Abstract
Infection of C3HeB/FeJ and C57BL/6 mice with Leishmania major stimulates a healing cell-mediated immune response, while Leishmania amazonensis infection leads to chronic disease. Here we show C3HeB/FeJ mice co-infected with both species of Leishmania heal, while co-infected C57BL/6 mice do not. Using an in vitro killing assay we determined B cells from infected C57BL/6 mice are ineffective in promoting parasite killing compared with B cells from infected C3HeB/FeJ mice. Furthermore, infected C57BL/6 mice produce less antigen-specific antibodies compared with infected C3HeB/FeJ mice. These findings suggest B cells play a required role in the cell-mediated immune response against L. amazonensis.
Keywords: Cutaneous leishmaniasis, Murine model, Co-infection, B cells, Macrophages
Leishmaniasis is a zoonotic, vector-borne disease caused by an obligate intracellular protozoan parasite of the genus Leishmania. Infection of C3HeB/FeJ (C3H) mice with Leishmania amazonensis (La) leads to chronic disease with large non-resolving cutaneous lesions and high parasite loads (Jones et al., 2000). The La-induced immune response is neither a T helper 1 (Th1) or a T helper 2 (Th2) response, as evidenced by unpolarized CD4+ T cells that fail to efficiently produce either IFN-γ or IL-4 and by dendritic cells that produce little IL-12 (Afonso and Scott, 1993; Jones et al., 2000; Ji et al., 2002; Vanloubbeeck et al., 2004; Ramer et al., 2006). Experimental evidence derived using Leishmania major (Lm) indicates that protection from these parasites requires establishment of a polarized Th1 immune response characterized by production of IL-12 and subsequent activation of IFN-γ -producing CD4+ T cells (Sacks and Noben-Trauth, 2002). More recent studies demonstrated that La-specific Th1 CD4+ T cells were ineffective in killing La in vivo and did not promote lesion resolution (Qi et al., 2004; Vanloubbeeck et al., 2004). However, our laboratory and others have shown that TH1 immunity associated with Lm infection provided significant protection against subsequent La infection (Veras et al., 1999; Vanloubbeeck and Jones, 2004; Gonzalez-Lombana et al., 2008).
Similar to the cross-protection observed in C3H mice, C57BL/6 (B6) mice first infected with 1 × 104 Lm and subsequently challenged with La also heal the infection, but interestingly, these mice do not heal a simultaneous infection with both Lm and La (Gonzalez-Lombana et al., 2008). To better understand the cellular mechanism underlying the productive healing response provided during co-infection of C3H mice and not B6 mice, we performed a simultaneous co-infection with Lm and La in both the C3H and B6 mouse models. La (MHOM/BR/0016/LTB) and Lm (MHOM/IL/80/Friedlin) promastigotes were grown in complete Grace's Insect medium (Atlanta Biologicals, Lawrenceville, GA, USA) to stationary phase, harvested, washed in endotoxin-free PBS (Cellgro, Herdon, VA, USA) and diluted to a concentration of 1 × 108 parasites per ml. Female C3HeB/FeJ mice (6-8 weeks of age) were bred in-house in a specific pathogen-free environment. The Institutional Animal Care and Use Committee at Iowa State University, USA, approved all protocols involving animals. Female C57BL/6 mice of the same age were obtained from Jackson Laboratories (Bar Harbor, Maine, USA). Mice with a single infection were inoculated with 5 × 106 La or Lm stationary phase promastigotes, while co-infected mice were inoculated with 2.5 × 106 stationary phase Lm promastigotes plus 2.5 × 106 La promastigotes, totaling 5 × 106 parasites in the left hind footpad. Mice were infected for 12 weeks with weekly monitoring of lesion size. At 12 weeks p.i. the mice were euthanized and parasite quantification in the infected footpad was performed using limiting dilution.
After co-infection with La and Lm, C3H mice had negligible footpad lesions by 12 weeks p.i., similar to infection with Lm alone (Fig. 1A). Parasite load from infected footpads was between 102 and 103 parasites (Fig. 1C). In contrast, co-infected B6 mice developed large footpad lesions that persisted for the 12 week period (Fig. 1B) and parasite burdens were 106 to 107 parasites per footpad, similar to infection with La alone (Fig. 1C). Therefore, co-infection with Lm leads to decreased footpad lesion size during concurrent La infection in C3H mice, but not in B6 mice.
Fig. 1.
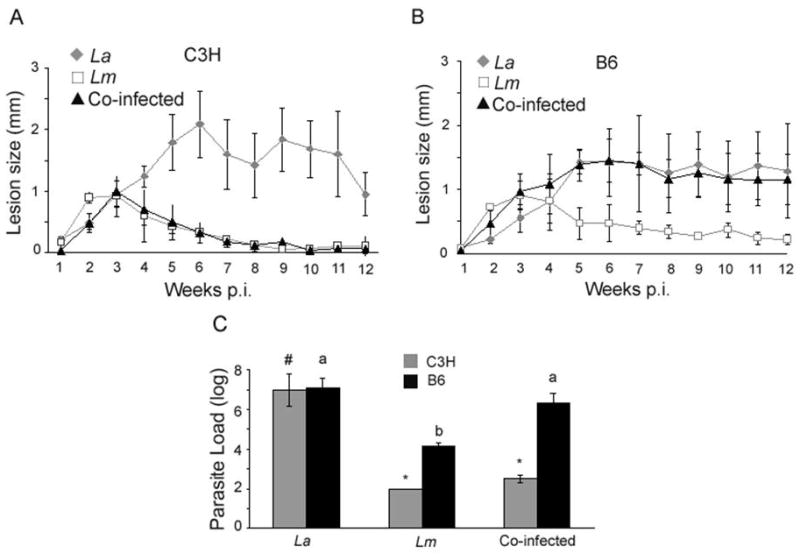
Simultaneous co-infection with both Leishmania major (Lm) and Leishmania amazonensis (La) allows for lesion resolution in C3H but not B6 mice. A) Lesion size of co-infected C3H mice was significantly different from C3H mice infected with La alone (grey diamonds) (P < 0.001), while B) co-infected B6 mice were significantly different from the B6 mice infected with Lm alone (open squares) (P < 0.001). Lesion size was determined by measuring the infected footpad and comparing that with the non-infected footpad. Repeated measure ANOVA was used for statistical analysis. Results are representative of three separate experiments. C) The number of parasites in the lesions of co-infected C3H and B6 mice. Infected footpads were harvested and parasite suspensions were serially diluted in Complete Grace's medium and incubated at 27°C for 5 to 7 days. Different symbols (*, #) represent a statistically significant difference (P < 0.001) within the C3H infection groups and different letters (a, b) represent significant differences (P < 0.001) within the B6 infection groups. ANOVA and Scheffe pair-wise comparisons using Stat View software were used for statistical analyses. Results are the mean and standard error from three separate experiments.
To better understand the underlying cellular mechanism of the difference observed after co-infection of C3H versus B6 mice, we employed an in vitro assay developed in our laboratory (Mukbel et al., 2006) to assess which immune cell-types, derived from the draining lymph node (DLN) of Lm-infected C3H mice, are required to deplete La infection. This assay utilizes bone marrow-derived macrophages (BMDM) from C3H mice infected with La amastigotes for 24 h in vitro. These infected macrophages are then co-cultured with DLN cells harvested from C3H mice that had been infected with Lm for 4 weeks, the point at which footpad lesions are resolving. Our previous studies determined that although antigen-specific CD4+ T cells are required, they are not sufficient to promote macrophage killing of intracellular La. Antigen-specific CD19+ B cells and their antibodies were found to be additional necessary components for macrophage killing of intracellular La amastigotes in this assay (Mukbel et al., 2006).
Using transwell chambers we showed that direct contact between infected macrophages and the lymphocytes was not required for the observed parasite depletion, allowing experiments with syngeneic cells. However, although B cells could be isolated to the upper chamber, CD4+ T cell function required cell contact with either the macrophage in the lower chamber or the B cell in the upper chamber. This fact precludes the ability to test syngeneic macrophages and B cells with allogeneic CD4+ T cells without the confounding influence of a mixed lymphocyte response. Based on these findings and the fact that co-infected B6 mice do not heal (Fig. 1B), we hypothesized that CD4+ T cells and B cells from the DLN of Lm-infected B6 mice would not be able to induce macrophages to kill La in our in vitro co-culture assay. To test this, BMDM were derived from naïve C3H and B6 mice as described previously (Mukbel et al., 2006). Briefly, after 6 days in culture BMDM were harvested, counted and 5 × 105 macrophages were added into the bottom compartment of 24 well transwell plates (Corning Costar, NY, USA), each containing a coverslip (Fisher Scientific, Hanover Park, IL, USA) in complete tissue culture media (CTCM). Macrophages were infected with La amastigotes at a 3:1 parasite to macrophage ratio. Popliteal lymph nodes were harvested from C3H or B6 mice infected for 4 weeks with Lm. CD4+ T cells (Miltenyi Biotech, Auburn, CA, USA) or CD19+ B cells (MagCellect, R&D system, Minneapolis, MN, USA) were purified via depletion using an autoMACS™ separator (Miltenyi Biotech, Auburn, CA, USA). The CD4+ T cells and B cells were added to the top compartment of a 0.4 μm diameter transwell together with freeze-thawed Lm promastigote antigen, as indicated in Fig. 2. When allogeneic B cells were assessed they were placed separately into the upper chamber and the syngeneic CD4+ T cells and macrophages were placed together in the bottom chamber, necessitating the use of BMDM from both C3H (Fig. 2A) or B6 (Fig. 2B) mice. All experimental conditions were performed in duplicate. Coverslips were harvested after 5 days of culture, fixed with 100% methanol and stained with the HEMA 3 stain set (Fisher Scientific, Hanover Park, IL, USA). Data analysis was performed by counting the coverslips via light microscopy and examining three areas at 100× magnification. In each area, 100 macrophages were examined assessing the number of infected macrophages and the number of parasites per 100 infected macrophages.
Fig. 2.
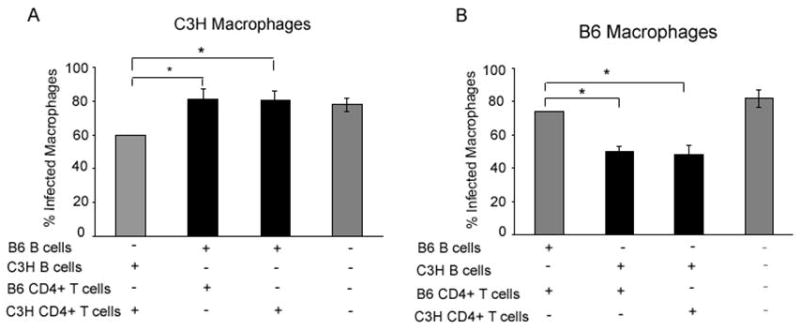
B cells isolated from Leishmania major (Lm)-infected C57BL/6 (B6) mice do not promote killing of Leishmania amazonensis (La) in vitro. A) CD19+ B cells and CD4+ T cells, purified from the draining lymph node (DLN) of C3H or B6 mice infected with Lm for 4 weeks, were placed into the upper chamber of a transwell plate with La-infected C3H-bone marrow-derived macrophages (BMDM) in the lower chamber as indicated above (first and second bars, respectively). B cells from B6 mice were isolated to the upper chamber and CD4+ T cells from C3H mice were placed in the bottom chamber (third bar). All wells contained Lm freeze-thawed antigen and were incubated for 5 days at 34°C. Black shading indicates the presence of B cells from B6 mice. B) Same as A except with BMDM derived from B6 mice and B cells from C3H mice were isolated to the upper chamber (second bar) as indicated. Black shading indicates the presence of B cells from C3H mice. * represent statistically significant differences (P < 0.001) as determined by ANOVA and Scheffe pair-wise comparison. Results are the mean and standard error from three separate experiments.
Fig. 2 shows CD4+ T cells and B cells isolated from Lm-infected B6 mice do not reduce the percentage of infected macrophages in vitro compared with the same cells isolated from Lm-infected C3H mice. This phenomenon is not affected by the mouse strain from which the macrophages are derived (Fig. 2A, first and second bars; Fig. 2B, first and third bars). Furthermore, B cells from C3H mice promoted killing of La even in combination with CD4+ T cells from B6 mice (Fig. 2B, second bar). In contrast, B cells from B6 mice did not promote killing even with CD4+ T cells isolated from C3H mice (Fig. 2A, third bar). These results demonstrate that the inability of B6 mice to control the co-infection tracks with an inability of B6 B cells to aid in the killing of La in our in vitro killing assay system. Thus B cells from Lm-infected B6 mice are not functionally equivalent to those from Lm-infected C3H mice.
Based on the conclusion that B cells from C3H and B6 mice did not demonstrate equivalent responses in vitro, we wanted to determine whether there were differences in their B cell responses in vivo. Therefore we compared parasite-specific total IgG and IgG2a or IgG2c (Martin et al., 1998) antibody production during infection of C3H or B6 mice, respectively. ELISA was performed on serum for antibody isotype titers. All samples from both C3H and B6 mice were positive for total IgG, IgG2a or IgG2c, at 1:10,000 dilutions at 5 and 12 weeks post-infection, indicating readily detectable serum antibodies. Western blot analysis was performed with serum (1:25 dilution) from mice infected for 5 or 12 weeks hybridized against freeze-thawed La and Lm stationary phase promastigote parasite antigen. C3H mice infected with Lm alone or co-infected produced readily demonstrable parasite-specific total IgG and the TH1-associated IgG2a isotype to both La and Lm antigens (Fig. 3) at both 5 and 12 weeks p.i. No parasite specific IgG2a was detected after La infection at 5 weeks with a small amount seen at 12 weeks p.i. (Fig. 3B). In contrast, B6 mice produced only small amounts of parasite-specific total IgG (Fig. 3A) and almost undetectable amounts of the Th1-associated IgG2c isotype, which was Lm-specific even after co-infection (Fig. 3B). These results were recapitulated using amastigote-derived antigen preparations for Western blot analyses (data not shown).
Fig. 3.
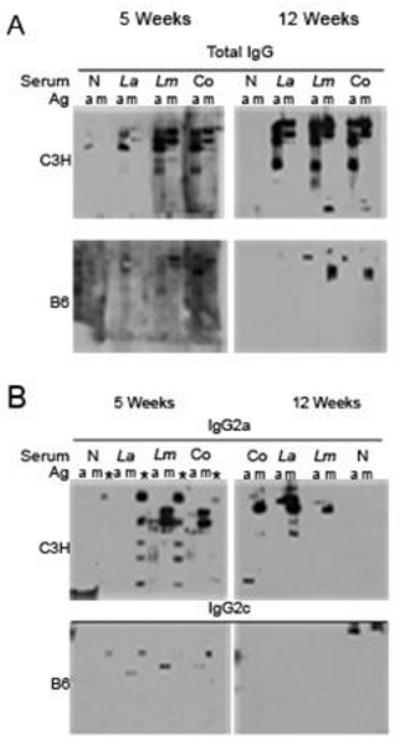
Western blot analyses of parasite-specific production of total IgG and IgG isotypes IgG2a and IgG2c. Freeze-thawed Leishmania amazonensis (La) (a) or Leishmania major (Lm) (m) antigen was separated on a polyacrylamide gel and protein was transferred to a polyvinylidene fluoride (PVDF) membrane. The blots were subsequently hybridized with mouse serum (1:25 dilution) pooled from four C3H mice or B6 mice (as indicated) that were non-infected (N) or infected for 5 or 12 weeks with Lm, La or both parasites (Co) as indicated. Following serum hybridization, the membranes were probed with goat anti-mouse antibodies to (A) total IgG or with goat anti-mouse antibodies to (B) IgG2a (C3H), or IgG2c. * identifies lanes with commercial molecular weight markers that cross-reacted with serum antibodies. Results are from one experiment at 5 weeks p.i. and representative of two experiments at 12 weeks p.i.
Our results support the premise that B cells play a necessary role in an effective Th1-mediated immune response towards La and that the inability of B6 mice to heal a co-infection correlates with a defect in the B cell response, rather than an exclusive defect in the CD4+ T cell response. Although B cells and the production of protective antibodies are classically considered part of a productive Th2 immune response, several reports indicate a protective role for B cells and/or antibodies during Leishmania infection. Recently, B cell production of antibodies has been shown to be important for phagocytosis of Lm by dendritic cells. Without antibodies, Lm-infected mice had larger lesion sizes, higher parasite loads, low IFN-γ production and a decreased T cell response, indicating antibodies supported resolution of the infection (Woelbing et al., 2006). In contrast, other studies have indicated the importance of B cells in enhancing immunopathology during Leishmania infection. Miles et al. (2005) showed that in the absence of IgG, Lm-infected BALB/c mice had smaller lesions with fewer parasites as compared with infected wild-type mice (Miles et al., 2005). Recently, another study using mice that lack functional B cells and antibodies showed La-infected mice had a delayed onset of disease and developed small lesions (Wanasen et al., 2008). Kima et. al. (2000) also described limited infections with both L. amazonensis and Leishmania pifanoi in the absence of circulating antibodies, and infection of Fc gamma receptor (Fc γR) knockout mice resulted in similarly limited lesions (Kima et. al., 2000). In addition, work by Thomas and Buxbaum showed Fc γRIII knockout mice were resistant to Leishmania mexicana infection. These mice healed their lesions with a high level of IFN-γ production, indicating antibody stimulation of Fc γRIII was detrimental during L mexicana infection (Thomas and Buxbaum, 2008).
Our results are not in disagreement with these studies. Our previous and current work suggests that any effective immune response against La in addition to other factors, must include a productive antigen-specific B cell response that can promote macrophage-mediated parasite killing. Following inoculation, Leishmania promastigotes are phagocytosed by mammalian macrophages and dendritic cells located at the site of infection. Macrophages express Fc γR on their surface that bind the Fc portion of antibodies; specifically IgG antibodies. After Fc γR-antibody binding, macrophages can become activated to produce effector molecules including NADPH-oxidase dependent superoxide production via immunoreceptor tyrosine-based activation motifs (ITAMs) (Hulett and Hogarth, 1994). A recent study demonstrated that superoxide generation was a required factor in killing La in human cases of chronic disease (Khouri et al., 2009). In the C3H mouse model, our laboratory has shown that B cells and IgG antibodies together with CD4+ T cells from an established Lm infection are necessary for providing effective stimulation to macrophages for superoxide dependent killing of La (Mukbel et al., 2006). Neutralization of IgG2a in vitro negates killing of La within infected macrophages (Supplementary Fig. S1). However, replacement of C3H B cells with serum from Lm-infected C3H mice is not sufficient for killing of La in vitro (unpublished observations).
Our results indicate B6 mice produce detectable but low levels of Lm parasite-specific total IgG and IgG2c antibodies during infection, compared with total IgG and parasite-specific IgG2a produced by C3H mice. This overall low level of parasite-specific IgG2c antibody production may result in inadequate stimulation of Fcγ receptors, limiting superoxide production. Overall, our findings indicate that antibodies are necessary, but not sufficient, for killing La and that antibodies are just one of several critical immune components required for killing. The known complexities of antigen-antibody and FcγR-mediated immustimulatory versus immunoregulatory mechanisms (reviewed in Hjelm et. al., 2006) suggests that simple serum or B cell transfer experiments may not adequately recapitulate all of the immune components required for killing La either in vivo or in vitro, consistent with our own unpublished observations.
In conclusion, the inability of B6 mice to heal a co-infection of Lm and La correlates with a defect in the B cell response, rather than the CD4+ T cell response, as defined by our in vitro killing assay. These results suggest that both effective CD4+ T cells and B cells are required for a protective, healing, cell-mediated immune response to La.
Supplementary Material
Supplementary Fig. S1. IgG2a antibodies are necessary in vitro to kill Leishmania amazonensis (La) in infected macrophages. Draining lymph node (DLN) cells from C3H mice infected with either La or Leishmania major (Lm) for 4 weeks were added to bone marrow-derived macrophages from C3H mice infected with La amastigotes on coverslips with Lm freeze-thawed antigen and incubated for 5 days at 34°C. Polystyrene beads were incubated with 12.5 μg/ml of either goat anti-mouse IgG2a or isotype control antibody, followed by two washing steps with PBS and a final concentration of 15 × 106 beads were added per well, as designated. * represent statistically significant differences (P < 0.05) as determined by unpaired student's t-test. Results are from two separate experiments.
Acknowledgments
This work was supported by National Institutes of Health Grants K08AI076616, AI48357 and the Fort Dodge Animal Health Fellowship in Veterinary Medicine. The authors would like to thank Dr. Amanda Ramer-Tait for her critical evaluation of the manuscript and Ms. Jenny Li for technical help.
Footnotes
Note: Supplementary data associated with this article
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afonso LC, Scott P. Immune responses associated with susceptibility of C57BL/10 mice to Leishmania amazonensis. Infect Immun. 1993;61:2952–2959. doi: 10.1128/iai.61.7.2952-2959.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Lombana CZ, Santiago HC, Macedo JP, Seixas VA, Russo RC, Tafuri WL, Afonso LC, Vieira LQ. Early infection with Leishmania major restrains pathogenic response to Leishmania amazonensis and parasite growth. Acta Trop. 2008;106:27–38. doi: 10.1016/j.actatropica.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Hjelm F, Carlsson F, Getahun A, Heyman B. Antibody-Mediated Regulation of the Immune Response. Scand J Immunol. 2006;64:177–184. doi: 10.1111/j.1365-3083.2006.01818.x. [DOI] [PubMed] [Google Scholar]
- Hulett MD, Hogarth PM. Molecular basis of Fc receptor function. Adv Immunol. 1994;57:1–127. doi: 10.1016/s0065-2776(08)60671-9. [DOI] [PubMed] [Google Scholar]
- Ji J, Sun J, Qi H, Soong L. Analysis of T helper cell responses during infection with Leishmania amazonensis. Am J Trop Med Hyg. 2002;66:338–345. doi: 10.4269/ajtmh.2002.66.338. [DOI] [PubMed] [Google Scholar]
- Jones DE, Buxbaum LU, Scott P. IL-4-independent inhibition of IL-12 responsiveness during Leishmania amazonensis infection. J Immunol. 2000;165:364–372. doi: 10.4049/jimmunol.165.1.364. [DOI] [PubMed] [Google Scholar]
- Kima PE, Constant SL, Hannum L, Colmenares M, Lee KS, Haberman AM, Shlomchik MJ, McMahon-Pratt D. Internalization of Leishmania mexicana complex amastigotes via the Fc receptor is required to sustain infection in murine cutaneous leishmaniasis. J Exp Med. 2000;191:1063–1068. doi: 10.1084/jem.191.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khouri R, Bafica A, Silva Mda P, Noronha A, Kolb JP, Wietzerbin J, Barral A, Barral-Netto M, Van Weyenbergh J. IFN-beta impairs superoxide-dependent parasite killing in human macrophages: evidence for a deleterious role of SOD1 in cutaneous leishmaniasis. J Immunol. 2009;182:2525–2531. doi: 10.4049/jimmunol.0802860. [DOI] [PubMed] [Google Scholar]
- Martin RM, Brady JL, Lew AM. The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. J Immunol Methods. 1998;212:187–192. doi: 10.1016/s0022-1759(98)00015-5. [DOI] [PubMed] [Google Scholar]
- Miles SA, Conrad SM, Alves RG, Jeronimo SM, Mosser DM. A role for IgG immune complexes during infection with the intracellular pathogen Leishmania. J Exp Med. 2005;201:747–754. doi: 10.1084/jem.20041470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukbel R, Petersen CA, Jones DE. Soluble factors from Leishmania major-specific CD4+T cells and B cells limit L. amazonensis amastigote survival within infected macrophages. Microbes Infect. 2006;8:2547–2555. doi: 10.1016/j.micinf.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Qi H, Ji J, Wanasen N, Soong L. Enhanced replication of Leishmania amazonensis amastigotes in gamma interferon-stimulated murine macrophages: implications for the pathogenesis of cutaneous leishmaniasis. Infect Immun. 2004;72:988–995. doi: 10.1128/IAI.72.2.988-995.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramer AE, Vanloubbeeck YF, Jones DE. Antigen-responsive CD4+ T cells from C3H mice chronically infected with Leishmania amazonensis are impaired in the transition to an effector phenotype. Infect Immun. 2006;74:1547–1554. doi: 10.1128/IAI.74.3.1547-1554.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002;2:845–858. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- Thomas BN, Buxbaum LU. FcgammaRIII mediates immunoglobulin G-induced interleukin-10 and is required for chronic Leishmania mexicana lesions. Infect Immun. 2008;76:623–631. doi: 10.1128/IAI.00316-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanloubbeeck Y, Jones DE. Protection of C3HeB/FeJ mice against Leishmania amazonensis challenge after previous Leishmania major infection. Am J Trop Med Hyg. 2004;71:407–411. [PubMed] [Google Scholar]
- Vanloubbeeck YF, Ramer AE, Jie F, Jones DE. CD4+ Th1 cells induced by dendritic cell-based immunotherapy in mice chronically infected with Leishmania amazonensis do not promote healing. Infect Immun. 2004;72:4455–4463. doi: 10.1128/IAI.72.8.4455-4463.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veras P, Brodskyn C, Balestieri F, Freitas L, Ramos A, Queiroz A, Barral A, Beverley S, Barral-Netto M. A dhfr-ts- Leishmania major knockout mutant cross-protects against Leishmania amazonensis. Mem Inst Oswaldo Cruz. 1999;94:491–496. doi: 10.1590/s0074-02761999000400011. [DOI] [PubMed] [Google Scholar]
- Wanasen N, Xin L, Soong L. Pathogenic role of B cells and antibodies in murine Leishmania amazonensis infection. Int J Parasitol. 2008;38:417–429. doi: 10.1016/j.ijpara.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woelbing F, Kostka SL, Moelle K, Belkaid Y, Sunderkoetter C, Verbeek S, Waisman A, Nigg AP, Knop J, Udey MC, von Stebut E. Uptake of Leishmania major by dendritic cells is mediated by Fcgamma receptors and facilitates acquisition of protective immunity. J Exp Med. 2006;203:177–188. doi: 10.1084/jem.20052288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. S1. IgG2a antibodies are necessary in vitro to kill Leishmania amazonensis (La) in infected macrophages. Draining lymph node (DLN) cells from C3H mice infected with either La or Leishmania major (Lm) for 4 weeks were added to bone marrow-derived macrophages from C3H mice infected with La amastigotes on coverslips with Lm freeze-thawed antigen and incubated for 5 days at 34°C. Polystyrene beads were incubated with 12.5 μg/ml of either goat anti-mouse IgG2a or isotype control antibody, followed by two washing steps with PBS and a final concentration of 15 × 106 beads were added per well, as designated. * represent statistically significant differences (P < 0.05) as determined by unpaired student's t-test. Results are from two separate experiments.


