Abstract
We examined the relationship between neuropsychological performance and magnetic resonance imaging (MRI) of the orbital frontal cortex (OFC) and diffusion tensor imaging (DTI) of the cingulum bundle (CB) within groups of patients with schizophrenia and healthy participants. We analyzed data from subjects, who had participated in prior MRI, DTI, and neuropsychological studies (Nakamura et al., 2008; Nestor et al., 2008). In comparison to healthy participants, patients showed the expected reductions across CB fractional anisotropy (white matter) and OFC gray matter volume as well as lower neuropsychological scores. In addition, in comparison to healthy participants, patients showed a very different pattern of functional-anatomical correlates. For patients, CB white matter but not OFC gray matter correlated with various aspects of intelligence, including general abilities and working memory. For controls, OFC gray matter but not CB white matter correlated with scores on tests of intelligence and decision making. These results point to the potentially important role of CB white matter in the neuropsychological disturbance in schizophrenia.
Keywords: schizophrenia, cingulum bundle, white matter, orbital frontal cortex, gray matter
Introduction
Schizophrenia is a heterogeneous disorder that is marked by considerable variation in both its expression and pathophysiology. Central to the illness are widespread cognitive difficulties with reasoning, perceiving, remembering, and concentration, all of which are often evident by a general diminution of scores across neuropsychological tests of intelligence, memory, executive function, and attention. These neuropsychological impairments have long been presumed to reflect underlying neuropathology. But only relatively recently with the advent of high resolution brain imaging studies has the nature of the relationship between disease-related changes in both brain anatomy and cognitive function begun to unfold. These combined brain imaging and neuropsychological studies have helped to establish cognitive deficits as the “primary expression of the schizophrenic brain” (p. 299), a conclusion Heinrichs (2005) offered on the basis of his meta-analysis of the extant literature.
Schizophrenia lacks an established pathognomonic signature. Diverse brain areas are affected, just as various domains of cognitive function are compromised. Moreover, neuropsychological correlates of disease-related brain changes do not always comport with those that would be predicted on the basis of either lesion (e.g., Swayze, & Andreasen, 1997; Szeszko et al., 2002; Torres et al., 1997) or functional imaging (Weis & Heckers, 2002) studies. Indeed, neuropsychological deficits in schizophrenia fit neither a localized nor a lateralized pattern of cortical damage, but rather have been seen increasingly as reflecting underlying abnormalities in widely distributed networks of brain regions (Andreasen et al., 1999; McGlashan & Hoffman, 2000; Nestor et al., 1998; Nestor et al., 2004; Stephan, et al., 2006; Weinberger et al., 1992; Winterer et al., 2003). From this perspective, schizophrenia compromises the neural circuitry of anatomically and functionally distinct networks of brain regions, disrupting both processing and transmission of informational signals across the wide expanse of the cortex.
Consider the findings of two recent brain structural imaging studies, both of which have been cited as evidence of disease-related disturbance of neural networks (Csernansky & Cronenwett, 2008). In a meta-analysis of MRI schizophrenia studies, Ellison-Wright et. al. (2008) identified gray matter volume loss across specific cortical-subcortical networks, originating with limbic input to the striatum, traversing through the thalamus, and continuing to sites in the prefrontal and cingulate cortex. In a related study using diffusion tensor imaging (DTI), Friedman et al. (2008) traced widespread white matter changes in chronic schizophrenia spanning the left inferior longitudinal fasiculus as well as the genu and splenium of the corpus callosum. Unknown, however, is how these disease-related gray matter and white matter changes might contribute to neuropsychological disturbance. To date, no neuropsychological study has yet to compare the functional associates of these disease related changes in white and gray matter within the same group of patients. Indeed, what is needed is to examine both MRI and DTI brain changes within-groups of patients and healthy participants, such that associations can be directly tested between imaged brain regions and neuropsychological functioning.
In two separate studies, we recently examined neuropsychological-DTI associations with the cingulum bundle (CB) white matter (Nestor et al., 2008) and neuropsychological-MRI associations of orbital frontal cortex (OFC) gray matter (Nakamura et al., 2008) in patients with schizophrenia. Our findings suggested that structural abnormalities in each of these brain regions may make differential contributions to distinct core features of the clinical schizophrenia phenotype. That is, for patients with schizophrenia, reduced DTI CB white matter uniquely and specifically accounted for a significant source of variance for measures of intelligence and executive functioning, but not for tests of general memory for such items as stories and word pairs (Nestor et al., 2004, 2008). Conversely, reduced MRI OFC gray matter volume, particularly of the middle orbital gyrus, correlated with symptoms of formal thought disorder and illness duration but not with neuropsychological measures of intelligence, executive functioning, and decision making (Nakamura et al., 2008). In addition, in contrast to the patient group, for healthy participants, OFC gray matter volume predicted performance for tests of intelligence and decision making (Nakamura et al., 2008). When taken together, these findings may be viewed as pointing to a hypothesis linking disease-related abnormalities in CB white matter but not in OFC gray matter with reduced levels of intelligence and executive functioning.
In the current study, we continue to focus on these two brain regions, but we now examine the contribution of each to neuropsychological functioning within the same group of patients with schizophrenia. Our rationale is that both the OFC and CB represent two key sites that belong to widely distributed, functionally diverse neural networks that are thought to be compromised by schizophrenia (e.g., Andreasen et al., 1999; Csernansky & Cronenwett, 2008; McGlashan & Hoffman, 2000; Nakamura et al., 2008; Nestor et al, 2004; Nestor et al., 2008). We focus on the structural integrity of these two sites, comparing OFC MRI gray matter volume and DTI CB white matter within- and between-groups. While each of these brain imaging modalities offer different information, combining them in the same study provides a framework to examine the extent to which these regions might enter into specific relationships with different aspects of neuropsychological functioning in schizophrenia and in healthy participants.
Two aspects of neuropsychological functioning are addressed: intelligence and decision making. First with respect to intelligence, Jung and Haier (2007) recently proposed that frontal areas interact with parietal and temporal sites to form rich and complex cognitive systems that support psychometric intelligence as measured by the Wechsler Adult Intelligence Scale-Third Edition (WAIS-III, Wechsler 1997). Consistent with Jung and Haier (2007) theorizing, several empirical studies have pointed to an important role of frontal lobe structures in intelligence (e.g, Duncan, Emslie, Williams, Johnson, & Freer, 1996; Duncan et al., 2000; Gray, Chabris, & Braver, 2003, Obonsawin, et al., 2002). For patients with schizophrenia, reduced levels of intelligence represent a core aspect of the clinical phenotype (e.g., Koenen et al., 2009). For example, in a recent meta-analysis, Woodberry, Giuliano, and Seidman (2008) demonstrated a reliable, medium-sized impairment in premorbid IQ in schizophrenia samples. Similarly, lower childhood IQ has been associated with both increased risk for developing schizophrenia spectrum disorder (Koenen et al., 2009) as well as genetic susceptibility for the disease (Zinkstok et al., 2007). And while we have recently demonstrated strong relationship between reduced DTI CB white matter and lower levels of intelligence in schizophrenia (Nestor et al. 2008), it remains unknown as to whether for these same patients the role, if any, of MRI OFC gray matter volume in their depressed levels of intelligence.
Second, in addition to their link to intelligence, the CB and OFC along with the temporoparietal junction, the temporal sulcus and the temporal poles are thought to support the dynamic interplay of informational, motivational, and neural processes from which decision making about social matters emerges (Adolphs 2003; Amodio & Frith, 2006). Here representations of internal bodily states, knowledge of self, perceptions of others, and interpersonal motivations are carefully orchestrated to allow for healthy and adaptive social decision making (Amodio & Frith, 2006; Bar-Oh et al., 2004; Bechara et al., 1994). Among these neural sites, OFC circuitry has been ascribed an important role in sociability with damage linked to deleterious effects in conduct, comportment, and personality. Recent studies have suggested a relatively new neuropsychological measure of decision making, known as the Iowa Gambling Test (IGT) to be a sensitive behavioral probe of OFC pathology, with performance depending heavily on functions mediated by the OFC and medial prefrontal cortex, independently of dorsolateral prefrontal cortex-mediated functions (Bechara et al., 1994; Bechara, Tranel, & Damasio, 2000; Bechara et al., 2001). We recently reported that for healthy participants, but not for patients with schizophrenia, OFC gray matter predicted IGT performance and also intelligence (Nakamura, 2008).
The current study aims to test further the role of disease-related abnormalities in CB in neuropsychological disturbance of schizophrenia. To accomplish this aim we compare neuropsychological associations with CB white matter and OFC gray matter within the same group of patients. The research design allows for both critical within- and between-subject analyses aims towards addressing two pivotal questions: First, will those particular neuropsychological deficits in intellectual abilities that have been strongly linked to disease-related abnormalities in CB white matter also be related to OFC gray matter volume loss within the same group of patients with schizophrenia? Second will the patient and healthy participant groups show a different pattern of CB and OFC neuropsychological correlates?
To address these questions, we now combine, for this current investigation, samples from Nestor et al (2008) and Nakamura (2008) including only those patients and healthy participants who had available both prior DTI-CB and MRI-OFC studies. Two high-resolution structural brain imaging techniques, DTI of the CB and MRI of OFC are compared within each group of subjects in relation to two neuropsychological outcomes, WAIS-III and IGT. Thus, the design of the current study provides a strong and direct test of the hypothesis that reduced CB white matter but not reduced OFC gray matter contributes specifically to lower WAIS-III but not IGT scores in patients with schizophrenia. By contrast, for healthy controls, OFC gray matter but not CB white is expected to predict performance in both intelligence and decision making.
Method
Participants
All subjects were between the ages of 17 and 55 years, right-handed, native speakers of English, without histories of ECT, neurological illness, and without alcohol or drug abuse in the past 5 years, as assessed by the Addiction Severity Index (McClellan et al., 1992). Subjects were selected from samples used in two published studies that examined MRI gray matter volume of the OFC (Nakamura et al, 2008) and DTI fractional anisotropy of the CB (Nestor et al., 2008). From these two studies, 16 patients and 12 healthy participants had available a complete set of measures across MRI, DTI and neuropsychological tests of intelligence (WAIS-III) and decision making (IGT). For the patient group, diagnoses were ascertained by the Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID-P), along with chart review. All patients were part of an ongoing comprehensive, longitudinal study of schizophrenia, and all were receiving neuroleptic medication; the mean chlorpromazine (CPZ) equivalent daily dose was 436.10 mg (SD=369.56). The mean duration of illness was 15.9 years (SD = 10.33). Healthy participants, who had been recruited from newspaper advertisement, and had undergone the SCID-NP, were matched to patients on the basis of age, sex, handedness, and parental SES. Mean age did not differ significantly (t = −0.71, df = 46, p =. 484) between patient (39.1 years, SD = 9.11) and healthy participant (41.1 years, SD = 8.67) groups. Mean years of education did differ significantly (t = −4.01, <.001) between patient (12.8 years, SD = 1.76) and healthy participant (15.0 years, SD = 1.98). After the study was described to them, all subjects provided written informed consent.
Procedure
Neuropsychological Assessment
The neuropsychological assessment included the complete Wechsler Adult Intelligence Test-Third Edition (WAIS-III; Wechsler, 1997) and the Iowa Gambling Task (IGT; Bechara et al., 1994). The WAIS-III yielded composite measures of intelligence (FSIQ, VIQ, PIQ), and index scores of verbal comprehension, perceptual organization, working memory, and processing speed. The complete WAIS-III was available for 22 patients and 23 healthy participants taken from Nakamura et al. (2008). The IGT was administered using a computerized version (Rodriguez-Sanchez et al., 2005). Subject faced a computer screen where four decks of cards were shown (A, B, C, D). Subjects were asked to choose cards from the decks. When subjects clicked on a deck, the computer screen notified the subjects of the amount of money gained or lost – the task goal is to win as much money as possible. Subjects did not know that they would choose a total of 100 cards. Nor did subjects know that there are two advantageous decks, C and D, for which little money is won on a single choice but even less is lost (resulting in a net gain) and two disadvantageous decks, A and B, for which a lot of money is won but even more is lost (resulting in a net loss). In other words, the absolute magnitude of the award is large in the A and B decks, but money loss (punishment) occurs more frequently. In contrast, in the C and D decks, the absolute magnitude of the award is small but money gain (reward) occurs more frequently. The key to success in this gambling task is to eliminate the amount of money lost rather than to obtain a jackpot. The variable that measures total performance on this task was the difference between choices in advantageous decks minus choices in disadvantageous decks for the whole task [(C+D)−(A+B)]. The IGT was available for 23 patients and 24 healthy participants taken from Nakamura et al. (2008).
Diffusion Tensor Imaging
As described elsewhere in detail (Kubicki et al., 2002, Kubicki et al., 2003), we applied Line-Scan-Diffusion-Imaging (LSDI) to obtain fractional anisotropy maps in order to measure the integrity of fibers within the CB. MR scans used a quadrature head coil on a 1.5 Tesla GE Echospeed system (General Electric Medical Systems, Milwaukee, WI), which permits maximum gradient amplitudes of 40 mT/m. A set of three orthogonal T1-weighted images were used as localizers (sagittal, axial oblique aligned to the anterior commissure (AC-PC) line and another sagittal oblique aligned to the inter-hemispheric fissure). For each session, six images were collected with high (1000 s/mm2) diffusion weighting along six non-collinear directions. For low (5 s/mm2) diffusion weighting, two images were collected, an adequate sample in that diffusion related changes were minimal. Scan parameters were: rectangular FOV (field of view) 220×165mm; 128×128 scan matrix (256×256 image matrix); slice thickness 4 mm; inter-slice distance 1 mm; receiver bandwidth +/−4kHz; echo time 64 ms; TR (repetition time) 81 ms; (effective TR 2592 ms); scan time 60 seconds/section. The number of coronal slices acquired to cover the entire brain ranged from 31 to 35 slices depending upon brain size. After reconstruction, the diffusion-weighted images were transferred to a UNIX workstation for calculation of eigenvalue, eigenvector, and fractional anisotropy maps of diffusion. Details of the CB extraction, including out-of-plane diffusion maps and segmentation method can be found in our previous investigations (Kubicki et al., 2002; Kubicki et al., 2003; Nestor et al., 2004), as can the method of definition of the ROI for the CB. Figure 1 depicts image of right CB generated using fiber tractography and the region of interest method overlayed on sagittal fractional anisotropy map.
Figure 1.
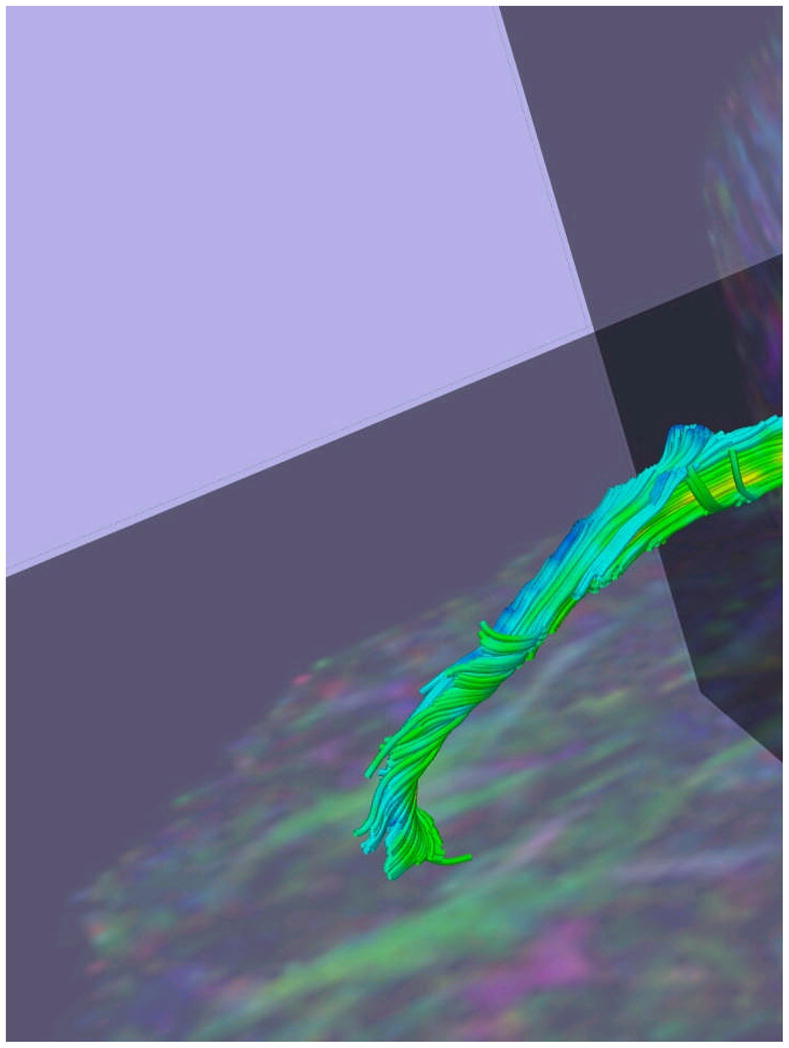
Results of fiber tractography for the left cingulum bundle. Figure represents the entire cingulum bundle; only anterior third of cingulum bundle was measured in the current study.
MRI Processing
The MRI processing is described in detailed in Nakamura et al. (2008). In brief, MR images were acquired with a 1.5-Tesla General Electric scanner (GE Medical Systems, Milwaukee) at the Brigham and Women’s Hospital in Boston. A three-dimensional Fourier transformed spoiled gradient-recalled (SPGR) acquisition sequence yielded a coronal series of contiguous 1.5 mm images (TE=5 msec, TR=35 msec, repetition=1, nutation angle=45°, field of view = 24 cm, acquisition matrix = 256×256×124, voxel dimension = 0.9375×0.9375×1.5 mm). Next, a double-echo spin-echo yielded 108 contiguous axial double-echo (proton-density- and T2-weighted) slices, with 54 levels, throughout the brain (TE =30 and 80msec, TR =3000msec, field of view = 24 cm, an interleaved acquisition with 3-mm slice thickness, voxel dimensions = 0.9375×0.9375×3.0 mm). The T2 information from the double-echo spin-echo axial slices was registered to the SPGR images. An expectation-maximization (EM) segmentation technique (Bioux et al., 2007; Pohl et al., 2007) was used to segment the images into three major tissue classes: gray matter; white matter; and CSF, using both SPGR and T2-weighted MR information as well as spatial priors. This technique was used to get Intra-Cranial Contents (ICC) volume. Manual tracing of OFC ROI was performed on non-segmented images to avoid segmentation errors due to susceptibility artifacts which are common in the OFC region.
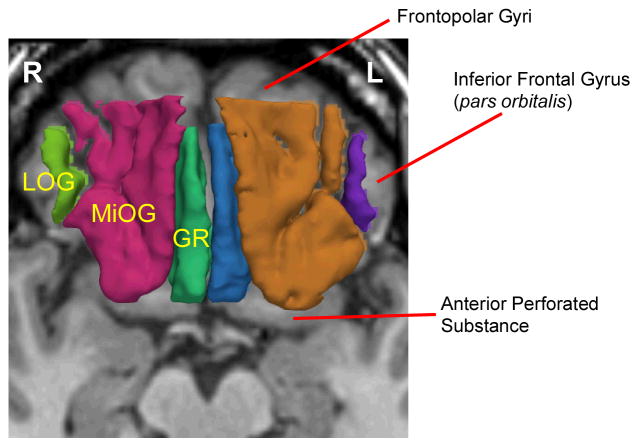
Images were realigned using the line between the anterior and posterior commissures and the sagittal sulcus to correct head tilt, and re-sampled into isotropic voxels (0.9375 mm3). This realignment procedure was essential for precise and consistent ROI delineation. Three-dimensional information was used to provide reliable delineation of the OFC ROI with a software package for medical image analysis [3D slicer, http://www.slicer.org] on a workstation. Definition and details of the method of region of interest for the OFC are provided in Nakamura et al. (2008). Figure 2 depicts image of the OFC divided into three sub-regions gyrus rectus, middle orbital gyri, and lateral orbital gyrus. These sub-regions are not used in the analysis for this current study.
Figure 2.
MR Images of Three Orbitofrontal Subregions. 3D reconstruction of the three orbitofrontal subregions of Gyrus Rectus (GR; left: blue, right: green), Middle Orbital Gyri (MiOG; left: brown, right: red), and Lateral Orbital Gyrus (LOG; left: purple, right: light green), superimposed on axial plane of SPGR image.
Results
Table 1 presents CB fractional anisotropy and OFC gray matter volumes measures (see Table 1.) Analysis of covariance (ANCOVA), controlling for parental SES, with one between-subjects factor of group (patients/control) and two within-subjects factors of region (CB/OFC) and side (left/right) revealed a significant group effect, F (1, 26) = 6.66, p = .016, partial eta squared = .204. None of the interaction terms with group was significant. As Table 1 shows, in relation to healthy controls, patients had both lower CB fractional anisotropy and OFC volume. For the patient group, antipsychotic medication dosage did not correlate with any of the anatomical measures.
Table 1.
MRI Gray Matter Volume for the Orbital Frontal Cortex and DTI Fractional Anisotropy Values for the Cingulum Bundle for Patient (n=17) and Control (n=12) Groups.
| Patients | Controls | |
|---|---|---|
| Orbital Frontal Cortex | ||
| Left | .6797 ± .0442 | .7058 ± .0685 |
| Right | .6650 ± .0520 | .7069 ± .0720 |
| Cingulum Bundle | ||
| Left | .4571 ± .0380 | .4900 ± .0374 |
| Right | .4341 ± .0397 | .4642 ± .0350 |
ote. Values are means plus or minus standard deviations
Neuropsychological Tests
Table 2 presents neuropsychological summary scores for the WAIS-III, and IGT. We performed mixed-model analyses of covariance to assess group differences in each test, covarying for parental SES, with group (patient, control) as a between-subjects factor and index measures for the WAIS-III and trial block for the IGT as the within-subjects factor. Table 2 shows lower full-scale, verbal, and performance IQ scores for patients (n=22) than for controls (n=23). ANCOVA revealed a highly significant group effect, F (1, 42) = 8.34, p =. 006, partial eta squared = .166, as well as a significant Group X WAIS-III IQ interaction, F (1, 42) = 6.83, p = .012, partial eta squared = .140. As shown in Table 2, whereas the control group earned similar IQ scores for both verbal and performance scales, the patient group did not, with performance IQ disproportionately lower than verbal IQ. For the patient group, antipsychotic medication correlated significantly with full scale (r = −.627, p = .003), verbal (r = −.566, p = .009), and performance (r = −.621, p = .003) IQ scores.
Table 2.
Neuropsychological scores for patients with schizophrenia for WAIS-III (n = 22) and IGT (n = 23) and for healthy participants for WAIS-III (n = 23) and IGT (n = 24).
| Demographic Information | Patients | Controls |
|---|---|---|
| Age | 38.78±9.90 | 41.5±7.35 |
| Education | 12.83±1.99 | 15.00±1.98 |
| SES | 3.75±1.22 | 2.42±1.06 |
| Parental SES | 2.83±1.11 | 2.71±1.27 |
| WAIS-III IQ | ||
| Full Scale | 91.83±13.74 | 105.22±16.24 |
| Verbal | 95.35±14.78 | 104.22±14.16 |
| Performance | 88.87±12.20 | 105.30±18.07 |
| WAIS-III Index | ||
| Verbal Comprehension | 99.96±15.88 | 103.13±14.47 |
| Perceptual Organization | 94.74±16.05 | 107.48±17.56 |
| Working Memory | 91.91±13.54 | 105.75±15.68 |
| Processing Speed | 81.04±10.1 | 101.87±14.48 |
| IGT | ||
| Block 1 | −2.17±12.02 | −1.17±10.87 |
| Block 2 | −1.25±10.01 | 4.33±9.08 |
| Block 3 | 1.25±9.97 | 6.91±9.65 |
| Block 4 | −1.58±9.55 | 3.83±10.30 |
| Block 5 | −0.08±9.16 | 4.67±11.31 |
Note. Values are means plus or minus standard deviations. SES = socioeconomic status; WAIS-III = Wechsler Adult Intelligence Scale---Third Edition; IGT= Iowa Gambling Test (IGT).
Likewise, for the WAIS-III index measures, the patient had lower scores, as revealed by the highly significant group effect, F (1, 42) = 11.34, p = .002, partial eta squared .213. A highly significant Group X WAIS-III Index interaction also emerged, reflected by disproportionately lower processing speed index score for the patients F (3, 126) = 6.82, p <.001, partial eta squared = .140. For the patient group, antipsychotic medication dosage correlated significantly with verbal comprehension (r = −.644, p = .002) and perceptual organization (r = −.628, p = .003) but not with working memory or processing speed. For the IGT, covarying for parental SES, patients had overall lower net scores across the five blocks of 20 trials, F (1, 44) = 5.36, p = .02, partial eta squared = .109. Antipsychotic medication dosage did not correlate with IGT performance for the patient group.
Neuropsychological and Brain Structure Correlates
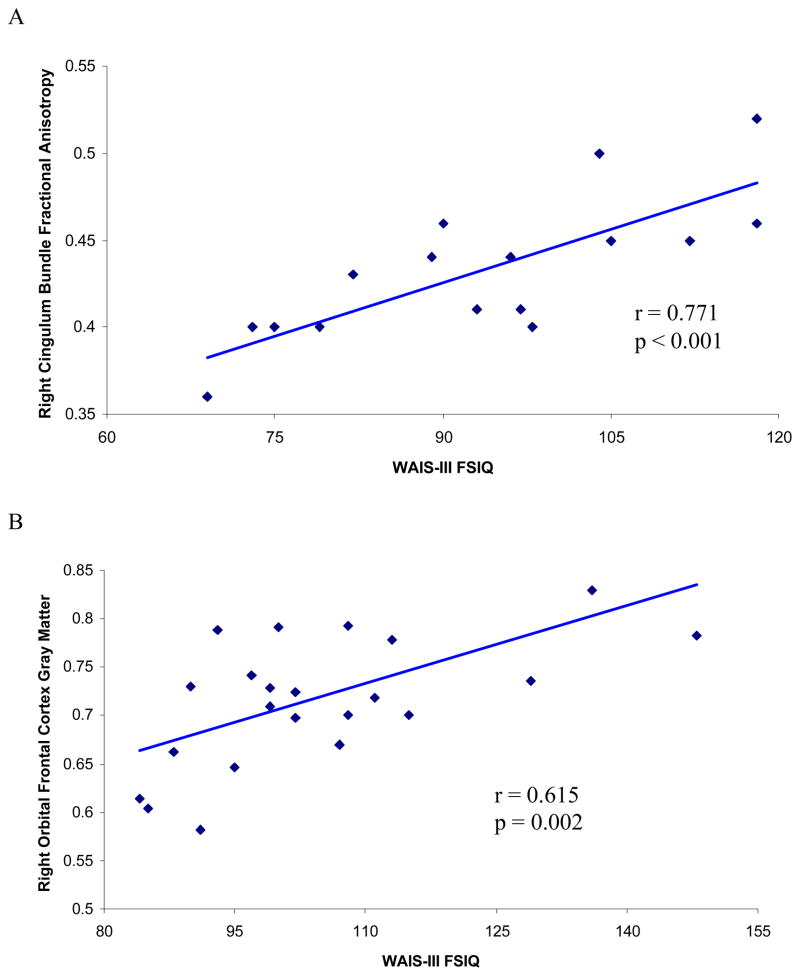
Table 3 presents structural anatomical-neuropsychological correlates for the patient sample. For the 16 patients who had both IQ and DTI measures, WAIS-III full-scale IQ correlated significantly with left (r = .581, p = .018) and right (r = .771, p < .001) CB, as did performance IQ with left (r=.701, p = .002) and right (r = .782, p <. 001) CB. Verbal IQ correlated significantly with right CB (r = .701, p = .002). When controlling for antipsychotic medication via partial correlation, right CB remained significantly associated with full-scale IQ (partial r = .670, p = .006), performance IO (partial r = 687, p = .005) and verbal IQ (partial r = .573, p = .026), whereas left CB remained significantly associated with performance IQ (partial r = .675, p = .006) and nearly significantly associated with full-scale IQ (partial r = .496, p = .006), but not with verbal IQ (p > .20). WAIS-III working memory also correlated significantly with left (r = .642, p = .007) and right (r = .740, p = .001) CB. IGT did not correlate with CB. IGT also did not correlate with OFC volume for the 23 patients who had both these measures. In addition, for the patient group, OFC volume measures did not correlate with any of the neuropsychological measures. Figure 3 presents a scatter plot of WAIS-III full-scale IQ and right CB for the patient group (see Panel A).
Table 3.
Pearson correlations of neuropsychological scores and orbital frontal cortex (OFC) for patients with schizophrenia (n=23) and healthy participants (n=22), and cingulum bundle (CB) for patients with schizophrenia (n=16) and healthy participants (n=12).
| Patients | Healthy Participants | |||||||
|---|---|---|---|---|---|---|---|---|
| OFC | CB | OFC | CB | |||||
| Left | Right | Left | Right | Left | Right | Left | Right | |
| WAIS-III IQ | ||||||||
| Full Scale | −.007 | −.135 | .581* | .771** | .435* | .615* | .106 | −.150 |
| Verbal | .089 | −.013 | .468 | .701** | .461* | .585** | −.050 | −.267 |
| Performance | −.135 | −.201 | .701** | .788** | .377 | .591** | .252 | −.016 |
| WAIS-III Index | ||||||||
| VC | .094 | −.009 | .447 | .607* | .586** | .632** | .234 | −.124 |
| PO | −.141 | −.259 | .642**. | 740** | .307 | .594** | .293 | .049 |
| WM | −159 | −.255 | .545* | .764** | .196 | .375 | .170 | −.363 |
| PS | −.226 | −.236 | .351 | .335 | .323 | .409 | .029 | −.345 |
| IGT | .204 | −.185 | −.125 | −.026 | .140 | .456* | −.240 | −.215 |
p< 0.05
p<.001
Figure 3.
Scatter plots of IQ with fractional anisotropy values of right cingulum bundle for the patient group (panel A) and gray matter volume of right orbital frontal cortex for healthy participant group (panel B).
The healthy participant group showed a different pattern of correlations from that of the patients. For the 23 healthy participants who had both MRI and IQ measures, WAIS-III full-scale IQ correlated significantly with left (r = .435, p = .038) and right (r = .615, p = .002) OFC. Verbal IQ correlated significantly with left (r = .461, p = .027) and right (r = .585, p = .003) OFC, as did performance IQ with right OFC (r = .585, p = .003). For the healthy participant sample, unlike the patient sample, right OFC correlated with overall earnings for the IGT (r = .456, p = .022). In addition, for the healthy participant sample unlike the patient sample, CB did not correlate with any of the neuropsychological measures. Figure 3 presents a scatter plot of WAIS-III full-scale IQ and right OFC for the healthy participants group (see Panel B).
To compare the specific and joint contributions of CB and OFC to general intelligence and decision making, both brain regions were entered as predictors into a hierarchical regression, first with WAIS-III full-scale IQ, and then with IGT, as the dependent variable. To be included in these analyses, subjects had to have available both MRI and DTI measures, which yielded groups of 16 patients and 12 healthy participants. As predicted, in the patient group, for WAIS-III full-scale IQ, right CB produced a highly significant R square change of .595 (F = 20.54, df = 1, 14, p < .001) in contrast to the absence of any contribution attributable to right OFC (p = .861). The right CB accounted for 59.14% and 58.52% of the variance in full-scale IQ of the patient group, as reflected by partial and semi-partial correlation values of .769 and .765. However, for the healthy participant group, right OFC, but not right CB, contributed significantly to full-scale IQ, as reflected by a significant R square change of .351 (F = 5.41, df = 1, 10, p=.04), in contrast to the absence of any significant contribution attributable to right CB (p = .35). Right OFC accounted for 40.07% and 39.06% of the variance in full-scale IQ of the healthy participant group in comparison to 9.67% and 6.3% of the variance attributable to right CB.
For the IGT in the patient group, neither right CB (p > .90) nor right OFC (p > .50) accounted for a significant portion of the variance in IGT scores. For the healthy participant group, however, right OFC produced an R square change of. 264 (F = 3.58, df =1, 10, p=.09), which, approached significance, accounting for 32.38% and 30.91% of variance in IGT scores, as reflected by partial and semi-partial correlation values of .569 and .556. These trend findings may reflect the restricted statistical power of the current study that was limited to multiple regression analyses on those 12 control subjects who had both OFC and CB measures. By comparison, right CB failed to account for a significant portion of variance in IGT scores in the healthy participant group.
Discussion
The study compared within a group of patients with schizophrenia the contributions of disease-related structural changes in CB white matter and OFC gray matter to performance on neuropsychological tests of intelligence and decision making. The WAIS-III served as a measure of general intelligence and the IGT measured motivated decision making. The structural imaging data revealed reductions in both CB white matter and OFC gray matter for the patients in comparison to healthy participants. These findings were consistent with between-subject analyses that have focused on either one of these brain regions in samples of patients with schizophrenia and healthy participants (Kubicki et al., 2003; Nakamura et al., 2008). Likewise, the patient group showed, as expected, lower WAIS-III scores, as well as lower overall earnings on the IGT, and these test data conformed well to previous studies that have emphasized generalized cognitive deficits in schizophrenia.
For the patients, reduced CB white matter integrity correlated significantly with poorer scores in general intelligence as well as for the WAIS-III derived measure of working memory. However, neither CB nor OFC measures correlated with IGT performance for the patient group. By contrast, for the healthy participants, larger right and left OFC volume measures correlated with higher verbal IQ, as did increased right OFC volume with higher performance IQ and with overall greater earnings for the IGT. For the patient group, then, multiple regression analysis indicated that reduced CB white matter but not reduced OFC gray matter predicted lower scores for WAIS-III measures of general intellectual abilities. Surprisingly, even though correlated with WAIS-III IQ, IGT failed to be linked with either reduced CB white matter or with reduced OFC gray matter in the patient group. For healthy participants, OFC gray matter correlated with both WAIS-III and IGT scores, whereas CB white matter did not.
The current results thus demonstrated that disease-related structural changes in CB white matter and OFC gray matter may contribute differently to functional impairment in schizophrenia. The data showed that within the same persons with schizophrenia, reductions in CB white matter but not in OFC gray matter correlated with neuropsychological outcome of general intelligence. As the most prominent white matter tract in the limbic system, the CB furnishes both input and output to the dopaminergic-rich anterior cingulate cortex and lateral frontal sites, as well as to the amygdala, nucleus accumbens, and medial dorsal thalamus (Goldman-Rakic, Selemon, & Schwartz, 1984; Pandya & Seltzer, 1982; Vogt, Rosene, & Pandya, 1979). By virtue of these extensive white matter connections, the CB may very well be intimately involved in supporting psychometric intelligence via a general executive process of monitoring and evaluation of performance (Nestor et al., 2008). Schizophrenia may disrupt these connections thereby leading to failures of executive functions of monitoring that in turn contribute to reductions in general intelligence. Thus, these within-subject comparisons of the functional significance of reductions in CB white matter and OFC gray matter pointed to an especially important role for a disruption in CB connections in diminished general intellectual abilities in this sample of patients with chronic schizophrenia.
Other research studies have offered evidence as to how the anterior cingulate cortex, including its white matter tract, CB, may be compromised by schizophrenia. For example, functional imaging studies have shown performance monitoring, especially in relation to sensitivity to errors, to be supported by the anterior cingulate cortex (Carter et al., 1998; Kiehl, Liddle, & Hopfinger, 2000). Event-related functional MRI studies involving patients with schizophrenia have demonstrated reduced error-related activity in the anterior cingulate and less performance adjustment after errors on a degraded continuous performance task (Carter, McDonald, Ross, & Stenger, 2001). Likewise, event-related potential studies have shown that performance errors on speeded response tasks elicit a specific brain wave, known as error-related negativity, which typically peaks about 40–70 ms after the commission of an error (Taylor, Stern, & Gehring, 2007). Westlye, Walhovd, Bjornerud, Due-Tonnessen, and Fjell (2008) recently correlated DTI fractional anisotropy values of anterior cingulate cortex regions with event-related brain potential recordings while healthy middle aged adults performed a version of the Eriksen flanker task. Their results pointed to the left posterior cingulate cortex as a principal neuronal generator for event-related negativity (Westlye, et al. 2008).
In a similar vein, modeling studies have suggested that the functional neuroanatomy of the anterior cingulate cortex to be ideally suited for implementation of a neural network that is dynamically set and flexibly tuned to select among existing codes and pathways in the service of processing dopaminergic “prediction error” signals that are ultimately used to modify behavior in response to direct feedback (e.g., Dias, Robbins, & Roberts, 1996; Schultz, Dayan, & Montague, 1997). Taken together, these studies, along with the current findings, converge upon the anterior cingulate cortex as a key pathophysiological site in schizophrenia. These accumulating and converging findings provide an empirical foundation for the development of a testable and falsifiable hypothesis for a disease-related disturbance in anterior cingulate cortex circuitry that is evident, anatomically, by reduced CB white matter integrity, neurophysiologically, by abnormalities in error-related negativity, and neuropsychologically, by diminished general intelligence.
On the other hand, the current findings found no association between reduced OFC volume and lower IGT performance in the patient sample. Early pioneering work revealed the IGT to be associated with lesions to the ventromedial prefrontal cortex in patients who were described as habitually making disadvantageous choices in their personal lives that negatively impacted how they related with others (Bechara et al., 1994; Bechara, Tranel, & Damasio, 2000). Persons with substance abuse problems, irrespective of substance, also perform poorly on the IGT, and these decision making deficits can occur with or without concomitant problems with executive functioning or working memory (e.g., see Paulus, 2007). In studies comparing IGT performance of patients with schizophrenia and healthy controls, however, the results are mixed (Sevy et al., 2007). Several studies have indeed shown impaired IGT performance in patients although the extent to which other disease-related deficits in executive function and working memory may have contributed to their impaired decision making is not entirely clear (Sevy et al., 2007). Finding of other studies have raised the question as to whether co-occurring substance abuse may be an important factor influencing IGT performance in patients with schizophrenia (Bechara et al., 2001; Mazas, Finn, & Steinmetz, 2000; Sevy et al., 2007).
For the healthy participants, both IGT and IQ scores correlated strongly with OFC gray matter. These data raised two important points. First, that IGT correlated significantly with OFC gray matter volume is consistent with the growing body of research demonstrating that this brain region plays a key role in the kind of decision making and reasoning assessed by this neuropsychological measure (Bechara et al., 1994; Bechara, Tranel, & Damasio, 2000; Bechara et al., 2001). Second, that OFC gray mater volume also correlated with IQ runs counter to studies that have long emphasized IQ to be reduced by damage to posterior brain areas in contrast to being generally spared by frontal lobe lesions (see Gray and Thompson, 2004). More recently, though, studies have examined frontal lobe contributions to IQ in reference to crystallized and fluid intelligences, with the former defined as over learned skills and static knowledge, such as vocabulary, and the latter defined as reasoning and novel problem solving ability, such as putting blocks together to make designs (Cattell, 1963). These studies have suggested that fluid intelligence is compromised more by damage to the frontal lobes than to posterior lobes (Duncan, 1996; Duncan, Burgess, & Emslie, 1995). We too found fluid intelligence, as measured by WAIS-III Block Design subtest, to be correlated significantly with right OFC gray matter volume (r=.536, p=.008). Moreover, we also found that unlike prior studies, crystallized intelligence, as measured by the WAIS-III Vocabulary subtest, correlated with right (r=.564, p=.006) and left OFC gray matter volumes (r=.502, p=.015). Whether this latter finding reflects the increased sensitivity of individual differences in quantitative measures derived from high resolution MRI awaits further research.
Overall, then, the healthy participants showed a strikingly different pattern of correlations from that evident in the patient group. This suggested that schizophrenia may alter the normal ordering and organization of brain-behavior relationships. The current findings further suggested that these abnormalities are expressed as reduced white matter microstructural integrity of the CB that disrupts long-range axonal communication among widespread networks of brain regions that are vital to thinking and intelligence. For the healthy participants, however, OFC but not CB correlated strongly with intelligence and decision making, and these findings comported to growing line of evidence from functional brain imaging that has suggested general intelligence and decision making to be closely related to cognitive functions that depend heavily on prefrontal lobe circuitry (e.g., Duncan, Emslie, Williams, Johnson, & Freer, 1996; Duncan et al., 2000; Gray, Chabris, & Braver, 2003, Jung & Haier, 2007).
In summary, the current study demonstrated that variation in intelligence could be accounted for only by variation in CB white matter but not OFC gray matter in this sample of patients with chronic schizophrenia. These findings thus pointed to a rather specific relationship between CB white matter and reduced intelligence. However, the current study relied on a retrospective analysis that combined data from two separate studies (Nakamura et al., 2008; Nestor et al., 2008), for the expressed purpose of providing a novel and rigorous quantitative within- and between-group comparison of CB and OFC involvement in schizophrenia neuropsychology. An additional limitation of the study is that these critical between-and within group comparisons were based on modest sample sizes of 16 patients and 12 controls who had complete CB, OFC, and neuropsychological measures. Replication of these findings is required using a prospective study that combines DTI-CB and MRI-OFC with neuropsychological measures. Future investigations are also needed in order to compare neuropsychological correlates of CB white matter to those of other brain structures in addition to the OFC region studied here. In this regard, gray matter volume of the anterior cingulate cortex might provide an excellent comparison to CB white matter. Other important factors that require further exploration include the roles of medication, disease duration, and family history in the functional neuroanatomy of schizophrenia.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/journals/neu.
References
- Adolphs R. Cognitive neuroscience of human social behavior. Nature Reviews Neuroscience. 2003;4(3):165–178. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Bar-On R, Tranel D, Denburg NL, Bechara A. Exploring the neurological substrate of emotional and social intelligence. Brain. 2003;126:1790–1800. doi: 10.1093/brain/awg177. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39(4):376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123(Pt 11):2189–202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- Bouix S, Martin-Fernandez M, Ungar L, Nakamura M, Koo MS, McCarley RW, et al. On evaluating brain tissue classifiers without a ground truth. Neuroimage. 2007;36:1207–1224. doi: 10.1016/j.neuroimage.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick M, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the on line monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Carter CS, McDonald AW, Ross LL, Stenger VA. Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event related fMRI study. American Journal of Psychiatry. 2001;158:1423–1428. doi: 10.1176/appi.ajp.158.9.1423. [DOI] [PubMed] [Google Scholar]
- Cattell RB. Theory of fluid and crystallized intelligence. A critical experiment. Journal of Educational Psychology. 1963;54:1–22. doi: 10.1037/h0024654. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Croonenwett WJ. Neural networks in schizophrenia. American Journal of Psychiatry. 2008;165:937–939. doi: 10.1176/appi.ajp.2008.08050700. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbibs TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behavioral Neuroscience. 1996;110:872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- Duncan J, Emslie H, Williams P, Johnson R, Freer C. Intelligence and the frontal lobe: The organization of goal-directed behavior. Cognitive Psychology. 1996;30:257–303. doi: 10.1006/cogp.1996.0008. [DOI] [PubMed] [Google Scholar]
- Duncan J, Seitz RJ, Kolodny J, Bor D, Herzog H, Ahmed A, Newell FN, Emslie H. A neural basis for general intelligence. Science. 2000;289(5478):457–460. doi: 10.1126/science.289.5478.457. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. American Journal of Psychiatry. 2008;165:1015–1023. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JI, Tang C, Carpenter D, Buchsbaum M, Schmeidleer J, Flanagan L, et al. Diffusion tensor imaging findings in first-episode and chronic schizophrenic patients. American Journal of Psychiatry. 2008;165:1024–1032. doi: 10.1176/appi.ajp.2008.07101640. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD, Schwartz ML. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12:719–743. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- Gray JR, Thompson PM. Neurobiology of intelligence: Science and ethics. Nature Reviews/Neuroscience. 2004;5:471–482. doi: 10.1038/nrn1405. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW. The primacy of cognition in schizophrenia. American Psychologist. 2005;60:229–242. doi: 10.1037/0003-066X.60.3.229. [DOI] [PubMed] [Google Scholar]
- Jung RE, Haier RJ. The parieto-frontal integration theory (P-FIT) of intelligence: Converging neuroimaging evidence. Behavioral and Brain Sciences. 2007;30:135–134. doi: 10.1017/S0140525X07001185. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, Hopfinger JB. Error processing and the rostral anterior cingulate: an event-related fMRI study. Psychophysiology. 2000;37:216–223. [PubMed] [Google Scholar]
- Koenen KC, Moffitt TE, Roberts A, Martin LT, Kubzansky L, Harrington H, Poulton R, Caspi A. Childhood IQ and adult mental disorders: A test of the cognitive reserve hypothesis. American Journal of Psychiatry. 2009;166:50–57. doi: 10.1176/appi.ajp.2008.08030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin C-F, Maier SE, Frumin M, Nestor PG, Salisbury DF, et al. Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. American Journal of Psychiatry. 2002;159(5):813–820. doi: 10.1176/appi.ajp.159.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Nestor PG, Wible CG, Frumin M, Maier SE, et al. Cingulate fasciculus integrity disruption in schizophrenia: a magnetic resonance diffusion tensor imaging study. Biological Psychiatry. 2003;54(11):1171–1180. doi: 10.1016/s0006-3223(03)00419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazas CA, Finn PR, Steinmetz JE. Decision-making biases, antisocial personality, and early-onset alcoholism. Alcoholism, Clinical, and Experimental Research. 2000;24(7):1036–1040. [PubMed] [Google Scholar]
- McClellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The fifth edition of the Addiction Severity Index. Journal of Substance Abuse Treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Nestor PG, Levitt JJ, Cohen AL, Kawashima T, Shenton ME, McCarley RW. Orbital frontal volume deficit in schizophrenia and thought disorder. Brain. 2008;131:180–195. doi: 10.1093/brain/awm265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor PG, Akdag SJ, O’Donnell BF, Niznikiewicz MA, Law S, Shenton ME, McCarley RW. Word recall in schizophrenia: A connectionist model. American Journal of Psychiatry. 1998;155:1685–1690. doi: 10.1176/ajp.155.12.1685. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Kubicki M, Gurrera RJ, Niznikiewicz M, Frumin M, McCarley RW, et al. Neuropsychological correlates of diffusion tensor imaging in schizophrenia. Neuropsychology. 2004;18:629–637. doi: 10.1037/0894-4105.18.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor PG, Kubicki M, Niznikiewicz M, Gurrera RJ, McCarley RW, Shenton ME. Neuropsychological disturbance in schizophrenia: A Diffusion Tensor Imaging Study. Neuropsychology. 2008;22(2):246–254. doi: 10.1037/0894-4105.22.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obonsawin MC, Crawford JR, Page J, Chalmers P, Cochrane R, Low G. Performance on tests of frontal lobe function reflect general intellectual ability. Neuropsychologia. 2002;40(7):970–977. doi: 10.1016/s0028-3932(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Seltzer B. Intrinsic connections and architectonics of posterior parietal cortex in the rhesus monkey. Journal of Comparative Neurology. 1982;204(2):196–210. doi: 10.1002/cne.902040208. [DOI] [PubMed] [Google Scholar]
- Paulus MP. Decision-making dysfunctions in psychiatry--altered homeostatic processing? Science. 2007;318(5850):602–606. doi: 10.1126/science.1142997. [DOI] [PubMed] [Google Scholar]
- Pohl KM, Bouix S, Nakamura M, Rohlfing T, McCarley RW, Kikinis R, et al. A hierarchical algorithm for MR brain image parcellation. IEEE T MED IMAGING. 2007;26(9):1201–12. doi: 10.1109/TMI.2007.901433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriquez-Sanchez JM, Crespo-Facorro B, Perez-Iglesias R, Gonzalez-Blanch C, Alvarez-Jimenez M, Llorca J, et al. Prefrontal cognitive functions in stabilized first-episode patients with schizophrenia spectrum disorders: a dissociation between dorsolateral and orbitofrontal functioning. Schizophrenia Research. 2005;77(2–3):279–288. doi: 10.1016/j.schres.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1597. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Sevy S, Burdick KE, Visweswaraiah H, Abdelmessih S, Lukin M, Yechiam E, et al. Iowa gambling task in schizophrenia: a review and new data in patients with schizophrenia and co-occurring cannabis use disorder. Schizophrenia Research. 2007;92(1–3):74–84. doi: 10.1016/j.schres.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeszko PR, Strous RD, Goldman RS, Ashtari M, Knuth KH, Lieberman JA, et al. Neuropsychological correlates of hippocampal volumes in patients experiencing a first episode of schizophrenia. The American Journal of Psychiatry. 2002;159(2):217–226. doi: 10.1176/appi.ajp.159.2.217. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Stern ER, Gehring WJ. Neural systems for error monitoring: recent findings and theoretical perspectives. Neuroscientist. 2007;1:160–172. doi: 10.1177/1073858406298184. [DOI] [PubMed] [Google Scholar]
- Torres IJ, Flashman LA, O’Leary DS, Swayze V, Andreasen NC. Lack of an association between delayed memory and hippocampal and temporal lobe size in patients with schizophrenia and healthy controls. Biological Psychiatry. 1997;42(12):1087–1096. doi: 10.1016/s0006-3223(97)00024-3. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Rosene DL, Pandya DN. Thalamic and cortical afferents differentiate anterior from posterior cingulate cortex in the monkey. Science. 1979;204(4389):205–207. doi: 10.1126/science.107587. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3. Texas: Harcourt, Brace; 1997. [Google Scholar]
- Weiss AP, Heckers S. Neuroimaging of declarative memory in schizophrenia. Scandinavian Journal of Psychiatry. 2001;42:239–250. doi: 10.1111/1467-9450.00234. [DOI] [PubMed] [Google Scholar]
- Westlye LT, Walhovd KB, Bjornerud A, Due-Tonnessen P, Fjell AM. Error-related negativity is mediated by fractional anisotropy in the posterior cingulated gyrus-A study combining diffusion tensor imaging and electrophysiology in health adults. Cerebral Cortex; 2008 doi: 10.1093/cercor/bhn084. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Woodberry KA, Giuliano AJ, Seidman LJ. Premorbid IQ in schizophrenia: A meta-analytic review. American Journal of Psychiatry. 2008;165:579–587. doi: 10.1176/appi.ajp.2008.07081242. [DOI] [PubMed] [Google Scholar]
- Zinkstok JR, de Wilde O, van Amelsvoort TAMJ, Tanck MW, Baas F, Linszen DH. Association between DTNB1 gene and intelligence: a case-controlled study in young patients with schizophrenia and related disorders and unaffected siblings. Behavior and Brain Functions. 2007;3 doi: 10.1186/1744-9081-3-19. [Open Access]. [DOI] [PMC free article] [PubMed] [Google Scholar]