Abstract
CD4+ CD25+ Foxp3+ Tregs are critical regulators of immune responses and autoimmune diseases. nTregs are thymically derived; iTregs are converted in the periphery from CD4+ CD25– Foxp3– Teffs. Recent studies reported that GALT CD103+ DCs mediated enhanced iTreg conversion via the secretion of RA. However, the factors regulating RA secretion and hence, the induction of iTregs by DCs are not yet clear. Activation of the nuclear hormone receptor PPARγ has been shown to induce RA expression in human DCs, and thus, we postulated that PPARγ activation in DCs may be an important regulator of RA secretion and iTreg generation. Using in vitro and in vivo approaches, we now demonstrate that PPARγ activation enhances iTreg generation through increased RA synthesis from murine splenic DCs. In addition, we demonstrate that inhibition of DC PPARγ decreases iTreg generation, suggesting a role for endogenous PPARγ ligands in this process. Overall, our findings suggest that PPARγ may be important as a factor that stimulates DCs to produce RA and as a potential mechanism by which PPARγ ligands ameliorate autoimmunity.
Keywords: nuclear hormone receptor, immunoregulation
Introduction
CD4+ CD25+ Foxp3+ Tregs have been shown to be important regulators of immune responses and are critical in inhibiting autoimmune diseases [1]. Two types of Tregs have been described: nTregs are thymically derived, and iTregs are “converted” in the peripheral immune system from CD4+ CD25– Foxp3– Teffs [1]. Recently, it was reported that conversion of Teffs to iTregs occurs in the GALT and is mediated by RA secreted by a specialized subset of CD103-expressing GALT DCs [2,3,4,5]. When compared with splenic DCs, CD103+ GALT DCs were found to be significantly more efficient in inducing iTreg generation, and this was mediated by increased secretion of RA. Importantly, however, the factors that regulate RA secretion and iTreg generation by DCs have not been defined.
One molecule potentially involved in regulating RA secretion by DCs is PPARγ, a ligand-dependent transcription factor and member of the nuclear hormone receptor family, which has been characterized primarily for its role in regulating insulin and glucose metabolism [6,7,8]. The ability of PPARγ ligands to enhance insulin sensitivity has led to its use in humans for the treatment of type 2 diabetes. However, we and others [7, 9,10,11,12,13,14] have demonstrated that PPARγ ligands are also capable of down-regulating most cells of the innate and adaptive immune system. This has led to numerous studies demonstrating the efficacy of PPARγ ligands in treating animal models of autoimmunity, including experimental allergic encephalomyelitis, asthma, arthritis, colitis, and diabetes [13, 15,16,17,18,19,20,21,22,23].
More recently, we and others [24, 25] have identified a role for PPARγ in nTreg function and iTreg generation. We demonstrated that activation of PPARγ in nTregs using a synthetic PPARγ ligand in vivo enhanced regulatory function of these cells in a murine model of graft-versus-host disease [25]. This effect on nTregs was PPARγ-dependent. We also reported that high concentrations of this synthetic PPARγ ligand in vitro enhanced the generation of iTregs. Importantly, this effect was demonstrated to be mediated directly on T cells in the absence of APCs and was PPARγ-independent [25].
In addition to its T cell effect, PPARγ has been shown to affect DC function, including having a possible inhibitory effect on DC maturation, IL-12 secretion, and expression of costimulatory molecules [26, 27]. Pertinent to the present studies, Szatmari et al. [28] reported that activation of PPARγ in human blood monocyte-derived DCs resulted in RA secretion by these cells. Thus, it is possible that PPARγ could play an important role in regulating RA production in murine DCs as well. In addition, it has been documented that PPARγ ligands are expressed in the gut [29, 30]. These reports have led us to postulate that PPARγ activation may be an important regulator of RA secretion and iTreg generation in CD103+ GALT DCs and in DCs from other sites. In the present studies, using in vitro and in vivo approaches, we now demonstrate that PPARγ activation enhances iTreg generation through increased RA synthesis from murine splenic DCs and that endogenous PPARγ ligands may play a critical role in regulating iTreg generation.
MATERIALS AND METHODS
Mice
C57BL/6 and B6.C-Hbm12/KhEgJ (Bm12) mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). OT-II Rag−/− mice were obtained originally from Dr. Francis Carbone, University of Melbourne, Melbourne, Australia. All mice were maintained and bred in accordance with University of Connecticut Center for Laboratory Animal Care regulations (Farmington, CT, USA).
OT-II, anti-CD3 antibody-stimulated, and mixed lymphocyte reaction iTreg conversion assay
CD11c+ splenic DCs were purified from C57BL/6 or Bm12 mice using murine CD11c-conjugated beads (Miltenyi Biotec, Germany). DCs were irradiated (2600 R) and for the OT-II studies, pulsed with 100 μM OVA323–339 peptide (GenScript, Piscataway, NJ, USA). OT-II Rag−/− CD4+, C57BL/6 CD4+CD25–, or Bm12 CD4+CD25– T cells were purified from spleens using magnetic bead purification (Miltenyi Biotec). DCs (1×104 cells/well) and T cells (1×105 cells/well) were plated in round-bottom 96-well plates with TGF-β (1, 2, or 3 ng/ml; R&D Systems, Minneapolis, MN, USA), VC (DMSO) or Cig (5 μM; Biomol, Plymouth Meeting, PA, USA), LE 540 (Wako, Richmond, VA, USA), GW9662 (Biomol), Rosi (Cayman Chemical, Ann Arbor, MI, USA), 15d-PGJ2 (Enzo Life Sciences, Plymouth Meeting, PA, USA), or Citral (Sigma-Aldrich, St. Louis, MO, USA) as indicated. On Day 4 of culture, a final concentration of 100 U/ml IL-2 was added to all wells. Cells were harvested and stained on Day 5. For plate-bound α-CD3 antibody experiments, C57BL/6 CD4+CD25– Teffs were cultured as above in the absence of DCs in 96-well plates coated with 5 μg/ml α-CD3 antibody.
Staining
Cells were stained with the following mAb: FITC α-CD4 (GK1.5), PE α-A4B7 (DATK32), α-B7.2 (GL1), α-B7.1 (16-10A1), and FITC α-I-Ab (MHC II; 25-9-17; BD PharMingen, San Diego, CA, USA); PE α-CD103 (2E7) and allophycocyanin α-Foxp3 (FJK-16s; e-Bioscience, San Diego, CA, USA); and PE CCR9 (R&D Systems). FACS analysis was performed on a FACS Calibur (Becton Dickinson, San Jose, CA, USA).
Preparation of DC RNA for qPCR
For overnight in vitro splenic DC culture, CD11c+ splenic DCs were purified from C57BL/6 mice and cultured at 1 × 106 cells/ml in media containing TGF-β (3 ng/ml), GM-CSF (20 ng/ml; Pierce, Rockford, IL, USA), and VC or Cig (5 μM). DCs were cultured for 18 h, and RNA was prepared using an RNeasy mini kit (Qiagen, Valencia, CA, USA). For derivation of ex vivo RNA from in vivo Cig or VC-treated C57BL/6 mice (see below), CD11c+ splenic DCs were purified, and RNA was prepared using an RNeasy mini kit (Qiagen).
qPCR for aldh1a2, ltbp3, and plat
cDNA was generated with an iScript RT kit (BioRad, Hercules, CA, USA). qPCR was performed by SYBR green incorporation (BioRad) and normalized to HPRT. Primers for HPRT, aldh1a2, ltbp3, and plat, were obtained from Qiagen, and running conditions were those described by Coombes et al. [5].
In vivo treatment of mice and ex vivo analysis of splenic DCs
C57BL/6 mice were treated for 1, 2, 5, or 6 days with a daily gavage of VC, Cig (300–400 μg), or GW9662 (331 μg). In some experiments, mice also received daily s.c. injections of VC, Cig (1.4 mg), or GW9662 (1.3 mg). Gavage was given as VC, GW9662, or Cig (20 μl) in a total volume of 200 μl vegetable oil. s.c. injections were given as 70–80 μl VC, GW9662, or Cig. After in vivo treatment, spleens were removed, and CD11c+ splenic DCs were derived using magnetic beads. Such splenic DCs were then used as a source of RNA (as above), stained for surface marker expression (as above), or used in iTreg conversion cultures: Cells/well (1×105) magnetically purified splenic CD4+ CD25– Teffs derived from Bm12 or C57BL/6 mice were cultured for 5 days with 1 × 104 CD11c+ splenic DCs derived from VC, GW9662-treated, or Cig-treated allogeneic (Bm12 or C57BL/6) mice in the presence of TGF-β (1, 2, or 3 ng/ml). IL-2 (100 U/ml) was added to the cultures on Day 4. After 5 days of culture, Teffs were stained for CD4 and Foxp3.
RESULTS
Cig enhances DC-dependent iTreg generation
To investigate whether PPARγ activation in murine splenic DCs could enhance iTreg generation, we first studied iTreg generation in vitro using CD4+ Rag−/− OT-II T cells, which were cultured for 5 days with TGF-β and OVA323–339 peptide-pulsed C57BL/6 splenic DCs (or alternatively, with DCs and soluble OVA323–339 peptide). In addition, Cig (5 μM), a thiazolidinedione class of PPARγ ligand, or VC was added to the cultures. We have reported previously that Cig is capable of enhancing in vitro iTreg generation in an APC-independent manner but primarily at concentrations higher than 20 μM. We have reported further that this direct effect of Cig on T cells is PPARγ-independent [25]. In the present studies, we chose a concentration of Cig (5 μM), which we determined previously did not have a direct effect on T cell conversion to iTregs, and we used culture systems that required APCs for T cell activation.
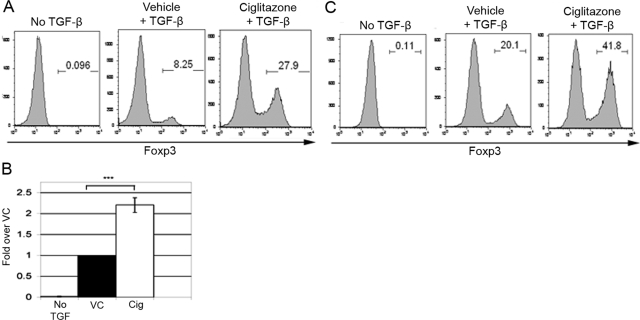
Using these culture conditions, we found that in the absence of TGF-β, DC-OVA-stimulated Foxp3– OT-II T cells did not convert to Foxp3+ iTregs (Fig. 1, A and B). This was true with or without Cig (data not shown). In the presence of TGF-β and VC, we found typically that 4–20% of the DC-OVA-stimulated OT-II cells became Foxp3+. When TGF-β was combined with 5 μM Cig, OT-II conversion to Foxp3+ iTregs increased two- to 3.5-fold consistently over that seen with TGF-β and VC (Fig. 1, A and B).
Figure 1.
Cig (5 μM) enhances splenic DC-dependent iTreg generation. (A) CD4+ Rag−/− OT-II T cells (1×105) were cultured with 1 × 104 CD11c+ splenic DCs in the presence or absence of TGF-β and with VC or 5 μM Cig. The splenic DCs were pulsed with 100 μM OVA323–339 peptide. The cells were stained for CD4 and Foxp3 expression on Day 5. Results are shown gated on CD4+ cells. (B) Compilation of OT-II experiments (n=18); statistical significance was determined by unpaired Student’s t-test (***, P<0.0001). (C) CD4+CD25– C57BL/6 splenic Teffs (1×105) were cultured with 1 × 104 CD11c+ Bm12 splenic DCs in the presence or absence of TGF-β and with VC or 5 μM Cig. The cells were stained for CD4 and Foxp3 expression on Day 5. Results are shown gated on CD4+ cells.
To investigate whether 5 μM Cig could also enhance iTreg generation in polyclonal Teff populations stimulated in an APC-dependent manner, we stimulated C57BL/6-derived CD4+ CD25– Teffs with allogeneic Bm12 DCs in the presence of TGF-β and 5 μM Cig. After 5 days in culture, only a small percentage of Teff stimulated by Bm12 DCs in the absence of TGF-β was Foxp3+ (Fig. 1C). As expected, stimulation of Teff with Bm12 DCs in the presence of TGF-β and VC increased the percentage of CD4+ Foxp3+ cells. Importantly, stimulation with Bm12 DCs in the presence of TGF-β and 5 μM Cig typically more than doubled the percentage of cells expressing Foxp3 (Fig. 1C). The Teff population at the initiation of the cultures contained a small population of contaminating nTregs (data not shown). However, it has been shown previously that such contaminating nTregs do not proliferate significantly under these culture conditions, and it is therefore unlikely that the Cig-induced enhancement of Foxp3-expression is a result of expansion of this small percentage of nTregs [31]. Therefore, 5 μM Cig also has the capability of enhancing iTreg generation in an allogeneic APC-stimulated polyclonal response.
Cig-induced enhancement of iTreg generation is inhibited by the RA inhibitor LE 540
We next asked whether the enhancement by Cig of iTreg generation is mediated through RA. As in the studies characterizing GALT CD103+ DCs [2,3,4,5], we approached this question by examining the effects of the competitive RA inhibitor LE 540 on the enhancement by Cig of iTreg generation. The addition of LE 540 reduced the low level of conversion found in cultures of TGF-β and VC by 50–70% (Fig. 2A). This suggests that RA normally plays a role even in the low level of iTreg generation mediated by splenic DCs in the absence of Cig. This low level of “background” RA is most likely present in the culture serum. Importantly, we found that LE 540 resulted in a significant reversal of Cig-induced enhancement, typically reducing the expression of Foxp3+ cells in Cig cultures by ∼85% (Fig. 2, A and B). This inhibition by LE 540 indicates that Cig-induced enhancement of conversion is mediated through a RA-dependent mechanism.
Figure 2.
Cig-induced enhancement of iTreg generation is blocked by the RA inhibitor LE 540. (A) CD4+ Rag−/− OT-II T cells were stimulated as in Figure 1 but with or without 0.25 μM RA inhibitor LE 540. Cells were stained for CD4 and Foxp3 on Day 5. Results of one representative experiment are shown. Results are shown gated on CD4+ cells. (B) Compilation of experiments as in A (n=5) represented graphically as percent Foxp3-expressing cells after 5 days in culture. Statistical significance was determined by one-way ANOVA and Scheffé test to compare VC versus Cig and LE 540 versus LE 540 + Cig (*, significance set at P<0.05). NS, Not significant.
Cig enhancement of iTreg generation is associated with T cell expression of gut-homing receptors
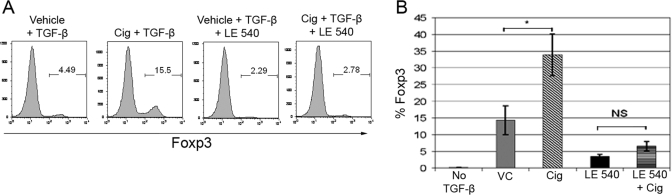
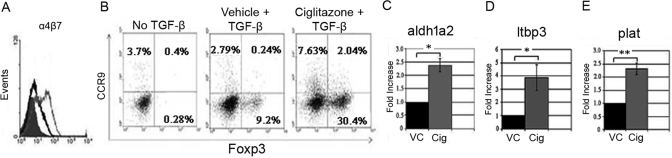
To understand further the relevance of RA in the Cig-mediated enhancement in iTreg generation, we next asked whether culture with Cig induced or increased T cell expression of CD103, α4β7, or CCR9. T cell expression of CD103 has been associated with TGF-β signaling, and T cell expression of the gut-homing receptors, α4β7 and CCR9, has been associated with RA signaling [4, 32]. We examined the expression of CD103, α4β7, and CCR9 on OT-II cells after 5 days of culture with TGF-β and VC, Cig, or RA. We found that Cig together with TGF-β induced a slight increase in percentage of Foxp3+ T cells expressing CD103 when compared with that seen with VC and TGF-β (data not shown). Cig induced a small but consistent up-regulation in the percentage of Foxp3+ T cells expressing α4β7 in comparison with VC (Fig. 3A). As expected, RA together with TGF-β induced expression of both of these molecules on OT-II cells. Significantly, Cig also increased the expression of CCR9 on Foxp3+ T cells 6.4–8.5 times compared with control cultures (Fig. 3B). These results suggest further that Cig enhancement of iTreg generation involves a RA-dependent mechanism.
Figure 3.
Cig up-regulates T cell expression of gut-homing surface markers and DC expression of mRNA for RA and TGF-β-related enzymes. (A) OT-II cells, cultured as in Figure 1, were stained for α4β7 and Foxp3 and analyzed by FACS, gating on Foxp3+ T cells. Filled histogram, Cells cultured with TGF-β and VC; open black histogram, cells cultured with TGF-β and Cig; open gray histogram, cells cultured with TGF-β and RA (10 nm). (B) OT-II cells cultured for 5 days as in Figure 1 were stained for CCR9 and Foxp3 and analyzed by FACS after gating on CD4+ cells. (C–E) CD11c+ splenic DCs were cultured for 18 h with TGF-β, GM-CSF, and VC or Cig (5 μM), harvested and RNA-derived, and the expression of mRNA for (C) aldh1a2, (D) ltbp3, and (E) plat was assessed by qPCR. All results are normalized to HPRT, and the mRNA expression in VC-cultured DCs was assigned a value of 1. Results represent two or more determinations for each gene. Statistical significance was determined by unpaired Student’s t-test (*, P<0.05; **, P<0.001).
Short-term culture of splenic DCs with Cig induces mRNA for RA and TGF-β-related genes
The conversion of retinal to RA is mediated by retinal dehydrogenase enzymes. Iwata et al. [32] found that DCs from Peyer’s patches and mesenteric lymph nodes expressed significant levels of mRNA for aldh1a2 and that this correlated closely with DC RA levels as measured by HPLC. Consistent with these studies, Coombes et al. [5] demonstrated that mRNA for aldh1a2 was constituitively increased in GALT CD103+ DCs. Interestingly, Szatmari et al. [28] demonstrated that culture of human DCs with PPARγ ligands induced the expression of mRNA for aldh1a2 and that this correlated with enhanced DC levels of RA as measured by liquid chromatography-mass spectrometry. We next asked whether culturing splenic DCs with Cig resulted in an induction of mRNA for aldh1a2. CD11c+ splenic DCs were cultured with TGF-β, GM-CSF, and Cig or VC. (In some experiments, agonistic anti-CD40 antibody was added to the cultures, resulting in no change in viability or experimental outcome; data not shown.) We cultured such splenic DCs under these conditions for 18 h, derived RNA from these cells, and assessed the expression of aldh1a2 using qPCR. We found that when 5 μM Cig was included in such short-term cultures of splenic DCs, there was a 2.0- to 2.5-fold increase in expression of mRNA for aldh1a2 compared with that seen with VC (Fig. 3C). In addition, using RT-PCR, we have found a slight increase in expression of mRNA for aldh1a1 but no increase in mRNA for aldh1a3 in Cig-treated splenic DCs (data not shown). These results demonstrate for the first time that Cig can regulate the production of RA by murine splenic DCs.
Coombes et al. [5] found that in addition to an increase in mRNA for aldh1a2, GALT CD103+ DCs expressed increased levels of mRNA for enzymes related to TGF-β availability: LTBP3, PLAT, and TGF-β2. We therefore assessed the expression of mRNA for ltbp3, plat, and tgfβ2 in splenic DCs cultured for 18 h with VC or Cig. Even in short-term culture, Cig induced an approximate fourfold increase in mRNA expression for ltbp3 and an ∼2.3-fold increase in mRNA expression for plat (Fig. 3, D and E). No increase was found in mRNA for tgfβ2 (data not shown). Overall, these results suggest that even short-term culture of splenic DCs with Cig induces a pattern of gene activation that is similar to that seen in CD103+ GALT DCs.
Cig enhancement of iTreg generation is mediated through increased RA synthesis in APC-containing cultures
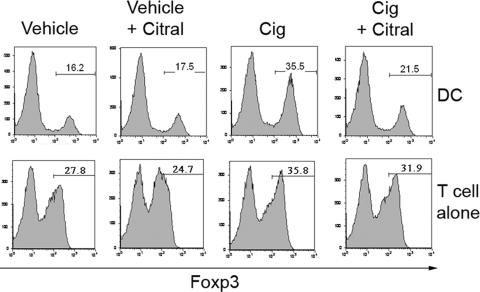
To characterize further the RA dependency of the Cig-induced enhancement in iTreg generation, we used Citral, an inhibitor of retinaldehyde dehydrogenases, which blocks de novo RA synthesis from vitamin A [32]. We examined the effect of Citral on the Cig enhancement of iTreg generation in APC-containing and APC-free cultures. We found that the Cig-induced enhancement seen in DC-containing cultures was inhibited consistently by Citral. Thus, as seen in Figure 4, although the conversion in the VC DC-containing cultures was not affected by the addition of Citral (14.5–16% Foxp3+), the enhancement induced by Cig was reduced almost to VC levels by Citral (32% Foxp3+ with Cig to 19.2% Foxp3+ with Cig and Citral). Similar inhibition of the effect of Cig occurred when we used another RA synthesis inhibitor, 4-(diethylamino)benzaldehyde (data not shown). We chose to use 5 μM Cig in the present studies, as our previous work had shown that this concentration does not have a significant effect on cultures of T cells alone. We occasionally noted a small increase in iTreg generation induced by 5 μM Cig in cultures of T cells in the absence of APCs, however this effect was inconsistent across experiments and much lower than the effect seen in APC-containing cultures. Importantly, when this enhancement was observed in cultures of T cells alone, it was not inhibited by Citral (Fig. 4). These results suggest that the effect of Cig in DC-containing cultures is mediated by an increase in RA synthesis (i.e., Citral-sensitive), and any direct T cell effect is not (i.e., Citral-insensitive). Therefore, Citral sensitivity distinguishes the effect of Cig on DCs from its effect on T cells and also confirms the RA dependence of the DC effects.
Figure 4.
Cig-induced enhancement of DC-mediated iTreg generation is inhibited by Citral. CD4+CD25– C57BL/6 splenic Teffs (1×105) were cultured with TGF-β and with 1 × 104 CD11c+ Bm12 splenic DCs (DC cultures) or on plate-bound α-CD3 (T cell alone cultures). Some wells contained VC, Cig (5 μM), or Citral (20 μM). The cells were stained for CD4 and Foxp3 expression on Day 5. Results are shown gated on CD4+ cells.
In vivo treatment with Cig results in increased aldh1a2 mRNA expression and iTreg generation by ex vivo splenic DCs
We have reported previously that high concentrations of Cig enhance in vitro iTreg generation through a direct T cell effect [25]. Given our present results demonstrating an enhancing effect of low-dose Cig on DC-mediated iTreg generation, it was apparent that the in vivo administration of PPARγ ligands could potentially alter in vivo iTreg generation via direct effects on DCs or T cells. To focus solely on the in vivo effects on splenic DCs, we treated mice with Cig, isolated the splenic DCs, and characterized their ex vivo phenotype, expression of aldh1a2 mRNA, and ex vivo function.
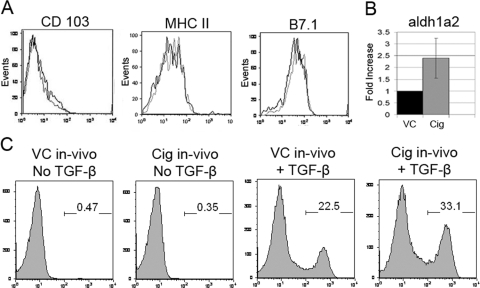
C57BL/6 mice were treated for 1–6 consecutive days by gavage with Cig or VC. In some experiments, mice also received a daily s.c. injection of Cig or VC. The spleens and splenic CD11c+ populations derived from Cig-treated mice (Cig-treated splenic DCs) showed no difference in cell numbers or viability when compared with spleens and splenic DCs derived from VC-treated mice (VC-treated splenic DCs; data not shown). It has been reported that costimulation may affect iTreg generation [2] and that PPARγ ligands may affect DC expression of MHC II and costimulatory molecules [26, 27]. In light of these findings and reports suggesting the relevance of the CD103+ DC subset on iTreg conversion [2,3,4,5], we examined the expression of these molecules by the Cig-treated splenic DCs using flow cytometry. In vivo Cig-treated splenic DCs demonstrated a small decrease in percent of cells expressing B7.1 but otherwise showed no difference in expression of CD103 or MHC II when compared with VC-treated splenic DCs (Fig. 5A).
Figure 5.
In vivo treatment with Cig results in increased aldh1a2 mRNA expression and iTreg generation by ex vivo splenic DCs. C57BL/6 mice were treated in vivo by gavage and s.c. injection with Cig or VC. (A) Splenocytes from Cig-treated mice (black lines) or VC-treated mice (gray lines) were stained with antibodies to CD11c, CD103, MHC II, and B7.1 and analyzed by FACS after gating on CD11c+ cells. (B) mRNA was derived immediately after magnetically purifying CD11c+ splenic DCs from Cig-treated and VC-treated mice and assessed for expression of aldh1a2 mRNA using qPCR as described in Figure 3. Results are normalized to HPRT, and the mRNA expression in VC-cultured DCs was assigned a value of 1. Results represent two determinations. (C) CD4+ CD25– Teffs derived from Bm12 mice (1×105 cells/well) were cultured for 5 days in the presence or absence of TGF-β and with CD11c+ splenic DCs (1×104/well) derived from in vivo Cig-treated or VC-treated C57BL/6 mice (2-day gavage and s.c.-injected). After 5 days of culture, Bm12 Teffs were stained for CD4 and Foxp3 and analyzed by FACS. Results are shown gated on CD4+ cells.
To examine differences in regulation of RA production after in vivo treatment, we derived mRNA from the Cig-treated and VC-treated splenic DCs immediately ex vivo and assessed their expression of aldh1a2 mRNA using qPCR. This analysis was performed using a 5-day and 2-day in vivo Cig-treatment protocol. qPCR analysis demonstrated an increase in expression of aldh1a2 mRNA in Cig-treated splenic DCs after both in vivo treatment protocols, with a mean increase of ∼2.5-fold over levels seen in VC-treated splenic DCs (Fig. 5B). These results indicate that in vivo activation with Cig can regulate RA production by splenic DCs.
Next, we examined the ability of ex vivo Cig-treated splenic C57BL/6 DCs to mediate in vitro conversion of allogeneic Bm12 CD4+ CD25– Teffs to iTregs. Consistent with their increase in expression of aldh1a2 mRNA, in vivo Cig-treated splenic DCs were enhanced in mediating ex vivo iTreg generation when compared with VC-treated splenic DCs. It is important to note that Cig was not added to the 5-day iTreg conversion cultures. The percentage of Foxp3-expressing T cells found after 5 days in culture without TGF-β did not differ between Cig-treated and VC-treated spleens (Fig. 5C). In contrast, Cig-treated splenic DCs together with TGF-β consistently demonstrated an ∼50% enhancement in iTreg generation from Bm12 Teff when compared with VC-treated splenic DCs with TGF-β (Fig. 5C). This enhanced iTreg generation was seen for all of the in vivo Cig-treatment protocols examined and was not associated with an alteration of the ability of Cig-treated splenic DCs to stimulate Bm12 Teffs in culture. Thus, there was no significant difference in total numbers or viability of Bm12 T cells after 5 days in culture with Cig-treated splenic DCs compared with VC-treated splenic DCs (data not shown). Overall, these results confirm that administration of Cig in vitro and in vivo can enhance the ability of splenic DCs to mediate iTreg generation.
The Cig enhancement of iTreg generation is PPARγ-dependent
We and others [25, 33] have reported previously that many of the effects of PPARγ ligands on immune responses result from PPARγ-dependent and -independent effects. Therefore, to further characterize the role of PPARγ in splenic DC-mediated iTreg generation, we assessed the effect of the PPARγ-specific inhibitor GW9662 on iTreg generation. GW9662 has been demonstrated to bind PPARγ irreversibly and with high affinity and to inhibit the binding of other ligands competitively [34].
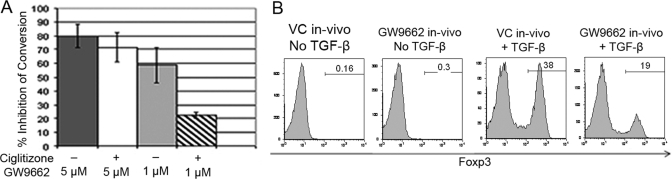
We added GW9662 to the 5-day in vitro cultures of C57BL/6 DC and OVA-stimulated OT-II T cells and found that the addition of 5 μM GW9662 inhibited the VC and Cig-enhanced iTreg conversion by 70–80% (Fig. 6A). The inhibition of the VC iTreg conversion was an unexpected finding, and this inhibition was not a result of a toxic effect of GW9662, as we found no decrease in absolute numbers or viability of OT-II after culture with 5 μM GW9662 (data not shown). Importantly, when the concentration of GW9662 was decreased from 5 μM to 1 μM, the VC conversion was still inhibited significantly (50–68%), but the Cig-enhanced conversion was inhibited by only 20–25% (Fig. 6A). These results suggest that the Cig enhancement in iTreg conversion is PPARγ-dependent. Furthermore, the finding that the VC iTreg conversions could be inhibited by even low concentrations of GW9662 suggests that endogenous PPARγ ligand-mediated PPARγ activation is required in the baseline splenic DC-mediated iTreg generation.
Figure 6.
The PPARγ-specific inhibitor GW9662 inhibits iTreg generation in vitro and in vivo. (A) CD4+ Rag−/− OT-II T cells were cultured as in Figure 1, and the PPARγ-specific inhibitor GW9662 was added at 5 μM or 1 μM as indicated. In addition, Cig was added where indicated and always used at 5 μM. The results represent the composite of two representative experiments and are presented as “% Inhibition of Conversion” mediated by GW9662. (B) CD4+ CD25– Teffs derived from C57BL/6 mice (1×105 cells/well) were cultured for 5 days in the presence or absence of TGF-β with CD11c+ splenic DCs (1×104/well) derived from in vivo GW9662-treated or VC-treated Bm12 mice (2-day gavage and s.c.-injected). After 5 days of culture, C57BL/6 Teffs were stained for CD4 and Foxp3 and analyzed by FACS. Results are shown gated on CD4+ cells.
These in vitro studies showed that inhibiting PPARγ blocked the basic ability of splenic DCs to mediate iTreg generation and also blocked the Cig enhancement. However, these results did not allow us to differentiate the effect of GW9662 on T cells versus DCs. To determine if blocking PPARγ had a direct effect on DCs, we treated Bm12 mice in vivo with GW9662 or VC via gavage and s.c. injections. Splenic CD11c+ cells were then derived from these mice, and their ability to mediate iTreg generation in vitro was assessed. Importantly, GW9662 was not added to the 5-day in vitro cultures, and the responding C57BL/6 CD4+ CD25– Teffs were derived from nontreated mice. Using this approach, we consistently found a 30–50% decrease in the ability of the in vivo-treated DCs to mediate iTreg generation in vitro (Fig. 6B). In vivo treatment with GW9662 did not affect the ability of the ex vivo DCs to prime T cell responses as cell numbers, and viability of responding T cells was similar in cultures stimulated with GW9662-treated or VC-treated DCs (data not shown). The results of these in vivo studies indicate that the inhibitory effect of GW9662 on splenic DCs is sufficient to decrease iTreg generation and suggest that PPARγ activation by endogenous PPARγ ligands in splenic DCs plays a critical role in iTreg conversion.
Additional PPARγ agonists enhance iTreg conversion
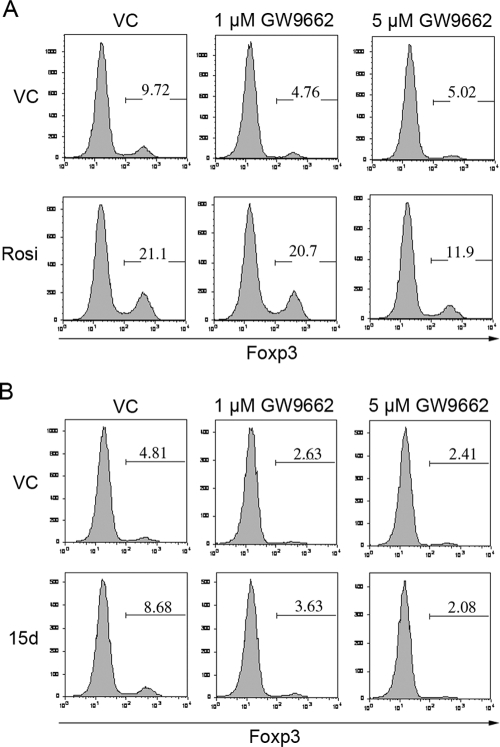
To confirm the PPARγ dependence of the Cig effect further, we tested a second synthetic PPARγ agonist, Rosi. We added 40 μM Rosi to a polyclonal Bm12-stimulated Treg conversion assay and found that like Cig, Rosi was able to increase iTreg conversion approximately twofold over VC (Fig. 7A). To further characterize the PPARγ dependence of the enhancement of iTreg conversion by Rosi, we added Rosi to a polyclonal Bm12 Treg conversion assay in the presence of 5 μM or 1 μM GW9662. Once again, we found that 5 μM and 1 μM GW9662 blocked the baseline iTreg conversion (Fig. 7A). Importantly, as with Cig, we found that 5 μM GW9662 inhibited the Rosi-enhanced conversion but that 1 μM GW9662 did not (Fig. 7A). The finding that a second synthetic PPARγ ligand is also capable of enhancing iTreg conversion and that this enhancement is inhibited by GW9662 strongly suggests that this enhancement is PPARγ-dependent.
Figure 7.
Additional PPARγ agonists enhance iTreg conversion. CD4+CD25– C57BL/6 splenic Teffs (1×105) were cultured with 1 × 104 CD11c+ Bm12 splenic DCs in the presence of TGF-β and VC or 40 μM Rosi (A) or VC or 15d-PGJ2 (15d; B). Some wells contained 1 μM or 5 μM GW9662. The cells were stained for CD4 and Foxp3 expression on Day 5. Results are shown gated on CD4+ cells.
Finally, to determine the effect of an endogenous ligand of PPARγ on iTreg conversion, we used 100 nM 15d-PGJ2 and found that this ligand increased conversion by almost twofold (Fig. 7B). Importantly, as with Cig and Rosi, the iTreg-enhancing effect of 15d-PGJ2 was also blocked by GW9662 (Fig. 7B). Taken together, these results confirm that the enhancement in iTreg conversion seen with Cig, Rosi, and 15d-PGJ2 is PPARγ-dependent and that the suppression of background iTreg conversion by GW9662 is likely a result of blocking of endogenous PPARγ activation.
DISCUSSION
The role of iTregs is presently an area of intense investigation, and recent reports have shown that Teff-to-iTreg conversion occurs in the GALT and is mediated by RA secreted by a specialized subset of CD103-expressing GALT DCs [2,3,4,5]. When compared with splenic DCs, these CD103+ GALT DCs were found to be enhanced significantly in their ability to induce iTreg generation as a result of increased RA secretion. A significant question posed by these studies is the nature of the intrinsic or extrinsic factors that regulate RA secretion by DCs, not only in the GALT but from other sites as well.
In the present studies, we postulated that PPARγ activation may be an important regulator of RA production and iTreg generation in CD103+ GALT DCs and in DCs from other sites. A number of the documented characteristics of PPARγ make it a likely candidate to play such a role. In addition to its role in the function of conventional T cells and Tregs documented by our lab and others [9, 24, 25], PPARγ has been shown to have a role in DC function and maturation. Initial studies demonstrated that although PPARγ ligation inhibited CD40-induced IL-12 secretion, it did not affect DC maturation, antigen processing, antigen presentation, MHC class II expression, or costimulatory molecule expression [26, 27]. Subsequent studies reported that prolonged PPARγ ligation did decrease the expression of DC costimulatory molecules and the ability of DCs to prime a naïve T cell response [27]. Of interest is the recent report by Szatmari et al. [28] showing that the PPARγ agonist Rosi induced the secretion of RA in human blood monocyte-derived DCs. Thus, as recently suggested by Svensson et al. [35], CD103+ GALT DCs may have enhanced the ability to metabolize vitamin A in vivo, and PPARγ may be one of the signals that regulates this ability. This possibility is made even more intriguing given that natural PPARγ ligands have been shown to be highly expressed in the gut, and their expression is dependent on the intestinal microflora [29, 30]. Given these characteristics of PPARγ, in the present studies, we asked whether PPARγ activation in splenic DCs can result in the enhanced ability to induce iTreg conversion in a RA-dependent manner.
We have reported previously that when used at high concentrations in vitro, the synthetic PPARγ ligand Cig enhances the conversion of Teffs to iTregs. This enhanced conversion using high concentrations of Cig is mediated directly through the Teff, is independent of APCs, and is mediated in a PPARγ-independent manner [25]. In contrast to these earlier findings, we now report that when Cig is used at lower concentrations (5 μM), optimal enhancement of iTreg generation requires the presence of DCs. When this lower concentration of Cig is used in cultures of T cells alone, enhancement of iTreg generation occurs only inconsistently, and when noted, the increase is less than that seen in DC-containing cultures.
We next asked whether the Cig-induced, DC-dependent enhancement of iTreg generation is mediated by RA. We found that a competitive inhibitor of RA (LE 540) reverses the Cig-induced enhancement in splenic DC-mediated iTreg generation, that Cig up-regulates RA-associated gut-homing receptors on T cells, and that Cig induces the expression of aldh1a2 mRNA, an enzyme involved in the production of RA, in splenic DCs. Most notably, we demonstrated that Cig-induced enhancement of iTreg generation involves increased splenic DC RA synthesis by showing that this enhancement was inhibited by the RA synthesis inhibitor Citral. However, the inhibition by Citral was noted only in DC-containing cultures and not in cultures of T cells alone. This finding not only confirms that Cig-induced enhancement of iTreg generation involves increased splenic DC RA synthesis but also clearly distinguishes a DC-mediated (Citral-sensitive) versus a T cell-mediated (Citral-insensitive) enhancing effect of Cig on iTreg generation. Finally, we found that splenic DCs derived from mice treated in vivo with Cig demonstrated increased expression of aldh1a2 mRNA and increased ability to mediate in vitro iTreg generation. These in vivo studies further confirm that the effects of Cig are mediated through increased DC RA synthesis and are distinct from effects mediated through the T cells.
Although PPARγ has been demonstrated previously to play a role in many cells involved in immune responses, it has become increasingly clear that many effects of PPARγ ligands are PPARγ-independent [25, 33]. To characterize whether the Cig-enhancing effect on iTreg generation was PPARγ-dependent, we used the PPARγ inhibitor GW9662 and found that GW9662 was able to block the iTreg-enhancing effect of Cig. A second synthetic PPARγ ligand, Rosi, was also capable of enhancing iTreg conversion, and GW9662 also inhibited Rosi-induced enhancement. Surprisingly, iTreg enhancement required a higher concentration of Rosi than Cig, although Rosi is a more potent activator. Nevertheless, our results indicate that at the concentration of Rosi used, its iTreg-enhancing effect is inhibited by the PPARγ inhibitor GW9662, and the level of inhibition noted is similar to that for Cig. Finally, we found that a low concentration of an endogenous ligand, 15d-PGJ2, was also able to enhance iTreg conversion and was also inhibited by GW9662. The inhibition by GW9662 of the Cig-, Rosi-, and 15d-PGJ2-induced enhancement strongly suggests that the iTreg-enhancing effects of all three of these compounds are PPARγ-dependent.
Interestingly, using in vitro and in vivo approaches, we also found that GW9662 inhibited the baseline splenic DC-mediated iTreg conversion in the absence of synthetic PPARγ ligands. Taken together, these results suggest that activation of PPARγ in splenic DCs by endogenous ligands likely plays an important role in the mediation of iTreg generation, and PPARγ ligands mediate enhancement of splenic DC-induced iTreg generation through a PPARγ- and RA-dependent mechanism.
It is not yet clear what role PPARγ activation plays in splenic DCs, allowing them to mediate the baseline generation of iTregs. Future studies will further characterize this requirement for PPARγ activation in splenic DC-mediated iTreg generation. Overall, our present results suggest that PPARγ ligation may be an important factor in stimulating DCs to produce RA and that the associated ability to enhance generation of iTregs may be one mechanism by which PPARγ ligands can ameliorate autoimmune diseases.
ACKNOWLEDGMENTS
The authors thank Dr. Leo Lefrançois for his critical reading of the manuscript.
DISCLOSURE
The authors have no financial conflict of interest.
Footnotes
Abbreviations: 15d-PGJ2=15-deoxy-Δ-12,14-prostaglandin J2, ALDH1A2 or aldh1a2=aldehyde dehydrogenase family, member 2, Cig=Ciglitazone, DC=dendritic cell, Foxp3=forkhead box p3, HPRT=hypoxanthine guanine phosphoribosyl transferase, iTreg=induced Treg, LTBP3 or ltbp3=latent TGF-β-binding protein 3, nTreg=natural Treg, PLAT or plat=plasminogen tissue activator, PPARγ=peroxisome proliferator-activated receptor γ, qPCR=quantitative PCR, RA=retinoic acid, Rosi=Rosiglitazone, Teff=effector T cell, Treg=regulatory T cell, VC=vehicle control
References
- Tang Q, Bluestone J A. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson M J, Pino-Lagos K, Rosemblatt M, Noelle R J. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C M, Hall J A, Blank R B, Bouladoux N, Oukka M, Mora J R, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- Coombes J L, Siddiqui K R, Arancibia-Carcamo C V, Hall J, Sun C M, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun R P, Spiegelman B M. PPAR γ and the molecular control of adipogenesis. J Endocrinol. 1997;155:217–218. doi: 10.1677/joe.0.1550217. [DOI] [PubMed] [Google Scholar]
- Chinetti G, Fruchart J C, Staels B. Peroxisome proliferator-activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm Res. 2000;49:497–505. doi: 10.1007/s000110050622. [DOI] [PubMed] [Google Scholar]
- De Vos P, Lefebvre A M, Miller S G, Guerre-Millo M, Wong K, Saladin R, Hamann L G, Staels B, Briggs M R, Auwerx J. Thiazolidinediones repress ob gene expression in rodents via activation of peroxisome proliferator-activated receptor γ. J Clin Invest. 1996;98:1004–1009. doi: 10.1172/JCI118860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R B, Bishop-Bailey D, Estrada-Hernandez T, Hla T, Puddington L, Padula S J. The nuclear receptor PPAR γ and immunoregulation: PPAR γ mediates inhibition of helper T cell responses. J Immunol. 2000;164:1364–1371. doi: 10.4049/jimmunol.164.3.1364. [DOI] [PubMed] [Google Scholar]
- Clark R B. The role of PPARs in inflammation and immunity. J Leukoc Biol. 2002;71:388–400. [PubMed] [Google Scholar]
- Delerive P, Fruchart J C, Staels B. Peroxisome proliferator-activated receptors in inflammation control. J Endocrinol. 2001;169:453–459. doi: 10.1677/joe.0.1690453. [DOI] [PubMed] [Google Scholar]
- Daynes R A, Jones D C. Emerging roles of PPARs in inflammation and immunity. Nat Rev Immunol. 2002;2:748–759. doi: 10.1038/nri912. [DOI] [PubMed] [Google Scholar]
- Natarajan C, Bright J J. Peroxisome proliferator-activated receptor-γ agonists inhibit experimental allergic encephalomyelitis by blocking IL-12 production, IL-12 signaling and Th1 differentiation. Genes Immun. 2002;3:59–70. doi: 10.1038/sj.gene.6363832. [DOI] [PubMed] [Google Scholar]
- Ray D M, Akbiyik F, Bernstein S H, Phipps R P. CD40 engagement prevents peroxisome proliferator-activated receptor γ agonist-induced apoptosis of B lymphocytes and B lymphoma cells by an NF-κB-dependent mechanism. J Immunol. 2005;174:4060–4069. doi: 10.4049/jimmunol.174.7.4060. [DOI] [PubMed] [Google Scholar]
- Natarajan C, Muthian G, Barak Y, Evans R M, Bright J J. Peroxisome proliferator-activated receptor-γ-deficient heterozygous mice develop an exacerbated neural antigen-induced Th1 response and experimental allergic encephalomyelitis. J Immunol. 2003;171:5743–5750. doi: 10.4049/jimmunol.171.11.5743. [DOI] [PubMed] [Google Scholar]
- Shiojiri T, Wada K, Nakajima A, Katayama K, Shibuya A, Kudo C, Kadowaki T, Mayumi T, Yura Y, Kamisaki Y. PPAR γ ligands inhibit nitrotyrosine formation and inflammatory mediator expressions in adjuvant-induced rheumatoid arthritis mice. Eur J Pharmacol. 2002;448:231–238. doi: 10.1016/s0014-2999(02)01946-5. [DOI] [PubMed] [Google Scholar]
- Saubermann L J, Nakajima A, Wada K, Zhao S, Terauchi Y, Kadowaki T, Aburatani H, Matsuhashi N, Nagai R, Blumberg R S. Peroxisome proliferator-activated receptor γ agonist ligands stimulate a Th2 cytokine response and prevent acute colitis. Inflamm Bowel Dis. 2002;8:330–339. doi: 10.1097/00054725-200209000-00004. [DOI] [PubMed] [Google Scholar]
- Woerly G, Honda K, Loyens M, Papin J P, Auwerx J, Staels B, Capron M, Dombrowicz D. Peroxisome proliferator-activated receptors α and γ down-regulate allergic inflammation and eosinophil activation. J Exp Med. 2003;198:411–421. doi: 10.1084/jem.20021384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad H, de Heer H J, Soullie T, Angeli V, Trottein F, Hoogsteden H C, Lambrecht B N. Activation of peroxisome proliferator-activated receptor-γ in dendritic cells inhibits the development of eosinophilic airway inflammation in a mouse model of asthma. Am J Pathol. 2004;164:263–271. doi: 10.1016/s0002-9440(10)63116-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller C, Weaver V, Vanden Heuvel J P, August A, Cantorna M T. Peroxisome proliferator-activated receptor γ ligands attenuate immunological symptoms of experimental allergic asthma. Arch Biochem Biophys. 2003;418:186–196. doi: 10.1016/j.abb.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Ueki S, Matsuwaki Y, Kayaba H, Oyamada H, Kanda A, Usami A, Saito N, Chihara J. Peroxisome proliferator-activated receptor γ regulates eosinophil functions: a new therapeutic target for allergic airway inflammation. Int Arch Allergy Immunol. 2004;134:30–36. doi: 10.1159/000077790. [DOI] [PubMed] [Google Scholar]
- Beales P E, Liddi R, Giorgini A E, Signore A, Procaccini E, Batchelor K, Pozzilli P. Troglitazone prevents insulin dependent diabetes in the non-obese diabetic mouse. Eur J Pharmacol. 1998;357:221–225. doi: 10.1016/s0014-2999(98)00574-3. [DOI] [PubMed] [Google Scholar]
- Augstein P, Dunger A, Heinke P, Wachlin G, Berg S, Hehmke B, Salzsieder E. Prevention of autoimmune diabetes in NOD mice by troglitazone is associated with modulation of ICAM-1 expression on pancreatic islet cells and IFN-γ expression in splenic T cells. Biochem Biophys Res Commun. 2003;304:378–384. doi: 10.1016/s0006-291x(03)00590-4. [DOI] [PubMed] [Google Scholar]
- Hontecillas R, Bassaganya-Riera J. Peroxisome proliferator-activated receptor {γ} is required for regulatory CD4+ T cell-mediated protection against colitis. J Immunol. 2007;178:2940–2949. doi: 10.4049/jimmunol.178.5.2940. [DOI] [PubMed] [Google Scholar]
- Wohlfert E A, Nichols F C, Nevius E, Clark R B. Peroxisome proliferator-activated receptor γ (PPARγ) and immunoregulation: enhancement of regulatory T cells through PPARγ-dependent and -independent mechanisms. J Immunol. 2007;178:4129–4135. doi: 10.4049/jimmunol.178.7.4129. [DOI] [PubMed] [Google Scholar]
- Faveeuw C, Fougeray S, Angeli V, Fontaine J, Chinetti G, Gosset P, Delerive P, Maliszewski C, Capron M, Staels B, Moser M, Trottein F. Peroxisome proliferator-activated receptor gamma activators inhibit interleukin-12 production in murine dendritic cells. FEBS Lett. 2000;486:261–266. doi: 10.1016/s0014-5793(00)02319-x. [DOI] [PubMed] [Google Scholar]
- Klotz L, Dani I, Edenhofer F, Nolden L, Evert B, Paul B, Kolanus W, Klockgether T, Knolle P, Diehl L. Peroxisome proliferator-activated receptor γ control of dendritic cell function contributes to development of CD4+ T cell anergy. J Immunol. 2007;178:2122–2131. doi: 10.4049/jimmunol.178.4.2122. [DOI] [PubMed] [Google Scholar]
- Szatmari I, Pap A, Ruhl R, Ma J X, Illarionov P A, Besra G S, Rajnavolgyi E, Dezso B, Nagy L. PPARγ controls CD1d expression by turning on retinoic acid synthesis in developing human dendritic cells. J Exp Med. 2006;203:2351–2362. doi: 10.1084/jem.20060141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D, Campbell J I, King T P, Grant G, Jansson E A, Coutts A G, Pettersson S, Conway S. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-γ and RelA. Nat Immunol. 2004;5:104–112. doi: 10.1038/ni1018. [DOI] [PubMed] [Google Scholar]
- Dubuquoy L, Rousseaux C, Thuru X, Peyrin-Biroulet L, Romano O, Chavatte P, Chamaillard M, Desreumaux P. PPARγ as a new therapeutic target in inflammatory bowel diseases. Gut. 2006;55:1341–1349. doi: 10.1136/gut.2006.093484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Jin W, Hardegen N, Lei K J, Li L, Marinos N, McGrady G, Wahl S M. Conversion of peripheral CD4+CD25– naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song S Y. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Ray D M, Akbiyik F, Phipps R P. The peroxisome proliferator-activated receptor gamma (PPARgamma) ligands 15-deoxy-Delta12,14-prostaglandin J2 and ciglitazone induce human B lymphocyte and B cell lymphoma apoptosis by PPARgamma-independent mechanisms. J Immunol. 2006;177:5068–5076. doi: 10.4049/jimmunol.177.8.5068. [DOI] [PubMed] [Google Scholar]
- Leesnitzer L M, Parks D J, Bledsoe R K, Cobb J E, Collins J L, Consler T G, Davis R G, Hull-Ryde E A, Lenhard J M, Patel L, Plunket K D, Shenk J L, Stimmel J B, Therapontos C, Willson T M, Blanchard S G. Functional consequences of cysteine modification in the ligand binding sites of peroxisome proliferator activated receptors by GW9662. Biochemistry. 2002;41:6640–6650. doi: 10.1021/bi0159581. [DOI] [PubMed] [Google Scholar]
- Svensson M, Johansson-Lindbom B, Zapata F, Jaensson E, Austenaa L M, Blomhoff R, Agace W W. Retinoic acid receptor signaling levels and antigen dose regulate gut homing receptor expression on CD8+ T cells. Mucosal Immunol. 2008;1:38–48. doi: 10.1038/mi.2007.4. [DOI] [PMC free article] [PubMed] [Google Scholar]