Abstract
Introduction
Sonic hedgehog (SHH) is an essential regulator of smooth muscle apoptosis in the penis that has significant clinical potential as a therapy to suppress post-prostatectomy apoptosis, an underlying cause of erectile dysfunction (ED). Thus an understanding of how SHH signaling is regulated in the adult penis is essential to move the field of ED research forward and to develop new treatment strategies. We propose that hedgehog-interacting protein (HIP), which has been shown to bind SHH protein and to play a role in SHH regulation during embryogenesis of other organs, is a critical regulator of SHH signaling, penile morphology, and apoptosis induction.
Aims
We have examined HIP signaling in the penis and cavernous nerve (CN) during postnatal differentiation of the penis, in CN-injured, and a diabetic model of ED.
Methods
HIP localization/abundance and RNA abundance were examined by immunohistochemical (IHC) analysis and real-time reverse transcriptase-polymerase chain reaction (RT-PCR) in Sprague-Dawley rats between the ages of 7 and 92 days old, in CN-injured Sprague-Dawley rats and in BioBreeding/Worcester diabetic rats. HIP signaling was perturbed in the pelvic ganglia and in the penis and TUNEL assay was performed in the penis. CN tie, lidocaine, and anti-kinesin experiments were performed to examine HIP signaling in the CN and penis.
Results
In this study we are the first to demonstrate that HIP undergoes anterograde transport to the penis via the CN, that HIP perturbation in the pelvic ganglia or the penis induces apoptosis, and that HIP plays a role in maintaining CN integrity, penile morphology, and SHH abundance.
Conclusions
These studies are significant because they show HIP involvement in cross-talk (signaling) between the pelvic ganglia and penis, which is integral for maintenance of penile morphology and they suggest a mechanism of how nerves may regulate target organ morphology and function.
Keywords: Cavernous Nerve, Hedgehog-Interacting Protein, Erectile Dysfunction, Penile Differentiation, Sonic Hedgehog, Nitric Oxide
Introduction
Erectile dysfunction (ED) is a serious medical condition that affects 52% of men between the ages of 40 and 70 years [1], and 22% of men under 40 [2]. Prostatectomy and diabetic patients have an increased risk for developing ED with the prevalence of ED in diabetic men ranging from 20% to 71% (Massachusetts Male Aging Study), and from 30% to 87% in radical prostatectomy patients [3–6]. Current treatment options for ED, including oral therapy with phosphodiesterase type 5 (PDE5) inhibitors, are ineffective in 29–86% of prostatectomy patients [6–8] and in 30–50% of types I and II diabetic men [9–12]. The reduced efficacy of treatments makes novel therapeutic approaches to treat ED essential. Peripheral nerve injury to the cavernous nerve (CN) is common in diabetic and prostatectomy patients with ED. Patients and animal models of neuropathy-induced ED experience increased smooth muscle apoptosis and fibrosis of the penis, decreased penile size, and impaired erectile function [13–20] and in patients that do not respond to PDE5 inhibitors, smooth muscle cell atrophy is abundant [21]. Thus understanding the mechanisms that regulate smooth muscle apoptosis in the penis is critical for development of new therapies for ED treatment and prevention.
An essential regulator of penile smooth muscle, apoptosis, and erectile function is the secreted protein Sonic hedgehog (SHH) [15,22,23]. When SHH function is inhibited in the adult penis, there is a significant 12-fold increase in smooth muscle apoptosis [15], which causes ED [24], and SHH protein treatment of the CN-injured penis is able to suppress CN injury induced apoptosis [15], indicating that SHH has significant potential to be developed as a treatment to prevent post-prostatectomy apoptosis. However, the mechanism of how SHH signaling is regulated in the adult penis remains unclear. It is likely that neural input and/or trophic factors from the pelvic ganglia regulate SHH in the penis as SHH protein is significantly decreased in two models of neuropathy-induced ED and apoptosis [15,23,24], and lidocaine and colchicine treatment of the CN, which inhibit neural activity and transport, cause decreased SHH protein and increased apoptosis in the penis [23]. Examination of the factors transported by the CN that may regulate SHH abundance in the penis are critical for understanding the mechanism(s) of apoptosis regulation in the penis.
As decreased SHH protein is a cause of smooth muscle apoptosis in the penis and of ED, it would be highly beneficial to have a better understanding of the mechanisms that transduce the SHH signal and which regulate SHH abundance in the penis. The SHH signal is transduced through the interplay between patched (PTCH1) and smoothened (SMO). PTCH1 is a 12-transmembrane protein [25–27] that functions as a receptor for SHH. PTCH1 binds to SHH but does not transduce the intracellular signal (Figure 1). SMO, a 7-transmembrane protein that forms a receptor complex with PTCH1 [28], does not bind to SHH but transduces the SHH signal through activation of the GLI family of transcriptional factors (Figure 1). In the absence of SHH protein bound to PTCH1, PTCH1 represses down stream targets of SHH signaling by inhibiting the activity of SMO at the substoichiometric level [29]. When SHH protein binds to PTCH1, this relieves the repression of PTCH1 on SMO and allows transcription of SHH’s downstream targets [30–35] (Figure 1). A transcriptional target of SHH signaling is PTCH1 itself. When SHH binds to PTCH1, PTCH1 expression is increased [36]. By increasing the level of PTCH1 protein in responding cells, SHH signaling attenuates its own activity in a negative feedback loop [37]. Under normal conditions, PTCH1 competes with hedgehog-interacting protein (HIP) for SHH ligand, in SHH target cells (Figure 1). The interaction of PTCH1 and HIP modulate SHH signaling by limiting its diffusion and range of signaling.
Figure 1.
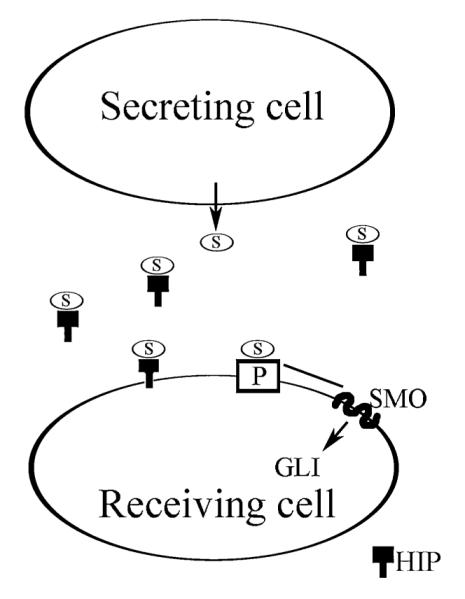
Diagram of Sonic hedgehog, patched (PTCH1), smoothened (SMO), and hedgehog-interacting protein (HIP) interactions. S = SHH; P = PTCH1.
We propose that the glycoprotein HIP is a potential regulator of SHH signaling in the penis. HIP is a type I membrane glycoprotein that acts as a negative regulator of the SHH pathway in many organs during embryogenesis by binding to the active form of SHH protein in vivo and sequestering SHH [38]. Overexpression of HIP in cells making SHH reduce the amount of SHH secreted into the media and overexpression of HIP in mouse epithelial cells attenuate their response to SHH, demonstrating that HIP can antagonize Hedgehog signaling when expressed in the responding cell. Ectopic expression of Shh leads to ectopic Hip expression in the embryo and in Shh mutants Hip expression is lost [38], indicating that HIP is not only a regulator of SHH but also a transcriptional target of SHH signaling in the developing embryo. Very little is known about HIP-SHH interactions in adult tissues. HIP is present in adult testis [39], lung [39], liver [40,41], brain [42,43], and endothelial cells of blood vessels and vascular-rich tissues [39]. The most information about HIP signaling in adult organs comes from studies of the brain in which HIP has been identified in both a membrane-associated and a soluble form [42]. HIP transcription does not occur upon SHH protein injection in the adult brain, suggesting different mechanisms of SHH regulation by HIP during embryonic development and in the mature brain [42]. The mechanism of how HIP attenuates SHH signal transduction in the adult remains unclear and is the focus of these studies. We showed that HIP is localized in penile smooth muscle and endothelium and in neurons of the pelvic ganglia/CN, that HIP undergoes anterograde transport from the pelvic ganglia to the penis via the CN, that perturbation of HIP signaling both in the pelvic ganglia and in the penis induces penile apoptosis, and that HIP is decreased in CN injury models of ED. To our knowledge this is the first report of HIP transport by peripheral nerves and these findings are significant because they provide a potential mechanism of how nerves regulate target organ morphology and function.
Materials and Methods
Animals
Sprague-Dawley rats postnatal day P115-120 (P115-P120) were obtained from Charles River (Wilmington, MA, USA). BioBreeding/Worcester (BB/WOR) diabetic and diabetes resistant control rats were obtained from an established breeding colony (Biomedical Research Models, Inc., Worcester, MA, USA). Rats were sacrificed for tissue collection 233 to 256 days after birth with 150 to 160 days of diabetes duration. Control rats were age-matched. Penises were harvested from euthanized males and were either frozen in liquid nitrogen or fixed in 4% paraformaldehyde.
All animals were cared for in accordance with the National Research Council publication Guide for Care and Use of Laboratory Animals.
CN Injury
P120 Sprague-Dawley rats were randomized into two groups: bilateral CN resection (N = 8) and sham abdominal exploration (control, N = 4). A 5-mm section of the CN was removed bilaterally using a KAPS (Asslar, Germany) Industrial microscope under direct vision through a midline abdominal incision. The prostatic capsule was manipulated in control animals without resecting the CN. Stress-related fluctuations of serum testosterone were minimized at the time of abdominal exploration through bilateral epididymo-orchiectomy and subcutaneous placement of a 2-cm piece of medical grade silastic tubing (Dow Corning Corp., Midland, MI, USA) filled with crystalline testosterone [44,45]. This method ensures reliable, uniform serum testosterone levels for both the control and CN-cut groups up to 28 days after placement [46]. Penises were harvested from euthanized males by sharp dissection 2 and 5 days after CN resection and were frozen in liquid nitrogen.
Time Course
Penises were harvested from euthanized males by sharp dissection in postnatal day 7, (P7, N = 7), P12 (N = 7), P22 (N = 7), P42 (N = 5), P62 (N = 4), and P92 (N = 4) Sprague-Dawley rats and were frozen in liquid nitrogen for real-time reverse transcriptase-polymerase chain reaction (RT-PCR) and immunohistochemical (IHC) analysis.
HIP Inhibitor and HIP Treatment of the Pelvic Ganglia
Affi-Gel beads (100–200 mesh, Bio-Rad Laboratories, Hercules, CA, USA) were equilibrated with HIP inhibitor (N = 6; 200 μg/mL, Santa Cruz Biotechnology, Santa Curz, CA, USA), HIP protein (N = 4, 0.13 μg/μL, R&D Systems [Minneapolis, MN, USA]), or Dulbecco’s phosphate-buffered saline (PBS) (control, N = 6) overnight at 4°C. Approximately 10–20 beads were injected directly under the pelvic ganglia bilaterally in adult Sprague-Dawley rats. Injection was not made into the ganglia itself as this would likely destroy the ganglia. Rats were sacrificed at 2 and 5 days following bead injection and the penis tissue was either frozen in liquid nitrogen for IHC analysis, or fixed in 4% paraformaldehyde and embedded in paraffin prior to TUNEL analysis. Pelvic ganglia and CN from HIP inhibited rats were also isolated for electron microscopy (EM).
HIP Inhibitor and HIP Treatment of the Corpora Cavernosa
Affi-Gel beads (100–200 mesh, Bio-Rad Laboratories) were equilibrated with HIP inhibitor (N = 3, 200 μg/mL, Santa Cruz Biotechnology; sc-6159, affinity-purified goat polyclonal antibody raised against a peptide mapping at the N-terminus of HIP protein of rat origin; the antibody binds to HIP in vivo and prevents HIP binding to SHH), HIP protein (N = 3, 0.13 μg/μL, R&D Systems), or PBS (control, N = 3) overnight at 4°C. Approximately 30–40 beads were injected directly into the corpora cavernosa of P120 Sprague-Dawley rat penises. Rats were sacrificed at 2 days following bead injection. Penises were excised and either frozen for IHC or fixed in 4% paraformaldehyde for TUNEL assay.
CN Tie Experiments
A midline abdominal incision was made with a scalpel under direct vision through a KAPS Industrial microscope in adult (P115-120) Sprague-Dawley rats. The pelvic ganglia and CN were exposed and surgical silk (9-0) was used to tie off the CN (double knot). Two (N = 9) and 3 (N = 3) days after the tie was placed, rats were sacrificed and the pelvic ganglia and CN were excised and frozen in OCT. The contralateral pelvic ganglia/CNs, which did not have a tie, were used as controls. IHC for HIP was performed on sectioned CNs (14-μm sections). Alexa Fluor 488 rabbit anti-goat, chicken anti-goat, and donkey anti-goat secondary antibodies were used at 1/400 (Molecular Probes, Eugene, OR, USA). Potential buildup of HIP in the CN was analyzed using a fluorescent microscope (Leitz, Jasper, IN, USA) and a Nikon (Tokyo, Japan) digital camera.
Lidocaine Treatment of the CN
Two percent lidocaine HCl (Hospira, Inc., Lake Forest, IL, USA) and PBS (which was used as a control) were soaked in Gel-Foam (Ferrsan, Soeborg, Denmark). A midline abdominal incision was made with a scalpel under direct vision through a KAPS Industrial microscope. The lidocaine-treated (N = 3) and control PBS-treated (N = 3) Gel-Foam was placed on top of the CN bilaterally, of adult Sprague-Dawley rats (P115-120). After 2 days of lidocaine or PBS treatment penises were harvested from euthanized males by sharp dissection and were frozen in liquid nitrogen for quantitative IHC.
Anti-Kinesin Treatment of the CN
Affi-Gel beads (100–200 mesh, Bio-Rad Laboratories) were equilibrated with mouse anti-kinesin (1.8 mg/mL, Sigma [St. Louis, MO, USA], N = 4) and PBS (which was used as a control, N = 3) overnight at 4°C. Approximately 10–20 beads were injected directly under the pelvic ganglia bilaterally in adult Sprague-Dawley rats. Rats were sacrificed at 3 days following bead injection/antikinesin treatment and the penis tissue was frozen in liquid nitrogen or fixed in 4% paraformaldehyde. Frozen penis sections (16 μm) were treated with 1/100 goat polyclonal HIP (D-15, Santa Cruz Biotechnology) and with the Alexa Fluor 488 chicken anti-goat secondary antibody (1/400, Molecular Probes) in order to quantify HIP in the corpora cavernosa after anti-kinesin treatment of the pelvic ganglia.
5E1 SHH Inhibitor Treatment of the Pelvic Ganglia
Affi-Gel beads (100–200 mesh, Bio-Rad Laboratories) were equilibrated with 5E1 SHH inhibitor (N = 5, 378 μg/mL, Jessel, Hybridoma Bank at the University of Iowa), PBS (control, N = 3), or mouse IgG (378 μg/mL, control, N = 4) overnight at 4°C. Approximately 10–20 beads were injected directly under the pelvic ganglia bilaterally in adult Sprague-Dawley rats. Rats were sacrificed at 1 day following bead injection/SHH inhibition, and the penis tissue was frozen in liquid nitrogen. Frozen penis sections (16 μ) were treated with 1/100 goat polyclonal HIP (D-15, Santa Cruz Biotechnology) and with the Alexa Fluor 488 rabbit anti-goat secondary antibody (1/400, Molecular Probes) in order to quantify HIP in the corpora cavernosa after SHH inhibition in the pelvic ganglia.
TUNEL Assay for Apoptosis
TUNEL assay was performed using the Apoptag kit (Chemicon International, Temecula, CA, USA) on isolated penis tissue fixed over night at 4°C in 4% paraformaldehyde, embedded in paraffin, and sectioned 16 μ in thickness as described previously [15]. All cells were stained for comparison using propidium iodide (0.02 Lg/mL). Fluorescent apoptotic cells were observed under a fluorescent microscope (Leitz) and photographed using a Nikon digital camera. Quantification of apoptosis was performed by counting the total number of cells and the number of apoptotic cells in a given field selected at random by visual observation. The number of apoptotic cells/all cells in five fields from each section and five sections for each penis were counted. Statistics were performed using the Excel program (Microsoft) and the ratio of apoptotic cells/all cells was reported ± the standard error of the mean (SEM). A t-test was performed to determine significant differences in apoptosis. Analysis of variance (anova) was performed to determine significant differences in apoptosis between HIP-inhibited and HIP-treated tissues using SPSS software version 17 (SPSS Inc., Chicago, IL, USA).
IHC Analysis and Confocal Microscopy
IHC was performed as previously outlined [15,22,24] on penis tissue assaying for goat polyclonal SHH (1/100,N-19, SC-1194, Santa Cruz Biotechnology), HIP (D-15, Santa Cruz Biotechnology, SC-6159, affinity-purified goat polyclonal antibody raised against a peptide mapping at the N-terminus of HIP of rat origin), PTCH1 (Santa Cruz Biotechnology, SC-6149, affinity-purified goat polyclonal antibody raised against a peptide mapping at the amino terminus of PTCH1 of mouse origin), and mouse monoclonal ACTA1 (Sigma). Secondary antibodies used were Alexa Fluor 488 rabbit anti-goat, chicken anti-goat, donkey anti-goat, and Alexa Fluor 594 chicken anti-mouse (1/300 or 1/400, Molecular Probes). Negative controls were performed with secondary only (without primary) to test for non-specific staining and autofluorescence. Sections were mounted using Pro-Tex Mounting Medium (Baxter Diagnostics, Inc., Pittsburgh, PA, USA). Microscopy was performed using a fluorescent microscope (Leitz) and photographed using a Nikon digital camera. Confocal microscopy was performed using a Zeiss LSM laser scanning confocal microscope and Zeiss LSM 510 software. SHH and HIP proteins were quantified using the Image J program [47]. Total fluorescence was measured in five fields from each section and five sections for each penis. Statistics were performed using the Excel program (Microsoft) and the results were reported ± SEM. A t-test was performed to determine significant differences in SHH and HIP abundance. anova was performed to determine significant differences in SHH, PTCH1, and HIP proteins between 2 and 5 days after CN injury using SPSS software version 17. Two-way anova was performed to determine significant differences in HIP proteins between 2 and 5 days of HIP inhibition using SPSS software version 17.
EM
EM was performed as described previously [48] on corpora cavernosa and CN from adult Sprague-Dawley rats that had their pelvic ganglia treated with PBS as a control (N = 3) or HIP inhibitor (N = 3, 200 μg/mL, Santa Cruz Biotechnology) via Affi-Gel beads for 5 days (N = 3). Isolated corpora cavernosa and CN were fixed in 2.5% glutaraldehyde, postfixed in 1% OsO4, dehydrated and embedded in Epon resin. These sections were cut and stained with 2% uranyl acetate and 3% lead citrate. EM was performed using a Zeiss Electron Microscope 900 to examine which cell type was undergoing apoptosis in the corpora cavernosa and to examine axonal degeneration in the CN.
Quantification of RNA Expression by Real-Time RT-PCR
RNA from penis tissue was isolated with TRIzol (Invitrogen, Carlsbad, CA, USA) and DNase treated as described previously [15]. CDNA was synthesized from 300 ng of RNA using the Gene Amp RNA PCR Core kit (Perkin-Elmer, Branchburg, NJ, USA). Real-time RT-PCR was performed on the Opticon Monitor system (Bio-Rad, Irvine, CA, USA) using SYBR Green Super UDG mix (Invitrogen) according to the manufacturer’s recommendations. RT-PCR reactions were set up in microtubes in a volume of 20 μL. The reaction components included 4 uL of cDNA and 20 nM of each primer (Integrated DNA Technologies, Coralville, IA, USA). For primer sequences see Table 1. Negative controls with out cDNA were run to ensure the absence of contamination. Amplification was performed with the following temperature cycling: an initial step at 50°C for 2 minutes, 95°C for 2 minutes, and then 40 cycles of 95°C for 15 seconds, 58°C for 30 seconds, and 72°C for 30 seconds. Fluorescent readings were completed after each cycle. The threshold cycle was automatically calculated by the Opticon Monitor and a melt curve was generated from 65° to 90°C with fluorescent readings every 0.2°C. Specificity of primers was verified by melt curve analysis and sequencing at the Northwestern University Center for Genetic Medicine Sequencing Core Facility. Shh, Ptch1, and Hip were normalized to Ribosomal subunit L-19 (Rpl19), malate dehydrogenase (Mdh), and Ribosomal subunit L-32 (Rpl32) housekeepers by the 2−ΔΔCT method [49]. Assays were performed in triplicate on individual tissue specimens, the results averaged and the product ratios reported as the mean plus or minus the standard error of the mean. Excel (Microsoft) was used for statistical analysis and a t-test was used to determine significant changes in RNA expression. anova was performed to determine significant differences in Shh, Ptch1, and Hip expression between 2 and 5 days after CN injury using SPSS software version 17.
Table 1.
Real-time RT-PCR Primer Sequences
| HIP-F: 5′-CTATTGGGCCTCACGACCAC-3′ |
| HIP-R: 5′-TTCCAGAAACACCCTGGCTG-3′ |
| MDH-F: 5′-TCTGCCACTCTGTCCATGGCTTAT-3′ |
| MDH-R: 5′-TGCCAATGCCTAGGTTCTTCTCCA-3′ |
| PTCH-F: 5′-GTACATGGACCGGCCTTGC-3′ |
| PTCH-R: 5′-TCAAAACAAGGGCCACATCA-3′ |
| RPL19-F: 5′-GAGCACATCCACAAACTGAAGGCA-3′ |
| RPL19-R: 5′-TGATCTCCTCCTTCTTGGCTTGGA-3′ |
| RPL32-F: 5′-TCTGGTGAAGCCCAAGATC-3′ |
| RPL32-R: 5′-CTCTGGGTTTCCGCCAGT-3′ |
| SHH-F: 5′-AGTTTATCCCCAACGTAGCC-3′ |
| SHH-R: 5′-TTGGGGGTGAGTTCCTTAAA-3′ |
Results
Localization of HIP in the Developing Penis
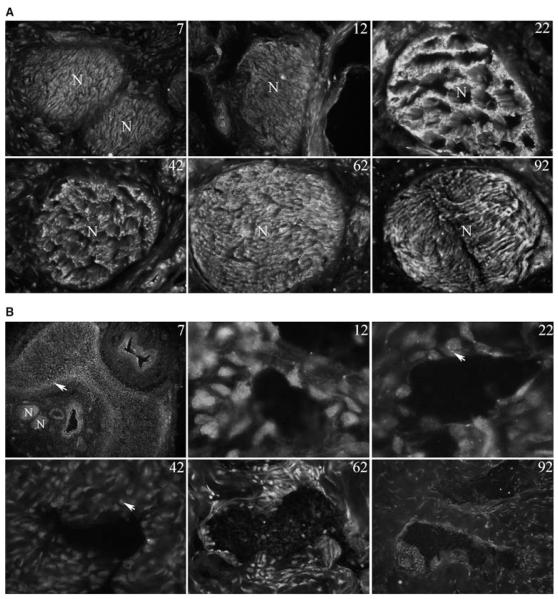
IHC analysis was performed on penis tissue assaying for HIP in postnatal day 7–92 (P7-P92, N = 4–7 for each age) rat penis. HIP was abundant in the nerves at all ages assayed (Figure 2). In P22 and P42 penis the staining pattern in the nerve appeared unusual, with suggestion of a higher order of structure not present at other ages in the nerve (Figure 2). HIP was also present in a layer under the tunica at P7 (Figure 2). As differentiation into smooth muscle and endothelium occurred HIP became localized in the lining of the sinusoidal tissue of the corpora cavernosa (Figure 2) and in the urethra (not shown). The morphology of the sinusoid lining is not exclusively of the adult configuration until P62 in the rat. At this time smooth muscle and endothelium are clearly differentiated (Figure 2). At P12–P42, intense HIP staining was observed in small structures within and between the developing smooth muscle cells, which suggests HIP involvement in intercellular signaling in penile smooth muscle.
Figure 2.
Immunohistochemical analysis of hedgehog-interacting protein (HIP) in the dorsal nerve bundle (A) and in the corpora cavernosa (B) of the penis of P7-P92 Sprague-Dawley rats. (a) HIP protein is localized in the nerves of the penis at all ages assayed. An unusual ultrastructure was observed in the nerves at P22 and P42. X250. (B) HIP was observed in a layer under the tunica at P7 (×1,000). HIP localization was restricted to the developing sinuses of the corpora cavernosa and individual cells between the sinuses that are likely fibroblasts at P12–P22 (×1,000) and at P42–P92 (×400). At P12, P22, and P42, intense HIP staining was observed in small structures within and between the developing smooth muscle cells. Arrows indicate HIP staining.
Time Course of Hip Expression in the Developing Penis
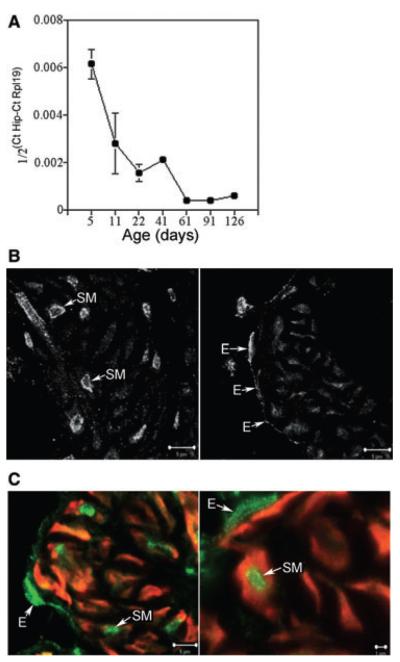
Hip expression was quantified by real-time RT-PCR (N = 4–7 for each age), and was found to be most abundant during the first 2 weeks after birth when sinusoidal differentiation takes place in the corpora cavernosa. Hip expression decreased consistently with age as sinusoidal differentiation was completed (Figure 3). A small spike in Hip expression was observed with the onset of puberty (P40-60, Figure 3), which may suggest hormonal regulation at this time by testosterone. Hip expression in the adult penis remained constant but low (Figure 3).
Figure 3.
Quantification of Hip RNA and localization of the hedgehog-interacting protein (HIP) in penis tissue. (A) Time course of Hip RNA expression quantified by real-time reverse transcriptase-polymerase chain reaction in Sprague-Dawley rat penises. Hip expression was most abundant during the first week after birth. Hip expression remained constant but low by comparison in the adult penis. A small spike in Hip expression was observed with the onset of puberty (P40-P60). (B) Confocal microscopy of normal Sprague-Dawley rat penises assayed for HIP protein. HIP was abundant in the nucleus and cytoplasm of endothelial cells and in the cellular membrane and cytoplasm of smooth muscle cells of the corpora cavernosal sinusoidal tissue. (C) Dual ACTA1/HIP IHC confirms the presence of HIP protein in penile smooth muscle of control and 2 days cavernous nerve-cut rats. E = endothelium; SM = smooth muscle.
Localization of HIP in the Adult Penis
IHC analysis and confocal microscopy were performed on normal adult Sprague-Dawley rat penis assaying for HIP (N = 5). HIP was abundant in the nucleus and cytoplasm of endothelial cells and in the cellular membrane and cytoplasm of smooth muscle cells of the corpora cavernosa (Figure 3). Dual ACTA1/HIP IHC confirms the presence of HIP in penile smooth muscle of control and 2 day CN-cut rats (Figure 3, N = 4 for each group). The localization of HIP appeared unaltered at 5 days after CN injury although grossly, HIP staining appeared less intense and in a smaller number of smooth muscle cells (data not shown).
HIP protein was Decreased and Hip RNA Expression was Increased with CN Injury
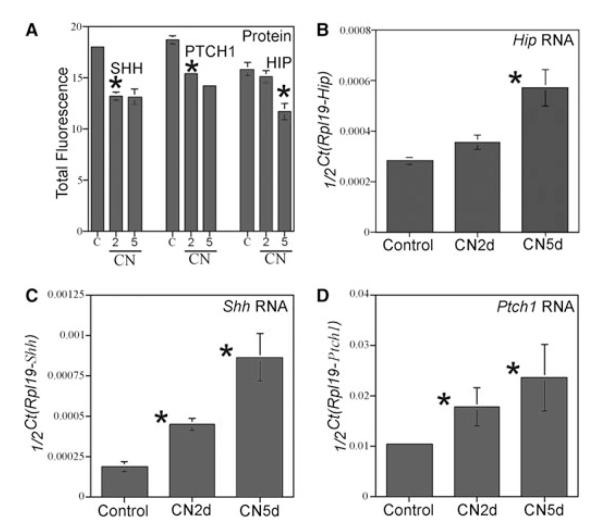
HIP protein was quantified in control and CN-injured penises at 2 and 5 days after CN injury (N = 4 for each group). HIP was unchanged at 2 days (P value = 0.22) but was significantly decreased 1.4-fold 5 days after CN injury (P value = 0.004, Figure 4A). anova shows that HIP was significantly decreased between 2 and 5 days after CN injury (P value = 0.006, Figure 4A). SHH and PTCH1 proteins were significantly decreased at 2 (1.4- and 1.2-fold, respectively) and at 5 days (1.4- and 1.3-fold, respectively) after CN injury (SHH P values = 1.3 × 10−5 and 0.0004, PTCH1 P values = 3.21 × 10−5 and 0.0003, Figure 4A). anova shows that SHH and PTCH1 proteins were unchanged between 2 and 5 days after CN injury (P value = 0.97 and 0.35, respectively, Figure 4A).
Figure 4.
Quantification of Hip, Shh, and Ptch1 RNA and hedgehog-interacting protein (HIP) in cavernous nerve (CN)-cut rats. (A) Quantification of SHH, PTCH1, and HIP proteins in control, 2 and 5 days CN-injured Sprague-Dawley rat penises. SHH and PTCH1 proteins were significantly decreased at 2 and 5 days after CN injury. HIP was unchanged at 2 days after CN injury but was significantly decreased 1.4-fold 5 days after CN injury. (B) Hip RNA was quantified by real-time reverse transcriptase-polymerase chain reaction (RT-PCR) using Rpl19 as an internal standard. There was no change in Hip expression at 2 days after CN injury however Hip expression was significantly increased twofold at 5 days after CN injury (P value = 0.03). (C) Shh RNA was quantified by real-time RT-PCR using Rpl19 as an internal standard. Shh expression significantly increased 2.4 and 1.7-fold at 2 and 5 days after CN injury (P values = 0.001 and 0.002). (D) Ptch1 RNA was quantified by real-time RT-PCR using Rpl19 as an internal standard. Ptch1 expression significantly increased 4.6- and 2.3-fold at 2 and 5 days after CN injury (P values = 0.05 and 0.03).
Hip RNA was quantified by real-time RT-PCR, which showed no change in Hip expression at 2 days after CN injury; however, Hip was significantly increased twofold (P value = 0.03) at 5 days after CN injury (Figure 4B, N = 4 for each group). anova shows no difference in Hip expression between 2 and 5 days after CN injury (P value = 0.06, Figure 4A). Shh and Ptch1 expression were increased 2.4-, 4.6-, and 1.7-, and 2.3-fold, respectively, at 2 and 5 days after CN injury (Shh P value = 0.001 and 0.002, Ptch1 P values = 0.05 and 0.03, Figure 4C,D). anova shows that Shh expression was significantly increased between 2 and 5 days after CN injury (P value = 0.005, Figure 4C), however, Ptch1 expression remained unaltered between 2 and 5 days after CN injury (P value = 0.34, Figure 4D).
Perturbation of HIP Signaling in the Pelvic Ganglia Caused Apoptosis Induction and Decreased SHH Protein in the Corpora Cavernosa
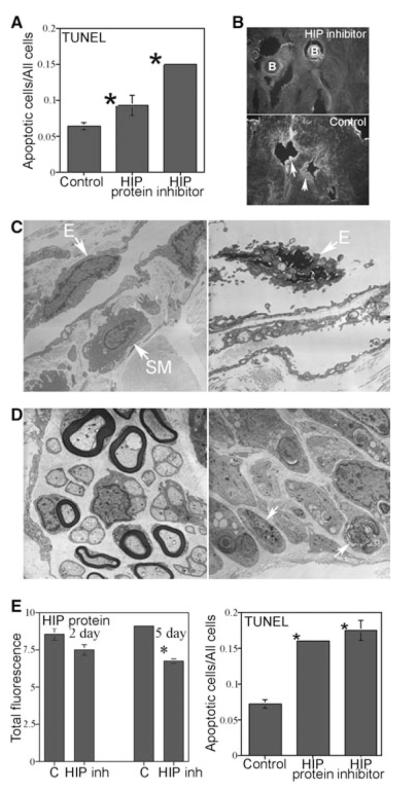
Affi-Gel beads soaked in HIP inhibitor, HIP protein, or PBS (control) were implanted under the pelvic ganglia of adult Sprague-Dawley rats (N = 6 for each group). Verification of HIP inhibition was performed in vivo by staining the HIP inhibited penis tissue with a second HIP antibody (Abcam ab39208). As the HIP inhibitor was already bound to HIP in the corpora cavernosal tissue, the Abcam antibody should not stain in the region of the Affi-Gel beads; however, staining should be present in untreated tissue away from the beads as was observed (Figure 5). The TUNEL assay showed that apoptosis was increased 2.4-fold in the corpora cavernosa after 2 days of HIP inhibition in the pelvic ganglia (P value = 2.9 × 10−5, Figure 5) and 1.5-fold in the corpora cavernosa after HIP treatment of the pelvic ganglia (P value = 0.02, Figure 5). anova showed a significant difference in apoptosis when comparing effects of HIP inhibitor vs. HIP (P value < 0.0001, Figure 5A). EM of penis from rats that were treated with HIP inhibitor in the pelvic ganglia showed much less abundant smooth muscle upon visual observation, and endothelial apoptosis was easily identifiable (Figure 5). CNs of the rats that were treated with HIP inhibitor in the pelvic ganglia displayed axonal degeneration and demyelination of CN fibers in comparison with controls (Figure 5).
Figure 5.
Affi-Gel beads soaked in hedgehog-interacting protein (HIP) inhibitor, HIP protein, and phosphate-buffered saline (control) were implanted under the pelvic ganglia of Sprague-Dawley rats and TUNEL, confocal microscopy, and HIP protein quantification were performed in the penis. (A) TUNEL assay showed that apoptosis was increased 2.4-fold in the corpora cavernosa after 2 days of HIP inhibition in the pelvic ganglia and 1.5-fold in the corpora cavernosa after HIP protein treatment of the pelvic ganglia. (B) Verification of HIP inhibitor function in vivo in the corpora cavernosa was performed by staining the HIP inhibited penis tissue with a second HIP antibody (Abcam ab39208). As the HIP inhibitor was already bound to HIP in the corpora cavernosal tissue, the Abcam antibody should not stain in the region of the Affi-Gel beads; however, staining should be present in untreated tissue away from the beads as was observed. Arrows indicate HIP protein. (X160). (C) Electron microscopy (EM) of HIP inhibited corpora cavernosa showed much less smooth muscle abundance by comparison with controls (×44,000) and endothelial apoptosis was evident (×70,000). (D) EM of HIP inhibited CN (×30,000) showed axonal degeneration and demyelination of nerve fibers by comparison with controls (×44,000). Arrows indicate degenerating axons. (E) HIP protein in the corpora cavernosa remained unchanged at 2 days of HIP inhibition in the pelvic ganglia but was significantly decreased 1.4-fold after 5 days of HIP inhibition in the pelvic ganglia. TUNEL assay showed that apoptosis was increased 2.4-fold in the corpora cavernosa after 2 days of HIP inhibition directly in the penis and 2.2-fold in the corpora cavernosa after direct HIP protein treatment of the penis via Affi-Gel beads. B = Affi-Gel bead; E = endothelium; SM = smooth muscle.
HIP protein in the corpora cavernosa remained unchanged after 2 days of HIP inhibition in the pelvic ganglia (P value = 0.06) but was significantly decreased 1.4-fold after 5 days of HIP inhibition in the pelvic ganglia (P value = 0.0002, Figure 5E). Two-way anova showed a significant difference in HIP protein when comparing effects of treatment (P value < 0.0001) but not of day (P value = 0.71, Figure 5E).
HIP inhibition in the pelvic ganglia for 2 days caused a 1.5-fold decrease in SHH protein in the corpora cavernosa (control = 21.47 ± 1.26, HIP inhibited = 14.06 ± 0.52, P value = 0.003). After 5 days of HIP inhibition in the pelvic ganglia, SHH protein was decreased 1.2-fold in the corpora cavernosa (control = 9.22 ± 0.37, HIP inhibited = 7.63 ± 0.11, P value = 0.007). However, HIP protein treatment of the pelvic ganglia did not alter SHH protein in the corpora cavernosa (control = 25.54 ± 0.40, HIP = 25.30 ± 0.44, P value = 0.36).
HIP Protein Remained Unchanged in the Corpora Cavernosa After SHH Inhibition in the Pelvic Ganglia
Affi-Gel beads, soaked in 5E1 SHH inhibitor (N = 5), PBS (control, N = 3), or mouse IgG (control, N = 4), were implanted under the pelvic ganglia. After 1 day of SHH inhibition in the pelvic ganglia, SHH protein was decreased 1.1-fold in the corpora cavernosa (control PBSN = 17.93 ± 0.19, SHH inhibited = 16.35 ± 0.34, P value = 0.007, control mouse IgG = 16.84 ± 0.4); however, HIP protein remained unchanged in the corpora cavernosa (control = 15.31 ± 0.53, SHH inhibited = 14.61 ± 0.38, P value = 0.12).
Perturbation of HIP Signaling in the Penis Caused Apoptosis Induction in the Corpora Cavernosa
Affi-Gel beads soaked in HIP inhibitor, HIP protein, or PBS (control, N = 3 for each group) were injected into the corpora cavernosa of adult Sprague-Dawley rats. TUNEL assay showed that apoptosis was increased 2.4- and 2.2-fold in the corpora cavernosa after 2 days of HIP inhibition (P value = 0.001) and HIP treatment respectively in the penis (P value = 0.0007, Figure 5E). anova showed no significant difference in apoptosis when comparing effects of HIP inhibitor vs. HIP protein (P value = 0.37, Figure 5E).
CN Tie Experiments Identify Anterograde HIP Transport
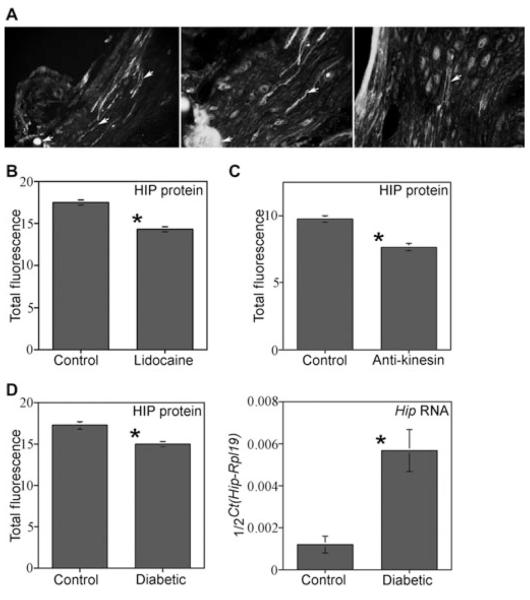
A tie was placed bilaterally on CNs of adult Sprague-Dawley rats in order to determine if HIP protein was being transported from the pelvic ganglia to the penis via the CN. IHC of the CN/pelvic ganglia assayed for HIP show buildup of HIP protein on the ganglia side of the tie 2 (N = 9) and 3 (N = 3) days after tie placement (Figure 6), indicating that HIP under goes anterograde transport from the pelvic ganglia to the penis via the CN.
Figure 6.
Hedgehog-interacting protein (HIP) localization and quantification in the cavernous nerve (CN) tie, lidocaine, anti-kinesin-treated Sprague-Dawley rats, and in diabetic rats. (A) A tie was placed on the CNs in order to determine if HIP protein was under going anterograde transport to the penis via the CN. HIP protein built up on the ganglia side of the tie after 2 days (left and middle, ×100, and right, ×250) showing that HIP protein was transported by the CN. HIP protein was observed originating in neurons of the pelvic ganglia (Right, ×160). (B) HIP protein was significantly decreased 1.2-fold in corpora cavernosa of rats in which their CN’s were treated with lidocaine. (C) HIP was significantly decreased 1.3-fold in corpora cavernosa of rats in which their CN’s were treated with anti-kinesin. (D) HIP protein was significantly decreased 1.2-fold in diabetic penises and Hip RNA expression was increased 3.1-fold in diabetic penises.
Lidocaine Treatment of the CN Caused Decreased HIP in the Corpora Cavernosa
HIP was quantified in penis tissue isolated from adult Sprague-Dawley rats in which the CN’s were treated bilaterally with either lidocaine or PBS (N = 3 for each group) in order to determine the effect of cessation of neural activity and transport on penile HIP abundance. HIP protein was significantly decreased 1.2-fold in the corpora cavernosa after lidocaine treatment of the CN (P value = 0.015, Figure 6). We have previously shown that apoptosis is increased 1.6-fold after 2 days of lidocaine treatment of the CN [23].
Anti-Kinesin Treatment of the CN Decreases HIP in the Corpora Cavernosa
HIP protein was quantified in penis tissue isolated from adult Sprague-Dawley rats in which their CN’s were treated bilaterally with either anti-kinesin (N = 4) or PBS (N = 3) in order to determine the effect of blocking anterograde transport on penile HIP abundance. HIP protein was significantly decreased in the corpora cavernosa after 3 days of anti-kinesin treatment of the pelvic ganglia (anti-kinesin = 7.61 ± 0.27, PBS = 9.72 ± 0.27, P value = 0.002, Figure 6).
HIP Protein was Decreased and Hip RNA was Increased in a Diabetic Model of Neuropathy, the BB/WOR Rat
HIP protein and Hip RNA were quantified in BB/WOR diabetic and BB/WOR control penis tissue (N = 4 for each group). HIP protein was significantly decreased 1.2-fold in diabetic penis (P value = 0.001) and Hip RNA was significantly increased 3.1-fold in diabetic penises (P value = 0.012, Figure 6).
Discussion
We propose that HIP protein is transported from pelvic ganglia neurons to the penis via the CN. This hypothesis is supported by CN tie experiments in which HIP protein built up on the ganglia side of the tie, indicating anterograde transport of HIP in the CN. Lidocaine and antikinesin treatment of the CN also decreased HIP protein in the penis, confirming HIP transport by the CN. This is an important finding because it may shed light on one way in which peripheral nerves communicate with tissues that they innervate and how nerves maintain end organ morphology in adult organs. Only one other study in adult tissues has suggested that HIP was transported between tissues. The authors based their observation on comparison of HIP protein and RNA localization in the adult brain, in which regions were detected with only the transcript or only the protein, such as the olfactory bulbs or the cortex, suggesting that HIP may be transported to projection areas of HIP-synthesizing cells [42]. These findings in the adult brain are suggestive of HIP transport and consistent with our findings of HIP anterograde transport by the CN from the pelvic ganglia to the penis in the adult rat. Also consistent with observations in the adult brain, we did not observe HIP upregulation in response to SHH protein in the corpora cavernosa (unpublished observation), further supporting the hypothesis that SHH and HIP signaling are not identical during embryogenesis and in adult organs. In our studies, when the CN was cut or when HIP signaling was inhibited in the pelvic ganglia, it took 5 days before a decrease in HIP protein was observed in the corpora cavernosa of the penis. This suggests that HIP may be similar to NOS1 in the CN, such that HIP/NOS1 may build up on the nerve terminals and take several days to be fully depleted when the CN is injured [50]. As SHH and PTCH1 proteins are decreased significantly 2 days after CN injury, and possibly sooner because this was the earliest time assayed, it may be that normal HIP physiological levels in the penis out compete PTCH1 (which is decreased in abundance after CN injury), for SHH binding. Unbound PTCH1 might then induce apoptosis through its dependence receptor capacity [51]. By 5 days after CN injury, HIP levels are similarly decreased to that of SHH and PTCH1 and apoptosis abundance is much decreased [14], suggesting that competition of HIP and PTCH1 has returned to a more physiologic balance. This hypothesis is one potential mechanism of how perturbation of HIP in the CN may induce penile apoptosis.
Our results suggest that HIP in the CN serves more than one function. HIP regulates SHH in the corpora cavernosa and penile morphology, as was shown when HIP inhibition in the pelvic ganglia caused decreased SHH protein and increased apoptosis in the penis. HIP also plays a role in maintaining CN integrity as was observed by HIP inhibition in the pelvic ganglia causing axonal degradation and demyelination of the CN. This function is distinct from HIP transport to the penis. HIP is normally localized in adult neurons of the pelvic ganglia and fibers of the CN. In embryonic sciatic nerve, Hip RNA was elevated in response to Desert hedgehog (DHH) treatment of cultured fibroblasts, suggesting HIP involvement in other peripheral nerve signaling [52]. We did not observe HIP protein in Schwann cells or the perineurium, which is surprising considering the role of DHH in forming the perineurium of the sciatic nerve. We have previously identified SHH protein in Schwann cells of the CN [23], which are involved in maintaining and regenerating the CN, suggesting a role for the SHH pathway in maintaining neural integrity. As HIP inhibition in the pelvic ganglia has such a profound and specific effect on CN morphology, it is likely that HIP plays a role in maintaining CN axons and in myelination of the nerve fibers.
Perturbation of physiological HIP in either the pelvic ganglia or the penis induces apoptosis in the corpora cavernosa, indicating the importance of HIP to maintaining penile morphology. Disturbing physiological HIP levels in the pelvic ganglia may either affect CN integrity, which causes down stream induction of apoptosis in the penis or may directly affect some factor in the penis, which regulates apoptosis. It is also possible that a combination of these mechanisms takes place upon HIP perturbation in the pelvic ganglia. It is interesting that HIP inhibition in the pelvic ganglia decreases SHH protein as well as increases apoptosis in the penis, suggesting that HIP inhibition in the pelvic ganglia induces apoptosis in the penis through a SHH dependent mechanism. This is in keeping with our previous results that show that SHH inhibition causes smooth muscle apoptosis in the penis [15,22]. Excess HIP protein treatment of the pelvic ganglia does not alter SHH protein abundance in the penis however it does induce apoptosis (to a lesser extent than HIP inhibition), suggesting a SHH-independent mechanism. It is possible that adding extra HIP to a nerve that already has abundant HIP protein may have a toxic effect on the CN similar to HIP inhibition effects on CN morphology, and may thus induce apoptosis in the target organ of innervation. HIP perturbation directly in the penis, also induced apoptosis in the corpora cavernosa, indicating that HIP effects on penile morphology are not only caused by disruption of CN signaling/integrity, but also results because of the direct interactions on factors in the penis. Whereas HIP has been demonstrated to have a negative effect on SHH signaling during embryogenesis of other organs, the mechanism of HIP–SHH interactions in the adult penis does not appear as straightforward.
Conclusions
In this study we show that HIP protein is transported by the CN to the penis, that HIP perturbation in the pelvic ganglia or the penis induce apoptosis and that HIP plays a role in maintaining both CN integrity and corpora cavernosal morphology. These studies are significant because they show HIP involvement in the cross-talk between the pelvic ganglia and penis which is integral for maintenance of penile morphology, and they suggest a mechanism of how nerves may regulate end organ morphology.
Statement of Authorship.
Category 1
-
Conception and Design
Carol A. Podlasek
-
Acquisition of Data
Nicholas L. Angeloni; Christopher W. Bond; Diana Monsivais; Yi Tang
-
Analysis and Interpretation of Data
Carol A. Podlasek; Nicholas L. Angeloni; Christopher W. Bond; Yi Tang
Category 2
-
Drafting the Manuscript
Carol A. Podlasek; Nicholas L. Angeloni; Christopher W. Bond; Diana Monsivais; Yi Tang
-
Revising It for Intellectual Content
Carol A. Podlasek
Category 3
-
Final Approval of the Completed Manuscript
Carol A. Podlasek; Nicholas L. Angeloni; Christopher W. Bond; Diana Monsivais; Yi Tang
Acknowledgments
Grant Sponsor: National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases, Grant number DK068507 and DK079184.
Footnotes
Conflict of Interest: None declared.
References
- 1.Johannes CB, Araujo AB, Feldman HA, Derby CA, Kleinman KP, McKinlay JB. Incidence of erectile dysfunction in men 40 to 69 years old: Longitudinal results from the Massachusetts male aging study. J Urol. 2000;163:460–3. [PubMed] [Google Scholar]
- 2.Heruti R, Shochat T, Tekes-Manova D, Ashkenazi I, Justo D. Prevalence of erectile dysfunction among young adults: Results of a large-scale survey. J Sex Med. 2004;1:284–91. doi: 10.1111/j.1743-6109.04041.x. [DOI] [PubMed] [Google Scholar]
- 3.Katz A. What happened? Sexual consequences of prostate cancer and its treatment. Can Fam Physician. 2005;51L:977–82. [PMC free article] [PubMed] [Google Scholar]
- 4.Alivizatos G, Skolarikos A. Incontinence and erectile dysfunction following radical prostatectomy: A review. Sci World J. 2005;5:747–58. doi: 10.1100/tsw.2005.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kendirci M, Hellstrom WJ. Current concepts in the management of erectile dysfunction in men with prostate cancer. Clin Prostate Cancer. 2004;3:87–92. doi: 10.3816/cgc.2004.n.017. [DOI] [PubMed] [Google Scholar]
- 6.Vale J. Erectile dysfunction following radical therapy for prostate cancer. Radiother Oncol. 2000;57:301–5. doi: 10.1016/s0167-8140(00)00293-0. [DOI] [PubMed] [Google Scholar]
- 7.Perimenis P, Markou S, Gyftopoulos K, Athanaso-poulos A, Giannitsas K, Barbalias G. Switching from long-term treatment with self-injections to oral sildenafil in diabetic patients with severe erectile dysfunction. Eur Urol. 2002;41:387–91. doi: 10.1016/s0302-2838(02)00032-5. [DOI] [PubMed] [Google Scholar]
- 8.Kalsi JS, Ralph DJ, Thomas P, Bellringer J, Minhas S, Kell PD, Cellek S. A nitric oxide-releasing PDE5 inhibitor relaxes human corpus cavernosum in the absence of endogenous nitric oxide. J. Sex Med. 2005;2:53–7. doi: 10.1111/j.1743-6109.2005.20105.x. [DOI] [PubMed] [Google Scholar]
- 9.Montorsi F, McCullough A. Efficacy of sildenafil citrate in men with erectile dysfunction following radical prostatectomy: A systematic review of clinical data. J Sex Med. 2005;2:658–67. doi: 10.1111/j.1743-6109.2005.00117.x. [DOI] [PubMed] [Google Scholar]
- 10.de Tejada I Saenz, Anglin G, Kinght JR, Emmick JT. Effects of tadalafil on erectile dysfunction in men with diabetes. Diabetes Care. 2002;25:2159–64. doi: 10.2337/diacare.25.12.2159. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein I, Young JM, Fischer J, Bangerter K, Segerson T, Taylor T. Vardenafil, a new phosphodiesterase type 5 inhibitor, in the treatment of erectile dysfunction in men with diabetes. Diabetes Care. 2003;26:777–83. doi: 10.2337/diacare.26.3.777. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein I, Lue TF, Padma-Nathan H, Rosen RC, Steers WD, Wicker PA. Oral sildenafil in the treatment of erectile dysfunction. N Engl J Med. 1998;338:1397–404. doi: 10.1056/NEJM199805143382001. [DOI] [PubMed] [Google Scholar]
- 13.Klein LT, Miller MI, Buttyan R, Raffo AJ, Burchard M, Devris G, Cao YC, Olsson C, Shabsigh R. Apoptosis in the rat penis after penile denervation. J Urol. 1997;158:626–30. [PubMed] [Google Scholar]
- 14.User HM, Hairston JH, Zelner DJ, McKenna KE, McVary KT. Penile weight and cell subtype specific changes in a post-radical prostatectomy model of erectile dysfunction. J Urol. 2003;169:1175–9. doi: 10.1097/01.ju.0000048974.47461.50. [DOI] [PubMed] [Google Scholar]
- 15.Podlasek CA, Meroz CL, Tang Y, McKenna KE, McVary KT. Regulation of cavernous nerve injury-induced apoptosis by Sonic hedgehog. Biol Reprod. 2007;76:19–28. doi: 10.1095/biolreprod.106.053926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lysiak JJ, Yang SK, Klausner AP, Son H, Tuttle JB, Steers WD. Tadalafil increases Akt and extracellular signal-regulated kinase 1/2 activation, and prevents apoptotic cell death in the penis following denervation. J Urol. 2008;179:779–85. doi: 10.1016/j.juro.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 17.Iacono F, Giannella R, Somma P, Manno G, Fusco F, Mirone V. Histological alterations in cavernous tissue after radical prostatectomy. J Urol. 2005;173:1673–6. doi: 10.1097/01.ju.0000154356.76027.4f. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz EJ, Wong P, Graydon RJ. Sildenafil preserves intracorporeal smooth muscle after radical retropubic prostatectomy. J Urol. 2004;171:771–4. doi: 10.1097/01.ju.0000106970.97082.61. [DOI] [PubMed] [Google Scholar]
- 19.Yaman O, Yilmaz E, Bozlu M, Anafarta K. Alterations of intracorporal structures in patients with erectile dysfunction. Urol Int. 2003;71:87–90. doi: 10.1159/000071101. [DOI] [PubMed] [Google Scholar]
- 20.Fraiman MC, Lepor H, McCullough AR. Changes in penile morphometrics in men with erectile dysfunction after nerve-sparing radical retropublic prostatectomy. Mol Urol. 1999;3:109–15. [PubMed] [Google Scholar]
- 21.Wespes E, Rammal A, Garbar C. Sildenafil non-responders: Haemodynamic and morphometric studies. Eur Urol. 2005;48:1061–2. doi: 10.1016/j.eururo.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 22.Podlasek CA, Zelner DJ, Harris JD, Meroz CL, Tang Y, McKenna KE, McVary KT. Altered Sonic hedgehog signaling is associated with morphological abnormalities in the penis of the BB/WOR diabetic rat. Biol Reprod. 2003;69:816–27. doi: 10.1095/biolreprod.102.013508. [DOI] [PubMed] [Google Scholar]
- 23.Bond C, Tang Y, Podlasek CA. Neural influences on Sonic hedgehog and apoptosis in the rat penis. Biol Reprod. 2008;78:947–56. doi: 10.1095/biolreprod.107.064766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Podlasek CA, Zelner DJ, Jiang HB, Tang Y, Houston J, McKenna KE, McVary KT. Sonic hedgehog cascade is required for penile postnatal morphogenesis, differentiation, and adult homeostasis. Biol Reprod. 2003;68:423–8. doi: 10.1095/biolreprod.102.006643. [DOI] [PubMed] [Google Scholar]
- 25.Hooper JE, Scott MP. The drosophila patched gene encodes a putative membrane protein required for segmental patterning. Cell. 1989;59:751–65. doi: 10.1016/0092-8674(89)90021-4. [DOI] [PubMed] [Google Scholar]
- 26.Nakano Y, Guerrero I, Hidalgo A, Taylor A, Whittle JRS, Ingham PW. A protein with several possible membrane-spanning domains encoded by the drosophila segment polarity gene patched. Nature. 1989;341:508–13. doi: 10.1038/341508a0. [DOI] [PubMed] [Google Scholar]
- 27.Goodrich LV, Johnson RL, Milenkovic L, McMahon JA, Scott MP. Conservation of the hedgehog/patched signaling pathway from flies to mice: Induction of a mouse patched gene by hedgehog. Genes Dev. 1996;10:301–12. doi: 10.1101/gad.10.3.301. [DOI] [PubMed] [Google Scholar]
- 28.Stone DM, Hynes M, Armanini M, Swanson TA, Gu Q, Johnson RL, Scott MP, Pennica D, Goddard A, Phillips H, Noll M, Hooper JE, de Sauvage F, Rosenthal A. The tumor-suppressor gene patched encodes a candidate receptor for sonic hedgehog. Nature. 1996;14:129–34. doi: 10.1038/384129a0. [DOI] [PubMed] [Google Scholar]
- 29.Taipale J, Cooper MK, Maiti T, Beachy PA. Patched acts catalytically to suppress the activity of smoothened. Nature. 2002;418:892–7. doi: 10.1038/nature00989. [DOI] [PubMed] [Google Scholar]
- 30.Hidalgo A, Ingham P. Cell patterning in the drosophila segment: Spatial regulation of the segment polarity gene patched. Development. 1990;110:291–301. doi: 10.1242/dev.110.1.291. [DOI] [PubMed] [Google Scholar]
- 31.Ingham PW, Taylor AM, Nakano Y. Role of the drosophila patched gene in positional signaling. Nature. 1991;353:184–7. doi: 10.1038/353184a0. [DOI] [PubMed] [Google Scholar]
- 32.Capdevila J, Estrada MP, Sanchez-Herrero E, Guerrero I. The drosophila segment polarity gene patched interacts with decapentaplegic in wing development. EMBO J. 1994;13:71–82. doi: 10.1002/j.1460-2075.1994.tb06236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alexandre C, Jacinto A, Ingham PW. Transcriptional activation of hedgehog target genes in Drosophila is mediated directly by the cubitus interruptus protein, a member of the GLI family of Zinc finger DNA-binding proteins. Genes Dev. 1996;10:2003–13. doi: 10.1101/gad.10.16.2003. [DOI] [PubMed] [Google Scholar]
- 34.Dominguez M, Brunner M, Hafen E, Basler K. Sending and receiving the hedgehog signal: Control by the Drosophila gli protein cubitus interruptus. Science. 1996;272:1621–5. doi: 10.1126/science.272.5268.1621. [DOI] [PubMed] [Google Scholar]
- 35.Hepker J, Wang I-T, Motzny CK, Holmgran R, Orenic TV. Drosophila Cubitus Interruptus forms a negative feedback loop with Patched and regulates expression of hedgehog target genes. Development. 1997;124:549–58. doi: 10.1242/dev.124.2.549. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y, Struhl G. Dual roles for patched in sequestering and transducing hedgehog. Cell. 1996;87:553–63. doi: 10.1016/s0092-8674(00)81374-4. [DOI] [PubMed] [Google Scholar]
- 37.Karahira H, Ma NH, Tzanakakis ES, McMahon AP, Chuang P-T, Hebrok M. Combined activities of hedgehog signaling inhibitors regulate pancreas development. Development. 2003;130:4871–9. doi: 10.1242/dev.00653. [DOI] [PubMed] [Google Scholar]
- 38.Chuang P, McMahon AP. Vertebrate Hedgehog signaling modulated by induction of a Hedgehog-binding protein. Lett Nat. 1999;397:617–21. doi: 10.1038/17611. [DOI] [PubMed] [Google Scholar]
- 39.Olsen CL, Hsu P-P, Glienke J, Rubanyi GM, Brooks AR. Hedgehog-interacting protein is highly expressed in endothelial cells but down-regulated during angiogenesis and in several human tumors. BMC Cancer. 2004;4:43–54. doi: 10.1186/1471-2407-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shackel NA, McGuinness PH, Abbott CA, Gorrell MD, McCaughan GW. Novel differential gene expression in human cirrhosis detected by suppression subtractive hybridization. Hepatology. 2003;38:577–88. doi: 10.1053/jhep.2003.50376. [DOI] [PubMed] [Google Scholar]
- 41.Omenetti A, Popov Y, Jung Y, Choi SS, Witek RP, Yang L, Brown KD, Schuppan D, Diehl AM. The hedgehog pathway regulates remodeling responses to biliary obstruction in rats. Gut. 2008;57:1275–82. doi: 10.1136/gut.2008.148619. [DOI] [PubMed] [Google Scholar]
- 42.Coulombe J, Traiffort E, Loulier K, Faure H, Ruat M. Hedgehog interacting protein in the mature brain: membrane-associated and soluble forms. Mol Cell Neurosci. 2004;25:323–33. doi: 10.1016/j.mcn.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 43.Loulier K, Ruat M, Traiffort E. Analysis of hedgehog interacting protein in the brain and its expression in nitric oxide synthase-positive cells. Neuroreport. 2005;16:1959–62. doi: 10.1097/01.wnr.0000187632.91375.81. [DOI] [PubMed] [Google Scholar]
- 44.Dziuk PJ, Cook B. Passage of steroids through silicone rubber. Endocrinology. 1966;78:208–11. doi: 10.1210/endo-78-1-208. [DOI] [PubMed] [Google Scholar]
- 45.Stratton LG, Ewing LL, Desjardins C. Efficacy of testosterone-filled polydimethylsiloxane implants maintaining plasma testosterone in rabbits. J Reprod Fertil. 1973;35:235–44. doi: 10.1530/jrf.0.0350235. [DOI] [PubMed] [Google Scholar]
- 46.Wang JM, McKenna KE, Lee C. Determination of prostatic secretion in rats: Effect of neurotransmitters and testosterone. Prostate. 1991;18:289–301. doi: 10.1002/pros.2990180403. [DOI] [PubMed] [Google Scholar]
- 47.Rasband WS, Image J, Bethesda MD, U.S. National Institutes of Health [accessed September 1, 2007];1997–2007 Available at: http://rsb.info.nih.gov/ij/
- 48.Tang Y, Rampkin O, Calas A, Facchinetti P, Giuliano F. Oxytocinergic and serotonergic innervation of identified lumbosacral nuclei controlling penile erection in the male rat. Neuroscience. 1998;82:241–54. doi: 10.1016/s0306-4522(97)00290-x. [DOI] [PubMed] [Google Scholar]
- 49.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2ΔΔCT method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 50.Podlasek CA, Gonzalez CM, Zelner DJ, Jiang HB, McKenna KE, McVary KT. Analysis of NOS isoform changes in a post radical prostatectomy model of erectile dysfunction. Int J Impot Res. 2001;13(5 suppl):S1–15. doi: 10.1038/sj.ijir.3900772. [DOI] [PubMed] [Google Scholar]
- 51.Thibert C, Teillet MA, Lapointe F, Mazelin L, Le Douarin NM, Mehlen P. Inhibition of neuroepithelial patched-induced apoptosis by Sonic hedgehog. Science. 2003;301:843–6. doi: 10.1126/science.1085405. [DOI] [PubMed] [Google Scholar]
- 52.Parmantier E, Lynn B, Lawson D, Turmaine M, Namini SS, Chakrabarti L, McMahon AP, Jessen KR, Mirsky R. Schwann cell-derived desert hedgehog controls the development of peripheral nerve sheaths. Neuron. 1999;23:713–24. doi: 10.1016/s0896-6273(01)80030-1. [DOI] [PubMed] [Google Scholar]