Abstract
Zebrafish has been gaining increasing amount of interest in behavioral neuroscience as this species may represent a good compromise between system complexity and practical simplicity. Particularly successful have been those studies that utilized zebrafish as a screening tool. Given the complexity of the mechanisms of learning, for example, forward genetic screens with zebrafish could potentially reveal previously unknown genes and molecular pathways that subserve this function. These screens, however, require appropriate phenotypical (e.g. behavioral) paradigms. A step in this direction is the characterization of learning abilities of zebrafish. Here we employ two classical learning tasks in a plus maze. In the first, zebrafish are required to associate a visible cue with food reward irrespective of the location of this pairing. In the second, zebrafish are required to associate the spatial location of food reward irrespective of intra-maze cues. Our results demonstrate that zebrafish perform well in both tasks and show significant acquisition of the association between cue and reward as well as between location and reward. We conclude that zebrafish, similarly to classical laboratory rodents, may have utility in the biological analysis of simple as well as complex forms of associative learning.
Keywords: associative learning, Danio, plus maze, relational learning, spatial learning, zebrafish
INTRODUCTION
For the past three decades the zebrafish has been one of the favorite species of developmental biologists [34] due to the small size and prolific nature of this fish and to the fact that its development is fast (takes about 5 days from fertilization to get a free swimming little fish) and its embryo is mainly transparent throughout its development. As a result of these features numerous genetic tools have been developed and a substantial amount of genetic information has been amassed for zebrafish. By now the zebrafish is perhaps the third most genetically well characterized animal species after the mouse and the fruit fly [19]. Due to this accumulated knowledge on, and the available tool set for the zebrafish, disciplines other than developmental genetics have also taken notice of this fish. For example, behavior and behavioral neuroscience related publications on the zebrafish appear at an exponential rate [30]. Nevertheless, compared to the mouse, the main biomedical research species in the laboratory, the number of zebrafish behavioral tests is orders of magnitude fewer. For example, a PubMed (Medline) search with keywords “learning” and “mouse” returns 13520 publications whereas a similar search but with the keywords “learning” and “zebrafish” gives 82 publications, only less than one fourth of which is actually about some form, or mechanism, of learning in zebrafish.
Learning and memory are highly complex brain processes and despite decades of successful research of these phenomena, the number of molecular mechanisms discovered every year does not seem to plateau out. According to some estimates, about 30–40% of our genes are expressed in the brain and many of these genes are expected to be involved in some form of neuronal plasticity subserving different forms, types and/or phases of learning and memory [32]. Calculating with the conservative estimate of about 30 thousand mammalian genes (for mouse and human, for example), one may expect as much as 9–12 thousand genes potentially involved in learning and memory, a staggering number compared to the known few hundred genes so far associated with these phenomena. How can one investigate such mechanistically complex functions at the molecular, i.e. genetic, level? Some have argued that forward genetics, i.e. large scale mutagenesis screens may have utility [7]. These screens have the potential to cover the entire genome and identify numerous mutations leading to the localization and identification of the genes and molecular pathways involved. The cornerstone of such screens is the phenotypical screening and characterization tools [30; 13]. The zebrafish is particularly suitable for forward genetics [1] given its small size (4 cm when adult), ease of maintenance, prolific nature (2–300 eggs per spawning per female every other day), and the fact that numerous genetic markers have been developed for this species [21]. Also important is the translational relevance of zebrafish, i.e. the high nucleotide sequence homology and functional similarities between mammalian and zebrafish genes (for review see [25]). However, there is a serious bottleneck in this research: the phenotypical testing tools that would be required for the screening are often rudimentary or not available at all [30]. The current paper is a step towards addressing this need.
An important step in this research is the characterization of learning abilities of zebrafish. For example, can zebrafish perform well in simple and complex associative learning tasks? Here we analyze associative learning in zebrafish in two tasks using a maze design principally similar to the classical radial arm maze employed for the analysis of spatial and non-spatial associative learning of rats and mice before [10]. In one task the fish are required to associate a visible cue with food reward while other intra-maze as well as extra-maze cues are made irrelevant, a single cue-based, simple, associative learning task. In the other task, the fish are required to find the location of the food reward irrespective of intra-maze cues. The latter task is often regarded as “spatial learning” [10; 22], a form of complex associative learning termed relational learning [9]. Relational (and spatial) learning has been attributed to the function of the mammalian hippocampus [11]. However, the typical hippocampal anatomy, including the trisynaptic (dentate gyrus – CA3 – CA1) circuitry is missing from the teleost brain, including the zebrafish brain [27]. Nevertheless, spatial learning has been demonstrated in a cyprinid species, the carp [28], closely related to zebrafish. Thus it appears that spatial (and thus relational) learning is not the distinct property of the mammalian hippocampal circuitry, but rather perhaps depends, at least partly, on mechanisms lower in the organization levels of the brain: for example, molecular pathways and/or synaptic function. Briefly, zebrafish represents a reductionist tool that does not possess the complex hippocampal circuitry of a mammal (nor does it have the cortex, the place where relational memories are believed to be stored [27; 33; 8]. Despite this simplicity, zebrafish have been found to perform well in learning tasks including a one trial avoidance learning paradigm [4], olfactory conditioning [6], shuttle box active appetitive conditioning [24], place conditioning [12], appetitive choice discrimination [3], active avoidance conditioning [36], alternation memory task, and even an automated learning paradigm [20] to mention but the most recent examples.
Here we propose that analysis of the zebrafish may allow one to identify the molecular and synaptic mechanisms fundamental to forms of associative learning including simple two-cue association and more complex relational associations at an evolutionary stage that long preceded the mammals. The goal of this paper is to demonstrate that zebrafish are capable of acquiring such associations and show therefore that it may have utility as a tool for the analysis of the mechanisms of these higher brain functions.
METHODS
Animals and Housing
Adult zebrafish (Danio rerio) were purchased at their age of 3 months from a local pet supplier (Big Al’s Aquarium Warehouse Inc., Mississauga, Ontario, Canada). All fish were housed in 143 liter glass tanks (91cm length × 30cm width × 50cm height) at the University of Toronto Mississauga Vivarium (20 fish per tank). The holding tanks were filled with aged (biologically active) tap water (which was filtered in a system water cylinder for at least one week before its use in the holding tanks). The holding tanks were filtered by canister filters containing a biological (high surface area bacterial), chemical (activated carbon) and physical (porous sponge) filter media (EHEIM Classic Filter, Model 2213, Deizisau, Germany). The water temperature was kept constant at 27 C°. The holding tanks were illuminated by 13W overhead fluorescent light placed directly above the tanks, and a 13h/11h light/dark cycle was maintained with lights on at 7 am. Fish were fed a 50–50% mix of ground freeze-dried krill (Aquatic Ecosystems, Florida)and TetraMin flakes (Melle, Germany) twice a day. All fish were allowed to acclimatize for one month in their holding tanks. After this period the fish were transferred to 10 liter tanks (31cm length × 16cm width × 21cm height, 1 fish per tank) that were filtered using Tetra-Whisper Power Filters (Model#1295520, VA, USA). The tanks were placed adjacent to each so that experimental zebrafish were not visually isolated from each other. In the 10 liter holding tanks experimental zebrafish were fed Gelly Belly (Aquatic Eco-Systems Inc., Dade City, Florida, USA), a gelatinous mixture of krill, kelp and fish meal. Using this food substance allowed us to precisely determine the exact amount of food delivered to the fish during learning trials and we could also control the exact location of the food reward. Habituation to this novel food substance took one month, after which the experimental zebrafish entered the learning trials.
Apparatus and Procedure
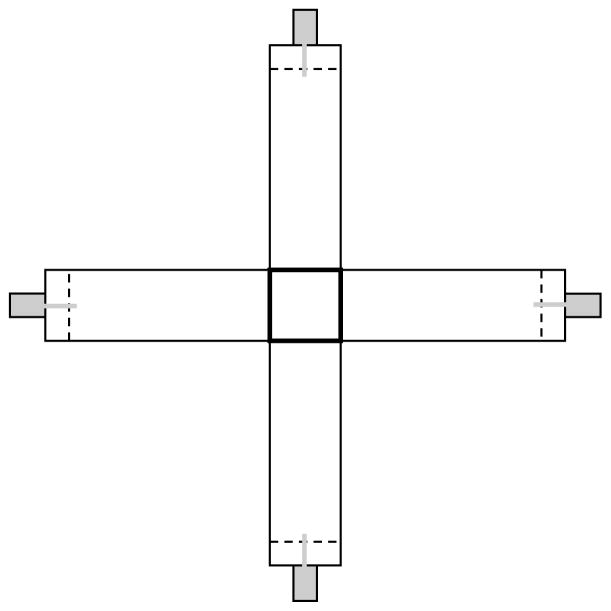
The test apparatus (Figure 1) was a four-arm, plus-shaped transparent Plexiglas maze similar to that employed by others [26; 28]. Each arm of the maze was 35 cm long, 11 cm wide, and 20 cm high. The arms were connected to each other by an 11 × 11 cm center square into which a start box could be lowered and could be made accessible by lifting the start box. The maze was placed inside a large transparent Plexiglas tank (86 × 86 × 20 width × length × depth) filled with water with temperature maintained at 27 C° by four symmetrically positioned 50-watt thermostat controlled aquarium heaters (EHEIM JAGER Model 7357890, Deizisau, Germany). This arrangement allowed us to maintain constant water temperature inside the plus maze without having to put any objects (heaters and air-stones) that would disturb the fish or obscure them from the overhead digital camera (Optura 30, Canon, Japan) that monitored their behavior. The entire apparatus was placed on a rotating circular platform that allowed us to position the maze as required by the trials with ease.
Figure 1.
The plus maze (4 arm radial maze). Note that each arm is equipped with a food dispensing syringe (grey rectangles) attached to Teflon tubing. The end of the Teflon tube may be positioned just behind or just in front of perforated plastic sheets (broken line). If the end of the tube is in front of the perforated sheet, the fish have access to food. Also note the center square piece (thick black line), which allowed us to place the fish in the maze and which could be lifted remotely using a pulley and nylon string system thereby allowing the fish to start the exploration of the maze with minimal experimenter interference.
Habituation trials
To acclimatize fish to the maze, they were administered four 2 hour long habituation trials (one trial per day on consecutive days). On the 1st day 20 fish were placed in the maze at a time, on the 2nd day 10 fish, on the 3rd day 5, and on the 4th day 1 fish was placed in the maze at a time. For the 2h long habituation trials all arms of the maze were baited, i.e. access to the food reward was allowed.
Shaping
In order to facilitate successful acquisition of the association between a visual cue (conditioned stimulus) and the food reward (unconditioned stimulus) a shaping procedure was conducted. The 2 hour long habituation trials were followed by 12 short (5 minute long) habituation trials (4 trials per day on 3 consecutive days) during which only one fish explored the maze at a time. On the first day of the short habituation trials all arms of the maze was baited with food and next to the food a red plastic cue card was also placed. This stimulus was chosen as it is expected to be clearly distinguishable for the tetrachromatic zebrafish and has been successfully utilized in the past [35]. On the following day three of the four arms were baited and marked by the visual cue and on the third day two of the arms were baited and marked with the visual cue. The gradual decrease of shoal size in the maze and the multiple exposures to the maze environment and cues was designed to minimize the possible stress associated with placing the fish in a novel test environment singly [18] and also to gradually shape the acquisition of the association between cue and reward. The position of the red cue card and the food reward when not present in all arms was rotated semi-randomly, and the amount of time each arm was paired with the stimuli was evenly distributed throughout the habituation trials to minimize preference for one arm over the other arms.
Simple Associative Learning: Training
Following habituation and shaping, experimental zebrafish were divided randomly into two groups: paired (for which the cue and the food reward were presented in close physical proximity to each other) and unpaired (for which the cue and food reward were presented at independent random locations). All fish were food deprived for 24 hours immediately before the first training trial, and remained on food deprivation until the last training day and could only eat in the plus maze [35]. During the training trials, the syringes filled with Gelly Belly (food reward) were placed in all four arms but food was accessible only in one arm. A red cue card (a 7 cm wide and 10 cm high plastic sheet) was also placed in only one arm. Fish in the “paired” group received the cue card always next to the food dispensing tube. Fish in the “unpaired” group received the food reward and the cue card at locations independent of each other. For all fish the food rewarded arm changed randomly from trial to trial. Each day, four 5-minute long training trials were administered between 9:00h and 19:00h for a total of 5 consecutive days, i.e. training consisted of 20 trials in total. For each trial, fish were removed individually from their holding tank and transferred into the centre start box of the maze, where they acclimatized for 10 seconds. The box was then lifted using a nylon string and pulley system from a remote location, and the fish was allowed to explore the maze. Each session was videotaped and the recordings later replayed for analysis.
Probe Trial
The probe trial is the most important part of the behavioral procedure as it allowed us to quantify acquisition of the association between cue and reward. During the probe trial only the conditioned stimulus, the cue card, was presented, i.e., all procedures were the same as in the training trials except that no food was accessible in any arm of the maze. The length of the probe trial was also 5 minutes.
Spatial learning task
Following the above simple associative learning task another group of zebrafish was measured in a spatial learning task. The procedures, including the habituation and shaping trials, employed for this task were identical to those used for the simple associative learning paradigm described above except that here zebrafish were required to find the fixed location of the food reward. The location of this reward was not marked by an intra-maze visible cue but instead it was supposed to be identified based upon external visual cues that surrounded the maze. These cues included equipment in the room, racks containing fish tanks, cues on the ceiling e.g. fluorescent light fixtures etc. The diversity of these cues made it unlikely that zebrafish could pick a single salient guiding stimulus. Also, to ascertain that intra-maze cues are made irrelevant, the maze, which was placed on a rotating circular turn-table, was turned in a randomized order so that the location of the food reward remained fixed only in terms of the constellation of external visual cues, but the actual arm of the maze in which the food reward was presented varied randomly. Fish in the “paired group” received this training. Fish in the “unpaired” group found the food reward in random spatial locations, i.e. the target arm was not fixed according to external visual cues for these fish. Last, olfactory cues associated with the food reward were also made irrelevant, i.e. consistently present in all locations, by providing inaccessible food in all arms except the rewarded one.
Fish in the paired and unpaired groups were tested in a randomized order. The experimenter quantifying the behavior of the fish was unaware of the group designation of the experimental animals.
Quantification of Behavior and statistical analysis
The location of fish was measured using the event recording software application Observer ColorPro 5.0 (Noldus, Info Tech., Wageningen, The Netherlands). We quantified the percent of time the fish spent in the target arm, in the center square, and in the other arms of the maze during the probe trial. We also recorded the number of times the fish entered the target arm and other arms of the maze during the probe trial. We statistically analyzed the following measures: Percent of time the fish stayed in the target arm, this measure quantifies the preference of the target arm relative to other locations, which is regarded as a sensitive measure of remembering the conditioned stimulus (the visual cue or the location associated with the food reward); The total number of visits to the target arm was also analyzed as it quantifies general locomotory activity, an important component of an active appetitive learning task; Last the number of entries to the target arm relative to the total number of arm visits was analyzed as this measure reflects exploratory motivation to look for cues and locations associated with the food reward.
No gender differences or gender × group interaction was found and data are pooled for sexes. To compare the performance of fish in the paired and unpaired groups independent sample t-tests were conducted. Where appropriate, one sample t-tests were also employed to compare performance of certain groups to chance level.
RESULTS
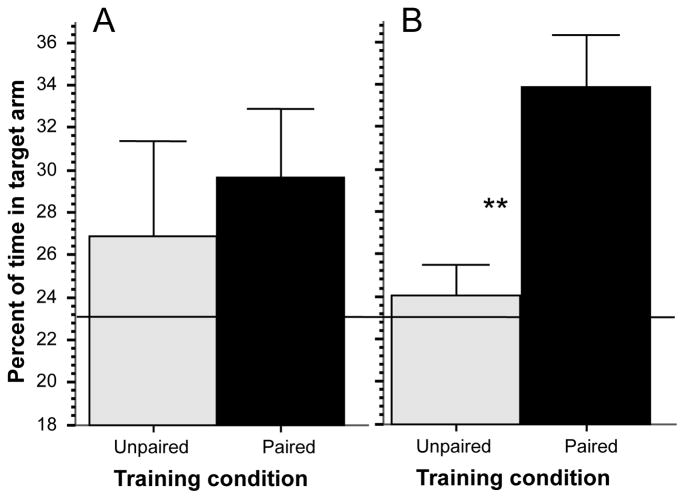
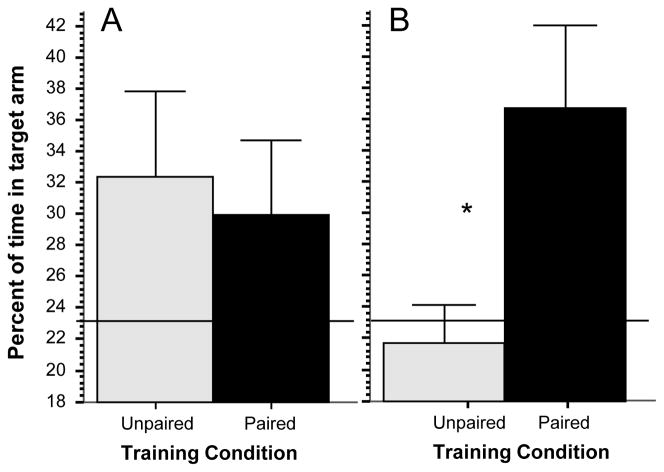
Zebrafish appeared active, explored the plus maze, and exhibited no signs of fear [31; 2; 23]. For example, erratic movement (zig-zagging) or jumping (leaping) or freezing (complete immobility) was not observed. The percent of time the experimental fish spent in the target arm containing the food reward at the first trial of the simple associative learning task (Figure 2, panel A) did not significantly differ between the paired and unpaired group (t = 0.498, df = 18, p > 0.50) as expected. From the figure it appears that both groups showed some preference toward the target arm. This may be because the target arm was the only location where food was accessible and thus zebrafish may have spent slightly more time there actually eating the food (in the other arms access to food was blocked). Nevertheless, comparison of performance levels of the two groups to random chance (23.14%, a value that is based upon the area of the target arm relative to the total area of the maze) was not significantly different (unpaired group t = 0.848, df = 9, p > 0.40; paired group t = 1.997, df = 9, p > 0.05). The difference between the unpaired and paired group (Figure 2, Panel B), however, became robust and significant (t = 3.42, df = 18, p < 0.01) at the probe trial, i.e. after 20 training trials. Also importantly, comparison of performance to chance revealed that the fish in the unpaired group remained at chance (t = 0.59, df = 9, p > 0.50), whereas fish in the paired group spent significantly more time in the target arm than chance (t = 4.32, df = 9, p < 0.01). Briefly, the zebrafish that received the visual cue – food reward pairing responded with strong preference towards the visual cue alone during the probe trial, and the fish in the unpaired group did not.
Figure 2.
Simple associative learning: Percent of time in the target arm significantly differs between the groups at the probe trial. Panel A, performance at the first training trial. Panel B, performance at the probe trial, i.e. after 20 training trials. Mean ± SEM are shown. Sample sizes nunpaired = 10, npaired = 10. The solid horizontal line represents random chance level performance. Significant difference (p < 0.01) between the groups is indicated by two asterisks. For methodological details see Methods. For details of statistical analyses, see Results.
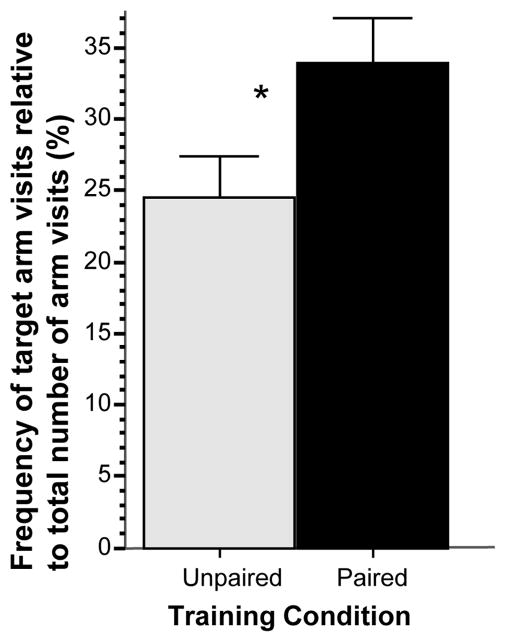
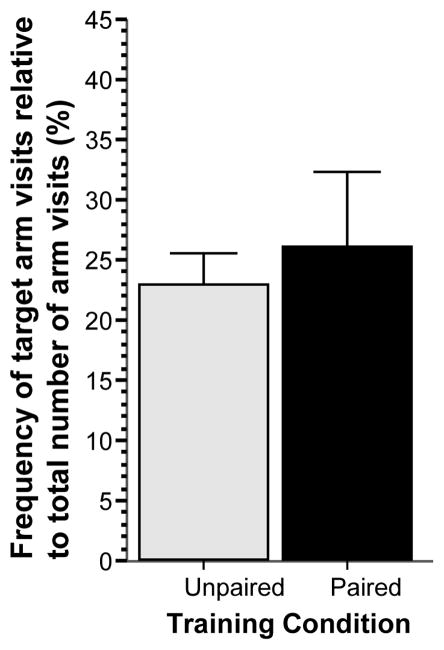
The frequency of target arm visits during the probe trial relative to the total number of arm visits (Figure 3) was also significantly larger in the paired group as compared to the unpaired group (t = 2.228, df = 18, p < 0.05), i.e. fish of the paired group not only spent more time in the vicinity of the red cue card during the probe trial but also visited (explored) it preferentially more frequently, a conditioned active appetitive response.
Figure 3.
Simple associative learning: Frequency of target arm visits relative to total number of arm visits, expressed as percentage, significantly differs between the groups at the probe trial. Mean ± SEM are shown. Sample sizes nunpaired = 10, npaired = 10. The single asterisk indicates significant difference at p < 0.05. For methodological details see Methods. For details of statistical analyses, see Results.
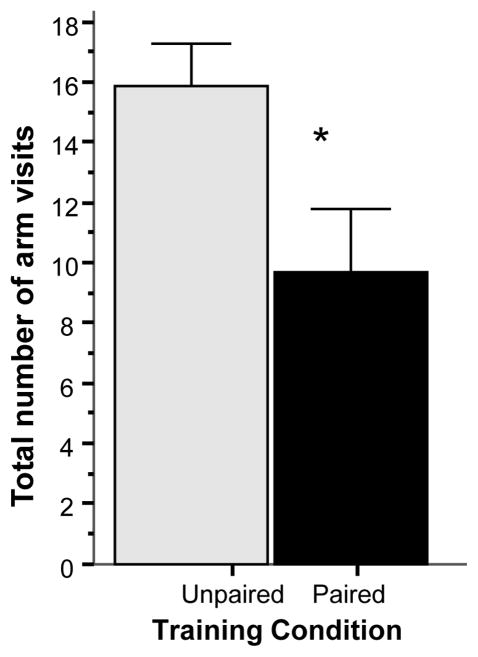
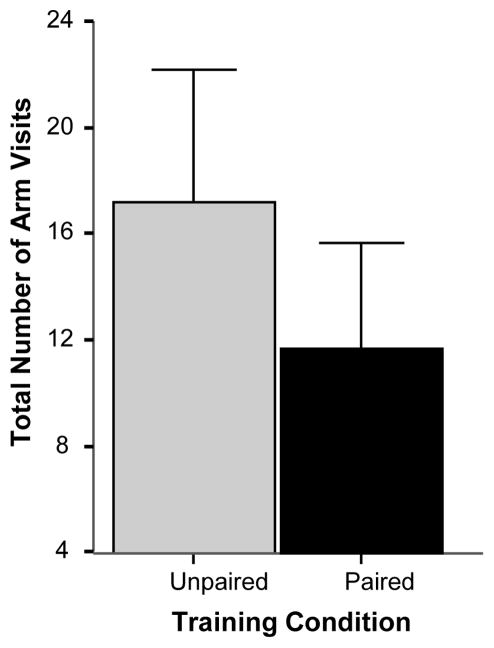
Analysis of the total number of visits to all arms (Figure 4) also revealed an interesting finding. Fish of the unpaired group showed a significantly increased number of total visits (t = 2.501, df = 18, p < 0.05) suggesting an overall, non-arm specific enhancement of exploratory activity in these fish.
Figure 4.
Simple associative learning: The total number of arm visits significantly differs between the groups at the probe trial. Mean ± SEM are shown. Sample sizes nunpaired = 10, npaired = 10. The single asterisk indicates significant difference at p < 0.05. For methodological details see Methods. For details of statistical analyses, see Results.
The spatial learning task also resulted in significant findings. Figure 5 shows the percent of time the fish spent in the target arm during the very first training trial of this paradigm (Figure 5 panel A) and during the probe trial (Figure 5 panel B), i.e. after 20 trials of training. During the first training trial the performance of fish in the two groups did not differ from each other (t = 0.336, df = 22, p > 0.70). Also, neither the unpaired (t = 1.697, df = 9, p > 0.40) nor the paired group (t = 1.435, df = 11, p > 0.05) differed significantly from random chance. However, after the 20 training trials, fish that received the food at a consistent, spatially defined, location (the paired group) showed a significantly enhanced preference (t = 2.577, df = 22, p < 0.05) for the target location as compared to fish of the unpaired group that received the food at random locations. Also importantly, fish of the paired group spent significantly (t = 2.57, df = 11, p < 0.05) above chance (23.14%) percent of time in the target arm, but fish from the unpaired group did not (t = − 0.597, df = 11, p > 0.50).
Figure 5.
Spatial learning: Percent of time in the target arm significantly differs between the groups at the probe trial. Panel A, performance at the first training trial. Panel B, performance at the probe trial, i.e. after 20 training trials. Mean ± SEM are shown. Sample sizes nunpaired = 10, npaired = 12. The solid horizontal line represents random chance level performance. Significant difference (p < 0.05) between the groups is indicated by an asterisk. For methodological details see Methods. For details of statistical analyses, see Results.
Analysis of the frequency of target arm visits relative to total number of arm visits (Figure 6) revealed no significant difference between the two groups (t = 1.08, df = 22, p > 0.25), a finding that suggests some potentially noteworthy difference between the cue-based and spatial learning task performance in zebrafish (compare figures 3 and 6).
Figure 6.
Spatial learning: Frequency of target arm visits relative to total number of arm visits, expressed as percentage, does not significantly differ between the groups at the probe trial. Mean ± SEM are shown. Sample sizes nunpaired = 10, npaired = 12. For methodological details see Methods. For details of statistical analyses, see Results.
The total number of arm entries is shown on Figure 7. Analysis of this measure showed no significant difference between the two groups (t = 1.91, df = 22, p = 0.069) but the figure and the borderline p value imply again that fish of the paired group somewhat reduced their exploratory activity perhaps as a result of finding the target location better than the fish that received the food at random locations during training.
Figure 7.
Spatial learning: The total number of arm visits does not significantly differ between the groups at the probe trial. Mean ± SEM are shown. Sample sizes nunpaired = 10, npaired = 12. For methodological details see Methods. For details of statistical analyses, see Results.
DISCUSSION
The significant increase of time in the target arm demonstrated that zebrafish of the paired groups have learned and remembered the association between the single visual cue and the food reward (simple associative learning), and in the following task, the location of the food reward (spatial learning). The results thus suggest that the zebrafish is capable of attaining good performance in these associative learning tasks. The improved performance is not likely to be due to factors other than the establishment of associative memory. For example, it is unlikely that zebrafish developed a non-specific preference for particular arms in the maze (e.g. entering certain arms due to stereotypy, or staying in a particular arm due to immobility as a result of enhanced fear) because the unpaired groups, which underwent a training and handling procedure identical to that of the paired groups, did not show the improved performance. Associative learning has been shown in zebrafish [24; 3] but spatial learning has only been demonstrated so far in a related cyprinid, the gold fish [28].
It is also interesting to note the results we obtained for the total number of entries to the arms. Although the effect was significant only in the simple associative learning task (and only bordered significance in the spatial learning task) the total number of arm entries appeared to increase in the unpaired group as compared to the paired group (Figures 4 and 7). This increase is noteworthy because it suggests that the fish of the unpaired groups were motivated to explore the maze and their worse performance relative to that of the paired group in finding the target arm was not associated with passivity. In principle, these results demonstrate that training of the fish of the unpaired groups was also successful: these fish have learned that the plus maze environment represents the possibility to find food, and thus they responded with increased exploration of the maze.
The frequency of target arm visits relative to the total number of arm visits shows a notable pattern too. While the paired group showed a significantly larger relative frequency compared to the unpaired group in the probe trial after the simple associative learning task, such a difference was not found after the spatial learning. It appears that the single cue-based task required the zebrafish to visit the target arm frequently, presumably because the red cue card was only visible once the fish entered the arm. However, the spatial task did not require such frequent visits to the target arm, presumably because the location of this arm was determined by the fish by attending to external visual cues visible from most locations in the maze.
The latter argument brings up an important question. In the mammalian spatial learning literature, acquisition of a spatial task, e.g. the Morris Water Maze hidden platform task, is usually taken as evidence for spatial learning [22]. However, these tasks may be solved using non-spatial strategies [17; 15]. The issue is whether the experimental subjects have learned the constellation of, and dynamic relationships among, the external visual stimuli (relational learning) and indeed used these relationships to navigate through the maze and find the target, or, alternatively, just memorized a single salient external cue next to the target location and solved the task using a single cue-based non-spatial strategy. For example, mice of the DBA/2 inbred strain, which have impaired hippocampal function and relational learning abilities, could respond to the location of previous fear training using a single cue-based strategy (for discussion and experimental examples see [14]). Could zebrafish in the current spatial task employ such a non-spatial strategy? Our data only provide indirect evidence to the contrary and thus future studies will need to ascertain whether the excellent spatial learning performance of our fish was indeed the result of true relational learning.
The last point we want to consider is the use of learning tasks in high throughput screening. For such screens, most believe the task needs to be simple and fast. But perhaps the most important parameter is whether the task is scaleable. If a paradigm can be run parallel, i.e. multiple pieces of test equipment each running an individual subject can work at the same time, throughput can be increased dramatically even if the test is slow. Parallel running is only possible if the task is automated, i.e. if it does not require the constant presence and attention of a human experimenter. Our current behavioral tasks clearly do not fall into this category. The plus maze is labor intensive. Although the actual trial is short (only 5 minutes), the experimenter needs to move the fish in and out of the maze manually. Quantification of behavior may easily be automated with the use of video-tracking [5], but the required human handling makes the tasks inappropriate for screening purposes. Nevertheless, if the findings of the current work with regard to both simple associative and complex relational learning are upheld, perhaps automatable versions of such tasks may be designed for zebrafish. For example, we have found that zebrafish respond to computer animated images of shoal mates [29] as well as to animated images of predatory fish [15]. As a diurnal species with excellent vision, zebrafish may respond to numerous other computer presented stimuli. Thus, one can envision paradigms where stimuli presented on the computer screen are paired or shown at particular locations around the test tank, thereby mimicking numerous aspects of simple associative as well as spatial learning tasks. Furthermore, relational learning may be tested by the presentation of visual cues on the computer screen in particular order or combination (see for example the principle of transitive inference [11]). Given that the presentation of the visual cues involved can be computerized and the behavior of the fish can also be recorded automatically using videotracking, many such test apparati may be run in parallel, and even if several trials may be required to reach appropriate level of learning performance, throughput may be dramatically increased. We have already utilized a conceptually similar and fully automated learning task, an appetitive reward based shuttle box paradigm in which zebrafish are rewarded by the sight of conspecifics [24] and others have also described the prototype of an automatable learning task for zebrafish [20]. In summary, it is likely automated test paradigms capable of eliciting and quantifying associative learning in zebrafish will be developed for high throughput screening, and once implemented, with the help of these paradigms zebrafish will advance the discovery of novel genes involved in vertebrate learning and memory processes.
Acknowledgments
This study was supported by an NIH/NIAAA grant (1R01AA015325-01A2) and an NSERC discovery grant (#311637-06) to RG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amsterdam A, Hopkins N. Mutagenesis strategies in zebrafish for identifying genes involved in development and disease. Trends Genet. 2006;22:473–478. doi: 10.1016/j.tig.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Bass SLS, Gerlai Zebrafish (Danio rerio) responds differentially to stimulus fish: The effects of sympatric and allopatric predators and harmless fish. Behav Brain Res. 2008;186:107–117. doi: 10.1016/j.bbr.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 3.Bilotta J, Risner ML, Davis EC, Haggbloom SJ. Assessing appetitive choice discrimination learning in zebrafish. Zebrafish. 2005;2:259–268. doi: 10.1089/zeb.2005.2.259. [DOI] [PubMed] [Google Scholar]
- 4.Blank M, Guerim LD, Cordeiro RF, Vianna MR. A one-trial inhibitory avoidance task to zebrafish: rapid acquisition of an NMDA-dependent long-term memory. Neurobiol Learn Mem. 2009;92:529–534. doi: 10.1016/j.nlm.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Blaser R, Gerlai R. Behavioral phenotyping in Zebrafish: Comparison of three behavioral quantification methods. Behavior Research Methods. 2006;38:456–469. doi: 10.3758/bf03192800. [DOI] [PubMed] [Google Scholar]
- 6.Braubach OR, Wood HD, Gadbois S, Fine A, Croll RP. Olfactory conditioning in the zebrafish (Danio rerio) Behav Brain Res. 2009;198:190–198. doi: 10.1016/j.bbr.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 7.Burgess HA, Granato M. The neurogenetic frontier--lessons from misbehaving zebrafish. Brief Funct Genomic Proteomic. 2008;7:474–482. doi: 10.1093/bfgp/eln039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgess N, Becker S, King JA, O’Keefe J. Memory for events and their spatial context: models and experiments. Philos Trans R Soc Lond B Biol Sci. 2001;356:1493–1503. doi: 10.1098/rstb.2001.0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen NJ, Poldrack RA, Eichenbaum H. Memory for items and memory for relations in the procedural/declarative memory framework. Memory. 1998;6:689–699. [Google Scholar]
- 10.Crusio WE, Schwegler H, Lipp HP. Radial-maze performance and structural variation of the hippocampus in mice: a correlation with mossy fibre distribution. Brain Res. 1987;425:182–185. doi: 10.1016/0006-8993(87)90498-7. [DOI] [PubMed] [Google Scholar]
- 11.Dusek JA, Eichenbaum H. The hippocampus and memory for orderly stimulus relations. Proc Natl Acad Sci U S A. 1997;94:7109–7114. doi: 10.1073/pnas.94.13.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eddins D, Petro A, Williams P, Cerutti DT, Levin ED. Nicotine effects on learning in zebrafish: the role of dopaminergic systems. Psychopharmacology. 2009;202:103–109. doi: 10.1007/s00213-008-1287-4. [DOI] [PubMed] [Google Scholar]
- 13.Gerlai R. Phenomics: Fiction or the Future? Trends Neurosci. 2002;25:506–509. doi: 10.1016/s0166-2236(02)02250-6. [DOI] [PubMed] [Google Scholar]
- 14.Gerlai R. Contextual Learning and Cue Association in Fear Conditioning in Mice: A Strain Comparison and a Lesion Study. Behav Brain Res. 1998;95:191–203. doi: 10.1016/s0166-4328(97)00144-7. [DOI] [PubMed] [Google Scholar]
- 15.Gerlai R. Behavioral tests of hippocampal function: Simple paradigms, complex problems. Behav Brain Res. 2001;125:269–277. doi: 10.1016/s0166-4328(01)00296-0. [DOI] [PubMed] [Google Scholar]
- 16.Gerlai R, Fernandes Y, Pereira T. Zebrafish (Danio rerio) responds to the animated image of a predator: Towards the development of an automated aversive task. Behav Brain Res. 2009;201:318–324. doi: 10.1016/j.bbr.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerlai R, McNamara A, Williams S, Phillips HS. Hippocampal dysfunction and behavioral deficit in the water maze in mice: An unresolved issue? Brain Res Bull. 2002;57:3–9. doi: 10.1016/s0361-9230(01)00630-x. [DOI] [PubMed] [Google Scholar]
- 18.Gleason PE, Weber PG. Effect of group size on avoidance learning in zebrafish, Brachydanio rerio (Pisces: Cyprinidae) Animal Learning and Behavior. 1977;5:213–216. [Google Scholar]
- 19.Grunwald DJ, Eisen JS. Timeline: Headwaters of the zebrafish—emergence of a new model vertebrate. Nat Rev Genet. 2002;3:717–724. doi: 10.1038/nrg892. [DOI] [PubMed] [Google Scholar]
- 20.Hicks C, Sorocco D, Levin M. Automated analysis of behavior: a computer-controlled system for drug screening and the investigation of learning. J Neurobiol. 2006;66:977–990. doi: 10.1002/neu.20290. [DOI] [PubMed] [Google Scholar]
- 21.Meli R, Prasad A, Patowary A, Lalwani MK, Maini J, Sharma M, Singh AR, Kumar G, Jadhav V, Scaria V, Sivasubbu S. FishMap: a community resource for zebrafish genomics. Zebrafish. 2008;5:125–130. doi: 10.1089/zeb.2008.0531. [DOI] [PubMed] [Google Scholar]
- 22.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 23.Parra KV, Adrian JC, Jr, Gerlai R. The synthetic substance hypoxanthine 3-N-oxide elicits alarm reactions in zebrafish (Danio rerio) Behav Brain Res. 2009 doi: 10.1016/j.bbr.2009.06.037. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pather S, Gerlai R. Shuttle box learning in zebrafish. Behav Brain Res. 2009;196:323–327. doi: 10.1016/j.bbr.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roest Crollius H, Weissenbach J. Fish genomics and biology. Genome Res. 2005;15:1675–1682. doi: 10.1101/gr.3735805. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez F, Lopez JC, Vargas JP, Broglio C, Gomez Y, Salas C. Spatial memory and hippocampal pallium through vertebrate evolution: insights from reptiles and teleost fish. Brain Research Bulletin. 2002;57:449–503. doi: 10.1016/s0361-9230(01)00682-7. [DOI] [PubMed] [Google Scholar]
- 27.Salas C, Broglio C, Durán E, Gómez A, Ocaña FM, Jiménez-Moya F, Rodríguez F. Neuropsychology of learning and memory in teleost fish. Zebrafish. 2006;3:157–171. doi: 10.1089/zeb.2006.3.157. [DOI] [PubMed] [Google Scholar]
- 28.Salas C, Rodríguez F, Vargas JP, Durán E, Torres B. Spatial learning and memory deficits after telencephalic ablation in goldfish trained in place and turn maze procedures. Behav Neurosci. 1996;110:965–980. doi: 10.1037//0735-7044.110.5.965. [DOI] [PubMed] [Google Scholar]
- 29.Saverino C, Gerlai R. The social zebrafish: Behavioral responses to conspecific, heterospecific, and computer animated fish. Behav Brain Res. 2008;191:77–87. doi: 10.1016/j.bbr.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sison M, Cawker J, Buske C, Gerlai R. Fishing for genes influencing vertebrate behavior: zebrafish making headway. Lab Anim. 2006;35:33–39. doi: 10.1038/laban0506-33. [DOI] [PubMed] [Google Scholar]
- 31.Speedie N, Gerlai R. Alarm substance induced behavioral responses in zebrafish (Danio rerio) Behav Brain Res. 2008;188:168–177. doi: 10.1016/j.bbr.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sweatt D. Mechanisms of Memory. Amsterdam: Elsevier; 2003. p. 400. [Google Scholar]
- 33.Vargas JP, López JC, Portavella M. What are the functions of fish brain pallium? Brain Res Bull. 2009;79:436–440. doi: 10.1016/j.brainresbull.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 34.Vascotto SG, Beckham Y, Kelly GM. The zebrafish’s swim to fame as an experimental model in biology. Biochem Cell Biol. 1997;75:479–485. [PubMed] [Google Scholar]
- 35.Williams FE, White D, Messer WS., Jr A simple spatial alternation task for assessing memory function in zebrafish. Behavioural Processes. 2002;58:125–132. doi: 10.1016/s0376-6357(02)00025-6. [DOI] [PubMed] [Google Scholar]
- 36.Xu X, Scott-Scheiern T, Kempker L, Simons K. Active avoidance conditioning in zebrafish (Danio rerio) Neurobiol Learn Mem. 2007;87:72–77. doi: 10.1016/j.nlm.2006.06.002. [DOI] [PubMed] [Google Scholar]