Abstract
Attaching and effacing Escherichia coli (AEEC) share the ability to induce pedestal formation and intimate adherence of the bacteria to the intestinal epithelial cell and effacement of microvilli of epithelial tissue. The Locus of Enterocyte Effacement (LEE) pathogenicity island encodes the ability to induce attaching and effacing (A/E) lesions and contains the gene eae, which encodes intimin, an outer membrane protein that is an adhesin for A/E lesion formation. Here we show the utility of using intimin as a vaccine to protect rabbits from challenge with rabbit Enteropathogenic E. coli (REPEC), a member of the AEEC family. The C-terminal portion of intimin was delivered by the attenuated Vibrio cholerae vaccine strain CVD 103-HgR. To export intimin, a fusion was engineered with ClyA, a secreted protein from Salmonella enterica serovar Typhi. After immunization, antibodies specific to intimin from serum and bile samples were detected and moderate protection against challenge with a virulent REPEC strain was observed. Compared to animals immunized with vector alone, intimin-immunized rabbits exhibited reduced fecal bacterial shedding, milder diarrheal symptoms, lower weight loss, and reduced colonization of REPEC in the cecum. V. cholerae CVD 103-HgR shows promise as a vector to deliver antigens and confer protection against AEEC pathogens.
Keywords: EHEC, EPEC, AEEC, REPEC, intimin, vaccine
1. Introduction
Attaching and effacing Escherichia coli (AEEC) represent a group of human and animal pathogens that produce a characteristic intestinal histopathology defined by attaching and effacing (A/E) lesions on epithelial cells in culture and in the intestine of experimentally inoculated animals [1, 2]. A/E histopathology results from intimate attachment of the bacteria to the epithelial cells, effacement of the microvilli, and rearrangement of the host cell actin cytoskeleton [3, 2]. Among AEEC are pathotypes such as Enterohemorrhagic E. coli (EHEC), Enteropathogenic E. coli (EPEC), atypical EPEC, rabbit EPEC (REPEC), and Citrobacter rodentium [1, 4, 5, 6, 7]. Together, these pathotypes form a family of pathogens able to cause disease in humans and a variety of animal hosts. The genes encoding the A/E phenotype are contained on a pathogenicity island called the Locus of Enterocyte Effacement (LEE) [8]. The LEE encodes proteins with a range of functions, including a type III secretion system (TTSS), various secreted effectors proteins, and their chaperones [8, 9]. The central region of the LEE contains the eae (E. coli attachment and effacement) gene encoding the 94 to 97 kDa outer membrane protein known as intimin [10]. This protein mediates close contact between the bacteria and the target cell upon interaction with its translocated receptor Tir (Translocated intimin receptor) [11]. The TTSS is responsible for secreting effector proteins into epithelial cells and modulating eukaryotic pathways to produce pedestal-like structures and effaced microvilli [2].
All eae alleles described to date demonstrate high similarity to each other in their N-terminal regions [12-15], but great diversity in their C-terminal regions, the region essential for Tir recognition and binding. Different intimin proteins are designated by using a Greek letter. Intimins of AEEC, including EPEC O127:H6 (α intimin), EHEC O157:H7 (γ intimin), and REPEC O15:H- (β intimin), show greater than 94% protein identity over two-thirds of the molecule on the N-terminal end, while demonstrating only 55% protein identity over the remaining C terminus, the region responsible for subtype classification [15, 16, 17]
To date, no other adhesin have been implicated as strongly as intimin in both epidemiological surveys and animal models against AEEC strains. Further evidence of the crucial role of intimin in immunogenicity is provided by studies in mice that show the last 280 amino acids of the C-terminal fragment (Int280) of EHEC can protect against homologous challenge [18, 12, 19, 20]. Passive immunization of neonatal piglets from dams immunized with an intact intimin molecule exhibited protection against EHEC O157:H7 colonization and intestinal damage [19]. All of these studies used vaccines made from purified C terminus of intimin, demonstrating that this domain is sufficient for protective immunity.
One approach to induce mucosal immunity is to delivery antigens via attenuated enteric vaccine strains such as the attenuated Salmonella enterica serovar Typhi live-vector vaccine strain CVD 908-htrA. Such an approach was successfully utilized to induce antibodies to Bacillus anthracis protective antigen (PA) [21]. This study used a new antigen export system engineered from an endogenous cryptic hemolysin (ClyA), of Salmonella enterica serovar Typhi, fused to the domain 4 (D4) moiety of (PA).
Vibrio cholerae has previously been used as a vector to deliver EHEC antigens. Butterton et al [22, 23] used the attenuated Peru-2 Vibrio cholerae strain carrying a chromosomal copy of the complete eae gene and found that one of two rabbits immunized with this strain developed antibodies to intimin. However, Peru2 transformed with a plasmid carrying stxB1 and eae under the transcriptional control of the V. cholerae heat shock promoter PhtpG was unstable and produced low levels of StxB1 in vitro. Animals immunized with this construct did not develop antibodies against intimin following oral immunization.
Here we report the use of an alternative attenuated V. cholerae vaccine strain, CVD 103-HgR, as a vector to deliver intimin derived from rabbit-specific E. coli strain RDEC-1. A relevant animal challenge model was used to demonstrate immunogenicity and partial protection in rabbits immunized with this construct.
2. Results
2.1 Construction and expression of ClyA fused to intimin
To export intimin across the bacterial cellular membrane, we employed the plasmid vector pSEC91 which contains the cytolysin A hemolysin of Salmonella enterica serovar Typhi (ClyA). Wai et al demonstrated that the ClyA is exported from the bacterial cell in outer-membrane vesicles and that this vesicle-mediated transport mechanism may contribute to the activation and delivery of pathogenic effector proteins [24]. An antigen fusion to ClyA has been shown to effectively deliver the domain 4 (D4) of the protective antigen (PA) from Bacillus anthracis to mice [21].
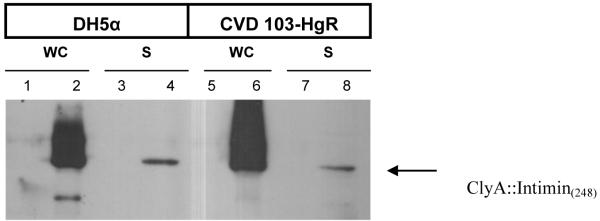
The 772 bp fragment encoding the C-terminus of the β-eae gene from REPEC strain RDEC-1- (GenBank accession no. U59503) was amplified using PCR and ligated into the Nhe I site of pSEC91 (~15 copies/chromosomal equivalent) immediately downstream of the ompC promoter (Fig. 1A). This region contains the immunodominant region of intimin and the resulting construct was named pInt248 as it contains first 248 residues of the Int280 C-terminal region (Fig. 1B). Plasmid pInt248 was electroporated into V. cholerae CVD 103-HgR, and the resulting strain was examined for β-intimin expression Whole-cell lysates from both E. coli DH5α pInt248 and V. cholerae CVD 103-HgR pInt248 were analyzed by western blot with anti-β intimin antisera and expression of β -intimin was observed from both hosts (Fig. 1C). ClyA::β intimin fusion protein was also detected in the supernatant from both strains, although in lower levels in comparison to the whole-cell extract (Fig. 1C). No protein was recognized by antiserum in whole-cell lysates from CVD 103-HgR (pSEC91) or DH5α (pSEC91) controls containing only the plasmid vector without eae sequences (Fig. 1C).
Figure 1.
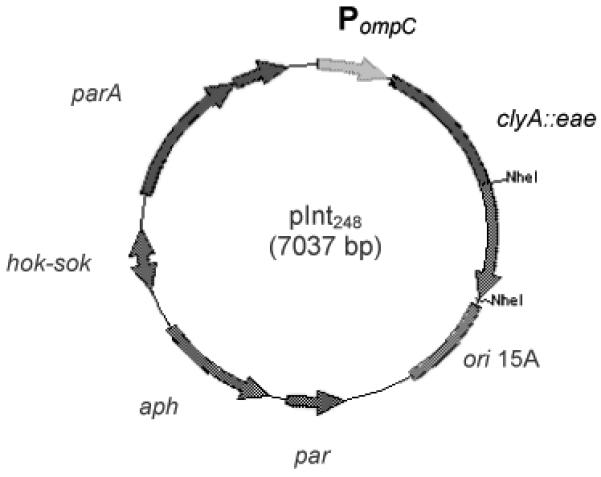
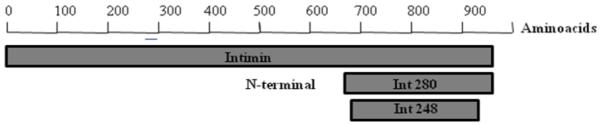
Intimin constructs (A) Genetic map of expression plasmid pInt248 derived from pSEC91encoding ClyA::Int248 fusion protein. Abbreviations: PompC, modified osmotically controlled ompC promoter from E. coli; clyA::eae(248), gene encoding intimin(248) of RDEC-1 fused to the carboxyl terminus of ClyA from Salmonella serovar Typhi; ori15A, origin of replication from p15A providing an expected copy number of ~15 per chromosomal equivalent; par, passive partitioning system from pSC101; aph, gene encoding the aminoglycoside 3′-phosphotransferase conferring resistance to kanamycin; hok-sok, postsegragational killing locus from the antibiotic resistance R plasmid pR1; and parA, gene encoding the active partitioning system from pR1. (B) Diagram showing the localization of Intimin248 in the complete intimin protein. (C) Western immunoblot analysis of bacterial whole cell fraction (WC) and supernatants(S). Whole cell fractions (WC) and supernatants (S) from either DH5α (pSEC91) (lanes 1 and 3), DH5α (pInt248) (lanes 2 and 4), CVD 103-HgR(pSEC91) (lanes 5 and 7), CVD 103-HgR (pInt248) (lanes 6 and 8) were transferred to a nitrocellulose membrane. After the transfer, the membranes were blocked and incubated with β-intimin antiserum.. ClyA::Intimin fusion protein was detected in the supernatant from both strains, although in lower levels in comparison to the whole-cell extract.
2.2. Assessment of immunogenicity of intimin in rabbits using CVD 103-HgR (pInt248)
To determine the ability of intimin to confer immunity in rabbits, experiments were performed examining the IgA levels in serum after oral vaccination with CVD 103-HgR (pInt248). Three groups of six rabbits were primed on day 1 with one of three inocula: PBS, CVD 103-HgR (pSEC91), or CVD 103-HgR (pInt248) and boosted on day 15 with the identical inoculum. Serum collected after inoculation (day 28) exhibited significantly higher levels of specific anti-intimin IgA antibodies than serum collected before exposure (day 0) in the rabbits who received CVD 103-HgR (pInt248) (p = 0.0046) (Fig. 2). There was no difference in the day 0 and day 28 responses in the rabbits who received CVD 103-HgR containing the vector only [CVD 103-HgR (pSEC91)]. Fecal shedding was examined for seven consecutive days for both strains after inoculation and booster to confirm delivery of bacteria to the rabbit intestinal track (data not shown).
Figure 2.
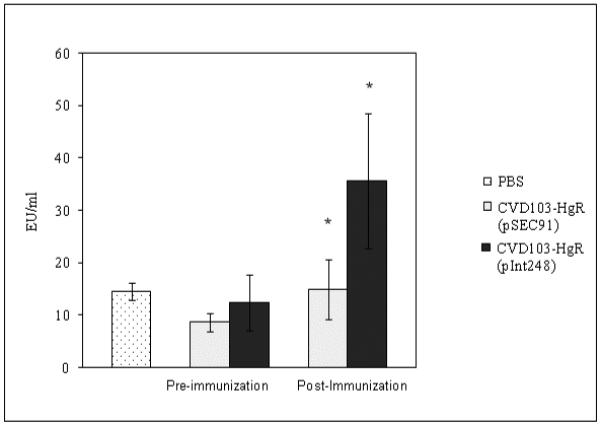
Serum anti-intimin IgA titers pre-immunization and post-immunization. Immunoglobulin A (IgA) antibodies specific for β-intimin measured by enzyme-linked immunosorbent assay (ELISA). Serum collected before exposure (day 0) and post-immunization (day 28th ) exhibited significantly higher levels of anti-intimin IgA specific antibodies in the rabbits who received CVD 103-HgR (pInt248) compared to CVD 103-HgR alone ( * p = 0.0046).
2.3. Protection against REPEC challenge after oral immunization
To ascertain whether intimin offers protection to rabbits immunized using our antigen delivery system, rabbits were challenged on day 29 with wild type REPEC strain E22 (6 × 107 CFU). Fecal characteristics, bacterial shedding in feces and weight loss were examined over a course of 6 consecutive days after challenge. All rabbits immunized with control construct CVD 103-HgR (pSEC91) developed diarrhea by the third day and two developed symptoms as early as day 1. Three rabbits were euthanized on day 3 and one on day 4 due to severity of the diarrhea, with only two surviving to the sixth day. Conversely, the subset of rabbits immunized with CVD 103-HgR (pInt248) did not develop diarrhea until the third day; one rabbit was euthanized at day 3 and one on day 4. The four remaining rabbits survived with diarrhea symptoms throughout the course of the study (6 days). Overall, throughout the course of infection, animals immunized with intimin before the challenge exhibited milder diarrheal symptoms than the group receiving the vector alone (Table 2), with the major difference being the number of animals having to be euthanized because of severe diarrhea being lower in the intimin-immunized group (2 of 6) compared to the sham-immunized group (4 of 6).
Table 2.
Characteristics of feces after vaccination with CVD 103-HgR (pSEC91) and CVD103-HgR (pInt248)*
| Rabbit Number |
Plasmid utilized |
Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 |
|---|---|---|---|---|---|---|---|
| 228 | N | N | BD † | ||||
| 229 | SS | N | DM | DM | D | D | |
| 230 | pSEC91 | DM | D | SD | SD † | ||
| 231 | N | SS | SD † | ||||
| 232 | N | SS | SD † | ||||
| 233 | N | N | D | DM | D | D | |
| 234 | N | SS | SD † | ||||
| 235 | N | SS | DM | DM | SS | D | |
| 236 | pInt248 | N | N | DM | SD † | ||
| 237 | N | N | SS | DM | SS | D | |
| 238 | N | N | DM | DM | D | D | |
| 239 | N | N | DM | DM | D | D |
Abbreviations: soft stools (SS), diarrhea (D), diarrhea with mucus (DM), bloody diarrhea (BD), and severe diarrhea (SD).
indicate that the rabbit had to be euthanized
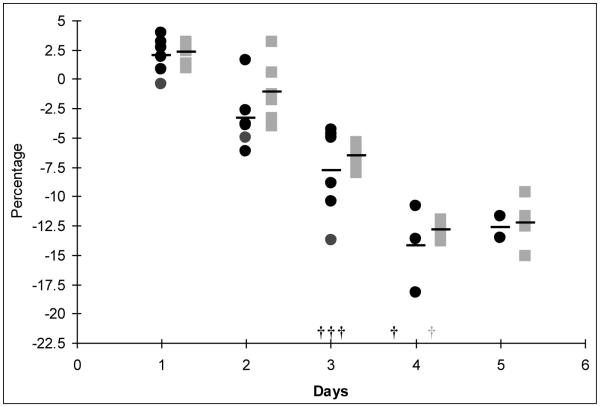
After challenge with REPEC E22, the feces were collected over a period of 5 days and bacterial shedding was assessed (Figure 3A). Shedding of REPEC E22 was lower in the rabbits immunized with intimin, and the difference was statistically significant at day 3 (p =0.03). The overall trend of REPEC E22 fecal shedding was consistently lower in the group who received intimin compared to vector alone, although a statistically significant difference was seen only on day 3.
Figure 3A.
REPEC shedding from rabbits. After challenge with the wild-type REPEC E22 feces were collected and plated to determine bacterial colony forming units (CFUs) per gram of feces. Rabbits inoculated with CVD 103-HgR (pSEC91) are depicted in black and those inoculated with CVD 103-HgR (pInt248) depicted in grey. The symbols indicate individual animals; the average in each group of rabbits is indicated by horizontal bars. Shedding of REPEC E22 was lower in the rabbits immunized with intimin, and was statistically significant at day 3 (p =0.03).
The percentage of weight loss was studied over a period of seven days post REPEC infection among animals primed with PBS (data not shown), CVD 103-HgR (pSEC91), and CVD 103-HgR (pInt248). Although the population immunized with intimin lost less weight than the other groups, the differences were not statistically significant using t-test analysis (Fig. 3B).
Figure 3B.
Percentage of weight loss in rabbits after challenge with REPEC E22. The rabbits were weighed every day after challenge with REPEC E22 and the weight loss or gain was represented as a percentage of their original weight. The group of rabbits receiving CVD 103-HgR(pSEC91) is presented in black and the group of rabbits receiving CVD 103-Hg (pInt248) is in grey. Each symbol indicates an individual animal; the average of each group is indicated by horizontal bars. Mortality is represented by crosses. Although the population immunized with intimin lost less weight than the other groups, the differences were not statistically significant.
To test for antibody levels against intimin, IgA levels in the bile were evaluated after euthanization (Fig. 3C). Rabbits immunized with intimin had IgA levels eight times higher than those seen in animals immunized with the vector alone (p = 0.032) or PBS (p = 0.033) following challenge.
Figure 3C.
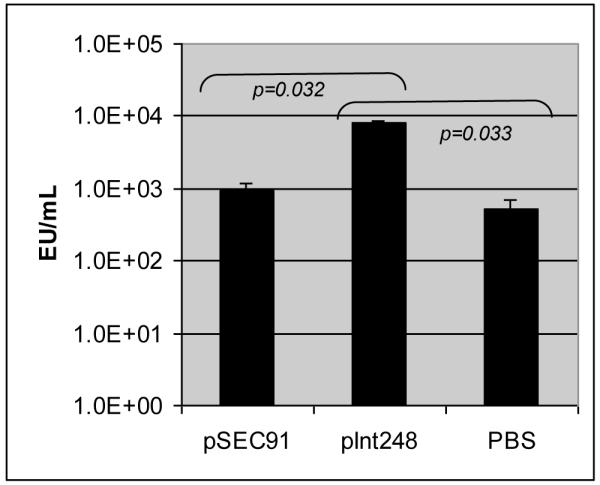
Bile anti-intimin IgA titers after REPEC challenge. Animals were immunized with CVD 103-HgR (pSEC91), CVD 103-Hg (pInt248) or PBS. Significant differences were found between animals immunized with CVD 103-Hg (pInt248) and those immunized with CVD 103-HgR (pSEC91) or PBS. The results shown are means of each with standard deviation indicated. Bile samples were collected either on day 6 or on earlier days, if the animals required euthanasia. Animals treated with PBS were not challenged with REPEC and bile samples were collected on day 6.
The amount of non-adherent REPEC E22 was assessed from the cecal contents post-mortem. Colony forming units were counted, and no statistically significant difference was found between rabbits exposed to CVD 103-HgR (pInt248) and CVD 103-HgR (pSEC91) (p =0.126) (data not shown).
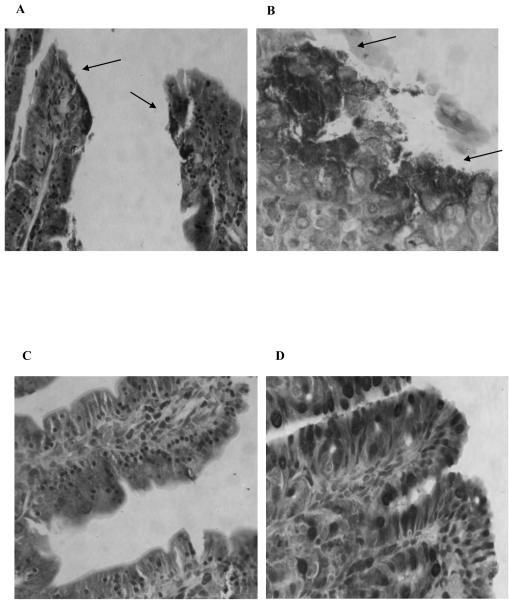
A powerful way to assess REPEC diarrheal disease is the evaluation of REPEC adherence, particularly in the cecum of the rabbits after challenge, in a quantitative manner. Bacterial adherence of the challenge organism was determined post-mortem by histological examination of intestinal sections and graded as the percentage of surface area in the microscopic field that was covered by closely adherent bacteria. The level of bacterial adherence in the cecum was lower (9%) in the group who received CVD 103-HgR expressing intimin than in the group who received the vector-only control (23%) (Fig. 3D). These results correlated with the level of microvilli effacement seen post mortem in the ileum, proximal cecum and distal cecum (Fig. 4). Microvilli effacement beneath adherent bacteria and irregular mucosal surfaces were seen in rabbits immunized with the vector control and then challenged with REPEC E22 (Fig. 4A and 4B) while normal mucosal surfaces were seen in animals vaccinated with intimin and then challenged (Fig. 4C and 4D).
Figure 3D.
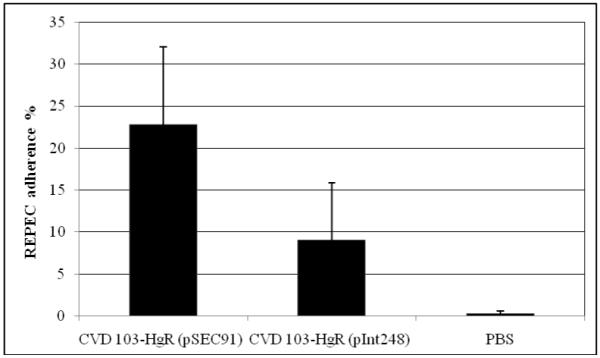
REPEC adherence in the cecum measured post mortem. Although the population immunized with intimin had less REPEC adhered than the other group the differences were not statistically significant. The results shown are means of each with standard deviation indicated. Bile samples were collected either on day 6 or on earlier days, if the animals required euthanasia. Animals treated with PBS were not challenged with REPEC and bile samples were collected on day 6.
Figure 4.
Representative histological sections of ileum (A) and proximal cecum in (B) of a rabbit from the control group immunized with CVD 103-HgR (pSEC91) and challenged with REPEC E22 showing effacement of microvilli (arrows). Note that the mucosal surface has become irregular. In contrast, sections from the ileum (C) and from the proximal cecum (D) of a rabbit from the group who received CVD 103-HgR (pInt248) and challenged with REPEC E22 strain showed normal histology of the mucosa. Giemsa staining: magnification X 400
3. Discussion and Conclusion
Antibodies against intimin are induced after both human and animal infections and are related to protection as well as inhibition of bacterial adhesion to epithelial cells in vitro [25, 26]. The carboxyl end of intimin (Int280) can be divided into two immunoglobulin-like domains (D1 and D2) and one lectin-like domain (D3) [27, 28]. Despite the variability of these regions among the intimin subtypes, amino acid residues C860, W899 and C937, located in D3, are highly conserved and important for protein stability, Tir binding, and A/E lesion induction [29, 28]. A minimal intimin fragment capable of binding Tir is composed of the last 190 amino acids in the Int280 sequence, but this smaller fragment binds with less affinity than the larger 280-residue fragment of Int280 [29]. Although the Tir-binding site is located at the C-terminal end of Int280, analysis of the immunodominant regions show that antibodies are directed towards two regions in the N-terminal of Int280 (residues 1 – 80 and 80 – 130) [12]. Human colostrum samples specifically recognize these fragments, but not the C-terminal region of Int280 [12]. Thus, natural exposure to intimin-bearing pathogens seems to elicit antibodies that are reactive only to the N-terminal region of Int280. In the present work, the intimin fragments expressed in V. cholerae CVD 103-HgR include the first 248 residues of Int280, and therefore the immunodominant region.
V. cholerae has been used to delivery a variety of foreign antigens including Shiga toxin (Stx) B subunit, fragment C of tetanus toxin, tracheal colonization factor of B. pertussis, Shigella sonnei O antigen, the immunogenic portion of Clostridium difficile toxin A, the intimin colonization factor of enterohemorrhagic E. coli, hepatitis B middle surface antigen, gp120 from HIV, and the serine-rich Entamoeba histolytica protein (reviewed in [30]). Although no human studies have yet been reported with any of these constructs, protective immunity has been demonstrated in a rabbit model against challenge with C. difficile toxin A and Stx holotoxin (reviewed in [30]).
CVD 103-HgR is a live, oral attenuated V. cholerae vaccine strain that is immunogenic, safe, and protective in humans and was licensed in several countries including Switzerland, Canada, and Australia [31, 32]. Previous research utilized this strain to induce protective antibodies in rabbits with a vector containing Shiga-like toxin 1B subunit (Stx-1B) [33]. Butterton et al used attenuated V. cholerae strain Peru-2 to express a variety of heterologous antigens such as fragment C of tetanus toxin and C. difficile toxin A and intimin of EHEC [22] [23]. In the present study, we utilized an antigen delivery system comprised of the cryptic hemolysin ClyA from Salmonella serovar Typhi Ty2 to express V. cholerae CVD 103-HgR. This system was previously reported by Galen et al to provide excellent secretion from Salmonella serovar Typhi of a Clya::D4 fusion of 50.2kDA encoding Protective Antigen (PA) of Bacillus anthracis, and the exported Clya::D4 induced an immune response in mice [21]. Our data demonstrates Int248 was highly expressed in V. cholerae CVD 103-HgR using the pSEC91 vector but was minimally secreted into the surrounding medium (Fig. 1C). The reason for the poor extracellular secretion seen is not known but may possibly be due to the hydrophobicity of the intimin fragment.
Although poorly secreted, the intimin fusion construct in V. cholerae was found to be immunogenic in rabbits as noted by bile and serum IgA response against intimin orally inoculated with this contruct. When the immunized animals were challenged with the wild type REPEC only partial protection against REPEC challenge was demonstrated as all rabbits developed diarrhea during the course of study. However, IgA levels were significantly increased, death and severity of illness much reduced, and histological samples indicated less tissue damage in the animals inoculated with CVD103-HgR (pInt248) compared to the CVD103-HgR (pSEC91) vector control. Considering the high mortality associated with the REPEC E22 strain, the challenge of 107 CFU may have been too high to observe full protection as it has a lethal dose of 6.7 × 103 CFU [34].
Although conclusive demonstration of protective efficacy was not demonstrated in this sturdy, our results suggest that attenuated V. cholerae expressing ClyA::intimin merits further study as a tool to confer protection against AEEC strains. The demonstration of protective efficacy may be improved by some changes in the experimental design. For example, we administered only two doses of the vaccine but three doses might prove to be more protective. A lower challenge dose more reflective of the natural infectious dose may also show increased protection. In the future additional proteins should be considered as potentially protective antigens such as EspA, EspB, or EspD to induce a broader immune response. In conclusion, V. cholerae CVD 103-HgR (pInt248) has potential as a delivery vehicle for a new generation of live oral attenuated heterologous AEEC vaccine candidates for animals and humans but further work is required to optimize the antigen delivery system.
4. Materials and Methods
4.1. Strains
Strains and plasmids used in this study are detailed in Table 1. The antibiotics Ampicillin (Ap) and Kanamycin (Km) were used at concentrations of 100 and 50 μg/ml respectively in Luria-Bertani (LB) medium or agar plates.
Table 1.
Strains and plasmids utilized in this study
4.2. Cloning of the beta-intimin fragment into pSEC91
The intimin fragment (Int248) was PCR amplified using primers K3894 (5′-ctagctagcagcattactgagattaaggc) and K3895 (5′-ctagctagctcatatgtgcttgatacwcc) that span the C terminal- end (D1, D2 and part of D3) of β intimin (residues 652 – 908 of RDEC-1 strain; GenBank accession no. U59503). The PCR protocol included the following cycles: denaturation at 94°C for 2 minutes followed by 34 cycles of 94°C for 30 seconds; 50°C for 30 seconds, 72°C for 1 minute, and final extension cycle at 72°C for 8 minutes. The 772 bp amplicon was purified using the Qiaquick PCR purification Kit (Qiagen). The β-intimin fragment and pSEC91 vector were digested with Nhe I and ligated using T4 DNA ligase (BioLabs). The ligation was transformed into competent E. coli DH-5α (Invitrogen) and V. cholerae CVD 103-HgR.
4.3. Screening of transformants by PCR
Both E. coli DH5α and V. cholerae CVD 103-HgR transformants were screened for the presence of clyA::int248 fusion by PCR using the clyA forward primer from pSEC91 (K 3901: 5′-tactagcttatcagctacag) and K3895. The PCR cycles remained the same as above for Int248 amplification. DNA sequence analysis was performed to confirm the constructs.
4.4. Cloning the beta-intimin fragment into pBAD102
The intimin fragment (Int248) was PCR amplified using primers K4096 (5′-caccgccagyattactgagattaaggct) and K4095 (5′-atcatatgtgcttgatacacc) of β intimin (residues 658 – 911 of RDEC-1 strain; GenBank accession no. U59503). PCR included the following cycles: denaturation at 94°C for 2 minutes followed by 34 cycles of 94°C for 15 seconds; 55°C for 30 seconds, 68°C for 1 minute, and final extension cycle at 68°C for 8 minutes. The 732 bp amplicon was purified using the Qiaquick PCR purification Kit (Qiagen). The β-intimin fragment was cloned into the pBAD102 vector following the manufacturer’s instruction for the pBAD Directional TOPO Expression Kits (Invitrogen).
4.5 Expression and Purification of His-tagged beta-intimin from pCVD473
The expression and purification of His-tagged beta-intimin protein was performed using Ni-NTA Purification System (Invitrogen).
4.6 Secreted protein by Western Blot analysis
CVD 103-HgR (pInt248), was grown overnight in 2ml LB and centrifuged at 3500 rpm for 10 minutes at 4° C (in a microcentrifuge). (The pellet was retained for the whole cell lysate analysis). Deoxycholic acid (5%) was added to the supernatant (10 μl) and mixed. Trichloroacetic acid solution 10% (Sigma Diagnostic, St. Louis, 200 μl) was added and the preparation was kept on ice for 15 min. The sample was centrifuged at 15,000 rpm for 10 min. at 4° C and the pellet was suspended in 2x SDS sample buffer and 2M Tris base (4:1). After boiling for 5 min., the samples were subjected to SDS-12% PAGE and transferred to Hybond-ECL ™ Nitrocellulose membranes (GE Healthcare) following the standard western blot procedure [35]. After the transfer, the membranes were blocked with 3% bovine serum albumin [BSA] and incubated with β-intimin antiserum (1:5000) (kindly provided by Flavia A. Lima from Institute Butantan, São Paulo, Brazil) diluted in PBS-0.1% Tween-20 for 1 hr with constant shaking at room temperature. The membranes were then incubated with secondary antibody (Goat α-rabbit IgG [diluted 1:10 000]) conjugated with horse-radish peroxidase [HRP] (Sigma). Membranes were washed three times consecutively after primary and secondary antibody incubations with PBS-0.1% Tween-20 for 5 min. each. The blots were developed with ECL plus Western Blotting Detection System (Amersham Biosciences) followed by X-ray film exposure.
4.7. Rabbit inoculation and immunization
The protocols for rabbit inoculation and immunization were done according to Zhu et al with some modifications [36]. Briefly, twelve male New Zealand White rabbits (approximately 7 weeks old) were anesthetized with xylazine and ketamine after a 12 hr fasting period. The rabbits were then given 50 mg cimetidine intravenously. After 15 minutes, a stomach tube was inserted in each rabbit and 10 ml of a 10% sodium bicarbonate solution was administered, followed by 3 ml of the bacterial inoculation (2 ×109 CFU) and 5 ml of PBS; 30 minutes later, each animal was given 1 ml of tincture of opium intraperitoneally. Three groups of six rabbits received one of the following: CVD 103-HgR (pSEC91), CVD 103-HgR (pInt248), or PBS. Rabbits were given a second oral inoculation of the same inoculum (1 ×1010 CFU) 15 days after the first inoculation. Blood was drawn from the rabbits prior to the inoculation at days 0, 14, and 28.
4.8. Challenge studies
Rabbits were challenged with REPEC E22 (6 × 107 CFU) 29 days after the first inoculation. Rabbits were weighed daily and observed for clinical signs of illness and stool characteristics six consecutive days post challenge, and euthanized at day 6. Stool characteristics were classified as: soft stools (SS), diarrheic (D), diarrhea with mucus (DM), bloody diarrhea (BD), and severe diarrhea (SD). Rabbits that lost greater than 20% body weight or experienced severe or bloody diarrhea were euthanized. Bile was aspirated from the rabbit gall bladder at euthanization, and bacterial counts of REPEC in cecal contents were determined by plating serial 10-fold dilutions of weighed cecal contents on LB agar supplemented with appropriate antibiotics.
4.9. Evaluation of IgA serum and bile antibody titers
Total immunoglobulin A (IgA) antibodies specific for β-intimin were measured by enzyme-linked immunosorbent assay (ELISA). Briefly, microtiter plates were coated with purified recombinant protein β-intimin at 5 μg/ml (purified from pCVD473) in 100 μl of PBS (pH=7.4 for 3 hrs. at 37° C). The plates were then blocked with 10% skim milk (Nestle USA, Inc., Glendale, Calif) in PBS overnight at 4° C. After each incubation, plates were washed six times with PBS containing 0.05% Tween-20 (PBST). To determine endpoint titers, eight two-fold dilutions of sera (or bile) in 10% milk PBST were tested. After washing, plates were incubated with 100 μl of peroxidase-conjugated goat serum specific for rabbit IgA (Zymed, San Francisco, Calif.) and diluted 1/1,000 in the same diluent (1 hr incubation at room temperature). The substrate solution used was TMB Microwell Peroxidase (KPL Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.). After a 15 min. incubation at room temperature, the reaction was stopped by the addition of 100 μl of 1 M H2PO4, and the OD450 nm was determined in an ELISA microplate reader (Multiskan Ascent; Thermo Labsystems, Helsinki, Finland). Serum samples were run in duplicate with negative and positive control sera included in each assay. Linear regression curves were plotted for each serum sample, and titers were calculated through equation parameters as the inverse of the serum dilution that produces an OD of 0.2 above the blank and expressed as ELISA units (EU)/ml.
4.10. Histological preparations
Transmural sections from the ileum, cecum, and colon were excised and fixed in 10% buffered formalin for sectioning. Tissues were stained with hematoxylin and eosin or with Giemsa stain. Microscopic examination of histopathology was based on 10 sequential, well-oriented, 400× fields from each sample. Bacterial enteroadherence was graded as the percentage of surface area in the field that is covered by closely adherent bacteria.
4.11. Statistical Analysis
The Student t-test was used as statistical analysis and a P value of <0.05 was considered to be statistically significant.
Acknowledgements
The authors would like to thank Flavia A. Lima for supplying the β-intimin antisera. Additionally, we would like to thank Jane Michalski for editorial assistance on the manuscript. This study was supported by NIH grants DK58957 and AI19716 (to JBK), DK 059012 and VA Merit Award to ECB, and the Mid-Atlantic Regional Center for Excellence for Biodefense and Emerging Infectious Diseases Research grant U54 AI57168 (to M. M. Levine).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Moon HW, Whipp S, Argenzio RA, Levine MM, Giannella RA. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983;41:1340–51. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Knutton S, Baldwin T, Williams PH, McNeish AS. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989;57:1290–8. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].An H, Fairbrother JM, Dubreuil JD, Harel J. Cloning and characterization of the eae gene from a dog attaching and effacing Escherichia coli strain 4221. FEMS Microbiol Lett. 1997;148:239–45. doi: 10.1111/j.1574-6968.1997.tb10295.x. [DOI] [PubMed] [Google Scholar]
- [5].Cantey JR, Blake RK. Diarrhea due to Escherichia coli in the rabbit: a novel mechanism. J Infect Dis. 1977;135:454–62. doi: 10.1093/infdis/135.3.454. [DOI] [PubMed] [Google Scholar]
- [6].Zhu C, Agin TS, Elliott SJ, Johnson LA, Thate TE, Kaper JB, Boedeker EC. Complete nucleotide sequence and analysis of the locus of enterocyte effacement from rabbit diarrheagenic Escherichia coli RDEC-1. Infect Immun. 2001;69:2107–15. doi: 10.1128/IAI.69.4.2107-2115.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Schauer DB, Falkow S. The eae gene of Citrobacter freundii biotype 4280 is necessary for colonization in transmissible murine colonic hyperplasia. Infect Immun. 1993;61:4654–61. doi: 10.1128/iai.61.11.4654-4661.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Elliott SJ, Wainwright LA, McDaniel TK, Jarvis KG, Deng YK, Lai LC, McNamara BP, Donnenberg MS, Kaper JB. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic Escherichia coli E2348/69. Mol Microbiol. 1998;28:1–4. doi: 10.1046/j.1365-2958.1998.00783.x. [DOI] [PubMed] [Google Scholar]
- [9].Donnenberg MS, Kaper JB, Finlay BB. Interactions between enteropathogenic Escherichia coli and host epithelial cells. Trends Microbiol. 1997;5:109–14. doi: 10.1016/S0966-842X(97)01000-7. [DOI] [PubMed] [Google Scholar]
- [10].Jerse AE, Yu J, Tall BD, Kaper JB. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci U S A. 1990;87:7839–43. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kenny B, Devinney R, Stein M, Reinscheid DJ, Frey EA, Finlay BB. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–20. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- [12].Adu-Bobie J, Trabulsi LR, Carneiro-Sampaio MMS, Dougan G, Frankel G. Identification of immunodominant regions within the C-terminal cell binding domain of intimin alpha and intimin beta from enteropathogenic Escherichia coli. Infect Immun. 1998;66:5643–9. doi: 10.1128/iai.66.12.5643-5649.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Oswald E, Schmidt H, Morabito S, Karch H, Marchès O, Caprioli A. Typing of intimin genes in human and animal enterohemorrhagic and enteropathogenic Escherichia coli: characterization of a new intimin variant. Infect Immun. 2000;68:64–71. doi: 10.1128/iai.68.1.64-71.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ramachandran V, Brett K, Hornitzky MA, Dowton M, Bettelheim KA, Walker MJ, Djordjevic SP. Distribution of intimin subtypes among Escherichia coli isolates from ruminant and human sources. J Clin Microbiol. 2003;41:5022–32. doi: 10.1128/JCM.41.11.5022-5032.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhang WL, Kohler B, Oswald E, Beutin L, Karch H, Morabito S, Caprioli A, Suerbaum S, Schmidt H. Genetic diversity of intimin genes of attaching and effacing Escherichia coli strains. J Clin Microbiol. 2002;40:4486–92. doi: 10.1128/JCM.40.12.4486-4492.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tarr CL, Whittam TS. Molecular evolution of the intimin gene in O111 clones of pathogenic Escherichia coli. J Bacteriol. 2002;184:479–87. doi: 10.1128/JB.184.2.479-487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ito K, Iida M, Yamazaki M, Moriya K, Moroishi S, Yatsuyanagi J, Kurazono T, Hiruta N, Ratchtrachenchai OA. Intimin types determined by heteroduplex mobility assay of intimin gene (eae)-positive Escherichia coli strains. J Clin Microbiol. 2007;45:1038–41. doi: 10.1128/JCM.01103-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Judge NA, Mason HS, O’Brien AD. Plant cell-based intimin vaccine given orally to mice primed with intimin reduces time of Escherichia coli O157:H7 shedding in feces. Infect Immun. 2004;72:168–75. doi: 10.1128/IAI.72.1.168-175.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dean-Nystrom EA, Gansheroff LJ, Mills M, Moon HW, O’Brien AD. Vaccination of pregnant dams with intiminO157 protects suckling piglets from Escherichia coli O157:H7 infection. Infect Immun. 2002;70:2414–8. doi: 10.1128/IAI.70.5.2414-2418.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ghaem-Maghami M, Simmons CP, Daniell S, Pizza M, Lewis D, Frankel G, Dougan G. Intimin-specific immune responses prevent bacterial colonization by the attaching-effacing pathogen Citrobacter rodentium. Infect Immun. 2001;69:5597–605. doi: 10.1128/IAI.69.9.5597-5605.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Galen JE, Zhao L, Chinchilla M, Wang JY, Pasetti MF, Green J, Levine MM. Adaptation of the endogenous Salmonella enterica serovar Typhi clyA-encoded hemolysin for antigen export enhances the immunogenicity of anthrax protective antigen domain 4 expressed by the attenuated live-vector vaccine strain CVD 908-htrA. Infect Immun. 2004;72:7096–106. doi: 10.1128/IAI.72.12.7096-7106.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Butterton JR, Beattie DT, Gardel CL, Carroll PA, Hyman T, Killeen KP, Mekalanos JJ, Calderwood SB. Heterologous antigen expression in Vibrio cholerae vector strains. Infect Immun. 1995;63:2689–96. doi: 10.1128/iai.63.7.2689-2696.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Butterton JR, Ryan ET, Acheson DW, Calderwood SB. Coexpression of the B subunit of Shiga toxin 1 and EaeA from enterohemorrhagic Escherichia coli in Vibrio cholerae vaccine strains. Infect Immun. 1997;65:2127–35. doi: 10.1128/iai.65.6.2127-2135.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wai SN, Lindmark B, Söderblom T, Takade A, Westermark M, Oscarsson J, Jass J, Richter-Dahlfors A, Mizunoe Y, Uhlin BE. Vesicle-mediated export and assembly of poreforming oligomers of the enterobacterial ClyA cytotoxin. Cell. 2003;115:25–35. doi: 10.1016/s0092-8674(03)00754-2. [DOI] [PubMed] [Google Scholar]
- [25].Cravioto A, Tello A, Villafán H, Ruiz J, del Vedovo S, Neeser JR. Inhibition of localized adhesion of enteropathogenic Escherichia coli to HEp-2 cells by immunoglobulin and oligosaccharide fractions of human colostrum and breast milk. J Infect Dis. 1991;163:1247–55. doi: 10.1093/infdis/163.6.1247. [DOI] [PubMed] [Google Scholar]
- [26].Camara LM, Carbonare SB, Silva ML, Carneiro-Sampaio MM. Inhibition of enteropathogenic Escherichia coli (EPEC) adhesion to HeLa cells by human colostrum: detection of specific sIgA related to EPEC outer-membrane proteins. Int Arch Allergy Immunol. 1994;103:307–10. doi: 10.1159/000236645. [DOI] [PubMed] [Google Scholar]
- [27].Frankel G, Candy DCA, Fabiani E, Adu-Bobie J, Gil S, Novakova M, Phillips AD, Dougan G. Molecular characterization of a carboxy-terminal eukaryotic-cell-binding domain of intimin from enteropathogenic Escherichia coli. Infect Immun. 1995;63:4323–8. doi: 10.1128/iai.63.11.4323-4328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Luo Y, Frey EA, Pfuetzner RA, Creagh AL, Knoechel DG, Haynes CA, Finlay BB, Strynadka NC. Crystal structure of enteropathogenic Escherichia coli intimin-receptor complex. Nature. 2000;405:1073–7. doi: 10.1038/35016618. [DOI] [PubMed] [Google Scholar]
- [29].Batchelor M, Prasannan S, Daniell S, Reece S, Connerton I, Bloomberg G, Dougan G, Frankel G, Matthews S. Structural basis for recognition of the translocated intimin receptor (Tir) by intimin from enteropathogenic Escherichia coli. EMBO J. 2000;19:2452–64. doi: 10.1093/emboj/19.11.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kaper JB, Tacket C. Attenuated Vibrio cholerae strains as live oral cholerae vaccines and vectors. In: Levine MM, Kaper JB, Rappuoli R, Liu M, Goods M, editors. New Generation Vaccines. Marcel Dekker Inc; New York: 2004. pp. 511–7. [Google Scholar]
- [31].Ketley JM, Michalski J, Galen J, Levine MM, Kaper JB. Construction of genetically marked Vibrio cholerae O1 vaccine strains. FEMS Microbiol Lett. 1993;111:15–21. doi: 10.1111/j.1574-6968.1993.tb06355.x. [DOI] [PubMed] [Google Scholar]
- [32].Levine MM, Kaper JB, Herrington D, Ketley J, Losonsky G, Tacket CO, Tall B, Cryz S. Safety, immunogenicity, and efficacy of recombinant live oral cholera vaccines, CVD 103 and CVD 103-HgR. Lancet. 1988;2:467–70. doi: 10.1016/s0140-6736(88)90120-1. [DOI] [PubMed] [Google Scholar]
- [33].Acheson DW, Levine MM, Kaper JB, Keusch GT. Protective immunity to Shiga-like toxin I following oral immunization with Shiga-like toxin I B-subunit-producing Vibrio cholerae CVD 103-HgR. Infect Immun. 1996;64:355–7. doi: 10.1128/iai.64.1.355-357.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Boullier S, Nougayrède JP, Marchès O, Tasca C, Boury M, Oswald E, De Rycke J, Milon A. Genetically engineered enteropathogenic Escherichia coli strain elicits a specific immune response and protects against a virulent challenge. Microbes Infec. 2003;5:857–67. doi: 10.1016/s1286-4579(03)00175-8. [DOI] [PubMed] [Google Scholar]
- [35].Sambrook J, Russel DW. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor: 2001. [Google Scholar]
- [36].Zhu C, Feng S, Thate TE, Kaper JB, Boedeker EC. Towards a vaccine for attaching/effacing Escherichia coli: a LEE encoded regulator (ler) mutant of rabbit enteropathogenic Escherichia coli is attenuated, immunogenic, and protects rabbits from lethal challenge with the wild-type virulent strain. Vaccine. 2006;24:3845–55. doi: 10.1016/j.vaccine.2005.07.019. [DOI] [PubMed] [Google Scholar]
- [37].Marchès O, Nougayrède JP, Boullier S, Mainil J, Charlier G, Raymond I, Pohl P, Boury M, De Rycke J, Milon A, Oswald E. Role of tir and intimin in the virulence of rabbit enteropathogenic Escherichia coli serotype O103:H2. Infect Immun. 2000;68:2171–82. doi: 10.1128/iai.68.4.2171-2182.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]