Abstract
A paradox of plant hormone biology is how a single small molecule can affect a diverse array of growth and developmental processes. For instance, brassinosteroids (BRs) regulate cell elongation, vascular differentiation, senescence and stress responses. BRs signal through the BES1/BZR1 (bri1-EMS-suppressor 1/Brassinazole-Resistant 1) family of transcription factors, which regulate hundreds of target genes involved in this pathway; yet little is known of this transcriptional network. By microarray and chromatin immunoprecipitation (ChIP) experiments, we identified a direct target gene of BES1, AtMYB30, which encodes a MYB family transcription factor. AtMYB30 null mutants display decreased BR responses and can enhance the dwarf phenotype of a weak allele of the BR receptor mutant bri1. Many BR-regulated genes have reduced expression and/or hormone-induction in AtMYB30 mutants, indicating that AtMYB30 functions to promote the expression of a subset of BR-target genes. AtMYB30 and BES1 bind to a conserved MYB-binding site and E-box sequences, respectively, in the promoters of genes that are regulated by both BRs and AtMYB30. Finally, AtMYB30 and BES1 interact with each other both in vitro and in vivo. These results demonstrated that BES1 and AtMYB30 function cooperatively to promote BR target gene expression. Our results therefore establish a new mechanism by which AtMYB30, a direct target of BES1, functions to amplify BR signaling by helping BES1 activate downstream target genes.
Keywords: Brassinosteroids, BES1, MYB30, transcription, target genes
Introduction
Brassinosteroids (BRs) play important roles in many plant growth and developmental processes, including germination, cell expansion and division, photomorphogenesis, vascular differentiation, senescence and stress/disease resistance (Clouse, 1996; Li and Chory, 1999; Krishna, 2003). Mutants defective in BR biosynthesis or perception display dwarf phenotypes in both light and dark conditions (Clouse et al., 1996; Li et al., 1996; Szekeres et al., 1996; Li and Chory, 1997).
Genetic and molecular studies in Arabidopsis have greatly advanced our understanding of the BR signaling pathway (Clouse, 2002; Thummel and Chory, 2002; Belkhadir and Chory, 2006; Li and Jin, 2007). The BR receptor was identified by many mutant alleles in a single gene, BRASSINOSTEROID INSENSITIVE1 (BRI1), which encodes a leucine rich repeat (LRR) receptor kinase (Clouse et al., 1996; Li and Chory, 1997). BRI1 binds BRs through a novel 100 amino acid subdomain embedded within the LRRs and transduces the hormone signal to downstream targets through its intracellular kinase domain (Friedrichsen et al., 2000; He et al., 2000; Oh et al., 2000; Wang et al., 2001; Kinoshita et al., 2005; Wang et al., 2005; Wang et al., 2005). In the absence of BRs, a novel protein, BKI1, binds to BRI1 and inhibits its function (Wang and Chory, 2006). BR binding to BRI1 leads to dissociation of BKI1 (Wang and Chory, 2006) and increased association of BRI1 with BAK1, another LRR receptor kinase (Li et al., 2002; Nam and Li, 2002). BRI1 may signal through BSK kinases (Tang et al., 2008).
The output of the signaling pathway is the dephosphorylation of closely-related transcription factors including BES1, BZR1 and 4 other members in Arabidopsis (Wang et al., 2002; Yin et al., 2002; Zhao et al., 2002). BES1 was identified by a gain-of-function mutation in the BES1 gene, which suppresses bri1 dwarfism. The gain-of-function mutant bes1-D has a constitutive BR response phenotype, including excessive stem elongation, early senescence, resistance to a BR-biosynthesis inhibitor Brassinazole (BRZ) in both dark and light-grown seedlings, as well as up-regulation of BR-induced gene expression (Yin et al., 2002), most likely due to increased BES1 protein levels. BZR1 was identified by a gain-of-function dominant mutation that leads to its over-accumulation; bzr1-D seedlings are resistant to BRZ in the dark but hypersensitive to BRZ in the light, due to increased feedback inhibition of BR biosynthesis (Wang et al., 2002). Consistent with the difference in the mutant phenotypes in the light, BES1 was shown to be a transcriptional activator while BZR1 was a transcription repressor (He et al., 2005; Yin et al., 2005).
BES1 and BZR1 activities are regulated by a GSK3-like kinase BIN2 (Choe et al., 2002; Li and Nam, 2002; Pérez-Pérez et al., 2002). BIN2 phosphorylates BES1 and BZR1 and negatively regulates their function (He et al., 2002; Yin et al., 2002; Vert and Chory, 2006; Gampala et al., 2007; Gendron and Wang, 2007; Ryu et al., 2007). BR signaling through BRI1 inhibits BIN2 function by an unknown mechanism, leading to the accumulation of unphosphorylated BES1/BZR1 in the nucleus. The dephosphorylation of BES1 is facilitated by BSU1 phosphatase, which is required for accumulation of unphosphorylated BES1 (Mora-Garcia et al., 2004). The unphosphorylated forms are capable of binding promoter elements in BR-regulated genes.
Several genome-wide microarray analyses performed in Arabidopsis indicated that BRs regulate several classes of target genes, including many cell wall organization enzymes required for cell expansion and division, genes involved in ethylene biosynthesis and many other metabolic pathways, transcription factors, signaling molecules and genes with unknown functions (Goda et al., 2002; Mussig et al., 2002; Yin et al., 2002; Goda et al., 2004; Nemhauser et al., 2004). In light-grown seedlings, BR treatment for 2.5 hr induces the expression of about 342 genes and represses the expression of 296 genes (Nemhauser et al., 2004). Longer BR treatment (12-24 hr) affected many more genes (Goda et al., 2004). Reduction of BRs in root tissues in the brevis radix mutant (brx, which has reduced root growth) affected about 4000 (or ∼15%) of Arabidopsis genes, indicating a more profound effect of BRs on long-term gene expression (Mouchel et al., 2006). How BRs regulate different subsets of target genes in different tissues, organs, developmental stages and environmental conditions is largely unknown.
BR-induced genes include more than 29 transcription factors, many of which are also up-regulated in the bes1-D mutant (Nemhauser et al., 2004, our unpublished results). This observation raises the possibility that BES1 directly regulates some of these transcriptional factors, which either mediate or modulate BR-target gene expression. Here, we report the characterization of one BES1-induced transcription factor, AtMYB30, which was previously found to be involved in cell death in hypersensitive response (HR) during pathogen attack in adult Arabidopsis plants (Daniel et al., 1999; Raffaele et al., 2006; Raffaele et al., 2008). At the young seedling stage, knock-out of the AtMYB30 gene can cause altered BR responses and target gene expression. We also found that AtMYB30 protein binds to a conserved MYB-binding site in BR-target gene promoters and interacts with BES1. Thus, AtMYB30 is a node in a transcriptional circuit by which BRs regulate target gene expression.
Results
AtMYB30 is a BES1 direct target
Recently published microarray studies identified 342 BR-induced genes of which 29 encode putative transcription factors (Nemhauser et al., 2004). We have been studying the functions of several BR-regulated transcription factors. Here we present the characterization of AtMYB30 (At3g28910) since its expression level increased about 1.5 fold after BR treatment in Arabidopsis seedlings (Nemhauser et al., 2004). To confirm the microarray result, we examined AtMYB30 expression levels using quantitative real time PCR (Figure 1a). As expected, AtMYB30 was induced about 1.5 fold in wild-type seedlings after treatment with BL, the most active BR. Moreover, in bes1-D mutants (in which BES1 protein levels are increased), AtMYB30 expression levels were significantly higher than in wild-type plants treated with or without BL. In the dark, the expression level of AtMYB30 was about 2 times higher than that of seedlings grown under light and is reduced by BRZ, which specifically blocks BR biosynthesis at the C-22 hydroxylation step, thereby decreasing endogenous BR level (Asami et al., 2000). The induction of AtMYB30 by BRs was also reported in the Arabidopsis gene expression eFP browser (http://bbc.botany.utoronto.ca/efp/cgi-bin/efpWeb.cgi). All of these results indicate that AtMYB30 is regulated by BRs / BES1 and may function in the BR pathway.
Figure 1. AtMYB30 is a BES1 direct target.
(a) The expression levels of AtMYB30 were determined using quantitative real time PCR with RNA prepared from 10-day-old light-grown Col (WT) or bes1-D seedlings treated with or without 1 μM BL for 3 hr and 5-day-old dark-grown Col-0 (WT) in the absence or presence of 1 μM BRZ. The number on each column represents the relative expression levels compared to an UBQ5 (At5g15400) gene as internal control.
(b) CHIP assay indicated that BES1 is associated with the promoter of AtMYB30 in vivo. BES1 antibody and GFP antibody (as control) were used to immunoprecipitate chromatin prepared from 10-day-old light-grown bes1-D seedlings. Quantitative real-time PCR was performed with primers from indicated positions at the AtMYB30 promoter. The fold changes were calculated based on the relative change of anti-BES1 comparing with anti-GFP, after normalized by TA3 internal control.
(c-e) AtMYB30 expression patterns in 5-day-old dark-grown (c), 5-day-old (d), or 10-day-old (e) light-grown seedlings, detected by GUS reporter gene.
Chromatin Immunoprecipitation (ChIP) experiments were used to determine if BES1 directly regulates AtMYB30 expression levels. ChIP was performed using anti-BES1 antibody and a control antibody. TA3, a retrotransposable element in Arabidopsis, which does not respond to BRs or contain BES1 binding sites, was used as the internal control (Yu et al., 2008). It has been demonstrated that BES1 can bind to E-boxes (CANNTG) to regulate target gene expression (Yin et al., 2005). Quantitative real time PCR was carried out with ChIP products and PCR primers flanking E-boxes at -2.3kb and -1.1kb of the AtMYB30 promoter (Figure 1b). BES1 was enriched significantly at -2.3kb but not at -1.1kb, suggesting that BES1 binds to -2.3kb promoter region in vivo. We therefore conclude that BES1 activates AtMYB30 expression by directly binding to the AtMYB30 promoter, likely through the E-box sequences.
AtMYB30 knock-out mutants show altered BR response phenotypes
To study the function of AtMYB30 in the BR pathway, we first determined its expression pattern using the β-glucuronidase (GUS) reporter gene fused to the AtMYB30 promoter fragment. Consistent with our earlier quantitative PCR analysis (Figure 1a), AtMYB30 is strongly expressed in dark-grown seedlings (Figure 1c). In light-grown seedlings, AtMYB30 was expressed highly in roots, cotyledons and hypocotyls, and relatively weaker in leaves (Figure 1 d and e). The strong expression of AtMYB30 in young seedlings and no detectable expression in adult plants (Daniel et al., 1999) suggest that the gene plays an important role during early stages of plant development. It is worth noting that BES1 is ubiquitously expressed throughout the entire seedlings, which overlaps with the AtMYB30 expression regions (Yin et al., 2002).
To investigate the role of AtMYB30 in BR response, we obtained two T-DNA knock-out lines, atmyb30-1, with a T-DNA insertion located in the third exon and atmyb30-2 with a T-DNA inserted in the 5′ UTR region (Figure 2a). No transcripts of AtMYB30 gene were detected by RT-PCR in either line (Figure 4, and data not shown), suggesting that they are null alleles. The knock-out lines showed altered BR response phenotypes as measured by hypocotyl elongation assays in the absence or presence of BRZ (Figure 2). Because the main function of BRs is to regulate cell elongation, the hypocotyl elongation assays have been routinely used to determine BR activity (e.g. Li and Chory 1997, Nemhauser et al., 2004, Yin et al., 2005).
Figure 2. AtMYB30 knock-out and over-expression lines show altered BR response phenotype.
(a) Schematic representation of the two T-DNA knock-out alleles of AtMYB30 gene.
(b) 5-day-old dark-grown seedlings of Col-0 (WT), atmyb30-1 and atmyb30-2 grown on ½ MS media with or without 1μM BRZ.
(c) The hypocotyl lengths of 5-day-old dark-grown seedlings in the absence or presence of 1 μM BRZ. Averages and standard deviations were calculated from 15-30 seedlings.
(d) 6-day-old light-grown seedlings grown on ½ MS media with or without 1μM BRZ.
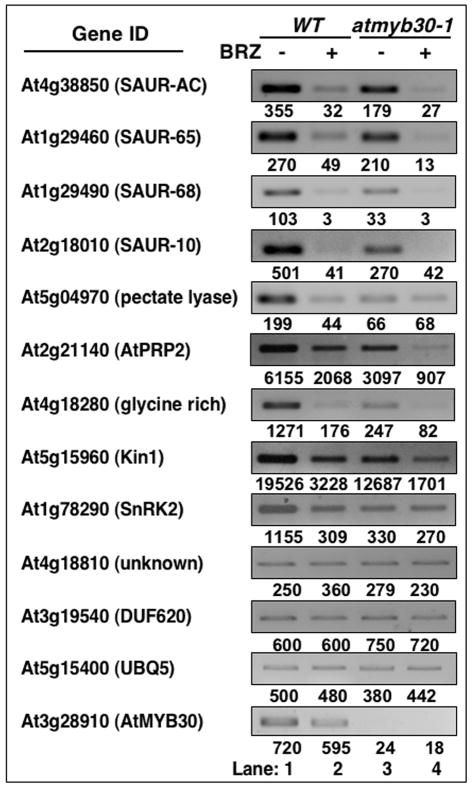
Figure 4. The expression of a subset of BR-regulated genes is reduced in atmyb30.
(a) RT-PCR were carried out with a biological replicate of material used in microarray experiment using 5-day-old dark grown seedlings in the absence or presence of 1 μM BRZ. Nine BRZ-regulated genes, 3 control genes (At4g18810, At3g19540 and At5g15400/UBQ5) and AtMYB30 were shown. The numbers below the gel panel (lanes 1-4) represented the expression levels from the microarray experiment.
In the dark, the knock-out mutants had slightly shorter hypocotyls compared to wild-type control (Figure 2b and c). More significantly, in the presence of 1μM BRZ, the knock-out lines were about 30% shorter than wild-type (Figure 2b and c). The experiments have been repeated more than 5 times with similar results. The differences are significant as determined by Student's t-test (p<0.01). The results indicate that AtMYB30 knock-out lines are more sensitive to BRZ compared to wild-type seedlings, suggesting that AtMYB30 is involved in BR responses.
The light grown seedlings of AtMYB30 mutants were not obviously different from the wild-type plants, however, the mutants were more sensitive to BRZ with shorter hypocotyls and darker green leaves (Figure 2d). Taking together, these results demonstrated that AtMYB30 plays a positive role in the BR signaling pathway.
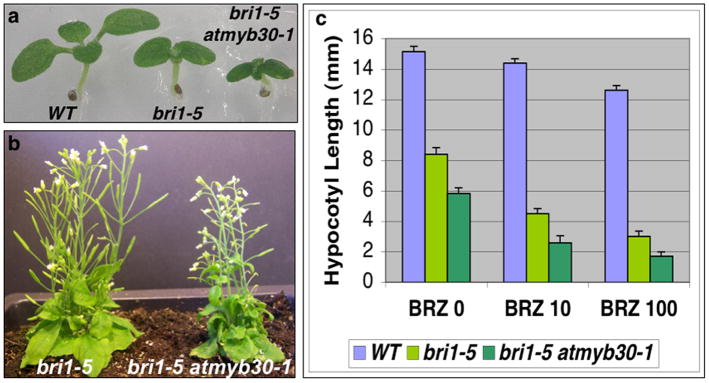
To test if there is any genetic interaction between BRI1 and AtMYB30, we crossed the atmyb30-1 with bri1-5, a weak allele of bri1, and generated a bri1-5 atmyb30-1 double mutant (Figure 3). bri1-5 has a mutation in the extracellular domain of the BR receptor BRI1 and displays a semi-dwarf phenotype (Noguchi et al. 1999). The double mutant significantly enhanced the bri1-5 phenotype, including reduced hypocotyl and leaf petiole length, and rounder, curlier and darker-green leaves under light (Figure 3a and 3b, Supplementary Figure S1). The inflorescence stems of double mutants were shorter than bri1-5 (Figure 3b), although the difference became much more subtle as plants matured (data not shown). We also tested BRZ response with dark-grown seedlings of wild-type, bri1-5 and bri1-5 atmyb30-1 double mutants. As shown in Figure 3c, the bri1-5 atmyb30-1 double mutant was more sensitive to BRZ than bri1-5. The results further suggest that AtMYB30 is involved in BR pathway.
Figure 3. AtMYB30 knock-out mutants enhance bri1-5 mutant phenotype.
(a) 6-day-old light-grown WT, bri1-5 and bri1-5 atmyb30-1 seedlings grown on ½ MS plates.
(b) Adult phenotype of bri1-5 and bri1-5 atmyb30-1 in soil.
(c) The hypocotyl length measurements of 5-day-old dark-grown seedlings in the presence of different concentrations of BRZ (nM).
AtMYB30 regulates a subset of BR-induced genes
The fact that AtMYB30 is a direct target of BES1 and that loss-of-function mutants displayed altered BR response phenotypes suggests that AtMYB30 may function to modulate BR-regulated gene expression. We first used Affymetrix Arabidopsis genome arrays to examine BR-regulated gene expression in atmyb30-1 in the absence or presence of BRZ in the dark, conditions in which the mutants showed most clear and consistent phenotypes (Figure 2b). A western blotting experiment indicated that unphosphorylated BES1 (the active form) was reduced significantly in the presence of BRZ in the dark (Supplementary Figure S2), suggesting that BRZ can reduce BES1 activity. In addition, previous microarray experiments indicated that there is a strong inverse correlation between BR- and BRZ-regulated genes (Goda et al., 2002), we therefore consider the absence of BRZ as plus BR and presence of BRZ as minus BR conditions, respectively. Since BRs usually induce gene expression by a small fold (1.4 to 5 fold) (Nemhauser et al., 2004), we set the cutoff ratio to 2-fold change and only chose genes with expression level above 100 in wild-type without BRZ treatment for further analysis. About 400 genes were down-regulated (>2-fold reduction) by BRZ treatment in wild-type and therefore are likely BR-induced genes in the dark. More importantly, about 25% of those 400 genes were reduced at least 2-fold in atmyb30-1 mutants compared with wild-type seedlings grown on either control or BRZ medium (data not shown). Nine genes that showed most significant changes were chosen for semi-quantitative RT-PCR with independent biological samples. All the nine genes were clearly reduced in the atmyb30 mutant, in good agreement with the microarray results (Figure 4). BRZ treatment also appeared to reduce target gene expression in light grown seedlings. The expression level of TCH4 (At5g57560), known to be involved in BR-regulated cell elongation (Xu et al., 1995), was reduced in atmyb30-1 in 6-day-old light-grown seedlings without or with BRZ treatment (Supplemental Figure 3). These results indicate that BES1 and AtMYB30 act cooperatively to activate a subset of BR-target gene expression in young seedlings.
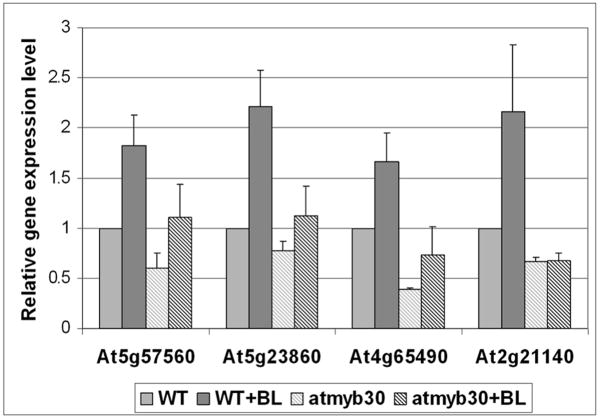
We also examined BR responses with several BR marker genes in light grown seedlings using quantitative real-time PCR (Figure 5). In general, the expression levels of BR-induced genes were reduced either in the absence or presence of BL in atmyb30 mutant. The BL induction was significantly decreased in at least two genes tested (At5g23860 and At2g21140). Taking together, the gene expression studies suggest that AtMYB30 is required for the optimal expression and/or BL-induction of a subset of BR target genes in both dark- and light-grown seedlings.
Figure 5. BR-induced gene expression was reduced in atmyb30 mutant.
Quantitative real time PCR was performed using 6-day-old light-grown atmyb30-1 or WT seedlings treated with or without 1 μM BL for 3h on At5g57560 (TCH4), At5g23860, At4g65490 and At4g21140. UBQ5 was used as the reference gene. The gene expression levels were significantly reduced either in the absence or presence of BL in atmyb30-1 mutant, as tested by one-way ANOVA F-test (P<0.1).
AtMYB30 binds to a conserved MYB site in target gene promoters
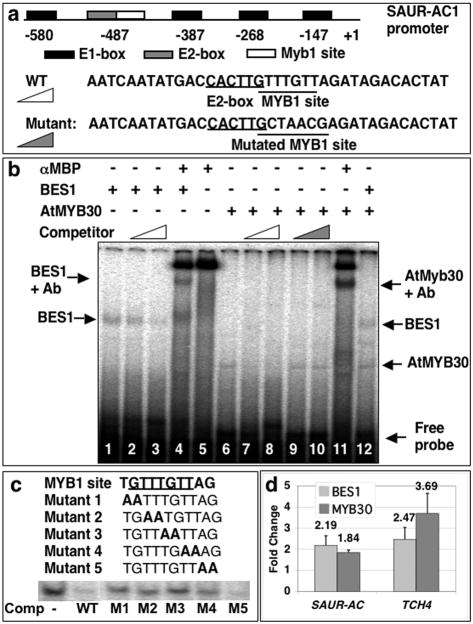
To study how AtMYB30 regulates BR-induced (or BRZ-repressed) gene expression, we examined the promoter of SAUR-AC1, a well-established BES1 target gene (Yin et al., 2005) that is also regulated by AtMYB30 (Figure 4). In the ∼600 bp SAUR-AC1 promoter region, there are five E-box sequences, including four CACATG E-boxes (designated as E1) that have been previously shown to bind BES1 (Yin et al., 2005) and one CACTTG E-box (designated as E2) (Figure 6a). Adjacent to the E2 site, there is a putative MYB binding site (AACAAAC, designated as MYB1), which was predicted by AGRIS (http://arabidopsis.med.ohio-state.edu/AtcisDB/) (Davuluri et al., 2003; Palaniswamy et al., 2006) (Figure 6a). This observation raises the possibility that AtMYB30 directly binds to BR-target gene promoters with BES1 to activate gene expression. We first used Gel Mobility Shift Assays (GMSAs) to test the hypothesis. DNA probes containing both E2 and MYB1 sites or a mutated MYB1 site with 6 of 7 base-pairs changed were first used in the binding assay with recombinant BES1 and AtMYB30 proteins, both fused with MBP (maltose binding protein) at their N-termini (Figure 6a). BES1 bound to the probe specifically, and its binding can be competed by unlabeled probe (Figure 6b, lanes 1-3) and super-shifted by anti-MBP antibody (lane 4). AtMYB30 also bound to the probe (lane 6) and the binding can be competed by unlabeled wild-type probe (lanes 7 and 8), but not by mutant probe in which the MYB1 site is totally disrupted (lanes 9 and 10). AtMYB30 binding was super-shifted by anti-MBP antibody (lane 11). Moreover, when both BES1 and AtMYB30 were present, they appeared to bind to the probe independently (lane 12).
Figure 6. AtMYB30 and BES1 bind to promoters of target genes in vitro and in vivo.
(a) Schematic representations of SAUR-AC1 gene promoter with E1 (CACATG), E2 (CACTTG) boxes and MYB1 (GTTTGTT/AACAAAC) binding site. Sequences of wild-type probe with both E2 and MYB1 sites and mutant probe with mutated MYB1 site were shown.
(b) Gel mobility shift assays (GMSAs) with BES1 and AtMYB30 proteins using probe derived from SAUR-AC1 promoter. Arrows, on the left and right, indicated the BES1 and AtMYB30 bands respectively. In lanes 4, 5 and 11, 5 μl anti-MBP antibody (NEB) was added and BES1 or AtMYB30 complexes with antibody (Ab) were also indicated. Notice that anti-MBP alone produced a background band (lane 5) that was also present in lanes 4 and 11. The triangles represented competition with increasing concentrations (50× and 250×) of unlabeled wild-type (open) or mutant (filled) probes.
(c) Competition of AtMYB30 binding with different mutations in the MYB1 site. AtMYB30 protein bound to the wild-type probe (lane1). The binding can be competed by 100 × cold self-competitor (lane 2) and Mutant 5 with mutation outside the MYB1 site, but not by mutants 1-4 with consecutive two residues mutated within MYB1 sites.
(d) Quantitative real-time CHIP PCR assay showed that AtMYB30 and BES1 interact with SAUR-AC1 and TCH4 gene promoters in vivo. Anti-Myc and anti-BES1 antibodies were used to precipitate chromatin prepared from 5-day-old dark-grown or 6-day-old light grown seedlings. IgG was used as antibody control. Primers from SAUR-AC1, TCH4 or TA3 (internal control) were used to detect the corresponding promoters in ChIP products. The fold changes were calculated based on the relative change of either anti-Myc or anti-BES1 comparing with anti-IgG, after normalized with TA3 internal control.
To further confirm the AtMYB30 binding site, we performed competition with a set of mutants, in which every two residues were mutated to “AA” within the MYB1 binding site (Figure 6c). Mutations of the residues within the predicated MYB1 site (Mutant 1-4) significantly reduced their ability of AtMYB30 binding, while a mutation (Mutant 5) outside the MYB1 site (Figure 6c) did not affect its binding. These results indicate that AtMYB30 specifically binds to the AACAAAC site found in the SAUR-AC1 promoter.
AtMYB30 and BES1 bind to and regulate target gene promoters in vivo
We then used a ChIP assay to test if BES1 and AtMYB30 can bind to SAUR-AC1 and other target gene promoters in vivo using AtMYB30-Myc transgenic plants (Figure 6d). We generated transgenic tagged lines with AtMYB30-MYC driven by the strong and constitutive BRI1 promoter (Li and Chory, 1997). Anti-BES1 and anti-Myc antibodies were used to immunoprecipitate BES1- and AtMYB30-associated chromatin, respectively, with IgG as antibody control. TA3 was used as the internal control. When dark-grown seedlings were used to prepare chromatin, the SAUR-AC1 promoter was enriched in both anti-Myc and anti-BES1 samples compared to the TA3 control (Figure 6d, left bars). On the other hand, as shown in Figure 6d (right bars), the TCH4 gene promoter is clearly enriched in light-grown seedlings (in which TCH4 is reduced in atmyb30-1, Figure 5) by both BES1 and MYB30. These results suggest that AtMYB30 and BES1 can bind to target gene promoters in vivo.
AtMYB30 interacts with BES1 in vitro and in vivo
The functional interaction of BES1 and AtMYB30 prompted us to examine whether they physically interact with each other. We first examined the interaction using bimolecular fluorescence complementation (BiFC) assay. In the assay, EYFP is split into N terminal (YFP-N) and C terminal (YFP-C) parts (Citovsky et al., 2006). We fused YFP-C downstream of BES1 and YFP-N upstream of AtMYB30. When both constructs were transformed into Arabidopsis protoplasts, strong fluorescence was observed in the nucleus (Figure 7a and b), indicating the reconstruction of EYFP protein and a physical interaction between BES1 and AtMYB30 in vivo. About 2-5% protoplasts we observed showed positive signals, depending on the quality of protoplasts. As negative controls, BES1-YFP-C plus YFP-N or YFP-N-AtMYB30 plus YFP-C were also co-transformed into protoplasts and no positive signal was observed (Figure 7c).
Figure 7. AtMYB30 interact with BES1 in vitro and in vivo.
(a-b) Co-expression of BES1-EYFP-C and EYFP-N-AtMYB30 lead to the reconstitution of EYFP activity as observed under an OLYMPUS IX71 fluorescence microscope with a YFP filter. The protoplasts with positive signals in the nuclei are indicated by arrows.
(c) Co-expression of BES1-YFP-C plus YFP-N did not produce any positive signal.
(d) BES1 interacts with AtMYB30 by GST pull-down assay. Full-length BES1 (aa 1-335), including DNA BD (DNA binding domain), Phospho (BIN2 phosphorylation domain), PEST motif, and the C-terminus (C), as well as various deletions are shown. Approximately equal amounts of GST, GST-AtMYB30 and MBP-BES1 (top gel) or GST, GST-BES1 deletions and MBP-AtMYB30 (middle gel) proteins were used in the assays as shown by Comassie stained gel (bottom gel). The proteins were detected by Western blotting with anti-MBP antibody (NEB).
(e) Models AtMYB30 function in BES1-regulated gene expression. BES1 activates the expression of AtMYB30, which bind to the target gene promoters together with BES1 to synergistically activate a subset of BR target genes. Other BES1 induced transcription factors (TFs) may function similarly as AtMYB30 to regulate other BR-target genes.
To further test if there is direct interaction between AtMYB30 and BES1, we performed a GST pull-down assay with purified proteins expressed from E. coli. As shown in Figure 7d, GST-AtMYB30 can pull-down significantly more MBP-BES1 than the GST control (Figure 7d, top gel), suggesting that BES1 interacts with AtMYB30 directly in vitro. To identify the domain of BES1 required for the interaction, we examined the binding between AtMYB30-MBP and a series of truncated GST-BES1 proteins (Figure 7d, middle gel). While deletion to BES1 amino acid (aa) position 89 and 140 had no effect on AtMYB30 binding, deletion to aa 198 of BES1 reduced the interaction. Further deletion of BES1 to aa 272 abolished the interaction with AtMYB30. These results suggest that the central part of BES1 (aa 140-272) is important for interaction with AtMYB30.
Discussion
BES1 is a key transcription factor in the BR signaling pathway, regulating the expression of many genes. However, relatively little is known about how BES1 regulates target gene expression and BR responses. In this study, we demonstrate that BES1 interacts with one of its target transcription factors to regulate the expression of a subset of BR-induced genes. We previously found that BES1 binds E-boxes with its partner BIM1, a bHLH protein (Yin et al., 2002; Yin et al., 2005). There are extensive E-box elements predicted in the promoter regions of Arabidopsis genes. However, only a small portion of these genes are regulated by BES1, implying that BES1 needs other functional partners to regulate target genes at certain developmental or environmental stages. The requirement of AtMYB30 for optimal BES1-induced gene expression at seedling stage provides an important mechanism to modulate BES1 function, i.e. a promoter needs to have both BES1 and AtMYB30 binding sites to achieve a high level of gene expression. The direct interaction of BES1 and AtMYB30 may explain, at least in part, the synergistic activation of the subset of BR target genes. Since BES1 and AtMYB30 do not bind DNA cooperatively (Figure 6), their interactions may lead to synergistic transcriptional activation. Considering the rather weak direct interaction between BES1 and AtMYB30 in vitro (i.e. only about 2-3% of the input, Figure 6d), it is possible that additional transcriptional factors or cofactors are involved in the formation of a transcriptional activation complex for BR target gene expression, like the enhanceosome in the activation of IFNβ gene expression (Thanos and Maniatis, 1995). Several transcriptional factors (IRF-1, ATF-2/C-Jun, HMG, and NF-κB) bind to different DNA elements in the IFNβ promoter to form a transcriptional activation complex (enhanceosome) for the synergistic induction of the target gene.
MYB transcription factors are one of the largest transcription factor families in Arabidopsis, and are involved in a broad spectrum of physiological processes (Jin and Martin, 1999). MYB transcription factors are known to cooperate with other transcription factors to control developmental processes and hormone-regulated gene expression. In maize, MYB transcriptional factor C1 and bHLH factor R interact to regulate genes in the anthocyanin pathway (Grotewold et al., 1998). In addition, AtMYB2 and AtMYC2 (bHLH) function together to activate genes in the Abscisic Acid (ABA) signaling pathway (Abe et al., 2003). More recently, it has been reported that AtMYB77 could interact with auxin response factor ARF7 to synergistically activate target genes in the auxin response pathway (Shin et al., 2007). Our study provides a new example in which a MYB factor (AtMYB30) functionally and physically interacts with an atypical bHLH transcription factor (BES1) to regulate BR target gene expression (Figure 7e left).
Based on our observations that BES1 directly regulates AtMYB30 gene expression and that BES1 and AtMYB30 act cooperatively to regulate BR target gene expression, we propose that AtMYB30 functions as a BES1 target to amplify the BR signal (Figure 7e right). This model correlates well with the kinetics of BR-regulated gene expression. Both auxin and BRs function to promote cell elongation, however, it has long been recognized that the kinetics of BR promoted elongation is much slower than that of auxin (Clouse et al., 1992; Zurek et al., 1994). The model that BES1 activates and cooperates with secondary transcription factors to activate target genes may partially explain the longer time required for maximum BR-regulated gene expression.
BRs appear to regulate different sets of genes in different conditions (Nemhauser et al., 2004; Goda et al., 2004). This differential gene expression can be achieved by interactions of BES1 or its family members with different sets of partners. AtMYB30, the partner of BES1 identified in this study, appears to function during very early stages of plant development, which is in good agreement with a previous report that the mRNA level of AtMYB30 was relatively high in seedlings, but hardly detectable at adult stages (Daniel et al., 1999). Consistent with this developmental expression pattern, the BR response phenotype in AtMYB30 mutants is strongest in young seedlings (Figure 2) and there is no obvious phenotype difference between AtMYB30 knock-out and wild-type plants in adult stage (our unpublished observation). Since BRs regulate cell elongation in both seedling and adult stages, other factors may function during the later stages to amplify a BR signal. In light of this, at least 28 other transcription factors are induced by BRs (Nemhauser et al., 2004) and some of them may function in different tissues and/or developmental stages in a similar manner to AtMYB30 (Figure 7e).
AtMYB30 itself appears to act in different pathways at different developmental stages. While AtMYB30 is involved in BR-regulated gene expression at early stages, it is implicated in pathogen response in adult plants. Although AtMYB30 is not expressed at adult stages under normal conditions, its expression is rapidly induced during the hypersensitive response (HR) to a bacterial pathogen (Daniel et al., 1999). While over-expression of AtMYB30 increases HR response and pathogen resistance, suppression of the gene causes the opposite phenotype, indicating that AtMYB30 is a positive regulator of hypersensitive response (HR) (Vailleau et al., 2002). Most recently, AtMYB30 was shown to regulate the biosynthesis of very-long-chain fatty acids, which function as the cell death messengers in plants (Raffaele et al., 2008).
In summary, our study establishes a new mechanism by which BRs function through BES1 and one of its targets, AtMYB30, to cooperatively activate target gene expression and amplify the hormone signal. The results not only provide important insights into the network of BR-regulated gene expression, but also help explain the general mechanisms through which a specific signal can be amplified in growth, development and responses to a changing environment.
Materials and Methods
Plant Materials, Growth Conditions and Hypocotyl Elongation Assay
Arabidopsis thaliana ecotype Columbia (Col-0) was used as wild-type control. T-DNA insertion mutants, atmyb30-1 and atmyb30-2, were obtained from ABRC (Arabidopsis Biological Resource Center) and correspond to lines SALK_122884 and SALK_027644 respectively. Plants were grown in plates or soil under long-day (15h light/9h dark) conditions at 22°C with 70% humidity. BRZ was added to the ½ MS agar medium during the assays. In the hypocotyl elongation assay, seeds collected from plants grown synchronously were planted in ½ MS agar plates and kept at 4 °C in the dark for 5 days to ensure equal germination time. For the dark treatment hypocotyl measurement, seeds were exposed to light for 12 hr before kept in dark at room temperature for 5 more days. Hypocotyls were measured from the root of the seedlings to the base of cotyledons. 15-30 seedlings were measured in each assay, which were repeated more than 3 times. For BL treatment, about 100mg 6-day-old light grown seedlings were treated with 1μM BL or DMSO (as control) in liquid ½ MS medium for 3 hours.
Plasmid Construction
All the primers used in this study are listed in the Supplementary Table 1. For recombinant protein and GST pull-down assay, AtMYB30 and BES1 coding regions were amplified from Col-0 cDNA and incorporated into the pETMALc-H vector (Pryor and Leiting, 1997). AtMYB30 and BES1 deletion constructs were cloned into pET42a(+) (Novagen). For transgenic over expression plants, AtMYB30 ORF was fused with 2×MYC tag flanked by BRI1 promoter (Li and Chory, 1997) and RBCS terminator in pZP211 (Hajdukiewicz et al., 1994).
For BiFC assay, the constructs of N or C -terminus of EYFP were described previously (Yu et al., 2008). The coding region of AtMYB30 and BES1 were inserted downstream of YFP-N or upstream YFP-C constructs, respectively.
Plant Transformation and Analyses of Transgenic Plants
Agrobacterium tumefaciens (stain GV3101) containing plasmid constructs were used to transform plants by the floral dip method (Clough and Bent, 1998). Transgenic lines were identified by selection in 1/2 MS medium plus 50 mg/l kanamycin. Transgenic plants were further identified by semi-quantitative RT-PCR and western blotting.
Gene Expression Analysis
Total RNA was extracted and purified from seedlings of different genotypes and treatments using RNeasy Mini Kit (Qiagen) with on-column DNase digestion, following the manufacturers' instructions. RNA samples were processed by GeneChip Facility at Iowa State University (http://www.biotech.iastate.edu/facilities/genechip/Genechip.htm/). For RT-PCR, 2 μg total RNA was reverse-transcribed by SuperScript II Reverse transcriptase (Invitrogen) following the product instructions. Equal amounts of RT products were used for PCR reaction with 25∼31 cycles within linear amplification range. PCR products were resolved by electrophoresis on 2% agarose gel and images were captured by the AlphaImager 3400 system (Alpha Innotech). For quantitative real-time PCR, SYBR GREEN PCR Master Mix (Applied Biosystems) and Mx4000 multiplex quantitative PCR system (Stratagene) were used following the product instructions. Two biological replicates and 2-3 technical replicates (for each biological replicate) were used for each treatment. The averages and standard deviations were calculated from biological replicates.
Chromatin Immunoprecipitation (ChIP)
Chromatin immunoprecipitation was performed as previously described (Johnson et al., 2002) with 5-day-old Col or AtMYB30 over expression lines. Antibodies against BES1, Myc tag (for AtMYB30) and IgG (Sigma) control were used for immunoprecipitation. Equal amounts of starting plant material and ChIP products were used for quantitative real-time PCR reaction. The ChIP experiments were performed 2-4 independent times. The averages and standard deviations were calculated from biological repeats.
Gel Mobility Shift Assays (GMSAs)
Gel shift mobility assay were performed as described (Yin et al., 2005). Briefly, oligonucleotide probes were synthesized, annealed, and labeled with P32-ϒ-ATP using T4 nucleotide kinase (NEB). The binding reactions were carried out in 20 μl binding buffer (25 mM HEPES- KOH [pH 8.0], 50 mM KCL, 1 mM DTT, and 10% glycerol) with about 1 ng probe (10,000 cpm) and about 200ng recombinant proteins purified from E. coli. After 30 min incubation on ice, the reactions were resolved by 5% native polyacrylamide gels with 1×TGE buffer (6.6g/l Tris, 28.6g/l glycine, 0.78 g/l EDTA, pH 8.7) and exposed to a phosphorimaging screen.
GST Pull-down Assay
GST pull-down assays were performed as described previously (Yin et al., 2002). AtMYB30 and BES1 fragments fused with Glutathione-S-transferase (GST) were purified with Glutathione beads (Sigma). AtMYB30 or BES1 fused with Maltose Binding Protein (MBP) were purified with Amylose resin (NEB). About 5 μg proteins were used in the assay each time. The pull-down assays were repeated 2 times with similar results.
Bimolecular Fluorescence Complementation (BiFC)
Arabidopsis mesophyll protoplasts were prepared and transformed by PEG-mediated transfection (Yoo et al., 2007). Protoplasts were observed under an OLYMPUS IX71 fluorescence microscope with an YFP filter, 16 -24 hours after transformation. The assay was repeated more than 3 times with different positive rates, depending on the quality of the protoplasts.
Supplementary Material
Figure S1: atmyb30-1 enhances bri1-5 mutant phenotype.
Figure S2: BRZ reduces active (unphosphorylated) BES1 in both light- and dark-grown seedlings.
Figure S3: BRZ reduces the expression of TCH4 gene in light-grown seedlings
Supplementary Table 1: Primers Used in This Study
Acknowledgments
We thank Steve Rodermel for comments on the manuscripts and Yi Hou for helping with the BiFC assay. These studies were supported by NSF grants (IOS-0546503 to Y. Y. and IOS-0649389 to J.C.) as well as a faculty start-up fund from Iowa State University to Y.Y. J.C. is an investigator of the Howard Hughes Medical Institute.
References
- Abe H, Urao T, Ito T, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell. 2003;15:63–78. doi: 10.1105/tpc.006130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asami T, Min YK, Nagata N, Yamagishi K, Takatsuto S, Fujioka S, Murofushi N, Yamaguchi I, Yoshida S. Characterization of brassinazole, a triazole-type brassinosteroid biosynthesis inhibitor. Plant Physiol. 2000;123:93–100. doi: 10.1104/pp.123.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkhadir Y, Chory J. Brassinosteroid signaling: a paradigm for steroid hormone signaling from the cell surface. Science. 2006;314:1410–1411. doi: 10.1126/science.1134040. [DOI] [PubMed] [Google Scholar]
- Choe S, Schmitz RJ, Fujioka S, Takatsuto S, Lee MO, Yoshida S, Feldmann KA, Tax FE. Arabidopsis brassinosteroid-insensitive dwarf12 mutants are semidominant and defective in a glycogen synthase kinase 3beta-like kinase. Plant Physiol. 2002;130:1506–1515. doi: 10.1104/pp.010496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citovsky V, Lee LY, Vyas S, Glick E, Chen MH, Vainstein A, Gafni Y, Gelvin SB, Tzfira T. Subcellular localization of interacting proteins by bimolecular fluorescence complementation in planta. J Mol Biol. 2006;362:1120–1131. doi: 10.1016/j.jmb.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Clouse SD. Molecular genetic studies confirm the role of brassinosteroids in plant growth and development. Plant J. 1996;10:1–8. doi: 10.1046/j.1365-313x.1996.10010001.x. [DOI] [PubMed] [Google Scholar]
- Clouse SD. Brassinosteroid signal transduction: clarifying the pathway from ligand perception to gene expression. Mol Cell. 2002;10:973–982. doi: 10.1016/s1097-2765(02)00744-x. [DOI] [PubMed] [Google Scholar]
- Clouse SD, Langford M, McMorris TC. A brassinosteroid-insensitive mutant in Arabidopsis thaliana exhibits multiple defects in growth and development. Plant Physiol. 1996;111:671–678. doi: 10.1104/pp.111.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse SD, Zurek DM, McMorris TC, Baker ME. Effect of brassinolide on gene expression in elongating soybean epicotyls. Plant Physiol. 1992;100:1377–1383. doi: 10.1104/pp.100.3.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel X, Lacomme C, Morel JB, Roby D. A novel myb oncogene homologue in Arabidopsis thaliana related to hypersensitive cell death. Plant J. 1999;20:57–66. doi: 10.1046/j.1365-313x.1999.00578.x. [DOI] [PubMed] [Google Scholar]
- Davuluri RV, Sun H, Palaniswamy SK, Matthews N, Molina C, Kurtz M, Grotewold E. AGRIS: Arabidopsis gene regulatory information server, an information resource of Arabidopsis cis-regulatory elements and transcription factors. BMC Bioinformatics. 2003;4:25. doi: 10.1186/1471-2105-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrichsen DM, Joazeiro CA, Li J, Hunter T, Chory J. Brassinosteroid-insensitive-1 is a ubiquitously expressed leucine-rich repeat receptor serine/threonine kinase. Plant Physiol. 2000;123:1247–1256. doi: 10.1104/pp.123.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gampala SS, Kim TW, He JX, Tang W, Deng Z, Bai MY, Guan S, Lalonde S, Sun Y, Gendron JM, Chen H, Shibagaki N, Ferl RJ, Ehrhardt D, Chong K, Burlingame AL, Wang ZY. An essential role for 14-3-3 proteins in brassinosteroid signal transduction in Arabidopsis. Dev Cell. 2007;13:177–189. doi: 10.1016/j.devcel.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron JM, Wang ZY. Multiple mechanisms modulate brassinosteroid signaling. Curr Opin Plant Biol. 2007;10:436–441. doi: 10.1016/j.pbi.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda H, Sawa S, Asami T, Fujioka S, Shimada Y, Yoshida S. Comprehensive comparison of auxin-regulated and brassinosteroid regulated genes in Arabidopsis. Plant Physiol. 2004;134:1555–1573. doi: 10.1104/pp.103.034736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda H, Shimada Y, Asami T, Fujioka S, Yoshida S. Microarray analysis of brassinosteroid-regulated genes in arabidopsis. Plant Physiol. 2002;130:1319–1334. doi: 10.1104/pp.011254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotewold E, Chamberlin M, Snook M, Siame B, Butler L, Swenson J, Maddock S, St Clair G, Bowen B. Engineering secondary metabolism in maize cells by ectopic expression of transcription factors. Plant Cell. 1998;10:721–740. [PMC free article] [PubMed] [Google Scholar]
- Hajdukiewicz P, Svab Z, Maliga P. The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol Biol. 1994;25:989–994. doi: 10.1007/BF00014672. [DOI] [PubMed] [Google Scholar]
- He JX, Gendron JM, Sun Y, Gampala SS, Gendron N, Sun CQ, Wang ZY. BZR1 is a transcriptional repressor with dual roles in brassinosteroid homeostasis and growth responses. Science. 2005;307:1634–1638. doi: 10.1126/science.1107580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He JX, Gendron JM, Yang Y, Li J, Wang ZY. The GSK3-like kinase BIN2 phosphorylates and destabilizes BZR1, a positive regulator of the brassinosteroid signaling pathway in Arabidopsis. Proc Natl Acad Sci U S A. 2002;99:10185–10190. doi: 10.1073/pnas.152342599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Wang ZY, Li J, Zhu Q, Lamb C, Ronald P, Chory J. Perception of brassinosteroids by the extracellular domain of the receptor kinase BRI1. Science. 2000;288:2360–2363. doi: 10.1126/science.288.5475.2360. [DOI] [PubMed] [Google Scholar]
- Jin H, Martin C. Multifunctionality and diversity within the plant MYB-gene family. Plant Mol Biol. 1999;41:577–585. doi: 10.1023/a:1006319732410. [DOI] [PubMed] [Google Scholar]
- Johnson L, Cao X, Jacobsen S. Interplay between two epigenetic marks: DNA methylation and histone H3 lysine 9 methylation. Curr Biol. 2002;12:1360–1367. doi: 10.1016/s0960-9822(02)00976-4. [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Caño-Delgado A, Seto H, Hiranuma S, Fujioka S, Yoshida S, Chory J. Binding of brassinosteroids to the extracellular domain of plant receptor kinase BRI1. Nature. 2005;433:167–171. doi: 10.1038/nature03227. [DOI] [PubMed] [Google Scholar]
- Krishna P. Brassinosteroid-Mediated Stress Responses. J Plant Growth Regul. 2003;22:289–297. doi: 10.1007/s00344-003-0058-z. [DOI] [PubMed] [Google Scholar]
- Li J, Chory J. A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell. 1997;90:929–938. doi: 10.1016/s0092-8674(00)80357-8. [DOI] [PubMed] [Google Scholar]
- Li J, Chory J. Brassinosteroid actions in plants. J Exp Botany. 1999;50:332–340. [Google Scholar]
- Li J, Jin H. Regulation of brassinosteroid signaling. Trends Plant Sci. 2007;12:37–41. doi: 10.1016/j.tplants.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Li J, Nagpal P, Vitart V, McMorris TC, Chory J. A role for brassinosteroids in light-dependent development of. Arabidopsis Science. 1996;272:398–401. doi: 10.1126/science.272.5260.398. [DOI] [PubMed] [Google Scholar]
- Li J, Nam KH. Regulation of brassinosteroid signaling by a GSK3/SHAGGY-like kinase. Science. 2002;295:1299–1301. doi: 10.1126/science.1065769. [DOI] [PubMed] [Google Scholar]
- Li J, Wen J, Lease KA, Doke JT, Tax FE, Walker JC. BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell. 2002;110:213–222. doi: 10.1016/s0092-8674(02)00812-7. [DOI] [PubMed] [Google Scholar]
- Mora-Garcia S, Vert G, Yin Y, Cano-Delgado A, Cheong H, Chory J. Nuclear protein phosphotases with Kelch-repeat domains modulate the response to bassinosteroids in Arabidopsis. Genes Dev. 2004;18:448–460. doi: 10.1101/gad.1174204. 18: 448-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchel CF, Osmont KS, Hardtke CS. BRX mediates feedback between brassinosteroid levels and auxin signalling in root growth. Nature. 2006;443:458–461. doi: 10.1038/nature05130. [DOI] [PubMed] [Google Scholar]
- Mussig C, Fischer S, Altmann T. Brassinosteroid-regulated gene expression. Plant Physiol. 2002;129:1241–1251. doi: 10.1104/pp.011003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam KH, Li J. BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell. 2002;110:203–212. doi: 10.1016/s0092-8674(02)00814-0. [DOI] [PubMed] [Google Scholar]
- Nemhauser JL, Mockler TC, Chory J. Interdependency of brassinosteroid and auxin signaling in Arabidopsis. PLoS Biol. 2004;2:E258. doi: 10.1371/journal.pbio.0020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T, Fujioka S, Choe S, Takatsuto S, Yoshida S, Yuan H, Feldmann KA, Tax FE. Brassinosteroid-insensitive dwarf mutants of Arabidopsis accumulate brassinosteroids. Plant Physiol. 1999;121:743–752. doi: 10.1104/pp.121.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MH, Ray WK, Huber SC, Asara JM, Gage DA, Clouse SD. Recombinant brassinosteroid insensitive 1 receptor-like kinase autophosphorylates on serine and threonine residues and phosphorylates a conserved peptide motif in vitro. Plant Physiol. 2000;124:751–766. doi: 10.1104/pp.124.2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniswamy SK, James S, Sun H, Lamb RS, Davuluri RV, Grotewold E. AGRIS and AtRegNet. a platform to link cis-regulatory elements and transcription factors into regulatory networks. Plant Physiol. 2006;140:818–829. doi: 10.1104/pp.105.072280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor KD, Leiting B. High-level expression of soluble protein in Escherichia coli using a His6-tag and maltose-binding-protein double-affinity fusion system. Protein Expr Purif. 1997;10:309–319. doi: 10.1006/prep.1997.0759. [DOI] [PubMed] [Google Scholar]
- Pérez-Pérez JM, Ponce MR, Micol JL. The UCU1 Arabidopsis gene encodes a SHAGGY/GSK3-like kinase required for cell expansion along the proximodistal axis. Devel Biol. 2002;242:161–173. doi: 10.1006/dbio.2001.0543. [DOI] [PubMed] [Google Scholar]
- Raffaele S, Rivas S, Roby D. An essential role for salicylic acid in AtMYB30-mediated control of the hypersensitive cell death program in Arabidopsis. FEBS Lett. 2006;580:3498–3504. doi: 10.1016/j.febslet.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Raffaele S, Vailleau F, Leger A, Joubes J, Miersch O, Huard C, Blee E, Mongrand S, Domergue F, Roby D. A MYB Transcription Factor Regulates Very-Long-Chain Fatty Acid Biosynthesis for Activation of the Hypersensitive Cell Death Response in Arabidopsis. Plant Cell. 2008;20:752–767. doi: 10.1105/tpc.107.054858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu H, Kim K, Cho H, Park J, Choe S, Hwang I. Nucleocytoplasmic Shuttling of BZR1 Mediated by Phosphorylation Is Essential in Arabidopsis Brassinosteroid Signaling. Plant Cell. 2007 doi: 10.1105/tpc.107.053728. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin R, Burch AY, Huppert KA, Tiwari SB, Murphy AS, Guilfoyle TJ, Schachtman DP. The Arabidopsis Transcription Factor MYB77 Modulates Auxin Signal Transduction. Plant Cell. 2007;19:2440–2453. doi: 10.1105/tpc.107.050963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekeres M, Németh K, Koncz-Kálmán Z, Mathur J, Kauschmann A, Altmann T, Rédei GP, Nagy F, Schell J, Koncz C. Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell. 1996;85:171–182. doi: 10.1016/s0092-8674(00)81094-6. [DOI] [PubMed] [Google Scholar]
- Tang W, Kim TW, Oses-Prieto JA, Sun Y, Deng Z, Zhu S, Wang R, Burlingame AL, Wang ZY. BSKs mediate signal transduction from the receptor kinase BRI1 in Arabidopsis. Science. 2008;321:557–560. doi: 10.1126/science.1156973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos D, Maniatis T. Virus induction of human IFN beta gene expression requires the assembly of an enhanceosome. Cell. 1995;83:1091–1100. doi: 10.1016/0092-8674(95)90136-1. [DOI] [PubMed] [Google Scholar]
- Thummel CS, Chory J. Steroid signaling in plants and insects-common themes, different pathways. Genes Dev. 2002;16:3113–3129. doi: 10.1101/gad.1042102. [DOI] [PubMed] [Google Scholar]
- Vailleau F, Daniel X, Tronchet M, Montillet JL, Triantaphylides C, Roby D. A R2R3-MYB gene, AtMYB30, acts as a positive regulator of the hypersensitive cell death program in plants in response to pathogen attack. Proc Natl Acad Sci U S A. 2002;99:10179–10184. doi: 10.1073/pnas.152047199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vert G, Chory J. Downstream nuclear events in brassinosteroid signalling. Nature. 2006;441:96–100. doi: 10.1038/nature04681. [DOI] [PubMed] [Google Scholar]
- Wang X, Chory J. Brassinosteroids regulate dissociation of BKI1, a negative regulator of BRI1 signaling, from the plasma membrane. Science. 2006;313:1118–1122. doi: 10.1126/science.1127593. [DOI] [PubMed] [Google Scholar]
- Wang X, Goshe MB, Soderblom EJ, Phinney BS, Kuchar JA, Li J, Asami T, Yoshida S, Huber SC, Clouse SD. Identification and Functional Analysis of in Vivo Phosphorylation Sites of the Arabidopsis BRASSINOSTEROID-INSENSITIVE1 Receptor Kinase. Plant Cell. 2005a;17:1685–1703. doi: 10.1105/tpc.105.031393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li X, Meisenhelder J, Hunter T, Yoshida S, Asami T, Chory J. Autoregulation and homodimerization are involved in the activation of the plant steroid receptor BRI1. Dev Cell. 2005b;8:855–865. doi: 10.1016/j.devcel.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Nakano T, Gendron J, He J, Chen M, Vafeados D, Yang Y, Fujioka S, Yoshida S, Asami T, Chory J. Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev Cell. 2002;2:505–513. doi: 10.1016/s1534-5807(02)00153-3. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Seto H, Fujioka S, Yoshida S, Chory J. BRI1 is a critical component of a plasma-membrane receptor for plant steroids. Nature. 2001;410:380–383. doi: 10.1038/35066597. [DOI] [PubMed] [Google Scholar]
- Xu W, Purugganan MM, Polisensky DH, Antosiewicz DM, Fry SC, Braam J. Arabidopsis TCH4, regulated by hormones and the environment, encodes a xyloglucan endotransglycosylase. Plant Cell. 1995;7:1555–1567. doi: 10.1105/tpc.7.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Vafeados D, Tao Y, Yokoda T, Asami T, Chory J. A new class of transcription factors mediate brassinosteroid-regulated gene expression in Arabidopsis. Cell. 2005;120:249–259. doi: 10.1016/j.cell.2004.11.044. [DOI] [PubMed] [Google Scholar]
- Yin Y, Wang ZY, Mora-Garcia S, Li J, Yoshida S, Asami T, Chory J. BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell. 2002;109:181–191. doi: 10.1016/s0092-8674(02)00721-3. [DOI] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Sheen J. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc. 2007;2:1565–1572. doi: 10.1038/nprot.2007.199. [DOI] [PubMed] [Google Scholar]
- Yu X, Li L, Li L, Guo M, Chory J, Yin Y. Modulation of Brassinosteroid-Regulated Gene Expression by Jumonji Domain-Containing Proteins ELF6 and REF6 in Arabodopsis. Proc Natl Acad Sci U S A. 2008;105:7618–7623. doi: 10.1073/pnas.0802254105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Peng P, Schmitz RJ, Decker AD, Tax FE, Li J. Two putative BIN2 substrates are nuclear components of brassinosteroid signaling. Plant Physiol. 2002;130:1221–1229. doi: 10.1104/pp.102.010918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurek DM, Rayle DL, McMorris TC, Clouse SD. Investigation of gene expression, growth Kinetics, and wall extensibility during brassinosteroid-regulated stem elongation. Plant Physiol. 1994;104:505–513. doi: 10.1104/pp.104.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: atmyb30-1 enhances bri1-5 mutant phenotype.
Figure S2: BRZ reduces active (unphosphorylated) BES1 in both light- and dark-grown seedlings.
Figure S3: BRZ reduces the expression of TCH4 gene in light-grown seedlings
Supplementary Table 1: Primers Used in This Study