Abstract
Although cranial sutures presumably play a role in absorbing and/or transmitting loads applied to the skull, loading patterns on facial sutures are poorly understood. The zygomatic arch provides a comparatively isolated mechanical part of the skull containing a single suture, the zygomatico-squamosal. In pigs the zygomatico-squamosal suture has a short vertical segment located within the postorbital process and a longer horizontal segment which extends posteriorly. In anesthetized pigs single-element high-elongation strain gages were bonded over both segments of the suture. Strain was recorded during stimulation of the masseter muscles and while the lightly anesthetized animals masticated food pellets. The predominant strain patterns differed in the two segments of the suture. During mastication compressive strains predominated in the vertical segment, but tensile strains predominated in the horizontal segment. The same patterns were also produced by stimulation of the ipsilateral masseter muscle. Contraction of the contralateral masseter reversed the strain pattern, but strain levels were low and during mastication such reversals occurred only transiently. The two segments of the suture have contrasting morphologies. The vertical segment has broad, interdigitating contacts with fibers arranged in a compression-resisting orientation. The horizontal segment has a simple tongue and groove structure with fibers arranged to resist tension. Thus, the structure of the suture reflects the predominant strain pattern.
A considerable body of literature suggests that both the morphology and the persistence of cranial sutures are influenced by mechanical stress. For the most part, however, the normal loading environment of sutures is unknown. Loading has often been inferred from sutural anatomy. For example, the degree of interdigitation of the adjoining bony surfaces has been correlated with levels of cranial stress (Herring, '72; Jaslow, '89). Interpretation of the nature of the load (e.g., compression vs. tension) is not possible using bony morphology alone, however, as the loads may be borne by intrasutural fibers that can be arranged in various orientations. Even if the arrangement of fibers is known, the loading pattern may not be clear. For example, the histological organization of braincase sutures has been used to argue both that the vault is loaded under tension (Smith and McKeown, '74) and that it is loaded under compression (Prahl-Andersen, '68).
Only a few studies on in vivo loading of sutures have been carried out, and these have involved sutures of the braincase rather than of the face. Behrents et al. ('78) reported tensile strain in the sagittal sutures of two juvenile rhesus monkeys whenever the temporalis muscles were stimulated. In an in vitro study of strain in the goat skull during impact loading, Jaslow ('87) found that braincase sutures were either compressed or bent, depending on the direction of the impact. She found higher levels of strain across the sutures than on adjacent bone and suggested that the sutures functioned to dissipate stresses transmitted through the skull. Bending was also found by Smith and Hylander ('85) in the mesokinetic joint (fronto-parietal suture) during feeding in the savanna monitor lizard. In none of these studies was the histological structure of the suture reported, so it is not possible to reconcile these loading patterns with previous controversies on cranial stress.
The present study was undertaken as part of a systematic investigation of bone strain and muscle function in a fairly simple segment of the face, the zygomatic arch of pigs (Sus scrofa). The pig zygomatic arch is a beam-like structure interrupted by a single suture between the zygomatic bone and the zygomatic process of the squamosal bone. Whereas the arch could theoretically be loaded solely through its roots (e.g., rostrally from the dentition or caudally from the jaw joint), the major mechanical influence on the arch is the attaching musculature, mm. masseter and zygomaticomandibularis (Hylander and Johnson, '89). The activity patterns of these muscles are comparatively well studied (Herring et al., '79; Herring and Wineski, '86). Thus, its mechanical isolation and known sources of loading make the zygomatico-squamosal suture a good model for this initial investigation of in vivo strain levels in facial sutures.
The purposes of this contribution are 1) to report loading patterns on the zygomatico-squamosal suture of pigs during normal and stimulated muscle function and 2) to correlate loading patterns with the histological structure of the suture. In brief, we find that the strain pattern is indeed dominated by the attaching masseter muscle, that one part of the suture is loaded under compression while another is tensed, and that sutural morphology correlates well with the predominant loading pattern.
Materials and Methods
Experimental animals
All observations were made on male miniature pigs of the Hanford strain, obtained from Charles River Laboratories (Wilmington, MA). Five animals, ranging in weight from 12 to 48 kg, were used for recordings of sutural strain during stimulation of the masseter muscles. Three of these also were used for in vivo recordings of strain during reflexive mastication. Simultaneous electromyographic recordings (EMG) of the masseters were made in two of these three. A sixth animal (6.5 kg) was used for histological studies. Further anatomical observations were made on the dried skulls of pigs ranging in weight from 2 to 60 kg (corresponding to ages of 2 weeks to about 1 year).
Recording procedures
Single-element foil strain gages were prepared by soldering 36-gauge leadwires in a three-wire configuration. Gage insulation was provided by successive coatings of polyurethane and acrylic lacquer (M-Coat A, M-Coat D, Micro-Measurements, Raleigh, NC). In the first two experiments, general purpose gages (CEA-13-250UW-120, Micro-Measurements) were used. Because of concerns that these gages would not accurately record the high strains expected in the suture, all later experiments used high-elongation gages (EP-08-125BT-120, Micro-Measurements).
Pigs were anesthetized with halothane-nitrous oxide. Body temperature was controlled with a heating pad. The skin over the zygomatic arch was incised and reflected. The periosteum was removed from the lateral surface of the zygomatic and squamosal bones. The musculature of the arch, which attaches only to its medial and ventral sides, was not exposed or affected by this procedure. The surfaces were scraped, cauterized, abraded smooth, degreased with ether, and then neutralized (M-Prep Neutralizer 5, Micro-Measurements). Prepared strain gages were bonded using cyanoacrylate cement and catalyst (M-Bond 200, Micro-Measurements). Except for the first two experiments, the gages were bonded to the adjacent bones but were isolated from the suture by a 3.175 mm (the length of the element) wide strip of teflon tape (Fig. 1). Thus, displacement of the suture resulted in uniform deformation of the foil element, and a reading of 1,000 microstrain (0.1% strain) corresponded to a movement of 3.175 μm across the suture. In these experiments three gages were applied to each suture, one gage over the vertical segment and two over the horizontal segment (Fig. 1). In two pigs both zygomatic arches were instrumented.
Fig. 1.
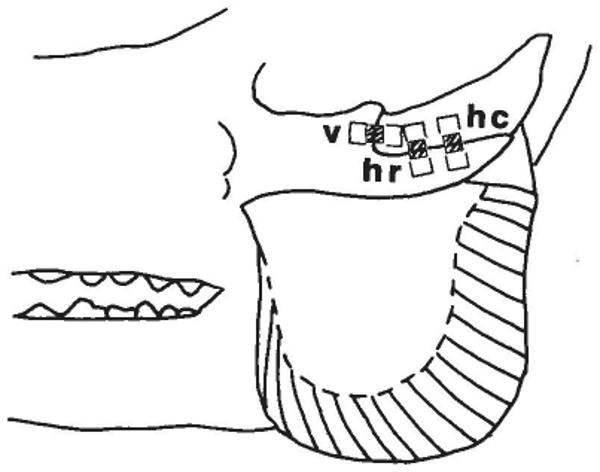
Diagram of the zygomatic arch and masseter muscle of the pig, showing the location of uniaxial strain gages placed over the zygomatico-squamosal suture. Note differing muscle fiber orientations along the rostrocaudal axis of the masseter. The gage elements were aligned along the long axis of each rectangle. The hatched area in the center of each rectangle shows the area (underlying the gage element) which was not bonded to the underlying tissue. v = vertical segment; hr = horizontal segment, rostral portion; hc = horizontal segment, caudal portion.
Gages were excited at 400 mV DC and connected to Wheatstone bridge balance boxes. Output was amplified with op-amp IC's wired as instrumentation amplifiers and the gain adjusted so that an output of 1 V corresponded to 1,000 microstrain (shunt calibrated with precision resistors). Gage excitation and amplifier power were provided by dry cells with common center ground; drift and AC noise were thus negligible even with this high gain. Strain signals were displayed on Tektronix 5113 dual-beam storage oscilloscopes and recorded magnetically via FM modules (DC-1250 Hz) installed in a Hewlett Packard tape recorder. Up to six strain channels were recorded simultaneously. For analysis, the recorded signals were played back and strain measurements were made from photographs of the oscilloscope screen. Compressive strains were expressed as negative values, tensile strains as positive values.
EMG activity from the center of the masseter muscles was recorded with bipolar electrodes (0.05 mm nickel-chromium wire, 1 mm exposed tip). The two wires were glued together along their length and inserted using 27-gauge hypodermic needles. Electrodes were led to a Grass polygraph. Signals were amplified to 1–2 V, displayed on the oscilloscope, and recorded via direct record modules (50–16,000 Hz) installed in the tape recorder. The recordings were used for temporal correlation with strain signals.
For in vivo recordings the anesthetic level was allowed to lighten until the pigs became responsive to food (pig chow pellets) placed within their mouths and masticated it. Chewing movements and the screens of the oscilloscopes were videotaped as a split-screen image using two video cameras and a special effects generator (Panasonic WJ 545P). Under this light anesthesia chewing cycles varied from small amplitude opening-and-closing movements to mastication that appeared generally normal but was somewhat slower and less vigorous than in alert animals (Herring and Wineski, '86). No signs of discomfort or favoring one side were seen. After several sequences of mastication had been recorded, the anesthetic level was deepened again for muscle stimulation.
Two needle electrodes were inserted percutaneously into each masseter muscle for electrical stimulation. One electrode was placed near the entry point of the masseteric nerve, near the posterosuperior corner of the muscle (Herring et al., '89) and the other was placed diagonally opposite, at the anterioinferior corner. Tetanus was produced by 500–1,000 msec trains of 0.5 msec pulses at 40 Hz (Grass stimulator with isolation unit). Voltage was adjusted to produce a maximal contraction of masseter and zygomaticomandibularis muscles (typically 20–40 V) without affecting neighboring jaw muscles such as the temporalis (although facial muscles contracted due to the path of the facial nerve across the masseter). This stimulation protocol is assumed to activate the entire muscle complex through the masseteric nerve. Most stimulations were performed with the teeth in occlusion, but trials with bite blocks 2–5 mm thick placed unilaterally or bilaterally between the anterior premolars were also carried out. In the first two experiments the effect of contracting the fibers of the masseter differentially was also investigated. The stimulating electrodes were placed superiorly and inferiorly along 1) the most anterior fibers of the muscle, 2) the central fibers, or 3) the most posterior fibers. The stimulation protocol was similar to that used for the whole muscle except a longer pulse length (5 msec) was used to stimulate the muscle fibers directly, and lower voltage (typically 10 V) was used to assure regional contractions. Following the muscle stimulations the animals were sacrificed; the skulls were cleaned with the gages in place.
Histology
The right zygomatic arch was removed from a freshly killed pig (6.4 Kg) and cleaned of soft tissue. After decalcification in formic acid and sodium citrate, it was embedded in nitrocellulose and sectioned in the horizontal plane at approximately 25 μm. Sections were stained using a variety of connective tissue demonstration methods, including hematoxylin and eosin, picro-indigo carmine, and van Gieson's stain. Picro-indigo carmine was found to be most helpful for showing the orientation of intrasutural fibers.
Results
Anatomy
The external morphology of the zygomatico-squamosal suture is shown in Figure 2. As described previously (Watzek et al., '82), the suture is sharply angled. The rostral part (“vertical segment”) is roughly perpendicular to the tooth row and extends from the superior edge to about the middle of the arch. The two bones are beveled, the zygomatic lying lateral to the squamosal. Therefore, although in lateral view the vertical segment of the suture corresponds in position to the postorbital process, in medial view it is more rostral. The overlapping bony surfaces have interdigitating laminae arranged generally in a chevron pattern (Fig. 3a). The caudal part of the suture (“horizontal segment”) is parallel to the tooth row, the squamosal bone sitting in a shallow groove on the zygomatic. The abutting bony surfaces are smooth, without interdigitations (Fig. 3b). This general morphology was found in all specimens regardless of age.
Fig. 2.
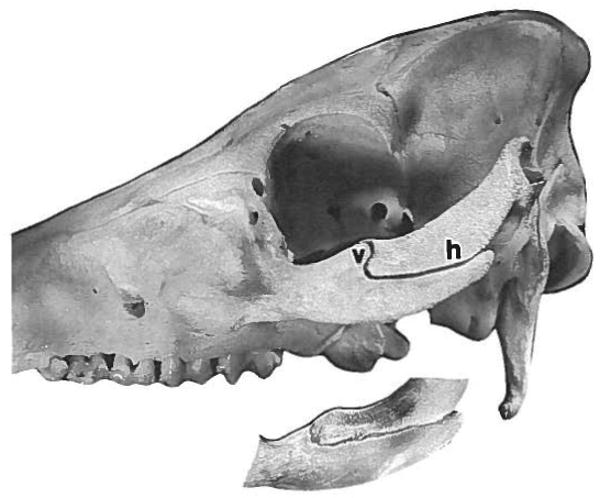
Photograph of the zygomatico-squamosal suture, showing the vertical segment (v) and the horizontal segment (h). The inset shows a medial view of the same suture, taken using a mirror placed in the infratemporal fossa. Note the more rostral position of the vertical segment on the medial side of the zygomatic arch.
Fig. 3.
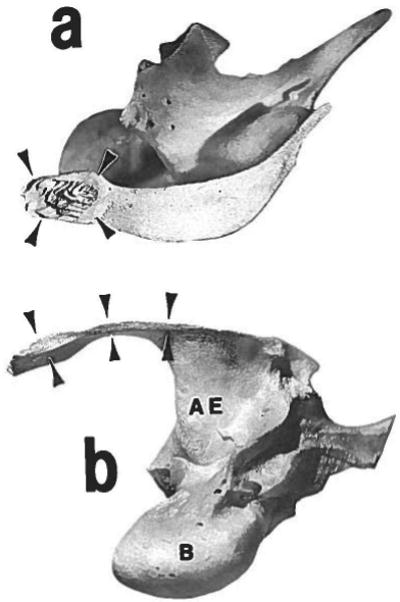
Disarticulated left squamosal bone, seen in lateral aspect (a) and inferior aspect (b). Rostral is to the left. The sutural articular surfaces are marked with arrowheads. The surface for the vertical segment is seen in the lateral view (a). Note the chevron pattern of bony laminae; these interdigitate with similar laminae on the medial side of the zygomatic bone. The surface for the horizontal segment is seen in the inferior view (b); it is nearly smooth, as is the superior surface of the zygomatic bone at this location. AE = articular eminence; B = auditory bulla.
The histological sections showed a strikingly different arrangement of tissues in the two segments of the suture. In the vertical segment (Fig. 4A) the projections of each bone fit conformably into cavities of the other bone. Zygomatic and squamosal bones were closely approximated throughout the segment. Although in some areas the orientation of the intrasutural fibers was complex, in most areas the fibers ran diagonally, attaching rostrally to the squamosal bone and caudally to the zygomatic bone. These fibers would be placed under tension if the squamosal bone were moved rostral relative to the zygomatic bone, i.e., if the vertical segment were under compression. The overall pattern is quite similar to the periodontal ligament, in which fibers are oriented to resist compressive loads tending to force the tooth into the alveolar bone. In contrast, in the horizontal segment the adjacent bony surfaces did not interdigitate and were situated relatively far apart (Fig. 4B). Wherever fiber direction was clear, the fibers ran directly between the territories of the zygomatic and squamosal bones. Thus, the fibers would resist tensile or shearing loads tending to pull the two bones apart.
Fig. 4.
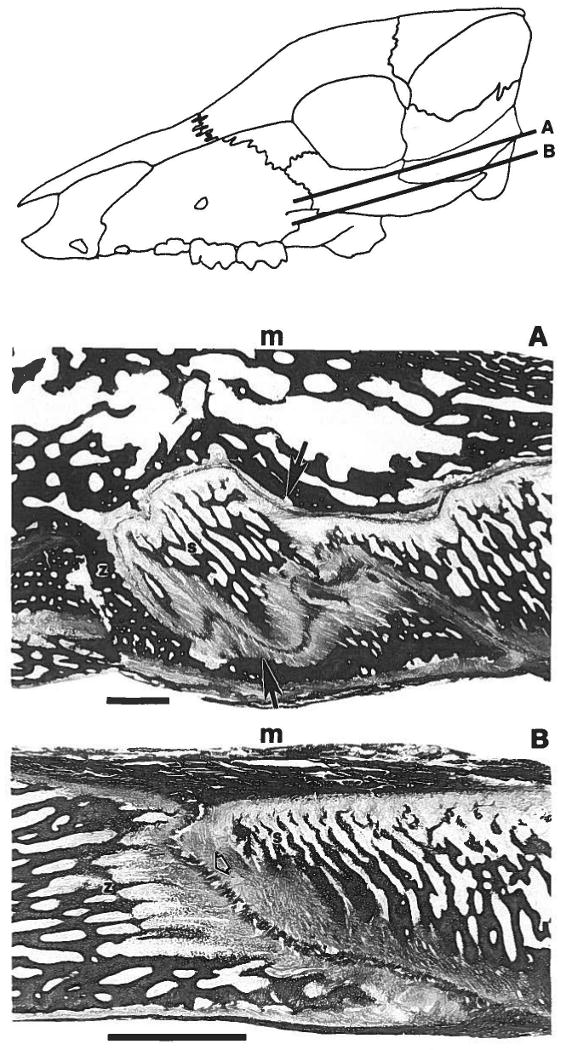
Low power histological sections of the zygomatico-squamosal suture, picro-indigocarmine stain for connective tissue. The drawing at the top of the figure shows the orientation of the horizontal sections shown in A and B. Rostral is to the left in all panels. In A and B medial (m) is toward the top. A: Vertical segment. A projection from the squamosal bone (s) is surrounded by the zygomatic bone (z). The rostral attachment of the sutural fibers (solid arrows) is to the squamosal, the caudal attachment to the zygomatic. These fibers would be placed under tension if the squamosal were moved toward the zygomatic (compressive load to the suture). B: Horizontal segment. Zygomatic (z) and squamosal (s) bones do not interdigitate, and the sutural fibers (open arrowhead) attach rostrally to the zygomatic and caudally to the squamosal. These fibers would be tensed if the squamosal were moved away from the zygomatic (tensile load to the suture). Calibration bars = 1 mm.
Strain during masseter stimulation
Although the patterns of strain were generally clear, the absolute values of strain varied, not only among individuals, but also within individuals whenever measurements were repeated at different times. This variation arose from several sources. Muscle stimulation altered baseline strain values, perhaps because slack sutural fibers were stretched and/or because hemodynamic events occurred in the sutural vessels. The baseline changes were maximal at the beginning of stimulation or after rest periods of 15 or more minutes, but some alterations during the course of a sequence were also observed. Whenever baselines changed, they were re-zeroed. In two cases a decrease in strain was due to deterioration of the gage attachments due to moisture from underlying tissues. Some of the repeated insertions of the stimulating electrodes may not have recruited all masseteric fibers. In addition, the posture of the pig was found to be important. Strain levels changed when the pig was moved from lateral recumbency to a prone position, presumably because transmission of the weight of the head to the table also deforms the skull. For all of these reasons, although we present and discuss absolute values of strain, we emphasize patterns of tension and compression, which were very consistent.
No strain differences were found between standard gages and high elongation gages, so results were grouped. Also, strain values were independent of body size, although a relationship may have been obscured by the variation mentioned above. For all three gage sites, strain levels were similar whenever the ipsilateral masseter was stimulated by itself and whenever both masseters were stimulated; data from these two modes of stimulation were therefore grouped.
Results from the masseteric stimulations are shown in Table 1. The largest strains were always found during ipsi/bilateral stimulations. The gages over the horizontal segment of the suture consistently registered tension (range 250 to 2,500 microstrain for prone animals). Readings with a bite block positioned at the anterior premolars on either or both sides were the same as with the teeth in occlusion. Further, there were no statistically significant differences between the rostral and caudal horizontal segment sites under any conditions. In contrast, strain in the vertical segment of the suture was compressive when the teeth were occluded (range −150 to −1,000 microstrain for prone animals). However, if a bite block was used, strain became more tensile in the vertical segment (range 0 to 750 microstrain). The difference between occlusal and bite block stimulations was significant (P < 0.01; Welch's t-test for samples with unequal variance).
TABLE 1. Maximum strains in the zygomatico-squamosal suture: stimulation of masseters (microstrain: positive = tension; negative = compression).
| Ipsilateral1 | Contralateral | Ipsi vs contra2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Gage site | Mean | SD | n3 | Mean | SD | n | p | ||
| Vertical segment | |||||||||
| Occlusion | −750 | 410 | 7 | 3174 | 161 | 3 | <.01 | ||
| Bilateral bite block | 295 | 324 | 4 | NS | |||||
| [Occlusion vs. bite block2: p < .01] | |||||||||
| Horizontal segment | |||||||||
| Rostral4 | 1,472 | 732 | 10 | −467 | 153 | 3 | <.02 | ||
| Caudal4 | 1,022 | 418 | 9 | −200 | 218 | 3 | <.01 | ||
| [Rostral vs. caudal2: | NS | NS] | |||||||
Including bilateral stimulations, since these did not differ from ipsilateral stimulations.
t-test for two samples with unequal variances. Because the individual measurements within samples were not always independent (some were from the same gage under different modes of stimulation), levels of significance should be taken as indicating trends only.
Maximum values of strain for each mode of stimulation. Although there were 5–6 gages for each site, only a few modes of stimulation were recorded per gage.
Strain values with and without the bite block did not differ and were combined.
Strain patterns were strikingly different when only the contralateral masseter was stimulated (Table 1). The vertical segment registered tension (range 30 to 750 microstrain), while the horizontal segment registered compression (range −50 to −800 microstrain). Thus, the pattern was essentially reversed from that observed during ipsi/bilateral stimulation (in occlusion), although strain levels were much reduced, particularly in the horizontal segment. This contrast is illustrated in Figure 5. The presence of a bite block did not alter these results.
Fig. 5.
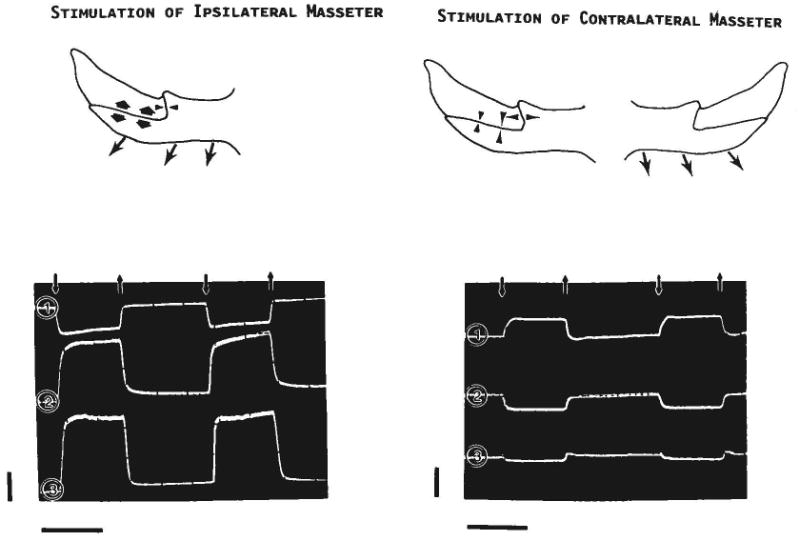
Results of stimulating the ipsilateral (left) or contralateral (right) masseter. The drawings depict strains (arrowheads) seen in the right zygomatico-squamosal suture when the masseter (arrows) contracts. The lower tracings were photographed from the oscilloscope screen (pig 105). Downward small arrows indicate the onset of the stimulus, upward small arrows its offset. Channel 1 is from the gage on the vertical segment of the suture and shows compression (negative values) during ipsilateral stimulation and tension (positive values) during contralateral stimulation. Channels 2 and 3 are, respectively, from the gages at the rostral and caudal locations along the horizontal segment; both show tension during ipsilateral stimulation and compression during contralateral stimulation. Calibration bars: vertical = 1,000 microstrain; horizontal = 1 second.
The effects of altering the line of action of the ipsilateral masseter using partial stimulations with the jaws in occlusion were examined in the first two experiments (pig 87, 48 kg, and pig 90, 12.2 kg, Table 2). Table 2 also compares the most restricted contractions (10 V stimulations) with more general contractions (20 V stimulations) and whole muscle stimulations. Because only one gage per site was used in these studies, the results must be considered tentative even though trends are clear. In direction the strain pattern was the same as that produced by whole muscle stimulation: compression in the vertical segment and tension in the horizontal segment. However, there were differences in strain magnitude. For the vertical segment, strain was least when only the rostral fibers of the masseter were stimulated. These fibers are the most vertical (relative to the tooth row); they pull only slightly backward on the zygomatic bone. When the more backward-pulling middle and caudal fibers were stimulated, compressive strain was roughly doubled, reaching levels that equaled or exceeded those observed during whole muscle stimulation. As in other procedures, the two gages on the horizontal segment registered very similar patterns: rostral fiber stimulation caused the smallest strains and middle fiber stimulation caused the largest strains. Strains in the horizontal segment resulting from partial contractions of the masseter were always smaller than those from whole muscle stimulation.
TABLE 2. Strain in the zygomatico-squamosal suture: effect of partial muscle contraction, jaws in occlusion.
| Ipsilateral Masseter (microstrain) | ||||||
|---|---|---|---|---|---|---|
| Pig No. | Gage site | Voltage (V) | Rostral fibers | Middle fibers | Caudal fibers | Whole muscle |
| 87 | Vertical | 10 | −500 | −1,250 | −1,000 | −950 |
| 20 | −600 | −1,300 | −1,300 | −1,350 | ||
| 90 | Horizontal rostral | 10 | 50 | 450 | 160 | 850 |
| 20 | 80 | 800 | 250 | 850 | ||
| 90 | Horizontal caudal | 10 | 50 | 400 | 150 | 1,200 |
| 20 | 120 | 800 | 250 | 1,200 | ||
Strain during mastication
The three pigs recorded during mastication varied in their performance while under light anesthesia; thus, these results will be discussed separately.
Pig 105 (16.5 kg) made regular but very low amplitude jaw movements with no detectable lateral deviations. Electromyography was not carried out on this animal. As shown in Table 3, the strains recorded from the suture were small, especially compared to the values recorded during ipsilateral stimulation of the masseter muscle. The strain patterns, however, were the same as those observed during ipsilateral stimulation: compression in the vertical segment and tension in the horizontal segment. Right and left sides of this bilaterally instrumented animal were similar in pattern. Interestingly, the level of compression in the vertical part was higher than the level of tension in the horizontal part; this is in contrast to the results for stimulations.
TABLE 3. Comparison of peak sutural strains during mastication and during bilateral masseter stimulation in occlusion (means and standard deviation in microstrain, sample sizes).
| Gage Site | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vertical | Horizontal rostral | Horizontal caudal | ||||||||
| Animal/side | Mast | Stim1 | p | Mast | Stim1 | p | Mast | Stim1 | p | |
| 105 left | M | −142 | −194 | NS | 81 | 1,325 | <.001 | 38 | 950 | <.05 |
| SD | 48 | 116 | 53 | 275 | 48 | 477 | ||||
| n | 8 | 4 | 8 | 4 | 4 | 3 | ||||
| 105 right | M | −84 | −600 | — | 67 | 1,017 | <.02 | 0 | 617 | <.05 |
| SD | 20 | — | 32 | 457 | 35 | 340 | ||||
| n | 6 | 1 | 7 | 3 | 5 | 3 | ||||
| 104 left | M | −778 | 253 | 416 | ||||||
| SD | 467 | 249 | 176 | |||||||
| n | 16 | 9 | 16 | |||||||
| 104 right | M | −302 | 915 | 1,723 | ||||||
| SD | 141 | 740 | 1,425 | |||||||
| n | 16 | 13 | 11 | |||||||
| 107 right | M | −1,118 | −800 | <.01 | 1,075 | 1,800 | <.05 | 1,476 | 550 | NS |
| SD | 251 | 141 | 385 | 424 | 678 | 71 | ||||
| n | 31 | 2 | 31 | 2 | 31 | 2 | ||||
A series of stimulations always resulted in identical strain values. Sample size for stimulations refers to the no. of separate insertions of stimulating electrodes into the masseter.
Pig 104 (32 kg) chewed more vigorously than Pig 105, and showed distinct lateral movements. Electromyography indicated a rather unusual contraction pattern of the masseters (Fig. 6; compare with Fig. 7). Right and left masseters were frequently asynchronous. Many cycles, such as the ones shown in Figure 6, began with activity in the right masseter (corresponding to a closing and leftward movement of the mandible), which was followed by a burst from the left masseter (mandible moving to the right), and then a final burst from the right masseter (mandible moving leftward again before opening). Because this pattern incorporates an extra lateral deviation from normal pig mastication (cf. Herring, '76) and the position of the bolus cannot be seen on the videotape, it is difficult to specify the working side. Masticatory strains were much higher than in Pig 105, although the directionality of the strain pattern was the same—compression in the vertical segments and tension in the horizontal segments. Unfortunately, the gages on this animal deteriorated before the muscles could be stimulated, so spontaneous and stimulated strain values could not be directly compared. However, comparisons can be made with the average stimulation values shown in Table 1. On the left side, peak strain in the vertical segment was the same as average stimulation values, while strains in the horizontal segment were much lower than average stimulation values (P < 0.001 at both locations, t-test adjusted for unequal variance). On the right side, compression in the vertical segment was somewhat less than average stimulation values (P < 0.02), but tension in the horizontal segment did not differ from stimulation values.
Fig. 6.
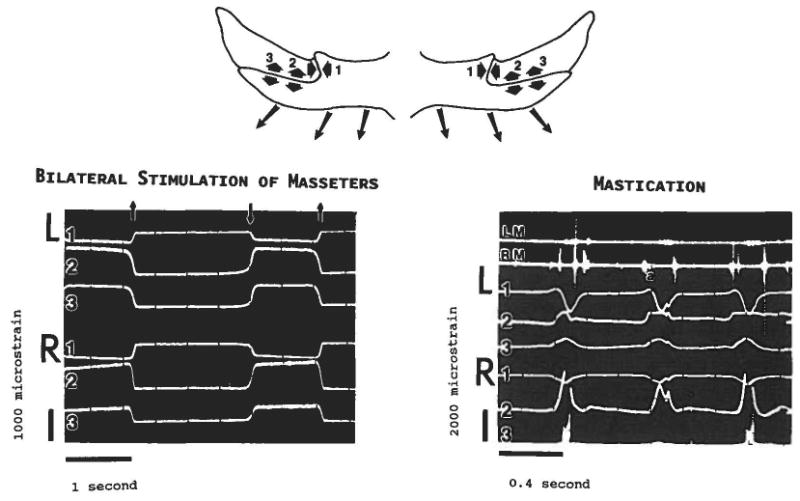
Comparison of bilateral stimulation of the masseters (pig 105) with spontaneous masticatory activity (pig 104). Conventions as in Figure 5. Strains from both left (L) and right (R) sutures are shown. Note the difference in calibration bars. In the oscilloscope tracing of mastication, the top two channels are EMG from the left (LM) and right (RM) masseters. Three masticatory cycles are included. The predominant strain pattern for mastication is the same as for bilateral stimulation: compression in the vertical segments (left and right channel 1) and tension in the horizontal segments (left and right channels 2 and 3 [right channel 3 is partly cut off on the mastication tracing]). However, because the masseter activity is asynchronous during spontaneous movements, the “reversed” pattern can be briefly detected. For example, at a the right masseter has a burst of activity; this activity is accompanied by a small tensile transient in the vertical segment of the left (contralateral) suture (channel L1).
Fig. 7.
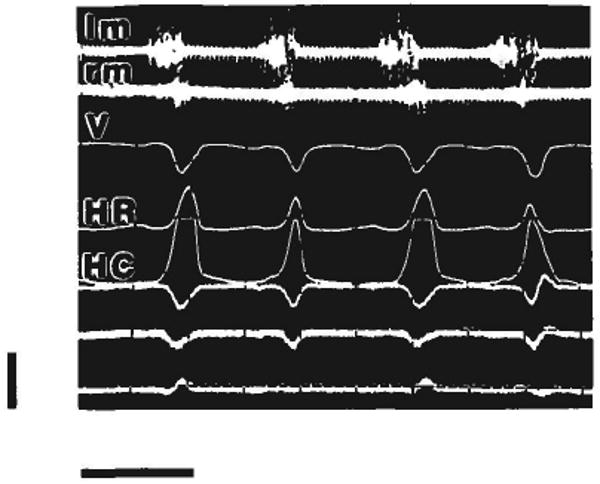
Mastication in pig 107, showing an essentially normal pattern of muscle coordination. Strain gages were on the right suture, lm and rm = left and right masseter EMG; V = strain from vertical segment; HR = strain from rostral part of horizontal segment; HC = strain from caudal part of horizontal segment. The bottom three channels register strain from another area of the skull and are not considered here. Of the four masticatory cycles included, the working side was on the right for the first and third and on the left for the second and fourth. When the working side was on the right (ipsilateral to the gages), horizontal segment strains were larger and vertical segment strains were earlier. Calibration bars: vertical = 2,000 microstrain; horizontal = 0.4 second.
The asynchronous nature of muscle contraction in pig 104 allows a more detailed view of strain in the zygomatico-squamosal suture. When the contralateral masseter was active, transient opposite-sign strains could be seen in most channels. For example, in Figure 6, when the right masseter begins the cycle (e.g., at “a” in the second of three cycles in Fig. 6), a tensile transient is seen in the vertical segment of the left suture (channel L1), while the gages on the left horizontal segment (channels L2, L3) show a slight compressive dip. These patterns resemble the strains produced by stimulation of the contralateral masseter (Fig. 5).
Pig 107 (13 kg) showed the most normal chewing pattern of the three animals, with overlapping bursts of activity in the right and left masseters and regular alternation of the working side (Fig. 7 and compare with Herring et al., '79). However, mastication was slower than in unanesthetized animals (about 2 Hz, as opposed to 3 Hz) and may not have been as forceful. Once again, a predominant pattern of compression in the vertical segment of the suture and tension in the horizontal segment was found. Surprisingly, compression in the vertical segment was greater during mastication than during muscle stimulation (Table 3). For the rostral gage on the horizontal segment, tension levels were higher during stimulation than during mastication. No difference could be shown for the caudal gage, which sometimes gave underestimates due to accidental saturation of the channel.
Right and left working cycles of pig 107 are compared in Table 4. No differences in strain level were seen except for the rostral gage on the horizontal segment, which registered greater strain when the working side was ipsilateral to the gage (on the right). As in pig 104, opposite-sign transients can be seen when the contralateral (left) masseter begins the cycle (for example, the first and third cycles in Fig. 7; in these cycles the left masseter is moving the mandible toward the working [right] side). A difference in timing can also be seen in Figure 7. Peak strain in the vertical segment precedes that in the horizontal segment when the chewing side is ipsilateral to the gage (right side, cycles 1 and 3 in Fig. 7) but is later when chewing is contralateral (left side, cycles 2 and 4).
TABLE 4. Sutural strain in pig 107 right arch: comparison of right- and left-sided chewing cycles (means and standard deviations in microstrain, sample sizes).
| Gage Site | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Vertical | Horizontal rostral | Horizontal caudal | |||||||
| Working side | Mean | SD | n | Mean | SD | n | Mean | SD | n |
| Right | −1,079 | 229 | 14 | 1,353 | 281 | 14 | 1,539 | 698 | 14 |
| Left | −1,150 | 270 | 17 | 847 | 302 | 17 | 1,424 | 678 | 17 |
| Right vs. left | NS | p < .001 | NS | ||||||
Discussion
Predominant strain pattern
The masseter originates from the zygomatic bone and pulls it ventrally, caudally and medially. The vertical segment of the zygomatico-squamosal suture apposes the squamosal bone to both medial and caudal surfaces of the zygomatic. If the strains across this segment are caused by the masseter, these contacts should be loaded compressively. In the horizontal segment of the suture, the squamosal is situated dorsal to the zygomatic, and thus the ventral pull of the masseter on the zygomatic should cause tension. The medial pull of the masseter would tend to shear the zygomatic medially relative to the squamosal; on the lateral surface of the arch such a shearing load would also register as tension. The zygomaticomandibularis muscle was presumably stimulated as well, as it has a common innervation with the masseter (Herring et al., '89). This smaller muscle originates from both bones and therefore is not expected to deform the suture predictably.
Stimulations of the musculature uniformly resulted in the strain pattern predicted for the masseter muscle. The fact that strain during spontaneous activity was for the most part indistinguishable from ipsilateral muscle stimulation indicates that the strain pattern in the zygomatic arch is indeed dominated by the attaching musculature. Loads transmitted from more distant parts of the skull could only be detected when the ipsilateral masseter was inactive (see below), a comparatively rare occurrence for pigs.
It is possible to compare our results qualitatively with the only previous strain measurements on pig facial bones (Fisher et al., '76). That study of simulated muscle forces on skulls included rosette gages on the zygomatic bone; the directions of strain resemble our findings: compression along the rostrocaudal axis of the arch and tension along the dorsoventral axis.
The absolute values of sutural strain during muscle stimulation were typically 1,000–2,000 microstrain, corresponding to absolute deformations on the order of 2–6 μm. However, these values are underestimates of true deformation because 1) we did not compensate for the resistance of the lead wires, which could have resulted in measurements 5–10% lower than the actual strains; 2) the strain gages were positioned orthogonal to the suture, whereas the postulated load from the masseter is at an angle; thus only the craniocaudal (for the vertical segment) or dorsoventral (for the horizontal segment) component of the actual deformation was measured; shearing deformations parallel to the suture segments would appear as tension; and 3) the total area of the gage element was not bonded, and thus during compressive movements the element may have bent slightly. Strains of 1,000–2,000 microstrain were also recorded in Jaslow's ('87) in vitro study of impact loading in goat braincase sutures, although those reported by Behrents et al. ('78) in the macaque sagittal suture after stimulation of the temporalis were an order of magnitude smaller. Our values form a reasonable intermediate between the low strains (hundreds of microstrain) usually reported from facial bones (e.g., Hylander and Johnson, '89) and the high strains (up to 14,000 microstrain) found by Smith and Hylander ('85) in the fronto-parietal sutural “joint” of the savanna monitor lizard. Thus, the zygomatico-squamosal suture of pigs appears to be a location where limited movement occurs in the skull. In the absence of information about the modulus of elasticity of the suture as compared to the bone, it is impossible to say whether the suture functions to dissipate stresses transmitted to the skull, as suggested by Jaslow ('87).
Influence of muscle fiber direction
The masseter of the pig has fibers of varying orientation, and previous studies have shown that the fibers are not simultaneously recruited. Rather, different groups of muscle fibers are activated, altering the fine of action of the muscle for different jaw movements (Herring et al., '79). The rostral fibers are used for simple vertical movements and for producing occlusal force, whereas the caudal fibers are recruited only when the mandible is moving toward the opposite side, i.e., the contraction of caudal fibers on the right side is associated with deviations to the left. These variations should affect the strain pattern in the zygomatic arch.
Although the muscle fiber directions change gradually (Fig. 1), as a first approximation the fibers can be grouped into three categories. 1) The rostral fibers pull ventrally on the zygomatic bone well in front of the suture (Fig. 8a). Because of their vertical orientation, they should primarily load the horizontal segment; however, they are remote from this part of the suture. The results in Table 2 suggest that the rostral fibers are not particularly effective in loading either sutural segment. In particular, the horizontal segment is nearly unstrained. 2) The middle fibers represent the average fiber direction of the whole muscle and should strongly load both segments (Fig. 8b), as Table 2 confirms. 3) The caudal fibers pull in a direction close to the long axis of the arch. Thus they should primarily load the vertical segment of the suture (Fig. 8c), as also is indicated by Table 2.
Fig. 8.
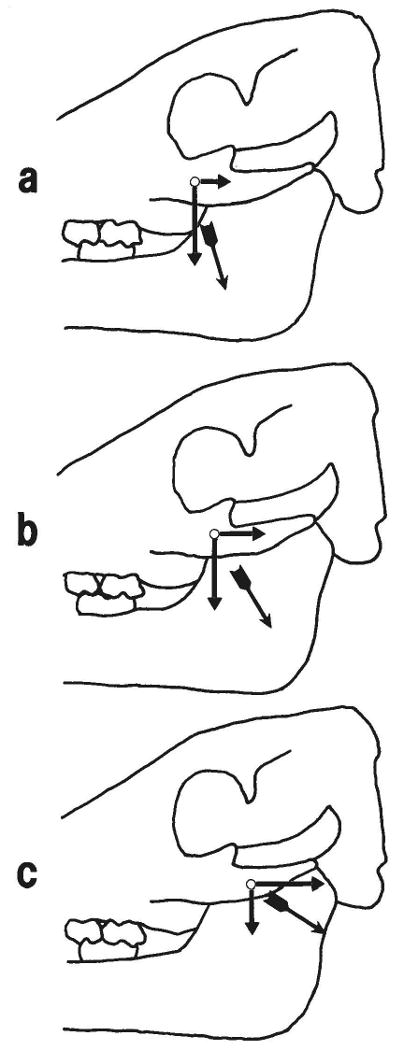
Interpretation of the effect of partial masseter contraction on strain in the zygomatico-squamosal suture. a: Contraction of rostral fibers, such as occurs during simple closing movements of the mandible (e.g., pig 105) or on the balancing side in unilateral mastication (e.g., left side in pig 104). Caudal and ventral components of the muscle force are shown acting on the zygomatic bone. The horizontal component, assumed to be responsible for compression in the vertical segment of the suture, is relatively small. The ventral component, assumed to be responsible for tension in the horizontal segment of the suture, is large, but it is distant from the horizontal segment. Thus strains are low in both parts of the suture. b: Contraction of middle fibers. These fibers represent the average pull of the whole muscle; thus strains produced are similar to those produced by the masseter on the working side during mastication (e.g., right side of pig 104; pig 107). Both caudal (compressing the vertical segment) and ventral (tensing the horizontal segment) components of the muscle force are large, c: Contraction of caudal fibers, seen when the working side masseter moves the mandible toward the balancing side at the end of the power stroke of mastication. The caudal component of force is large, while the ventral component is small, accounting for high compressive strain in the vertical segment of the suture but low tensile strain in the horizontal segment.
Although we were unable to record from multiple sites in the masseter in this study, the recordings from masticating animals provide many examples in which differential contraction of the masseter almost certainly modified the observed strains. In pig 105 vertical segment strains were larger than horizontal strains (Table 3), and the overall pattern is similar to that produced by contraction of the rostral fibers only (Fig. 8a; Table 2). This pig performed simple vertical movements. In the illustrated sequence from pig 104 (Fig. 6), the left suture shows a similar pattern, suggesting that only the rostral fibers were active, whereas the strain pattern of right suture suggests that the whole muscle was active (Fig. 8b).
Pig 107 exhibited normal chewing coordination with nearly simultaneous activation of the right and left sides, so the within-masseter contraction sequences were probably normal as well. In the power stroke of normal mastication, the middle and caudal fibers of the masseter are progressively recruited on the working side only, corresponding to the movement toward the opposite side (Herring et al., '79; Herring and Wineski, '86). This differential activity accounts for the higher horizontal segment strains seen in ipsilateral (right side working) chews (Table 4). Furthermore, the sequential recruitment of working-side caudal fibers (i.e., the change from Fig. 8b to c) accounts for the later peak strains in the vertical segment when the working side was ipsilateral to the gage (Fig. 7).
Thus, although all fibers of the masseter contribute to the predominant strain pattern in the zygomatico-squamosal suture, the pattern is apparently modulated by the details of differential activation.
Other strain patterns
Although compression in the vertical segment and tension in the horizontal segment was the predominant strain pattern, it was not the only one we observed.
During ipsilateral masseter stimulations, if a bite block was used at the premolars of either or both sides, tensile strain was registered in the vertical as well as the horizontal segment. This pattern could have been produced by a dorsal movement of the squamosal bone relative to the zygomatic bone. One possible explanation is that the bite block establishes a more rostral bite point than does occlusion (anterior premolars rather than molars). Given third-class lever mechanics, the more distal bite point should decrease bite force. However, the stimulated muscle force is constant, and it must be balanced by an increased dorsal load from the mandibular condyle onto the articular surface of the squamosal bone. Such an increased joint load could have caused the observed strain pattern. This all-tension pattern was not seen during mastication; however, the pellets eaten by the pigs required no processing by the anterior teeth. Possibly the pattern would have been seen if we had observed incision, The all-tension pattern might also have been produced by a lateral movement of the zygomatic relative to the squamosal; however, it is not obvious how a bite block could cause such a movement.
The second unusual strain pattern was produced spontaneously as well as during stimulations. This was the reversal pattern seen when only the contralateral masseter was active, with tension in the vertical segment and compression in the horizontal segment. Strains in the reversal pattern were always low, suggesting dissipation of loads from distant parts of the skull. In mastication the reversal pattern was fleeting, occurring primarily at the beginning of asymmetric movements when only the contralateral masseter was active. Evidently, the reversal pattern was soon overpowered by contraction of the ipsilateral masseter. The reversal pattern suggests that the squamosal bone was moving ventrally and caudally relative to the zygomatic. Such a pattern might result from the asymmetrical movement of the mandible caused by contralateral masseteric activity (Fig. 9) or from torsion of the braincase due to the upward joint force on the contralateral squamosal bone.
Fig. 9.
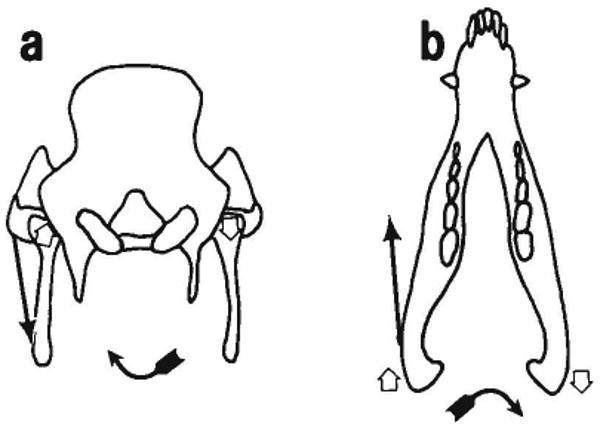
Suggested cause of the reversal strain pattern (tension in the vertical segment and compression in the horizontal segment). Contraction of the left masseter causes the reversal pattern on the right zygomatico-squamosal suture. In occipital view (a) the masseter causes the mandible to rotate about a sagittal axis. The left condyle is forced dorsad against the left squamosal, causing tension in the horizontal segment (the dominant pattern). However, the right condyle comes away from the right squamosal; tension in the capsule would place a ventral load on the right squamosal, compressing the horizontal segment (the reversal pattern). In occlusal view (b) it can be seen that contraction of the left masseter also causes the mandible to rotate around a vertical axis. The left condyle pushes the left squamosal rostrally, compressing the vertical segment (the dominant pattern). However, the right condyle moves caudally, putting caudal loads on the squamosal through articular soft tissues and tensing the vertical segment (the reversal pattern).
Correlation with structure
The predominant pattern of compression in the vertical segment of the suture and tension in the horizontal segment corresponds very closely with the differing morphologies of the two segments. The critical feature is the orientation of the intrasutural fibers. In the compression-resisting segment they run obliquely between interdigitating laminae, analogously to the periodontal ligament; whereas in the tension-resisting segment they run directly from bone to bone (cf. Herring, '72). It should be noted that tension resistance includes resistance to shearing loads tending to move the bones past each other. In both segments, the fibers would be placed under tension by the predominant strains. This finding supports the use of fiber orientation as an indicator of functional stress as suggested by Prahl-Andersen ('68). An analogous correlation in the human frontozygomatic suture was reported by Kokich ('76), who found fibers running parallel to the masseter muscle, presumably the source of a net tensile load in that suture. An association between fiber orientation and functional stress is also consistent with in vivo studies showing that suture morphology remodels readily in response to extrinsic forces (Southard and Forbes, '88; for a review see Wagemans et al., '88) and with in vitro studies reporting increases in the synthesis of type III collagen under tensile loads (Meikle et al., '82; Yen et al., '90). Nevertheless, the alternative suggestion, that fiber direction reflects growth trajectories of the bones (Koskinen et al., '76), is not refuted by our results.
In the vertical segment the bony arrangement also reflects the compressive load. This is true both on a gross level, with the overlap of the squamosal onto the medial and caudal surfaces of the zygomatic, and on a histological level, with the interdigitating processes that would block slippage in most directions. Furthermore, the interdigitations increase the surface area available for fibrous attachments (Herring, '72). Surprisingly, the horizontal segment shows no such bony adaptations, although the tensile strains observed here were as large or larger than the compressive strains in the vertical segment. This result is contrary to the prevailing opinion that sutural complexity is related to the level, but not to the direction, of cranial stress (Moss, '57; Herring, '72; Gottlieb, '78; Jaslow, '89). Several interpretations are possible, including 1) tensile strains require less morphological adaptation in sutures than do compressive strains, and 2) the actual compressive strain in the vertical segment would have been much greater had it lacked bony complexity. Distinguishing among these and other hypotheses will have to be the work of future studies.
It is remarkable that even though the predominant strain pattern is not the only strain pattern that occurs in this suture, the fibrous morphology appears to be specifically adapted to it. That is, we detected no fibers in the vertical segment that would resist tension (although there were some areas without clear orientation), and the bony morphology of the horizontal segment does not permit any useful arrangement of fibers to resist compression. It seems unlikely that the bones themselves would be subjected to important compressive loads, but the bony arrangement in both segments could serve the purpose. The interdigitations of the vertical segment block cranial as well as caudal slippage of the zygomatic bone, while the juxtaposition of the bones in the horizontal segment provides a compressive surface. Most probably, strains other than the predominant pattern are so minor and so infrequent that no particular morphological adaptation is necessary to accommodate them.
Acknowledgments
This work was supported by PHS DE08513. We thank Al Obrez for help with the experiments, Ona Kotov for histology, William Winn for photography, Walter Greaves for insights into jaw mechanics, and Dean Dessem and Walter Greaves for a critical reading of the manuscript.
Literature Cited
- Behrents RG, Carlson DS, Abdelnour T. In vivo analysis of bone strain about the sagittal suture in Macaca mulatta during masticatory movements. J Dent Res. 1978;57:904–908. doi: 10.1177/00220345780570091401. [DOI] [PubMed] [Google Scholar]
- Fisher JL, Godfrey K, Stephens RI. Experimental strain analysis of infant, adolescent and adult miniature swine skulls subjected to simulated mastication forces. J Biomech. 1976;9:333–338. doi: 10.1016/0021-9290(76)90054-3. [DOI] [PubMed] [Google Scholar]
- Gottlieb K. Artificial cranial deformation and the increased complexity of the lambdoid suture. Am J Phys Anthropol. 1978;48:213–214. doi: 10.1002/ajpa.1330480215. [DOI] [PubMed] [Google Scholar]
- Herring SW. Sutures—a tool in functional cranial analysis. Acta Anat (Basel) 1972;83:222–247. doi: 10.1159/000143860. [DOI] [PubMed] [Google Scholar]
- Herring SW. The dynamics of mastication in pigs. Arch Oral Biol. 1976;21:473–480. doi: 10.1016/0003-9969(76)90105-9. [DOI] [PubMed] [Google Scholar]
- Herring SW, Wineski LE. Development of the masseter muscle and oral behavior in the pig. J Exp Zool. 1986;237:191–207. doi: 10.1002/jez.1402370206. [DOI] [PubMed] [Google Scholar]
- Herring SW, Grimm AF, Grimm BR. Functional heterogeneity in a multipinnate muscle. Am J Anat. 1979;154:563–576. doi: 10.1002/aja.1001540410. [DOI] [PubMed] [Google Scholar]
- Herring SW, Wineski LE, Anapol FC. Neural organization of the masseter muscle in the pig. J Comp Neurol. 1989;280:563–576. doi: 10.1002/cne.902800407. [DOI] [PubMed] [Google Scholar]
- Hylander WL, Johnson KR. The relationship between masseter force and masseter electromyogram during mastication in the monkey Macaca fascicularis. Arch Oral Biol. 1989;34:713–722. doi: 10.1016/0003-9969(89)90078-2. [DOI] [PubMed] [Google Scholar]
- Jaslow CR. PhD Dissertion. Univ. of Chicago; 1987. A functional analysis of skull design in the Caprini. Unpubl. [Google Scholar]
- Jaslow CR. Sexual dimorphism of cranial suture complexity in wild sheep (Ovis orientalis) J Zool. 1989;95:273–284. [Google Scholar]
- Kokich VG. Age changes in the human frontozygomatic suture from 20 to 95 years. Am J Orthod. 1976;69:411–430. doi: 10.1016/0002-9416(76)90209-8. [DOI] [PubMed] [Google Scholar]
- Koskinen L, Isotupa K, Koski K. A note on craniofacial sutural growth. Am J Phys Anthropol. 1976;45:511–516. doi: 10.1002/ajpa.1330450312. [DOI] [PubMed] [Google Scholar]
- Meikle MC, Heath JK, Hembry RM, Reynolds JJ. Rabbit cranial suture fibroblasts under tension express a different collagen phenotype. Arch Oral Biol. 1982;27:609–613. doi: 10.1016/0003-9969(82)90078-4. [DOI] [PubMed] [Google Scholar]
- Moss MX. Experimental alteration of sutural area morphology. Anat Rec. 1957;127:569–589. doi: 10.1002/ar.1091270307. [DOI] [PubMed] [Google Scholar]
- Prahl-Andersen B. PhD Dissertation. Univ. of Nijmegen; The Netherlands: 1968. Sutural growth. Unpubl. [Google Scholar]
- Smith KK, Hylander WL. Strain gauge measurement of mesokinetic movement in the lizard Varanus exanthematicus. J Exp Biol. 1985;114:53–70. doi: 10.1242/jeb.114.1.53. [DOI] [PubMed] [Google Scholar]
- Smith HG, McKeown M. Experimental alteration of the coronal sutural area: a histological and quantitative microscopic assessment. J Anat. 1974;118:543–559. [PMC free article] [PubMed] [Google Scholar]
- Southard KA, Forbes DP. The effects of force magnitude on a sutural model: A quantitative approach. Am J Orthod Dent Orthop. 1988;93:460–466. doi: 10.1016/0889-5406(88)90074-1. [DOI] [PubMed] [Google Scholar]
- Wagemans PAHM, van de Velde JP, Kuijpers-Jagtman AM. Sutures and forces: A review. Am J Orthod Dent Orthop. 1988;94:129–141. doi: 10.1016/0889-5406(88)90361-7. [DOI] [PubMed] [Google Scholar]
- Watzek G, Grundschober F, Plenk H, Jr, Eschberger J. Experimental investigations into the clinical significance of bone growth at viscerocranial sutures. J Maxillofac Surg. 1982;10:61–79. doi: 10.1016/s0301-0503(82)80016-7. [DOI] [PubMed] [Google Scholar]
- Yen EHK, Pollit DJ, Whyte WA, Suga DM. Continuous stressing of mouse interparietal suture fibroblasts in vitro. J Dent Res. 1990;69:26–30. doi: 10.1177/00220345900690010301. [DOI] [PubMed] [Google Scholar]


