Abstract
Operant conditioning paradigms are useful for studying factors involved in reward, particularly when combined with the tools of genetic manipulation in mice. Published operant studies involving mice vary widely with respect to design, and insight into the consequences of design choices on performance in mice is limited. Here, we evaluated the impact of five design variables on the performance of inbred male mice in operant tasks involving solid food pellets as reinforcing agents. We found that the use of lever-press or nose-poke during FR1 sessions did not impact the performance of C57BL/6 mice, but that the lever-press approach correlated with enhanced performance during PR testing. While FR1 session duration had a notable impact on the rate of acquisition of food-maintained responding, performance during FR1 and PR sessions was largely unaffected. Higher order schedules of reinforcement (FR3 and FR5) led to elevated responding during both FR and PR sessions, and improved the correspondence between rewards earned and consumed. Single and group-housed mice performed indistinguishably during FR1 and PR sessions, while environmental enrichment combined with group housing accelerated the rate of acquisition of food-maintained responding while decreasing responding during PR testing. Finally, while C57BL/6 and 129/Sv mice exhibited comparable behavior during FR1 sessions, C57BL/6 mice tended to acquire food-maintained responding faster than 129/Sv counterparts, and exhibited elevated responding during PR testing. Altogether, our findings indicate that while operant performance for food in mice is relatively insensitive to many study parameters, experimental outcomes can be shaped predictably with proper design decisions.
Keywords: reinforcement, reward, instrumental learning, enrichment, strain
Introduction
Operant conditioning refers to the use of positive or negative reinforcement to modify the frequency of a particular voluntary behavior. In the 1950s, B.F. Skinner and colleagues pioneered automated operant conditioning tasks involving the pigeon as subject, and early studies from this group shed important light on the impact of behavioral variables such as the schedule of reinforcement and their influence on operant behavior [1-3]. Automated operant tasks have also been adapted for rodents, which exhibit several reproductive and biological characteristics advantageous for high-throughput experimentation. Rats have been particularly common subjects in operant studies, and have helped to provide critical insights into the neurochemical and anatomic basis of reward (e.g., [4-10]).
The use of mice as subjects in operant conditioning studies accelerated in the 1970s when groups began to evaluate the impact of pharmacologic interventions on operant responding in mice. Several groups, for example, studied the effect of drugs of abuse and related agents on food-maintained operant responding in mice [11-22]. Operant-based approaches have more recently been adapted for drug self-administration studies in mice. Mice, like rats and primates, will self-administer nicotine [23], ethanol [24], cocaine [25, 26], opioids [27], and cannabinoids [28].
Differences between inbred and outbred mouse strains with respect to operant responding were noted in early studies, highlighting genetic influences on instrumental learning [29, 30]. In the last two decades, investigators have generated and utilized mice harboring targeted genetic mutations in operant studies in an attempt to better understand the genetic and molecular underpinnings of drug reward. Approaches involving knockout mice in particular have highlighted the significance of specific receptor types, such as the serotonin 5-HT1B and D2 dopamine receptors, to the reinforcing effects of cocaine [31, 32], ethanol [33, 34], and morphine [35]. Similarly, the D1 dopamine receptor and melanocortin receptor types 3 and 4, among other targets, have been implicated in the reinforcing effects of food and sweet rewards [36-38]. As the spatial and temporal resolution of gene ablation or suppression strategies in mice improves, operant-based studies will be particularly useful for delineating the cellular basis of reward.
A careful comparison of protocols employed in mouse-based operant conditioning studies reveals striking variability with respect to many experimental design variables. For example, published studies differ with respect to the use of lever press or nosepoke to earn rewards, session numbers and duration, schedule(s) of reinforcment, housing conditions, genetic background, and nature and composition of the reinforcing agent. To date, a systematic evaluation of the influence of most such design variables on operant performance in mice is lacking. As such, the goal of this study was to quantify the influence of manipulanda, session length (30-90 min), schedule of reinforcement (FR1, FR3, FR5), housing status (isolation, group-housing, and environmental enrichment), and mouse strain (C57BL/6J and 129/SvJ) on the performance of mice in an operant task involving food as the reinforcing agent.
Materials and Methods
Subjects
All studies were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23; revised 1996), and were granted formal approval by the Institutional Animal Care and Use Committee of the University of Minnesota. Efforts were made to minimize the pain and discomfort of the animals throughout the study, and when possible, to reduce the number of animals used in each test. Male C57BL/6 and 129/Sv mice were purchased from Jackson Laboratories (Bar Harbor, ME) and were group-housed for 2-4 weeks on a 12-h light/dark cycle prior to testing. Prior to testing, animals were given ad libitum access to standard rodent chow (#2018, Harlan Teklad Global Diets; Madison, WI). Unless otherwise noted, subjects were single-housed at 7-8 wks of age. Single-housed mice were given ad libitum water access and 1.8 g of standard rodent chow per day until each subject reached 85-95% of their free-feeding weight (∼3d). Subsequently, subjects were given 2.0-2.3 g of rodent chow per day to maintain stable bodyweights throughout testing.
General Experimental Design
Five separate studies were conducted, each involving a distinct cohort of mice. Experiments were conducted in six mouse operant chambers as described previously [39]. Subjects were handled individually in the testing room for 3 d prior to the first test session. One day prior to testing, 20 reward pellets (20 mg PJA/100020 dust-free Noyes Precision pellets, Bio Serv; Frenchtown, NJ) were placed into the home cage to blunt the potential effect of food neophobia on operant performance [40]. All subjects were tested in 10 sessions occurring over an 11-d period using a fixed ratio 1 (FR1) schedule of reinforcement unless stated otherwise. Testing was conducted between 1100 and 1600 h. The house light was illuminated throughout the session. The active lever/nose-poke hole was counter-balanced between subjects for each study. A response on the active manipulandum resulted in stimulus light illumination (3 s) above the lever or inside the nose-poke hole; a single pellet was immediately delivered to the food cup. A response on the inactive manipulandum was recorded but did not lead to pellet delivery or stimulus light illumination. Prior to the start of the session on Day 1, reward pellets were crushed to create powder that was sprinkled on the active lever or in the active nose poke hole to facilitate instrumental responding on the first training day. Upon session completion, the house light was turned off and the mice were returned to their home cage. After completing the 10 FR1 sessions, mice that had acquired food-maintained operant responding behavior were evaluated in a single 1-h PR test wherein delivery of a food pellet was contingent upon a progressive ratio of active responding (e.g., 1, 2, 4, 6, 9, 12, 15, 20, etc.), as described previously [39, 41].
Analysis
Data are presented throughout as the mean ± SEM and were analyzed using Prism 5 software (GraphPad, La Jolla Ca). Acquisition rate was defined as the first day of the first block of 3 consecutive days during which all acquisition criteria were met (see Results section for the criteria). Responding (active and inactive) and the number of pellets (earned, consumed, and uneaten) were determined for each subject by averaging values measured over the 3-d stability period. Active and inactive responding, rewards earned and breakpoint values were recorded from the PR data. The breakpoint is defined as the value associated with the last completed set of active responses that resulted in a reward in a 60-min session. Data were compared between groups using Student's t-test or one-way ANOVA followed by Tukey's multiple comparison tests, as appropriate. The threshold for statistical significance in all instances was P<0.05.
Results
Study 1. Manipulanda
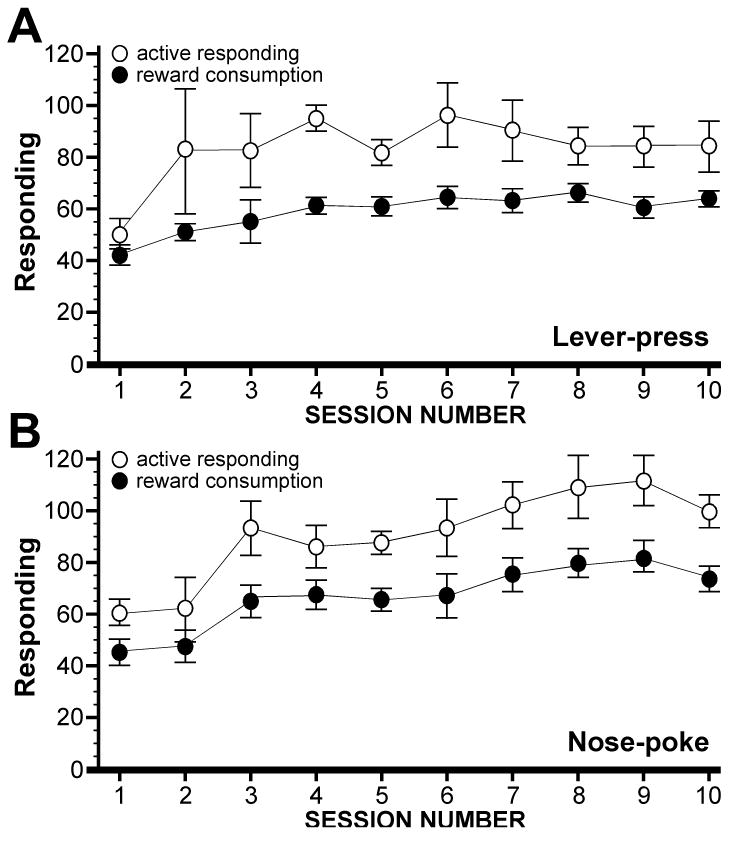
To determine whether food-reinforced operant responding in mice is influenced by the manipulanda employed during testing, we randomly assigned male C57BL/6 mice to either lever-press (LP) or nose-poke (NP) groups (n=10 per group). The number of active and inactive responses, as well as the number of pellets earned, consumed, and uneaten under a fixed ratio 1 (FR1) schedule of reinforcement were tabulated for ten days during 90-min test sessions. As shown in Fig. 1, both LP and NP groups engaged in robust active responding and reward consumption over the 10 FR1 sessions.
Figure 1. Active responding and reward consumption for lever-press and nose-poke groups.
Active responding and reward consumption for male C57BL/6 mice assigned to lever-press (A, n=10) and nose-poke (B, n=9) groups over 10 days of FR1 training. While no obvious differences between NP and LP groups were noted with respect to these measures, the relative stability of consumption over active responding within groups is evident (note the smaller error bars), particularly for the LP group.
Criteria used to determine whether and when a particular subject acquires food-maintained operant responding include a minimum number of active responses or rewards earned, a measure of discrimination between active and inactive levers or nose-poke holes, and a measure of performance stability (e.g., [42-44]). Prior to establishing the acquisition criteria for our studies, we carefully examined the performance of all subjects over the ten FR1 sessions. Within the first 3 sessions, all subjects in both the LP and NP groups routinely engaged in active responding >30 times per session. Moreover, all subjects in both groups exhibited reasonable discrimination (>3:1) between active and inactive levers or nose-poke holes within 3 sessions. Active responding and pellets earned, however, varied dramatically between sessions for many subjects (see Supplemental Fig. S1). Indeed, the coefficient of variation for active responding ranged from 16-94% and 17-59% for the LP and NP groups, respectively, over the 10 test sessions. In contrast, reward consumption was a more stable within- and between-subjects parameter for the subjects, particularly for the LP group (%CV=14-38) (Fig. 1; Supplemental Fig. S1).
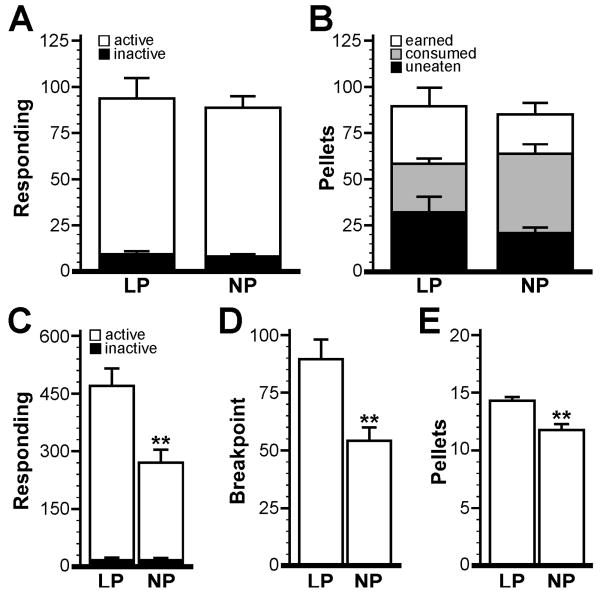
Given these observations, we used the following criteria to assess whether and when subjects successfully acquired food-maintained operant responding: 1) Responding: 30+ active responses per session, 2) Discrimination: a 3:1 ratio of active-to-inactive responding, and 3) Stability: 3 consecutive sessions during which reward consumption fell within ±15% of the mean for the 3-session block. Only one mouse in this study (from the NP group) failed to acquire food-maintained operant responding according to these criteria, and data from this subject were not included in the analysis. There was no difference between LP (2.9±0.5 d, n=10) and NP (2.7±0.4 d, n=9; P=0.74) groups with respect to acquisition rate, identified as the 1st day of the 3-day period during which all acquisition criteria were met. Similarly, active responding (LP: 93±12 vs. NP: 88±7; P=0.71), inactive responding (LP: 9±2 vs. NP: 8±1; P=0.61), rewards earned (LP: 89±11 pellets vs. NP: 85±7; P=0.76), rewards consumed (LP: 58±3 pellets vs. NP: 64±5; P=0.28), and the number of uneaten pellets (LP: 32±9 vs. NP: 21±3; P=0.28) did not differ between groups (Fig. 2A,B).
Figure 2. Impact of manipulanda on operant performance in mice.
A) Active and inactive responding for the lever-press (LP, n=10) and nose-poke (NP, n=9) groups. B) Pellets earned, consumed, and uneaten for the LP and NP groups. Group summary data presented in (A) and (B) were obtained by averaging parameters for each subject over the first block of 3 consecutive days wherein acquisition criteria were satisfied. Operant responding (C), breakpoint (D), and pellets earned (E) for the LP and NP groups during the 60-min PR test. Statistical symbols: ** P<0.01 vs. LP group.
After completing the FR1 sessions, all 19 subjects that successfully acquired food-maintained operant responding were evaluated in a single 60-min test employing a progressive ratio (PR) schedule of reinforcement. In this test, the LP group exhibited elevated active responding (467±49 lever presses, n=10; P<0.01), breakpoint (89±9 lever presses; P<0.01), and rewards earned (14.2±0.5 pellets; P<0.01) as compared to the NP group (270±32 nose-pokes, 54±5 breakpoint, and 11.8±0.4 pellets earned, n=9) (Fig. 2C-E). Inactive responding during the PR test did not differ between LP (12±2 presses) and NP (17±3 nose-pokes; P=0.11) groups. Thus, the lever-press design compared favorably with the nose-poke design with respect to the acquisition of food-maintained operant responding, while promoting elevated responding levels in a PR test.
Study 2. Session duration
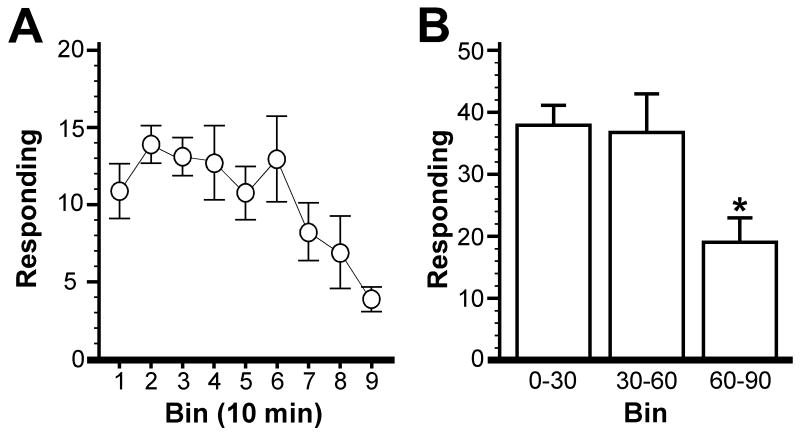
We noted in Study 1 that many pellets earned during FR1 sessions were not consumed and that responding on the active lever declined over the final 30 min of testing (Fig. 3A,B). Furthermore, visual monitoring of several experienced mice revealed that pellets earned early in the FR1 sessions were consumed almost immediately, while pellets earned later in the session tended to accumulate in the dispenser cup. As the subjects retrieved pellets later in the session, some pellets were dropped into the collection tray beneath the grid floor, while others were simply left in the dispenser cup. We speculated that 90-min testing sessions were excessive for mice under an FR1 schedule of reinforcement. Thus, we next probed the impact of session duration on operant performance in C57BL/6 mice.
Figure 3. Active responding for the lever-press group as a function of session time.
A) Average active responding at acquisition for mice in the lever-press group, divided into 10-min bins. Note the decline in active responding during the final 30 min of the session. B) Average active responding during the 0-30, 30-60, and 60-90 min intervals. Statistical symbols: * P<0.05 vs. 0-30 & 30-60 min bins.
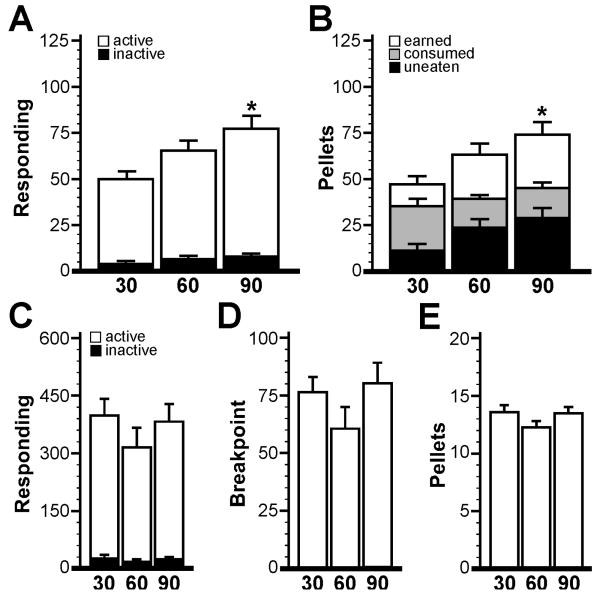
Male C57BL/6 mice were randomly assigned to 30-min, 60-min, and 90-min session groups (n=10 per group) and were evaluated using a lever-press design as in Study 1. Data from two mice in the 30-min session group were excluded from analysis due to technical problems with the chamber/computer interface. While all remaining mice in this study satisfied the acquisition criteria defined in Study 1, acquisition rates did differ substantially between groups (F2,25=13.8; P<0.0001). Subjects in the 90-min session group acquired food-maintained operant responding significantly faster (2.8±0.2 d, n=10; P<0.001) than the other groups, while subjects in the 60-min session group (4.6±0.6 d, n=10; P<0.05) reached acquisition faster than the 30-min session group (6.4±0.5 d, n=8). The delayed acquisition rates noted in the 30- and 60-min groups were attributable to an increase in the number of sessions required to achieve and maintain the minimum responding criterion (30+ active responses per session), as well as a delay in the number of sessions required to achieve consumption stability; in contrast, session length did not significantly impact the rate at which subjects achieved and maintained discrimination between active and inactive levers (Supplemental Fig. S2). Interestingly, an impact of session duration on acquisition rate (F2,25=14.4; P<0.0001) was observed even when the minimum responding criterion was scaled according to session duration (i.e., subjects in the 30-, 60-, and 90-min groups needed to exceed 10, 20, and 30 active presses, respectively). Thus, the delayed acquisition rates noted for subjects trained in the shorter FR1 sessions were not due solely to the imposition of an arbitrarily high minimum response criterion.
Analysis of operant performance at acquisition using the criteria defined in Study 1 revealed relatively modest differences between the 30-, 60-, and 90-min groups. While there was an impact of session duration on active responding (F2,25=4.4; P=0.02), the difference was only seen between the 90-min (77±7 active presses; P<0.05) and 30-min (50±4 active presses) groups (Fig. 4A). Similarly, session duration impacted rewards earned (F2,25=4.8; P=0.02), with mice in the 90-min session group earning more pellets (74±7 pellets, P<0.05) than mice in the 30-min group (47±4 pellets) (Fig. 4B). Consistent with the contention that most responding and consumption occurs during the early stages of a session, however, we observed no significant impact of session duration on pellet consumption (F2,25=2.5; P=0.10). Moreover, a trend was observed with respect to the number of uneaten pellets (F2,25=2.8; P=0.08), with mice in the 30-min session group leaving fewer than half of the number of pellets left by the 60- and 90-min groups (Fig. 4B). Regardless of session duration, subjects who satisfied acquisition criteria performed indistinguishably during the 60-min PR test in terms of active (F2,25=0.9; P=0.44) and inactive (F2,25=2.2; P=0.13) responding, breakpoint (F2,25=1.5; P=0.24), and rewards earned (F2,25=1.6; P=0.22) (Fig. 4C-E). Thus, while 90-min session lengths promote faster acquisition of food-maintained operant responding, extending test sessions beyond 60 min does little to improve performance during FR1 and subsequent PR testing. Moreover, 30 min sessions appear to promote a better (though not perfect) correspondence between rewards earned and consumed, while maintaining reasonable responding levels.
Figure 4. Impact of session duration on operant performance in mice.
A) Active and inactive responding for the 30-min (30), 60-min (60), and 90-min (90) session groups (n=8-10 per group) at acquisition. B) Pellets earned, consumed, and uneaten for the three groups at acquisition. Operant responding (C), breakpoint (D), and pellets earned (E) for the three groups during the 60-min PR test. Statistical symbols: * P<0.05 vs. 30-min group.
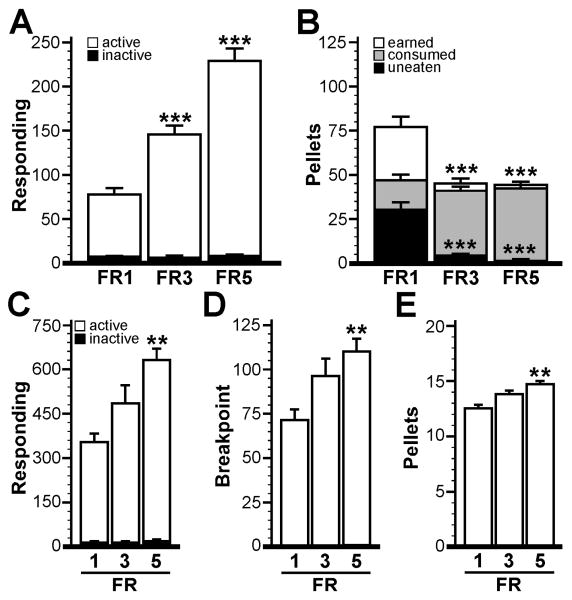
Study 3. Schedule of reinforcement
While slightly better than seen for mice trained in 60- and 90-min sessions, the fraction of unconsumed rewards was still substantial for the 30-min session group (∼25%). Thus, we next evaluated operant responding and reward consumption using FR3 and FR5 schedules of reinforcement, reasoning that by increasing the unit cost of each reward [45], we might improve the correspondence between rewards earned and consumed. On Days 1 and 2 of testing, male C57BL/6J mice (n=36) were acclimated to operant testing during 60-min sessions using an FR1 schedule of reinforcement. On Day 3, subjects were randomly assigned to three groups, one of which continued for the next 8 days on an FR1 schedule of reinforcement, while the other two groups continued with either FR3 or FR5 scheduling. Assessments of acquisition rate and stability began on Day 3 with subject assignment to their permanent fixed ratio scheduling groups. One subject in each of the FR1 and FR3 groups, and two subjects in the FR5 group, failed to meet acquisition criteria. All data pertaining to these animals were excluded from analysis.
There were no differences between FR1 (2.9±0.4 d, n=11), FR3 (2.4±0.4 d, n=11), and FR5 (2.1±0.4 d, n=10) groups with respect to acquisition rate (F2,29=1.1; P=0.34). Consistent with published observations (e.g., [46]), mice in the FR3 (146±9 presses) and FR5 (229±13 presses) groups exhibited elevated active responding (F2,29=62.2; P<0.0001) as compared to mice in the FR1 group (78±6 presses) (Fig. 5A). No group-dependent differences were observed for inactive lever responding (F2,29=0.4; P=0.71). Interestingly, whereas mice in the FR1 group (77±6 pellets) earned significantly more pellets than subjects in the FR3 (45±3 pellets) and FR5 (44±2 pellets) groups (F2,29=23.0; P<0.0001), there were few if any pellets remaining at the end of sessions for mice in the FR3 (4±1) and FR5 (1±0.4) groups, in contrast to the FR1 group (30±4 uneaten pellets) (Fig. 5B). Indeed, there was no difference between groups with respect to reward consumption (F2,29=1.7; P=0.19).
Figure 5. Impact of the response-reward contingency on operant performance in mice.
A) Active and inactive responding for the FR1, FR3, and FR5 groups (n=10-11 per group) at acquisition. B) Pellets earned, consumed, and uneaten for the three groups at acquisition. Note that there was no difference between groups with respect to pellets consumed during testing; statistical symbols in B refer to the comparison between groups with respect to pellets earned and uneaten during testing. Operant responding (C), breakpoint (D), and pellets earned (E) for the three groups during the 60-min PR test. Statistical symbols: **,*** P<0.01 and 0.001, respectively, vs. FR1 group.
During the 60-min PR test, clear differences were observed between groups with respect to active responding (F2,29=8.7; P=0.001), breakpoint (F2,29=6.2; P=0.006), and pellets earned (F2,29=8.2; P=0.002). Mice in the FR5 group outperformed mice in the FR1 group for active responding (631±40 vs. 353±31 lever presses; P<0.001), breakpoint (110±7 vs. 71±6 lever presses; p<0.01), and pellets earned (14.7±0.3 vs. 12.5±0.4; P<0.001) (Fig. 5C-E). In all instances, the performance of the FR3 group fell in between measures for the FR1 and FR5 groups. No group differences were observed with respect to inactive responding during the PR test (F2,29=0.9; P=0.40).
Study 4. Housing environment
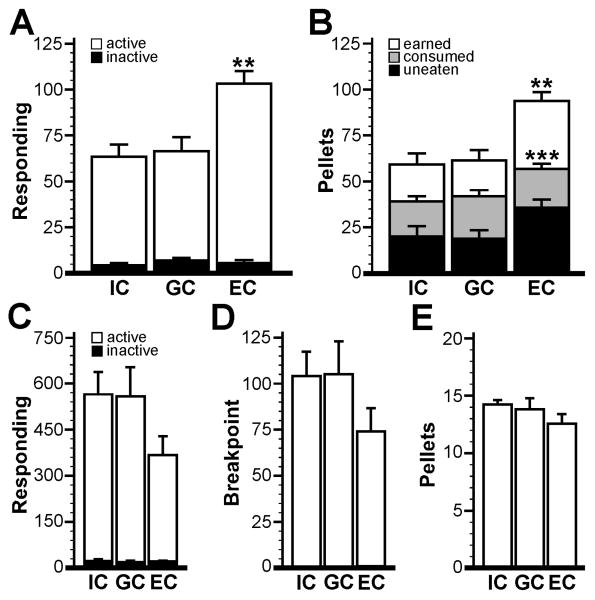
Single-housing facilitates controlled food deprivation and body weight regulation in rodents, important considerations for operant studies. Single-housing, however, is also a significant stressor for mice, leading to up-regulation of the HPA axis and altered behavior [47]. Group housing and environmental enrichment, in contrast, are correlated with reduced stress levels in mice and have been shown to decrease the reinforcing effects of addictive drugs and to promote faster learning in operant tasks [48]. To evaluate the impact of housing status on operant performance for food in mice, C57BL/6 mice were randomly assigned to one of three groups at 3-4 weeks of age: 1) Isolated condition (IC). IC subjects were group-housed in small rodent cages (n=3/cage, 3 separate cages) containing only bedding and nesting material for 1 month prior to testing. 2) Grouped condition (GC). Mice in the GC were group-housed (n=9) in a large rodent housing cage containing only bedding and nesting material for 1 month prior to testing. 3) Enriched grouped condition (EC). Mice in the EC were group-housed (n=9) for 1 month in a large rodent housing cage containing at least one running wheel at all times, along with several houses and toys of different shapes and colors, as described [49]. Enrichment materials were switched and rotated once per week to provide a fresh layout and color scheme. Whereas IC mice were single-housed from the point of food deprivation to the end of the operant study, GC and EC subjects remained group-housed throughout the study. For the GC and EC groups, food deprivation was accomplished by eliminating food from the housing cage. After each FR1 session, each subject was isolated for 2 h in a small cage containing 2.2 g of rodent chow. No differences in body weights (absolute or relative to free-feeding weights) were observed between groups (not shown).
Mice were tested in 60-min sessions using an FR1 schedule of reinforcement. All mice acquired food-maintained operant responding in the IC group. One subject in the EC group died within a week of arrival to the mouse colony, while 3 animals (1 GC and 2 EC) failed to satisfy acquisition criteria. Data from these mice were not included in the final dataset. No impact of housing condition on acquisition rate was observed (F2,20=1.8; P=0.19), though mice in the EC group (2.5±0.3 d, n=6) tended to acquire operant responding faster than subjects in the GC (4.6±0.9 d, n=8) or the IC (3.9±0.6 d, n=9) groups. Significant housing-dependent differences were observed, however, with respect to active lever responding (F2,20=8.1; P=0.002), rewards earned (F2,20=9.4; P=0.001), and rewards consumed (F2,20=14.7; P=0.0001) (Fig. 6A,B); the EC group outperformed both the GC and IC groups, which exhibited comparable performance. Neither inactive responding (F2,20=1.0; P=0.40, Fig. 6A) nor uneaten pellets (F2,20=3.2; P=0.06, Fig. 6B) differed significantly between groups. Similarly, no group differences were observed during the 60-min PR test with respect to active (F2,20=1.4; P=0.26) and inactive (F2,20=0.8; P=0.48) responding, breakpoint (F2,20=1.5; P=0.24), or pellets earned (F2,20=1.2; P=0.31); the EC group did, however, tend to exhibit lower responding (Fig. 6C-E).
Figure 6. Impact of housing condition on operant performance in mice.
A) Active and inactive responding at acquisition for mice housed individually (IC, n=9), in groups (GC, n=8), and in groups with environmental enrichment (EC, n=6). B) Pellets earned, consumed, and uneaten at acquisition for the IC, GC, and EC groups. Operant responding (C), breakpoint (D), and pellets earned (E) for the three groups during the 60-min PR test. Statistical symbols: **,*** p<0.01 and 0.001, respectively, vs. the IC and GC groups.
Study 5. Strain
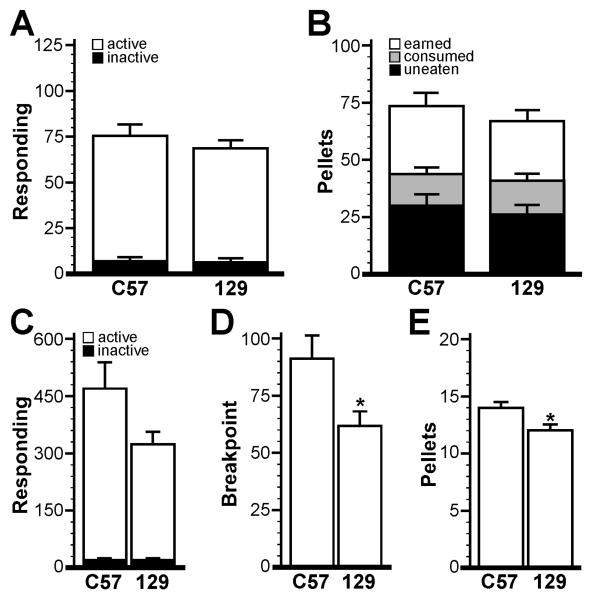
Most existing knockout mouse lines were generated by manipulation of embryonic stem cells derived from 129/Sv mice [50]. These targeted ES cells were used to generate chimeric mice that were typically bred with animals of the C57BL/6 strain to facilitate detection of germ-line transmission of the mutant allele. Despite extensive backcrossing of the null or targeted mutations against the C57BL/6 strain, significant 129/Sv-based genomic sequence flanking the targeted gene (differential chromosome segment) exists which can influence behaviors independent of the targeted mutation. As such, it is important to ascertain the baseline behaviors of the default genetic background on which the missing genes are developed prior to analyzing mutant animals. Thus, we next evaluated C57BL/6 and 129/Sv mice (n=9 per strain) using 60-min FR1 sessions and isolated housing conditions.
All of the 129/Sv mice reached acquisition while one C57BL/6 mouse did not; data from this subject were excluded from analysis. C57BL/6 mice (2.8±0.3 d; P=0.08) and 129/Sv mice (3.9±0.4 d) did not differ with respect to rate of acquisition of food-maintained operant responding. Similarly, the performance of the two strains during the 3-d acquisition period was indistinguishable (Fig. 7A,B). C57BL/6 mice, however, did tend toward elevated active responding (471±67 presses; P=0.06) as compared to 129/Sv mice (321±37), and exhibited elevated breakpoints (91±12 vs. 62±6; P<0.05) and earned more rewards (14±0.5 vs. 12±0.5; P<0.05) than 129/Sv mice during the 60-min PR test (Fig. 7C-E). Inactive responding during PR training was no different between the two strains.
Figure 7. Impact of strain on operant performance in mice.
A) Active and inactive responding at acquisition for C57BL/6 (n=8) and 129/Sv (n=9) groups. B) Pellets earned, consumed, and uneaten at acquisition for C57BL/6 and 129/Sv groups. Operant responding (C), breakpoint (D), and pellets earned (E) for the two groups during the 60-min PR test. Statistical symbols: * p<0.05 vs. the C57BL/6 group.
Discussion
While mice are certainly capable of performing instrumental learning tasks involving both natural and drug rewards, surprisingly few studies have systematically evaluated the impact of study design variables on operant performance. Here, we evaluated the influence of five factors that often differ across laboratories on the performance of mice in an operant task involving solid food pellets as the reinforcing agent. With some important exceptions (discussed below), we found that operant performance was relatively insensitive to manipulation of these study design variables.
Some published studies have suggested that nose-poke designs favor elevated rates of acquisition of food-maintained responding in mice relative to comparable studies involving lever-press [43, 51]. Other studies have concluded that the lever-press is comparable to nose-poke in operant responding tasks maintained by appetitive stimuli [44, 52]. We observed no difference in acquisition rate, responding, discrimination, reward consumption, or stability between the lever-press and nose-poke groups during FR1 sessions. We did find, however, that the inter-subject variability in reward consumption was slightly lower for mice in the lever-press group, and that mice in the lever-press group exhibited elevated active responding and rewards earned during a 60-min PR test. Thus, we suggest that lever press designs compare favorably with nose-poke designs during FR1 training sessions involving food as reinforcer, and may prove beneficial for studies culminating in PR testing.
A wide range of training session durations (15-min to 3-h) has been employed for food-maintained operant studies involving mice (e.g., [39, 46]). Previous food-maintained operant responding studies from our lab involved 3-h sessions, which imposed a significant practical constraint on the daily throughput of the assay [39, 41]. Here, we found slight differences in responding between groups tested using 60- and 90-min FR1 sessions, and no difference in reward consumption across groups tested using 30-, 60-, or 90-min FR1 sessions. Indeed, mice in our studies adapted to different session durations (and response-reward contingencies) to ensure consumption of ∼40 pellets (∼0.8 g), close to the meal size reported for male mice with experimental conditions similar to those described herein [37, 53]. Thus, one might reasonably conclude that the food-restricted mice in our studies behaved in a manner that allowed consumption sufficient to achieve satiation, and that 30-min under FR1 scheduling (and 60-min under higher order FR1 schedules) is sufficient to allow for completion of a single meal.
It is important to note that mice trained in 90-min FR1 sessions did acquire food-maintained operant responding significantly faster than mice in the 30- and 60-min session groups. As such, 90-min sessions (perhaps combined with FR3 or FR5 scheduling) may be warranted if faster acquisition of operant behavior is preferred, such as in studies of factors (genetic, pharmacologic, biological, social) that might delay acquisition, or for operant training prior to intravenous cannulation surgery and subsequent intravenous drug self-administration studies. In contrast, shorter 30-min sessions could be employed in studies examining factors that might increase the acquisition of food-maintained responding. In our experience, 60-min test sessions struck a suitable balance between experimental throughput and responding, while facilitating a moderate acquisition rate.
The relationship between rewards earned and consumed during testing was one of the more interesting aspects of this study. In intravenous drug self-administration studies, the schedule of reinforcement strictly defines the relationship between active responding and administration. In an operant task involving solid food pellets as the reinforcing agent, however, the subject can decide whether or not to “administer” the reward. As such, the relationship between the behavior and the biological actions of the reward is not necessarily simple or constant, and is subject to influence by many factors including satiety. In this regard, the composition of the food reinforcer may have a significant impact on operant performance. Indeed, humans sate more slowly with liquid as compared to solid food reinforcers (e.g., [54-59]). If satiety underlies the disconnect between rewards earned and consumed seen in our studies, then perhaps the use of liquid food reinforcers may promote a stronger correspondence between these two parameters.
Given that FR1 scheduling is often used for training purposes, the significance of an imperfect relationship between rewards earned and consumed is unclear. Surplus or wasted rewards during FR1 sessions may constitute an important facet of reward-related behavior. Accordingly, by tracking both the number of rewards earned and consumed during FR1 sessions, one might gain additional insight into the influence of genetic, biological, social, or pharmacological manipulations on reward-related behavior. From another perspective, designs that facilitate or ensure a strict correspondence between rewards earned and consumed might be preferable for some studies. Our work is consistent with published observations showing that a strict correspondence can be achieved with higher-order FR scheduling [45]. Moreover, training mice on FR3 or FR5 schedules of reinforcement may be useful for studies culminating in PR testing. Indeed, our findings show that subjects trained in higher order FR schedules exhibited significantly elevated responding, breakpoint, and rewards earned during PR testing.
The precise impact of housing conditions on experimental outcomes in behavioral studies involving mice is unresolved and likely to vary depending upon strain, gender, age, and task. In many instances, mice are housed individually, a condition contrary to their normal social structure and which may trigger stress reaction not seen in group-housed counterparts [60-62]. Elevated stress responses in single-housed mice could, for example, explain abnormal behaviors seen in behavioral tests of anxiety in mice [63, 64]. We found that maintenance of subject body weight at a desired target (∼90% of free feeding weight) was easier when mice were housed individually, in part due to the superior control over food allotment per subject as compared to group-housed conditions. Importantly, male C57BL/6 mice housed in isolation or in groups performed indistinguishably during FR1 sessions and the PR test, not unlike observations involving this strain of mice and several other behavioral tests (e.g., [64, 65]). Thus, isolation housing does not compromise performance in a food-maintained operant task, at least for male C57BL/6 mice.
While social isolation in rodents has been linked to increased reinforcing effects of drugs and vulnerability to drug addiction [66-68]), environmental enrichment has been linked to improved brain function and prevention/reversal of several pathological conditions, including drug dependence [69-73]. Indeed, environmental enrichment was shown to reduce the reinforcing effects of drugs of abuse, and to reverse evidence of cocaine dependence in mice [49, 74, 75]. We found that environmental enrichment for C57BL/6 mice increased the acquisition rate of food-maintained operant responding while decreasing responding during the PR test. Moreover, mice housed in enriched conditions dramatically out-performed animals housed in either unenriched group or single-housing conditions with respect to active responding, rewards earned, and rewards consumed during the FR1 test. Altogether, these observations are consistent with the documented impact of environmental enrichment on food-maintained operant responding in the rat [48, 70, 76, 77].
Strain can dramatically influence the performance of mice in many behavioral tests [78, 79]. C57BL/6 mice tend to exhibit average responses in a wide variety of behavioral tests, one reason why this strain is a popular choice for line propagation and back-crossing [79]. 129/Sv mice, in contrast, tend to perform poorly in many tasks, particularly in studies of learning and memory [78, 80]. Consistent with these tendencies, C57BL/6 mice demonstrated elevated responding in a nose-poke task for liquid food as compared to 129 mice [81]. In our hands, C57BL/6 and 129/Sv mice behaved similarly during FR1 sessions, though 129/Sv mice did tend to exhibit slower acquisition of food-maintained operant responding. Strain-dependent differences in PR performance were observed, however, with C57BL/6 mice outperforming 129/Sv mice. Altogether, our observations suggest that epistatic genes from the 129/Sv strain are unlikely to explain elevated responding for food during PR testing reported in some knockout mouse lines [39, 41].
In summary, we conclude that mice tolerate wide variation in multiple design elements in food-maintained operant testing paradigms. For some variables (housing, session duration, manipulanda), practical considerations such as cost, time, and cage space can drive experimental design without significant impact on study outcomes. In other cases (session duration, environmental enrichment, and schedule of reinforcement), designs can be manipulated to create a wider dynamic window for evaluation of factors that may either increase or decrease operant performance.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Stan Floresco for his assistance with Med-PC programming and for his helpful suggestions throughout the course of the study. We would also like to thank members of the Wickman laboratory for providing helpful input on the manuscript, and Tristan Driscoll for his assistance with the mouse colony. This project was funded by NIH grants MH061933 and DA011806 (KW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Skinner BF, Morse WH. Concurrent activity under fixed-interval reinforcement. J Comp Physiol Psychol. 1957;50:279–281. doi: 10.1037/h0047021. [DOI] [PubMed] [Google Scholar]
- 2.Morse WH, Skinner BF. Some factors involved in the stimulus control of operant behavior. J Exp Anal Behav. 1958;1:103–107. doi: 10.1901/jeab.1958.1-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skinner BF. Diagramming schedules of reinforcement. J Exp Anal Behav. 1958;1:67–68. doi: 10.1901/jeab.1958.1-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amit Z, Smith BR, Gill K. Serotonin uptake inhibitors: effects on motivated consummatory behaviors. J Clin Psychiatry. 1991;52(Suppl):55–60. [PubMed] [Google Scholar]
- 5.Self DW, Stein L. Pertussis toxin attenuates intracranial morphine self- administration. Pharmacol Biochem Behav. 1993;46:689–695. doi: 10.1016/0091-3057(93)90563-9. [DOI] [PubMed] [Google Scholar]
- 6.Rudski JM, Billington CJ, Levine AS. Naloxone's effects on operant responding depend upon level of deprivation. Pharmacol Biochem Behav. 1994;49:377–383. doi: 10.1016/0091-3057(94)90437-5. [DOI] [PubMed] [Google Scholar]
- 7.Self DW, Belluzzi JD, Kossuth S, Stein L. Self-administration of the D1 agonist SKF 82958 is mediated by D1, not D2, receptors. Psychopharmacology (Berl) 1996;123:303–306. doi: 10.1007/BF02246638. [DOI] [PubMed] [Google Scholar]
- 8.Salamone JD, Correa M, Mingote S, Weber SM. Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: implications for studies of natural motivation, psychiatry, and drug abuse. J Pharmacol Exp Ther. 2003;305:1–8. doi: 10.1124/jpet.102.035063. [DOI] [PubMed] [Google Scholar]
- 9.Kelley, AE Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Floresco SB, McLaughlin RJ, Haluk DM. Opposing roles for the nucleus accumbens core and shell in cue-induced reinstatement of food-seeking behavior. Neuroscience. 2008;154:877–884. doi: 10.1016/j.neuroscience.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Wenger GR, Dews PB. The effects of phencyclidine, ketamine, delta- amphetamine and pentobarbital on schedule-controlled behavior in the mouse. J Pharmacol Exp Ther. 1976;196:616–624. [PubMed] [Google Scholar]
- 12.Harris AD, Snell D, Loh HH. Effects of stimulants, anorectics, and related drugs on schedule-controlled behavior. Psychopharmacology (Berl) 1978;56:49–55. doi: 10.1007/BF00571408. [DOI] [PubMed] [Google Scholar]
- 13.McKim WA. The effect of caffeine, theophylline and amphetamine on operant responding of the mouse. Psychopharmacology (Berl) 1980;68:135–138. doi: 10.1007/BF00432130. [DOI] [PubMed] [Google Scholar]
- 14.Hendry JS, Rosecrans JA. The development of pharmacological tolerance to the effect of nicotine on schedule-controlled responding in mice. Psychopharmacology (Berl) 1982;77:339–343. doi: 10.1007/BF00432767. [DOI] [PubMed] [Google Scholar]
- 15.Katz JL. Effects of clonidine and some alpha-adrenergic antagonists alone and in combination on schedule-controlled behavior in pigeons and mice. Psychopharmacology (Berl) 1984;83:38–43. doi: 10.1007/BF00427419. [DOI] [PubMed] [Google Scholar]
- 16.Glowa JR. Some effects of d-amphetamine, caffeine, nicotine and cocaine on schedule-controlled responding of the mouse. Neuropharmacology. 1986;25:1127–1135. doi: 10.1016/0028-3908(86)90160-7. [DOI] [PubMed] [Google Scholar]
- 17.Rosecrans JA, Glennon RA. The effect of MDA and MDMA (“Ecstasy”) isomers in combination with pirenpirone on operant responding in mice. Pharmacol Biochem Behav. 1987;28:39–42. doi: 10.1016/0091-3057(87)90008-6. [DOI] [PubMed] [Google Scholar]
- 18.Solomon RE, Goodrich JE, Katz JL. Opioid receptor subtype-specific cross-tolerance to the effects of morphine on schedule-controlled behavior in mice. Psychopharmacology (Berl) 1988;96:218–222. doi: 10.1007/BF00177563. [DOI] [PubMed] [Google Scholar]
- 19.Katz JL. Interactions of clonidine and naloxone on schedule-controlled behavior in opioid-naive mice. Psychopharmacology (Berl) 1989;98:445–447. doi: 10.1007/BF00441939. [DOI] [PubMed] [Google Scholar]
- 20.Tidey JW, Miczek KA. Effects of SKF 38393 and quinpirole on aggressive, motor and schedule-controlled behaviors in mice. Behav Pharmacol. 1992;3:553–565. [PubMed] [Google Scholar]
- 21.Miczek KA, Haney M. Psychomotor stimulant effects of d- amphetamine, MDMA and PCP: aggressive and schedule-controlled behavior in mice. Psychopharmacology (Berl) 1994;115:358–365. doi: 10.1007/BF02245077. [DOI] [PubMed] [Google Scholar]
- 22.Miczek KA, de Almeida RM. Oral drug self-administration in the home cage of mice: alcohol-heightened aggression and inhibition by the 5-HT1B agonist anpirtoline. Psychopharmacology (Berl) 2001;157:421–429. doi: 10.1007/s002130100831. [DOI] [PubMed] [Google Scholar]
- 23.Martellotta MC, Kuzmin A, Zvartau E, Cossu G, Gessa GL, Fratta W. Isradipine inhibits nicotine intravenous self-administration in drug-naive mice. Pharmacol Biochem Behav. 1995;52:271–274. doi: 10.1016/0091-3057(95)00096-f. [DOI] [PubMed] [Google Scholar]
- 24.Elmer GI, Meisch RA, George FR. Oral ethanol reinforced behavior in inbred mice. Pharmacol Biochem Behav. 1986;24:1417–1421. doi: 10.1016/0091-3057(86)90204-2. [DOI] [PubMed] [Google Scholar]
- 25.Carney JM, Landrum RW, Cheng MS, Seale TW. Establishment of chronic intravenous drug self-administration in the C57BL/6J mouse. Neuroreport. 1991;2:477–480. doi: 10.1097/00001756-199108000-00017. [DOI] [PubMed] [Google Scholar]
- 26.George FR, Elmer GI, Meisch RA, Goldberg SR. Orally delivered cocaine functions as a positive reinforcer in C57BL/6J mice. Pharmacol Biochem Behav. 1991;38:897–903. doi: 10.1016/0091-3057(91)90260-9. [DOI] [PubMed] [Google Scholar]
- 27.Criswell HE, Ridings A. Intravenous self-administration of morphine by naive mice. Pharmacol Biochem Behav. 1983;18:467–470. doi: 10.1016/0091-3057(83)90471-9. [DOI] [PubMed] [Google Scholar]
- 28.Mendizabal V, Zimmer A, Maldonado R. Involvement of kappa/dynorphin system in WIN 55,212-2 self-administration in mice. Neuropsychopharmacology. 2006;31:1957–1966. doi: 10.1038/sj.npp.1300957. [DOI] [PubMed] [Google Scholar]
- 29.Goodrick CL. Learning and retention of a light contingent bar press response for three inbred strains of mice. J Psychol. 1967;67:191–199. doi: 10.1080/00223980.1967.10544916. [DOI] [PubMed] [Google Scholar]
- 30.Padeh B, Wahlsten D, DeFries JC. Operant discrimination learning and operant bar-pressing rates in inbred and heterogeneous laboratory mice. Behav Genet. 1974;4:383–393. doi: 10.1007/BF01066158. [DOI] [PubMed] [Google Scholar]
- 31.Rocha BA, Ator R, Emmett-Oglesby MW, Hen R. Intravenous cocaine self-administration in mice lacking 5-HT1B receptors. Pharmacol Biochem Behav. 1997;57:407–412. doi: 10.1016/s0091-3057(96)00444-3. [DOI] [PubMed] [Google Scholar]
- 32.Caine SB, Negus SS, Mello NK, Patel S, Bristow L, Kulagowski J, Vallone D, Saiardi A, Borrelli E. Role of dopamine D2-like receptors in cocaine self-administration: studies with D2 receptor mutant mice and novel D2 receptor antagonists. J Neurosci. 2002;22:2977–2988. doi: 10.1523/JNEUROSCI.22-07-02977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Risinger FO, Doan AM, Vickrey AC. Oral operant ethanol self- administration in 5-HT1b knockout mice. Behav Brain Res. 1999;102:211–215. doi: 10.1016/s0166-4328(99)00012-1. [DOI] [PubMed] [Google Scholar]
- 34.Risinger FO, Freeman PA, Rubinstein M, Low MJ, Grandy DK. Lack of operant ethanol self-administration in dopamine D2 receptor knockout mice. Psychopharmacology (Berl) 2000;152:343–350. doi: 10.1007/s002130000548. [DOI] [PubMed] [Google Scholar]
- 35.Elmer GI, Pieper JO, Rubinstein M, Low MJ, Grandy DK, Wise RA. Failure of intravenous morphine to serve as an effective instrumental reinforcer in dopamine D2 receptor knock-out mice. J Neurosci. 2002;22 doi: 10.1523/JNEUROSCI.22-10-j0004.2002. RC224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El-Ghundi M, O'Dowd BF, Erclik M, George SR. Attenuation of sucrose reinforcement in dopamine D1 receptor deficient mice. Eur J Neurosci. 2003;17:851–862. doi: 10.1046/j.1460-9568.2003.02496.x. [DOI] [PubMed] [Google Scholar]
- 37.Vaughan CH, Moore MC, Haskell-Luevano C, Rowland NE. Meal patterns and foraging in melanocortin receptor knockout mice. Physiol Behav. 2005;84:129–133. doi: 10.1016/j.physbeh.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 38.Vaughan C, Moore M, Haskell-Luevano C, Rowland NE. Food motivated behavior of melanocortin-4 receptor knockout mice under a progressive ratio schedule. Peptides. 2006;27:2829–2835. doi: 10.1016/j.peptides.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 39.Pravetoni M, Wickman K. Behavioral characterization of mice lacking GIRK/Kir3 channel subunits. Genes Brain Behav. 2008;7:523–531. doi: 10.1111/j.1601-183X.2008.00388.x. [DOI] [PubMed] [Google Scholar]
- 40.Hayward MD, Low MJ. The contribution of endogenous opioids to food reward is dependent on sex and background strain. Neuroscience. 2007;144:17–25. doi: 10.1016/j.neuroscience.2006.08.067. [DOI] [PubMed] [Google Scholar]
- 41.Perry CA, Pravetoni M, Teske JA, Aguado C, Erickson DJ, Medrano JF, Lujan R, Kotz CM, Wickman K. Predisposition to late-onset obesity in GIRK4 knockout mice. Proc Natl Acad Sci U S A. 2008;105:8148–8153. doi: 10.1073/pnas.0803261105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rocha BA, Scearce-Levie K, Lucas JJ, Hiroi N, Castanon N, Crabbe JC, Nestler EJ, Hen R. Increased vulnerability to cocaine in mice lacking the serotonin-1B receptor. Nature. 1998;393:175–178. doi: 10.1038/30259. [DOI] [PubMed] [Google Scholar]
- 43.Caine SB, Negus SS, Mello NK. Method for training operant responding and evaluating cocaine self-administration behavior in mutant mice. Psychopharmacology (Berl) 1999;147:22–24. doi: 10.1007/s002130051134. [DOI] [PubMed] [Google Scholar]
- 44.Colwill RM, Triola SM. Instrumental responding remains under the control of the consequent outcome after extended training. Behav Processes. 2002;57:51–64. doi: 10.1016/s0376-6357(01)00204-2. [DOI] [PubMed] [Google Scholar]
- 45.Chaney MA, Rowland NE. Food demand functions in mice. Appetite. 2008;51:669–675. doi: 10.1016/j.appet.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heyser CJ, McDonald JS, Beauchamp V, Koob GF, Gold LH. The effects of cocaine on operant responding for food in several strains of mice. Psychopharmacology (Berl) 1997;132:202–208. doi: 10.1007/s002130050337. [DOI] [PubMed] [Google Scholar]
- 47.Belz EE, Kennell JS, Czambel RK, Rubin RT, Rhodes ME. Environmental enrichment lowers stress-responsive hormones in singly housed male and female rats. Pharmacol Biochem Behav. 2003;76:481–486. doi: 10.1016/j.pbb.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 48.Wood DA, Rebec GV. Environmental enrichment alters neuronal processing in the nucleus accumbens core during appetitive conditioning. Brain Res. 2009;1259:59–67. doi: 10.1016/j.brainres.2008.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Solinas M, Chauvet C, Thiriet N, El Rawas R, Jaber M. Reversal of cocaine addiction by environmental enrichment. Proc Natl Acad Sci U S A. 2008;105:17145–17150. doi: 10.1073/pnas.0806889105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lariviere WR, Chesler EJ, Mogil JS. Transgenic studies of pain and analgesia: mutation or background genotype? J Pharmacol Exp Ther. 2001;297:467–473. [PubMed] [Google Scholar]
- 51.Goeders JE, Murnane KS, Banks ML, Fantegrossi WE. Escalation of food-maintained responding and sensitivity to the locomotor stimulant effects of cocaine in mice. Pharmacol Biochem Behav. 2009;93:67–74. doi: 10.1016/j.pbb.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McKerchar TL, Zarcone TJ, Fowler SC. Differential acquisition of lever pressing in inbred and outbred mice: comparison of one-lever and two-lever procedures and correlation with differences in locomotor activity. J Exp Anal Behav. 2005;84:339–356. doi: 10.1901/jeab.2005.95-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fox EA, Phillips RJ, Baronowsky EA, Byerly MS, Jones S, Powley TL. Neurotrophin-4 deficient mice have a loss of vagal intraganglionic mechanoreceptors from the small intestine and a disruption of short-term satiety. J Neurosci. 2001;21:8602–8615. doi: 10.1523/JNEUROSCI.21-21-08602.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haber GB, Heaton KW, Murphy D, Burroughs LF. Depletion and disruption of dietary fibre. Effects on satiety, plasma-glucose, and serum-insulin. Lancet. 1977;2:679–682. doi: 10.1016/s0140-6736(77)90494-9. [DOI] [PubMed] [Google Scholar]
- 55.Tournier A, Louis-Sylvestre J. Effect of the physical state of a food on subsequent intake in human subjects. Appetite. 1991;16:17–24. doi: 10.1016/0195-6663(91)90107-4. [DOI] [PubMed] [Google Scholar]
- 56.Hulshof T, De Graaf C, Weststrate JA. The effects of preloads varying in physical state and fat content on satiety and energy intake. Appetite. 1993;21:273–286. doi: 10.1006/appe.1993.1045. [DOI] [PubMed] [Google Scholar]
- 57.Mattes RD. Dietary compensation by humans for supplemental energy provided as ethanol or carbohydrate in fluids. Physiol Behav. 1996;59:179–187. doi: 10.1016/0031-9384(95)02007-1. [DOI] [PubMed] [Google Scholar]
- 58.Mattes RD, Rothacker D. Beverage viscosity is inversely related to postprandial hunger in humans. Physiol Behav. 2001;74:551–557. doi: 10.1016/s0031-9384(01)00597-2. [DOI] [PubMed] [Google Scholar]
- 59.Tsuchiya A, Almiron-Roig E, Lluch A, Guyonnet D, Drewnowski A. Higher satiety ratings following yogurt consumption relative to fruit drink or dairy fruit drink. J Am Diet Assoc. 2006;106:550–557. doi: 10.1016/j.jada.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 60.Brain PF, Nowell NW. The effects of differential grouping on endocrine function of mature male albino mice. Physiol Behav. 1970;5:907–910. doi: 10.1016/0031-9384(70)90180-0. [DOI] [PubMed] [Google Scholar]
- 61.Brain PF, Nowell NW. The effects of isolation as opposed to grouping on adrenal and gonadal function in male and female mice. J Endocrinol. 1970;46:xvi–xvii. [PubMed] [Google Scholar]
- 62.Valzelli L. The “isolation syndrome” in mice. Psychopharmacologia. 1973;31:305–320. doi: 10.1007/BF00421275. [DOI] [PubMed] [Google Scholar]
- 63.Ferrari PF, Palanza P, Parmigiani S, Rodgers RJ. Interindividual variability in Swiss male mice: relationship between social factors, aggression, and anxiety. Physiol Behav. 1998;63:821–827. doi: 10.1016/s0031-9384(97)00544-1. [DOI] [PubMed] [Google Scholar]
- 64.Voikar V, Polus A, Vasar E, Rauvala H. Long-term individual housing in C57BL/6J and DBA/2 mice: assessment of behavioral consequences. Genes Brain Behav. 2005;4:240–252. doi: 10.1111/j.1601-183X.2004.00106.x. [DOI] [PubMed] [Google Scholar]
- 65.Arndt SS, Laarakker MC, van Lith HA, van der Staay FJ, Gieling E, Salomons AR, van't Klooster J, Ohl F. Individual housing of mice--impact on behaviour and stress responses. Physiol Behav. 2009;97:385–393. doi: 10.1016/j.physbeh.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 66.Goeders NE, Clampitt DM. Potential role for the hypothalamo- pituitary-adrenal axis in the conditioned reinforcer-induced reinstatement of extinguished cocaine seeking in rats. Psychopharmacology (Berl) 2002;161:222–232. doi: 10.1007/s00213-002-1007-4. [DOI] [PubMed] [Google Scholar]
- 67.Marinelli M, Piazza PV. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur J Neurosci. 2002;16:387–394. doi: 10.1046/j.1460-9568.2002.02089.x. [DOI] [PubMed] [Google Scholar]
- 68.Lu L, Shepard JD, Hall FS, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neurosci Biobehav Rev. 2003;27:457–491. doi: 10.1016/s0149-7634(03)00073-3. [DOI] [PubMed] [Google Scholar]
- 69.van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 70.Bardo MT, Klebaur JE, Valone JM, Deaton C. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology (Berl) 2001;155:278–284. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- 71.Nithianantharajah J, Hannan AJ. Enriched environments, experience- dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7:697–709. doi: 10.1038/nrn1970. [DOI] [PubMed] [Google Scholar]
- 72.Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447:178–182. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- 73.Laviola G, Hannan AJ, Macri S, Solinas M, Jaber M. Effects of enriched environment on animal models of neurodegenerative diseases and psychiatric disorders. Neurobiol Dis. 2008;31:159–168. doi: 10.1016/j.nbd.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 74.Solinas M, Thiriet N, El Rawas R, Lardeux V, Jaber M. Environmental enrichment during early stages of life reduces the behavioral, neurochemical, and molecular effects of cocaine. Neuropsychopharmacology. 2009;34:1102–1111. doi: 10.1038/npp.2008.51. [DOI] [PubMed] [Google Scholar]
- 75.El Rawas R, Thiriet N, Lardeux V, Jaber M, Solinas M. Environmental enrichment decreases the rewarding but not the activating effects of heroin. Psychopharmacology (Berl) 2009;203:561–570. doi: 10.1007/s00213-008-1402-6. [DOI] [PubMed] [Google Scholar]
- 76.van der Harst JE, Baars AM, Spruijt BM. Standard housed rats are more sensitive to rewards than enriched housed rats as reflected by their anticipatory behaviour. Behav Brain Res. 2003;142:151–156. doi: 10.1016/s0166-4328(02)00403-5. [DOI] [PubMed] [Google Scholar]
- 77.Wood DA, Siegel AK, Rebec GV. Environmental enrichment reduces impulsivity during appetitive conditioning. Physiol Behav. 2006;88:132–137. doi: 10.1016/j.physbeh.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 78.Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- 79.Crawley JN. Behavioral phenotyping strategies for mutant mice. Neuron. 2008;57:809–818. doi: 10.1016/j.neuron.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 80.Livy DJ, Wahlsten D. Tests of genetic allelism between four inbred mouse strains with absent corpus callosum. J Hered. 1991;82:459–464. doi: 10.1093/oxfordjournals.jhered.a111128. [DOI] [PubMed] [Google Scholar]
- 81.Thomsen M, Caine SB. Cocaine self-administration under fixed and progressive ratio schedules of reinforcement: comparison of C57BL/6J, 129X1/SvJ, and 129S6/SvEvTac inbred mice. Psychopharmacology (Berl) 2006;184:145–154. doi: 10.1007/s00213-005-0207-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.