Abstract
The energetic resources in an organism’s environment are essential for executing a wide range of life history functions, including immunity and reproduction. Most energetic budgets, however, are limited, which can lead to trade-offs among competing functions. Increasing reproductive effort tends to decrease immunity in many cases; and increasing total energy via supplemental feedings can eliminate this effect. Testosterone (T), an important regulator of reproduction, and food availability are thus both potential factors regulating life-history processes, yet they are often tested in isolation of each other. In this study, we considered the effect of both food availability and elevated T on immune function and reproductive behavior in sagebrush lizards, Sceloporus graciosus, to assess how T and energy availability affect these trade-offs. We experimentally manipulated diet (via supplemental feedings) and T (via dermal patches) in males from a natural population. We determined innate immune response by calculating the bacterial killing capability of collected plasma exposed to E. coli ex vivo. We measured reproductive behavior by counting the number of courtship displays produced in a 20-min sampling period. We observed an interactive effect of food availability and T-patch on immune function, with food supplementation increasing immunity in T-patch lizards. Additionally, T increased courtship displays in control food lizards. Lizards with supplemental food had higher circulating T than controls. Collectively, this study shows that the energetic state of the animal plays a critical role in modulating the interactions among T, behavior and immunity in sagebrush lizards and likely other species.
Keywords: Context-dependent, Energy allocation, Innate immunity, Life history, Resources, Sceloporus, Trade-offs
Introduction
Animals often use behavioral and hormonal modifications to moderate the impact of variation in food availability in their natural environment. When food is limited, animals may experience trade-offs between survival and fecundity (e.g., Therrien et al., 2008; Waelti and Reyer, 2007). These effects, however, are sometimes modulated by behavioral or hormonal shifts that buffer the animal from sudden shifts in food supply (Guo et al., 2008, Mattila and Otis, 2007). For example, hormones have been implicated in producing the alternative reproductive strategies exhibited by different male side-blotched lizard morphs (Mills et al., 2008). Here, we begin to tease apart the effects of food resources and hormones by manipulating breeding male sagebrush lizards (Sceloporus graciosus) in the field and simultaneously assessing the effects of our manipulations on both immune function and reproductive condition.
The nutritional resources available to organisms in their natural environment determine the energy available at a given time (Owen et al., 1992). This energy is essential for an organism to carry out the cellular processes required for survival or reproduction (e.g., Hill et al., 2008). However, energy is not limitless; finite energy reserves are required to maintain a wide range of physiological and behavioral functions, often resulting in trade-offs among competing functions (Klein and Nelson, 1999; Marler and Moore, 1989). Thus, changes in food availability in an organism’s environment could lead to effects on survival and fecundity of these organisms (Houston et al., 2007, Macdonald and Bayne, 1993). Fecundity is reduced in organisms with low food supply (Crawford et al., 2006; Nagy and Holmes, 2005), and this could possibly be due to behavioral or hormonal modifications produced by food limitation. Survival is also impacted by food availability (Le Galliard et al., 2005; Marler and Moore, 1988; Marler and Moore, 1991), likely due to effects on immunity and/or energy stores.
Trade-offs between reproduction and immunity are not always observed (e.g., Greenman et al., 2005; Nakazawa et al., 1997). This is most likely due to the large differences in the amount of resources available to individuals within the population (McDade 2003). For example, when energy is not limiting (e.g., when animals are fed ad libitum), trade-offs between reproduction and immune function may not occur because individuals can simply increase their energy expenditure to accommodate increased energetic demands. Trade-offs may therefore be a plastic response to limited diet (Owen-Ashley et al., 2004). In support of this idea, limited resources decrease immune function in reproductively active female tree lizards (Urosaurus ornatus), but this trade-off is not apparent in lizards provided with ample food resources (French et al., 2007a). We thus expect individuals to increase investment into both reproductive and immune functions when resources are plentiful, as trade-offs are expected to be relaxed when energy availability is high.
The gonadal steroid hormone testosterone (T) serves as the predominant hormone regulating reproduction in males in a large variety of vertebrate taxa and is an important factor regulating life-history processes. High circulating T levels during the breeding season are correlated with an increase in testis size (Dixson and Anderson, 2004), induce male copulatory behavior (James and Nyby, 2002), activate elaboration of male ornamental traits or reproductive coloration (Cooper et al., 1987; Eens et al., 2000), and increase courtship song rate (Ketterson et al., 2001; Sartor et al., 2005). Individuals with high T are thus expected to be allocating large amounts of energetic output towards reproductive physiology and behavior. Additionally, T can increase metabolism and energy expenditure (Marler et al., 1995; Tobler et al., 2007). Consequently, maintenance of high T levels could significantly reduce the energy available for immune function (Muehlenbein and Bribiescas, 2005). Testosterone is indeed immunosuppressive in some cases. (Cox and John-Alder, 2007; Derting and Virk, 2005; Greives et al., 2006; Opplinger et al., 2004). Thus under high levels of T, we expect elevated reproductive investment to come at a cost to immune function. Many species exhibit a trade-off between reproductive effort and immunity by decreasing one or more functions when another is enhanced (see Ardia, 2005; Ahtiainen et al., 2005; Tomas et al., 2007; Uller et al., 2006 for examples).
Resource availability and T may both act separately to determine how much an individual should invest into reproductive versus immune processes, or they may interact. In this study, we examined the effects of both T and food supplementation on trade-offs between reproduction and immunity. Specifically, we predicted that increasing reproduction and courtship behavior via T treatment will impair immune function in sagebrush lizards. Furthermore, if this effect is due to an energetic trade-off, then food supplementation should attenuate T-induced immunosuppression by increasing energy availability. Considering these factors simultaneously allows us to better understand how life-history processes are mediated through both environmental and endocrine factors.
Methods
Overview
We conducted a field study on a natural population of male Sceloporus graciosus in southern California USA (33°N, 116°W). S. graciosus are small (50–70mm snout-to-vent length; 5–11g mass), territorial lizards with overlapping male and female territories (Martins, 1993). Their robust site fidelity makes this a particularly useful species for conducting field studies and for manipulating resources. We studied these animals in May and June 2008, at the peak of their breeding season (Martins, 1993). We captured and paint-marked individual lizards with a unique 3-color code, and gave each lizard a dermal patch (see testosterone manipulation below) before releasing them at the site of capture.
Seven to ten days after initial capture, we recaptured lizards in the afternoon hours (12:30 – 17:20 PST, except for one individual recaptured at 10:53 PST) immediately following behavioral observations. We immediately took blood samples from each lizard via rupturing the post-orbital sinus with a heparin-coated microcapillary tube. We measured mass upon initial capture and recapture to confirm the effectiveness of food manipulation, and then released the lizards at the site of capture. Within 8 h of collection, we centrifuged the blood at 4000 rpm for 10 min, and stored plasma samples at −20C for subsequent endocrine and immune assays. We treated all animals in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and as approved by the Bloomington Institutional Animal Care and Use Committee (BIACUC) and by the California Department of Fish and Game.
Testosterone manipulation
During the initial capture, we manipulated hormone levels by placing an external dermal patch on the lower back of each lizard. This is a non-invasive technique that has been shown to effectively elevate hormone levels over a 24-h period (Knapp and Moore 1997). Patches contained either 5 ul of 4ug testosterone (Sigma-Aldrich, Saint Louis, MO) suspended in sesame oil (T-manipulated group, N=21) or sesame oil vehicle alone (control group, N=19). We assembled patches from small pieces (approximately 0.3 cm2) of adhesive bandages (Johnson and Johnson©) and electrical tape (approximately 1.0 cm2), as described in Knapp and Moore (1997). We attached patches to the backs of lizards with SC Nexaband glue.
Resource manipulation
During the week between captures, we placed a petri-dish on the territories of each marked individual. We fed 3–6 vitamin-dusted (Herptivite® Multivitamins, Rep-Cal) mealworms and/or 2–4 vitamin-dusted crickets to individuals in the food supplemented group (N= 20, vs. N=20 without food supplementation) every day until the day of recapture. On days in which marked lizards in the food-supplemented group were spotted on their territories, we noted that these lizards readily consumed the prey. We additionally used mass increases as an indirect measure of food intake. Lizards in the non-supplemented group remained with empty petri-dishes in their home ranges until recapture.
Behavioral observations
Seven to ten days after initial capture, we located marked individuals in the afternoon hours (noon – 16:30 PST, except for one individual re-spotted at 10:30 PST) and conducted behavioral observations in the field prior to recapture. Elevated T is expected to increase reproductive behaviors, such as courtship (Woolley et al. 2004). We thus conducted behavioral observations in order to confirm the effect of the T-patch. We recorded behaviors produced by the target individual for 20 min, paying particular attention to headbob and shudder displays (Martins 1994). During the breeding season, S. graciosus males engage in frequent pushup (stereotyped single and double headbobs) and shudder (short, rapid headbobs with little fine-scale structure) displays (Kelso and Martins, 2008). These displays require considerable energy expenditure to perform (Brandt 2003). In other lizards, display frequency is also T-dependent (Tokarz et al., 2002; Watt et al., 2003).
We also recorded foraging behavior performed during this time, specifically the number of times a focal individual was spotted consuming prey. We considered foraging behavior in addition to changes in mass as factors for validating food manipulation in the field.
Bacteria killing assay
We determined innate immune response by calculating the bacterial killing capability of collected plasma exposed to E. coli (ATCC#8739, Microbiologics, St. Cloud, MN) ex vivo. This assay assesses the ability of complement in the blood to kill a bacterial colony. To do this, we diluted plasma in a 1:5 dilution with glutamine enriched CO2-independent media under laminar-flow conditions (Sigma-Aldrich). We used a 1:5 dilution due to preliminary dilution-response curve results for sagebrush lizard plasma. We added a working bacteria solution of E. coli in PBS to our diluted plasma samples (ratio 1:10 bacteria:plasma). We then incubated the plasma/bacteria cocktails for 30 min at 37°C and subsequently plated each sample on agar, in duplicate, including a positive control (of only bacteria) and a negative control (with no bacteria). Plates were incubated overnight at 37°C to allow colony growth. We quantified percent bacteria killed with the equation 100% - [(# of colonies on sample plated / # of colonies on positive control plate)*100]. No colonies formed on negative control plates.
Hormone analysis
We analyzed circulating levels of testosterone (T) and corticosterone (CORT) for each lizard using radioimmunoassay (RIA). We measured CORT since T manipulation has been shown to elevate CORT in other species (Ketterson et al., 2001, Zysling et al., 2006); we therefore considered CORT as a possible mediator of T effects. Samples were assayed as described under a previously published protocol (Moore, 1986) with slight modifications, and performed in the Ketterson laboratory at Indiana University. Briefly, we extracted samples with diethyl ether, separating the ether phase by snap freezing in a bath of dry ice in methanol, and subsequently resuspended samples in 10% ethyl acetate in isooctane. We separated individual hormones using column chromatography (T and CORT), with short-columns composed of one layer celite (Celite 521, Sigma-Aldrich) evenly mixed with water and a second layer of celite evenly mixed with glycol. We separated T from the column with an elution of 20% ethyl acetate: isooctane; and CORT with 52% ethyl acetate: isooctane. Separated hormones were collected, dried, resuspended in PBS, and assayed in duplicate. For each sample, we used an aliquot of the resuspended fractions to measure individual recoveries following extraction and chromatography. These recoveries were used to adjust the final sample concentration values to account for any losses during these procedures. Intra-assay coefficients of variation were 6% for T and 13% for CORT.
Statistical analysis
We performed multiple regression analyses using SAS (2002) to test whether lizard mass and food consumption during behavioral observations were predictors of food supplementation and whether headbob and shudder displays were predictors of patch group. Once manipulations were confirmed, we conducted two-way analysis of variance (ANOVA) with an interaction effect to test the ability of food supplementation, dermal patch group, and the interaction between food and patch group to predict each of our response measures (separately). Specifically, these response measures were immunity (percent of bacteria killed by the lizard’s plasma), and circulating hormone levels (T and CORT, separately).
Results
Mass and foraging behavior confirmed resource manipulation
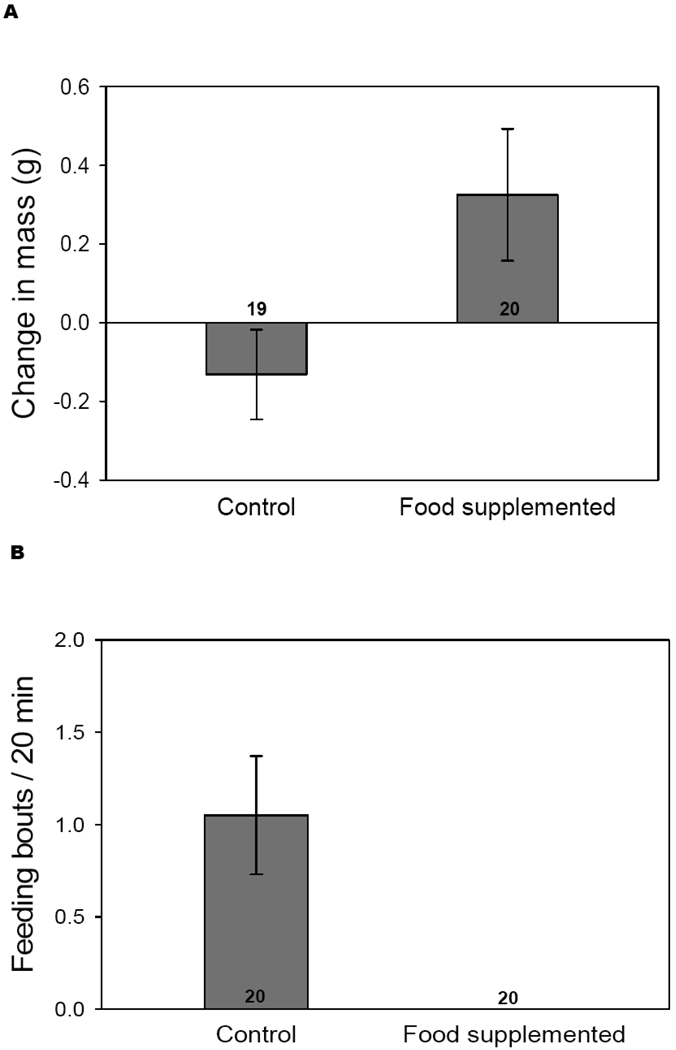
We used lizards in which we did not manipulate T-levels to determine the effectiveness of our food manipulation for use in the field. Food-supplemented lizards gained an average of 0.3 gm (SE = 0.26) during the week of resource manipulation, whereas controls lost an average of 0.3 gm (SE = 0.17). (F1,35 = 4.60, p < 0.05; Fig. 1a). Foraging behavior also differed between groups. Food supplemented lizards did not eat during behavioral assays, whereas we usually observed control individuals eating at least once during the 20 min trial (F1,36 = 10.99, p < 0.01; Fig. 1b). Together, these observations suggest that the food manipulation was successful, and individuals in the food-supplemented group were in fact supplied with enhanced energy resources.
Figure 1.
Mean (+/− SEM) weight change (a) and number of times observed eating (b) in control and food supplemented lizards
Courtship behavior confirmed testosterone manipulation
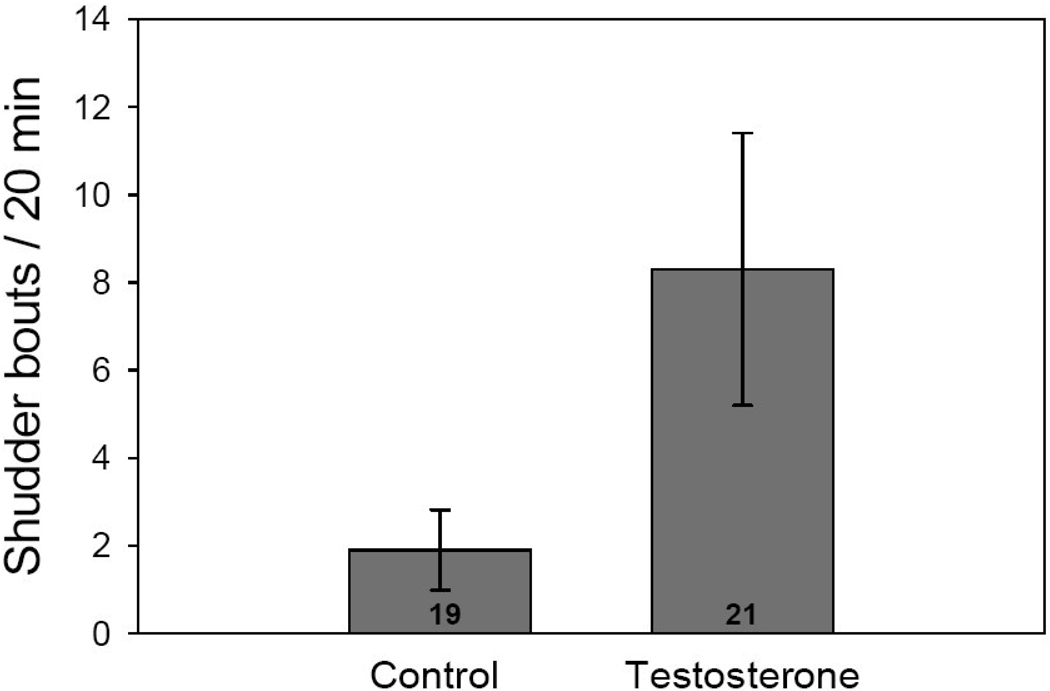
Considering only lizards that did not receive supplemental food, males that were provided with a short-term burst of T (T-patch) produced over 4 times more courtship shudder bouts (mean = 8.3 shudder displays/20 min, SE = 3.1) than did males that received only a sesame oil patch (mean = 1.9 shudder displays/20 min, SE = 0.9). (F1,36 = 5.60, p < 0.03; Fig. 2). However, we did not observe a similar effect of dermal patch treatment on headbob displays (F1,36 = 0.00, p = 0.98).
Figure 2.
Mean (+/− SEM) number of shudder bouts in vehicle or testosterone treated lizards without food supplementation
Food supplementation and T manipulation interact to affect immune function
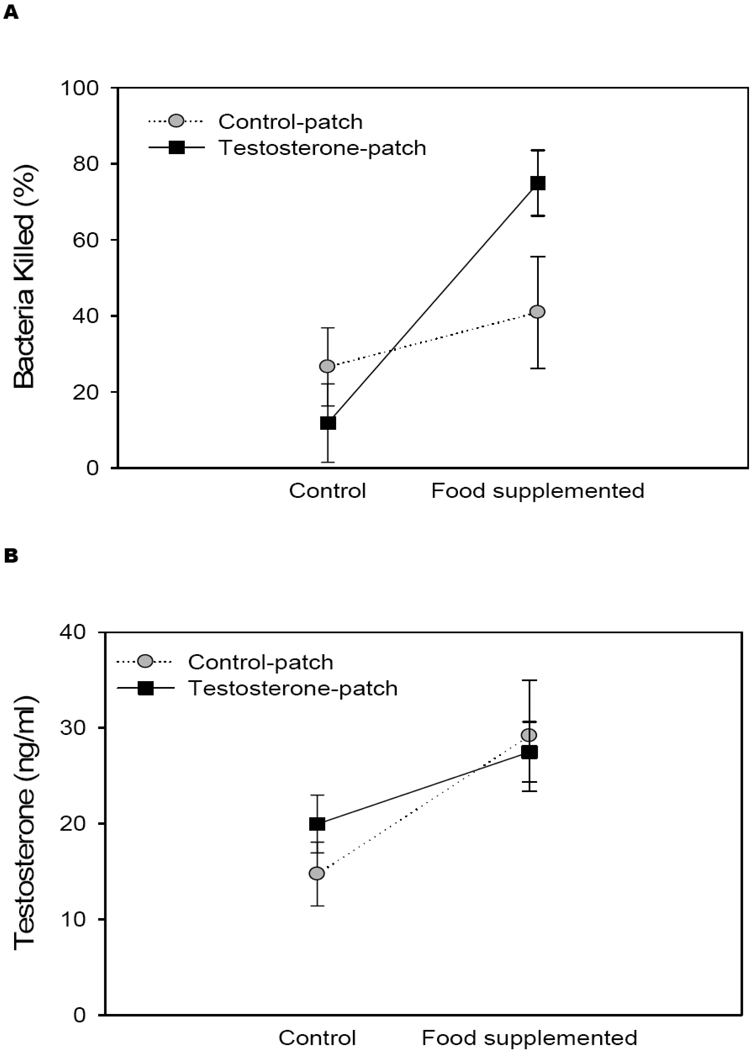
Food supplementation and T manipulation interacted to affect bacteria killing capability (Table 1, Fig. 3a). Food supplemented lizards had 3 times greater immune activity than control lizards (Table 1, Fig. 3a). This effect was mainly due to a significant increase in immune function for food-supplemented lizards that also received a T-patch. T-patch lizards had elevated immune function with food-supplementation when compared to T-patch individuals that were not fed (t = 2.12, df = 16, p < 0.001). This effect of food supplementation on immunity was not pronounced in control-patch lizards, leading to a significant interaction effect between food and patch in the two-way ANOVAs (Table 1).
Table 1.
Mean squares and F-values from two-way ANOVAS of immune (df = 1,31) and hormonal (df = 1,36) responses to food and patch (testosterone) manipulations
| Bacteria killed (%) | Circulating T | Circulating CORT | ||||
|---|---|---|---|---|---|---|
| Effect | MS | F | MS | F | MS | F |
| Food | 12909.05* | 12.67* | 1197.93* | 8.14* | 0.01 | 0.00 |
| T-Patch | 794.90 | 0.78 | 31.17 | 0.21 | 24.81 | 2.72 |
| Interaction | 5121.94* | 5.03* | 119.24 | 0.81 | 4.11 | 0.45 |
| Full model | 6608.08* | 6.49* | 445.50* | 3.03* | 9.62 | 1.05 |
p<0.05
Figure 3.
Mean (+/− SEM) percent bacteria killed (a), circulating T (b) and CORT (c) in food supplemented or control lizards treated with exogenous testosterone (black squares) or vehicle (gray circles)
Food supplementation increases circulating testosterone
Food, but not patch, manipulation predicted average circulating T levels between groups (Table 1). Lizards provided with additional food resources had 1.5 times higher circulating T than control lizards (Fig. 3b). This was true even when considering only control-patch individuals. Although there was a trend towards a weak correlation between T and CORT (r = 0.277, df = 38, p = 0.08), food supplementation was not a good predictor of CORT levels (Table 1, Fig. 3c). Within each food treatment group, circulating T and immune function were not correlated (food-supplemented: r = 0.153, df = 14, p = 0.57; control: r = 0.025, df = 17, p = 0.92).
Discussion
We found significant interactions between the reproductive system, the immune system, and food availability. Food supplementation and T manipulation interacted to influence immune function in male sagebrush lizards. Consistent with our predictions, food supplementation increased immune response in lizards and this response was more pronounced in T-treated compared with control animals. Furthermore, food supplementation increased plasma T levels in both T-treated and control lizards. In contrast to our predictions, however, T enhanced immunity in food supplemented lizards. These results suggest that T can act differentially to alter immune function, depending on the energetic state of the animal. For example, T may enhance both reproduction and immunity when energy is abundant, but T may also mediate trade-offs between these two physiological systems when resources are limiting. These context-dependent effects of T may reconcile the conflicting evidence for both immunosuppression and immunoenhancement of T in the literature (Muehlenbein and Bribiescas, 2005). Variability in lab or field conditions or in species requirements of energetic resources may account for these variable effects of T. Trade-offs between reproduction (as measured by T) and immunity may also be due to the indirect effects of energy allocation (Owen-Ashley et al., 2004), explaining our lack of immunosuppression in food supplemented lizards. Nevertheless, we only considered one area of immunity, namely innate immune function, and may have attained different responses (ie., noted immunosuppression with T) if we had measured acquired or cell-mediated immunity.
Although T-treated individuals had increased courtship behavior when food-limited, T-patch did not produce long-term elevation of circulating levels of T, as plasma T was not significantly elevated at the time of blood sampling. The fact that T-patches increased courtship behavior in food-limited lizards one week after patch administration even without a measured effect on circulating T levels, suggests that some behavioral regulation may be in response to previous hormone levels. Similar to the way in which CORT-patches acted on circulating CORT levels (Knapp and Moore, 1997), T-patches likely produced a short-term elevation of T. This may have up-regulated androgen receptors, producing a prolonged behavioral and immune response.
One of the most pronounced effects of our study is that food supplementation increased levels of circulating testosterone in both T-patch and control individuals, as well as was involved in the enhancement of immune function. Mechanisms are now unclear but may involve differences in clearance rates or conversion from other precursors. An increase in energy resources provided by an increase in food may have allowed for greater energy distribution to multiple functions, including reproduction and immunity (French et al., 2007b). The fact that increasing food intake allowed for the elevation of both reproductive and immune processes suggests that these processes are not mutually exclusive and thus trade-offs may exist due to limitations in energy uptake. Female snow skinks in good condition may produce high levels of both reproductive investment and immune function, whereas costs arise in females that do not have the resources to produce high levels of both processes (Olsson et al., 2001). Tropical pythons with low body condition have decreased immunity (Ujvari and Madsen, 2006). Food availability may be one factor affecting individual quality. High levels of activity have immunosuppressive effects in female mice on a restricted diet (Schubert et al., 2008), suggesting energetic requirements are important for allocation towards multiple functions.
In addition to acting internally to increase energy stores, food supplementation may impose other externally-derived motives for increasing circulating testosterone. Increases in food availability in some territories may affect social interactions within those territories. Androgen levels can then be modulated by the social environment (Oliveira et al., 2002). This may occur due to an increase in density of, and thus behavioral encounters within, these now higher quality territories, promoting elevated levels of T (Beletsky et al., 1992). Increasing food resources in some territories may also elevate aggression to retain high quality territories. We witnessed one highly aggressive encounter between a focal food supplemented lizard and a conspecific male (pers. obs.). In this case, food supplementation may have elevated T due to the increased potential for aggressive interactions in defense of high food resources. Variation in food abundance causes changes in home ranges and associated social structures of mice (Schradin and Pillay, 2006) and red squirrels (Wauters et al., 2005). The role of food availability on social structures has yet to be studied, however, in sagebrush lizards.
Supplemental food items may have also provided a stimulus for increased testosterone. Lizards in food-supplemented sites were supplied with vitamin-dusted crickets and mealworms, which are notably larger than most of the natural prey of these lizards (personal observation). The size of prey items correlates with rump coloration (a signal of condition) in kestrel fledglings (Vergara and Fargallo, 2008). Similarly, the interaction with larger prey objects, most likely also indicative of greater food intake, may have allowed for an elevation of T due to enhancement of individual quality. Furthermore, providing lizards in supplemented group with vitamin-dusted crickets and mealworms may not only increase the amount of available food but also the quality of the food by providing essential vitamins and both a protein source (crickets) and a fat source (mealworms). In this way, lizards were able to intake their required nutrients without much effort. Diet quality has the potential to influence the direction of trade-offs (Naya et al., 2007). This may have allowed for the increase of T in these higher quality individuals. Nevertheless, calorie restriction, not diet quality, is associated with suppression of reproduction (including T levels) in other studies (Govic et al., 2008, Santos et al., 2004).
In conclusion, the present findings demonstrate that several key factors may interact to influence important life history functions, such as immunity and reproduction. Here we considered testosterone, a reproductive mediator, in the context of energetic resources. Testosterone was found to influence both immune function and courtship behavior differently depending on the energetic state of the animal. Thus, T is not necessarily immunosuppressive in all species and across all conditions. Rather, the effects of T on immunity appear depend on the environmental condition of the animal. For example, T can be immunosuppressive when food is limited; however, our results also support T-induced immunoenhancement when food availability is high. These findings support the idea of an energetic trade-off between reproduction and immune function, at least when environmental resources are limiting. Furthermore, this study suggests a role for food availability in enhancing immune function (in presence of T) and on reproduction (by elevating circulating T). Further research is needed to determine the mechanism regulating hormone levels by energetic state.
Acknowledgements
We thank Arián Avalos for endless hours of field assistance. We also thank David Kabelik for training and assistance, and Ellen Ketterson for the use of her lab in conducting RIAs. We thank Tim Grieves for training in bacteria killing assays. The research reported in this article conformed to all laws of the United States, and was approved through Bloomington IACUC # 08-010. MR was funded by the Common Themes in Reproductive Diversity Training Grant, awarded to Indiana University Bloomington by the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Ahtiainen JJ, Alatalo RV, Kortet R, Rantala MJ. A trade-off between sexual signalling and immune function in a natural population of the drumming wolf spider Hygrolycosa rubrofasciata. J. Evol. Biol. 2005;18:985–991. doi: 10.1111/j.1420-9101.2005.00907.x. [DOI] [PubMed] [Google Scholar]
- Ardia DR. Individual quality mediates trade-offs between reproductive effort and immune function in tree swallows. J. Anim. Ecol. 2005;74:517–524. [Google Scholar]
- Beletsky LD, Orians GH, Wingfield JC. Year-to-year patterns of circulating levels of testosterone and corticosterone in relation to breeding density, experience, and reproductive success of the polygynous red-winged blackbird. Horm. Behav. 1992;26:420–432. doi: 10.1016/0018-506x(92)90011-j. [DOI] [PubMed] [Google Scholar]
- Brandt Y. Lizard threat display handicaps endurance. Proc. R. Soc. Lond. B. 2003;270:1061–1068. doi: 10.1098/rspb.2003.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper WE, Jr, Mendonca MT, Vitt LJ. Induction of orange head coloration and activation of courtship and aggression by testosterone in the male broad-headed skink (Eumeces laticeps) J. Herpetol. 1987;21:96–101. [Google Scholar]
- Cox RM, John-Alder HB. Increased mite parasitism as a cost of testosterone in male striped plateau lizards Sceloporus virgatus. Funct. Ecol. 2007;21:327–334. [Google Scholar]
- Crawford RJM, Barham PJ, Underhill LG, Shannon LJ, Coetzee JC, Dyer BM, Leshoro TM, Upfold L. The influence of food availability on breeding success of African penguins Spheniscus demersus at Robben Island, South Africa. Biol. Conserv. 2006;132:119–125. [Google Scholar]
- Derting TL, Virk MK. Positive effects of testosterone and immunochallenge on energy allocation to reproductive organs. J. Comp. Physiol. B. 2005;175:543–556. doi: 10.1007/s00360-005-0015-1. [DOI] [PubMed] [Google Scholar]
- Dixson AF, Anderson MJ. Sexual behavior, reproductive physiology and sperm competition in male mammals. Physiol. Behav. 2004;83:361–371. doi: 10.1016/j.physbeh.2004.08.022. [DOI] [PubMed] [Google Scholar]
- Eens M, Van Duyse E, Berghman L, Pinxten R. Shield characteristics are testosterone-dependent in both male and female moorhens. Horm. Behav. 2000;37:126–134. doi: 10.1006/hbeh.1999.1569. [DOI] [PubMed] [Google Scholar]
- French SS, DeNardo DF, Moore MC. Trade-offs between the reproductive and immune systems: facultative responses to resources or obligate responses to reproduction? Am. Nat. 2007a;170:79–89. doi: 10.1086/518569. [DOI] [PubMed] [Google Scholar]
- French SS, Johnston GIH, Moore MC. Immune activity suppresses reproduction in food-limited female tree lizards Urosaurus ornatus. Funct. Ecol. 2007b;21:1115–1122. [Google Scholar]
- Govic A, Levay EA, Hazi A, Penman J, Kent S, Paolini AG. Alterations in male sexual behavior, attractiveness and testosterone levels induced by an adult-onset calorie restriction regimen. Behav. Brain Res. 2008;190:140–146. doi: 10.1016/j.bbr.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Greenman CG, Martin LB, II, Hau M. Reproductive state, but not testosterone, reduces immune function in male house sparrows (Passer domesticus) Physiol. Biochem. Zool. 2005;78:60–68. doi: 10.1086/425194. [DOI] [PubMed] [Google Scholar]
- Greives TJ, McGlothlin JW, Jawor JM, Demas GE, Ketterson ED. Testosterone and innate immune function inversely covary in a wild population of breeding Dark-Eyed Juncos (Junco hyemalis) Funct. Ecol. 2006;20:812–818. [Google Scholar]
- Guo ST, Ji WH, Li BG, Li M. Response of a group of Sichuan snub-nosed monkeys to commercial logging in the Qinling Mountains, China. Cons. Biol. 2008;22:1055–1064. doi: 10.1111/j.1523-1739.2008.00975.x. [DOI] [PubMed] [Google Scholar]
- Hill JW, Elmquist JK, Elias CF. Hypothalamic pathways linking energy balance and reproduction. Amer. J. Physiol. Endocrinol. Met. 2008;294:E827–E832. doi: 10.1152/ajpendo.00670.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston AI, McNamara JM, Barta Z, Klasing KC. The effect of energy reserves and food availability on optimal immune defence. Proc. R. Soc. Lond., Ser. B: Biol. Sci. 2007;274:2835–2842. doi: 10.1098/rspb.2007.0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James PJ, Nyby JG. Testosterone rapidly affects the expression of copulatory behavior in house mice (Mus musculus) Physiol. Behav. 2002;75:287–294. doi: 10.1016/s0031-9384(01)00666-7. [DOI] [PubMed] [Google Scholar]
- Kelso EC, Eklund AC, Martins EP. Fecal measures of testosterone in sagebrush lizards (Sceloporus graciosus) In review. [Google Scholar]
- Ketterson ED, Nolan V, Jr, Casto JM, Buerkle CA, Clotfelter E, Grindstaff JL, Jones KJ, Lipar JL, McNabb FMA, Neudorf DL, Parker-Renga I, Schoech SJ, Snajdr E. Testosterone, phenotype and fitness: a research program in evolutionary behavioral endocrinology. In: Dawson A, Chaturvedi CM, editors. Avian Endocrinology. New Delhi, India: Narosa Publishing House; 2001. pp. 19–40. [Google Scholar]
- Klein SL, Nelson RJ. Influence of social factors on immune function and reproduction. Rev. Reprod. 1999;4:168–178. doi: 10.1530/ror.0.0040168. [DOI] [PubMed] [Google Scholar]
- Knapp R, Moore MC. A non-invasive method for sustained elevation of steroid hormone levels in reptiles. Herp. Rev. 1997;28:33–36. [Google Scholar]
- Le Galliard J-F, Ferriere R, Clobert J. Juvenile growth and survival under dietary restriction: are males and females equal? Oikos. 2005;111:368–376. [Google Scholar]
- Macdonald BA, Bayne BL. Food availability and resource allocation in scenescent Placopecten magellanicus: Evidence from field populations. Funct. Ecol. 1993;7:40–46. [Google Scholar]
- Marler CA, Moore MC. Evolutionary costs of aggression revealed by testosterone manipulations in free-living male lizards. Behav. Ecol. Sociobiol. 1988;23:21–26. [Google Scholar]
- Marler CA, Moore MC. Time and energy costs of aggression in testosterone-implanted free-living male mountain spiny lizards (Sceloporus jarrovi) Physiol.Zool. 1989;62:1334–1350. [Google Scholar]
- Marler CA, Moore MC. Supplemental feeding compensates for testosterone-induced costs of aggression in male mountain spiny lizards (Sceloporus jarrovi) Anim. Behav. 1991;42:209–219. [Google Scholar]
- Marler CA, Walsberg G, White ML, Moore M, Marler CA. Increased energy expenditure due to increased territorial defense in male lizards after phenotypic manipulation. Behav. Ecol. Sociobiol. 1995;37:225–231. [Google Scholar]
- Martins EP. Contextual use of the push-up display by the sagebrush lizard, Sceloporus graciosus. Anim. Behav. 1993;45:25–36. [Google Scholar]
- Martins EP. Structural complexity in a lizard communication system – the Sceloporus graciosus push-up display. Copeia. 1994;1994:944–955. [Google Scholar]
- Mattila HR, Otis GW. Dwindling pollen resources trigger the transition to broodless populations of long-lived honeybees each autumn. Ecol. Entomol. 2007;32:496–505. [Google Scholar]
- McDade TW. Life history theory and the immune system: Steps toward a human ecological immunology. Yearbook. Phys. Anthropol. 2003;46:100–125. doi: 10.1002/ajpa.10398. [DOI] [PubMed] [Google Scholar]
- Mills SC, Hazard L, Lancaster L, Mappes T, Miles D, Oksanen TA, Sinervo B. Gonadotropin hormone modulation of testosterone, immune function, performance, and behavioral trade-offs among male morphs of the lizard Uta stansburiana. Am. Nat. 2008;171:339–357. doi: 10.1086/527520. [DOI] [PubMed] [Google Scholar]
- Moore MC. Elevated testosterone levels during nonbreeding season territoriality in a fall-breeding lizard, Sceloporus jarrovi. J. Comp. Physiol. A. 1986;158:159–163. doi: 10.1007/BF01338559. [DOI] [PubMed] [Google Scholar]
- Muehlenbein MP, Bribiescas RG. Testosterone-mediated immune functions in male life histories. Am. J. Hum. Biol. 2005;17:527–558. doi: 10.1002/ajhb.20419. [DOI] [PubMed] [Google Scholar]
- Nagy LR, Holmes RT. Food limits annual fecundity of a migratory songbird: an experimental study. Ecology. 2005;86:675–681. [Google Scholar]
- Nakazawa M, Fantappie MR, Freeman GL, Eloi-Santos S, Olsen NJ, Kovacs WJ, Secor WE, Colley DG. Schistosoma mansoni: susceptibility differences between male and female mice can be mediated by testosterone during early infection. Exp. Parasitol. 1997;85:233–240. doi: 10.1006/expr.1997.4148. [DOI] [PubMed] [Google Scholar]
- Naya DE, Lardies MA, Bozinovic F. The effect of diet quality on physiological and life-history traits in the harvestman Pachylus paesslerik. J. Insect Physiol. 2007;53:132–138. doi: 10.1016/j.jinsphys.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Oliveira RF, Hirschenhauser K, Carneiro LA, Canario AVM. Social modulation of androgen levels in male teleost fish. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 2002;132:203–215. doi: 10.1016/s1096-4959(01)00523-1. [DOI] [PubMed] [Google Scholar]
- Olsson M, Shine R, Wapstra E. Costs of reproduction in a lizard species: a comparison of observational and experimental data. Oikos. 2001;93:121–125. [Google Scholar]
- Opplinger A, Giorgi MS, Conelli A, Nembrini M, John-Alder HB. Effects of testosterone on immunocompetence, parasite load, and metabolism in the common wall lizard (Podarsis muralis) Can. J. Zool. 2004;82:1713–1719. [Google Scholar]
- Owen-Ashley NT, Hasselquist D, Wingfield JC. Androgens and the immunocompetence handicap hypothesis: unraveling direct and indirect pathways of immunosuppression in song sparrows. Am. Nat. 2004;164:490–505. doi: 10.1086/423714. [DOI] [PubMed] [Google Scholar]
- Owen M, Wells RL, Black JM. Energy budgets of wintering barnacle geese: The effects of declining food resources. Ornis. Scand. 1992;23:451–458. [Google Scholar]
- Santos AMD, Ferraz MR, Teixeira CV, Sampaio FJB, Ramos CD. Effects of undernutrition on serum and testicular testosterone levels and sexual function in adult rats. Horm. Metab. Res. 2004;36:27–33. doi: 10.1055/s-2004-814198. [DOI] [PubMed] [Google Scholar]
- Sartor JJ, Balthazart J, Ball GF. Coordinated and dissociated effects of testosterone on singing behavior and song control nuclei in canaries (Serinus canaria) Horm. Behav. 2005;47:467–476. doi: 10.1016/j.yhbeh.2004.12.004. [DOI] [PubMed] [Google Scholar]
- SAS. SAS for Windows 9.1. Cary (NC): SAS Institute, Inc.; 2002. [Google Scholar]
- Schradin C, Pillay N. Female striped mice (Rhabdomys pumilio) change their home ranges in response to seasonal variation in food availability. Behav. Ecol. 2006;17:452–458. [Google Scholar]
- Schubert KA, Vaanholt LM, Stavasius F, Demas GE, Daan S, Visser GH. Female mice respond differently to costly foraging versus food restriction. J. Exp. Biol. 2008;211:2214–2223. doi: 10.1242/jeb.017525. [DOI] [PubMed] [Google Scholar]
- Sheldahl LA, Martins EP. The territorial behavior of the western fence lizard, Sceloporus occidentalis. Herpetologica. 2000;56:469–479. [Google Scholar]
- Smith LC, John-Alder HB. Seasonal specificity of hormonal, behavioral, and coloration responses to within- and between-sex encounters in male lizards (Sceloporus undulatus) Horm. Behav. 1999;36:39–52. doi: 10.1006/hbeh.1999.1523. [DOI] [PubMed] [Google Scholar]
- Therrien JF, Cote SD, Festa-Bianchet M, Ouellet JP. Maternal care in white-tailed deer: trade-off between maintenance and reproduction under food restriction. Anim. Behav. 2008;75:235–243. [Google Scholar]
- Tobler M, Nilsson JK, Nilsson JF. Costly steroids: egg testosterone modulates nestling metabolic rate in the zebra finch. Biol Lett. 2007;3:408–410. doi: 10.1098/rsbl.2007.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokarz RR, McMann S, Smith LC, John-Alder H. Effects of testosterone treatment and season on the frequency of dewlap extensions during male-male interactions in the lizard Anolis sagrei. Horm. Behav. 2002;41:70–79. doi: 10.1006/hbeh.2001.1739. [DOI] [PubMed] [Google Scholar]
- Tomas G, Merino S, Moreno J, Morales J, Martinez-de la Puente J. Impact of blood parasites on immunoglobulin level and parental effort: a medication field experiment on a wild passerine. Funct. Ecol. 2007;21:125–133. [Google Scholar]
- Ujvari B, Madsen T. Age, parasites, and condition affect humoral immune response in tropical pythons. Behav Ecol. 2006;17:20–24. [Google Scholar]
- Uller T, Isaksson C, Olsson M. Immune challenge reduces reproductive output and growth in a lizard. Funct. Ecol. 2006;20:873–879. [Google Scholar]
- Vergara P, Fargallo JA. Sex, melanic coloration, and sibling competition during the postfledging dependence period. Behav. Ecol. 2008 doi:10.1093/beheco/arn035. [Google Scholar]
- Waelti MO, Reyer HU. Food supply modifies the trade-off between past and future reproduction in a sexual parasite-host system (Rana esculenta, Rana lessonae) Oecologia. 2007;152:415–424. doi: 10.1007/s00442-007-0671-9. [DOI] [PubMed] [Google Scholar]
- Watt MJ, Forster GL, Joss JMP. Steroid correlates of territorial behavior in male jacky dragons, Amphibolurus muricatus. Brain Behav. Evol. 2003;61:184–194. doi: 10.1159/000070702. [DOI] [PubMed] [Google Scholar]
- Wauters LA, Bertolino S, Adamo M, Van Dongen S, Tosi G. Food shortage disrupts social organization: the case of the red squirrel in conifer forests. Evol. Ecol. 2005;19:375–404. [Google Scholar]
- Woolley SC, Sakata JT, Crews D. Evolutionary insights into the regulation of courtship behavior in male amphibians and reptiles. Physiol. Behav. 2004;83:347–360. doi: 10.1016/j.physbeh.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Zysling DA, Grieves TJ, Breuner CW, Casto JM, Demas GE, Ketterson ED. Behavioral and physiological responses to experimentally elevated testosterone in female dark-eyed juncos (Junco hyemalis carolinensis) Horm Behav. 2006;50:200–207. doi: 10.1016/j.yhbeh.2006.03.004. [DOI] [PubMed] [Google Scholar]