Abstract
Few studies have reported the reduced suppression of brain activity within the default network in schizophrenia. The relationship, however, between task-specific activation and default network suppression, as well as impact of this relationship on brain function is still not clear, and has not been studied in schizophrenia so far. We used previously published data showing relationship between semantic encoding and white matter integrity in schizophrenia (Jeong et al., 2009), and reanalyzed the data using independent component analysis (ICA). Ten healthy control subjects and ten chronic schizophrenics underwent an fMRI scan during which they performed Levels of Processing paradigm. The semantic processing related independent components were compared between two groups using tensor ICA. An independent component of semantic repetition priming showed a significant difference between two groups. The component consisted of both less activated and less suppressed regions within the schizophrenics’ brain. The less activated regions included the bilateral inferior frontal gyri and the supramarginal gyri. The less suppressed regions included the medial frontal gyrus, the posterior cingulate gyrus, precuneus and right cerebellum. Our results suggest two components of semantic repetition priming deficit in schizophrenia. One related to weaker suppression of default network, mainly precuneus and medial frontal gyrus, the other related to weaker activation of regions directly involved in semantic repetition priming.
Keywords: Semantic processing, Functional MRI, Schizophrenia, Independent component analysis, Default network
1. Introduction
Schizophrenia is a psychiatric disorder in which deficits in thought and perception alter patients’ awareness of themselves as well as of the outside world. Cognitive deficits affecting working memory (Perlstein et al., 2003), attention (Choi et al., 2008), verbal memory (Bilder et al., 2000) have been attributed to the dysfunction of the brain, and tracked down to the first-episode, drug-naïve patients. The brain dysfunction in this disease is attributed not only to local brain abnormalities related to cognitive function-specific regions (Choi et al., 2008) but also linked to inter-regional brain connectivity (Schlosser et al., 2003) in schizophrenia. According to this theory, as well as our previous findings, the deficit in semantic processing, for example, may be related to both the dysfunction of left inferior frontal gyrus (Kubicki et al., 2003) and anatomo-functional connectivity within the semantic network (Jeong et al., 2009).
One specific element of semantic processing, semantic priming, has been implicated in several schizophrenia publications, as being highly predictive of clinical symptoms. A meta-analysis using behavioral data reported that schizophrenics with thought disorder showed an increased semantic priming, when compared with healthy controls (Pomarol-Clotet et al., 2008). In terms of priming effect, words with high connectivity between shared semantic associates are considered to produce the larger semantic priming effects, faster response in lexical decision and less brain activation in region that includes the left inferior frontal gyrus (Tivarus et al., 2006; Weems and Zaidel, 2005), while words with low semantic connectivity are considered to produce a relatively smaller effect in healthy subjects (Han et al., 2007). In an event-related potential (ERP) study, the negative relationship between N400 amplitude and the degree of the association of prime-to-target stimulus, even though evident in control subjects, was not present in schizophrenia (Kiang et al., 2008). Also, the N400 amplitude was positively correlated with positive psychotic symptoms (Kiang et al., 2008). The late information processing responsible for N400 during semantic priming task in ERP study could be associated with the dysfunction in left inferior frontal gyrus, as suggested by a functional magnetic resonance imaging (fMRI) study (Kubicki et al., 2003). Schizophrenics also showed a differential increase in activation of both left frontal and temporal cortices from high connectivity to low connectivity to unrelated word pairs (Han et al., 2007). In addition, semantic repetition priming effect (Guillaume et al., 2009) observed with the left inferior frontal gyrus (Bergerbest et al., 2009; Wig et al., 2009) has been also reported to be decreased in schizophrenia (Matsuoka et al., 1999). The behavioral, ERP and fMRI studies suggest that the abnormal semantic and repetition priming is related to abnormalities in functional connectivity among brain regions in schizophrenia.
The default network has been thought to mediate task-independent brain function during a resting state, with its activity being suppressed during a cognitive task. The abnormal activity in the default network both at rest (Bluhm et al., 2007; Zhou et al., 2007) and during cognitive task has been reported in schizophrenia (Garrity et al., 2007; Whitfield-Gabrieli et al., 2009), and was correlated with positive psychotic symptoms (Whitfield-Gabrieli et al., 2009; Zhou et al., 2008). Since activations in the default network are usually negatively correlated with activations in regions typically involved in stimulus processing (McKiernan et al., 2003), this might suggest that both task-related as well as the default networks are involved simultaneously in cognitive task with the efficacy of interaction between both networks possibly influencing the task performance. Thus, the cognitive deficits in schizophrenia could arise from both the dysfunction of task-related regions as well as the default network. Little, however, is known about the relationship between the default network and the regions that show a stimulus processing-related activation during cognitive task in schizophrenia. Here we used the independent component analysis (ICA) to examine the status of both task-related activations and deactivations during semantic processing in schizophrenia. Using ICA for fMR data, we investigated the activity of regions related to semantic repetition priming as well as default network related regions in patients with chronic schizophrenia and healthy controls. We hypothesized that schizophrenics will show less suppression of the activity in default network as well as less activation of task-related activated regions.
2. Methods
2.1. Subjects
Ten male patients with schizophrenia and ten male healthy control subjects were enrolled in this study. The possible confounding factors including handedness, parental socioeconomic status (PSES) (Hollingshead, 1965), and age were matched between groups. Verbal IQ was lower in the schizophrenia group (84.9 ± 13.4), compared to control subjects (109.0 ± 9.5) (t = 4.7, P < 0.001). However, WRAT3 Reading scores representing premorbid IQ were not different between both groups (schizophrenics vs. controls = 91.9 ± 17.9 vs. 102.3 ± 8.2). All patients were diagnosed with chronic schizophrenia using DSM-IV criteria. No control subjects had an Axis-I psychiatric disorder. The study was approved by the VA Boston Healthcare System Human Subjects Committee and by the Brigham and Women’s Institutional Review Board. The written informed consent was provided by all participants in the study, and all were compensated for their time.
2.2. The levels-of-processing paradigm (Semantic paradigm)
The main purpose of this study was to investigate further the semantic priming network in schizophrenia, by independent component analysis (ICA). In the current analysis, we used semantic processing fMRI data published previously (Kubicki et al., 2003). During the fMRI session of two 9 minutes runs, patients and controls performed both semantic (deep) and perceptual (shallow) encoding tasks and also, in recognition condition, subjects were requested to judge as to whether the word presented on the screen was used before, under the encoding condition, or was new (Fig. 1). The semantic repetition of words facilitates the ability to recognize consecutive word stimuli. The semantic repetition priming decreases both response time and the activity of left inferior frontal gyrus (Bergerbest et al., 2009). In our experiment, the semantic repetition priming was defined by the difference between two consecutive semantic tasks. The contrast for semantic repetition priming was as follows: (semantic first - second block) – (perceptual first – second block) see Figure 1 and original paper for more details (Fig. 1). In this study, first one of two runs was used for ICA. Full details regarding data acquisition and behavioral paradigm can be found in original paper (Kubicki et al., 2003).
Fig. 1.
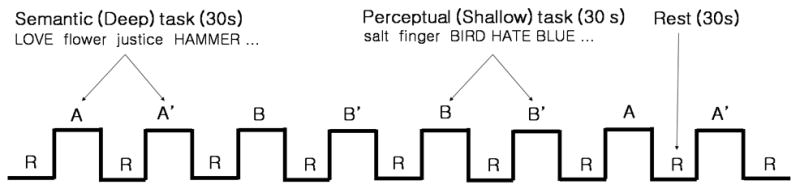
The levels-of-processing paradigm. Subjects performed abstract or concrete judgment task during semantic block and upper or lower case judgment task during perceptual block in encoding condition. In recognition condition, subjects were requested to judge as to whether the word presented on the screen was used before, under the encoding condition, or was new. Twelve words were presented in each block during 1.5 sec per a word. The Contrasts of A–B, A′–B′, (A–B)–(A′–B′) represent semantic encoding, recognition and repetition priming, respectively.
2.3. Data acquisition
All subjects underwent fMRI procedure on 1.5-T whole body MRI Echospeed system (General Electric Medical Systems, Milwaukee, WI). The total of 174 EPI BOLD scans (24 oblique coronal slices, 6 mm thick, TR 3 s; TE 40 ms; flip angle 90°; 64×64 matrix) were acquired perpendicular to the long axis of the hippocampus. A three-dimensional Fourier transformed spoiled-gradient-recalled (SPGR) acquisition sequence yielded a coronal series of contiguous 1.5-mm images (TE = 5 ms, TR = 35 ms, repetition = 1, field of view= 24 cm, acquisition matrix = 256 × 256 × 124, voxel dimension = .9375 × .9375 × 1.5 mm).
2.4. Data pre-processing
FMRI data was processed and analyzed with MELODIC (Multivariate Exploratory Linear Optimized Decomposition into Independent Components) module of the FSL software (http://www.fmrib.ox.ac.uk/fsl/feat5/index.html). The first 4 scans were discarded. The remaining 170 images were spatially realigned using FLIRT (FMRIB’s Linera Image Registration Tool), applying rigid-body transformation. Both scanner-related and physiological artifacts were removed using MELODIC. A brain mask from the first volume in fMR data was created for removal of signal outside of brain in each subject. A spatial smoothing was performed using 5mm FWHM for reducing noise. FMR images were filtered with 128 seconds high pass filter. The realigned images were coregistered to the Montreal Neurological Institute (MNI) T1 template through their SPGR images.
2.5. Tensor independent component analysis (multivariate analysis)
In existing group-ICA methodology, the data in space or time is simply concatenated to perform a single two-dimensional ICA decomposition and then meta-analysis is performed to estimate the variation between subjects (Calhoun et al., 2001; Svensen et al., 2002). The tensor-ICA approach, however, directly estimates separate modes in the three domains consisting of space, time and subject. In order to do that, as stated in methods paper “the iteration is being performed between estimating a 2-D ICA model on the data concatenated in time and a rank-1 decomposition of the resulting estimate of the mixing matrix which can be used to identify the temporal and subject factor matrices” (Beckmann and Smith, 2005). This methodology of tensor-ICA can be extended to higher dimensions (e.g., two group comparisons), where rank-2 approximation is used for each mixing matrix (space/time/subject component matrices) to allow for different temporal characteristics. Finally, to characterize components which differ significantly between two groups, the values of the subject domain are compared within the general linear model framework by performing t tests on the values between groups (Rombouts et al., 2009).
Since fMR data of the current study was acquired using a consistent stimulus timing across subjects, the temporal response pattern was the same for all the subjects and thus provided a single decomposition for the entire original data set. The pre-processed fMRI data were decomposed into three component matrices of time courses, spatial maps and subject domains using Tensor-ICA as described by a Beckmann and Smith (Beckmann and Smith, 2005). The number of components was automatically estimated from the fMR data by MELODIC. Tensor-ICA allowed for grouping similar brain activation patterns across the spatial, temporal and subject domains. The time course analysis for temporal domain was performed on the time courses of the independent component to calculate the BOLD amplitude difference both over time and among different condition blocks. We identified task-condition specific independent components including a semantic encoding, recognition and repetition priming, and compared groups based on these components. Finally, we identified commonly less deactivated regions among the independent networks in schizophrenia. To correct for false positives, we used an alternative hypothesis test based on fitting a Gaussian/Gamma mixture model to the distribution of voxel intensities within spatial maps and a posterior probability threshold of P = 0.5 (Beckmann et al., 2003). As opposed to most popular multiple comparison correction methods (including family-wised error and false discovery rate) that assume Gaussian distribution, the Gaussian mixture model does not make such assumption. Since the individual IC spatial statistical maps do not seem to follow Gaussian distribution, Gaussian mixture model seems to be more appropriate to inference statistical map from ICA (Beckmann and Smith, 2004).
3. Results
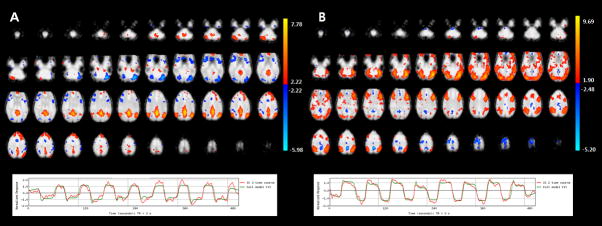
MELODIC produced 26 independent components (ICs). Two ICs among 26 components showed semantic repetition priming (IC 1: Z value = 1.87, P = .031; IC 2: Z value = 1.68, P = .022) as well as semantic encoding (IC 1: Z value = 2.47, P = .006; IC 2: Z value = 2.02, P = .047) effects. Group comparison, however, revealed that only one, IC 1, of the two ICs showed a group difference for priming (P < .001), but not for encoding effect (P = .457). The spatial map of the IC 2 is provided as a supplemental material (Supplemental fig. 1). Further, a specific component of semantic repetition priming that showed a significant group difference consisted of both activated as well as deactivated regions (Table 1, Fig. 1). The activated regions related with semantic repetition priming included bilateral frontal orbital cortices, bilateral paracingulate gyri and anterior cingulate cortices, right subcallosal cortex, left precentral gyrus, right middle frontal gryus, bilateral lateral occipital cortices (inferior division), right superior parietal lobule, left supramarginal gyrus and bilateral inferior temporal gyrus (posterior division). The deactivated regions related with semantic priming included bilateral precuneus and posterior cingulate cortices, bilateral superior and middle frontal gyri, bilateral medial frontal and paracingulate cortices, bilateral occipital cortices (superior division), right parahippocampal gyrus (posterior division), bilateral middle temporal gyri (posterior division), right cerebellum IX and bilateral Crus II. The time course of the IC of semantic repetition priming showed two opposite patterns of activation/deactivation (see Fig. 2). There was no semantic encoding specific component that would show group difference.
Table 1.
Significantly less deactivated or activated regions during the semantic repetition priming in schizophrenics, compared to controls,.
| Region | Cluster size (ml3) | Peak voxel coordinate |
Z-score | ||
|---|---|---|---|---|---|
| X | y | z | |||
| Less deactivated regions | |||||
| precuneus, posterior cingulate cortex, B | 77611 | −1 | −56 | 23 | 7.78 |
| Superior & middle frontal gyrus, B | 25497 | −25 | 29 | 43 | 4.38 |
| Medial frontal and paracingulate cortex, B | 6594 | −1 | 53 | −6 | 4.31 |
| Lateral occipital cortex, superior division, L | 20608 | −41 | −72 | 35 | 6.39 |
| R | 13440 | 56 | −68 | 35 | 5.86 |
| Parahippocampal gyrus, posterior division | 2176 | 20 | −27 | −18 | 3.53 |
| Middle temporal gyrus, posterior division, R | 1728 | 58 | 5 | −26 | 3.17 |
| L | 1024 | −61 | −19 | −14 | 3.08 |
| Cerebellum, IX, R | 6496 | 8 | −60 | −46 | 4.51 |
| Cerebellum, Crus II, L | 1344 | −33 | −84 | −42 | 3.16 |
| R | 11008 | 40 | −76 | −46 | 4.21 |
| Less activated regions | |||||
| Frontal orbital cortex, L | 15046 | −29 | 25 | −2 | 4.48 |
| R | 27076 | 48 | 17 | −6 | 4.15 |
| Paracingulate and anterior cingulate cortex, B | 4848 | 4 | 13 | 47 | 4.11 |
| Subcallosal cortex | 3968 | 16 | 29 | −10 | 3.38 |
| Precentral gyrus, L | 1664 | −49 | −11 | 51 | 2.82 |
| Middle frontal gyrus, R | 640 | 44 | 1 | 55 | 2.5 |
| Lateral occipital cortex, inferior division, L | 25959 | −41 | −76 | −22 | 5.98 |
| R | 12192 | 40 | −88 | 3 | 4.19 |
| Superior parietal lobule, R | 4752 | 36 | −52 | 43 | 3.21 |
| Supramarginal gyrus, anterior division, L | 3552 | −53 | −31 | 47 | 2.9 |
| Inferior temporal gyrus, posterior division, L | 1472 | −37 | −11 | −42 | 2.91 |
| R | 2048 | 40 | −15 | −46 | 2.73 |
Notes: L = left, R = right, B = bilateral The coordinates of maximally activated voxels are given in MNI space. All activations identified at posterior probability threshold of p>0.5 for a spatial extent of at least 500ml3
Fig. 2.
Condition-specific independent components and their time courses. Spatial maps show the voxel-wise posterior probability of activation in the fitted mixture model. A. A specific component of semantic repetition priming demonstrates a significant group difference (P < .001). Red and yellow colored areas (default network) represent deactivation pattern of semantic repetition priming (and less suppression in schizophrenic group). Dark to light blue colors (task-related activated IC) represents an activation pattern of the IC (and less activation in schizophrenics). The time course of the IC shows the signal of red to yellow colored areas (default network) to be increased at rest and decreased during task blocks and the time course of dark to light blue colored area (repetition semantic priming) shows an opposite behavior. B. An independent component of semantic recognition showing a significant group difference. Red to yellow blue colored areas (task-related activated regions) represent activation pattern of IC of semantic recognition (and less activation during semantic recognition task in schizophrenics). Dark to light blue colored areas (default network) represents a Reduced task-related suppression in schizophrenia deactivation pattern of the IC (and less suppression during semantic recognition task in schizophrenics). Its worth noting that the time course of IC of semantic recognition shows the opposite behavior of task-related and default networks across the entire experiment, not only during semantic recognition block. Radiological orientation (Right side of the brain is displayed on the left side of the figure)
MELODIC also produced an independent component demonstrating semantic recognition effect (Z value = 4.71, P < .001), as well as a group difference for semantic recognition (Z value = 5.11, P < .001). The semantic recognition specific component that showed a significant group difference consisted of both activated as well as deactivated regions (Table 2). The activated regions related to semantic recognition included bilateral inferior frontal gyrus (pars opercularis), bilateral paracingulate gyri and anterior cingulate cortices, left central opercular cortex, left lateral occipital cortex (inferior division), bilateral lateral occipital cortex (superior division), left superior parietal lobule, right middle temporal gyrus and right cerebellum VIIb. The semantic recognition-related deactivated regions included bilateral precuneus, anterior cingulate and medial frontal cortices. The time course of the IC of semantic recognition also showed two opposite patterns of activation/deactivation (see Fig. 2).
Table 2.
Significantly less deactivated or activated regions during the semantic recognition in schizophrenics, compared to controls,
| Region | Cluster size (ml3) | Peak voxel coordinate |
Z-score | ||
|---|---|---|---|---|---|
| X | y | z | |||
| Less suppressed regions | |||||
| Precuneus, B | 30912 | 5.2 | 0 | −48 | 54 |
| 8192 | 3.99 | 8 | −60 | 14 | |
| Anterior cingulated/Medial frontal cortices, B | 4672 | 3.94 | 4 | 40 | 10 |
| Less activated regions | |||||
| Lateral occipital cortex, inferior division, L | 132224 | 9.69 | −44 | −72 | −18 |
| Inferior frontal gyrus, pars opercularis, L | 68288 | 7.54 | −48 | 20 | 22 |
| R | 36864 | 6.32 | 52 | 16 | 26 |
| Lateral occipital cortex, superior division, superior parietal lobule, L | 31232 | 6.67 | −32 | −60 | 50 |
| Lateral occipital cortex, superior division, R | 23232 | 5.94 | 36 | −60 | 42 |
| Middle temporal gyrus, R | 23104 | 5.24 | 63 | −52 | −10 |
| Paracingulate gyrus, Anterior cingulate gyrus | 5120 | 3.82 | −4 | 20 | 42 |
| Right cerebellum, right VIIb | 1472 | 3.27 | 28 | −76 | −54 |
| Central opercular cortex, L | 704 | 2.67 | −56 | −16 | 14 |
Notes: L = left, R = right, B = bilateral The coordinates of maximally activated voxels are given in MNI space. All activations identified at posterior probability threshold of p>0.5 for a spatial extent of at least 500ml3
Finally, regions that demonstrated less deactivation (suppression), common to both conditions (semantic repetition priming and recognition) in schizophrenia included bilateral precuneus (Z value = 6.9, MNI: x = −8, y = −48, z = 50; Z value = 7.64, MNI: x = 4, y = −56, z = 16) and medial frontal cortices (Z value = 4.31, MNI: x = −1, y = 53, z = −6) (Fig. 3).
Fig. 3.
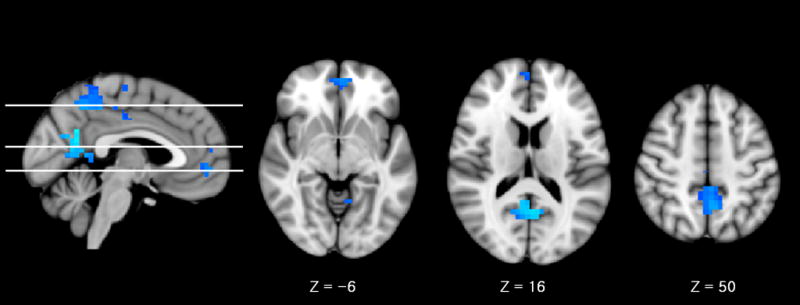
A commonly less deactivated region in both semantic repetition priming and recognition in schizophrenia, including bilateral precuneus (Z value = 6.9, MNI: x = −8, y = −48, z = 50; Z value = 7.64, MNI: x = 4, y = −56, z = 16) and medial frontal cortices (Z value = 4.31, MNI: x = −1, y = 53, z = −6)
4. Discussion
There are three major findings related to our ICA semantic processing study. First, as expected, default network and semantic network are both active during both cognitive processes associated with semantic task, as well as during rest. These two networks work with a reciprocal manner in that if one network increases its activity, the other’s activity decreases. Second, in schizophrenia, the reciprocal manner of these two networks even more attenuated. This means that schizophrenics, compared with healthy controls, show both less activation in task-related region and less suppression in default network during cognitive task and vice versa during rest. Third, the reciprocal manner of these two networks in schizophrenia is confined during semantic repetition priming as well as during recognition, but not during the encoding condition. Finally, the two regions that consistently demonstrate less deactivation during semantic repetition priming and recognition in schizophrenia (namely medial frontal and precuneus) are both considered main default network structures.
Previous studies of semantic priming have focused on the language network that includes left inferior frontal and left temporal regions (Bergerbest et al., 2009; Han et al., 2007; Tivarus et al., 2006; Wig et al., 2009). Current study, however, demonstrated an independent component showing semantic repetition priming-related activation being broader, and consisting of bilateral orbitofrontal, superior parietal and inferior temporal regions. Our ICA results suggest the semantic processing involves both hemispheres, where the activations of all the elements of this network are positively correlated with each other. Moreover, our ICA analysis also revealed brain regions (network) that demonstrate negative correlation (or anti-correlation) with semantic repetition priming condition. Interestingly, while this network showed decreased activity during semantic task, it also showed an increased activity during rest in schizophrenia. This anticorrelated network includes medial frontal gyrus, precuneus, posterior cingulate corex, the superior division of bilateral occipital cortices and cerebellum. Most regions of this brain network are consistently considered elements of the default network (Buckner et al., 2008; Greicius et al., 2003; McKiernan et al., 2003; Raichle et al., 2001). Thus, our study indicates that the default network is less suppressed during semantic repetition priming in schizophrenia, than in control subjects, which is consistent with previous studies reporting less suppression during auditory oddball task (Garrity et al., 2007) or working memory (Whitfield-Gabrieli et al., 2009) within the default network in schizophrenia. We also found that regions most consistently less deactivated across two different conditions (semantic repetition priming and recognition) in schizophrenia were located within the bilateral precuneus and medial frontal cortices, both prominent elements of the default network.
In addition to tensor ICA analysis, we also performed model-based analyses looking at the activation at rest (fixation block vs. semantic task) as well as at the deactivation during task (perceptual task vs. semantic task). Schizophrenics, compared with controls, showed significantly less deactivation at precuneus/posterior cingulate cortex and medial frontal cortex/anterior cingulate cortex during semantic block and showed less activation at medial frontal cortex/anterior cingulate cortex during fixation block (see supplemental fig. 2). These results from model-based analyses would suggest that the anti-correlation pattern may occur at medial frontal cortex/anterior cingulate cortex and/or precuneus/posterior cingulate cortex of default network. The anti-correlated areas were larger in tensor-ICA than in model-based analysis, which is consistent with previous report also showing tensor-ICA as being more sensitive to signal group differences than model-based analysis (Rombouts et al., 2009). Few possible explanations of differences between model-based analysis and tensor-ICA include the fact that in model-based analysis, the hemodynamic function for deactivation might not be as easy to estimate as the one for activation. Also, small sample size of ten of each group in the present study might affect statistical significance in model-based analysis using correction for multiple comparisons more than the T-ICA using Gaussian mixture model and t tests between groups.
One of the major findings of this study is the attenuated reciprocal manner, in which task-related and default networks interact in schizophrenia, and that this manner is more apparent during semantic repetition priming and semantic recognition than during semantic encoding. Since brain regions within a single independent component are characterized by a high temporal correlation with each other, and this is considered the evidence of their functional connection (connectivity), the attenuated reciprocal activity pattern in schizophrenia may be due to the decrement of the functional connectivity between the activated and the deactivated networks. Our previous study using both diffusion tensor and fMRI data of the same subjects reported decreased anatomical connectivity in left inferior frontal white matter was associated with the decreased functional connectivity during semantic processing within the language network in schizophrenia (Jeong et al., 2009). Indeed, in our previous work, patients with schizophrenia showed decreased anatomical connectivity in various white matter regions including the splenium of corpus callosum, left inferior frontal white matter, anterior limb of left internal capsule, right precuneus and bilateral cerebellum in schizophrenia (Jeong et al., 2009). Both these studies taken together might suggest the association between the abnormal anatomical connection and the attenuated reciprocal activity pattern of functional networks in schizophrenia (see supplemental fig. 3), where decreased anatomical connectivity among precuneus, left inferior frontal and cerebellar white matter (including anterior limb of left internal capsule) may be related to the decreased reciprocal functional activity pattern between less activated left inferior frontal gyrus, and less deactivated precuneus and cerebellum. The attenuated anatomo-functional connectivity between task-related and default networks might suggest “cognitive dysmetria” which is the hypothesis of the deficits in the cortico-cerebellar-thalamic-cortical circuit in schizophrenia (Andreasen, 1999). A previous neuroimaging study reported the cerebellum playing an important role in various higher cognitive functions in healthy controls, as it demonstrated diminished association between cerebellar volume and function in first-episode schizophrenia (Szeszko et al., 2003). However, to confirm our interpretation, the further studies are needed for exploring the region-specific relationship of white matter deficits and functional abnormalities.
The current study has several limitations. First, sample size was relatively small and only chronic, medicated males were involved in the study. Some of the disease related abnormalities could be thus masked, in our sample, by decreased sensitivity, medication effects, or possible gender effects. Thus the generalizability of our results need further confirmation with large, first-episode and/or drug naïve schizophrenic sample. IQ could also possibly affect the group difference in semantic priming. However, relatively low IQ may be related to disease itself and WRAT3 Reading scores suggesting premorbid intelligence did not differ between groups. We previously reported the relationship of anatomical and functional connectivity of left inferior frontal area (Jeong et al., 2009), which, combining with current data, suggests relationship between the abnormal anatomical connectivity and functional connectivity within and/or between task-related or default network. Further studies, however, are needed using both large sample data and other tools such as tractography or correlational analysis to test our hypothesis further.
In summary, our study using ICA demonstrates that semantic repetition priming induces functional abnormalities within both semantic as well as default networks in schizophrenia.
Supplementary Material
Acknowledgments
We gratefully acknowledge the support of the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MOST) (No.2006-05372: B.J.). This work is also, in part, funded by the National Alliance for Medical Image Computing (NAMIC), funded by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant U54 EB005149 (MK).
Footnotes
Conflict of Interest Statements
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen NC. A unitary model of schizophrenia: Bleuler’s “fragmented phrene” as schizencephaly. Archieves of General Psychiatry. 1999;56:781–787. doi: 10.1001/archpsyc.56.9.781. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Transactions on Medical Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM. Tensorial extensions of independent component analysis for multisubject FMRI analysis. Neuroimage. 2005;25:294–311. doi: 10.1016/j.neuroimage.2004.10.043. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Woolrich MW, Smith SM. Gaussian/Gamma mixture modelling of ICA/GLM spatial maps. Neuroimage. 2003;19:S985. [Google Scholar]
- Bergerbest D, Gabrieli JD, Whitfield-Gabrieli S, Kim H, Stebbins GT, Bennett DA, Fleischman DA. Age-associated reduction of asymmetry in prefrontal function and preservation of conceptual repetition priming. Neuroimage. 2009;45:237–246. doi: 10.1016/j.neuroimage.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder RM, Goldman RS, Robinson D, Reiter G, Bell L, Bates JA, Pappadopulos E, Willson DF, Alvir JM, Woerner MG, Geisler S, Kane JM, Lieberman JA. Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. American Journal of Psychiatry. 2000;157:549–559. doi: 10.1176/appi.ajp.157.4.549. [DOI] [PubMed] [Google Scholar]
- Bluhm RL, Miller J, Lanius RA, Osuch EA, Boksman K, Neufeld RW, Theberge J, Schaefer B, Williamson P. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophrenia Bulletin. 2007;33:1004–1012. doi: 10.1093/schbul/sbm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Human Brain Mapping. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Jeong B, Kim J-W. Dysfunction of the Left Dorsolateral Prefrontal Cortex is Primarily Responsible for Impaired Attentional Processing in Schizophrenia. Psychiatry Investigation. 2008;5:52–59. doi: 10.4306/pi.2008.5.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. American Journal of Psychiatry. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaume C, Guillery-Girard B, Chaby L, Lebreton K, Hugueville L, Eustache F, Fiori N. The time course of repetition effects for familiar faces and objects: an ERP study. Brain Research. 2009;1248:149–161. doi: 10.1016/j.brainres.2008.10.069. [DOI] [PubMed] [Google Scholar]
- Han SD, Nestor PG, Hale-Spencer M, Cohen A, Niznikiewicz M, McCarley RW, Wible CG. Functional neuroimaging of word priming in males with chronic schizophrenia. Neuroimage. 2007;35:273–282. doi: 10.1016/j.neuroimage.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Two-Factor Index of Social Position. Yale Station; New Haven, Conn: 1965. [Google Scholar]
- Jeong B, Wible CG, Hashimoto RI, Kubicki M. Functional and anatomical connectivity abnormalities in left inferior frontal gyrus in schizophrenia. Human Brain Mapping. 2009 doi: 10.1002/hbm.20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang M, Kutas M, Light GA, Braff DL. An event-related brain potential study of direct and indirect semantic priming in schizophrenia. American Journal of Psychiatry. 2008;165:74–81. doi: 10.1176/appi.ajp.2007.07050763. [DOI] [PubMed] [Google Scholar]
- Kubicki M, McCarley RW, Nestor PG, Huh T, Kikinis R, Shenton ME, Wible CG. An fMRI study of semantic processing in men with schizophrenia. Neuroimage. 2003;20:1923–1933. doi: 10.1016/s1053-8119(03)00383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka H, Matsumoto K, Yamazaki H, Sakai H, Miwa S, Yoshida S, Numachi Y, Saito H, Ueno T, Sato M. Lack of repetition priming effect on visual event-related potentials in schizophrenia. Biological Psychiatry. 1999;46:137–140. doi: 10.1016/s0006-3223(98)00330-8. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. Journal of Cognitive Neuroscience. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Dixit NK, Carter CS, Noll DC, Cohen JD. Prefrontal cortex dysfunction mediates deficits in working memory and prepotent responding in schizophrenia. Biological Psychiatry. 2003;53:25–38. doi: 10.1016/s0006-3223(02)01675-x. [DOI] [PubMed] [Google Scholar]
- Pomarol-Clotet E, Oh TM, Laws KR, McKenna PJ. Semantic priming in schizophrenia: systematic review and meta-analysis. British Journal of Psychiatry. 2008;192:92–97. doi: 10.1192/bjp.bp.106.032102. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts SA, Damoiseaux JS, Goekoop R, Barkhof F, Scheltens P, Smith SM, Beckmann CF. Model-free group analysis shows altered BOLD FMRI networks in dementia. Human Brain Mapping. 2009;30:256–266. doi: 10.1002/hbm.20505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser R, Gesierich T, Kaufmann B, Vucurevic G, Hunsche S, Gawehn J, Stoeter P. Altered effective connectivity during working memory performance in schizophrenia: a study with fMRI and structural equation modeling. Neuroimage. 2003;19:751–763. doi: 10.1016/s1053-8119(03)00106-x. [DOI] [PubMed] [Google Scholar]
- Svensen M, Kruggel F, Benali H. ICA of fMRI group study data. Neuroimage. 2002;16:551–563. doi: 10.1006/nimg.2002.1122. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Gunning-Dixon F, Goldman RS, Bates J, Ashtari M, Snyder PJ, Lieberman JA, Bilder RM. Lack of normal association between cerebellar volume and neuropsychological functions in first-episode schizophrenia. American Journal of Psychiatry. 2003;160:1884–1887. doi: 10.1176/appi.ajp.160.10.1884. [DOI] [PubMed] [Google Scholar]
- Tivarus ME, Ibinson JW, Hillier A, Schmalbrock P, Beversdorf DQ. An fMRI study of semantic priming: modulation of brain activity by varying semantic distances. Cognitive and Behavioral Neurology. 2006;19:194–201. doi: 10.1097/01.wnn.0000213913.87642.74. [DOI] [PubMed] [Google Scholar]
- Weems SA, Zaidel E. The effect of response mode on lateralized lexical decision performance. Neuropsychologia. 2005;43:386–395. doi: 10.1016/j.neuropsychologia.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto-Castanon A, LaViolette P, Wojcik J, Gabrieli JD, Seidman LJ. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proceedings of the National Academy of Sciences. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wig GS, Buckner RL, Schacter DL. Repetition priming influences distinct brain systems: evidence from task-evoked data and resting-state correlations. Journal of Neurophysiology. 2009;101:2632–2648. doi: 10.1152/jn.91213.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Liang M, Tian L, Wang K, Hao Y, Liu H, Liu Z, Jiang T. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophrenia Research. 2007;97:194–205. doi: 10.1016/j.schres.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Shu N, Liu Y, Song M, Hao Y, Liu H, Yu C, Liu Z, Jiang T. Altered resting-state functional connectivity and anatomical connectivity of hippocampus in schizophrenia. Schizophrenia Research. 2008 doi: 10.1016/j.schres.2007.11.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.