release. Proceedings of the Royal Society B. 2006;273:751–756. doi: 10.1098/rspb.2005.3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soons MB, Heil GW, Nathan R, Katul GG. Determinants of long-distance seed dispersal by wind in grasslands. Ecology. 2004;85:3056–3068. [Google Scholar]
- Sundberg S. The ecological significance of sexual reproduction in peat mosses (Sphagnum) Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology. 2000;581:1–37. (available at: http://urn.kb.se/resolve?urn=urn:nbn:se:uu:diva-526. ) [Google Scholar]
- Sundberg S. Sporophyte production and spore dispersal phenology in Sphagnum: the importance of summer moisture and patch characteristics. Canadian Journal of Botany. 2002;80:543–556. [Google Scholar]
- Sundberg S. Larger capsules enhance short-range spore dispersal in Sphagnum, but what happens further away? Oikos. 2005;108:115–124. [Google Scholar]
- Sundberg S, Rydin H. Spore number in Sphagnum and its dependence on spore and capsule size. Journal of Bryology. 1998;20:1–16. [Google Scholar]
- Sundberg S, Rydin H. Experimental evidence for a persistent spore bank in Sphagnum. New Phytologist. 2000;148:105–116. doi: 10.1046/j.1469-8137.2000.00746.x. [DOI] [PubMed] [Google Scholar]
- Sundberg S, Hansson J, Rydin H. Colonization of Sphagnum on land uplift islands in the Baltic Sea: time, area, distance and life history. Journal of Biogeography. 2006;33:1479–1491. [Google Scholar]
- Szövényi P, Terracciano S, Ricca M, Giordano S, Shaw AJ. Recent divergence, intercontinental dispersal and shared polymorphism are shaping the genetic structure of amphi-Atlantic peatmoss populations. Molecular Ecology. 2008;17:5364–5377. doi: 10.1111/j.1365-294X.2008.04003.x. [DOI] [PubMed] [Google Scholar]
- Tackenberg O. Modeling long-distance dispersal of plant diaspores by wind. Ecological Monographs. 2003;73:173–189. [Google Scholar]
- Tesmer J, Schnittler M. Sedimentation velocity of myxomycete spores. Mycological Progress. 2007;6:229–234. [Google Scholar]
- Zeleny J, McKeehan LW. Die Endgeschwindigkeit des Falles kleiner Kugeln in Luft. Physikalische Zeitschrift. 1910;11:78–93. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.